Abstract
Key points
A physiological response to increase microcirculatory oxygen extraction capacity at high altitude is to recruit capillaries.
In the present study, we report that high altitude‐induced sublingual capillary recruitment is an intrinsic mechanism of the sublingual microcirculation that is independent of changes in cardiac output, arterial blood pressure or systemic vascular hindrance.
Using a topical nitroglycerin challenge to the sublingual microcirculation, we show that high altitude‐related capillary recruitment is a functional response of the sublingual microcirculation as opposed to an anatomical response associated with angiogenesis.
The concurrent presence of a low capillary density and high microvascular reactivity to topical nitroglycerin at sea level was found to be associated with a failure to reach the summit, whereas the presence of a high baseline capillary density with the ability to further increase maximum recruitable capillary density upon ascent to an extreme altitude was associated with summit success.
Abstract
A high altitude (HA) stay is associated with an increase in sublingual capillary total vessel density (TVD), suggesting microvascular recruitment. We hypothesized that microvascular recruitment occurs independent of cardiac output changes, that it relies on haemodynamic changes within the microcirculation as opposed to structural changes and that microcirculatory function is related to individual performance at HA. In 41 healthy subjects, sublingual handheld vital microscopy and echocardiography were performed at sea level (SL), as well as at 6022 m (C2) and 7042 m (C3), during ascent to 7126 m within 21 days. Sublingual topical nitroglycerin was applied to measure microvascular reactivity and maximum recruitable TVD (TVDNG). HA exposure decreased resting cardiac output, whereas TVD (mean ± SD) increased from 18.81 ± 3.92 to 20.92 ± 3.66 and 21.25 ± 2.27 mm mm−2 (P < 0.01). The difference between TVD and TVDNG was 2.28 ± 4.59 mm mm−2 at SL (P < 0.01) but remained undetectable at HA. Maximal TVDNG was observed at C3. Those who reached the summit (n = 15) demonstrated higher TVD at SL (P < 0.01), comparable to TVDNG in non‐summiters (n = 21) at SL and in both groups at C2. Recruitment of sublingual capillary TVD to increase microcirculatory oxygen extraction capacity at HA was found to be an intrinsic mechanism of the microcirculation independent of cardiac output changes. Microvascular reactivity to topical nitroglycerin demonstrated that HA‐related capillary recruitment is a functional response as opposed to a structural change. The performance of the vascular microcirculation needed to reach the summit was found to be associated with a higher TVD at SL and the ability to further increase TVDNG upon ascent to extreme altitude.
Keywords: microcirculation, hemodynamic monitoring, capillary density, hand‐held video microscopy, hypoxia, vascular reactivity
Key points
A physiological response to increase microcirculatory oxygen extraction capacity at high altitude is to recruit capillaries.
In the present study, we report that high altitude‐induced sublingual capillary recruitment is an intrinsic mechanism of the sublingual microcirculation that is independent of changes in cardiac output, arterial blood pressure or systemic vascular hindrance.
Using a topical nitroglycerin challenge to the sublingual microcirculation, we show that high altitude‐related capillary recruitment is a functional response of the sublingual microcirculation as opposed to an anatomical response associated with angiogenesis.
The concurrent presence of a low capillary density and high microvascular reactivity to topical nitroglycerin at sea level was found to be associated with a failure to reach the summit, whereas the presence of a high baseline capillary density with the ability to further increase maximum recruitable capillary density upon ascent to an extreme altitude was associated with summit success.
Introduction
Microcirculatory alterations play an important determinant role in states of compromised tissue oxygenation. Exposure to high altitude has been hypothesized to increase capillary total vessel density (TVD) as an adaptive microvascular response to augmenting microcirculatory diffusion capacity and thereby its oxygen extraction capacity. Recent human studies indicate that subjects indeed respond to high altitude by a recruitment of sublingual capillary TVD (Martin et al. 2009, 2010; Gilbert‐Kawai et al. 2017). However, it is unknown whether this increase TVD passively follows changes in cardiac output and arterial blood pressure, or whether it is the result of adaptive response of the microcirculation itself. In disease states such as septic and cardiogenic shock, divergence between micro‐ and macrocirculatory function has been reported (De Backer et al. 2004, 2006). Investigations in animal models have found both structural and functional adaptations of the microcirculation in response to hypoxia (Parthasarathi & Lipowsky, 1999; Deveci et al. 2001), although it remains uncertain which mechanism primarily explains high altitude‐induced microvascular alterations in humans. To examine these adaptive responses, measurement of microvascular reactivity in response to vasodilatators such as nitroglycerin and acetylcholine as direct and endothelium cell‐dependent nitric oxide donors, respectively, has been developed to identify maximal microcirculatory reserve capacity of capillaries previously unfilled with red blood cells. Intra‐arterial (Yoshida et al. 1991; Thanyasiri et al. 2005) and intra‐venous (Buise et al. 2006) application of nitroglycerin, as well as sublingual topical application of acetylcholine (De Backer et al. 2004, 2006) and nitroglycerin (Buise et al. 2005; Hilty et al. 2017), had been examined previously for this purpose. In one study, sublingual topical nitroglycerin stimuli were applied to measure microvascular reactivity in healthy subjects by assessing the response in TVD (Hilty et al. 2017). In such a way, a local vasodilatory challenge can be used to identify the maximum recruitable TVD in response to nitroglycerin.
Against this background, the hypotheses that we aimed to test in the present study were that (i) exposure to high altitude leads to an increase in sublingual capillary TVD independent of systemic haemodynamic changes; (ii) microvascular reactivity as assessed by topical application of nitroglycerin may identify the role of microcirculatory recruitment capacity in relation to the recruitment of capillary TVD in response to high altitude exposure; and (iii) that the individual ability to perform at high altitude is related to these functional properties of the microcirculation.
Methods
This prospective observational cohort study was performed during the Swiss High Altitude Medical Research Expedition 2013 to Mount Himlung Himal, Nepal (7126 m) and, to our knowledge, represents the largest cohort of mountaineers available for systematic examination of the sublingual microcirculation at such extreme altitudes. Informed consent for study participation and publication of anonymized data was obtained from each subject prior to enrollment. The study was approved by the ethics committee of the University of Bern (KEK 226/12, ClinicalTrials.gov Identifier: NCT01953198) and was conducted in accordance with the Declaration of Helsinki.
Study population and design
Forty‐one healthy Caucasian persons (age 45.8 ± 11.9 years, 22/41 (54%) male, weight 69.0 ± 11.4 kg, height 174 ± 9 cm, body mass index 23.1 ± 4.6 kg m−2) were included in the study. All were physically fit but not involved in elite sport and had previous mountaineering experience without any history of severe acute mountain sickness or high altitude pulmonary oedema. To avoid any influence from previous hypoxic exposure, subjects did not travel to altitudes >2500 m within 4 weeks before the onset of the study. Details on subject selection criteria are provided elsewhere (Kottke et al. 2015). All subjects underwent examination of the sublingual microcirculation by handheld vital microscopy (HVM) before and after topical application of nitroglycerin, alongside a clinical examination, echocardiography, and venous and arterial blood sampling for baseline assessment at the University Hospital of Bern (sea level, SL; 553 m). Six weeks later, all subjects ascended by foot within 1 week from Besisahar, Nepal (800 m) to Himlung Himal Base Camp (BC, 4844 m) and within the following week to Camp 2 (C2, 6022 m). After an additional acclimatization period of 1 week at BC, a final ascent to Camp 3 (C3, 7042 m) and the summit of mount Himlung Himal (7126 m) was attempted (Fig. 1). Measurements were repeated on the first day after arriving at C2 and C3. Microcirculatory measurements at C3 were performed in 10 out of 15 subjects. The systemic haemodynamic variables determined included cardiac output, systemic vascular hindrance and oxygen delivery to allow comparison with the properties of the microcirculation. Ascent profile, accommodation and the availability of food and fluids were standardized across all subjects. Ambient temperature in the examination tents was kept between 17 and 22°C, except at C3 (Fig. 1). Exposure to environmental factors throughout the expedition, such as temperature and precipitation, was comparable in all subjects as a result of temporal synchronicity of their ascent within a time window of 48 h. Subjects were included in the study as long as they did not suffer from severe acute mountain sickness, high altitude cerebral oedema, high altitude pulmonary oedema or exhaustion leading to administration of bottled oxygen, medication for high altitude illness, premature descent or evacuation by helicopter. Subjects reaching C3 were assigned to the summiters group and all other subjects to the non‐summiters group, enabling comparison of measurements at SL, as well as C2, by performance at high altitude.
Figure 1. Ascent profile, ambient conditions and examination protocol.
