Abstract
Key points
Temporal imprecision leads to deficits in the comprehension of signals in cluttered acoustic environments, and the elderly are shown to use cognitive resources to disambiguate these signals.
To mimic ageing in young rats, we delivered sound signals that are temporally degraded, which led to temporally imprecise neural codes.
Instead of adaptation to repeated stimuli, with degraded signals, there was a relative increase in firing rates, similar to that seen in aged rats.
We interpret this increase with repetition as a repair mechanism for strengthening the internal representations of degraded signals by the higher‐order structures.
Abstract
To better understand speech in challenging environments, older adults increasingly use top‐down cognitive and contextual resources. The medial geniculate body (MGB) integrates ascending inputs with descending predictions to dynamically gate auditory representations based on salience and context. A previous MGB single‐unit study found an increased preference for predictable sinusoidal amplitude modulated (SAM) stimuli in aged rats relative to young rats. The results suggested that the age‐degraded/jittered up‐stream acoustic code may engender an increased preference for predictable/repeating acoustic signals, possibly reflecting increased use of top‐down resources. In the present study, we recorded from units in young‐adult MGB, comparing responses to standard SAM with those evoked by less salient SAM (degraded) stimuli. We hypothesized that degrading the SAM stimulus would simulate the degraded ascending acoustic code seen in the elderly, increasing the preference for predictable stimuli. Single units were recorded from clusters of advanceable tetrodes implanted above the MGB of young‐adult awake rats. Less salient SAM significantly increased the preference for predictable stimuli, especially at higher modulation frequencies. Rather than adaptation, higher modulation frequencies elicited increased numbers of spikes with each successive trial/repeat of the less salient SAM. These findings are consistent with previous findings obtained in aged rats suggesting that less salient acoustic signals engage the additional use of top‐down resources, as reflected by an increased preference for repeating stimuli that enhance the representation of complex environmental/communication sounds.
Keywords: top‐down, salience, Aging, Auditory pathways, medial geniculate body, cognitive effort, repetition, Adaptation
Key points
Temporal imprecision leads to deficits in the comprehension of signals in cluttered acoustic environments, and the elderly are shown to use cognitive resources to disambiguate these signals.
To mimic ageing in young rats, we delivered sound signals that are temporally degraded, which led to temporally imprecise neural codes.
Instead of adaptation to repeated stimuli, with degraded signals, there was a relative increase in firing rates, similar to that seen in aged rats.
We interpret this increase with repetition as a repair mechanism for strengthening the internal representations of degraded signals by the higher‐order structures.
Introduction
Age‐related hearing loss leads to a loss of speech understanding and affects 35–50% of the 43 million individuals aged 65 years or older in the US population (Humes et al. 2012; Ortman et al. 2014). A loss of speech understanding significantly impairs quality of life, frequently leading to social withdrawal and depression (Humes et al. 2012; Bainbridge & Wallhagen, 2014). Peripheral changes only partially account for speech understanding deficits in elderly listeners with mild to moderate hearing loss in complex listening environments. (Alain & Woods, 1999; Dalton et al. 2003; Anderson et al. 2012; Humes et al. 2012; Presacco et al. 2016b). In response to peripheral hair cell and/or acoustic nerve fibre losses associated with ageing, the entire central auditory pathway shows compensatory age‐related maladaptive changes (Caspary et al. 2008; Ouda et al. 2015; Caspary & Llano, 2018). Human and animal studies repeatedly show age‐related declines in the temporal reliability when coding ascending acoustic representations, partly as the result of a loss of normal adult inhibitory neurotransmitter function (Fitzgibbons & Gordon‐Salant, 1994; Caspary et al. 2008; Gordon‐Salant, 2014; Godfrey et al. 2017; Caspary & Llano, 2018). Previous human psychophysical and electrophysiological studies have modelled ageing in auditory system by temporally jittering stimuli (Pichora‐Fuller et al. 2007; Mamo et al. 2016). To compensate for these deficits with ageing, the elderly are known to use top‐down resources to improve performance in auditory tasks (Ostroff et al. 2003; Peelle et al. 2010; Fakhri et al. 2012; Leung et al. 2013). In essence, when speech signals are degraded, speech understanding can be improved by engaging the cortical pathways projecting to lower‐order cortical and subcortical structures that provide additional ‘meaning’/cognitive resources. These resources may provide linguistic or semantic context guiding the recognition of acoustically unclear speech (Harris et al. 2012; Mattys & Scharenborg, 2014; Sohoglu et al. 2014; Rogers & Wingfield, 2015; Peelle & Wingfield, 2016; Pichora‐Fuller et al. 2016). Early psychophysical studies on phenome restoration posited the role of top‐down resource usage that are now supported by modern imaging techniques (Thurlow, 1957; Warren, 1970; Vaden et al. 2016). This function is supported by the Bayesian properties of higher cortical areas in the prediction of sensory events using concurrent cognitive resources reflected in their top‐down projections, which in turn are corrected by bottom‐up circuits leading to enhancement or selective diminishment of the ascending acoustic code or prediction error (Rao & Ballard, 1999; Friston, 2009; Stebbings et al. 2014; Parras et al. 2017; Kuchibhotla & Bathellier, 2018; Wang et al. 2019). Examples of top‐down mediated processes include repetition enhancement effect for degraded signals, attended signals or speech in noise recognition (Luce & Pisoni, 1998; Eisenberg et al. 2002; Maunsell & Treue, 2006; Rivenez et al. 2006; Sheldon et al. 2008; Chandrasekaran et al. 2009; Peelle et al. 2010; Muller et al. 2013; Peelle & Wingfield, 2016; Helfer et al. 2018).
In the central auditory system, top‐down descending corticothalamic projections to the auditory thalamus or medial geniculate body (MGB) are more extensive than ascending reciprocal thalamocortical projections. MGB can be parsed into ventral, dorsal and medial subdivisions (Morest, 1964). The ventral division is lemniscal in nature, projecting principally to layers 3/4, whereas the dorsal and medial subdivisions receive inputs from dorsal and external cortices of the inferior colliculus, tegmentum, superior colliculus and spinal cord considered to be extra‐lemniscal and projecting to layers 1 and 6 of the primary auditory cortex, as well as belt areas of the auditory cortex and amygdala, amongst others (Winer et al. 2005; de la Mothe et al. 2006; Bartlett, 2013). The MGB also receives cholinergic projections that may further engage top‐down resources providing cognitive and attentional resources that shape the ascending code (Rouiller & Welker, 1991; Winer et al. 2001; Bartlett & Smith, 2002; He, 2003; Bartlett, 2013; Malmierca et al. 2015; Guo et al. 2017; Lesicko & Llano, 2017; Sottile et al. 2017a; Sottile et al. 2017b; Schofield & Hurley, 2018). The MGB also receives tectothalamic inputs that carry ascending sensory inputs and shows stimulus‐specific adaptation (SSA) to repeating stimuli, facilitating the detection of novel stimuli (Nelken, 2014; Malmierca et al. 2015), comprising a property considered to be of bottom‐up origin, independent of age and even enhanced by anaesthesia (Ulanovsky et al. 2003; von der Behrens et al. 2009; Richardson et al. 2013a; Malmierca et al. 2015; Nir et al. 2015). By contrast to afore‐mentioned studies, other studies have also shown changes in MGB unit tuning properties and gain via manipulation of the auditory cortex (Orman & Humphrey, 1981; Zhang et al. 1997; He, 2003; Malmierca et al. 2015). Increased detection of acoustic signals was shown to involve corticothalamic projections that increase neural representation in the MGB (Guo et al. 2017). However, our current understanding of how ageing affects top‐down influences at the level of the MGB is limited. A recent MGB single‐unit study showed an age‐related increasing preference (neuronal responses) for predictable/repeating stimuli relative to randomly presented sinusoidal amplitude modulated (SAM) stimuli. Rather than showing stimulus adaptation to repeating stimuli, MGB units from aged animals responded to repeating stimuli by increasing their discharge rates with each repeated trial, especially at higher modulation frequencies (f m) (Cai et al. 2016). These changes were not observed in MGB units from anaesthetized rats, possibly reflecting the relative weakening of top‐down circuits by anaesthesia (Ferrarelli et al. 2010; Casali et al. 2013; Mashour, 2014). Similar in concept to studies simulating central auditory ageing using temporal jitter, the present study hypothesized that less salient SAM stimuli will lead to a less‐precise ascending upstream code, similar to that seen in the elderly, and also that this change in salience would move the single‐unit preference toward predictable stimuli in young‐adult MGB neurons (Fig. 1) (Pichora‐Fuller et al. 2007; Mamo et al. 2016). To test this hypothesis, single unit recordings were made from awake and anaesthetized young rats that were presented with standard, 100% depth of modulation SAM and less salient SAM stimuli (decreasing modulation depth and ‘noisy’ SAM stimuli). We posited that less clear acoustic information will engender the use of top‐down, corticothalamic information in an effort to ‘better understand’ the ascending acoustic message leading to enhanced representation of predictable/repeating stimuli (Fig. 1).
Figure 1. Schematic representation of the study hypothesis.
