Abstract
Key points
Neurons of the retrotrapezoid nucleus (RTN) and medullary serotonin (5‐HT) neurons are both candidates for central CO2/pH chemoreceptors, but it is not known how interactions between them influence their responses to pH.
We found that RTN neurons in brain slices were stimulated by exogenous 5‐HT and by heteroexchange release of endogenous 5‐HT, and these responses were blocked by antagonists of 5‐HT7 receptors.
The pH response of RTN neurons in brain slices was markedly reduced by the same antagonists of 5‐HT7 receptors.
Similar results were obtained in dissociated, primary cell cultures prepared from the ventral medulla, where it was also found that the pH response of RTN neurons was blocked by preventing 5‐HT synthesis and enhanced by blocking 5‐HT reuptake. Exogenous 5‐HT did not enable latent intrinsic RTN chemosensitivity.
RTN neurons may play more of a role as relays from other central and peripheral chemoreceptors than as CO2 sensors.
Abstract
Phox2b‐expressing neurons in the retrotrapezoid nucleus (RTN) and serotonin (5‐HT) neurons in the medullary raphe have both been proposed to be central respiratory chemoreceptors. How interactions between these two sets of neurons influence their responses to acidosis is not known. Here we recorded from mouse Phox2b+ RTN neurons in brain slices, and found that their response to moderate hypercapnic acidosis (pH 7.4 to ∼7.2) was markedly reduced by antagonists of 5‐HT7 receptors. RTN neurons were stimulated in response to heteroexchange release of 5‐HT, indicating that RTN neurons are sensitive to endogenous 5‐HT. This electrophysiological behaviour was replicated in primary, dissociated cell cultures containing 5‐HT and RTN neurons grown together. In addition, pharmacological inhibition of 5‐HT synthesis in culture reduced RTN neuron chemosensitivity, and blocking 5‐HT reuptake enhanced chemosensitivity. The effect of 5‐HT on RTN neuron chemosensitivity was not explained by a mechanism whereby activation of 5‐HT7 receptors enables or potentiates intrinsic chemosensitivity of RTN neurons, as exogenous 5‐HT did not enhance the pH response. The ventilatory response to inhaled CO2 of mice was markedly decreased in vivo after systemic treatment with ketanserin, an antagonist of 5‐HT2 and 5‐HT7 receptors. These data indicate that 5‐HT and RTN neurons may interact synergistically in a way that enhances the respiratory chemoreceptor response. The primary role of RTN neurons may be as relays and amplifiers of the pH response from 5‐HT neurons and other chemoreceptors rather than as pH sensors themselves.
Keywords: Chemoreceptor, Raphe, Respiration, Ventilation
Key points
Neurons of the retrotrapezoid nucleus (RTN) and medullary serotonin (5‐HT) neurons are both candidates for central CO2/pH chemoreceptors, but it is not known how interactions between them influence their responses to pH.
We found that RTN neurons in brain slices were stimulated by exogenous 5‐HT and by heteroexchange release of endogenous 5‐HT, and these responses were blocked by antagonists of 5‐HT7 receptors.
The pH response of RTN neurons in brain slices was markedly reduced by the same antagonists of 5‐HT7 receptors.
Similar results were obtained in dissociated, primary cell cultures prepared from the ventral medulla, where it was also found that the pH response of RTN neurons was blocked by preventing 5‐HT synthesis and enhanced by blocking 5‐HT reuptake. Exogenous 5‐HT did not enable latent intrinsic RTN chemosensitivity.
RTN neurons may play more of a role as relays from other central and peripheral chemoreceptors than as CO2 sensors.
Introduction
Respiratory motor output is generated by a network of neurons in the caudal brainstem. Arterial is a major factor that determines the rate and depth of breathing, acting indirectly through changes in tissue pH (Pappenheimer et al. 1965; Fencl et al. 1966; Loeschcke, 1982; Nattie, 1999; Nattie & Li, 2012). Peripheral chemoreceptors contribute to the ventilatory response to CO2, but most of the response is mediated by central respiratory chemoreceptors (CRCs) in the brainstem. Identification of specific CRC neurons and/or glia responsible for sensing CO2/pH has been the focus of intense study (Mitchell et al. 1963; Nattie et al. 1991, 1993; Sato et al. 1992; Okada et al. 2002; Mulkey et al. 2004; Richerson, 2004; Richerson et al. 2005; Gourine et al. 2010). Within the medulla, neuronal candidates for CRCs that have been identified include: (1) 5‐HT neurons in the medullary raphe and ventrolateral medulla (VLM) (Richerson, 1995, 2004; Wang et al. 2001; Bradley et al. 2002; Richerson et al. 2005; Teran et al. 2014), and (2) neurons of the RTN that express Phox2b (Pattyn et al. 1999; Mulkey et al. 2004, 2007; Stornetta et al. 2006; Onimaru et al. 2012; Kumar et al. 2015). Phox2b is expressed in a limited subset of neurons, including visceral afferents, the central targets of these afferents, and many pre‐ and postganglionic sympathetic and parasympathetic neurons (Pattyn et al. 1999). Phox2b‐expressing neurons are particularly attractive as CRCs because >90% of patients with congenital central hypoventilation syndrome (CCHS), characterized by severe loss of central chemoreception, have mutations or an increased number of polyalanine repeats in this gene (Amiel et al. 2003; Weese‐Mayer et al. 2003).
The retrotrapezoid nucleus (RTN) has extensive projections to respiratory nuclei (Smith et al. 1989; Rosin et al. 2006) and overlaps with the parafacial respiratory group, which plays a role in respiratory rhythm generation (Onimaru et al. 2008, 2009, 2012; Guyenet & Mulkey, 2010; Pagliardini et al. 2011; Ikeda et al. 2015). The RTN was first identified as being involved in CO2 chemoreception on the basis of focal lesions and neuronal recordings in cats in vivo, although these results were not interpreted as direct evidence that chemoreception resides within the RTN (Nattie et al. 1991, 1993). Phox2b neurons in the RTN were proposed as CRCs when they were found to respond to acidosis with a large increase in firing rate in vivo and in brain slices (Mulkey et al. 2004), and additional data suggest they are critically important for the ventilatory response to hypercapnia in vivo (Kumar et al. 2015). However, to evaluate the relative importance of a neuron as a CRC it is necessary to know the magnitude of its intrinsic (cell‐autonomous) response to changes in pH across a physiological range (i.e. near 7.3) (Richerson et al. 2005). RTN neurons have been reported to have an intrinsic pH response (Onimaru et al. 2012; Wang et al. 2013), but it has not been determined what role synaptic input plays in the overall response. In fact, RTN neurons are highly chemosensitive in vivo (Mulkey et al. 2004), but less so in brain slices (Mulkey et al. 2004). After acute dissociation they are even less sensitive (Wang et al. 2013).
Some 5‐HT neurons lie close to the RTN (Mulkey et al. 2004), and other 5‐HT neurons project to the VLM including the RTN (Ptak et al. 2009; Brust et al. 2014). 5‐HT neurons are highly chemosensitive to acidosis, with a three‐fold average increase in firing rate in response to a decrease in pH from 7.4 to 7.2 in slices (Richerson, 1995; Bradley et al. 2002; Brust et al. 2014), when isolated in cell culture (Wang et al. 2001, 2002; Richerson, 2004; Teran et al. 2014) and after acute dissociation (Corcoran et al. 2009). Given that 5‐HT has been reported to stimulate RTN neurons (Mulkey et al. 2007) via 5‐HT2 and 5‐HT7 receptors (Hawryluk et al. 2012; Hawkins et al. 2015; Shi et al. 2017), it is possible that the response to CO2 of RTN neurons could be due in part to stimulation by 5‐HT released in proportion to the magnitude of acidosis. Here we tested this hypothesis using recordings from mouse RTN neurons in brain slices. We then replicated and extended these results using primary, dissociated cell culture. Our results suggest that at least a subset of RTN neurons owe a substantial component of their chemosensitivity to input from chemosensitive 5‐HT neurons, and may also act as a relay of chemosensory afferents from other CRCs and peripheral chemoreceptors.
Materials and methods
Ethical approval
All procedures and experiments involving mice were carried out with approval of the University of Iowa Institutional Animal Care and Use Committee (approval ref. no. 7111250), and in strict accordance with the recommendations given by Grundy (2015). The investigators understand the ethical principles under which the journal operates and this work complies with the animal ethics checklist. The minimum possible number of animals was used. All mice had free access to food and water. If not otherwise mentioned, animals were killed with an overdose of isoflurane (3–5%).
Reporter mice and genotyping
Experiments were performed using mice genetically expressing the fluorescent reporters tdTomato in Phox2b neurons, enhanced green fluorescent protein (eGFP) in cholinergic neurons, and/or enhanced yellow fluorescent protein (EYFP) in 5‐HT neurons. To generate mice, breeding stocks were obtained for the following four genetic constructs: (1) ePet‐EYFP mice (Scott et al. 2005) that express EYFP under control of the enhancer element for Pet1, a transcription factor found exclusively in 5‐HT neurons (Hendricks et al. 1999) (mice provided by Evan Deneris; Case Western Reserve University, Cleveland, OH, USA); (2) Phox2b‐Cre mice that express Cre recombinase in Phox2b neurons (B6(Cg)‐Tg(Phox2b‐cre)3Jke/J) (mice provided by Paul Gray; Washington University, St Louis, MO, USA); (3) floxed tdTomato mice (B6.Cg‐Gt(ROSA)26Sortm9(CAG‐tdTomato)Hze/J) (https://www.jax.org/strain/007909); and (4) ChAT−eGFP mice that express eGFP in choline acetyltransferase (ChAT)‐expressing neurons (ChATBAC‐eGFP) (https://www.jax.org/strain/007902). Mice from these strains were bred to each other to generate offspring with two to four of these alleles, as indicated. Genotypes were verified in all mice by obtaining tail samples that were digested and subjected to PCR using the following primers:
YFP1 – GAACTCCAGCAGGACCATGT; YFP2 – TATATCATGGCCGACAAGCA. Transgene1 – GGGGTGGGCAAAGATAAAG; Transgene2 – CTGCAGGCTAGAAGCAAATG. YFP product = 219 bp; transgene product = 588 bp.
Phox2b Common – CCGTCTCCACATCCATCTTT; Phox2b WT – GTACGGACTGCTCTGGTGGT; Phox2b Cre – ATTCTCCCACCGTCAGTACG; WT product = 300 bp, mutant product = 600 bp.
tdTomatoWTFor – AAGGGAGCTGCAGTGGAGTA; tdTomatoWTRev – CCGAAAATCTGTGGGAAGTC; tdTomatoMutantFor – CTGTTCCTGTACGGCATGG; tdTomatoMutantRev – GGCATTAAAGCAGCGTATCC; mutant product = 196 bp, WT product = 297 bp.
ChATFor – AGTAAGGCTATGGGATTCATTC; ChATRev – AGTTCACCTTGATGCCGTTC; Internal Positive Control Forward – CAAATGTTGCTTGTCTGGTG; Reverse – GTCAGTCGAGTGCACAGTTT; Transgene product = 600 bp; gontrol product 200 bp.
Agarose gel electrophoresis was used to detect PCR product of the expected sizes.
Brain slices and cell culture
For brain slices, juvenile mice (13–21 days old) of the appropriate genotype were killed by decapitation and their brains rapidly removed. A vibratome was used to make 200‐μm‐thick transverse slices from the medulla near the caudal pole of the VII nucleus. Verification that slices were prepared at the correct level was done by identifying VII motor neurons and RTN neurons using fluorescence microscopy to visualize the expected fluorophores.
To prepare cell cultures, tissue was harvested from the medulla of neonatal mice (postnatal days P1–P4) of the desired genotype. Pups were decapitated, their brainstems were rapidly removed, and the tissue was immersed in sterile‐filtered, ice‐cold HEPES buffer containing (in mm): NaCl 130, KCl 4, MgCl2 1, CaCl2 1.5, HEPES 10, dextrose 10 and NaOH 3. A region of tissue was prepared manually with a scalpel blade by making a transverse cut near the pontomedullary junction and another one approximately 2 mm more caudally. From this region a cut in the horizontal plane was made to isolate the ventral medulla including the RTN, raphe pallidus, raphe magnus, parapyramidal region, and parts of the raphe obscurus and VII motor nucleus (Fig. 1). This tissue was digested with papain in HEPES buffer for 30 min, then washed with complete MEM (SAFC Biosciences, Lenexa, KS, USA) with 0.15% trypsin inhibitor and 0.15% bovine serum albumin. Digested tissue was triturated with a fire‐polished Pasteur pipette, and plated as a cell suspension (of neurons and glia) onto 12 mm round coverslips coated with poly‐l‐ornithine. After 5–7 days, when a confluent glial bed had formed, cultured cells were fed with Neurobasal medium with 2% B‐27 supplement (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) containing cytosine arabinoside (1 μm) to suppress glial growth. At 21 days in vitro, and thereafter as needed, they were fed with half medium changes (49.5% Neurobasal medium, 49.5% complete MEM, 1% B‐27 supplement). Cultured cells were allowed to mature for at least 20 days prior to using them for experiments (Cerpa et al. 2017).
Figure 1. Characterization of Phox2b‐Cre::Floxed‐tdTomato mice and dissection for cell culture.
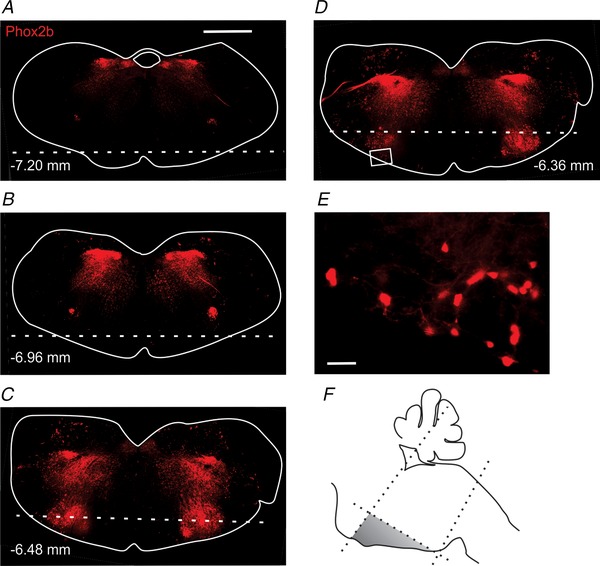
A–D, distribution of Phox2b neurons (red) in transverse slices. Tissue ventral to the dotted lines was harvested for culture. Numbers in each panel denote distance from Bregma. E, high‐power view of the inset in D. F, schematic of scalpel cuts made to tissue during dissection. Scale bars: 1 mm (A), 50 μm (E).