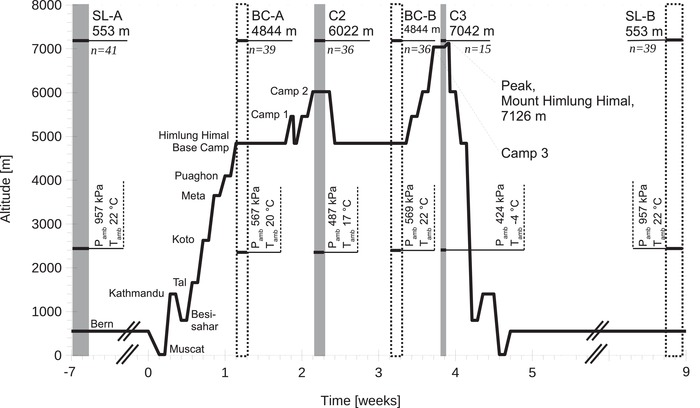
Grey areas denote measurement time points for macro‐ and microcirculation at different camps during ascent to 7126 m, dotted areas denote additional specification of ambient conditions. Microcirculatory and macrocirculatory measurements including echocardiography, as well as arterial and venous blood sampling, were performed at SL, 6022 m and 7042 m. SL, sea level; BC, base camp; C2, camp 2; C3, camp 3; Pamb, ambient atmospheric pressure; Tamb, ambient temperature.
Clinical and laboratory examination
Clinical examination, including chest auscultation and measurement of heart rate and systemic arterial blood pressure, was performed at SL, C2 and C3. Mean systemic arterial blood pressure was measured via an automated manometer using the oscillometric technique, identifying the sequentially decreased upper arm cuff pressure at the point where the amplitude of pulse wave‐induced oscillations were maximal. The measurement was not available at C3 for technical reasons. Venous blood was sampled from an antecubital vein into heparinized containers and was immediately centrifuged and deep frozen on site at all timepoints. The N‐terminal fragment of the pro‐peptide of brain natriuretic peptide was then measured with a fully automated sandwich electro‐chemiluminescence immunoassay method (Elecsys 2010 analyser; Roche Diagnostics, Basel, Switzerland), as described in more detail elsewhere (Prontera et al. 2004). Arterial blood was sampled from the radial artery after successful completion of Allen's test. Blood gas analysis on arterial blood samples was performed on site without delay (Epoc System; Alere, Waltham, MA, USA). The presence of acute mountain sickness was assessed using the Lake Louise Score for acute mountain sickness (LLS‐AMS) (Coates & Houston, 1992; Roach et al. 1993), as well as the environmental symptoms questionnaire acute mountain sickness cerebral sub score (AMS‐c) (Sampson et al. 1993), with cut‐off values for the presence of acute mountain sickness at LLS‐AMS ≥ 5 and AMS‐c ≥ 0.7.
Echocardiography and assessment of the macrocirculation
Echocardiography examinations were performed at SL, C2 and C3 using a portable device with tissue Doppler imaging capability (Vivid‐i ultrasound system; GE Healthcare, Chicago, IL, USA). Stroke volume was measured by multiplying left ventricular outflow tract velocity time integral as recorded in the parasternal short axis by pulsed wave Doppler and the left ventricular outflow tract area derived from its diameter. Cardiac output was obtained by multiplication of stroke volume and heart rate derived from the peak‐to‐peak distance of the outflow tract Doppler signal. Pulmonary vascular resistance was assessed using the tricuspid regurgitation maximum velocity to right ventricular outflow tract velocity time integral ratio without correction for right atrial pressure, yielding RV/RA (Rudski et al. 2010). Systolic right heart function was assessed by tricuspid annular plane systolic excursion. All measurements were performed in triplicate and recorded digitally for offline assessment, which was performed after removal of subject identifiables by a single operator. The mean of the three respective measurements is reported. Systemic vascular hindrance was calculated as Z = Pa Q−1 η−1 (Z, systemic vascular hindrance; Pa, mean systemic arterial pressure; Q, cardiac output; η, blood viscosity), as described in more detail elsewhere (Vázquez et al. 2010). Because of a linear relationship between haematocrit from 35% to 55% and blood viscosity as previously demonstrated (Cinar et al. 1999) and assuming a dynamic viscosity of water of 6.913 10−4 Pa s at 37°C, blood viscosity was calculated from haematocrit as η = 0.0909 Hct + 0.8849 Pa s (η, blood viscosity; Hct, haematocrit).
Assessment of the sublingual microcirculation
High resolution HVM image sequences of the sublingual microcirculation were obtained using the incident dark field technique (Aykut et al. 2015) with a CytoCam hand‐held microscope (Braedius Medical, Huizen, The Netherlands) connected to a portable computer via a custom built camera controller. Image sequences were recorded to a solid‐state storage device at a rate of 25 frames s–1 and a resolution of 2208 × 1648 pixels covering 1.242 mm2 of tissue. The device performed as expected even at extreme altitude. All examinations in the present study were performed by a single, experienced operator (MH) with the subject in supine position and at rest. Four image sequences of the sublingual area were recorded digitally to compensate for changes in microscope field‐of‐view inbetween measurements in compliance with international consensus on the measurement of the sublingual microcirculation (De Backer et al. 2007; Ince et al. 2018). To assess the microvascular reactivity, a drop (0.05 mL) of 1% (4.4 × 10−2 m) nitroglycerin solution (Perlinganit isotonic infusion solution; UBC Pharma, Bulle, Switzerland) diluted 1:102 with 0.9% sodium chloride, yielding 0.005 mg (2.2 × 10−2 μmol) per dose, was applied to the sublingual area. This technique has been previously demonstrated to only affect the local microcirculation and not the systemic circulation (Hilty et al. 2017). Two further image sequences at different sublingual locations were then recorded after 30 s. The number of video recordings obtained after topical application of nitroglycerin was restricted to two each to limit total examination time. Each image sequence was recorded for 10 s, within which a stable sequence of ≥150 frames was identified during offline analysis. In total, 696 video clips of the sublingual microcirculation obtained in this manner were graded for acceptable quality of image using Massey's scoring system (Massey et al. 2013) and selected for analysis if the Massey score <10. Overall, 694 image sequences that met these criteria were included for further analysis. The analysed image sequences had a mean Massey score of 1.1 ± 0.9 (illumination 0.2 ± 0.4, duration 0.3 ± 0.4, focus 0.3 ± 0.5, content 0.3 ± 0.4, stability 0.2 ± 0.4, pressure 0.2 ± 0.4). TVD was calculated from the video files as total length of all capillaries (<20 μm) divided by the field of view using CytoCam Tools 1.7.12 (Braedius Medical) image processing software. TVD was measured before and after topical application of nitroglycerin to assess maximum recruitable TVD in response to nitroglycerin (TVDNG), as well as microvascular reactivity (ΔTVDNG) comprising the difference between the two. Previous studies have demonstrated the validity of the software to reliably measure TVD (Carsetti et al. 2016; Gassmann et al. 2016; Hilty et al. 2017). Parameters are reported as the means of all obtained video clips per time point, intervention and subject.
Statistical analysis
Associations between macro‐ and microcirculatory parameters and altitude, as well as topical vasodilatory intervention, were tested using linear mixed effects model analysis (Bates et al. 2015). As independent variable fixed effects, high altitude exposure, topical vasodilatory intervention, sex and age were entered into the model without interaction terms. As random effects, intercepts for subjects and per‐subject random slopes for the effect on dependent variables were employed. Visual inspection of residual plots did not reveal any obvious deviations from homoscedasticity or normality. P values were calculated using a likelihood ratio test of the full model with the effect in question against a ‘null model’ without the effect in question (Baayen et al. 2008). Additionally, P values for fixed effects in a multidimensional model comprising high altitude exposure and topical vasodilatory intervention or the individual ability to reach the summit were obtained by Satterthwaite approximation (Barr et al. 2013). Pairwise related‐samples t tests with Benjamini & Hochberg's correction algorithm (Benjamini & Hochberg, 1995) were used in one‐dimensional models comparing effects of high altitude exposure on the change in microcirculatory parameters induced by topical vasodilatory intervention. P < 0.05 (two‐sided) was considered statistically significant. For all statistical analysis, a fully scripted data management pathway was created within the R environment for statistical computing, version 3.4.2 (R Development Core Team, 2011). Linear mixed effects modelling was performed using the R library lme4, version 1.1.13 (Bates et al. 2015). Graphical output was generated using the R library ggplot2, version 2.2.1 (Wickham, 2010). Values are given as the mean ± SD.
Availability of data and materials
The dataset supporting the conclusions of this article is available via the Zenodo research data repository (Hilty et al. 2018).
Results
Subject characteristics and macrocirculatory status at sea level and high altitude
The majority of the 41 subjects included in the study successfully reached C2 (n = 36), with two subjects being excluded before reaching base camp and the remaining three between base camp and C2. The 15 subjects reaching C3 were classified as the summiters group (Fig. 1). Overall, seven subjects dropped out of the study before reaching C3 as a result of acute mountain sickness, one each because of high altitude cerebral oedema and possible high altitude pulmonary oedema, and 17 because of physical exhaustion and an inability to proceed. These were classified as the non‐summiters group (n = 26). Exposure to high altitude at C2 and C3 led to a marked decrease in arterial haemoglobin oxygen saturation, arterial oxygen partial pressure, arterial oxygen content and delivery of oxygen, accompanied by an increase in serum lactate concentration (Table 1). Arterial haemoglobin oxygen saturation and arterial oxygen partial pressure at C3 ranged between 29.7% and 81.3% and between 3.29 kPa and 4.91 kPa (24.7–36.8 mmHg), respectively. Haemoglobin concentration and haematocrit both increased by 18% at C3 compared to SL (Fig. 2 and Table 1). Despite a high altitude‐related increase in heart rate, cardiac output decreased by 18% from SL to C3, associated with a decrease in stroke volume (Fig. 2 and Table 1). Mean arterial pressure, as well as systemic vascular hindrance, remained unchanged. Pulmonary artery pressure, as measured by echocardiography, brain natriuretic peptide and lactate, increased at C2 and C3. A measurable impairment in right ventricular function was present at C2 but reverted at C3. Similarly, the highest values for LLS‐AMS and AMS‐c were observed at C2 (Table 1).