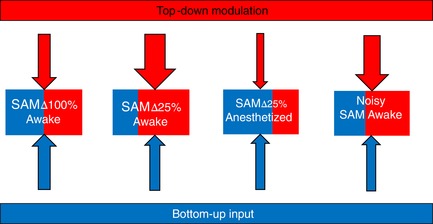
Less salient acoustic stimuli will produce a degraded ascending acoustic code, similar to the code seen in the ageing auditory system. In the MGB, this can engage an increased use of top‐down resources needed to maintain salience by enhancing responses to repeating/predictable over random stimuli. Bottom‐up (blue arrows) and top‐down (red arrows) MGB circuits work in concert to encode communication sounds and salient environmental information needed for survival. We hypothesize that temporally rich SAM stimuli with 100% modulation depth (SAM∆100%) balance the use of bottom‐up and top‐down resources to best code sounds in the MGB of young awake rats. We posit that decreasing the salience of SAM stimuli either by reducing the effective modulation depth (25% modulation depths: SAM∆25%) or by jittering the envelope of 100% modulation depth (Noisy SAM) leads to an increase in top‐down mediated processes in the awake but not in anaesthetized rat MGB.
Methods
Male Fischer 344 × Brown Norway (FBN) rats, aged 4–6 months old, were obtained from the National Institiute of Aging (NIA) Aging Rodent Resource Colony supplied by Charles River (Wilmington, MA, USA) and were housed individually under a reverse 12:12 h light/dark photocycle with access to food and water available ad libitum. FBN rats have a long life‐span and lower tumour load than other commonly used rat ageing models. They are available through rodent resources at the National Institute of Aging (nia.nih.gov/research/scientific‐resources#rodent) and age‐related changes in auditory structure and function have been studied extensively (Caspary et al. 2008; Cai et al. 2018; Caspary & Llano, 2018). Procedures were performed in accordance with guidelines and protocols approved by the Southern Illinois University School of Medicine Lab Animal Care and Use Committee, as well as in accordance with National Institute of Health guideline on minimizing animal usage and pain, and also conform with the regulations described by Grundy (2015).
Acoustic brain stem response (ABR) recording
To ensure normal hearing thresholds, before implantation surgery, ABR tests were completed on all rats as described previously (Wang et al. 2009). Briefly, rats were anaesthetized with an i.m. injection of a 3:1 mixture of ketamine and xylazine at a dose of 1.4 mL kg–1. Pure tones at 4, 8, 12, 16, 24, 28 and 32 kHz were presented 512 times at a rate of 20 s–1 with a duration of 3 ms and rise/decay clicks of 1 ms. A recording electrode was inserted into the skin over the vertex, a reference electrode was inserted under the left mastoid and a ground wire was attached to the hind leg. ABR signal gain totalled 200,000× with filtering between 0.3 and 3 kHz. Absolute thresholds were determined based on wave I at each frequency. ABRs and single unit recording experiments were completed in a double‐wall soundproof booth (Industrial Acoustic, Bronx, NY, USA).
Awake recordings
Rats recovered for 3 days after ABR testing before beginning acclimatization to the recording chamber. Starting at least 1 week prior to surgery, rats were acclimated to a modified experimental conditioning unit (Braintree Scientific, Braintree, MA, USA) with free access to water and a food reward (1/4 to 1/2 Froot Loop; Kellogg's; Battle Creek, MI, USA) until they could remain quiet/still for up to 3 h. VersaDrive4 tetrode drives (Neuralynx, Bozeman, MT, USA) were assembled and loaded with tetrodes as described previously (Richardson et al. 2013a; Kalappa et al. 2014). Each tetrode wire was gold‐electroplated to an impedance between 0.5 and 2.0 MΩ and sampled at 1 kHz (nanoZ; Neuralynx). Drives were sterilized with ethylene oxide before implantation. Surgery details were similar to those described in Richardson et al. (2013) and Kalappa et al. (2014). One day prior to surgery, acetaminophen (4.5 mg mL–1) was provided via drinking water and continued until day 2 after surgery to alleviate pain. Surgery was performed under anaesthesia and an i.m. injection of a ketamine/xylazine 3:1 mixture at a dose of 1.4 mL kg–1 was used for induction. Rats were given sterile saline (3 mL) s.c., placed on a thermostatically controlled heating pad (Harvard Apparatus, Holliston, MA, USA) and set in a Kopf stereotaxic apparatus (Kopf Instruments, Tujunga, CA, USA) with a nose cone and chin bars. Oxygen blood saturation levels and heart rate were monitored during the surgery using PulseSense Vet (Nonin Medical, Minneapolis, MN, USA). Oxygen was administered continuously to maintain 95–100% blood saturation and isoflurane (1–2.5%) was administered as needed until surgery is completed after induction with a ketamine/xylazine mixture (VetEquip, Pleasanton, CA, USA). The level of anaesthesia was adjusted based on the presence of pedal withdrawal or elevated heart rate.
Under sterile conditions, the skull surface was exposed and anchor screws were set in place. A craniotomy hole of 2.3 mm in diameter was drilled over the left occipitoparietal cortex, dorsal to the MGB (5.5 mm bregma and 3.5 mm of midline) and the dura was carefully removed. A ground wire was attached to a reference screw placed in the anterior right frontal bone that made contact with the dura, and the tetrode drive was slowly advanced (0.2 mm min–1) to a depth of 4.5–5 mm, placing the four tetrode tips just dorsal to the MGB. Dental acrylate cement was added around the anchor screws and the drive, encapsulating the entire drive with the exception of the advancing screws and pins. The total weight of tetrode drive and dental cement was less than 10 g. Mounting the tetrode drive did not appear to alter the behaviour or demeanor of an animal, with postmortem examination indicating little damage to the surface of the brain. Following surgery, triple antibiotic ointment was applied to the edge of the headcap and wound, and an additional 2–3 mL of sterile saline was administered s.c. The animal was exposed to 100% oxygen and kept on a heating pad throughout recovery until ambulatory. The tetrode drive was coupled to an 18 pin (16 single wires, 2 ground) VersaDrive4‐to‐Omnetics adaptor (Neuralynx) and connected to a unity gain 18‐channel headstage tethered to a preamplifier (2 × gain; 0.15 kHz high pass, 8 kHz low pass; Plexon Inc., Dallas, TX, USA). Sixteen channels of raw data were digitized using a multichannel acquisition processor (MAP) and visualized using Sort Client (Plexon Inc.). Tetrodes were advanced by turning a drive screw coupled to each tetrode and were advanced in increments of 1/4 turn (62.5 μm) with the distance recorded to aid in the localization of units (Richardson et al. 2013a). To avoid unit resampling, after a unit on a given tetrode was studied, the tetrode was advanced at least 125 μm. When auditory responsive units/field potentials were no longer present, tetrodes were left in position for marking.
Similar to Richardson et al. (2013a), spikes determined to be from single units were sorted using standard methods (amplitude threshold and principal component analysis) and saved as timestamps. Timestamps were relayed to a system running a custom program Auditory Neurophysiology Experiment Control Software (ANECS, Ken Hancock, Blue Hills Scientific, Boston, MA, USA) for stimulus generation and real‐time analysis of unit responses. During the recording period, the experimenter took precautions to ensure that the animal was not asleep. Whenever there was an unexpected change in the firing rates of a single unit under investigation, data collection was paused and the booth door was opened to ensure that the animal was awake and alert. As noted above, animals were kept on a reverse day/night cycle so their active period was during the recording sessions.
When recordings were complete (1–4 weeks), rats were anaesthetized with ketamine and xylazine as described above and current pulses (5–10 μA for 5 s) were passed through the tip of each tetrode wire, producing a small lesion. Rats were cardiac perfused with PBS (0.1 m, pH 7.4) followed by 4% paraformaldehyde (Sigma‐Aldrich, St Louis, MO, USA). The brain was removed, post‐fixed for 24 h in 4% paraformaldehyde at room temperature and tehn transferred to 20% sucrose and stored at 4°C until sectioned. Frozen coronal sections (thickness 30–35 μm) were taken and the electrode tracks and sites of lesion were visible without the need for staining, and these were then used to determine the position of each recording site relative to the final location of the tetrode tip (Paxinos & Watson, 1998).
Awake recordings and attention
As described in Richardson et al. (2013) and Kalappa et al. (2014), animals were placed in a darkened acoustic chamber under gentle orienting restraint (i.e. they could turn around but were pre‐trained not to do so) with only SAM stimuli to listen to. There were no other known distractors to divide their attention and so the sole activity of a rat was to attend to environmental sounds, which comprised the SAM stimuli presented from the speaker located above their heads.
Anaesthetized preparations
Rats recovered for at least 3 days following ABR testing prior to use in the anaesthesia study. Initial anaesthesia for surgery was the same as described for the awake preparation. Anaesthesia was then maintained with i.p. injections of 100% urethane (initially 1.3 mL kg–1, then booster doses at one‐third of the initial dose; Sigma‐Aldrich) (Cai et al. 2014; Cai & Caspary, 2015). Rats were placed in a modified (no ear bars) stereotaxic frame in an IAC sound‐attenuating booth (Industrial Acoustic Co., Inc., New York, NY, USA) with the body temperature maintained at 37 ± 0.5°C using a thermostatically controlled heating blanket. The skull surface and left occipito‐parietal cortex, dorsal to the MGB, were exposed. A tungsten electrode was gradually advanced into MGB by piezoelectric advancer (David Kopf Instruments, Tujunga, CA, USA). The electrodes were coupled to a headstage preamplifier, MAP system and a personal computer running MAP software and Sort Client (Plexon Inc.) for real‐time spike sorting.
