Abstract
Aims
ENSURE-AF (NCT 02072434) was the largest prospective randomized clinical trial of anticoagulation for cardioversion in atrial fibrillation (AF), which also provides the largest prospective dataset for transoesophageal echocardiography (TOE) prior to cardioversion. This ancillary analysis investigated determinants of TOE-detected left atrium thrombi (LAT) in patients scheduled for electrical cardioversion (ECV).
Methods and results
The ENSURE-AF multicentre PROBE evaluation trial compared edoxaban 60 mg once daily (QD) with enoxaparin/warfarin in 2199 subjects undergoing ECV of non-valvular AF. Patients were stratified by the use of TOE, anticoagulant experience, and selected edoxaban dose. Electrical cardioversion was cancelled or deferred when TOEdetected LAT. In total, 1183 subjects were stratified to the TOE arm and LAT was reported in 91 (8.2%). In univariate analysis, age ≥75 years (26.4% vs. 16.9%, P = 0.0308), lower weight (86.5 ± 15.0 vs. 90.7 ± 18.0 kg, P = 0.0309), lower creatinine clearance (80.1 ± 30.6 vs. 93.2 ± 33.9 mL/min, P = 0.0007), heart failure (59.3% vs. 43.0%, P = 0.0029), and diuretic treatment (53.9% vs. 40.1%, P = 0.0141) were more prevalent in the LAT group. Non-significant trends were seen for higher mean CHA2DS2-VASc score (3.0 ± 1.41 vs. 2.7 ± 1.48, P = 0.0571) and more prevalent anticoagulation use prior to enrolment (60.4% vs. 50.3%, P = 0.0795) in the LAT group. In logistic regression analysis, age (P = 0.0202) and heart failure (P = 0.0064) were independently associated with LAT.
Conclusion
Elective ECV is commonly cancelled or deferred due to TOE-detected LAT in patients with non-valvular AF. Age ≥75 years and heart failure were associated with the presence of LAT.
Keywords: Anticoagulation, Atrial fibrillation, Edoxaban, Electrical cardioversion, Left atrial thrombus, Transoesophageal echochardiography
What’s new?
The prevalence of left atrial thrombi (LAT) was found high in a contemporary population from the largest trial on electrical cardioversion (ECV) of atrial fibrillation.
Age and heart failure, but not the CHA2DS2-VASc score, were predictive of LAT.
Left atrial thrombus was found in some patients despite optimal anticoagulation but this did not appear to necessarily translate to the development of systemic embolism in case of ECV.
Introduction
Potential risk factors for stroke in patients with atrial fibrillation (AF) have been thoroughly studied and are well established.1 Stroke, however, may result from different mechanisms, such as left atrial thrombi (LAT) embolization or local brain thrombosis. Risk factors for LAT formation are not well understood and are not necessarily the same as the risk factors for stroke in patients with AF.2 The theory of endocardial remodelling describes the underlying molecular pathophysiology of atrial thrombogenesis in AF.3 Main factors of this process appear to be oxidative stress and inflammation in the fibrillating atrial tissue, which cause up-regulation of thrombogenic proteins at the endocardial surface. Furthermore, concomitant diseases and conditions such as ageing, arterial hypertension, diabetes mellitus, or congestive heart failure appear to have an important impact. Recognition of these factors is potentially important, especially for patients who are scheduled for cardioversion because the procedure may result in thrombi dislodgement and embolization.4
Multiple studies have reported the incidence of LAT in several clinical settings, including prior to AF cardioversion.5–8 These reports include prospective trials in the setting of cardioversion, as well as single-centre retrospective studies, which found different risk factors for LAT.9,10 The ENSURE-AF study (NCT 02072434) is the largest prospective, multicentre, randomized clinical trial of anticoagulation for cardioversion in non-valvular AF and demonstrated a comparable efficacy and safety of the oral factor Xa inhibitor edoxaban vs. enoxaparin–warfarin.11 This randomized trial also provides the largest prospective dataset for transoesophageal echocardiography (TOE) prior to cardioversion in this clinical setting.