Table 1.
Subject characteristics at sea level and high altitude
| SL | C2 (6022 m) | C3 (7042 m) | ||
|---|---|---|---|---|
| (n = 41) | (n = 36) | (n = 15) | P | |
| (%) | 97.6 ± 0.8 | 72.7 ± 8.8 | 65.8 ± 14.2 | <0.0001 C2, C3 |
| , kPa (mmHg) | 12.7 ± 1.1 (95.4 ± 7.9) | 4.6 ± 0.7 (34.3 ± 5.0) | 4.1 ± 0.6 (30.4 ± 4.4) | <0.0001 C2, C3 |
| , kPa (mmHg) | 5.1 ± 0.4 (38.0 ± 3.2) | 2.8 ± 0.3 (21.0 ± 2.6) | 1.8 ± 0.3 (13.8 ± 2.5) | <0.0001 C2, C3 |
| pH (art.) [1] | 7.44 ± 0.02 | 7.50 ± 0.02 | 7.53 ± 0.06 | <0.0001 C2, C3 |
| HCO3 − (art) (mmol L−1) | 25.6 ± 1.7 | 16.3 ± 1.6 | 11.6 ± 1.7 | <0.0001 C2, C3 |
| Heart rate (min−1) | 64 ± 11 | 83 ± 16 | 95 ± 12 | <0.0001C2, C3 |
| Stroke volume (mL) | 135 ± 50 | 87 ± 39 | 76 ± 22 | <0.0001 C2, C3 |
| η (mPa s) | 3.34 ± 0.18 | 3.58 ± 0.21 | 3.86 ± 0.20 | <0.0001 C2, C3 |
| Z (m−3) 104 | 2.79 ± 1.00 | 3.05 ± 0.80 | * | 0.10 |
| CaO2 (ml L−1) | 194 ± 12 | 154 ± 18 | 162 ± 24 | <0.0001 C2, C3 |
| DO2 (mL min−1) | 1612 ± 462 | 1034 ± 419 | 1174 ± 343 | <0.0001 C2, C3 |
| Mean Pa (mmHg) | 90 ± 10 | 87 ± 9 | * | 0.13 |
| RV/RA (mmHg) | 23 ± 4 | 49 ± 11 | 53 ± 10 | <0.0001 C2, C3 |
| TAPSE (mm) | 26 ± 4 | 24 ± 3 | 25 ± 4 | <0.01C2 |
| Lactate (art) (mmol L−1) | 0.8 ± 0.2 | 2.2 ± 0.8 | 2.6 ± 1.1 | <0.0001 C2, C3 |
| NT‐proBNP (ng L−1) | 53 ± 36 | 188 ± 214 | 615 ± 512 | <0.0001 C2, C3 |
| LLS‐AMS [1] | 0.13 ± 0.80 | 3.08 ± 3.07 | 1.67 ± 2.61 | <0.0001 C2, C3 |
| AMS‐c [1] | 0.01 ± 0.03 | 0.47 ± 0.56 | 0.39 ± 0.40 | <0.0001 C2, C3 |
Values are given as the mean ± SD. *Arterial pressure and systemic vascular hindrance at C3 are not available for technical reasons. Individual fixed effects analysis for intervention is represented by C2 where P < 0.05 for high altitude (6022 m) at C2 and C3 for high altitude (7042 m) at C3, respectively. SL, sea level; C2, camp 2; C3, camp 3; , arterial haemoglobin oxygen saturation; , arterial oxygen partial pressure; , arterial carbon dioxide partial pressure; HCO3 −, bicarbonate concentration; Hct, haematocrit; Q, cardiac output; η, blood viscosity; Z, systemic vascular hindrance; CaO2, arterial oxygen content; DO2, delivery of oxygen; Pa, systemic arterial pressure; RV/RA, right ventricular to right atrial pressure gradient; TAPSE, tricuspid annular plane systolic excursion; NT‐proBNP, N‐terminal fragment of the pro‐peptide of brain natriuretic peptide; LLS‐AMS, Lake Louise score for acute mountain sickness; AMS‐c, environmental symptoms questionnaire acute mountain sickness cerebral sub score; art, systemic arterial blood sample; SD, standard deviation.
Figure 2. Effect of ascent to 7042 m on blood haemoglobin concentration, TVD and cardiac output.
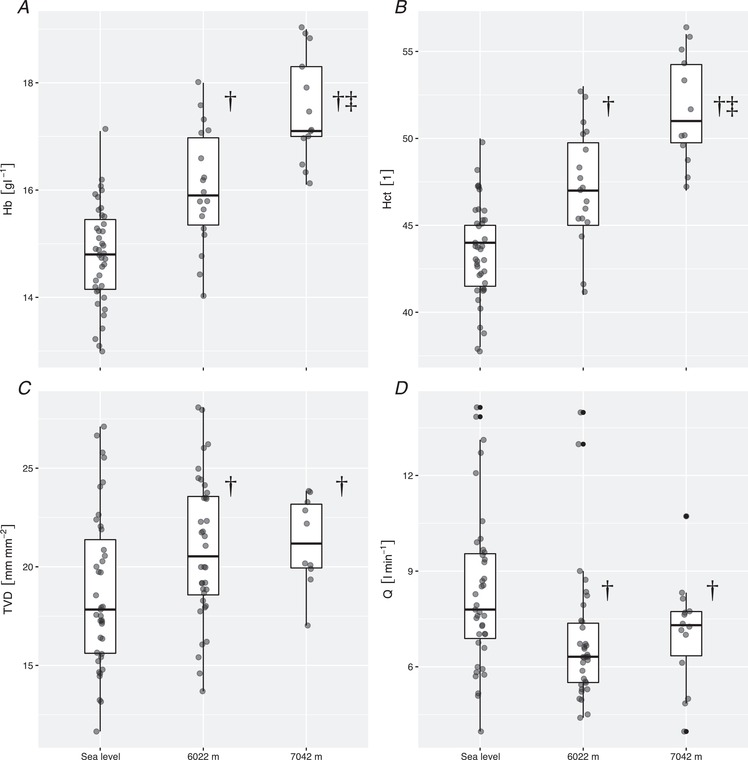
Ascent from sea level to 6022 m and 7042 m increased both haemoglobin concentration (A) and haematocrit (B). At the same time, it led to an increase in TVD (C) despite a decrease in global cardiac output (D). †Values differing during exposure to 6022 m vs. sea level (P < 0.05), ‡Values during exposure to 7042 m vs. 6022 m (P < 0.05). Boxplots represent median, interquartile range and range. Hb, haemoglobin concentration; Hct, haematocrit; Q, cardiac output.
Microcirculatory measurements and microvascular reactivity at sea level and high altitude
A gradual increase in sublingual capillary TVD was observed from SL to C2 and as a tendency at C3 (Fig. 2 and Table 2). Exposure to C3 increased TVDNG compared to SL and C2 (Table 2). Sublingual topical application of nitroglycerin led to an increase in microcirculatory vessel density represented by ΔTVDNG at SL but not at C2 or C3 (Table 2). Representative still images of the sublingual microcirculation throughout high altitude exposure and a nitroglycerin challenge are shown in Fig. 3. A close inverse correlation was found between ΔTVDNG and baseline TVD (r = 0.68, P < 0.0001). No correlation was found between systemic vascular hindrance and ΔTVDNG or TVD (r = 0.21, P = 0.63 and r = 0.07, P = 0.60, respectively), nor between haematocrit and ΔTVDNG or TVD (r = 0.12, P = 0.39 and r = 0.12, P = 0.38, respectively).
Table 2.