SAM stimuli paradigms and single‐unit recording procedures
Stimulus paradigms and single unit sorting/recording procedures were the same for awake and anaesthetized preparations. Acoustic signals were generated using a 16‐bit D/A converter (TDT RX6 for System III; Tucker Davis Technologies, Alachua, FL, USA) and transduced by a Fostex tweeter (model FT17H; Fostex, Tokyo, Japan) placed 30 cm above animal's head. The Fostex tweeter was calibrated off‐line using a ¼ inch microphone (model 4938; Brüel & Kjær, Naerum, Denmark) placed at the approximate location of the rat's head. Calibration tables in dB sound pressure level (SPL) were used to set programmable attenuators (PA5; Tucker Davis Technologies) to achieve pure tone levels accurate within 2 dB SPL for frequencies up to 45 kHz. Response maps were used to determine the characteristic frequency (CF) of sorted single units (Cai & Caspary, 2015). Random tone‐burst stimuli (duration of 50 ms, 4 ms rise/fall time, 2 Hz rate) were presented in 0.10 to 0.25 octave frequency steps (1–32 KHz) in 10 dB SPL steps (0–80 dB) to determine the response maps. Real‐time single unit activity was sampled at 100 kHz using ANECS and archived for off‐line analysis.
The modulation depth was decreased to 50% and 25% (SAM∆50% and 25%) to create less salient versions of 100% modulated SAM. In addition, we produced a signal termed ‘noisy SAM’, where 100% SAM signals were jittered by adding low‐pass filtered (1000 Hz) broadband noise (BBN) to the envelope of the SAM signal regardless of the carrier. The ratio of the carrier to noise rms was constant at 0, equal strength. The addition of BBN jitters the rising phase of the envelope and decreases the effective modulation depth (Fig. 2). There were no differences (<2 dB) in total energy levels for the standard and less salient SAM stimuli. SAM carrier (f c) was set at the unit's CF or BBN. Rate modulation transfer functions (rMTFs) and temporal modulation transfer functions (tMTFs) were collected at 30–35 dB above CF or BBN threshold. SAM unit data, 30–35 dB above threshold, were collected from young‐adult (aged 3–5 months) rats using either CF‐tones or BBN as the carrier and choosing the SAM carrier that best drove the unit under study. The findings reported in Cai et al. (2018) in an FBN rat model of auditory ageing mean that age‐related sensitivity changes at the apical end of the cochlea would probably have had no impact upon the previous results reported in Cai et al. (2016).
Figure 2. SAM stimuli waveforms and paradigms.
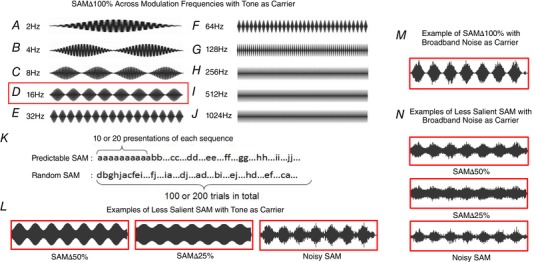
A‐J, SAM waveforms with tone as carrier at SAM∆100% in 500 ms epochs from 2 Hz to 1024 Hz modulation frequencies. K, each stimulus paradigm progressed from 2 Hz to 1024 Hz in a predictable repeated or random manner. L, exemplar waveforms of less salient SAM with tone as carrier at 16 Hz (SAM∆50%, SAM∆25% and noisy SAM). M and N, exemplar waveforms of salient and less salient SAM with BBN as carrier. Noisy SAM has jittered rising phase and decreased effective modulation depth. There were no differences (<2 dB) in total energy levels for the salient and less salient SAM stimuli regardless of carrier. [Color figure can be viewed at wileyonlinelibrary.com]
SAM stimuli were presented at 2 s–1, with a duration of 450 ms and a 4 ms raise–fall with f ms stepped between 2 and 1024 Hz. We tested whether f ms sequentially/predictably stepped in descending steps/reverse order, from 1024 Hz to 2 Hz, would have effect on the results. A descending sequential f m presentation order did not differ from the ascending sequential f m presentation order; hence, for all reported data, stimuli were stepped from 2 Hz to 1024 Hz. Spikes were collected over a period of 500 ms, following stimulus onset with 10 or 20 stimulus repetitions at each envelope frequency. SAM stimuli were presented as two separate sets: random across trials modulation frequencies (f ms) or sequential with f ms repeating (10 or 20 times) before being stepped to the next f m in an increasing predictable order (Fig. 2). Responses to less salient SAM stimuli (SAM∆50% and 25% and noisy SAM) were compared with SAM∆100%.
Statistical analysis
Responses were analysed offline. MTFs were determined using spike rate (rMTF) and temporal synchronization (tMTF) measurement at each f m tested.
Random preference ratio (RPR) (i.e. total spikes in predictable trials/total spikes in random trials) was calculated across all f ms, with a ratio of random preferring unit smaller than 0.95 and predictable preferring unit larger than 1.05. Ratios between the range of 0.95 and 1.05 were considered non‐selective units. A chi‐squared test was used to compare the sequence preference ratio.
A random preferring index (RPI) was calculated using the equation: RPI = [(AUCRAN – AUCSEQ)/(AUCRAN + AUCSEQ)], modified from the novelty response index (Lumani & Zhang, 2010; Cai et al. 2016) and the area under successive frequency segments of the rMTF curve (AUC) values were based on rMTF curve calculated using Prism (GraphPad Software Inc., San Diego, CA, USA). The range of RPI values varied between –1 and +1, with –1 representing a completely predictable preferring response and with +1 representing a completely random preferring response. Repeated‐measures ANOVA followed by post hoc Tukey correction for multiple comparisons was used to compare RPI values.
Trial‐to trial response analysis to predictable SAM presentation at 256 and 512 Hz was performed by comparing the difference in trend line slopes using two‐tailed analysis of covariance (ANCOVA). Phase locking ability was evaluated by standard vector strength (VS) equation: , where n is the total number of spikes and is the phase of observed spike relative to modulation frequency (Goldberg & Brown, 1969; Yin et al. 2011). Statistical significance was assessed using the Rayleigh statistic to account for differences in the number of driven spikes, with Rayleigh statistic values greater than 13.8 being considered statistically significant (Mardia & Jupp, 2000) (Fig. 8). To compare number of units showing phase locking ability and the quantitative vector strength data, a Wilcoxon test and a paired Student's t test were used followed by a Bonferroni correction for multiple comparisons.
Figure 8. Percentage of neurons showing temporal‐locking responses to random or predictable presentation of standard (SAM∆100%) and less salient (SAM∆50%, SAM∆25% and noisy SAM) stimuli at lower modulation frequencies (f m).
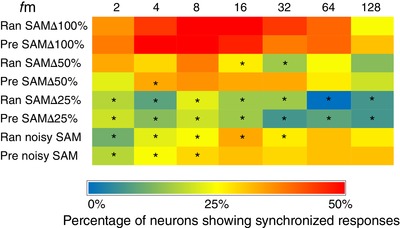
Each row in the heat map represents the presentation of salient or less salient stimuli either in a random or predictable manner, whereas each column represents the f m from 2 Hz to 128 Hz. The scale below the heat map shows the colour code for the percentage of neurons (out of 66 in total) showing temporal‐locking to the stimulus; hot colours (red) indicate a higher percentage of temporal‐locking, whereas cool colours (blue) indicate a lower percentage of temporal‐locking. Changes in the percentage of neurons showing temporal‐locking when switched from standard to less salient stimuli in either random or predictable presentations are compared. There was a significant decrease in the percentage of single‐units showing temporal‐locking with a decrease in salience predominantly at SAM∆25% and noisy SAM across both random and predictable presentations compared to SAM∆100% (Wilcoxon test followed by Bonferroni correction, P < 0.05).
Statistical analysis was performed using Prism, version 6 and SPSS, version 24 (IBM Corp., Armonk, NY, USA). All values are expressed as the mean ± SEM. * P < 0.05, ** P < 0.01, *** P < 0.001 were considered statistically significant.
Results
Ninety‐four carefully isolated units were recorded from the MGB of young‐adult (aged 4–6 months) rats. Sixty‐six units were recorded from six awake rats and 28 units from four anaesthetized rats. All units from awake and anaesthetized preparations were localized to the dorsal or ventral subdivisions of the MGB. Ninety‐five percent of the units recorded were determined to be clearly discriminated single units. The remaining units were clusters of 2–3 inseparable units, with responses consistent with the single‐unit findings and were included in the analyses. The number of recording sessions varied between 20 and 25 for each rat in the awake group. Between 0 and 2 units were recorded in each day's session as the electrodes were moved in small increments (62.5 microns) at the end of each day.
The tungsten electrodes used in the anaesthetized preparation (impedance 1–3 MΩ) were similar to the 0.5–2.0 MΩ impedance of VersaDrive microwires. The present study cannot rule out, with certainty, any population differences between the units sampled using different electrodes.
rMTFs and tMTFs were recorded in response to random or predictable presentation of standard (SAM∆100%) or less salient (SAM∆50%, SAM∆25% or noisy SAM) stimuli (Fig. 2). There were no differences in shapes of rMTFs with 10 or 20 repetitions and so 10 repetitions were used in most subsequent recordings.