The aim of this ancillary analysis was to investigate clinical determinants of TOE-detected LAT in patients scheduled for electrical cardioversion (ECV) of AF.
Methods
Study design
The ENSURE-AF trial design has been described elsewhere in detail.12 In brief, this was an open-label, randomized, multicentre, multinational, parallel-group study comparing edoxaban 60 mg QD with optimized anticoagulation by enoxaparin–warfarin in subjects with indication for ECV of AF.
The primary efficacy endpoint was the composite of stroke, systemic embolic event, myocardial infarction, and cardiovascular death from randomization until end of study, and the primary safety endpoint was the composite of major and clinically relevant non-major bleeding after TOE- or non–TOE-guided cardioversion in both treatment arms.
The study was conducted in compliance with the protocol, the ethical principles as outlined in the Declaration of Helsinki, the International Conference on Harmonization (ICH) consolidated Guideline E6 for Good Clinical Practice (CPMP/ICH/135/95), and applicable regulatory requirements. The protocol and its amendments were approved by ethics committees or institutional review boards. All patients provided written informed consent prior to participation in the study.
To comply with the Transparency and Openness Promotion Guidelines, data presented in this manuscript can be obtained from the corresponding author.
Patients
Patients were enrolled if they had ongoing non-valvular AF lasting for ≥48 h but ≤12 months, and were scheduled for ECV. Exclusion criteria included recent acute coronary syndrome or coronary intervention, thrombus or mass in the left cardiac chambers, left atrial appendage exclusion, mitral valve rheumatic disease or mechanical heart valve, formal indication for conventional anticoagulation, active or high-risk bleeding, severe renal insufficiency (calculated creatinine clearance [CrCl] <30 mL/min), treatment with dual antiplatelet therapy, or contraindication for anticoagulant agents. The sample size calculation for the main trial has been reported elsewhere, 11 and the present study represents a pre-specified ancillary analysis of those patients with left atrial thrombus present.
At randomization, all subjects were stratified based on the approach to cardioversion (TOE vs. non-TOE). The decision to enrol the patient in the TOE or non-TOE stratum was left to the local investigators.
Treatment and transoesophageal echocardiography
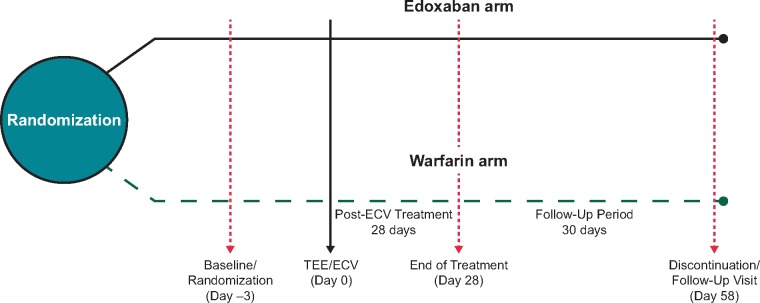
The study treatment with either edoxaban or enoxaparin–warfarin was started on the day of randomization and continued until day of ECV. Patients randomized to edoxaban received 60 mg once daily (QD) except for those with CrCl <50 mL/min, low body weight (≤60 kg), or concomitant use of P-glycoprotein inhibitors (with the exception of amiodarone) who received 30 mg of edoxaban. Patients randomized to warfarin also received enoxaparin until they had international normalized ratio (INR) ≥2.0. Both TOE and ECV were performed on the same day and within 3 days after randomization (Figure 1). Cardioversion was performed at least 2 h after the first dose of edoxaban or enoxaparin-warfarin. If LAT were identified during the TOE procedure, the patient was considered not eligible for subsequent cardioversion and the procedure was cancelled or postponed.
Figure 1.
Study design of the TOE stratum of the ENSURE-AF trial. ECV, electrical cardioversion; TOE, transoesophageal echocardiography.