Sublingual microcirculatory vessel density and microvascular reactivity during ascent to 7042 m
| SL | C2 (6022 m) | C3 (7042 m) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n = 41) | (n = 36) | (n = 10)* | ||||||||
| μ ± SD (SEM) | μ ± SD (SEM) | Δ high altitude (SE) | t | P | μ ± SD (SEM) | Δ high altitude (SE) | t | P | P (high altitude) | |
| TVD (mm mm−2) | 18.81 ± 3.92 (0.61) | 20.92 ± 3.66 (0.61) | 2.12 (0.73) | 2.93 | <0.01 | 21.25 ± 2.27 (0.72) | 1.70 (1.18) | 1.46 | 0.15 | <0.01 |
| TVDNG (mm mm−2) | 21.06 ± 3.60 (0.57) | 21.94 ± 2.79 (0.47) | 0.88 (0.70) | 1.26 | 0.21 | 23.59 ± 4.43 (1.40) | 2.56 (1.12) | 2.29 | 0.02 | |
| Δ nitroglycerin (SE), ΔTVDNG (mm mm−2) | 2.28 (0.71) | 1.05 (0.62) | 2.34 (1.50) | |||||||
| T | 3.20 | 1.69 | 1.56 | |||||||
| P | <0.01 | 0.10 | 0.13 | |||||||
| P (nitroglycerin) | <0.001 | |||||||||
Results are presented for total vessel density of capillaries defined as vessels with a diameter of <20 μm, of a two‐way linear mixed model analysis with nitroglycerin application, as well as exposure to high altitude as fixed effects and subject identity as a random effect. Values are given as the mean (μ) ± SD (SEM). P values for the effect of nitroglycerin application and exposure to high altitude represent likelihood ratio tests between a full mixed linear model and a null model without inclusion of the fixed effects. Data provided for each individual fixed effect are estimates across nitroglycerin application and exposure to high altitude (Δ, in the former case representing microvascular reactivity ΔTVDNG) with standard errors and t values, as well as P values obtained by Satterthwaite approximation. *Microcirculatory measurements at C3 were performed in 10 out of 15 subjects. TVD, total vessel density (capillaries <20 μm); TVDNG, maximum recruitable total vessel density by topical application of nitroglycerin; ΔTVDNG, microvascular reactivity in response to topical application of nitroglycerin; SE, standard error of the estimate; SD, standard deviation; SEM, standard error of the mean; NG, nitroglycerin; SL, sea level; C2, camp 2; C3, camp 3.
Figure 3. Representative images.
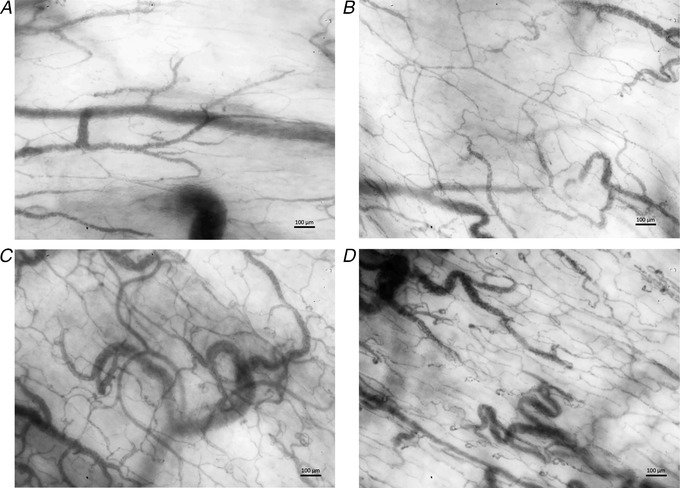
Representative still images of the sublingual microcirculation at sea level (A and C) and 7042 m (B and D), before and after recruitment of capillaries by topical application of nitroglycerin. Stimulation with topical nitroglycerin at sea level (C) lead to a recruitment of capillaries similar to that for exposure to high altitude (B); the highest capillary density was measured in summiters at 7042 m after stimulation with nitroglycerin (D). Moving image sequences are provided online via the Zenodo repository (Hilty et al. 2018).
Differences between summiters and non‐summiters
Comparable cardiac output and systemic vascular hindrance were observed in summiters and non‐summiters (Fig. 4). Similarly, arterial blood gas analysis, haematocrit, heart rate, systemic and pulmonary artery pressure and right heart function, as measured by echocardiography, as well as brain natriuretic peptide and serum lactate concentration, did not differ between both groups (Table 3). At SL, however, summiters had a higher sublingual capillary TVD than non‐summiters. The latter group increased their TVD at high altitude, reaching levels comparable to those measured in the summiters at SL and C2 (Fig. 5 and Table 3). An increase in microcirculatory recruitment following a nitroglycerin challenge was found at SL in non‐summiters but not in the summiters (Fig. 4 and Table 3). TVDNG in summiters, as well as in non‐summiters, reached comparable levels at SL and at C2 in both groups, corresponding to a decrease in ΔTVDNG in non‐summiters from SL to C2. The highest values for TVDNG were observed in summiters at C3 (Fig. 6 and Table 2).
Figure 4. Macro‐ and microcirculatory parameters in summiters and non‐summiters at sea level and 6022 m.
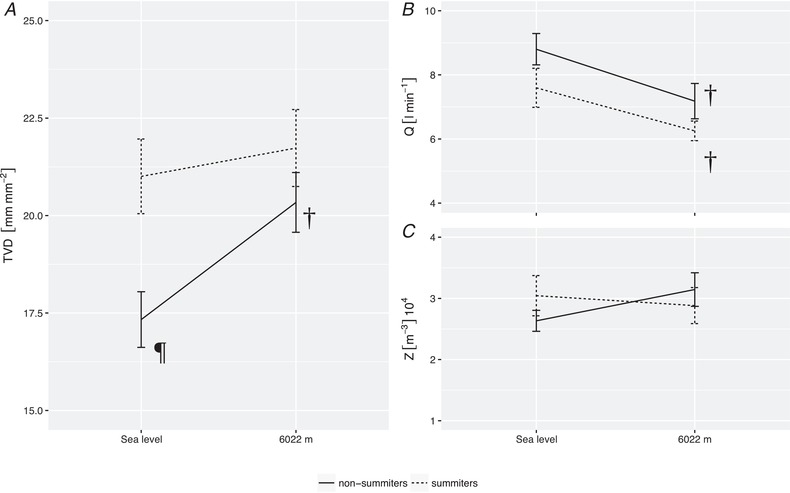
Subjects reaching the summit compared to those not reaching the summit demonstrated higher TVD at sea level, whereas both groups reached similar TVD at 6022 m (A). Differences between the two groups were reflected neither by cardiac output (B), nor systemic vascular hindrance (C). ¶Values differing after sublingual topical application of NG vs. native (P < 0.05), †Values differing during exposure to 6022 m vs. at sea level (P < 0.05). Lines and bars represent the mean; error bars represent the SEM. A common horizontal axis is used in (B) and (C). SEM, standard error of the mean; Q, cardiac output; Z, systemic vascular hindrance.
Table 3.
Differences in macrohaemodynamic parameters, sublingual microcirculatory vessel density and microvascular reactivity in subjects that have reached C3 (summiters) vs. those that have developed high altitude illness or physical exhaustion before reaching C3 (non‐summiters)
| SL | C2 (6022 m) | ||||||
|---|---|---|---|---|---|---|---|
| (n = 41) | (n = 36) | ||||||
| Non‐summiters | Summiters | Non‐summiters | Summiters | ||||
| (n = 26) | (n = 15) | (n = 21) | (n = 15) | P (summit) | P (nitroglycerin) | P (high altitude) | |
| , kPa (mmHg) | 12.8 ± 0.9 (95.8 ± 7.1) | 12.6 ± 1.2 (94.7 ± 9.2) | 4.7 ± 0.6 (35.3 ± 4.8) | 4.3 ± 0.7 (32.5 ± 5.4) | 0.46 | <0.0001 | |
| , kPa (mmHg) | 5.0 ± 0.4 (37.8 ± 2.6) | 5.1 ± 0.6 (38.4 ± 4.1) | 2.7 ± 0.3 (20.2 ± 2.5) | 3.0 ± 0.3 (22.4 ± 2.1) | 0.17 | <0.0001 | |
| Hct (art) (%) | 42.7 ± 3.2 | 44.7 ± 1.5 | 46.9 ± 3.5 | 47.7 ± 3.1 | 0.06 | <0.0001 | |
| Heart rate (min−1) | 64 ± 11 | 66 ± 10 | 82 ± 17 | 84 ± 15 | 0.52 | <0.0001 | |
| Stroke volume (mL) | 143 ± 49 | 121 ± 51 | 94 ± 45 | 78 ± 25 | 0.15 | <0.0001 | |
| η (mPa s] | 3.30 ± 0.20 | 3.42 ± 0.10 | 3.56 ± 0.22 | 3.61 ± 0.20 | 0.06 | <0.0001 | |
| CaO2 (mL L−1) | 191 ± 13 | 199 ± 7 | 156 ± 16 | 150 ± 23 | 0.30 | <0.0001 | |
| DO2 (mL min−1) | 1671 ± 430 | 1516 ± 510 | 1065 ± 487 | 977 ± 283 | 0.26 | <0.0001 | |
| RV/RA (mmHg) | 23.0 ± 4.9 | 21.9 ± 3.6 | 49.7 ± 10.1 | 48.4 ± 12.1 | 0.55 | <0.0001 | |
| TAPSE (mm) | 26.8 ± 3.8 | 25.3 ± 4.1 | 24.0 ± 4.1 | 24.0 ± 2.1 | 0.51 | <0.001 | |
| Lactate (art) (mmol L−1) | 0.8 ± 0.2 | 0.9 ± 0.3 | 2.0 ± 0.6 | 2.5 ± 1.1 | 0.17 | <0.0001 | |
| NT‐proBNP (ng L−1) | 56 ± 36 | 49 ± 37 | 157 ± 98 | 232 ± 312 | 0.37 | <0.001 | |
| LLS‐AMS [1] | 0.21 ± 1.02 | 0.00 ± 0.00 | 3.36 ± 2.50 | 2.67 ± 3.81 | 0.38 | <0.0001 | |
| AMS‐c [1] | 0.01 ± 0.03 | 0.01 ± 0.02 | 0.50 ± 0.40 | 0.44 ± 0.75 | 0.72 | <0.0001 | |
| TVD (mm mm−2) | 17.33 ± 3.50 (0.71) | 21.00 ± 3.71 (0.96)C2, S | 20.34 ± 3.51 (0.77)C2 | 21.73 ± 3.82 (0.99)C2 | <0.01 | 0.04 | <0.01 |
| TVDNG (mm mm−2) | 20.84 ± 4.01 (0.82)NG | 21.20 ± 2.99 (0.77) | 21.72 ± 3.02 (0.66) | 22.27 ± 2.49 (0.67) | |||
| Δ nitroglycerin (SE), ΔTVDNG (mm mm−2) | 3.52 (0.82)NG | 0.20 (1.27)C2, S | 1.39 (0.92) | 0.58 (1.01) | |||
Results are presented for macrohaemodynamic parameters of two‐way linear mixed model analysis with exposure to high altitude and the individual ability to reach the summit as fixed effects and subject identity as random effect. A three‐way linear mixed model analysis with nitroglycerin application as additional fixed effect was employed for the analysis of TVD of capillaries <20 μm. Values are given as the mean ± SD. SEM is additionally provided in parentheses for TVD and TVDNG. For microcirculatory analysis, individual fixed effects analysis for intervention is represented by C2 where P < 0.05 for high altitude (6022 m) at C2 vs. SL, by S where P < 0.05 in summiters vs. non‐summiters and by NG where P < 0.05 for nitroglycerin vs. native. , arterial oxygen partial pressure; , arterial carbon dioxide partial pressure; Hct, haematocrit; Q, cardiac output; η, blood viscosity; Z, systemic vascular hindrance; CaO2, arterial oxygen content; DO2, delivery of oxygen; RV/RA, right ventricular to right atrial pressure gradient; TAPSE, tricuspid annular plane systolic excursion; NT‐proBNP, N‐terminal fragment of the pro‐peptide of brain natriuretic peptide; TVD, total vessel density; TVDNG, maximum recruitable total vessel density by topical application of nitroglycerin; ΔTVDNG, microvascular reactivity in response to topical application of nitroglycerin; SE, standard error of the estimate; SD, standard deviation; SEM, standard error of the mean; NG, nitroglycerin; art, systemic arterial blood sample.