To maximize unit responses for the analyses, either a BBN or CF carrier was chosen based on which elicited the highest number of total spikes to SAM∆100%. Most units in this data set responded best to SAM with a BBN carrier (43 BBN and 23 CF). Units CFs and BFs ranged between 2 and 32 kHz. Similar to studies reported by Bartlett and Wang (2007, 2011) and Cai et al. (2014, 2015), SAM responses, based on rMTF shape across f m, conformed to previously described band‐pass, high‐pass, low‐pass, mixed (most common) or atypical types. BMFs ranged between 8 and 512 Hz, congruent with the rMTF profile types described above.
Preference for random or predictable stimuli with decreasing salience
We examined whether decreasing the salience of a SAM stimulus alters the MGB unit responses as reflected by an increase in the number of spikes when stimuli are presented in a random or predictable manner. An exemplar unit (Fig. 3) showed a slight preference for random presentation with SAM∆100%, although this same unit showed a clear preference for predictable stimuli at SAMΔ25%. Responses to random or predictable stimuli were compared when SAM salience was decreased. A difference criteria of greater than 10% change in total response (Ghitza et al. 2006; Cai & Caspary, 2015; Cai et al. 2016) was used as the qualifier to categorize the unit based on previous studies (random preference ratio = total spikes to predictable SAM/total spikes to random SAM). A qualitative comparison was made between standard SAM∆100% and less salient SAM for the units showing random, predictable and non‐selective responses. There was a significant increase in the number of units showing preference for predictable stimuli as the salience was decreased at SAMΔ50%, SAMΔ25% and noisy SAM (P < 0.05, chi‐squared test) (Fig. 4). The increase in the percentage of neurons showing predictable preference compared to SAMΔ100% was SAMΔ50%, (32% vs. 9%, P < 0.01), SAMΔ25% (46% vs. 9%, P < 0.0001) and noisy SAM (33% vs. 9%, P < 0.01) (Fig. 4). These preference changes were not seen in MGB units recorded in anaesthetized rats (SAMΔ100% vs. SAMΔ50%, SAMΔ25% and noisy SAM: 18% vs. 21%, 29% and 19%, respectively, P > 0.05).
Figure 3. A representative unit from an awake young‐adult rat responded differently to various stimulus paradigms.
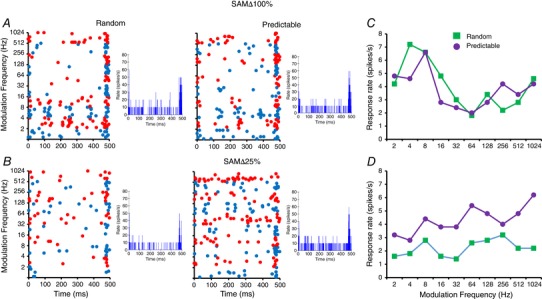
A and C, dot rasters, post‐stimulus time histogram and rMTFs from this unit show similar responses to either a random or a repeating predictable sequence of SAM∆100%. B and D, the same MGB unit showed increased responses to a predictable repeating SAM with less salient SAM∆25%. Responses to predictable SAM∆25% increased at the highest f ms. [Color figure can be viewed at wileyonlinelibrary.com]
Figure 4. Response ratio comparison across all modulation frequencies between SAM stimuli sets.
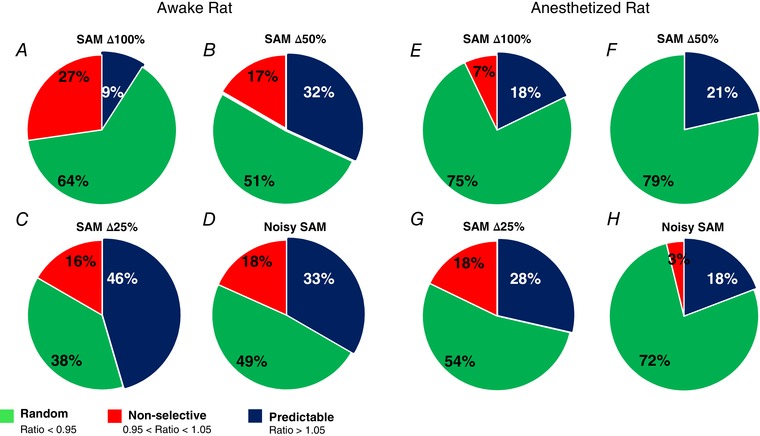
Percentage of MGB units showing response ratios (predictable/random) at SAM∆100% (A) and less salient SAM stimuli sets (B–D) in awake young‐adult rats. The percentage of units showing a preference for predictable SAM increased from 9% (6 of 66) at SAM∆100% to 32% (21 of 66) SAM∆50% and 33% percentage for noisy SAM (20 of 60). At SAM∆25%, MGB neurons from awake rats showed highest predictable‐preference 46% (30 of 66). Significant differences were seen between SAM∆100% vs. SAM∆50%, SAM∆25% and noisy SAM (chi‐squared test, P < 0.05). In anaesthetized rats, only non‐significant differences in preference for predictable stimuli were seen between SAM∆100% and less salient stimuli E–H, percentage of units showing preference for predictable SAM increased from 18% (5 of 28) to 21% (6 of 28) for SAM∆50%, 28% for SAM∆25% (8 of 28) and 18% for noisy SAM (5 of 26). [Color figure can be viewed at wileyonlinelibrary.com]
rMTF differences across f ms with decreasing salience (group data)
Similar to Cai et al.(2016), the AUC function and RPI values derived from the AUC, as described above, were used to compare responses between random and predictable stimuli across f ms (Figs 5 and 6). Higher values on RPI indicate a random preference, whereas lower or negative values of RPI indicate a decreased random preference/increased predictable preference. RPI values for SAMΔ100% were greater for randomly presented stimuli, whereas stimuli with a decreasing SAM salience were found to decrease the RPI values (i.e. increasing predictable preference for SAMΔ25% and for noisy SAM). These significant salience‐related changes in RPI values between SAMΔ100% vs. SAMΔ25% and noisy SAM are shown in Fig. 5 (P < 0.001 for SAMΔ100% vs. Δ25% and P < 0.05 for SAMΔ100% vs. noisy 100% depth). A similar analyses in anaesthetized rats showed no significant differences in RPI values between SAMΔ100% vs. SAMΔ50%, SAMΔ25% and noisy SAM (data not shown).
Figure 5. RPI for MGB units in awake rats across SAM stimulus sets.
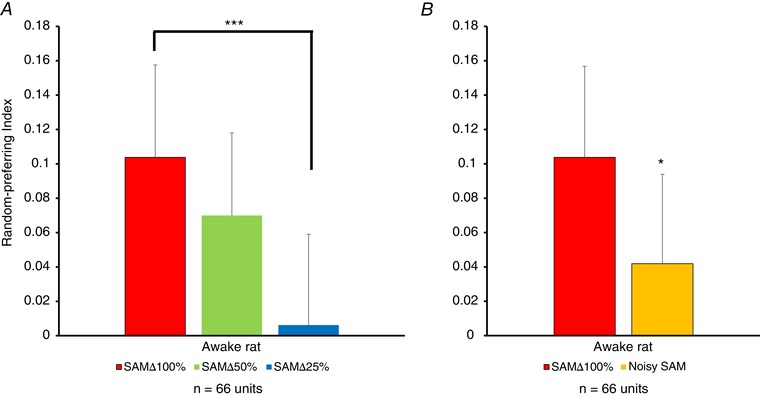
RPIs were calculated by finding the total AUC for random and predictable stimuli across all f ms. A, consistent with findings shown in Fig. 3, the RPI recorded from awake rat MGB neurons showed a significant decrease in RPIs as SAM salience was decreased by reducing modulation depth (SAM∆50% and SAM∆25%) B, similar to (A), jittering the stimulus (noisy SAM) showed a significant decrease in the RPI. Data are presented as the mean ± SEM; statistical analyses were repeated‐measures ANOVA followed by post hoc Tukey's correction using Graphpad. * P < 0.05; *** P < 0.01. [Color figure can be viewed at wileyonlinelibrary.com]
Figure 6. Comparison of RPIs across specific f ms for awake rat MGB neurons in response to SAM∆100%, SAM∆50%, SAM∆25% and noisy SAM.
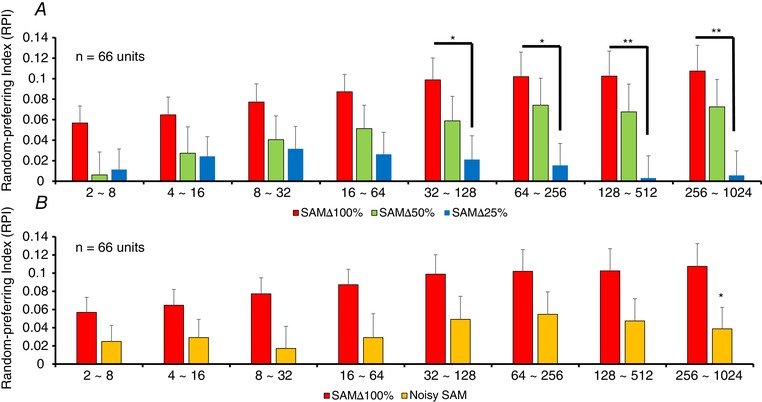
A, RPI for MGB neurons responding to SAM∆25% showed significant decreases across higher f ms of 32–128, 64–256, 128–512 and 256–1024 Hz compared to responses at SAM∆100% at the same f ms. There were no significant differences between SAM∆100% and SAM∆50% at any f ms. B, RPI for MGB neurons responding to noisy SAM were significantly different from SAM∆100% across f ms. The significant decrease in random‐preference with decreased temporal salience, when presented with SAM∆25% and noisy SAM, at higher f ms is suggestive of an increase in preference for predictable stimuli at higher f ms similar to previous findings in aged rats. Data are presented as the mean ± SEM; statistical analyses were repeated‐measures ANOVA with post hoc Tukey's correction using Graphpad. * P < 0.05; ** P < 0.01.