Statistical analysis
This ancillary analysis included all randomized patients in the TOE stratum who performed the TOE procedure. The data for patients in the non-TOE stratum can be found elsewhere.11 Patients were divided into two groups based on whether thrombi were identified by TOE. Demographic, clinical characteristics and prior anticoagulant treatment were summarized by TOE finding. Numerical variables were described as mean with standard deviation. Categorical variables were described by numbers and percentages of patients in each category. Data were compared using one-way analysis of variance for numerical data and Fisher’s exact test for categorical data. A logistic regression analysis with TOE finding as the response variable was performed. The independent variables included the demographic and baseline characteristics, which showed difference between LAT found and no LAT found groups (P < 0.1). Interaction term was not included in the analysis. Body weight and CrCl were excluded in the analysis due to their high correlations with age. Odds ratios and associated P-values were calculated.
Results
Some patients were excluded from the analysis because they did not receive at least one dose of the study drug (36 patients); converted spontaneously to sinus rhythm (20 patients); discontinued study treatment before TOE procedure (10 patients); or had TOE cancelled due to protocol deviation (1 patient), technical problem (1 patient), it was not tolerated (1 patient), or unknown reason (1 patient). The remaining TOE patients (n = 1113, 97.04%) finally had a TOE performed before ECV, and comprised the population used for the present analysis (Figure 2). The characteristics of these patients compared with those enrolled in the non-TOE stratum of the main study, which was not included in the present analysis, can be found elsewhere.11 LAT was found in 91 patients (8.2%). All these patients had ECV cancelled or delayed until LAT resolution was demonstrated. Demographic data of patients with and without LAT at TOE are shown in Table 1. Univariate analysis showed that age ≥75 years (26.4% vs. 16.9%, P = 0.0308), lower weight (86.5 ± 15.0 vs. 90.7 ± 18.0, P = 0.0309), lower CrCl (80.1 ± 30.6 vs. 93.2 ± 33.9, P = 0.0007), heart failure (59.3% vs. 43.0%, P = 0.0029), and diuretic treatment (53.9% vs. 40.1%, P = 0.0141) were more prevalent in the LAT group. Compared with patients with no LAT, those with LAT had a higher mean CHA2DS2-VASc score (3.0 vs. 2.7), but the differences were not statistically significant (P = 0.057); see Table 1.
Figure 2.
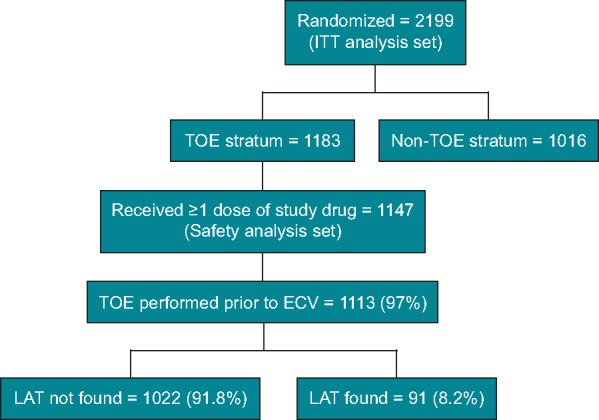
CONSORT figure for the TOE stratum of the ENSURE-AF trial. ECV, electrical cardioversion; ITT, intention-to-treat; LAT, left atrium thrombi; TOE, transoesophageal echocardiography.
Table 1.