Some cultured cells were treated with p‐chlorophenylalanine (PCPA), an antagonist of tryptophan hydroxylase, to deplete 5‐HT prior to recording. PCPA (Sigma Aldrich, St Louis, MO, USA) was dissolved in culture medium to a concentration of 100 μm and added to culture plates to a final concentration of 10 μm 24 h prior to recording. Control cells were treated with an equal volume of culture medium without PCPA. PCPA was included in artificial cerebrospinal fluid (aCSF) for recordings from neurons if it was present in culture medium.
Electrophysiology
Coverslips plated with cultured cells were transferred to a recording chamber on the stage of an inverted Axiovert 100 or an upright Axioskop FS2 microscope (Carl Zeiss USA, Thornwood, NY, USA). Brain slices were transferred to a recording chamber on an upright Axioskop FS2 microscope, and held down with nylon mesh. Cultures and brain slices were both superfused with aCSF containing (in mm): NaCl 124, KCl 3, NaHCO3 26, NaH2PO4 1.3, CaCl2 2, MgCl2 2 and dextrose 10, maintained at room temperature. The aCSF was continuously bubbled with 95% O2/5% CO2, resulting in a pH of 7.4. Acidosis was induced by bubbling the same solution in a separate reservoir with 9% CO2/91% O2, resulting in a pH of ∼7.2. Changes in pH were made by switching the superfusate rapidly between reservoirs. For all recordings, aCSF contained blockers of fast synaptic transmission [100 μm picrotoxin, 50 μm (±)‐2‐amino‐5‐phosphonopentanoic acid (AP‐5) and 10 μm 6‐cyano‐7‐nitroquinoxaline‐2,3‐dione (CNQX)]. The GABAB receptor antagonist CGP‐55845 (1 μm) was also included in aCSF for brain slice recordings.
For recordings from brain slices, RTN neurons were identified based on their location ventral and ventromedial to the VII motor nucleus, expression of red fluorescence (Phox2b‐tdTomato) and absence of green fluorescence (ChAT−GFP). For recordings from cell culture, RTN neurons constituted most of the neurons that expressed red, but not green, fluorescence that were included within the region of dissection, other than 22% of neurons with that pattern that were also immunoreactive for tyrosine hydroxylase (TH; see below).
The gramicidin perforated‐patch technique was used for recordings from cell culture (Ebihara et al. 1995), whereas whole‐cell recordings were used for brain slices. Patch clamp electrodes (7–12 MΩ; borosilicate glass) were pulled on a micropipette puller (Model #P‐97; Sutter Instrument Co., Novato, CA, USA) and filled with intracellular solution containing (in mm): 135 KOH, 135 methanesulfonic acid, 10 KCl, 5 HEPES and 1 EGTA (pH 7.2; osmolarity 275 ± 5 mOsm l–1). Recordings were performed with a Multiclamp 700B microelectrode amplifier (Molecular Devices, Sunnyvale, CA, USA) and data were collected using a Digidata 1440A acquisition system and PClamp software (Molecular Devices). All recordings were made in current clamp mode, typically with injection of constant current as necessary to establish a stable baseline firing rate between 0.1 and 2 Hz. The amount of current injection was then kept constant for the rest of the recording. Chemosensitivity was quantified by measuring the change in firing rate in response to an increase in CO2 from 5% to 9% (pH 7.4 to ∼7.2). RTN neurons in culture were classified as chemosensitive if they responded to this change in CO2/pH with an increase in firing rate of at least 20%, or by at least 0.2 Hz if baseline firing rate was less than 1 Hz.
Measurement of 5‐HT levels in vivo and in culture
CMA 7 microdialysis probes (2 mm diameter, CMA Microdialysis, Solna, Sweden) were implanted stereotaxically using sterile technique under anaesthesia (ketamine 100 mg kg−1, xylazine 10 mg kg−1; i.p.) targeting the amygdala, a region known to receive heavy innervation from 5‐HT neurons. Although the amygdala receives projections from a different subset of 5‐HT neurons than those that project to the RTN, all 5‐HT neurons express the same 5‐HT transporter, and thus undergo heteroexchange release (Rudnick & Wall, 1992; Rudnick, 2002) in response to the same stimuli. Due to the small size of the medulla and the loss of critical functions if there was damage to that area of the brain, the amygdala was used as a surrogate to measure heteroexchange release of 5‐HT. Implanted animals were placed in a CMA 120 freely moving collection system (CMA Microdialysis) and allowed to recover for at least 20 h. Basal monoamine dialysate samples were collected the following day every 30 min at a flow rate of 0.8 μl min−1 for 3 h in a refrigerated fraction collector (CMA 470, Harvard Apparatus, Holliston, MA, USA). 3,4‐Methylenedioxymethamphetamine (MDMA) 30 mg kg−1 was injected i.p. and microdialysate samples were collected for an additional 3 h. Microdialysis experiments were done at the same time of day for all animals to control for diurnal monoamine fluctuations. At the completion of microdialysis experiments, mice were killed with 5% isoflurane and their brain was extracted to confirm probe placement by cryostat sectioning and cresyl violet staining. Only data from accurate placements were used. The concentration of 5‐HT in the microdialysate was determined using a high‐performance liquid chromatography (HPLC) system (HTEC‐500, Eicom, Kyoto, Japan) coupled with an electrochemical detector (ECD) and autosampler (AS‐700, Eicom, San Diego, CA). A graphite working electrode was used (WE‐3G) and the applied voltage to the conditioning cell was +400 mV. Microdialysate samples were automatically injected into a CA‐ODS pre‐column and PP‐ODSII column (Eicom). The column temperature was set at 25°C and elution was performed with a mobile phase composed of 1.5% methanol in 0.1 m phosphate buffer (pH 5.4) containing 50 mg l−1 EDTA‐2Na and 500 mg l−1 sodium dodecylsulfate, at a flow rate of 500 μl min−1. The retention time of 5‐HT was ∼5.3 min. Concentrations were determined from calibration curves using a concentration range of 0.01–100 pg μl−1.
To measure 5‐HT release in culture, coverslips plated with cells cultured from the ventromedial medulla as described previously (Wang et al. 1998) were placed in 24‐well culture plates and rinsed three times with 0.5 ml aCSF containing (in mm): NaCl 124, KCl 3, NaHCO3 26, NaH2PO4 1.3, CaCl2 2, MgCl2 2 and dextrose 10 at room temperature and bubbled with 5% CO2 to a pH of 7.4. After the third rinse, aCSF was replaced with 100 μm or 500 μm MDMA in 200 μl aCSF or an equal volume of aCSF without MDMA. After 1 h of incubation at 37°C, aCSF was collected into vials and filtered through 0.22 μm filters. Aliquots were spiked with internal standard (isoproterenol) and vortexed with 30% perchloric acid to a final concentration of 0.2 m. Aliquots were placed on ice for 30 min and centrifuged at 14 000 g for 15 min at 4°C. The resultant supernatants were mixed with 1 m sodium acetate to adjust the pH to 3.0 and transferred to a 96‐well plate within a 4°C autosampler. Culture aCSF samples (12 μl) were automatically injected into a CA‐ODS pre‐column and a SC‐3ODS column (Eicom) and eluted with mobile phase (80% 0.1 m citrate‐acetate buffer, pH 3.5, 20% methanol, with 220 mg l−1 sodium octane sulfonate and 5 mg ml−1 EDTA) at a flow rate of 340 μl min−1. The retention time of 5‐HT was ∼17.6 min. 5‐HT concentrations were determined from calibration curves with a concentration range of 1–100 pg μl−1 and are presented as the percentage change from control. Statistical comparisons among groups were carried out with a Kruskal–Wallis one‐way ANOVA on ranks with a Dunn's multiple comparisons test (significance level set at P < 0.05).
Whole animal plethysmography
C57BL/6 mice underwent standard open‐flow (200 ml min−1), whole‐body plethysmography as previously described (Hodges et al. 2008). The plethysmograph chamber was a commercially available model (Buxco, Wilmington, NC, USA), but the remainder of the plethysmography equipment was custom‐designed and built (Kim et al. 2018). The chamber was maintained at 30°C with a heat lamp and a feedback controller (TCAT‐2AC; Physitemp Instruments, Clifton, NJ, USA). Body temperature was recorded by telemetry probes (IPTT‐300; BMDS, Seaford, DE, USA) inserted into the peritoneal cavity via a midline incision using sterile technique at least 3 days before experiments. Surgery was performed under isoflurane anaesthesia (1–2%) with the level assessed by response to toe pinch. The procedure typically lasted less than 1 min including stapling of the incision. All data were acquired with custom‐written MATLAB software that was also used to calculate breathing frequency, tidal volume and minute ventilation using established methods (Drorbaugh & Fenn, 1955). The protocol consisted of at least 20 min in 0% CO2, 50% O2, balance N2 followed by at least 5 min each of exposure to 5% and 7% CO2 with 50% O2 and balance N2. At 0% CO2, 15 min was allowed for mice to adjust to the chamber before gathering data. Under each condition, at least 90 s of data were gathered while mice were awake but sitting quietly without chewing, licking, scratching, walking or exhibiting other behaviours that disrupt normal eupnoea. Mice were then returned to their home cage for at least 3 h with access to food and water, after which they were given an injection of ketanserin (10 mg kg−1 , i.p.). After waiting 15 min, they were placed in the plethysmograph and the protocol was repeated to measure the response to hypercapnia. Data were analysed using MATLAB and GraphPad Prism 7 software (La Jolla, CA, USA). Ventilation under each condition was normalized to the value for each animal at baseline without drug at 0% CO2.
Anatomy and imaging
To characterize the anatomy of RTN and 5‐HT neurons, adult mice of the appropriate genotype were deeply anaesthetized with ketamine‐xylazine and perfused transcardially first with phosphate buffered saline (PBS) containing (in mm): 137 NaCl, 2.7 KCl, 10 Na2HPO4 and 1.8 KH2PO4, and then with 4% paraformaldehyde in phosphate buffer (PB) containing (in mm): 80.4 Na2HPO4 and 21.8 NaH2PO4. Paraformaldehyde was diluted from methanol‐free 16% ampules (ThermoFisher Scientific, Rockford, IL, USA). Brains were removed, post‐fixed by immersion in 4% paraformaldehyde in PB overnight, and cryoprotected in 30% sucrose prior to slicing with a cryostat (Leica CM 3050 S, Buffalo Grove, IL, USA). Tissue slices (35 μm thick) were mounted directly on microscope slides, washed briefly with PB and mounted with Vectashield hard‐set medium with DAPI (Vector Labs, Burlingame, CA, USA). They were imaged with a confocal microscope (TCS SP5 II, Leica, Buffalo Grove, IL, USA) to visualize tdTomato, GFP, YFP and/or DAPI.
In the rostral medulla of Phox2b‐Cre::Floxed‐tdTomato::ChAT−eGFP reporter mice, red fluorescent neurons were found only in those regions where Phox2b is known to be expressed at some point during embryonic or postnatal life (Pattyn et al. 1999; Dauger et al. 2003; Stornetta et al. 2006; Kang et al. 2007; Dubreuil et al. 2008), including the RTN, facial motor nucleus (VII), nucleus tractus solitarius (NTS), nucleus ambiguus pars compacta (NAc) and C1 adrenergic group (Fig. 1). Both RTN neurons and nearby motor neurons expressed tdTomato, but could be differentiated from each other as motor neurons also expressed eGFP. RTN neurons were more ventral and ventromedial, and were concentrated near the caudal pole of the VII nerve nucleus (Dubreuil et al. 2008).
Photomicrographs were taken of each neuron after patch clamp recording, including high power (40×) differential interference contrast (DIC) and fluorescence images, and for brain slice recordings low power (4×) brightfield images to document anatomical location. Some neurons recorded in acute brain slices were filled with biocytin during whole‐cell recording. They were transferred to cold 4% paraformaldehyde in PB overnight and stained with Cy5‐conjugated streptavidin (Jackson ImmunoResearch, West Grove, PA, USA). They were mounted with Vectashield hard‐set medium with DAPI. Cultured cells were transferred on coverslips to cold 4% paraformaldehyde in PB for 2–12 h. They were washed briefly with PBS prior to mounting with Vectashield hard‐set medium with DAPI. Cultured cells and neurons in brain slices were imaged using confocal microscopy to visualize Cy5, tdTomato and GFP.
Some RTN neurons have been described as non‐chemosensitive (Mulkey et al. 2004); however, catecholaminergic Phox2b neurons in the VLM are also reported to be non‐chemosensitive (Lazarenko et al. 2009). To discriminate between these two cell types in experiments studying non‐chemosensitive RTN neurons, cultured cells were fixed as described above, and immunostained for TH using a sheep primary anti‐TH (Novus Biologicals, Littleton, CO, USA), and a donkey anti‐sheep IgG secondary antibody conjugated to Cy5 (Jackson ImmunoResearch). Cells were identified based on their fluorescence and location, and assessed with confocal microscopy for the presence of Cy5. Immunohistochemistry revealed that neurons with TH immunoreactivity constituted 22% of Phox2b+/ChAT− neurons in our cultures.
Drug sources
CNQX, SB258719, SB269970, paroxetine maleate and CGP‐55845 were obtained from Bio‐Techne Corp. (Minneapolis, MN, USA). MDMA was obtained from Cayman Chemical (Ann Arbor, MI, USA). dl‐AP5 and serotonin hydrochloride were obtained from both Bio‐Techne Corp and Sigma‐Aldrich. All other drugs and chemicals were obtained from Sigma‐Aldrich. Stock solutions were made for SB258719, SB269970 and ketanserin using DMSO at the minimum volume possible, and DMSO was added to all control solutions at the same final concentration.
Analysis and statistics
Firing rate and pH were averaged in 10 s bins and plotted versus time while recording in real time, and again offline, using software custom written using Visual Basic. The response of neurons to acidosis and to pharmacological agents was calculated by comparing the mean firing rate for all except the first 1 min under each condition using a plot template developed for Origin Pro 7.0 (OriginLab, Northampton, MA, USA). The duration of exposure to each condition was decided based on whether firing rate had reached steady state. In a minority of cases, cells were exposed to acidosis more than once during exposure to drug and during wash‐out to reach a steady state, and in those cases the last exposure to acidosis was used to calculate the response. When that was the case, the responses changed consistent with gradual wash in or wash out of the drug being tested so the last exposure was a more accurate representation of the drug effects. In some cases, neurons were exposed to drugs more than once (e.g. Fig. 3 A), but in those cases only the first exposure to drug and wash out were used to calculate the summary data. Data were graphed and tested statistically using GraphPad Prism 7. Except where noted, data were analysed using a two‐way ANOVA with repeated measures and the Holm–Sidak test for multiple comparisons, or a paired two‐tailed t test, as appropriate. Prism does not report P values that are less than 0.0001, so those are expressed as P < 0.0001. Data expressed as X ± Y are mean ± SD. Error bars on plots are standard error of the mean.