To determine whether specific random vs. predictable differences in firing existed across f ms in awake rats, RPI values for three consecutive f m combinations were calculated as shown in Fig. 6. RPI values for units responding to SAMΔ100% were all positive, indicating a preference for random SAMΔ100% across all f m segments. By contrast, the same units showed significantly decreased RPI responses to less salient SAM, SAMΔ25% and noisy SAM for f ms above 128 and 256 Hz (Fig. 6). These data show a salience‐related preference shift from random toward predictable SAM stimuli, with the greatest changes for SAMΔ25% at the more challenging higher f ms including 32–128 Hz, 64–256 Hz (P < 0.05), 128–512 Hz and 256–1024 Hz (P < 0.01). RPI values to noisy SAM were also significantly decreased, suggesting an increased preference for predictable stimuli at 256–1024 Hz (P < 0.05). Because there was an increase in firing to predictably presented less salient SAM stimuli at higher f ms, we examined trial‐by‐trial differences between SAMΔ100% and SAMΔ25% at 256 and 512 Hz f m. Figure 7 shows trial‐by‐trial responses to SAMΔ100% and SAMΔ25% from a single MGB exemplar unit (A and B). Switching between repeating/predictable SAMΔ100% and SAMΔ25% (256 Hz f m) altered the unit responses from weakly adapting to increasing the discharge rate with each successive trial, as shown by the linear trend lines on the vertical histograms. Trial‐by‐trial responses for predicable SAMΔ100% and SAMΔ25% were calculated and compared at 256 Hz f m for all 66 MGB units (Fig. 7 C).
Figure 7. Trial‐by‐trial response analysis to predictable presentation of SAM∆100% and SAM∆25% at f m 256 and 512 Hz.
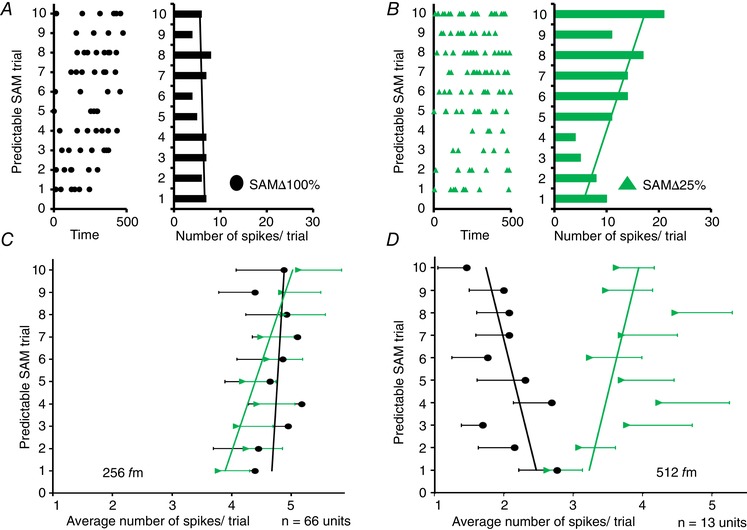
A, trial‐by‐trial measure of responses of an exemplar single‐unit from the MGB of an awake rat to 10 repeating presentations of a SAM∆100% at f m of 256 Hz presented in predictable manner. B, responses from the same unit to 10 repeating presentations of SAM∆25% at f m of 256 Hz presented in a predictable manner. C, comparison of group trial‐by‐trial data (mean ± SEM) of responses to predictable SAM at f m = 256 Hz. The trend line slopes are significantly different for SAM∆100% and SAM∆25% groups when plotting average spikes to predictable presentation of 256 Hz f m (ANCOVA, two‐tailed, P < 0.05). D, a subset of 13 neurons (these neurons show a decrease in RPI by >0.3 when switched from SAM∆100% to SAM∆25%) in response to a predictable presentation of f m at 512 Hz showed adaption with SAM∆100% and an increase in responses with each trial of SAM∆25% (ANCOVA, two‐tailed, P < 0.05). [Color figure can be viewed at wileyonlinelibrary.com]
Similar to the exemplar, the group data at 256 Hz f m showed an increase in discharge rate across successive repetitive SAMΔ25% stimuli as opposed to no change or adaptation to successive repetitive SAMΔ100% stimuli. Slopes comparing group data trend lines between SAMΔ100% and SAMΔ25% were significantly different (P < 0.05). MGB units recorded from anaesthetized rat at 256 Hz f m presented SAMΔ25% showed strong adaptation in the group data (n = 28) with the trend line slope being significantly different from SAMΔ 25% recorded from MGB units in awake rats (data not shown, P < 0.05). No significant changes between predictable SAMΔ100% and SAMΔ25% were observed at 512 Hz f m for all 66 neurons in awake rats. A subset of 13 single units, which showed decreases in RPI (Δ RPI > 0.3) when switched from SAMΔ100% to SAMΔ25%, showed a significant difference in group trend line slope (P < 0.05) (Fig. 7 D).
tMTF differences across f ms with decreasing SAM salience (group data)
The percentage of units showing temporal/envelope‐locking responses across f ms (2–128) during random or predictable presentation of SAMΔ100% stimuli were compared with random or predictable presentation of SAMΔ50%, SAMΔ25% and noisy SAM (Fig. 8). A Rayleigh statistic minimum of 13.8 was used as a qualifier for the envelope‐locking ability of each unit. The highest levels of locking to SAMΔ100% were observed for single‐unit responses near modulation frequencies centred between 8 and 16 Hz (Fig. 8). Compared to SAMΔ100%, there was a significant decrease in temporal‐locking ability at shallower modulation depths for both random and predictable stimuli: SAMΔ50% random (at f m 16 and 32 Hz, P < 0.05) and predictable stimuli presentation (at f m 4 Hz, P < 0.05). SAMΔ25% presented either in a random or predictable manner showed significant decreases at all f ms tested (P < 0.05). Temporal responses to noisy SAM showed significant decreases in the percentage of units showing envelope‐locking responses compared to SAMΔ100% for random (at f m 2, 4, 8, 16 and 32 Hz, P < 0.05) and predictable stimuli (at f m 2, 4 and 8 Hz, P < 0.05). Differences between predictable and random presentation of SAMΔ100%, SAMΔ50%, SAMΔ25% and noisy SAM stimuli were not significant. There were no significant differences in vector strength between predictable and random presentation of stimuli at different levels of salience and f ms between 2 and 128 Hz (data not shown).
Discussion
In the present study, the hypothesis that age‐related increases in preference coding for repeated/predictable acoustic stimuli are at least partially the result of a degraded temporal code seen in older rats and humans was tested using a degraded stimulus to simulate ageing in young animals (Tremblay et al. 2002; Caspary et al. 2008; Caspary & Llano, 2018). Consistent with our hypothesis, the presentation of less salient SAM stimuli led to a decrease in the number of neurons showing temporal/envelope locking, which mimics aged human/animal models (Caspary & Llano, 2018; Ng & Recanzone, 2018). A comparison of rate responses in single units from awake rat MGB showed a significant increase in preference for predictable stimuli and the number of spikes with each trial at a higher f ms (>128 Hz) when less salient SAM stimuli were presented but not with standard SAMΔ 100%. This change in preference coding was not seen in units recorded from anaesthetized rat MGB. The observed relative switch from adaptive to enhanced coding for repeating/predictable, less‐salient stimuli is considered to involve higher cognitive resources, which are brought into play to disambiguate the less salient acoustic message. This notion is supported by a lack of similar changes in units from anaesthetized animals in the present study, as well as by prior studies on top‐down effects over bottom‐up circuits and behaviour (Maunsell & Treue, 2006; Fritz et al. 2007; Chandrasekaran et al. 2009; Peelle & Wingfield, 2016; Homma et al. 2017; Helfer et al. 2018).