Demographic and clinical data of patients with and without LAT detected by TOE performed for electrical cardioversion of AF
| LAT found (n = 91) | No LAT found (n = 1022) | P-value | |
|---|---|---|---|
| Age (year) | |||
| Mean ± SD | 67.3 ± 9.4 | 64.2 ± 10.8 | 0.0095 |
| 65–74 | 33 (36.3) | 357 (34.9) | 0.8191 |
| >65 | 53 (58.2) | 500 (48.9) | 0.1007 |
| ≥75 | 24 (26.4) | 173 (16.9) | 0.0308 |
| Male | 55 (60.4) | 678 (66.3) | 0.2515 |
| Weight (kg) | |||
| Mean ± SD | 86.5 ± 15.05 | 90.7 ± 18.0 | 0.0309 |
| ≤60 kg | 1 (1.1) | 26 (2.5) | 0.7188 |
| BMI (mean ± SD) | 29.9 ± 5.1 | 30.4 ± 5.5 | 0.4312 |
| CHA2DS2-VASc score (mean ± SD) | 3.0 ± 1.4 | 2.7 ± 1.5 | 0.0571 |
| HAS-BLED score (mean ± SD) | 1.0 ± 0.8 | 0.9 ± 0.8 | 0.3713 |
| Current VKA user | 50 (54.9) | 469 (45.9) | 0.1011 |
| Current NOAC user | 14 (15.4) | 181 (17.7) | 0.6669 |
| CrCl (mL/min) | 80.1 ± 30.6 | 93.2 ± 33.9 | 0.0007 |
| TtTR (days) | 6.4 ± 3.3 | 7.6 ± 5.1 | 0.2658 |
| TiTR (% of time) | 74.5 (32.2) | 72.1 (28.9) | 0.6279 |
| TTR (% of time) | 62.1 (34.2) | 57.5 (33.2) | 0.4219 |
| Medical history | |||
| CHF | 54 (59.3) | 439 (43.0) | 0.0029 |
| CAD | 17 (18.7) | 174 (17.0) | 0.6647 |
| Diabetes | 19 (20.9) | 188 (18.4) | 0.5740 |
| Hypertension | 68 (74.7) | 825 (80.7) | 0.1708 |
| Myocardial infarction | 4 (4.4) | 63 (6.2) | 0.6477 |
| Valvular heart disease | 20 (22.0) | 302 (29.5) | 0.1477 |
| Peripheral artery disease | 8 (8.8) | 48 (4.7) | 0.1254 |
| Ischaemic stroke/TIA | 4 (4.4) | 78 (7.6) | 0.3989 |
| Intracranial bleeding | 0 | 1 (0.1) | 1.0000 |
| Life-threatening bleed | 1 (1.1) | 2 (0.2) | 0.2260 |
| AF history | |||
| Paroxysmal (≤7 days) | 17 (18.7) | 233 (22.8) | 0.4321 |
| Persistent (>7 days, <1 year) | 74 (81.3) | 789 (77.2) | |
| Drug treatmenta | |||
| Aspirin | 18 (19.8) | 194 (19.0) | 0.8892 |
| Lipid-modifying agents | 33 (36.3) | 392 (38.4) | 0.7365 |
| Beta blockers | 75 (82.4) | 797 (78.0) | 0.3556 |
| ACEI/ARB | 52 (57.1) | 660 (64.6) | 0.1718 |
| Diuretics | 49 (53.8) | 410 (40.1) | 0.0141 |
| VKA/NOAC | 55 (60.4) | 515 (50.3) | 0.0795 |
P-values are from 1-way ANOVA model for numerical data and Fisher’s exact test for categorical data. Data reported as n (%) unless otherwise noted.
During the 30 days before enrolment.
ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blockers; BMI body mass index; CAD, coronary artery disease; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75, diabetes mellitus, and prior stroke or transient ischaemic attack or thromboembolism, vascular disease, age 65–74 years, sex category; CHF, congestive heart failure; CrCl, creatinine clearance; HAS-BLED, hypertension, abnormal renal and liver function, stroke, bleeding history or disposition, labile INR, elderly, drugs or alcohol; INR, international normalized ratio; LAT, left atrium thrombi; NOAC, non-vitamin K antagonist oral anticoagulant; SD, standard deviation; TIA, transient ischaemic attack; TiTR, time in treatment range; TTR, time in treatment range calculated using the Rosendaal method; TtTR, time to treatment range; TOE, transoesophageal echocardiography; VKA, vitamin K antagonist.
Multivariable analysis showed that only age [odds ratio (OR): 1.055, P = 0.0013] and heart failure (OR: 1.97, P = 0.0064) were independently associated with the detection of LAT on TOE. Age ≥75 years (OR: 2.13, P = 0.0202) and heart failure were associated with the presence of LAT, which was found in 12.2% and 10.9% of patients with these variables, respectively. Left atrium thrombus was found in 6.0%, 7.3%, and 5.4% of patients with no heart failure, age <75 years, or both, respectively. In contrast, LAT was found in 18.7% of patients with heart failure and age ≥75 years (Figure 3).