Figure 3. In brain slices, RTN chemosensitivity was decreased by the 5‐HT7 receptor antagonist SB269970.
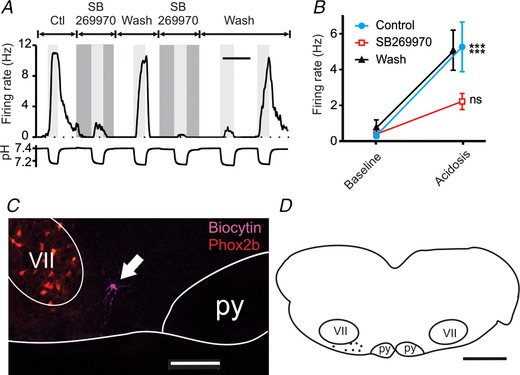
A, chemosensitivity of an RTN neuron in a brain slice was reversibly inhibited by the 5‐HT7 receptor antagonist SB269970 (10 μm). B, summary of responses to SB269970, which were consistent for RTN neurons (n = 7). The block of chemosensitivity by SB269970 was reversible in all neurons tested. C, example of a chemosensitive RTN neuron labelled during recording with biocytin (magenta). VII = facial motor nucleus, py = pyramidal tract. Phox2b (red). D, map of pH‐sensitive neurons inhibited by SB269970 showing their location in the RTN. For differences between firing rate at pH 7.4 (baseline) and pH ∼7.2 (acidosis): *** P < 0.001; ns, not significant. Scale bars: 10 min (A), 200 μm (C), 1 mm (D).
Results
In brain slices, 5‐HT neurons project to and stimulate Phox2b neurons
It has previously been shown that medullary 5‐HT neurons project to the RTN (Rosin et al. 2006; Mulkey et al. 2007; Brust et al. 2014). In Phox2b‐Cre::Floxed‐tdTomato::ePet‐EYFP::ChAT−eGFP mice, in which 5‐HT neurons are labelled with EYFP, Phox2b neurons are labelled with tdTomato, and cholinergic neurons are labelled with eGFP, there were abundant EYFP processes within the RTN near tdTomato+/eGFP− neurons (Fig. 2 A and B). Thus, the anatomical substrate exists for 5‐HT neurons to communicate with Phox2b RTN neurons through either synaptic or paracrine mechanisms.
Figure 2. In brain slices, RTN neurons are stimulated by 5‐HT neuron input.
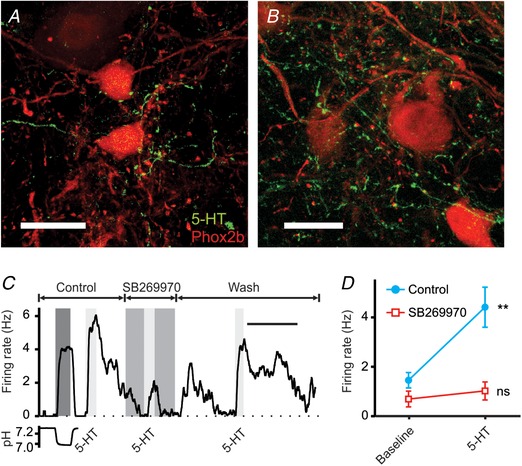
A and B, confocal z‐stack images of the VLM at the level of the caudal pole of the VII nucleus showing neurons in the region of the RTN that were Phox2b‐positive (red) and ChAT‐negative (channel not shown) with abundant 5‐HT neuron processes in close proximity (green). There were many en passant swellings and terminal boutons typical of neurotransmitter release sites. Scale bars: 20 μm. C, example of an RTN neuron that was chemosensitive (first stimulus) and also was stimulated by 5‐HT (10 μm). The response to 5‐HT was decreased by SB269970 (10 μm). D, summary of responses to 5‐HT and block by SB269970 (n = 9). For differences between baseline and 5‐HT: ** P = 0.002; ns, not significant. Scale bar: 10 min (C).
In brain slices, chemosensitive RTN neurons are stimulated by 5‐HT (Mulkey et al. 2007; Hawryluk et al. 2012; Hawkins et al. 2015), and this response is blocked by ketanserin, an antagonist of 5‐HT2 and 5‐HT7 receptors (Mulkey et al. 2007). RTN neurons are also stimulated by 5‐carboxamidotryptamine (5‐CT; 5 μm), a 5‐HT7 receptor agonist, and the response to 5‐CT is blocked by SB258719 (10 μm), a 5‐HT7 receptor antagonist (Hawkins et al. 2015). We performed whole‐cell patch clamp recordings from RTN neurons under direct visualization in brain slices prepared from Phox2b‐Cre::Floxed‐tdTomato::ChAT−eGFP mice. As above, RTN neurons were identified based on their location, their expression of tdTomato but not eGFP, and an increase in firing rate in response to acidosis (pH 7.4 to 7.2). Bath application of 5‐HT (10 μm) stimulated chemosensitive Phox2b+/Chat− neurons in the RTN, and this response was blocked by a third 5‐HT7 receptor antagonist, SB269970 (10 μm) (Fig. 2 C and D; n = 9).
SB258719 and SB269970 are both competitive antagonists of recombinant 5‐HT7 receptors (Romero et al. 2006), and are regarded as selective for 5‐HT7 receptors. SB258719 has no previously reported off‐target effects, whereas SB269970 does have low‐affinity antagonist activity at the α2‐adrenergic receptor (Foong & Bornstein, 2009). The results of Hawkins et al. (2015) with SB258719, Mulkey et al. (2007) with ketanserin, and those presented here with SB269970 are consistent with chemosensitive RTN neurons in brain slices being stimulated by 5‐HT acting on 5‐HT7 receptors.
In brain slices, RTN chemosensitivity was decreased by SB269970
It has been reported that RTN neurons have an intrinsic response to acidosis (Mulkey et al. 2004; Onimaru et al. 2012; Wang et al. 2013), but it is not clear whether the very large changes in firing rate induced in RTN neurons by changes in pH in vivo are entirely cell‐autonomous. In fact, the response of RTN neurons in brain slices is considerably smaller than their response in vivo (Mulkey et al. 2004). After acute dissociation their response is even smaller (Wang et al. 2013). For example, the firing rate of RTN neurons in vivo increases from approximately 0.5 to 8 Hz when end‐tidal CO2 increases from 5% to 8% (causing a decrease of 0.2 pH units) (Mulkey et al. 2004). In brain slices, a decrease of 0.2 pH units leads to an increase in firing rate from approximately 0.5 to only 1.5 Hz (Mulkey et al. 2004). After acute dissociation, a decrease of 0.2 pH units leads to an increase in firing rate from 2.8 to only 3.8 Hz in one group of RTN neurons and from 4.5 to 5.5 Hz in another (Wang et al. 2013). With greater physical isolation of RTN neurons, progressively larger changes in pH have been used to induce a measurable response. For example, acutely dissociated neurons have been stimulated by changing pH from 8.0 to 7.0 (Wang et al. 2013), which is much larger than the 0.1 pH units needed to strongly stimulate breathing in vivo (Pappenheimer et al. 1965; Fencl et al. 1966). These data suggest an alternative hypothesis that the pH response of RTN neurons is due in part to extrinsic effects.
We examined whether the hypercapnic response of RTN neurons is due in part to stimulation by 5‐HT released from chemosensitive serotonergic neurons. To test this, whole‐cell patch clamp recordings were made from RTN neurons in brain slices prepared from Phox2b‐Cre::Floxed‐tdTomato::ChAT−eGFP mice after blocking ionotropic glutamate, glycine and GABA receptors, and metabotropic GABAB receptors. Chemosensitivity was quantified by measuring the change in firing rate in response to hypercapnic acidosis. As previously reported from rat brain slices (Mulkey et al. 2004, 2007; Hawryluk et al. 2012; Hawkins et al. 2015), a subset of Phox2b RTN neurons were sensitive to acidosis, with an increase in CO2 from 5% to 9% (pH 7.4 to ∼7.2) causing an increase in firing rate from 0.73 ± 0.77 to 5.87 ± 3.40 Hz (n = 16) (Fig. 3 A and B). The response of these neurons was larger than previously reported for RTN neurons in brain slices, possibly because the age of the mice used here (P16.0 ± 1.5) was greater than that used previously for rats (P7 to P10; or P7 to P12) (Mulkey et al. 2004, 2007; Hawryluk et al. 2012; Hawkins et al. 2015), and the hypercapnic ventilatory response increases over this age range in rodents in vivo, as does chemosensitivity of 5‐HT neurons in vitro (Wang & Richerson, 1999; Cerpa et al. 2017).
To determine whether chemosensitivity of RTN neurons in brain slices was dependent on 5‐HT receptor activation, the response to acidosis was first examined in control aCSF, and then in aCSF with SB269970 (10 μm). SB269970 reduced chemosensitivity of RTN neurons in brain slices (Fig. 3 A and B). For this group of neurons (n = 7), firing rate increased from 0.33 ± 0.44 to 5.28 ± 3.68 Hz in response to acidosis in control aCSF (P = 0.0002). In aCSF with SB269970, firing rate was 0.39 ± 0.73 Hz at pH 7.4 and 2.22 ± 1.19 Hz at pH ∼7.2 (P = 0.14). The response to acidosis in SB269970 was 37% of the response in control aCSF (P = 0.009) (Fig. 3 B). The decrease in the response to acidosis by SB269970 was reversible in all neurons tested.
The locations of a subset of RTN neurons were documented either by biocytin injections (Fig. 3 C) or by brightfield images of the electrode tip, and are shown in Fig. 3 D. All recordings of RTN neurons in slices were in approximately this same region. These locations coincided with those previously described for chemosensitive RTN neurons (Mulkey et al. 2004).
The interaction between 5‐HT and RTN neurons was recapitulated in cell culture
Cell culture has advantages for studying neuronal electrophysiology compared to brain slices, such as lack of recent traumatic and ischaemic injury (Richerson & Messer, 1995), more stable and longer duration patch clamp recordings, ability to study neurons that are older and more developmentally mature (Cerpa et al. 2017), and better long‐term control of experimental conditions such as prolonged or repeated exposure to drugs. However, neuronal properties can sometimes be altered in cell culture, making it important to verify that the neuronal properties to be studied are comparable to those in brain slices.
Primary, dissociated cell cultures were prepared from the rostral ventral medulla of Phox2b‐Cre::Floxed‐tdTomato mice. Mice also carried the ChAT−eGFP allele, and sometimes ePet‐EYFP. These reporters allowed identification of Phox2b+ neurons, while permitting differentiation of motor neurons from the VII motor nucleus and the nucleus ambiguus. Those regions are the only ones included in our dissections for culture that contain Pho2b+, other than the C1 area that contains catecholaminergic neurons. Immunohistochemistry revealed that neurons with TH immunoreactivity constituted only 22% of Phox2b+/ChAT− neurons in our cultures. These catecholaminergic neurons have previously been reported to be not chemosensitive (Mulkey et al. 2004). The majority of neurons in our cultures that were Phox2b+/ChAT− were chemosensitive (56%) and were therefore identified as being from the RTN. In the following text, Phox2b+/ChAT− neurons in culture that were chemosensitive are referred to as ‘RTN neurons’.
Primary cell cultures contained neurons with four patterns of fluorescence: tdTomato+/GFP−; tdTomato+/GFP+; EYFP+; or not fluorescent. None of the EYFP+ neurons were tdTomato+ or GFP+. There were processes from some EYFP+ neurons that coursed along the processes of tdTomato+/GFP− neurons and formed close appositions (Fig. 4 A and B), suggesting that serotonergic neurons had synaptic connections with, or paracrine release sites near, RTN neurons in culture as they do in vivo (Fig. 2) (Rosin et al. 2006; Mulkey et al. 2007; Brust et al. 2014).
Figure 4. The interaction between 5‐HT and RTN neurons was recapitulated in cell culture.
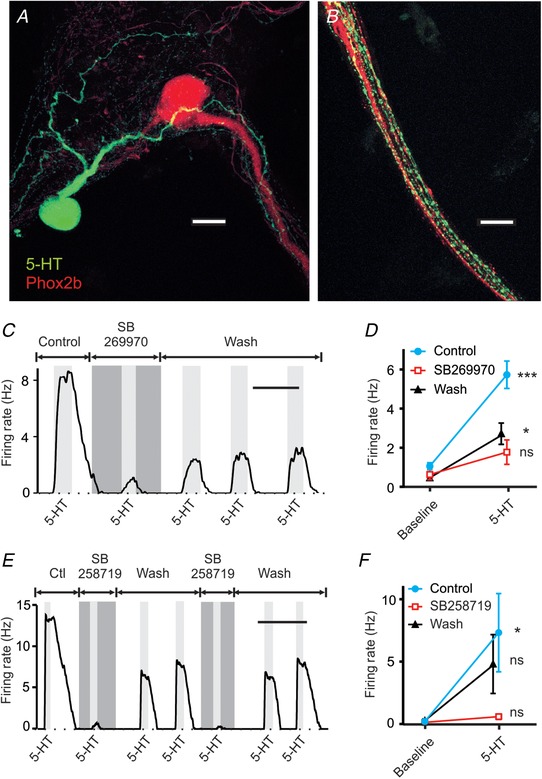
A, a 5‐HT neuron (green) has a large process which, along with multiple other neurites, forms close appositions with the major dendrite of a nearby RTN neuron (red). B, a bundle of processes from RTN neurons (red) is closely associated with multiple processes from 5‐HT neurons (green). C, a different cultured RTN neuron responded to exogenous 5‐HT (10 μm), and this was reversibly blocked by SB269970 (10 μm). D, summary data showing consistency of block of the 5‐HT response by SB269970 in culture (n = 5). E, an RTN neuron in cell culture responded to exogenous 5‐HT (10 μm), and the response was blocked by SB258719 (10 μm). F, summary data showing consistency of block of the 5‐HT response in culture by SB258719 (n = 5). For differences between 5‐HT response and baseline: * P < 0.05; *** P < 0.0003; ns, not significant. Scale bars: 20 μm (A and B), 10 min (C), 20 min (E).