Figure 5. Microvascular reactivity in summiters and non‐summiters at sea level and 6022 m.
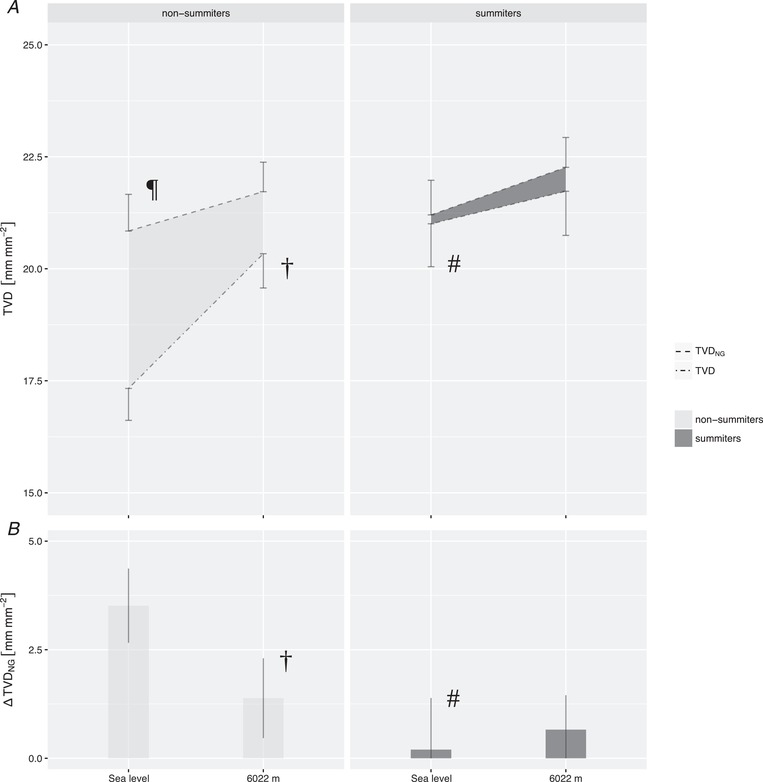
Sublingual topical application of nitroglycerin led to an increase in TVD at sea level predominantly in subjects who did not reach the summit (A). In both groups, maximum recruitable TVD (TVDNG) was similar at 6022 m. Microvascular reactivity in response to topical application of nitroglycerin, represented by ΔTVDNG, was depleted during ascent in subjects that did not reach the summit (B). #Values differing between summiters vs. non‐summiters (P < 0.05). ¶Values differing after sublingual topical application of NG vs. native (P < 0.05). †Values differing during exposure to 6022 m vs. at sea level (P < 0.05). Lines and bars represent the mean; error bars represent the SEM; the shaded area inbetween lines in (A) represents microvascular reactivity in response to topical application of nitroglycerin. SEM, standard error of the mean; ΔTVDNG, microvascular reactivity in response to topical application of nitroglycerin.
Figure 6. Effect of exposure to 7042 m on TVD and maximum recruitable total vessel density by topical application of nitroglycerin.
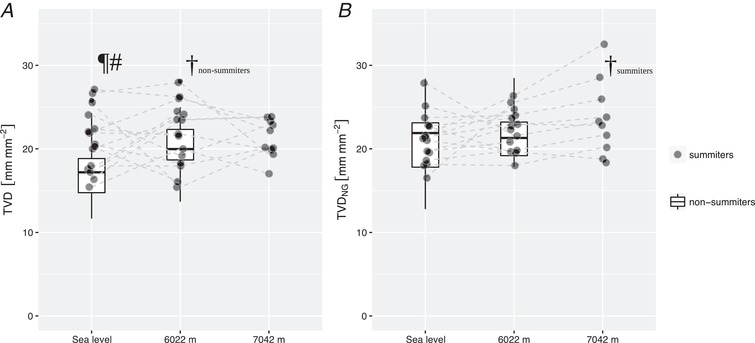
TVD did not further increase throughout ascent from sea level to 7042 m in summiters (A). Maximum recruitable total vessel density by topical application of nitroglycerin, however, was highest at 7042 m (B). #Values differing between summiters vs. non‐summiters (P < 0.05). ¶Values differing after sublingual topical application of NG vs. native (P < 0.05). †Values differing during exposure to 6022 m vs. at sea level (P < 0.05). Boxplots represent median, interquartile range and range. TVDNG, maximum recruitable total vessel density by topical application of nitroglycerin.
Discussion
The data obtained in the present study demonstrate that (i) high altitude associated recruitment of sublingual capillary TVD occurs despite a decrease in cardiac output, unchanged arterial pressure and systemic vascular hindrance and is thus an intrinsic mechanism of the microcirculation to increase oxygen extraction capacity; (ii) a nitroglycerin challenge to the microcirculation provokes the same maximum recruitable capillary TVD at SL, at high altitude, as well as when caused by a 2 week ascent to 6022 m; and (iii) non‐summiters have a lower TVD at SL than summiters when reaching a similar maximum recruitable capillary TVD upon a nitroglycerin challenge, suggesting that summiters have a reduced need to recruit their microcirculation because they are already functioning at an optimal oxygen extraction capacity with respect to delivery of oxygen carriers through the capillaries. Furthermore, the ability to increase maximum recruitable TVD at high altitude, in comparison with SL observed in summiters at 7042 m, is found to be associated with summit success, representing a way of further increasing microcirculatory oxygen extraction capacity as required.
Loss of haemodynamic coherence between macro‐ and microcirculation at extreme altitude
The increase in resting heart rate during exposure to 6022 m and 7042 m, as observed in the present study, corresponds with previous data (Lundby & van Hall, 2001) and has been attributed mostly to high altitude‐related sympathoexcitation and parasympathetic withdrawal (Siebenmann et al. 2017). Cardiac output at rest is expected to decrease towards SL values after an initial increase in well‐acclimatized lowlanders at moderate altitude (Hilty et al. 2016), as well as at extreme altitude (Reeves et al. 1987), whereas, in expedition settings, several previous studies have reported a decrease in cardiac output compared to baseline after 10–15 days (Wolfel et al. 1994; Sime et al. 1974), which is similar to the observation in the present study. Vascular hindrance, representing the contribution of vascular structure to the resistance to blood flow independent of high altitude‐related changes in blood viscosity effected by the increase in haematocrit, has been demonstrated to occur in an experimental animal model setting during 10 days of hypoxic exposure independent of changes in the systemic circulation (Pichon et al. 2012). Our data confirm the latter finding and show that systemic vascular hindrance stays constant in humans even at extreme altitude. The increase in brain natriuretic peptide probbaly represents strain exerted on the right ventricle as demonstrated by increased tricuspid annular plane systolic excursion. The observed increase in systolic pulmonary artery pressure was suggested to underlie this effect (Woods et al. 2013).