Figure 3.
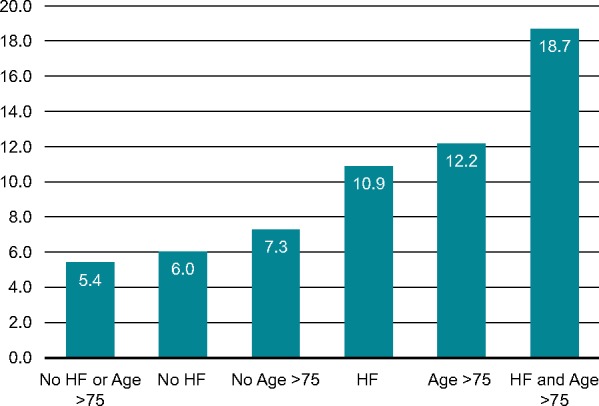
Prevalence of LAT according to age and history of heart failure in TOE stratum of the ENSURE-AF trial. HF, heart failure; LAT, left atrium thrombi; TOE, transoesophageal echocardiography.
Relationship to prior anticoagulation
The prevalence of LAT was not significantly different in relation to the anticoagulation use in the 30 days prior to TOE (Table 2). Compared with patients with no LAT, those with LAT were more frequently treated with vitamin K antagonists in the 30 days prior to enrolment; see Tables 1and2. No statistical differences were seen for mean INR at enrolment (1.6 ± 0.70 vs. 1.5 ± 0.65, P = 0.3381).
Table 2.
Differences in anticoagulation treatment between patients with and without LAT detected by TOE performed for electrical cardioversion of AF
| LAT found (n = 91) | No LAT found (n = 1022) | P-value | |
|---|---|---|---|
| Anticoagulation, n (%) | |||
| Experienced | 69 (75.8) | 745 (72.9) | 0.6221 |
| Naïve | 22 (24.2) | 277 (27.1) | |
| Prior 30-day anticoagulation | 55 (60.4) | 514 (50.3) | 0.0795 |
| VKA, n (%) | 43 (47.3) | 340 (33.3) | 0.0081 |
| NOAC, n (%) | 14 (15.4) | 183 (17.9) | 0.6671 |
| INR at enrolmenta | |||
| Mean ± SD | 1.6 ± 0.7 | 1.5 ± 0.6 | 0.3381 |
| ≥2, n (%) | 22 (27.2) | 213 (23.5) | 0.4955 |
Suboptimal anticoagulation was defined as missing ≥1 dose of NOAC or enoxaparin-warfarin with INR <2.
Denominators are the number of patients who took VKAs.
AF, atrial fibrillation; INR, international normalized ratio; LAT, left atrium thrombi; NOAC, non-vitamin K antagonist oral anticoagulant; SD, standard deviation; TOE, transoesophageal echocardiography; VKA, vitamin K antagonist.
Discussion
The main findings of this analysis, from a contemporary trial population, are the prevalence of LAT in patients scheduled for ECV. Age and heart failure, but not the CHA2DS2-VASc score, were predictive of LAT. Other studies have reported slightly lower and higher rates of LAT in patients scheduled for ECV of AF. However, most of these studies are single-centre retrospective cohorts or had low numbers of patients.5–8,13,14 Other randomized trials—such as the ACUTE trial, which enrolled 1222 patients with AF for ECV—included 549 patients who underwent TOE prior to ECV and found a higher rate of LAT (13.8%).15 However, this trial was terminated almost 20 years ago when the use of oral anticoagulation was less common and the non-VKA oral anticoagulants were not available. More recent trials, such as the X-VeRT trial,16 found LAT in 2.7% in 564 patients who underwent TOE prior to ECV of AF. The population of this trial was slightly younger, with lower prevalence of heart failure and prior stroke than that included in the ENSURE-AF trial, which may explain the three-fold higher prevalence of LAT in the ENSURE-AF trial.16 Data evaluating TOE prior to ECV of AF were obtained from more than 1100 patients from 239 study sites in 19 countries in Europe and the US in the ENSURE-AF trial. Therefore, it provides a contemporary and well-represented sample of the general population of patients scheduled for ECV. In addition, the EMANATE trial,17 which studied 840 patients with TOE prior to ECV of AF, found LAT in 7.3%, a figure similar to the present study. Age (64.5 ± 12.8 and 64.7 ± 12.2 years for heparin/warfarin and apixaban, respectively) and CHA2DS2-VASc score (2.4 ± 1.7 and 2.4 ± 1.7 for heparin/warfarin and apixaban, respectively) were slightly lower in the general population enrolled in the EMANATE trial compared with the ENSURE trial.