When 5‐HT (10 μm) was bath applied to cultured RTN neurons, it caused depolarization and a large increase in firing rate (Fig. 4 C–F). In all RTN neurons in which it was tested (n = 5), the response to 5‐HT (10 μm) was blocked by SB269970 (10 μm) (Fig. 4 C and D). In control aCSF, firing rate increased from 1.03 ± 0.40 to 5.71 ± 1.56 Hz in response to 5‐HT (P = 0.0003). When SB269970 was added to the bath solution firing rate was 0.61 ± 0.60 Hz at baseline and 1.75 ± 1.39 Hz in 5‐HT (P = 0.33). This response to 5‐HT in aCSF with SB269970 was 35.0 ± 14.4% of the response in control aCSF (P = 0.0009).
Exogenous 5‐HT was also applied to RTN neurons in culture (n = 5) in the presence and absence of SB258719 (Fig. 4 E and F). Firing rate in control aCSF increased from 0.17 ± 0.21 to 7.61 ± 7.33 Hz in response to 5‐HT (P = 0.03). As was reported in brain slices (Hawkins et al. 2015), the response to 5‐HT was blocked by SB258719 (10 μm) (0.09 ± 0.18 to 0.57 ± 0.52 Hz; P = 0.99), with a decrease in the response to 5‐HT to 8.26 ± 3.41% of the control response (P = 0.03). The decrease in the response to 5‐HT by SB258719 was reversible in all neurons tested.
These results demonstrate that the anatomical and physiological interactions of RTN neurons and 5‐HT neurons were recapitulated in culture, and the response to 5‐HT had similar pharmacology as in brain slices.
Release of endogenous 5‐HT from serotonergic neurons activates RTN neurons in slices and culture
It has previously been shown that MDMA causes heteroexchange release of 5‐HT via 5‐HT transporter reversal (Rudnick & Wall, 1992; Rudnick, 2002). To confirm that MDMA causes release of 5‐HT in the mouse brain in vivo, a microdialysis catheter was placed in the amygdala, a region that receives heavy innervation from 5‐HT neurons, and is known to be important for seizure generation and propagation (Dlouhy et al. 2015). Samples of dialysate were collected every 30 min, and 5‐HT content was measured using HPLC‐ECD. After treatment with MDMA (30 mg kg−1 i.p.), there was a rise in extracellular 5‐HT to more than 1000% of baseline (Fig. 5 A; n = 3 animals per group). MDMA also caused release of 5‐HT from cell cultures of the rostral ventral medulla. Coverslips containing cultured cells were treated for 1 h with MDMA (100 or 500 μm) added to aCSF. Sister cultures were treated identically except that MDMA was not added to the aCSF. MDMA caused a dose‐dependent increase in extracellular 5‐HT levels as measured with HPLC‐ECD (Fig. 5 B).
Figure 5. MDMA stimulated RTN neurons by inducing 5‐HT release from serotonergic neurons.
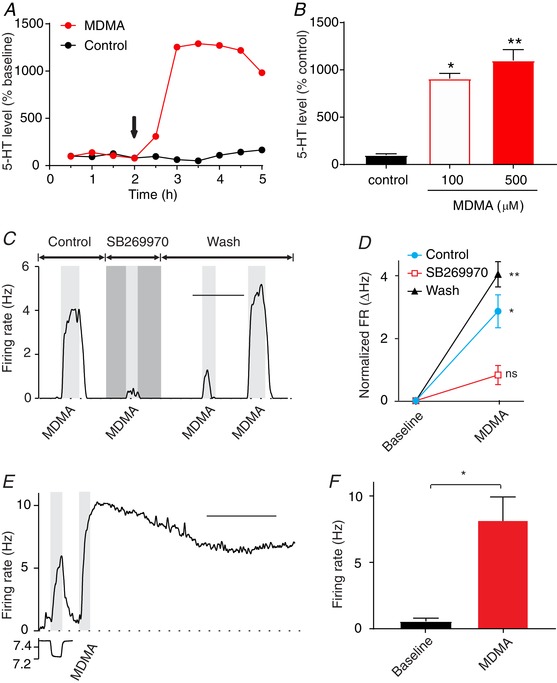
A, MDMA (30 mg kg−1, i.p.) caused a large increase in extracellular 5‐HT levels in the amygdala compared to saline controls as measured using in vivo microdialysis in C57Bl/6J mice (n = 3 males per group; 1 animal was excluded from each group in the plot due to discontinuous sample collection). The arrow indicates injection of MDMA or saline. Values are mean levels normalized to the baseline for each animal. B, treatment of cultured neurons from the ventral medulla with MDMA (100 or 500 μm) in aCSF for 1 h increased extracellular 5‐HT levels significantly. Values represent mean levels normalized to cultures treated with aCSF for 1 h. * P = 0.016; ** P = 0.003 compared with aCSF‐treated cultures (n = 6 coverslips per group). C, MDMA (100 μm) induced an increase in firing rate of an RTN neuron in culture, and this was blocked by the 5‐HT7 antagonist SB269970 (10 μm). D, summary data of MDMA responses showing that the responses, and the block by SB269970, were consistent in culture (n = 3). For differences between MDMA response and baseline: * P = 0.045; ** P = 0.001; ns, not significant. E, a chemosensitive RTN neuron in a brain slice responded to MDMA (40 μm) application. F, summary of the effect of MDMA (40 μm) on the firing rate of RTN neurons in brain slices (n = 4). * P = 0.03. Scale bars: 10 min (C), 20 min (E).
Based on these results, it was predicted that if 5‐HT neurons had synaptic terminals or other release sites in close apposition to dendrites of RTN neurons, 5‐HT receptors on those RTN neurons would be activated upon MDMA‐provoked release of 5‐HT. Recordings were made from cultured RTN neurons continuously superfused with aCSF at a rate of 1 ml min−1. Bath application of MDMA (100 μm; n = 3) induced a rapid and reversible increase in firing rate from 0.18 ± 0.27 to 3.09 ± 0.93 Hz (P = 0.045) (Fig. 5 C and D). MDMA (40 μm; n = 3) also induced a response, although it was smaller (from 0.34 ± 0.58 to 1.57 ± 1.1 Hz; P = 0.035). The response to 100 μm MDMA was blocked by SB269970 (10 μm) with firing rate 0.27 ± 0.32 Hz at baseline and 1.11 ± 0.75 Hz with MDMA (P = 0.60) (Fig. 5 C and D). These results are consistent with MDMA causing heteroexchange release of 5‐HT and activation of 5‐HT7 receptors. These results also indicate a close association of 5‐HT and RTN neurons, as continuous flow of bath solution across the cultured cells is expected to rapidly wash away 5‐HT from release sites. Thus, for 5‐HT to have an effect it must have been released from boutons of 5‐HT neurons that were closely associated with RTN neurons that express 5‐HT receptors. MDMA (40 μm) also induced a response in RTN neurons in brain slices (from 0.56 ± 0.49 to 8.19 ± 3.45 Hz; n = 4; P = 0.03) (Fig. 5 E and F).
Chemosensitivity of RTN neurons was blocked by SB269970 in culture
To study the effect of acidosis on RTN neurons in culture, perforated patch clamp recordings were made from RTN neurons after blocking ionotropic glutamate and GABA receptors. Hypercapnic acidosis (pH 7.4 to ∼7.2) caused firing rate to increase by more than 20% in 56% of cultured Phox2b+/ChAT− neurons (n = 123). In those neurons, firing rate increased from 0.73 ± 1.09 to 3.17 ± 2.97 Hz in response to acidosis (n = 68; P < 0.0001, paired t test). In contrast, a minority (n = 5/15) of Phox2b+/ChAT+ neurons (motor neurons) increased their firing rate by more than 20% in response to acidosis. However, as a group the average firing rate of these neurons (0.29 ± 0.31 Hz at pH 7.4; 1.34 ± 1.28 Hz at pH ∼7.2; n = 5) did not change significantly in response to acidosis (P = 0.13).
Synaptic connections often form between neurons grown in cell culture (Bekkers et al. 1990). Neurons and glia in culture can also communicate via non‐synaptic or non‐vesicular mechanisms (e.g. via paracrine release of neurotransmitter or reversal of transporters) (Wu et al. 2001, 2007). To determine whether chemosensitivity of RTN neurons was mediated via release of 5‐HT in cell culture, as in brain slices (Fig. 3), recordings were made from RTN neurons in culture and their response to acidosis was measured before and during exposure to 5‐HT7 receptor antagonists.
In RTN neurons in culture (n = 10), SB269970 (10 μm) caused a large reduction in the response to acidosis (Fig. 6 A–C). In control aCSF, firing rate increased in response to acidosis from 0.33 ± 0.45 to 3.66 ± 3.06 Hz (P = 0.0002), whereas in SB269970 firing rate did not change in response to acidosis (0.11 ± 0.25 to 0.39 ± 0.50 Hz; P = 0.95). The response in SB269970 was 8.2% of the response to acidosis in control solution (P = 0.0003). Thus, SB269970 caused a large decrease in the chemosensitivity of RTN neurons in culture, as in brain slices.
Figure 6. 5‐HT7 antagonists blocked chemosensitivity of RTN neurons in culture.
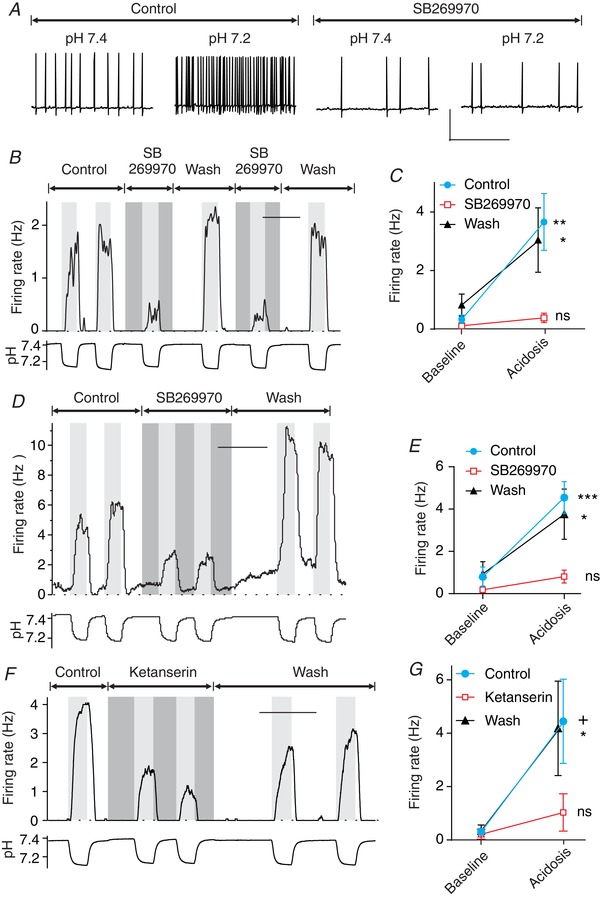
A, membrane potential of a cultured RTN neuron that responded to acidosis in aCSF by increasing its firing rate. Chemosensitivity was blocked by SB269970 (10 μm). B, firing rate plot of an RTN neuron showing that chemosensitivity was blocked by SB269970 (10 μm). C, summary of the effect of SB269970 on RTN neurons in culture (n = 10). D, example of an RTN neuron in culture recorded at a bath temperature of 31°C. The pH response and block by SB269970 were similar to recordings at room temperature. E, summary of recordings from RTN neurons at 31°C, showing consistent chemosensitivity and block by SB269970 (n = 9). F, chemosensitivity of an RTN neuron in culture was reduced by ketanserin (10 μm). G, summary of the effect of ketanserin on RTN neurons in cell culture (n = 7). For differences between firing rate at pH 7.4 (control) and pH ∼7.2 (acidosis): *** P < 0.0001; ** P = 0.0002, + P = 0.01; * P < 0.05; ns, not significant. Scale bars, 50 mV by 5 s (A), 10 min (B, D and F).
Previous in vitro experiments on RTN neuron chemosensitivity have been performed at room temperature (Mulkey et al. 2004, 2007; Hawkins et al. 2015), as have those on 5‐HT neuron chemosensitivity (Richerson, 1995; Bradley et al. 2002; Wang et al. 2002; Brust et al. 2014; Cerpa et al. 2017), in part to enhance stability of patch clamp recordings. To determine whether chemosensitivity was dependent on 5‐HT receptor activation at a temperature closer to normal body temperature, the above experiments were repeated with bath temperature = 31°C (Fig. 6 D and E). The response to acidosis was very similar to that when recording at room temperature, including block by SB269970. In control aCSF, firing rate increased from 0.79 ± 1.4 to 4.54 ± 2.2 Hz in response to acidosis (n = 9; P < 0.0001). In aCSF with SB269970, firing rate changed from 0.18 ± 0.26 to 0.81 ± 0.92 Hz in response to acidosis, which was not significant (P = 0.06). The response in SB269970 was 17% of the response in control aCSF (P = 0.0003). The decrease in chemosensitivity was reversible in all neurons tested.
Ketanserin is a less selective 5‐HT receptor antagonist with affinity for 5‐HT2 and 5‐HT7 receptors that has also been shown to block stimulation of RTN neurons by 5‐HT in brain slices (Mulkey et al. 2007). Here it was found that ketanserin decreased the response to acidosis of RTN neurons in culture (Fig. 6 F and G). Firing rate increased in response to acidosis from 0.30 ± 0.34 to 4.44 ± 4.16 Hz in control aCSF (P = 0.01; n = 7), whereas firing rate did not change in response to acidosis in 10 μm ketanserin (0.21 ± 0.48 to 1.03 ± 1.85 Hz; P = 0.98). The response to acidosis in ketanserin was 19.7% of the response in control solution (P = 0.045). Thus, 5‐HT7 receptor antagonists decreased chemosensitivity of RTN neurons in culture, as in brain slices. Taken together, these results support a role of 5‐HT7 receptors in chemosensitivity of RTN neurons. These data also validate the use of cell culture for studying the pH response of RTN neurons, as the results for the above experiments were the same as those in brain slices.
Chemosensitivity of RTN neurons was due to release of endogenous 5‐HT
If the response of RTN neurons to acidosis is due to release of 5‐HT from neighbouring 5‐HT neurons, then chemosensitivity of RTN neurons should be reduced if 5‐HT synthesis is inhibited. Patch clamp recordings were made from RTN neurons in cultures treated for 24 h before recording with either vehicle (n = 9) or the tryptophan hydroxylase inhibitor PCPA (10 μm; n = 8) (Fig. 7 A and B). Selection of neurons was made based only on whether they expressed tdTomato and not eGFP, and not on whether they had a response to acidosis. For neurons treated with vehicle, firing rate increased from 0.20 ± 0.26 to 5.54 ± 3.43 Hz in response to acidosis (P < 0.0001). RTN neurons treated with PCPA did not respond to acidosis (0.19 ± 0.35 Hz at pH 7.4; 1.15 ± 2.08 Hz at pH ∼7.2; P = 0.72). The response of PCPA‐treated cells was 18% of that in cells treated with vehicle (P = 0.0005).