Effects of ageing on central auditory system and top‐down processes
The present findings, obtained using temporally degraded modulated sounds in young‐adult MGB units, support our hypothesis that modelled ageing using temporally degraded stimuli in young rats. These findings are consistent with studies showing an age‐related increase in predictable preference or an increase in firing with repetition (de Villers‐Sidani et al. 2010; Cai et al. 2016; Cisneros‐Franco et al. 2018). We postulated this to be the result of a temporally jittered/less salient code in aged MGB that frequently led to increases in firing with repetition. We interpret this as a mechanism for strengthening the internal representations of temporally degraded signals by engaging top‐down cognitive resources. Congruent with these interpretations, previous human and animal studies also describe age‐related losses in auditory temporal processing and an inability to accurately localize sound in cluttered environments (Pichora‐Fuller et al. 2007; Dubno et al. 2008; Eddins & Hall, 2010; King et al. 2014; Harris & Dubno, 2017). Speech in noise conditions degrades speech understanding even in older individuals with healthy hearing (Pichora‐Fuller et al. 1995). This decline in speech understanding in older individuals correlates well with a decline in temporal processing with age (Fitzgibbons & Gordon‐Salant, 1994, 2011; Harris et al. 2012). Mechanistic insights into these deficits suggest a negative impact of ageing on the cochlea, where several studies have described some combination of sensory, neural, strial and conductive elements resulting in a decrease in the quality and intensity of sound signals reaching the central auditory system (Schuknecht & Gacek, 1993; Sergeyenko et al. 2013; Libreman & Kujawa, 2017; Cai et al. 2018). However, research conducted with normal hearing elderly humans has reported deficits in speech understanding, especially in complex acoustic environments, implicating central deficits in addition to peripheral deficits in presbycusis (Harris et al. 2012; Humes et al. 2012; Pichora‐Fuller et al. 2017). These findings are backed by evidence obtained from human psychoacoustic studies and animal studies demonstrating disruptive age‐related effects on the veracity of the ascending acoustic code in conjunction with age‐related peripheral losses at the cochlea and auditory nerve fibres (Willott et al. 1991; Gratton et al. 1997; Spongr et al. 1997; Tang et al. 2014; Cai et al. 2018). Previous studies have also shown de novo age‐related changes vs. deafferentation plasticity (Caspary & Llano, 2018; Occelli et al. 2019). Furthermore, neurochemical studies supporting the loss of speech understanding with ageing found an age‐related compensatory, net down‐regulation of GABAergic and glycinergic inhibition in the cochlear nucleus, inferior colliculus, MGB and auditory cortex (Stebbings et al. 2014; Gao et al. 2015; Ouda et al. 2015; Godfrey et al. 2017; Caspary & Llano, 2018). Rodent and primate neurophysiological ageing studies highlighted an increase in discharge rates and less precise temporal‐locking in central auditory structures, which are probably partly responsible for the loss of temporal processing in older humans (Pichora‐Fuller & Schneider, 1992; Palombi & Caspary, 1996; Frisina, 2001; Harris et al. 2012; Mattys & Scharenborg, 2014; Parthasarathy et al. 2016; Ng & Recanzone, 2018). Modelling these less precise temporal‐locked responses, as seen in aged populations, by jittering the temporal fine structure of the stimulus in young people led to decreased speech in noise recognition (Pichora‐Fuller et al. 2007; Mamo et al. 2016). Similar to jitter, decreasing the depth of SAM stimuli decreases the salience of the SAM, leading to decreased perceptual ability and phase locking (Pollack & Pickett, 1963; Joris & Yin, 1992; Wingfield et al. 1994; Shannon et al. 1995; Krishna & Semple, 2000; Jorgensen & Dau, 2011; Christiansen et al. 2013; Srinivasan & Zahorik, 2014). Despite such temporal deficits, many older individuals can maintain speech understanding, suggesting that compensatory mechanisms are in play (Dubno et al. 1984; Humes et al. 2012; Peelle & Wingfield, 2016). This points to cognitive based resources, including attention and context‐mediated enhancement of neural representations, with respect to identifying and extracting salient or difficult to identify signals in complex acoustic environments. By contrast to this compensatory strategy for enhancement, in young animals, neural representations of repeating stimuli are reduced and adapted with respect to the detection of novel stimuli (i.e. SSA) (Strange, 1989; Ohl et al. 1999; Ono et al. 2006; Davis & Johnsrude, 2007; Fritz et al. 2007; Shinn‐Cunningham & Wang, 2008; Tremblay et al. 2010; Duque et al. 2013; Obleser, 2014; Skoe et al. 2014; Malmierca et al. 2015; Cisneros‐Franco et al. 2018). Collectively, these studies suggest that adaptation may originate from lower structures (i.e. a bottom‐up process), whereas predictions are formed by higher‐order cortical structures (i.e. a top‐down process) (Rao & Ballard, 1999; Anderson & Malmierca, 2013; Peelle & Wingfield, 2016; Yun Rui et al. 2018). However cognitive deficits are also seen with ageing (Gazzaley & Nobre, 2012). Consistent with the study by Cai et al. (2016), responses to SAMΔ100% showed a balance between bottom‐up adaptation and descending top‐down influences, reflecting a mix of random and predictable preferring neurons. In response to relatively less salient SAMΔ25%, these same single units showed a clear preference for predictable signals, especially at higher f ms (Figs 6 and 7). An absence of similar changes in anaesthetized rats is consistent with anaesthesia‐related partial blockade of top‐down influences, as previously suggested by an absence of perceptual learning and cortical disconnectivity with anaesthesia (Aberg et al. 2009; Jordan et al. 2013; Mashour, 2014). These findings are in agreement with recent work showing context and experience‐based predictions in humans and animals (Ostroff et al. 2003; Fakhri et al. 2012; Leung et al. 2013; Skoe et al. 2014; Sohoglu & Chait, 2016; Parras et al. 2017; Schwartz & David, 2018). Furthermore, repetition‐induced increases in speech understanding, as well as improvements in the visual recognition of degraded objects with repetition, provide evidence in support of our interpretation of an increased response to degraded predictable stimuli (Rivenez et al. 2006; Muller et al. 2013; Helfer et al. 2018). Collectively, these results lend support to the hypothesis that less salient stimuli/degraded ascending acoustic code can be compensated for by an increased use of cortical cognitive (experience/contextual) and attentional resources (Bertoli et al. 2001; Alain et al. 2014; Bidelman et al. 2014; Presacco et al. 2016a; Lesicko & Llano, 2017).
Effects of ageing and top‐down processes in the medial geniculate body
In the present study, less salient stimuli not only altered the preference for predictable stimuli, but also showed a decrease in envelope‐locking. These results support our stimulus choice aimed at simulating temporal processing deficits observed with ageing, which are partly mediated by neurotransmitter‐deficits with ageing. Age‐related changes in neurotransmission impact upon the function of descending and ascending projections to the MGB, which include decreased tonic GABA currents, as well as pre‐ and post‐synaptic changes in cholinergic receptors, and are considered to be involved in gating/modulating MGB representations (Banay‐Schwartz et al. 1989; Richardson et al. 2013b; Godfrey et al. 2017; Sottile et al. 2017a; Sottile et al. 2017b). However, Richardson et al. (2013a) failed to find evidence of age‐related physiological changes in MGB function using short duration pure tone SSA paradigm (Richardson et al. 2013a). A second ageing MGB study by Cai et al. (2016) used complex longer duration SAM stimuli stepped between 2 and 1024 f m and found significant age‐related changes in the response properties of MGB neurons when a random presentation was compared with a predictable/repeating presentation of stimuli. These findings are consistent with studies performed in aged rat auditory cortex showing a decrease in SSA (de Villers‐Sidani et al. 2010; Cisneros‐Franco et al. 2018). The use of a relatively long SAM stimulus is more complex than the short pure tones used in most SSA studies and may more closely represent animal vocalizations and human speech. These results are consistent with previous studies using more complex (frequency modulated and harmonic complex tones) stimuli instead of pure tones, which also found an increased use of top‐down resources, possibly via corticothalamic projections for sound detection. Previous MGB studies on corticothalamic stimulation have shown simulated top‐down modulatory effects on responses in learning and discrimination studies in many species, including ferret, mouse, cat, gerbil, rat, guinea pig, bat and monkey (Orman & Humphrey, 1981; Zhang et al. 1997; Ohl et al. 1999; He, 2003; Ono et al. 2006; Rybalko et al. 2006; Wetzel et al. 2008; Guo et al. 2017; Homma et al. 2017; Barczak et al. 2018). Recently, Guo et al. (2017) examined the impact of corticothalamic stimulation on MGB discharge properties and a sound detection task. They found a gain in MGB activity that lasted for 150 ms post‐stimulation, which was associated with enhanced sound detection (Guo et al. 2017). Few studies have investigated the effect of corticothalamic stimulation with ageing on MGB physiology (Sottile et al. 2017a; Sottile et al. 2017b). Apart from modulating MGB response properties, corticothalamic projections are implicated in the switching from temporal to rate code, possibly involving some combination of short‐term NMDA/AMPA and long‐term mGluR‐dependent mechanisms (Lu et al. 2001; Bartlett & Smith, 2002; Bartlett & Wang, 2007; Wang et al. 2008; Rabang & Bartlett, 2011; Cai & Caspary, 2015). Although, in the present study, we found no differences in envelope‐locking between random and predictable presentation of less salient stimuli, we observed increases in the rate responses with repeated presentation. Differences between dorsal vs. ventral units should be carefully addressed in the future as a result of differences in afferent projections and the physiology between MGB subdivisions (Bartlett 2013). Future studies should aim to further examine our understanding of the top‐down effects on predictive coding using a direct activation/deactivation of cortiothalamic neurons that addresses the role of ageing in corticothalamic modulation at the MGB.
In conclusion, the present study found that the temporal degradation of a modulated stimulus appears to simulate the temporally degraded ascending acoustic code seen in the elderly, leading to an increased preference for repeating/predictable stimuli in young animals. Furthermore, temporally degraded stimuli increase firing responses to successive repetitions of the same stimulus, as observed in previous studies, suggesting that repetition as a top‐down strategy to better internalize representations (Muller et al. 2013; Helfer et al. 2018).
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
SPK was responsible for data acquisition, data analyses and drafting the manuscript. SPK, RC and DMC were responsible for the interpretation of data. SPK, RC, ELB and DMC were responsible for revising the manuscript. RC and ELB were responsible for the study concept. SPK, RC, ELB and DMC were responsible for the study design. All of the experiments were conducted in the laboratory of DMC at SIU School of Medicine. All of the authors have read and approved the final version of the manuscript and agree to be accountable for all aspects of the work with respect to ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved, and all persons designated as authors qualify for authorship, and all those who qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by National Institute on Deafness and Other Communication Disorders (DC000151‐34) to DMC.