Older age and heart failure were the only independent factors associated with LAT in this study. These were also among the most powerful determinants of LAT in a recent meta-analysis in patients with non-valvular AF.7 In contrast, previous stroke is the most powerful risk factor for stroke in the general population of patients with AF. This emphasizes that risk factors for stroke in AF are not necessarily the same as those for LAT development in AF patients scheduled for ECV. Some studies,5,13,14 but not others,18 have found the CHADS2 and CHA2DS2-VASc scores are the best determinants of LAT prior to ECV. Although there was a trend toward higher CHA2DS2-VASc in the present study, this was not statistically significant.
In addition, patients already treated with warfarin in the previous 30 days had significant higher prevalence of LAT than those naïve to it. Several factors could explain this apparent paradox. One reason could be that the decision of enrolment of patients in the TOE or non-TOE strata was left to the investigator; hence, there could be a potential bias that patients with higher perceived risk were placed in the TOE stratum despite prior optimal or close-to-optimal anticoagulation due to the fear of potential LAT. Indeed, patients enrolled in the TOE stratum have significantly higher CHA2DS2-VASc score than those enrolled in the non-TOE stratum.19 This probably was not the case for those not already treated with warfarin—either because of a low-risk profile or first AF episode—who could be equally enrolled in the TOE or non-TOE strata.
Left atrium thrombus was found in some patients despite optimal anticoagulation, as was found in other studies.13,14 This raises concerns about the safety of the non-TOE approach for ECV compared with the TOE approach. Indeed, there were non-significant trends towards lower cardiovascular events in the TOE strata compared with the non-TOE strata despite more cardiovascular risk factors in the former.19
Limitations
The main limitation of this study is that the decision to enrol patients in the TOE or non-TOE strata was left to the investigator. Therefore, the incidence of LAT in patients scheduled for ECV may have been overestimated due to the potential bias of patients with worse risk profile enrolled in the TOE stratum. A second limitation comes from an inconsistent follow-up of INR levels, time in therapeutic range and duration of anticoagulation before patient enrolment. Therefore, this study does not clarify the risk of LAT formation despite proper anticoagulation regime at the time of ECV and is unclear if age and heart failure will be more important determinants. Finally, TOE could have been performed up to 72 h after anticoagulation initiation. This may have resulted in some LATs having resolved prior to the TOE.
Conclusion
Elective ECV is commonly cancelled or deferred due to occurrence of TOE-detected LAT in patients with non-valvular AF. Age ≥75 years and heart failure are associated with the presence of LAT in these patient populations.
Acknowledgements
A.G.’s scientific contribution was supported by the Josef-Freitag-Stiftung Paderborn, Germany.
Funding
This work was supported by Daiichi Sankyo Pharma Development and Daiichi Sankyo Development, Ltd.