Figure 7. Chemosensitivity of RTN neurons is dependent on release of endogenous 5‐HT by neighbouring processes of 5‐HT neurons.
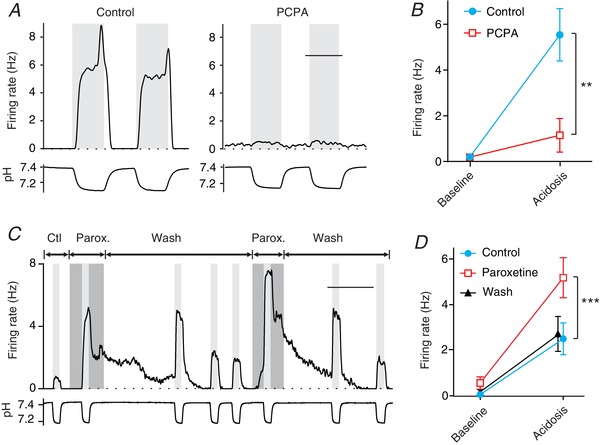
A, an RTN neuron in culture treated with the tryptophan hydroxylase antagonist PCPA (10 μm for 24 h) had a small response to acidosis (right) compared to an RTN neuron treated with control medium (left). B, summary data from PCPA‐treated RTN neurons (n = 8) compared to control neurons (n = 9) showing a smaller response to acidosis after depletion of 5‐HT. For differences between response to acidosis in PCPA compared to control: ** P = 0.0005. C, the SSRI paroxetine (1 μm) increased the amplitude of the response to acidosis of this RTN neuron in culture, and prolonged the recovery. D, summary data of CO2 responses of RTN neurons in culture (n = 9) showing that they were consistently larger in paroxetine: *** P = 0.0003 for control versus paroxetine. Scale bars: 5 min (A), 20 min (C).
If a large component of the response of RTN neurons to acidosis is due to vesicular 5‐HT release from chemosensitive 5‐HT neurons, then blocking 5‐HT reuptake should lead to a larger response to hypercapnic acidosis. Recordings were made from RTN neurons in culture before and during exposure to paroxetine (1 μm), a highly selective 5‐HT reuptake inhibitor (SSRI). Compared to the control response to acidosis (from 0.04 ± 0.07 to 2.49 ± 2.09 Hz), the response to acidosis during exposure to paroxetine was 88% larger (from 0.54 ± 0.82 to 5.17 ± 2.64 Hz) (Fig. 7 C and D; n = 9; P = 0.0003). In some cases (n = 2), the response to acidosis was prolonged after return to control pH (Fig. 7 C) consistent with accumulation of 5‐HT in the extracellular space near sites of vesicular 5‐HT release. In other cases a more rapid return to baseline occurred. This might happen if the processes of some RTN neurons are covered by glia and others are not. In the latter case, superfusion of monolayer cultures with bath solution would lead to rapid diffusion or convection of 5‐HT away from its receptors.
5‐HT does not enable latent intrinsic chemosensitivity of RTN neurons
It has previously been shown that Lmx1bf/f/p mice, which lack nearly all central 5‐HT neurons, have a blunted hypercapnic ventilatory response (HCVR) in vivo (Hodges et al. 2008). However, the HCVR can be restored by intracerebroventricular infusion of 5‐HT. This suggests that 5‐HT neurons may act to enhance the chemosensitivity of non‐serotonergic chemoreceptor neurons either instead of, or in addition to, having a role as CO2/pH sensors themselves. Given these alternative roles of 5‐HT neurons, the finding that 5‐HT7 receptor antagonists block RTN neuron chemosensitivity is consistent with two models that are not mutually exclusive: (1) 5‐HT neurons drive RTN neurons by releasing 5‐HT in proportion to the level of acidosis, or (2) tonic 5‐HT enables RTN neurons to express latent intrinsic chemosensitivity. Previous studies demonstrated that 5‐HT stimulates RTN neurons in brain slices, but does not enhance their chemosensitivity (Mulkey et al. 2007). We evaluated whether RTN neurons behaved in a similar way in culture.
A group of RTN neurons that were chemosensitive (n = 7) increased their firing rate from 1.80 ± 0.93 to 3.21 ± 1.62 Hz in response to acidosis (7.4 to 7.2, P = 0.0002). When 5‐HT (10 μm) was added to the bath solution, the baseline firing rate increased to 2.31 ± 1.64 Hz. However, 5‐HT did not potentiate the response of these chemosensitive neurons to acidosis as the slope of the firing rate versus pH curve did not change, with firing rate increasing to 4.11 ± 1.80 Hz in response to acidosis (P < 0.0001) (Fig. 8 A and B). This finding is the same as that previously reported for RTN neurons in brain slices (Mulkey et al. 2007). For Phox2b+/ChAT− neurons that were not chemosensitive (n = 5), 5‐HT did not confer chemosensitivity upon them (Fig. 8 C and D). All neurons that were evaluated for their 5‐HT response (chemosensitive and not chemosensitive) were subsequently found to be immunonegative for TH.
Figure 8. Exogenous 5‐HT did not reveal latent chemosensitivity of RTN neurons.
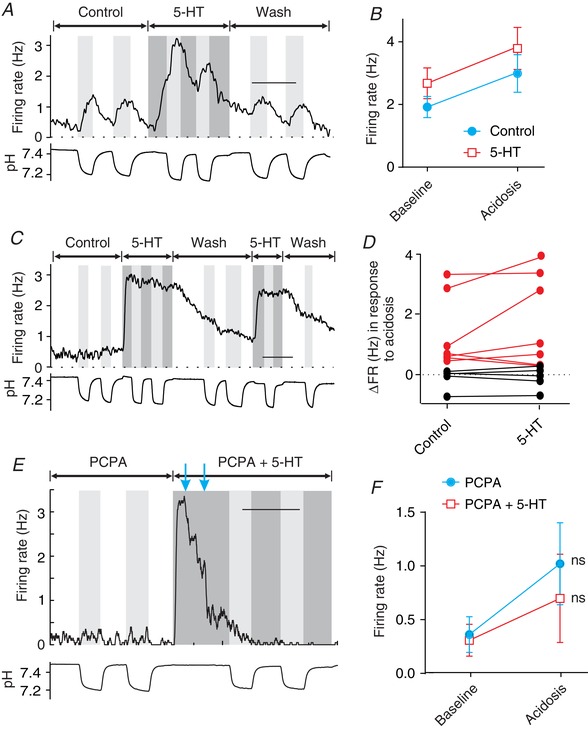
A, exogenous 5‐HT (10 μm) caused an increase in baseline firing rate of this chemosensitive RTN neuron but did not enhance the response to acidosis. B, bath‐applied 5‐HT induced an increase in firing rate of chemosensitive neurons (n = 7), but did not enhance chemosensitivity as indicated by a lack of change in the slope of the pH response. C, in this non‐chemosensitive RTN neuron, exogenous 5‐HT (10 μm) caused an increase in baseline firing rate but did not reveal a latent response to acidosis. D, in most RTN neurons, 5‐HT did not increase chemosensitivity (chemosensitive in red, n = 7; non‐chemosensitive in black, n = 5). E, after endogenous 5‐HT was decreased to near zero by pretreatment with PCPA (10 μm for 24 h), exogenous 5‐HT did not enable intrinsic RTN neuron chemosensitivity. Shown is the pH response of a PCPA‐treated neuron first in aCSF and then in aCSF with exogenous 5‐HT (10 μm). Blue arrows – the amount of depolarizing current injection was decreased in a stepwise fashion from 40 to 0 pA to counteract the excitatory effect of exogenous 5‐HT. F, summary of results from experiments like those in E (n = 15), showing that exogenous 5‐HT did not enable latent chemosensitivity in RTN neurons. Scale bars: 10 min (A, C and E). ns, not significant.
It is possible that the baseline level of extracellular 5‐HT present in our cultures was already sufficient to fully enable chemosensitivity of RTN neurons so that adding exogenous 5‐HT (Fig. 8 A–D) did not cause any additional effect. To rule out this possibility, ventral medullary cultures were first treated with PCPA (10 μm for 24 h as in Fig. 7). The response of RTN neurons (n = 15) to acidosis was then measured in control aCSF and aCSF containing exogenous 5‐HT (10 μm) (Fig. 8 E and F). In control aCSF, PCPA‐treated RTN neurons had a small increase in firing rate from 0.36 ± 0.65 to 1.02 ± 1.48 Hz that was not statistically significant (P = 0.13). When 5‐HT was added to the bath solution ( 5%), firing rate increased from 0.36 ± 0.70 to 7.26 ± 4.274 Hz. Depolarizing current was then decreased so that the baseline firing rate was close to that prior to the addition of 5‐HT. Then, in aCSF containing 10 μm 5‐HT, PCPA‐treated RTN neurons did not develop chemosensitivity, with firing rate 0.31 ± 0.57 Hz at baseline and 0.70 ± 1.59 Hz in acidosis (P = 0.38). The response in aCSF with 5‐HT was not significantly different from that in control aCSF (P = 0.57).
The effect of serotonergic drugs on 5‐HT neurons was fundamentally different than on RTN neurons
To determine if the response of 5‐HT neurons to serotonergic manipulations was different from the response of RTN neurons, we repeated some of the key experiments described above on 5‐HT neurons identified using ePet‐EYFP reporter mice. There was no effect of SB269970 on the chemosensitivity of ePet‐EYFP neurons in cell culture (n = 4) (Fig. 9 A), indicating that SB269970 did not directly affect chemosensitivity of 5‐HT neurons and that 5‐HT7 receptor activation was not required for a pH response. For this group of neurons in control aCSF, acidosis caused a change in firing rate from 0.06 ± 0.06 to 2.62 ± 1.48 Hz. In aCSF with SB269970 (10 μm), acidosis caused a change in firing rate from 0.24 ± 0.37 to 2.09 ± 1.26 Hz. There was no significant difference in these responses (P = 0.60).
Figure 9. The response of 5‐HT neurons to serotonergic agents was fundamentally different than that of RTN neurons.
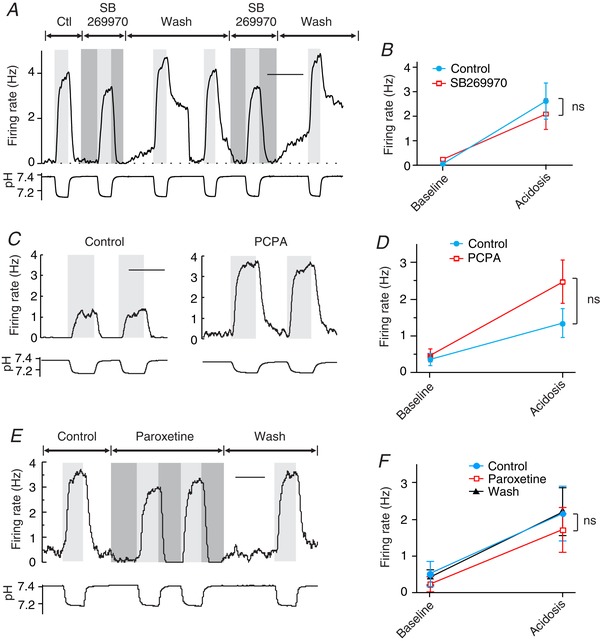
A, SB269970 did not affect chemosensitivity of this 5‐HT neuron cultured from the ventromedial medulla. B, summary of effect of SB269970 on chemosensitivity of serotonergic neurons in culture (n = 4). C, PCPA pretreatment did not reduce chemosensitivity of serotonergic neurons in culture. Shown are pH responses of a control neuron (left) and a PCPA‐treated neuron (right). D, summary of the effect of PCPA on the chemosensitivity of serotonergic neurons (n = 9 neurons per group). E, paroxetine did not increase the chemosensitivity of this serotonergic neuron in culture. F, summary of the effect of paroxetine on the chemosensitivity of serotonergic neurons (n = 4). Scale bars: 10 min (A), 5 min (C and E). ns, not significant.
Depletion of 5‐HT using pretreatment with PCPA (10 μm for 24 h) did not reduce chemosensitivity of ePet‐EYFP neurons (Fig. 9 C and D). In control neurons (n = 9), acidosis induced a change in firing rate from 0.36 ± 0.51 to 1.34 ± 1.20 Hz (P = 0.04). In neurons treated with PCPA (n = 9), acidosis induced a change in firing rate from 0.46 ± 0.55 to 2.47 ± 1.78 Hz (P = 0.005). The response to acidosis was not different between groups (P = 0.13). There was in fact a trend towards an increase in chemosensitivity in PCPA‐treated EYFP neurons, possibly due to a decrease in activation of inhibitory 5‐HT1a autoreceptors.
The SSRI paroxetine did not increase the response of 5‐HT neurons to acidosis (n = 4; Fig. 9 E and F). In control aCSF, acidosis caused a change in firing rate from 0.51 ± 0.68 to 2.15 ± 1.48 Hz. In aCSF with paroxetine, acidosis caused a change in firing rate from 0.22 ± 0.41 to 1.71 ± 1.23 Hz. Paroxetine did not cause a significant change in these responses (P = 0.66). In fact, there was a trend towards a decrease in chemosensitivity, this time possibly due to increased activation of inhibitory 5‐HT1a autoreceptors.
Thus, chemosensitive 5‐HT neurons had a very different response to serotonergic agents compared to RTN neurons. These results are consistent with chemosensitivity of 5‐HT neurons being cell‐autonomous and not due to release of 5‐HT onto each other.
The 5‐HT7 receptor antagonist SB258719 has off‐target effects
Previous experiments studying the effect of 5‐HT on RTN neurons used SB258719 (Hawkins et al. 2015), because it was considered to be a selective antagonist of 5‐HT7 receptors (Romero et al. 2006). We wanted to use this drug to replicate those findings, and confirmed that SB258719 does block the response of RTN neurons to 5‐HT, in our case using culture (Fig. 4). SB258719 has not been reported to have any off‐target effects, but in pilot experiments we found that it reduced chemosensitivity of 5‐HT neurons in brain slices (Fig. 10 A; n = 2) and cell culture (n = 3). There is no evidence that 5‐HT neurons express 5‐HT7 receptors, or that they are stimulated by 5‐HT, leading to the conclusion that SB258719 has an unreported off‐target effect. This prevented us from relying on SB258719 for our primary conclusions about RTN neuron chemosensitivity. However, we did perform the following experiments to be certain that its effects, including those on 5‐HT neurons, did not falsify our hypothesis.
