A central tenet of systems neuroscience is that neuronal firing is the physiological substrate of sensory‐perceptual experiences. This doctrine is the motivation for attempts to answer a number of fundamental questions: what are the codes employed by neuronal populations to carry sensory information? What are the transformations in stimulus representation within pathways leading from sensory receptors to high‐level cerebral cortical regions?
The starting point of modern explorations can be taken as the classical work of Hubel and Wiesel, wherein they mapped visual receptive fields in a series of studies leading to the 1981 Nobel Prize. Many of these studies asked the question, what are the features of the visual image that make a given neuron fire? Implicit in this question is the assumption that the neuron's firing constitutes one component of the many‐neuron representation of that visual feature. In other words, a stimulus is seen (felt, heard …) as soon as its presence activates the neurons whose receptive fields are well aligned with the features of the stimulus.
Receptive field mapping, while responsible for enormous strides forward in charting sensory systems, has a fundamental limitation that is not always taken into account: a neuron will not fire if the investigator fails to proffer the right stimulus. As simple as it may sound, the implications are enormous. The problem has been addressed computationally (Hong et al. 2007) and a simplified cartoon is given in Fig. 1. The sensory world is portrayed in the figure as occupying three feature dimensions. The investigators’ microelectrode encounters a neuron that is selective (unknown to them) to the ellipsoid feature space. The neuron will fire with high probability whenever the presented stimulus is composed of the feature combination aligned with the bright white region of the ellipsoid; it will fire with lower probability in the dark‐shaded feature space shown on the figure. The investigators now present their chosen stimulus set – the tested feature space – occupying the two‐dimensional square. Since the presented stimuli evoke a low probability of firing, and do so only for a small percentage of test trials, the neuron will fire sparsely, and its feature space will be largely unsampled.
Figure 1.
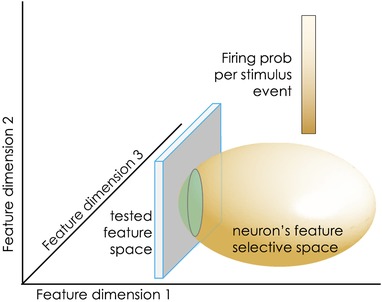
Schematic illustration of the estimation error that can occur when the stimulus space intersects with only the periphery of the studied neuron's feature‐selective space
With a fresh appreciation that sensory systems promote survival by extracting behaviourally relevant information about important (positive or negative) events in the surrounding world, in the last 20 or so years neuroscientists have begun to apply ‘naturalistic stimuli’: stimulus sets that contain innately meaningful features, for instance acoustic calls as a way to study auditory cortex (David et al. 2009). When whisker stimuli include the temporal noise that is characteristic of natural vibrations, rat barrel cortex firing rates are about 30% greater than those observed in response to unnatural, noise‐free stimuli (Lak et al. 2008).
The article by Ranjbar‐Slamloo & Arabzadeh (2019) in this issue of The Journal of Physiology adds a crucial piece to the puzzle. They performed loose cell‐attached recording across layers of the primary somatosensory cortex of awake mice. This method of recording allows blind targeting in a manner that is unbiased to a neuron's background rate of spiking. In the first step, the authors characterised neuronal activity evoked in response to well‐controlled whisker vibrations. Only about 1/4 of neurons responded to the strongest vibration – the ‘sharp’ stimulus. This was surprising given that under anaesthesia while using an identical method of stimulation and recording, the sharp stimulus activated a much higher proportion (about 3/4) of cortical neurons in the study of Ranjbar‐Slamloo & Arabzadeh (2017). So while whisker vibrations were represented by a dense code in the anaesthetised state, they were represented by a ‘sparse’ code in the awake state due to a seemingly narrower neuronal feature space. The authors then employed manual probing of whiskers to efficiently scan the stimulus space for any features that might activate a neuron, and to better capture the complex interactions between whiskers and objects that occur in natural settings. They found that cortical neurons which did not produce spiking activity in response to the sharp stimulus instead responded to a specific feature or a combination of features of manual stimulation. The manual stimulation revealed examples of tuning to non‐classical stimulus features such as a light tap on the whisker or pressure exerted along the whisker axis. A number of neurons responded exclusively when the mouse moved its whiskers against an object (generative mode), but did not exhibit any activity when the object was passively moved against whiskers. These examples demonstrate selectivity to stimulus dimensions that would have remained unexplored had the authors used only the low‐dimensional stimulus set.
Besides reminding neuroscientists that laboratory stimulus sets must be created with great attention to what might be natural and relevant for the sensory system, the present study forces us to think carefully about the functional role of the superficial layers of sensory cortex. While the common finding of sparse superficial layer firing has led neuroscientists to elaborate hypotheses about how sensory cortex processes and distributes information, the firing might not in fact be sparse when the right stimulus is encountered. Do natural stimuli excite superficial layer neurons, thus boosting the excitability and information processing capacity of the underlying layers?
Additional information
Competing interests
None declared.
Author contributions
Sole author.
Funding
EC | European Research Council (ERC): 294498; Human Frontier Science Program (HFSP): RGP0015/2013; Fifth Framework Programme (EC Fifth Framework Programme): 215910; Ministero dell'Istruzione, dell'Università e della Ricerca (Ministry of Education, Research and Universities): GA 280778.
Edited by: David Wyllie & Matthew Nolan
Linked articles This Perspectives article highlights an article by Ranjbar‐Slamloo & Arabzadeh. To read this article, visit https://doi.org/10.1113/JP277506. The article by Ranjbar‐Slamloo & Arabzadeh is also highlighted in a Journal Club article by Buchan et al. To read this article, visit https://doi.org/10.1113/JP278286.
References
- David SV, Mesgarani N, Fritz JB & Shamma SA (2009). Rapid synaptic depression explains nonlinear modulation of spectro‐temporal tuning in primary auditory cortex by natural stimuli. J Neurosci 29, 3374–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Agüera Y, Arcas B & Fairhall AL (2007). Single neuron computation: From dynamical system to feature detector. Neural Comput 19, 3133–3172. [DOI] [PubMed] [Google Scholar]
- Lak A, Arabzadeh E & Diamond ME (2008) Enhanced response of neurons in rat somatosensory cortex to stimuli containing temporal noise. Cereb Cortex 18, 1085–1093. [DOI] [PubMed] [Google Scholar]
- Ranjbar‐Slamloo Y & Arabzadeh E (2017). High‐velocity stimulation evokes “dense” population response in layer 2/3 vibrissal cortex. J Neurophysiol 117, 1218–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjbar‐Slamloo Y & Arabzadeh E (2019). Diverse tuning underlies sparse activity in layer 2/3 vibrissal cortex of awake mice. J Physiol 597, 2803–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]