Exposure to hypoxia in a rat model has been found to be associated with an increase in muscle TVD demonstrating hypoxia‐induced capillary recruitment (Parthasarathi & Lipowsky, 1999). This response effectively decreases the diffusion distance from the capillaries to the tissue, thereby increasing microcirculatory oxygen extraction capacity as a response to oxygen deficiency. However, two previous studies assessing the human sublingual microcirculation during ascent to Mount Everest base camp (5364 m) were unable to confirm an increase in capillary density. Their finding of an increased sublingual vessel density was caused by larger vessels within the field of view (Martin et al. 2009, 2010) and it was only observed after 8 weeks at high altitude and not following a 2 week ascent period. More recent data using upgraded equipment have shown an increase in sublingual capillary TVD as a secondary finding (Gilbert‐Kawai et al. 2017). The present study unequivocally confirms the recruitment of sublingual capillary TVD in human climbers associated with ascent to extreme altitude already after a 2 week ascent to C2. The highest mean and median values for TVD were observed at 7042 m after a total of 3 weeks of high altitude exposure.
Our observation of high altitude associated recruitment of sublingual capillary TVD, despite a decrease in cardiac output, as well as unchanged arterial pressure and systemic vascular hindrance, demonstrates an intrinsic response of the microcirculation as opposed to a passive recruitment as a result of an increase in global blood flow. Such a disassociation between the macro‐ and the microcirculation has been observed in other states of impaired tissue oxygenation or dysfunction and identifies the microcirculation as an independent physiological compartment responsible for regional homeostasis. In pathophysiological processes where damage to the capillary system is part of the primary disease process, such as septic shock and fulminant cardiogenic shock (De Backer et al. 2004, 2006), this ability to regulate regional homeostasis is impaired, resulting in a sustained reduction in oxygen extraction capacity. In other conditions, such as in a pig model of acute heart failure, recruitment of the sublingual microcirculation has been observed (Stenberg et al. 2014), indicating a compensation mechanism intrinsic to the intact microcirculation to improve microcirculatory function independent of dysfunction of the systemic circulation. Similarly, our data are consistent with a mechanism induced by exposure to high altitude where an increase in TVD is identified as an intrinsic capillary compensation response to the observed decrease in arterial oxygen content and global delivery of oxygen, as a means of increasing oxygen availability in the microcirculation alongside an increase in haematocrit. The modest rise in serum lactate concentration observed in the present study during hypoxic exposure could be related to increased glycolysis stimulated by respiratory alkalosis, high altitude‐induced sympathetic activation or an incomplete compensation mechanism of the microcirculation to the decrease in global delivery of oxygen.
Maximum recruitable sublingual TVD remains constant during ascent to high altitude
Currently, the mechanism that is primarily responsible for the recruitment of the microcirculation in response to high altitude exposure remains unclear. On the one hand, structural adaptation to the microcirculation has been described in a rat model of chronic hypoxia where systemic capillary angiogenesis was found to occur at week 3 following exposure to an atmosphere of 12% oxygen (Deveci et al. 2001). In humans, remodelling of the pulmonary arterioles has been indirectly demonstrated during constant exposure to a high altitude environment over several weeks (Hilty et al. 2016). However, remodelling of pulmonary arterioles and systemic capillary angiogenesis are not necessarily regulated by the same mechanisms, and the timeframe of such processes in animal and human models may diverge. On the other hand, high altitude associated recruitment of TVD could primarily be mediated by haemodynamic regulation within the microcirculation alongside a decrease in arterial oxygen content and global delivery of oxygen. Such an explanation is consistent with the findings in a rat cremaster muscle model where gradual acute hypoxia resulted in capillary recruitment (Parthasarathi & Lipowsky, 1999). In that previous study, total capillaries (e.g. those perfused by red blood cells and those perfused by plasma alone) were visualized via fluorescein fluorescence intravital microscopy. In another study, blood plasma filled capillaries were visualized via fluorescence labelling of platelets with the aim of investigating the properties of recruitable capillaries in an animal model of skeletal muscle capillary permeability (Cabrales et al. 2007). In the present study, however, because of the limitations of HVM, which only detects red blood cells, we were able to detect only red blood cell filled capillaries. For this reason, we used the nitroglycerin challenge test to identify the maximum number of capillaries present. Employing this methodology, we could investigate which of the two mechanisms is primarily responsible for high altitude associated increase in sublingual capillary TVD. Our observation that TVDNG remains unchanged throughout ascent to 6022 m, and that it corresponds to the extent of high altitude associated recruitment of capillary TVD when measured at SL, suggests that high altitude associated recruitment of sublingual capillary TVD is an intrinsic function of the microcirculation that is based on a haemodynamic property rather than an angiogenetic response. The possible mechanisms underlying this haemodynamic response are an increased haematocrit mediated capillary recruitment, which would be consistent with the observations found in the rat model of hypoxia (Parthasarathi & Lipowsky, 1999), or an increase in availability of nitric oxide as a result of hypoxia‐induced changes in the nitrate–nitrite–nitric oxide pathway as previously described in a rat model (Feelisch et al. 2008). Furthermore, the absence of a correlation between ΔTVDNG and systemic vascular hindrance supports our finding of high altitude associated loss of coherence between the macro‐ and microcirculation.
Sublingual capillary TVD at sea level is associated with performance at high altitude
The individual ability to reach the summit in an expedition setting appears to be influenced by factors such as physical fitness, psychological determination and, independently, susceptibility to high altitude illness, such as acute mountain sickness and high altitude pulmonary oedema (Milledge et al. 1991; Savourey et al. 1995; Gerard et al. 2000; Maggiorini et al. 2001). To our knowledge, whether physical performance at high altitude correlates with changes in microcirculatory function, as well as in microvascular reactivity, has not been investigated. In the present study, we found that climbers who successfully reached the summit had a higher sublingual capillary TVD at sea level than those that did not. Non‐summiters had lower a TVD and a higher microvascular reactivity upon a nitroglycerin challenge. In a parallel observation, hypertensive patients had a lower sublingual capillary TVD than healthy controls unless they were exercising for at least 150 min week–1, in which case they reached levels of TVD similar to those of healthy controls (Kanoore Edul et al. 2015). Also in patients with chronic mesenterial ischaemia, a lower TVD was found than in healthy controls, which increased in value upon caloric challenge, whereas healthy controls presented the same higher level of TVD at baseline, remaining unchanged after caloric challenge (Harki et al. 2018). Thus, the co‐existence of a lower TVD and higher microvascular reactivity suggests a lower functional state of the microcirculation in need of recruitment upon metabolic challenge. On the other hand, our observation of an increase of TVDNG during ascent from 6022 m to 7042 m is consistent with the idea that it is possible to further increase microcirculatory oxygen extraction capacity as required in summiters venturing to extreme altitude. Consequently, the ability to increase maximum recruitable TVD at high altitude compared to SL is found to be associated with good performance at high altitude. No difference was found between summiters and non‐summiters for parameters with regard to the macrocirculation, even when taking into consideration the effects of a high altitude associated increase in haematocrit and calculation of systemic vascular hindrance. Also worthy of note, our findings are independent of the presence of high altitude illness. Consistent with these observations, previous studies investigating the relationship between physical fitness and high altitude illness also showed no correlation (Milledge et al. 1991; Savourey et al. 1995).