Figure 10. The 5‐HT7 receptor antagonist SB258719 blocks the response of RTN neurons to acidosis, but also blocks 5‐HT neuron chemosensitivity.
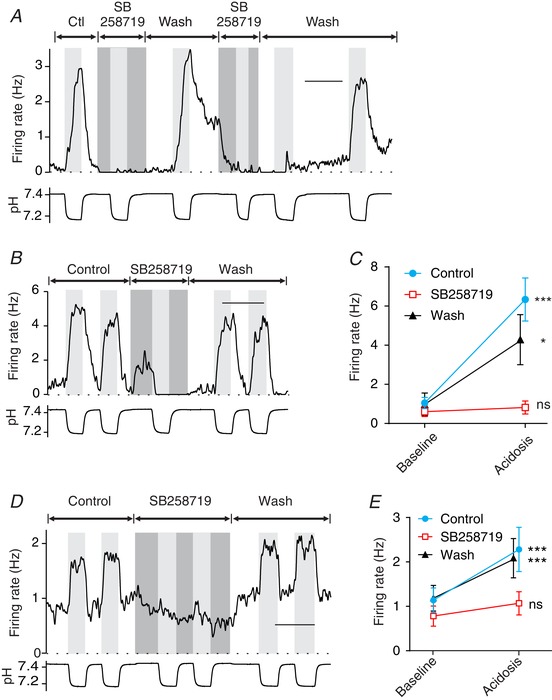
A, SB258719 blocked the chemosensitivity of this 5‐HT neuron in a brain slice. Similar results were seen in slices (n = 2) and cell culture (n = 3). SB258719 has been considered a highly selective 5‐HT7 antagonist, but was not known to have this off‐target effect. B, an RTN neuron in a brain slice was stimulated by hypercapnic acidosis. Chemosensitivity was reversibly inhibited by SB258719 (10 μm). C, the response to SB258719 was consistent for RTN neurons in brain slices (n = 9). Block of chemosensitivity by SB258719 was reversible in all neurons tested. D, an RTN neuron in cell culture was stimulated by hypercapnic acidosis, and this chemosensitivity was reversibly inhibited by SB258719 (10 μm). E, summary of the effect of SB258719 on chemosensitivity of RTN neurons in culture (n = 26). For differences between firing rate at pH 7.4 (control) and pH ∼7.2 (acidosis): *** P < 0.0001; * P < 0.05; ns, not significant. Scale bars: 10 min (A, C and E).
The effect of SB258719 (10 μm) was tested on chemosensitivity of RTN neurons in brain slices (Fig. 10 B and C). For a subset of RTN neurons (n = 9), firing rate increased from 1.05 ± 0.84 to 6.34 ± 3.31 Hz in response to acidosis in control aCSF (P < 0.0001), whereas there was no response to acidosis in SB258719, with firing rate 0.60 ± 0.74 Hz at pH 7.4 and 0.82 ± 0.99 Hz at pH ∼7.2 (P = 0.99). The response to acidosis in SB258719 was 4% of the response in control aCSF (P < 0.0001). The block of chemosensitivity by SB258719 was reversible in all neurons tested in slices.
The effect of SB258719 was also tested on chemosensitivity of RTN neurons in cell culture. When SB258719 (10 μm) was added to aCSF, there was a large reduction in the response of RTN neurons to acidosis (Fig. 10 D and E). In control aCSF, firing rate increased in response to acidosis from 1.14 ± 1.40 to 2.29 ± 2.54 Hz (n = 26; P < 0.0001), whereas in SB258719 firing rate did not change in response to acidosis (0.79 ± 1.16 to 1.07 ± 1.33 Hz; P = 0.25). The increase in firing rate in SB258719 was 25% of the response to acidosis in control aCSF (P < 0.0001). The block of chemosensitivity by SB258719 was reversible in all neurons tested in culture.
The decrease in chemosensitivity of RTN neurons seen here with SB258719 could be due to inhibition of chemosensitivity of 5‐HT or RTN neurons, block of 5‐HT7 receptors on RTN neurons, or a combination of these effects. These results, taken alone, do not prove that RTN neuron chemosensitivity is dependent on input from 5‐HT neurons, but neither do they alter the conclusions made from the other experiments.
The hypercapnic ventilatory response in vivo was markedly decreased by ketanserin
As described above, ketanserin is a 5‐HT receptor antagonist with affinity for both 5‐HT2 and 5‐HT7 receptors. Ketanserin has previously been shown to block stimulation of RTN neurons by 5‐HT in brain slices (Mulkey et al. 2007 a), and we showed here that it decreased the response of RTN neurons to acidosis in culture (Fig. 6 F and G). 5‐HT2 receptors are also expressed by other neurons that are part of the respiratory network (Ptak et al. 2009). Therefore, it was predicted that ketanserin would reduce the HCVR in vivo. When ketanserin (10 mg kg−1 i.p.) was administered to C57BL6 mice (n = 12) there was a decrease in minute ventilation at 0% CO2 (from 1.00 to 0.49 ± 0.23, P < 0.0001) (Fig. 11 A and B). The HCVR was also inhibited, with ventilation at 5% CO2 decreased from 2.58 ± 1.03 to 0.71 ± 0.34 (P < 0.0001) and at 7% CO2 decreased from 3.95 ± 1.90 to 1.04 ± 0.46 (P < 0.0001; two‐way ANOVA using the Holm–Sidak test for multiple comparisons). The slope of the ventilation response to 5% CO2 was decreased by 86%, and to 7% CO2 by 81%. The effect on ventilation was mediated by a decrease in both tidal volume and frequency (Fig. 11 C and D). The slope of the frequency curve decreased by 60% from 0% to 5% CO2, and by 53% from 0% to 7% CO2. The slope of the tidal volume curve decreased by 84% from 0% to 5% CO2, and by 63% from 0% to 7% CO2.
Figure 11. The HCVR in vivo was decreased by systemic ketanserin (10 mg kg−1 i.p.).
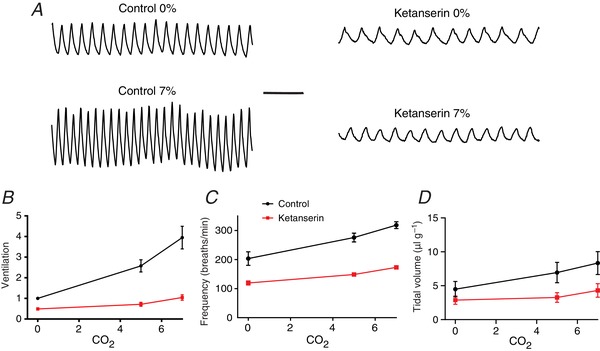
A, plethysmography traces from a mouse during exposure to 0% CO2 and 7% CO2 at baseline and after treatment with ketanserin. B, ketanserin significantly reduced minute ventilation at 0, 5 and 7% CO2 (n = 12 animals). The y‐axis represents ventilation normalized to baseline breathing at 0% CO2. Frequency (C) and tidal volume (D) were also significantly reduced by ketanserin. P < 0.0001 for ventilation in ketanserin compared to control.
Discussion
Breathing is highly sensitive to brain tissue acidosis, with ventilation approximately tripling in goats in response to a decrease of brain extracellular pH from 7.35 to 7.25 (Pappenheimer et al. 1965; Fencl et al. 1966). CRCs are assumed to have a cell‐autonomous (intrinsic) response to pH that approaches this degree of sensitivity, although such a large response may not be an absolute requirement for the respiratory system as a whole to respond adequately (Richerson et al. 2005). There are a number of groups of neurons in the brain, and some glia, that have been proposed to be CRCs, including Phox2b+ neurons in the RTN and 5‐HT neurons in the medullary raphe (Nattie, 1999; Mulkey et al. 2004; Richerson et al. 2005; Nattie & Li, 2009). However, the relative roles of these various candidates are not yet clear (Nattie, 1999), and it is not known how they interact with each other to produce a coordinated response.
The pH response of RTN neurons is mediated in part by synaptic input from chemosensitive 5‐HT neurons
It has previously been shown that RTN neurons in brain slices are stimulated by exogenous 5‐HT, and that this response is blocked by SB258719 and ketanserin (Mulkey et al. 2007), both of which are antagonists of 5‐HT7 receptors. We extended these results by showing in brain slices that: (1) RTN neurons are also stimulated by release of endogenous 5‐HT from neighbouring neurons; (2) the responses to exogenous and endogenous 5‐HT are blocked by SB269970 (a third 5‐HT7 receptor antagonist, and; (3) the pH response of RTN neurons is blocked by all three 5‐HT7 receptor antagonists. In addition to obtaining these new findings in brain slices, we replicated all of the above results in cell culture. Using cell culture, we also demonstrated that chemosensitivity of RTN neurons was markedly reduced by blocking synthesis of 5‐HT, and chemosensitivity was enhanced by blocking 5‐HT reuptake. 5‐HT neurons had a fundamentally different response to these experimental conditions, and their chemosensitivity had no dependence on endogenous 5‐HT. Taken together, these results indicate that a large component of the pH response of RTN neurons is due to input from chemosensitive 5‐HT neurons under these experimental conditions.
5‐HT neurons have many properties expected for CRCs (Richerson, 1995, 2004; Veasey et al. 1995; Bradley et al. 2002; Richerson et al. 2005; Hodges et al. 2008; Ray et al. 2011; Brust et al. 2014; Teran et al. 2014). Their firing rate in vitro increases 3‐fold (e.g. 1 to 3 Hz) with a decrease in pH from 7.4 to 7.2 (Wang et al. 1998, 2001, 2002), which approaches the degree of sensitivity of the response of the respiratory network in vivo (see above). RTN neurons receive abundant input from medullary 5‐HT neurons (Mulkey et al. 2007; Brust et al. 2014) and it has previously been shown that 5‐HT stimulates RTN neurons (Mulkey et al. 2007; Hawryluk et al. 2012; Hawkins et al. 2015). Therefore, the chemosensitivity of RTN neurons could be due in part to input from 5‐HT neurons, because brain slices containing the RTN also contain 5‐HT neurons. This possibility has previously been considered, and was tested in brain slices by measuring the pH response of RTN neurons in the presence and absence of exogenous 5‐HT (5 μm) (Mulkey et al. 2007). Consistent with our results in culture, this led to ‘a parallel upward shift in the pH sensitivity curve’ (Mulkey et al. 2007), which was interpreted as indicating that the ‘actions of pH and serotonin are independent and additive’. However, we would favour an alternative interpretation of that result, because that is what would be expected if RTN neurons are driven by 5‐HT neurons in proportion to the degree of acidosis.
The approaches we used to determine if chemosensitivity of RTN neurons is due to input from 5‐HT neurons (5‐HT receptor antagonists and 5‐HT depletion) had not previously been used. Our findings could be interpreted in one of two ways: (1) 5‐HT is released in response to acidosis, and stimulates RTN neurons proportionally; or (2) 5‐HT is released at a constant level and enables chemosensitivity of RTN neurons by activating a pH‐sensitive ion current in RTN neurons. The latter possibility is not consistent with the lack of an effect of exogenous 5‐HT on the pH response in brain slices (Mulkey et al. 2007) and in culture (Fig. 8), and also does not take into account that 5‐HT neurons increase their firing rate in response to hypercapnia. Thus, the present data indicate that a substantial component of chemosensitivity of RTN neurons is not cell‐autonomous.
Chemosensitivity of RTN neurons was reduced by 92–96% when treated with SB258719, by 63–92% with SB269970, and by 80% with ketanserin. It is unclear how much of the residual pH response is intrinsic. Some of the residual response could be due to stimulation of 5‐HT receptors unaffected by the above antagonists, release of thyrotropin releasing hormone (TRH) or substance P from 5‐HT neurons (Mulkey et al. 2007), or release of ATP by glia (Gourine et al. 2010). Thus, the upper limit of the cell‐autonomous response to a change in pH from 7.4 to 7.2 is 8–37% of the total response, but is likely to be less than that due to evidence for non‐5‐HT7 receptor‐mediated extrinsic mechanisms.
It is not yet clear why our results do not support a large role for intrinsic chemosensitivity in RTN neurons, in light of previous results (Mulkey et al. 2004). First, it is important to consider alternative interpretations of the current data. Recordings from brain slices were made in the region where chemosensitive RTN neurons were first described: ventral and ventromedial to the VII motor nucleus (Mulkey et al. 2004). It has subsequently been reported that there is also a subset of RTN neurons located ventrolateral to the VII motor nucleus that may be chemosensitive (Shi et al. 2017), although evidence for chemosensitivity in that region is indirect and not based on electrophysiological recordings. We did not sample these neurons in brain slices, so we cannot exclude the possibility that this subset of RTN neurons has intrinsic chemosensitivity. However, our cell cultures would have included each of the different populations of RTN neurons, and there was no evidence of a subpopulation whose chemosensitivity was not dependent on 5‐HT receptor activation.
The current set of recordings was made from brain slices older than P13 and from neurons of equivalent age in cell culture. It is possible that RTN neurons have intrinsic chemosensitivity in neonatal mice, and their response to pH becomes dependent on 5‐HT input only after serotonergic neuron chemosensitivity matures (Cerpa et al. 2017). There might be other explanations for the differences between our results and those reported previously, such as experimental conditions that might facilitate intrinsic chemosensitivity of RTN neurons. For example, there could be modulatory inputs that cause intrinsic chemosensitivity in vivo, and they may not be operative under the conditions of our in vitro experiments. Nonetheless, the results presented here demonstrate that a significant portion of the pH response of RTN neurons can be due to synaptic input from 5‐HT neurons under some conditions, and it remains to be determined whether there are other conditions in which intrinsic chemosensitivity is sufficiently robust in RTN neurons to make a major contribution to the ventilatory response to physiological changes in CO2.
Experimental proof of an intrinsic response to acidosis
As above, there are potential explanations for the current results that do not exclude a role of RTN neurons as chemoreceptors. However, it is possible that intrinsic chemosensitivity of RTN neurons is less important than previously believed, because there are methodological issues that are not widely considered when interpreting experiments designed to study intrinsic chemosensitivity.