Conflict of interest: J.L.M. reports personal fees from Abbott, Bayer, Biotronik, Boston Scientific, Bristol-Myers Squibb, Cardiome, Daiichi Sankyo LivaNova, Medtronic, Pfizer, and Sanofi outside the submitted work. G.Y.H.L.: Consultant for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon and Daiichi Sankyo; and Speaker fees from Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi Sankyo. No fees are directly received personally. H.H. has been a member of the scientific advisory boards and/or lecturer for Boehringer Ingelheim, Bayer, Bristol-Myers Squibb, Pfizer, Daiichi Sankyo, and Cardiome; and has received unconditional research grants through the University of Antwerp from Bracco Imaging Europe and through Hasselt University from Bayer. After 20 June 2017, he did not receive any personal honorarium (cf. President-Elect of European Heart Rhythm Association). A.-A.C. reports receiving research grants from ARS, RESICARD (research nurses), Bayer, and Boehringer Ingelheim; and consultant and lecture fees from Amgen, AstraZeneca, Bayer Pharma, BMS-Pfizer Alliance, Boehringer Ingelheim, Daiichi Sankyo, and Novartis. R.D.C. reports that his institution received research grant support from Boehringer Ingelheim, Bayer, Bristol‐Myers Squibb/Pfizer, Portola, and Roche; and honoraria for lectures and/or consulting from Boehringer Ingelheim, Bayer, Bristol‐Myers Squibb/Pfizer, Daiichi Sankyo, Lilly, AstraZeneca, Merck, and Novartis. J.R.d.G. is a consultant for Atricure, Novartis, and Daiichi Sankyo, and has received research funding outside the scope of this work from Abbot, Boston Scientific, and Medtronic. M.D.E. reports receiving speakers bureau fees from Boehringer Ingelheim and serving as a consultant/advisory board member for Pozen Inc, Eisai, AstraZeneca. Boehringer Ingelheim, ARYx Therapeutics, Pfizer, Sanofi, Bristol-Myers Squibb, Portola, Daiichi Sanko, Medtronics, Merck, Gilead, and Janssen Scientific Affairs. J.-Y.L.H. reports lecture and advisory board fees from Bayer, Boehringer Ingelheim, BMS/Pfizer and Daiichi Sankyo. S.T. has served as a consultant for Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, and Pfizer. J.J. and M.M. are employees of Daiichi Sankyo; S.M.W. was an employee of Daiichi Sankyo at the time of writing. B.M. reports lecture fees from Boehringer Ingelheim and MSD outside the submitted work. A.G. reports personal fees from AstraZeneca, Bayer, Berlin-Chemie, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Medtronic, Pfizer, and Sanofi-Aventis outside the submitted work.
References
- 1. Lip GYH, Freedman B, De Caterina R, Potpara TS.. Stroke prevention in atrial fibrillation: past, present and future. Comparing the guidelines and practical decision-making. Thromb Haemost 2017;117:1230–9. [DOI] [PubMed] [Google Scholar]
- 2. Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA. et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterisation, and clinical implication. Europace 2016;18:1455–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bukowska A, Hammwohner M, Corradi D, Mahardhika W, Goette A.. Atrial thrombogenesis in atrial fibrillation: results from atrial fibrillation models and AF-patients. Herzschr Elektrophys 2018;29:76–83. [DOI] [PubMed] [Google Scholar]
- 4. Lip GY. Cardioversion of atrial fibrillation. Postgrad Med J 1995;71:457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bejinariu AG, Hartel DU, Brockmeier J, Oeckinghaus R, Herzer A, Tebbe U.. Left atrial thrombi and spontaneous echo contrast in patients with atrial fibrillation: systematic analysis of a single-center experience. Herz 2016;41:706–14. [DOI] [PubMed] [Google Scholar]
- 6. Manning WJ, Silverman DI, Gordon SP, Krumholz HM, Douglas PS.. Cardioversion from atrial fibrillation without prolonged anticoagulation with use of transesophageal echocardiography to exclude the presence of atrial thrombi. N Engl J Med 1993;328:750–5. [DOI] [PubMed] [Google Scholar]