Limitations
In an expedition setting at extreme altitude, despite efforts to carefully standardize environmental factors and the availability of nutrients and fluids, some risk of bias remains. A main concern regarding blood circulation would be the potential of hypovolaemia as a result of excessive insensible fluid losses, which would be expected to deteriorate both macro‐ and microcirculatory function. However, in the present study, an increase in TVD was found to occur during a decrease in cardiac output, which is the opposite effect of what would be expected to occur as a result of hypovolaemia. Other factors, such as environmental temperature, were kept similar at SL and inside the research tent at C2, whereas, this was lower at C3. Although an effect of these lower environmental temperatures on the sublingual microcirculation may not entirely be excluded, it would be expected to be minimal because of the close embryological and physiological relationship of the sublingual microcirculation to the enteral microcirculation as opposed to the peripheral microcirculation. This is supported by previous findings obtained during a post‐resuscitation temperature management trial where no difference was found in the sublingual microcirculation between groups of patients with a core body temperature of 33 vs. 36°C (Koopmans et al. 2015). Besides environmental factors, the interpretability of microvascular reactivity in response to a topical vasodilatory stimulus as an estimate of recruitable microcirculation is naturally limited by the ability of the vasodilator to recruit the entire capillary bed. This can only be achieved by invasive methods such as labelling of platelets (Cabrales et al. 2007), which, for obvious reasons, was not possible to achieve in the present setting. High altitude exposure may also alter microvascular response to nitroglycerin. However, previous data suggest a decreased systemic vascular reactivity in response to acetylcholine but not sodium nitroprusside at high altitude, indicating a possible high altitude‐related impairment of the nitric oxide production pathway within the endothelial cells but not of the vascular response to nitric oxide (Berger et al. 2005). For nitroglycerin, where nitric oxide release is enzymatically mediated, a study in rabbits has reported unaltered nitric oxide release from nitroglycerin in hypoxia compared to normoxia (Agvald et al. 2002). It is therefore improbable that such mechanisms limit the interpretability of our results. Furthermore, in the present study, the microcirculation was examined sublingually. Although the sublingual microcirculation is not presumed to represent other parts of the body, its direct relationship with pathologies and divergence from the macrocirculation, as described previously (De Backer et al. 2004, 2006), demonstrate its relevance. Technical limitations of the HVM technology used in the present study include two main points. First, the use of a frame rate of 25 frames s–1 may reduce measurement accuracy in HVM image sequences under conditions of high flow velocity. Possible effects of a limited frame rate on measurement of capillary density are mitigated in the present study by a sufficiently high number of recorded frames in each image sequence. Second, only two image sequences were recorded per time point after the topical application nitroglycerin aiming to limit the total examination time and minimize confounding factors such as changes in the macrocirculation over time. In future studies, three image sequences should be recorded per measurement unless clips are recorded at the exact same mucosal location aiming to limit selection bias and fully comply with international consensus (De Backer et al. 2007; Ince et al. 2018). The impact of this limitation is partly mitigated by the larger field of view per location of the incident dark‐field camera setup used in the present study compared to the SDF imaging equipment used in previous studies (Aykut et al. 2015).
Conclusions
In the present study, we have demonstrated that a physiological response to increase microcirculatory oxygen extraction at high altitude is to recruit sublingual capillary TVD and that this is an intrinsic mechanism of the microcirculation independent of changes in cardiac output, arterial blood pressure or other parameters of the macrocirculation, such as systemic vascular hindrance. Using a nitroglycerin challenge to the microcirculation, we showed that high altitude‐induced recruitment of the TVD is a functional response as opposed to an anatomical response associated with angiogenesis. In addition, the concurrent presence of a low TVD and high microvascular reactivity at SL was found to be associated with a limited performance at high altitude, whereas the presence of a high TVD with the ability to further increase maximum recruitable TVD upon ascent to extreme altitude compared to SL was associated with good performance at high altitude.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
MPH, TMM, UH, MM and JP designed the study. MPH collected data. TMM, CI, MM and JP interpreted the data. MPH analysed data. MPH drafted the manuscript. TMM, UH, CI, MM and JP edited the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This research was supported by unrestricted research funds from the Swiss Society of Mountain Medicine, as well as a University of Zurich Walter und Gertrud Siegenthaler Foundation grant issued to MPH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgements
We are most grateful to the subjects who took part in the present study with great motivation and patience, the expedition team and the Sherpas for their invaluable organizational support, and Christiaan Boerma for many fruitful discussions.
Biography
Matthias Peter Hilty has found that, during his education and throughout his board certification in General Internal Medicine and Intensive Care Medicine, his main interests lie in the foundations of human physiology, in addition to their application in clinical practice. Studies of the response of the macro‐ and microcirculation to critical illness and high altitude environments have lead to his high appreciation of the contrast between witnessing cardiovascular pathophysiology during field studies, in laboratory settings and at the bedside. Hoping to support the upcoming challenge to translate these findings to clinical use, Dr Hilty is applying advanced computational techniques for the objective assessment of a patient's microcirculation.

Edited by: Harold Schultz & Bruno Grassi
This is an Editor's Choice article from the 15 May 2019 issue.
Linked articles: This article is highlighted in a Perspectives article by Kayser. To read this paper, visit https://doi.org/10.1113/JP277955.
References
- Agvald P, Adding LC, Artlich A, Persson MG & Gustafsson LE (2002). Mechanisms of nitric oxide generation from nitroglycerin and endogenous sources during hypoxia in vivo. Br J Pharmacol 135, 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aykut G, Veenstra G, Scorcella C, Ince C & Boerma C (2015). Cytocam‐IDF (incident dark field illumination) imaging for bedside monitoring of the microcirculation. Intensive Care Med Exp 3, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baayen RH, Davidson DJ & Bates DM (2008). Mixed‐effects modeling with crossed random effects for subjects and items. J Mem Lang 59, 390–412. [Google Scholar]
- Barr DJ, Levy R, Scheepers C & Tily HJ (2013). Random effects structure for confirmatory hypothesis testing: keep it maximal. J Mem Lang 68, 10.1016/j.jml.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B & Walker S (2015). Fitting linear mixed‐effects models using lme4. J Stat Softw 67, 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Benjamini Y & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 57, 289–300. [Google Scholar]
- Berger MM, Hesse C, Dehnert C, Siedler H, Kleinbongard P, Bardenheuer HJ, Kelm M, Bärtsch P & Haefeli WE (2005). Hypoxia impairs systemic endothelial function in individuals prone to high‐altitude pulmonary edema. Am J Respir Crit Care Med 172, 763–767. [DOI] [PubMed] [Google Scholar]
- Buise M, van Bommel J, Jahn A, Tran K, Tilanus H & Gommers D (2006). Intravenous nitroglycerin does not preserve gastric microcirculation during gastric tube reconstruction: a randomized controlled trial. Crit Care Lond Engl 10, R131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buise MP, Ince C, Tilanus HW, Klein J, Gommers D & van Bommel J (2005). The effect of nitroglycerin on microvascular perfusion and oxygenation during gastric tube reconstruction. Anesth Analg 100, 1107–1111. [DOI] [PubMed] [Google Scholar]
- Cabrales P, Vázquez BYS, Tsai AG & Intaglietta M (2007). Microvascular and capillary perfusion following glycocalyx degradation. J Appl Physiol Bethesda Md 1985 102, 2251–2259. [DOI] [PubMed] [Google Scholar]
- Carsetti A, Aya HD, Pierantozzi S, Bazurro S, Donati A, Rhodes A & Cecconi M (2016). Ability and efficiency of an automatic analysis software to measure microvascular parameters. J Clin Monit Comput 31, 669–676. [DOI] [PubMed] [Google Scholar]
- Cinar Y, Demir G, Paç M & Cinar AB (1999). Effect of hematocrit on blood pressure via hyperviscosity. Am J Hypertens 12, 739–743. [DOI] [PubMed] [Google Scholar]
- Coates G & Houston CS (1992). Hypoxia and Mountain Medicine, International Hypoxia Symposium 1991 (Lake Louise Alta), 1st edn, ed. Houston, CS. Pergamon Press, New York, NY. [Google Scholar]
- De Backer D, Creteur J, Dubois M‐J, Sakr Y, Koch M, Verdant C & Vincent J‐L (2006). The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit Care Med 34, 403–408. [DOI] [PubMed] [Google Scholar]
- De Backer D, Creteur J, Dubois M‐J, Sakr Y & Vincent J‐L (2004). Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am Heart J 147, 91–99. [DOI] [PubMed] [Google Scholar]