RTN neurons respond to acidosis in brain slices after blockade of glutamate and P2X receptors. This was interpreted as suggesting that they are intrinsically chemosensitive (Mulkey et al. 2004). However, this approach would not block synaptic input from many other neurotransmitters, including 5‐HT. In recognition of that fact, Mulkey et al. (2004) measured changes in input resistance of RTN neurons in brain slices in response to a change in pH from 7.5 to 6.9 during voltage clamp recordings in the presence of tetrodotoxin (TTX) in an attempt to block action potential‐dependent synaptic activity (Mulkey et al. 2004). Similarly, in an in vitro brainstem/spinal cord preparation from neonatal rats, a change in pH from 7.8 to 7.2 led to an increase in membrane potential in the presence of TTX and Cd2+, and in high Mg2+/low Ca2+ solution (Onimaru et al. 2012). These methods may be more effective at isolating neurons, but it is important to consider that none of them completely blocks release of neurotransmitters. For example, GABA, dopamine, 5‐HT and other neurotransmitters continue to be released in TTX, zero Ca2+, Cd2+, and/or high Mg2+/low Ca2+ solution, via a variety of vesicular and non‐vesicular mechanisms involving quantal release, graded potentials, transporter reversal, hemichannels, anion channels, etc. (Otis et al. 1991; Attwell et al. 1993; Levi & Raiteri, 1993; Falkenburger et al. 2001; Rossi et al. 2003; Ye et al. 2003; Borland & Michael, 2004; Wu et al. 2007; Lee et al. 2010). These alternative forms of release have been clearly demonstrated and can be surprisingly robust (Levi & Raiteri, 1993; Richerson & Wu, 2003; Rossi et al. 2003; Wu et al. 2007). Thus, the methods that have been used to block synaptic activity do not ensure that the pH response of RTN neurons is cell‐autonomous. Moreover, none of them would block glial release of signalling molecules (Gourine et al. 2010).
Focal acidosis within the RTN stimulates breathing (Li & Nattie, 2002; Nattie & Li, 2012), which is consistent with RTN neurons being intrinsically chemosensitive. However, focal acidosis would also stimulate glia in the RTN, and this would release ATP onto Phox2b neurons (Gourine et al. 2010). In addition, axonal processes of 5‐HT neurons within the RTN could respond to local changes in pH (directly or via effects on chemosensitive glia) and release 5‐HT onto Phox2b neurons. Thus, there are alternative explanations for these results other than intrinsic chemosensitivity of RTN neurons.
It has been reported that 91% of RTN neurons express the proton‐activated receptor Gpr4 (Kumar et al. 2015), which has been considered as evidence supporting a role of RTN neurons as pH sensors. However, the role of Gpr4 in chemoreception has recently been called into question (Hosford et al. 2018). Gpr4 is widely expressed, including in 100% of 5‐HT neurons in the medullary raphe and 83% of C1 adrenergic neurons, as well as by the carotid bodies (Kumar et al. 2015). Gpr4 is also expressed at high levels in vascular endothelia including in the brain (reviewed by Hosford et al. 2018), as detected using antibodies, in situ hybridization, GFP expression driven by the Gpr4 promoter, and cre‐mediated GFP expression in cells with a developmental history of Gpr4 expression (Qiao et al. 2006; Sun et al. 2016; Hosford et al. 2018). In contrast to the result of Kumar et al. (2015), Hosford et al. (2018) reported that Gpr4 is expressed in a sparse number of RTN neurons, and at low levels (5‐HT neurons in the dorsal raphe abundantly expressed Gpr4, but unlike Kumar et al (2015), they did not specifically report on the medullary raphe. As reported by Hosford et al. (2018), the sensitivity to pH of Gpr4 occurs across a range (7.4–8.0) that would not be suitable for CO2 monitoring for respiratory control. Finally, as reported by Kumar et al (2015), many RTN neurons remain chemosensitive after genetic deletion of Gpr4 (Kumar et al. 2015), which is not surprising given the current results. Although Kumar et al. (2015) reported that knockout of Gpr4 alone or with Task2 reduces the ventilatory response to CO2 in vivo, Hosford et al. (2018) found that pharmacological inhibition of Gpr4 does not affect the HCVR (Hosford et al. 2018). Thus, it remains an open question whether expression of Gpr4 supports the idea that RTN neurons have intrinsic chemosensitivity.
The most definitive method to demonstrate intrinsic chemosensitivity is to record from a neuron after acute dissociation. RTN neurons have been reported to retain pH sensitivity after they are physically isolated in this way (Wang et al. 2013), but the response is significantly smaller than in vivo or in brain slices (see above). It is possible that the residual response is entirely intrinsic, but acutely dissociated neurons can retain active synaptic boutons attached to the postsynaptic membrane (Yang et al. 2011; Zhou et al. 2014). These boutons could possibly still respond to acidosis by releasing neurotransmitters, so that even some of the residual pH response seen in dissociated RTN neurons (Wang et al. 2013) may be extrinsic. Thus, experiments used to test for intrinsic chemosensitivity of RTN neurons have relied on methods that do not necessarily block all extrinsic effects.
Use of cell culture to study the interaction of 5‐HT and RTN neurons
Many previous studies on RTN physiology have been performed using rodent brainstem slices. Slices have the advantage of maintaining tissue architecture, but they also have drawbacks in that cells are acutely damaged, and the tissue is thick so that changes in pH are buffered and pharmacological agents have restricted access to deep neurons and may not wash out easily (see Fig. 5). It is also common to use immature animals for brain slices to optimize tissue health, but their physiology may be different than in adults (Cerpa et al. 2017).
Cell culture has been used effectively across many fields of neuroscience to study neuronal physiology (Banker & Goslin, 1998). Cultured neurons recover from injury after plating and can be maintained for weeks during which many properties develop normally, as has been shown to be the case for pH sensitivity of 5‐HT neurons (Cerpa et al. 2017). In primary, dissociated cell culture, neurons grow on top of a thin layer of glia and are easily accessible to pharmacological agents and protons. Patch‐clamp recordings are often stable for hours, allowing complex experimental protocols with exposure to multiple conditions, including demonstration of reversibility and reproducibility of responses. The generalizability of data obtained from neuronal culture needs to be verified for each physiological process to be studied, but for those properties that are preserved, this method can allow rigorous and detailed studies to be performed.
Here we first used brain slices to identify a role of serotonergic signalling in chemosensitivity of RTN neurons, and then showed that these mechanisms are recapitulated in long‐term primary co‐cultures of dissociated medullary raphe and RTN tissue. The following results were found to be essentially identical in cell culture and brain slices (reported here and/or in the literature): (1) 5‐HT neurons form appositions with RTN neurons; (2) exogenous 5‐HT stimulates RTN neurons; (3) release of endogenous 5‐HT stimulates RTN neurons; (4) the responses to 5‐HT from either source are blocked by three different 5‐HT7 receptor antagonists; (5) acidosis stimulates approximately 50% of RTN neurons; (6) the response to acidosis is blocked by three 5‐HT7 receptor antagonists; and (7) exogenous 5‐HT does not induce latent chemosensitivity intrinsic to RTN neurons.
These findings provide confidence that results obtained in culture accurately reflect the biology of RTN neuron chemosensitivity and the influence of 5‐HT input. The use of cell culture allowed data to be obtained from a larger number of neurons for longer durations and with more stable responses than would have been possible using slices. Cell culture also allowed us to show that the chemosensitivity of RTN neurons was markedly reduced when synthesis of 5‐HT was blocked by PCPA, and that blocking 5‐HT reuptake increased the pH response. The use of cell culture for the study of chemosensitivity, including interactions between 5‐HT and RTN neurons, opens up a variety of new experiments that would not otherwise be possible.
RTN neurons: pH sensors or relays of chemoreceptor input?
The present data indicate that intrinsic chemosensitivity of mature RTN neurons is not as large as previously thought, and that a significant component of their pH response is due to synaptic input. These neurons might be most important as backup, or ‘emergency’, chemoreceptors (Nattie, 1999) that respond to larger changes in pH than used in the experiments presented here. Alternatively, they may play a greater role as a relay of input from other pH chemosensors, including peripheral chemoreceptors (Takakura et al. 2006), 5‐HT neurons (Rosin et al. 2006; Mulkey et al. 2007; Brust et al. 2014), other chemosensitive central neurons (Nattie & Li, 2009, 2012) and glia (Gourine et al. 2010). RTN neurons do receive input from many putative chemoreceptor sites (Rosin et al. 2006). If RTN neurons function as a relay, that alone could explain why chemoreception is decreased after genetic deletion of RTN neurons (Ramanantsoa et al. 2011; Ruffault et al. 2015) without requiring that they also have intrinsic chemosensitivity. The finding that about half the HCVR response is retained in adults after deletion of RTN neurons (Ramanantsoa et al. 2011) indicates that other chemoreceptors must be involved.
An additional function of RTN neurons might be to amplify the CO2/pH response of the network. The very large response of RTN neurons to hypercapnia in vivo relative to other CRC candidates has sometimes been cited as evidence that RTN neurons are respiratory chemoreceptors. This conclusion would be valid if their response to acidosis is intrinsic, but their large response when they are embedded within the network may provide an important clue to their function. Given that RTN neurons receive synaptic input from many putative chemoreceptors, they may relay this widespread chemoreceptor input, and possibly amplify it, leading to a much larger response to acidosis than that of any of the individual chemoreceptor neurons. This amplification could occur if RTN neurons have some low‐level intrinsic chemosensitivity, but could even occur without RTN neuron chemosensitivity via a non‐linear response to synaptic input. This possibility reinforces the argument that individual CRCs do not have to possess a pH response that is as sensitive to pH as the respiratory network as a whole (Richerson et al. 2005; Teran et al. 2014). The large response of RTN neurons to hypercapnia in adult rodents in vivo arising from these hypothetical amplification mechanisms could solve a biological problem where a respiratory network that must be exquisitely sensitive to pH needs to be built from individual components with inherent limitations on their sensitivity at the molecular/cellular level.
Future work will be needed to address a number of issues. The effect on RTN neurons of a larger range of pH changes should be examined, and it should be determined how much of the response is intrinsic versus being mediated by other signalling pathways (e.g. other 5‐HT receptors, substance P, TRH or ATP). The role of glia should also be defined, as well as whether 5‐HT input is monosynaptic, and whether 5‐HT release is synaptic or extrasynaptic. A molecular approach should be used to verify the identity of the 5‐HT receptor subtype involved instead of relying on pharmacology. Finally, it will be important to distinguish roles of the RTN in pH sensation versus relaying chemoreceptor information versus contributing to respiratory rhythm generation (Onimaru et al. 2009; Goridis et al. 2010; Ikeda et al. 2015; Ruffault et al. 2015). However, our findings show that a large component of the response of Phox2b‐expressing RTN neurons to pH within a narrow range expected to occur under physiological conditions in vivo is extrinsic – mediated in part by release of 5‐HT in proportion to the level of acidosis. This finding suggests that the RTN could be more important as an integrative centre, relay of chemoreceptor information or amplifier of the pH response, than as a chemoreceptor site.
Additional information
Competing interests
The authors declare no competing interests.
Author contributions
Y.W., K.L.P., F.A.T., R.J.L., H.K. and G.B.R. were responsible for the collection and analysis of data. K.L.P., F.A.T. and G.B.R. were responsible for the conception and design of the experiments. G.B.R. drafted the manuscript. All authors were responsible for interpretation of the data, and revised the manuscript critically for important intellectual content. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
The study was supported by US National Institutes of Health (NIH) grants U01NS090414, P01HD36379 and R01HD052772.
Acknowledgements
We thank Xiuqiong Zhou for mouse husbandry and genotyping, and Lori Smith‐Mellecker for technical contributions. We also thank Dr Paul Gray for providing Phox2b‐Cre mice and Dr Evan Deneris for providing ePet‐EYFP mice.
Biographies
Yuanming Wu, MD, received his MD from Hubei Medical College Xianning Branch, China, and his MS from Tongji Medical University, China. He joined the laboratory of George B Richerson, MD, PhD, in the neurology department at Yale University from 1997 to 2010. He then moved to the University of Iowa in 2010, where he has risen to the rank of Research Scientist. He is highly experienced and skilled at patch clamp recordings from neurons in cell culture and brain slices, and has a deep interest in synaptic transmission, chemoreception, respiratory control and epilepsy.

Katherine Proch received her BS in Neuroscience and German in 2008 from Allegheny College, Meadville, PA, where her senior thesis introduced her to CO2 chemosensation. She continued research in that field as a member of the Medical Scientist Training Program at the University of Iowa, and defended her thesis, Effects of serotonergic input on Phox2b neuron chemosensitivity in the RTN, in March 2018. She is expected to receive her medical degree and PhD in May 2019.
Edited by: David Wyllie & Gregory Funk
This is an Editor's Choice article from the 15 May 2019 issue.
Linked articles: This article is highlighted in a Perspectives article by Huckstepp. To read this article, visit https://doi.org/10.1113/JP277972.