Acknowledgements
The authors would like to thank Dr Kenneth E. Hancock for the design of the sound stimulus used in the present study and Lynne Ling for assistance with the surgery.
Biographies
Srinivasa P. Kommajosyula is a postdoctoral fellow at Southern Illinois University School of Medicine (SIU SOM) under the mentorship of Donald Caspary. His doctoral thesis on sudden death in epilepsy in audiogenic seizure models spurred his curiosity into maladaptive plasticity in networks witnessed in this model and central auditory system. His research interests include understanding the pathophysiological and compensatory changes in central auditory system with ageing and noise induced trauma. The present study highlights evidence of such changes in auditory thalamic coding of less salient stimuli that switch the preference of single unit to repeated stimuli.

Rui Cai is a Research Instructor at SIU SOM. She obtained her PhD in East China Normal University where she studied the environmental effects on the plasticity of auditory function. She continues her work in auditory system in SIU, with an emphasis on the mechanisms of age‐related hearing loss and tinnitus.
Edited by: Kim Barrett & Ian Forsythe
References
- Aberg KC, Albrecht E, Tartaglia EM, Farron A, Soom P & Herzog MH (2009). Anesthesia prevents auditory perceptual learning. Anesthesiology 111, 1010–1015. [DOI] [PubMed] [Google Scholar]
- Alain C, Roye A & Salloum C (2014). Effects of age‐related hearing loss and background noise on neuromagnetic activity from auditory cortex. Front Syst Neurosci 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alain C & Woods DL (1999). Age‐related changes in processing auditory stimuli during visual attention: evidence for deficits in inhibitory control and sensory memory. Psychol Aging 14, 507–519. [DOI] [PubMed] [Google Scholar]
- Anderson LA & Malmierca MS (2013). The effect of auditory cortex deactivation on stimulus‐specific adaptation in the inferior colliculus of the rat. Eur J Neurosci 37, 52–62. [DOI] [PubMed] [Google Scholar]
- Anderson S, Parbery‐Clark A, White‐Schwoch T & Kraus N (2012). Aging affects neural precision of speech encoding. J Neurosci 32, 14156–14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge KE & Wallhagen MI (2014). Hearing loss in an aging american population: extent, impact, and management. Annu Rev Public Health 35, 139–152. [DOI] [PubMed] [Google Scholar]
- Banay‐Schwartz M, Lajtha A & Palkovits M (1989). Changes with aging in the levels of amino acids in rat CNS structural elements. II. Taurine and small neutral amino acids. Neurochem Res 14, 563–570. [DOI] [PubMed] [Google Scholar]
- Barczak A, O'Connell MN, McGinnis T, Ross D, Mowery T, Falchier A & Lakatos P (2018). Top‐down, contextual entrainment of neuronal oscillations in the auditory thalamocortical circuit. Proc Natl Acad Sci U S A 115, E7605‐E7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett EL (2013). The organization and physiology of the auditory thalamus and its role in processing acoustic features important for speech perception. Brain Lang 126, 29–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett EL & Smith PH (2002). Effects of paired‐pulse and repetitive stimulation on neurons in the rat medial geniculate body. Neuroscience 113, 957–974. [DOI] [PubMed] [Google Scholar]
- Bartlett EL & Wang X (2007). Neural representations of temporally modulated signals in the auditory thalamus of awake primates. J Neurophysiol 97, 1005–1017. [DOI] [PubMed] [Google Scholar]
- Bartlett EL & Wang X (2011). Correlation of neural response properties with auditory thalamus subdivisions in the awake marmoset. J Neurophysiol 105, 2647–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoli S, Heimberg S, Smurzynski J & Probst R (2001). Mismatch negativity and psychoacoustic measures of gap detection in normally hearing subjects. Psychophysiology 38, 334–342. [PubMed] [Google Scholar]
- Bidelman GM, Villafuerte JW, Moreno S & Alain C (2014). Age‐related changes in the subcortical‐cortical encoding and categorical perception of speech. Neurobiol Aging 35, 2526–2540. [DOI] [PubMed] [Google Scholar]
- Cai R & Caspary DM (2015). GABAergic inhibition shapes SAM responses in rat auditory thalamus. Neuroscience 299, 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai R, Kalappa BI, Brozoski TJ, Ling LL & Caspary DM (2014). Is GABA neurotransmission enhanced in auditory thalamus relative to inferior colliculus? J Neurophysiol 111, 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai R, Montgomery SC, Graves KA, Caspary DM & Cox BC (2018). The FBN rat model of aging: investigation of ABR waveforms and ribbon synapse changes. Neurobiol Aging 62, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai R, Richardson BD & Caspary DM (2016). Responses to predictable versus random temporally complex stimuli from single units in auditory thalamus: impact of aging and anesthesia. J Neurosci 36, 10696–10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali AG, Gosseries O, Rosanova M, Boly M, Sarasso S, Casali KR, Casarotto S, Bruno MA, Laureys S, Tononi G & Massimini M (2013). A theoretically based index of consciousness independent of sensory processing and behavior. Sci Transl Med 5, 198–105. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG & Hughes LF (2008). Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol 211, 1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM & Llano DA (2018). Aging Processes in the Subcortical Auditory System In The Oxford Handbook of the Auditory Brainstem, ed. Kandler K. Oxford University Press, Oxford. [Google Scholar]
- Chandrasekaran B, Hornickel J, Skoe E, Nicol T & Kraus N (2009). Context‐dependent encoding in the human auditory brainstem relates to hearing speech in noise: implications for developmental dyslexia. Neuron 64, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen C, MacDonald EN & Dau T (2013). Contribution of envelope periodicity to release from speech‐on‐speech masking. J Acoust Soc Am 134, 2197–2204. [DOI] [PubMed] [Google Scholar]
- Cisneros‐Franco JM, Ouellet L, Kamal B & de Villers‐Sidani E (2018). A brain without brakes: reduced inhibition is associated with enhanced but dysregulated plasticity in the aged rat auditory cortex. eNeuro 5, ENEURO.0051‐18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton DS, Cruickshanks KJ, Klein BE, Klein R, Wiley TL & Nondahl DM (2003). The impact of hearing loss on quality of life in older adults. Gerontologist 43, 661–668. [DOI] [PubMed] [Google Scholar]
- Davis MH & Johnsrude IS (2007). Hearing speech sounds: top‐down influences on the interface between audition and speech perception. Hear Res 229, 132–147. [DOI] [PubMed] [Google Scholar]
- de la Mothe LA, Blumell S, Kajikawa Y & Hackett TA (2006). Thalamic connections of the auditory cortex in marmoset monkeys: core and medial belt regions. J Comp Neurol 496, 72–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villers‐Sidani E, Alzghoul L, Zhou X, Simpson KL, Lin RC & Merzenich MM (2010). Recovery of functional and structural age‐related changes in the rat primary auditory cortex with operant training. Proc Natl Acad Sci U S A 107, 13900–13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubno JR, Ahlstrom JB & Horwitz AR (2008). Binaural advantage for younger and older adults with normal hearing. J Speech Lang Hear Res 51, 539–556. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Dirks DD & Morgan DE (1984). Effects of age and mild hearing loss on speech recognition in noise. J Acoust Soc Am 76, 87–96. [DOI] [PubMed] [Google Scholar]
- Duque D, Malmierca MS & Caspary DM (2013). Modulation of stimulus‐specific adaptation by GABAA receptor activation or blockade in the medial geniculate body of the anaesthetized rat. J Physiol 592, 729–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddins DA & Hall JW (2010). Binaural Processing and Auditory Asymmetries In The Aging Auditory System, ed. Gordon‐Salant S, Frisina RD, Popper AN. & Fay RR, pp. 135–165. Springer, New York, New York, NY. [Google Scholar]
- Eisenberg LS, Martinez AS, Holowecky SR & Pogorelsky S (2002). Recognition of lexically controlled words and sentences by children with normal hearing and children with cochlear implants. Ear Hear 23, 450–462. [DOI] [PubMed] [Google Scholar]
- Fakhri M, Sikaroodi H, Maleki F, Ali Oghabian M & Ghanaati H (2012). Age‐related frontal hyperactivation observed across different working memory tasks: an fMRI study. Behav Neurol 25, 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli F, Massimini M, Sarasso S, Casali A, Riedner BA, Angelini G, Tononi G & Pearce RA (2010). Breakdown in cortical effective connectivity during midazolam‐induced loss of consciousness. Proc Natl Acad Sci U S A 107, 2681–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbons PJ & Gordon‐Salant S (1994). Age effects on measures of auditory duration discrimination. J Speech Hear Res 37, 662–670. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons PJ & Gordon‐Salant S (2011). Age effects in discrimination of repeating sequence intervals. J Acoust Soc Am 129, 1490–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisina RD (2001). Subcortical neural coding mechanisms for auditory temporal processing. Hear Res 158, 1–27. [DOI] [PubMed] [Google Scholar]
- Friston K (2009). The free‐energy principle: a rough guide to the brain? Trends Cogn Sci 13, 293–301. [DOI] [PubMed] [Google Scholar]