- De Backer D, Hollenberg S, Boerma C, Goedhart P, Büchele G, Ospina‐Tascon G, Dobbe I & Ince C (2007). How to evaluate the microcirculation: report of a round table conference. Crit Care Lond Engl 11, R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveci D, Marshall JM & Egginton S (2001). Relationship between capillary angiogenesis, fiber type, and fiber size in chronic systemic hypoxia. Am J Physiol Heart Circ Physiol 281, H241–H252. [DOI] [PubMed] [Google Scholar]
- Feelisch M, Fernandez BO, Bryan NS, Garcia‐Saura MF, Bauer S, Whitlock DR, Ford PC, Janero DR, Rodriguez J & Ashrafian H (2008). Tissue processing of nitrite in hypoxia: an intricate interplay of nitric oxide‐generating and ‐scavenging systems. J Biol Chem 283, 33927–33934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann NN, van Elteren HA, Goos TG, Morales CR, Rivera‐Ch M, Martin DS, Cabala Peralta P, Passano Del Carpio A, Aranibar Machaca S, Huicho L, Reiss IKM, Gassmann M & de Jonge RCJ (2016). Pregnancy at high altitude in the Andes leads to increased total vessel density in healthy newborns. J Appl Physiol Bethesda Md 1985 121, 709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard AB, McElroy MK, Taylor MJ, Grant I, Powell FL, Holverda S, Sentse N & West JB (2000). Six percent oxygen enrichment of room air at simulated 5,000 m altitude improves neuropsychological function. High Alt Med Biol 1, 51–61. [DOI] [PubMed] [Google Scholar]
- Gilbert‐Kawai E, Coppel J, Court J, van der Kaaij J, Vercueil A, Feelisch M, Levett D, Mythen M, Grocott MP, Martin D & Xtreme Everest 2 Research Group (2017). Sublingual microcirculatory blood flow and vessel density in Sherpas at high altitude. J Appl Physiol Bethesda Md 1985 122, 1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harki J, Suker M, Tovar‐Doncel MS, van Dijk LJ, van Noord D, van Eijck CH, Bruno MJ, Kuipers EJ & Ince C (2018). Patients with chronic mesenteric ischemia have an altered sublingual microcirculation. Clin Exp Gastroenterol 11, 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilty MP, Merz TM, Hefti U, Ince C, Maggiorini M & Pichler J (2018). Recruitment of non‐perfused sublingual capillaries increases microcirculatory oxygen extraction capacity throughout ascent to 7126 m – individual subject data. Zenodo Data Repos 10.5281/zenodo.1232321, March 1, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilty MP, Müller A, Flück D, Siebenmann C, Rasmussen P, Keiser S, Auinger K, Lundby C & Maggiorini M (2016). Effect of increased blood flow on pulmonary circulation before and during high altitude acclimatization. High Alt Med Biol 17, 305–314. [DOI] [PubMed] [Google Scholar]
- Hilty MP, Pichler J, Ergin B, Hefti U, Merz TM, Ince C & Maggiorini M (2017). Assessment of endothelial cell function and physiological microcirculatory reserve by video microscopy using a topical acetylcholine and nitroglycerin challenge. Intensive Care Med Exp 5, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince C, Boerma EC, Cecconi M, De Backer D, Shapiro NI, Duranteau J, Pinsky MR, Artigas A, Teboul JL, Reiss IKM, Aldecoa C, Hutchings SD, Donati A, Maggiorini M, Taccone FS, Hernandez G, Payen D, Tibboel D, Martin DS, Zarbock A, Monnet X, Dubin A, Bakker J, Vincent JL, Scheeren TWL & Cardiovascular Dynamics Section of the ESICM (2018). Second consensus on the assessment of sublingual microcirculation in critically ill patients: results from a task force of the European Society of Intensive Care Medicine. Intensive Care Med 44, 281–299. [DOI] [PubMed] [Google Scholar]
- Kanoore Edul VS, Ince C, Estenssoro E, Ferrara G, Arzani Y, Salvatori C & Dubin A (2015). The effects of arterial hypertension and age on the sublingual microcirculation of healthy volunteers and outpatients with cardiovascular risk factors. Microcirculation 22, 485–492. [DOI] [PubMed] [Google Scholar]
- Koopmans M, Kuiper MA, Endeman H, Veenstra G, Vellinga NAR, de Vos R & Boerma EC (2015). Microcirculatory perfusion and vascular reactivity are altered in post cardiac arrest patients, irrespective of target temperature management to 33°C vs 36°C. Resuscitation 86, 14–18. [DOI] [PubMed] [Google Scholar]
- Kottke R, Pichler Hefti J, Rummel C, Hauf M, Hefti U & Merz TM (2015). Morphological brain changes after climbing to extreme altitudes – a prospective cohort study. PloS ONE 10, e0141097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundby C & van Hall G (2001). Peak heart rates at extreme altitudes. High Alt Med Biol 2, 41–45. [DOI] [PubMed] [Google Scholar]
- Maggiorini M, Mélot C, Pierre S, Pfeiffer F, Greve I, Sartori C, Lepori M, Hauser M, Scherrer U & Naeije R (2001). High‐altitude pulmonary edema is initially caused by an increase in capillary pressure. Circulation 103, 2078–2083. [DOI] [PubMed] [Google Scholar]
- Martin DS, Goedhart P, Vercueil A, Ince C, Levett DZH & Grocott MPW (2010). Changes in sublingual microcirculatory flow index and vessel density on ascent to altitude. Exp Physiol 95, 880–891. [DOI] [PubMed] [Google Scholar]
- Martin DS, Ince C, Goedhart P, Levett DZH & Grocott MPW (2009). Abnormal blood flow in the sublingual microcirculation at high altitude. Eur J Appl Physiol 106, 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey MJ, Larochelle E, Najarro G, Karmacharla A, Arnold R, Trzeciak S, Angus DC & Shapiro NI (2013). The microcirculation image quality score: development and preliminary evaluation of a proposed approach to grading quality of image acquisition for bedside videomicroscopy. J Crit Care 28, 913–917. [DOI] [PubMed] [Google Scholar]
- Milledge JS, Beeley JM, Broome J, Luff N, Pelling M & Smith D (1991). Acute mountain sickness susceptibility, fitness and hypoxic ventilatory response. Eur Respir J 4, 1000–1003. [PubMed] [Google Scholar]
- Parthasarathi K & Lipowsky HH (1999). Capillary recruitment in response to tissue hypoxia and its dependence on red blood cell deformability. Am J Physiol 277, H2145–H2157. [DOI] [PubMed] [Google Scholar]
- Pichon A, Connes P, Quidu P, Marchant D, Brunet J, Levy BI, Vilar J, Safeukui I, Cymbalista F, Maignan M, Richalet J‐P & Favret F (2012). Acetazolamide and chronic hypoxia: effects on haemorheology and pulmonary haemodynamics. Eur Respir J 40, 1401–1409. [DOI] [PubMed] [Google Scholar]
- Prontera C, Emdin M, Zucchelli GC, Ripoli A, Passino C & Clerico A (2004). Analytical performance and diagnostic accuracy of a fully‐automated electrochemiluminescent assay for the N‐terminal fragment of the pro‐peptide of brain natriuretic peptide in patients with cardiomyopathy: comparison with immunoradiometric assay methods for brain natriuretic peptide and atrial natriuretic peptide. Clin Chem Lab Med 42, 37–44. [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2011). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. [Google Scholar]
- Reeves JT, Groves BM, Sutton JR, Wagner PD, Cymerman A, Malconian MK, Rock PB, Young PM & Houston CS (1987). Operation Everest II: preservation of cardiac function at extreme altitude. J Appl Physiol Bethesda Md 1985 63, 531–539. [DOI] [PubMed] [Google Scholar]
- Roach RC, Bartsch P, Hackett PH, Oelz O, Luks AM, MacInnis MJ, Baillie JK, Lake Louise AMS Score Consensus Committee & others (1993). The Lake Louise acute mountain sickness scoring system. Hypoxia Mol Med 272, 4. [Google Scholar]
- Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK & Schiller NB (2010). Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 23, 685–713. [DOI] [PubMed] [Google Scholar]
- Sampson JB, Kobrick JL & Johnson RF (1993). The Environmental Symptoms Questionnaire (ESQ): Development and Application . United States Army Research Institute of Environmental Medicine, Research, Development and Engineering Center, Soldier Science Directorate, Natick, Massachusetts 01760‐5000; https://apps.dtic.mil/dtic/tr/fulltext/u2/a264127.pdf.
- Savourey G, Moirant C, Eterradossi J & Bittel J (1995). Acute mountain sickness relates to sea‐level partial pressure of oxygen. Eur J Appl Physiol 70, 469–476. [DOI] [PubMed] [Google Scholar]
- Siebenmann C, Rasmussen P, Hug M, Keiser S, Flück D, Fisher JP, Hilty MP, Maggiorini M & Lundby C (2017). Parasympathetic withdrawal increases heart rate after 2 weeks at 3454 m altitude. J Physiol 595, 1619–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sime F, Peñaloza D, Ruiz L, Gonzales N, Covarrubias E & Postigo R (1974). Hypoxemia, pulmonary hypertension, and low cardiac output in newcomers at low altitude. J Appl Physiol 36, 561–565. [DOI] [PubMed] [Google Scholar]
- Stenberg TA, Kildal AB, Sanden E, How O‐J, Hagve M, Ytrehus K, Larsen TS & Myrmel T (2014). The acute phase of experimental cardiogenic shock is counteracted by microcirculatory and mitochondrial adaptations. PloS ONE 9, e105213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanyasiri P, Celermajer DS & Adams MR (2005). Endothelial dysfunction occurs in peripheral circulation patients with acute and stable coronary artery disease. Am J Physiol Heart Circ Physiol 289, H513–H517. [DOI] [PubMed] [Google Scholar]
- Vázquez BYS, Martini J, Tsai AG, Johnson PC, Cabrales P & Intaglietta M (2010). The variability of blood pressure due to small changes of hematocrit. Am J Physiol Heart Circ Physiol 299, H863–H867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H (2010). ggplot2: Elegant Graphics for Data Analysis, 1st edn 2009. Corrected 3rd printing 2010 edition Springer, New York, NY. [Google Scholar]
- Wolfel EE, Selland MA, Mazzeo RS & Reeves JT (1994). Systemic hypertension at 4,300 m is related to sympathoadrenal activity. J Appl Physiol Bethesda Md 1985 76, 1643–1650. [DOI] [PubMed] [Google Scholar]
- Woods DR, Mellor A, Begley J, Stacey M, O'Hara J, Hawkins A, Yarker J, Foxen S, Smith C & Boos C (2013). Brain natriuretic peptide and NT‐proBNP levels reflect pulmonary artery systolic pressure in trekkers at high altitude. Physiol Res 62, 597–603. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Imaizumi T, Ando S, Hirooka Y, Harada S & Takeshita A (1991). Impaired forearm vasodilatation by acetylcholine in patients with hypertension. Heart Vessels 6, 218–223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusions of this article is available via the Zenodo research data repository (Hilty et al. 2018).