References
- Amiel J, Laudier B, Attie‐Bitach T, Trang H, de Pontual L, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C & Lyonnet S (2003). Polyalanine expansion and frameshift mutations of the paired‐like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet 33, 459–461. [DOI] [PubMed] [Google Scholar]
- Attwell D, Barbour B & Szatkowski M (1993). Nonvesicular release of neurotransmitter. Neuron 11, 401–407. [DOI] [PubMed] [Google Scholar]
- Banker G & Goslin K (1998). Types of nerve cell cultures, their advantages and limitations In Culturing Nerve Cells, Second edn, ed. G Banker. & K Goslin, pp. 11–35. MIT Press, Cambridge, MA. [Google Scholar]
- Bekkers JM, Richerson GB & Stevens CF (1990). Origin of variability in quantal size in cultured hippocampal neurons and hippocampal slices. Proc Natl Acad Sci U S A 87, 5359–5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland LM & Michael AC (2004). Voltammetric study of the control of striatal dopamine release by glutamate. J Neurochem 91, 220–229. [DOI] [PubMed] [Google Scholar]
- Bradley SR, Pieribone VA, Wang W, Severson CA, Jacobs RA & Richerson GB (2002). Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nat Neurosci 5, 401–402. [DOI] [PubMed] [Google Scholar]
- Brust RD, Corcoran AE, Richerson GB, Nattie E & Dymecki SM (2014). Functional and developmental identification of a molecular subtype of brain serotonergic neuron specialized to regulate breathing dynamics. Cell Rep 9, 2152–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerpa VJ, Wu Y, Bravo E, Teran FA, Flynn RS & Richerson GB (2017). Medullary 5‐HT neurons: switch from tonic respiratory drive to chemoreception during postnatal development. Neuroscience 344, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran AE, Hodges MR, Wu Y, Wang W, Wylie CJ, Deneris ES & Richerson GB (2009). Medullary serotonin neurons and central CO2 chemoreception. Respir Physiol Neurobiol 168, 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauger S, Pattyn A, Lofaso F, Gaultier C, Goridis C, Gallego J & Brunet JF (2003). Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development 130, 6635–6642. [DOI] [PubMed] [Google Scholar]
- Dlouhy BJ, Gehlbach BK, Kreple CJ, Kawasaki H, Oya H, Buzza C, Granner MA, Welsh MJ, Howard MA, Wemmie JA & Richerson GB (2015). Breathing inhibited when seizures spread to the amygdala and upon amygdala stimulation. J Neurosci 35, 10281–10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drorbaugh JE & Fenn WO (1955). A barometric method for measuring ventilation in newborn infants. Pediatrics 16, 81–87. [PubMed] [Google Scholar]
- Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF & Goridis C (2008). A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnea, and specific loss of parafacial neurons. Proc Natl Acad Sci U S A 105, 1067–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara S, Shirato K, Harata N & Akaike N (1995). Gramicidin‐perforated patch recording: GABA response in mammalian neurones with intact intracellular chloride. J Physiol 484, 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenburger BH, Barstow KL & Mintz IM (2001). Dendrodendritic inhibition through reversal of dopamine transport. Science 293, 2465–2470. [DOI] [PubMed] [Google Scholar]
- Fencl V, Miller TB & Pappenheimer JR (1966). Studies on the respiratory response to disturbances of acid–base balance, with deductions concerning the ionic composition of cerebral interstitial fluid. Am J Physiol 210, 459–472. [DOI] [PubMed] [Google Scholar]
- Foong JP & Bornstein JC (2009). 5‐HT antagonists NAN‐190 and SB 269970 block α2‐adrenoceptors in the guinea pig. Neuroreport 20, 325–330. [DOI] [PubMed] [Google Scholar]
- Goridis C, Dubreuil V, Thoby‐Brisson M, Fortin G & Brunet JF (2010). Phox2b, congenital central hypoventilation syndrome and the control of respiration. Semin Cell Dev Biol 21, 814–822. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K & Kasparov S (2010). Astrocytes control breathing through pH‐dependent release of ATP. Science 329, 571–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology . J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG & Mulkey DK (2010). Retrotrapezoid nucleus and parafacial respiratory group. Respir Physiol Neurobiol 173, 244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins VE, Hawryluk JM, Takakura AC, Tzingounis AV, Moreira TS & Mulkey DK (2015). HCN channels contribute to serotonergic modulation of ventral surface chemosensitive neurons and respiratory activity. J Neurophysiol 113, 1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawryluk JM, Moreira TS, Takakura AC, Wenker IC, Tzingounis AV & Mulkey DK (2012). KCNQ channels determine serotonergic modulation of ventral surface chemoreceptors and respiratory drive. J Neurosci 32, 16943–16952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks T, Francis N, Fyodorov D & Deneris ES (1999). The ETS domain factor Pet‐1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J Neurosci 19, 10348–10356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF & Richerson GB (2008). Defects in breathing and thermoregulation in mice with near‐complete absence of central serotonin neurons. J Neurosci 28, 2495–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosford PS, Mosienko V, Kishi K, Jurisic G, Seuwen K, Kinzel B, Ludwig MG, Wells JA, Christie IN, Koolen L, Abdala AP, Liu BH, Gourine AV, Teschemacher AG & Kasparov S (2018). CNS distribution, signalling properties and central effects of G‐protein coupled receptor 4. Neuropharmacology 138, 381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Takahashi M, Sato S, Igarashi H, Ishizuka T, Yawo H, Arata S, Southard‐Smith EM, Kawakami K & Onimaru H (2015). A Phox2b BAC transgenic rat line useful for understanding respiratory rhythm generator neural circuitry. PLoS One 10, e0132475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BJ, Chang DA, Mackay DD, West GH, Moreira TS, Takakura AC, Gwilt JM, Guyenet PG & Stornetta RL (2007). Central nervous system distribution of the transcription factor Phox2b in the adult rat. J Comp Neurol 503, 627–641. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bravo E, Thirnbeck CK, Smith‐Mellecker LA, Kim SH, Gehlbach BK, Laux LC, Zhou X, Nordli DR, Jr & Richerson GB (2018). Severe peri‐ictal respiratory dysfunction is common in Dravet syndrome. J Clin Invest 128, 1141–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar NN, Velic A, Soliz J, Shi Y, Li K, Wang S, Weaver JL, Sen J, Abbott SB, Lazarenko RM, Ludwig MG, Perez‐Reyes E, Mohebbi N, Bettoni C, Gassmann M, Suply T, Seuwen K, Guyenet PG, Wagner CA & Bayliss DA (2015). Regulation of breathing by CO2 requires the proton‐activated receptor GPR4 in retrotrapezoid nucleus neurons. Science 348, 1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarenko RM, Milner TA, Depuy SD, Stornetta RL, West GH, Kievits JA, Bayliss DA & Guyenet PG (2009). Acid sensitivity and ultrastructure of the retrotrapezoid nucleus in Phox2b‐EGFP transgenic mice. J Comp Neurol 517, 69–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Yoon BE, Berglund K, Oh SJ, Park H, Shin HS, Augustine GJ & Lee CJ (2010). Channel‐mediated tonic GABA release from glia. Science 330, 790–796. [DOI] [PubMed] [Google Scholar]
- Levi G & Raiteri M (1993). Carrier‐mediated release of neurotransmitters. Trends Neurosci 16, 415–419. [DOI] [PubMed] [Google Scholar]
- Li A & Nattie E (2002). CO2 dialysis in one chemoreceptor site, the RTN: stimulus intensity and sensitivity in the awake rat. Respir Physiol Neurobiol 133, 11–22. [DOI] [PubMed] [Google Scholar]
- Loeschcke HH (1982). Central chemosensitivity and the reaction theory. J Physiol 332, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RA, Loeschcke HH, Massion WH & Severinghaus JW (1963). Respiratory responses mediated through superficial chemosensitive areas on medulla. J Appl Physiol 18, 523. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA & Guyenet PG (2007). Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH‐independent mechanism. J Neurosci 27, 14128–14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA & Guyenet PG (2004). Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7, 1360–1369. [DOI] [PubMed] [Google Scholar]
- Nattie E (1999). CO2, brainstem chemoreceptors and breathing. Prog Neurobiol 59, 299–331. [DOI] [PubMed] [Google Scholar]
- Nattie E & Li A (2009). Central chemoreception is a complex system function that involves multiple brain stem sites. J Appl Physiol 106, 1464–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E & Li A (2012). Central chemoreceptors: locations and functions. Compr Physiol 2, 221–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Fung ML, Li A & St John WM (1993). Responses of respiratory modulated and tonic units in the retrotrapezoid nucleus to CO2 . Respir Physiol 94, 35–50. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li AH & St John WM (1991). Lesions in retrotrapezoid nucleus decrease ventilatory output in anesthetized or decerebrate cats. J Appl Physiol (1985) 71, 1364–1375. [DOI] [PubMed] [Google Scholar]
- Okada Y, Chen Z, Jiang W, Kuwana S & Eldridge FL (2002). Anatomical arrangement of hypercapnia‐activated cells in the superficial ventral medulla of rats. J Appl Physiol 93, 427–439. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K & Kawakami K (2008). CO2‐sensitive preinspiratory neurons of the parafacial respiratory group express Phox2b in the neonatal rat. J Neurosci 28, 12845–12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K & Kawakami K (2009). Phox2b, RTN/pFRG neurons and respiratory rhythmogenesis. Respir Physiol Neurobiol 168, 13–18. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K & Kawakami K (2012). Postsynaptic mechanisms of CO2 responses in parafacial respiratory neurons of newborn rats. J Physiol 590, 1615–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, Staley KJ & Mody I (1991). Perpetual inhibitory activity in mammalian brain slices generated by spontaneous GABA release. Brain Res 545, 142–150. [DOI] [PubMed] [Google Scholar]
- Pagliardini S, Janczewski WA, Tan W, Dickson CT, Deisseroth K & Feldman JL (2011). Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J Neurosci 31, 2895–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer JR, Fencl V, Heisey SR & Held D (1965). Role of cerebral fluids in control of respiration as studied in unanesthetized goats. Am J Physiol 208, 436–450. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C & Brunet JF (1999). The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature 399, 366–370. [DOI] [PubMed] [Google Scholar]
- Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB & Smith JC (2009). Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci 29, 3720–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J, Huang F, Naikawadi RP, Kim KS, Said T & Lum H (2006). Lysophosphatidylcholine impairs endothelial barrier function through the G protein‐coupled receptor GPR4. Am J Physiol Lung Cell Mol Physiol 291, L91–101. [DOI] [PubMed] [Google Scholar]
- Ramanantsoa N, Hirsch MR, Thoby‐Brisson M, Dubreuil V, Bouvier J, Ruffault PL, Matrot B, Fortin G, Brunet JF, Gallego J & Goridis C (2011). Breathing without CO2 chemosensitivity in conditional Phox2b mutants. J Neurosci 31, 12880–12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E & Dymecki SM (2011). Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science 333, 637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB (1995). Response to CO2 of neurons in the rostral ventral medulla in vitro . J Neurophysiol 73, 933–944. [DOI] [PubMed] [Google Scholar]
- Richerson GB (2004). Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci 5, 449–461. [DOI] [PubMed] [Google Scholar]
- Richerson GB & Messer C (1995). Effect of composition of experimental solutions on neuronal survival during rat brain slicing. Exp Neurol 131, 133–143. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Hodges MR, Dohle CI & Diez‐Sampedro A (2005). Homing in on the specific phenotype(s) of central respiratory chemoreceptors. Exp Physiol 90, 259–266; discussion 266‐259. [DOI] [PubMed] [Google Scholar]
- Richerson GB & Wu Y (2003). Dynamic equilibrium of neurotransmitter transporters: not just for reuptake anymore. J Neurophysiol 90, 1363–1374. [DOI] [PubMed] [Google Scholar]
- Romero G, Pujol M & Pauwels PJ (2006). Reanalysis of constitutively active rat and human 5‐HT7a receptors in HEK‐293F cells demonstrates lack of silent properties for reported neutral antagonists. Naunyn Schmiedebergs Arch Pharmacol 374, 31–39. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Chang DA & Guyenet PG (2006). Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol 499, 64–89. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Hamann M & Attwell D (2003). Multiple modes of GABAergic inhibition of rat cerebellar granule cells. J Physiol 548, 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick G (2002). Mechanisms of biogenic amine neurotransmitter transporters In Contemporary Neuroscience: Neurotransmitter Transporters: Structure, Function, and Regulation, 2nd edn, ed. MEA Reith, pp. 25–52. Humana Press, Inc., Totowa, NJ. [Google Scholar]
- Rudnick G & Wall SC (1992). The molecular mechanism of “ecstasy” [3,4‐methylenedioxy‐methamphetamine (MDMA)]: serotonin transporters are targets for MDMA‐induced serotonin release. Proc Natl Acad Sci U S A 89, 1817–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffault PL, D'Autreaux F, Hayes JA, Nomaksteinsky M, Autran S, Fujiyama T, Hoshino M, Hagglund M, Kiehn O, Brunet JF, Fortin G & Goridis C (2015). The retrotrapezoid nucleus neurons expressing Atoh1 and Phox2b are essential for the respiratory response to CO2 . Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Severinghaus JW & Basbaum AI (1992). Medullary CO2 chemoreceptor neuron identification by c‐fos immunocytochemistry. J Appl Physiol 73, 96–100. [DOI] [PubMed] [Google Scholar]
- Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, Jiang W, Conlon RA, Strowbridge BW & Deneris ES (2005). A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci U S A 102, 16472–16477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Stornetta RL, Stornetta DS, Onengut‐Gumuscu S, Farber EA, Turner SD, Guyenet PG & Bayliss DA (2017). Neuromedin B expression defines the mouse retrotrapezoid nucleus. J Neurosci 37, 11744–11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR & Feldman JL (1989). Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol 281, 69–96. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA & Guyenet PG (2006). Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci 26, 10305–10314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Tommasi E, Molina D, Sah R, Brosnihan KB, Diz D & Petrovic S (2016). Deletion of proton‐sensing receptor GPR4 associates with lower blood pressure and lower binding of angiotensin II receptor in SFO. Am J Physiol Renal Physiol 311, F1260–F1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL & Guyenet PG (2006). Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2‐sensitive neurons in rats. J Physiol 572, 503–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teran FA, Massey CA & Richerson GB (2014). Serotonin neurons and central respiratory chemoreception: where are we now? Prog Brain Res 209, 207–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW & Jacobs BL (1995). Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci 15, 5346–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Shi Y, Shu S, Guyenet PG & Bayliss DA (2013). Phox2b‐expressing retrotrapezoid neurons are intrinsically responsive to H+ and CO2 . J Neurosci 33, 7756–7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Bradley SR & Richerson GB (2002). Quantification of the response of rat medullary raphe neurones to independent changes in pHo and P CO2 . J Physiol 540, 951–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Pizzonia JH & Richerson GB (1998). Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiol 511, 433–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W & Richerson GB (1999). Development of chemosensitivity of rat medullary raphe neurons. Neuroscience 90, 1001–1011. [DOI] [PubMed] [Google Scholar]
- Wang W, Tiwari JK, Bradley SR, Zaykin RV & Richerson GB (2001). Acidosis‐stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol 85, 2224–2235. [DOI] [PubMed] [Google Scholar]
- Weese‐Mayer DE, Berry‐Kravis EM, Zhou L, Maher BS, Silvestri JM, Curran ME & Marazita ML (2003). Idiopathic congenital central hypoventilation syndrome: analysis of genes pertinent to early autonomic nervous system embryologic development and identification of mutations in PHOX2b. Am J Med Genet A 123A, 267–278. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang W, Diez‐Sampedro A & Richerson GB (2007). Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT‐1. Neuron 56, 851–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang W & Richerson GB (2001). GABA transaminase inhibition induces spontaneous and enhances depolarization‐evoked GABA efflux via reversal of the GABA transporter. J Neurosci 21, 2630–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Buhlman L, Khan GM, Nichols RA, Jin G, McIntosh JM, Whiteaker P, Lukas RJ & Wu J (2011). Functional nicotinic acetylcholine receptors containing alpha6 subunits are on GABAergic neuronal boutons adherent to ventral tegmental area dopamine neurons. J Neurosci 31, 2537–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZC, Wyeth MS, Baltan‐Tekkok S & Ransom BR (2003). Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci 23, 3588–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JR, Shirasaki T, Soeda F & Takahama K (2014). Cholinergic EPSCs and their potentiation by bradykinin in single paratracheal ganglion neurons attached with presynaptic boutons. J Neurophysiol 112, 933–941. [DOI] [PubMed] [Google Scholar]