- 7. Di Minno MN, Ambrosino P, Dello Russo A, Casella M, Tremoli E, Tondo C.. Prevalence of left atrial thrombus in patients with non-valvular atrial fibrillation. A systematic review and meta-analysis of the literature. Thromb Haemost 2016;115:663–77. [DOI] [PubMed] [Google Scholar]
- 8. Yarmohammadi H, Klosterman T, Grewal G, Alraies MC, Varr BC, Lindsay B. et al. Efficacy of the CHADS(2) scoring system to assess left atrial thrombogenic milieu risk before cardioversion of non-valvular atrial fibrillation. Am J Cardiol 2013;112:678–83. [DOI] [PubMed] [Google Scholar]
- 9. Huang J, Wu SL, Xue YM, Fei HW, Lin QW, Ren SQ. et al. Association of CHADS2 and CHA2DS2-VASc scores with left atrial thrombus with nonvalvular atrial fibrillation: a single center based retrospective study in a cohort of 2695 Chinese subjects. BioMed Res Int 2017;2017:6839589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gunawardene MA, Dickow J, Schaeffer BN, Akbulak RO, Lemoine MD, Nuhrich JM. et al. Risk stratification of patients with left atrial appendage thrombus prior to catheter ablation of atrial fibrillation: an approach towards an individualized use of transesophageal echocardiography. J Cardiovasc Electrophysiol 2017;28:1127. [DOI] [PubMed] [Google Scholar]
- 11. Goette A, Merino JL, Ezekowitz MD, Zamoryakhin D, Melino M, Jin J. et al. Edoxaban versus enoxaparin-warfarin in patients undergoing cardioversion of atrial fibrillation (ENSURE-AF): a randomised, open-label, phase 3b trial. Lancet 2016;388:1995–2003. [DOI] [PubMed] [Google Scholar]
- 12. Lip GY, Merino J, Ezekowitz M, Ellenbogen K, Zamoryakhin D, Lanz H, et al. A prospective evaluation of edoxaban compared to warfarin in subjects undergoing cardioversion of atrial fibrillation: the edoxaban vs. warfarin in subjects undergoing cardioversion of atrial fibrillation (ENSURE-AF) study. Am Heart J. 2015;169: 597–604 e595 [DOI] [PubMed] [Google Scholar]
- 13. Barysiene J, Zebrauskaite A, Petrikonyte D, Marinskis G, Aidietiene S, Aidietis A.. Findings of transoesophageal echocardiogram in appropriately anticoagulated patients with persistent atrial fibrillation prior to planned cardioversion. BMC Cardiovasc Disord 2017;17:67.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bertaglia E, Anselmino M, Zorzi A, Russo V, Toso E, Peruzza F. et al. NOACs and atrial fibrillation: incidence and predictors of left atrial thrombus in the real world. Int J Cardiol 2017;249:179–83. [DOI] [PubMed] [Google Scholar]
- 15. Klein AL, Grimm RA, Murray RD, Apperson-Hansen C, Asinger RW, Black IW. et al. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med 2001;344:1411–20. [DOI] [PubMed] [Google Scholar]
- 16. Cappato R, Ezekowitz MD, Klein AL, Camm AJ, Ma CS, Le Heuzey JY. et al. Rivaroxaban vs. vitamin K antagonists for cardioversion in atrial fibrillation. Eur Heart J 2014;35:3346–55. [DOI] [PubMed] [Google Scholar]
- 17. Ezekowitz MD, Pollack CV, Sanders P, Halperin JL, Spahr J, Cater N. et al. Apixaban compared with parenteral heparin and/or vitamin k antagonist in patients with nonvalvular atrial fibrillation undergoing cardioversion: rationale and design of the EMANATE trial. Am Heart J 2016;179:59–68. [DOI] [PubMed] [Google Scholar]
- 18. Melduni RM, Gersh BJ, Wysokinski WE, Ammash NM, Friedman PA, Hodge DO. et al. Real-time pathophysiologic correlates of left atrial appendage thrombus in patients who underwent transesophageal-guided electrical cardioversion for atrial fibrillation. Am J Cardiol 2018;121:1540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Merino JL, Lip G, De Caterina R, Huber K, Jin J, Melino M. et al. Transesophageal echocardiography-guided strategy vs conventional anticoagulation for cardioversion of atrial fibrillation: an analysis of thromboembolic and bleeding events from the ENSURE-AF study. Circulation 2018;138:A14839. [Google Scholar]