- Fritz JB, Elhilali M, David SV & Shamma SA (2007). Auditory attention – focusing the searchlight on sound. Curr Opin Neurobiol 17, 437–455. [DOI] [PubMed] [Google Scholar]
- Gao F, Wang G, Ma W, Ren F, Li M, Dong Y, Liu C, Liu B, Bai X, Zhao B & Edden RA (2015). Decreased auditory GABA+ concentrations in presbycusis demonstrated by edited magnetic resonance spectroscopy. Neuroimage 106, 311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A & Nobre AC (2012). Top‐down modulation: bridging selective attention and working memory. Trends Cogn Sci 16, 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Prokopenko VF, West MO & Fabbricatore AT (2006). Higher magnitude accumbal phasic firing changes among core neurons exhibiting tonic firing increases during cocaine self‐administration. Neuroscience 137, 1075–1085. [DOI] [PubMed] [Google Scholar]
- Godfrey DA, Chen K, O'Toole TR & Mustapha A (2017). Amino acid and acetylcholine chemistry in the central auditory system of young, middle‐aged and old rats. Hear Res 350, 173–188. [DOI] [PubMed] [Google Scholar]
- Goldberg JM & Brown PB (1969). Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: some physiological mechanisms of sould localization. J Neurophysiol 32, 613–636. [DOI] [PubMed] [Google Scholar]
- Gordon‐Salant S (2014). Aging, hearing loss, and speech recognition: stop shouting, I can't understand you In Perspectives on Auditory Research, ed. Popper AN. & Fay RR, pp. 211–228. Springer, New York, NY. [Google Scholar]
- Gratton MA, Schulte BA & Smythe NM (1997). Quantification of the stria vascularis and strial capillary areas in quiet‐reared young and aged gerbils. Hear Res 114, 1–9. [DOI] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Clause AR, Barth‐Maron A & Polley DB (2017). A corticothalamic circuit for dynamic switching between feature detection and discrimination. Neuron 95, 180–194.e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KC & Dubno JR (2017). Age‐related deficits in auditory temporal processing: unique contributions of neural dyssynchrony and slowed neuronal processing. Neurobiol Aging 53, 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KC, Wilson S, Eckert MA & Dubno JR (2012). Human evoked cortical activity to silent gaps in noise: effects of age, attention, and cortical processing speed. Ear Hear 33, 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J (2003). Corticofugal modulation on both ON and OFF responses in the nonlemniscal auditory thalamus of the guinea pig. J Neurophysiol 89, 367–381. [DOI] [PubMed] [Google Scholar]
- Helfer KS, Freyman RL & Merchant GR (2018). How repetition influences speech understanding by younger, middle‐aged and older adults. Int J Audiol 57, 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma NY, Happel MFK, Nodal FR, Ohl FW, King AJ & Bajo VM (2017). A role for auditory corticothalamic feedback in the perception of complex sounds. J Neurosci 37, 6149–6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes LE, Dubno JR, Gordon‐Salant S, Lister JJ, Cacace AT, Cruickshanks KJ, Gates GA, Wilson RH & Wingfield A (2012). Central presbycusis: a review and evaluation of the evidence. J Am Acad Audiol 23, 635–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan D, Ilg R, Riedl V, Schorer A, Grimberg S, Neufang S, Omerovic A, Berger S, Untergehrer G, Preibisch C, Schulz E, Schuster T, Schroter M, Spoormaker V, Zimmer C, Hemmer B, Wohlschlager A, Kochs EF & Schneider G (2013). Simultaneous electroencephalographic and functional magnetic resonance imaging indicate impaired cortical top‐down processing in association with anesthetic‐induced unconsciousness. Anesthesiology 119, 1031–1042. [DOI] [PubMed] [Google Scholar]
- Jorgensen S & Dau T (2011). Predicting speech intelligibility based on the signal‐to‐noise envelope power ratio after modulation‐frequency selective processing. J Acoust Soc Am 130, 1475–1487. [DOI] [PubMed] [Google Scholar]
- Joris PX & Yin TC (1992). Responses to amplitude‐modulated tones in the auditory nerve of the cat. J Acoust Soc Am 91, 215–232. [DOI] [PubMed] [Google Scholar]
- Kalappa BI, Brozoski TJ, Turner JG & Caspary DM (2014). Single unit hyperactivity and bursting in the auditory thalamus of awake rats directly correlates with behavioural evidence of tinnitus. J Physiol 592, 5065–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, Hopkins K & Plack CJ (2014). The effects of age and hearing loss on interaural phase difference discrimination. J Acoust Soc Am 135, 342–351. [DOI] [PubMed] [Google Scholar]
- Krishna BS & Semple MN (2000). Auditory temporal processing: responses to sinusoidally amplitude‐modulated tones in the inferior colliculus. J Neurophysiol 84, 255–273. [DOI] [PubMed] [Google Scholar]
- Kuchibhotla K & Bathellier B (2018). Neural encoding of sensory and behavioral complexity in the auditory cortex. Curr Opin Neurobiol 52, 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesicko AMH & Llano DA (2017). Impact of peripheral hearing loss on top‐down auditory processing. Hear Res 343, 4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AW, He Y, Grady CL & Alain C (2013). Age differences in the neuroelectric adaptation to meaningful sounds. PLoS ONE 8, e68892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC & Kujawa SG (2017). Cochlear synaptopathy in acquired sensorineural hearing loss: manifestations and mechanisms. Hear Res 349, 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Liang L & Wang X (2001). Temporal and rate representations of time‐varying signals in the auditory cortex of awake primates. Nat Neurosci 4, 1131–1138. [DOI] [PubMed] [Google Scholar]
- Luce PA & Pisoni DB (1998). Recognizing spoken words: the neighborhood activation model. Ear Hear 19, 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumani A & Zhang H (2010). Responses of neurons in the rat's dorsal cortex of the inferior colliculus to monaural tone bursts. Brain Res 1351, 115–129. [DOI] [PubMed] [Google Scholar]
- Malmierca MS, Anderson LA & Antunes FM (2015). The cortical modulation of stimulus‐specific adaptation in the auditory midbrain and thalamus: a potential neuronal correlate for predictive coding. Front Syst Neurosci 9, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamo SK, Grose JH & Buss E (2016). Speech‐evoked ABR: effects of age and simulated neural temporal jitter. Hear Res 333, 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardia KV & Jupp PE (2000). Directional Statistics. Wiley, New York, NY. [Google Scholar]
- Mashour GA (2014). Top‐down mechanisms of anesthetic‐induced unconsciousness. Front Syst Neurosci 8, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattys SL & Scharenborg O (2014). Phoneme categorization and discrimination in younger and older adults: a comparative analysis of perceptual, lexical, and attentional factors. Psychol Aging 29, 150–162. [DOI] [PubMed] [Google Scholar]
- Maunsell JH & Treue S (2006). Feature‐based attention in visual cortex. Trends Neurosci 29, 317–322. [DOI] [PubMed] [Google Scholar]
- Morest DK (1964). The neuronal architecture of the medial geniculate body of the cat. J Anat 98, 611–630. [PMC free article] [PubMed] [Google Scholar]
- Muller NG, Strumpf H, Scholz M, Baier B & Melloni L (2013). Repetition suppression versus enhancement – it's quantity that matters. Cereb Cortex 23, 315–322. [DOI] [PubMed] [Google Scholar]
- Nelken I (2014). Stimulus‐specific adaptation and deviance detection in the auditory system: experiments and models. Biol Cybern 108, 655–663. [DOI] [PubMed] [Google Scholar]
- Ng CW & Recanzone GH (2018). Age‐related changes in temporal processing of rapidly‐presented sound sequences in the macaque auditory cortex. Cereb Cortex 28, 3775–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y, Vyazovskiy VV, Cirelli C, Banks MI & Tononi G (2015). Auditory responses and stimulus‐specific adaptation in rat auditory cortex are preserved across NREM and REM sleep. Cerebral cortex 25, 1362–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obleser J (2014). Putting the listening brain in context. Lang Linguist Compass 8, 646–658. [Google Scholar]
- Occelli F, Hasselmann F, Bourien J, Eybalin M, Puel JL, Desvignes N, Wiszniowski B, Edeline JM & Gourevitch B (2019). Age‐related changes in auditory cortex without detectable peripheral alterations: a multi‐level study in Sprague Dawley rats. Neuroscience 404, 184–204. [DOI] [PubMed] [Google Scholar]
- Ohl FW, Wetzel W, Wagner T, Rech A & Scheich H (1999). Bilateral ablation of auditory cortex in Mongolian gerbil affects discrimination of frequency modulated tones but not of pure tones. Learn Mem 6, 347–362. [PMC free article] [PubMed] [Google Scholar]
- Ono K, Kudoh M & Shibuki K (2006). Roles of the auditory cortex in discrimination learning by rats. Eur J Neurosci 23, 1623–1632. [DOI] [PubMed] [Google Scholar]
- Orman SS & Humphrey GL (1981). Effects of changes in cortical arousal and of auditory cortex cooling on neuronal activity in the medial geniculate body. Exp Brain Res 42, 475–482. [DOI] [PubMed] [Google Scholar]
- Ortman JM, Velkoff VA & Hogan H (2014). An Aging Nation: The Older Population in the United States, pp. 25–1140. Current Population Reports US Census Bureau, Washington, DC. [Google Scholar]
- Ostroff JM, McDonald KL, Schneider BA & Alain C (2003). Aging and the processing of sound duration in human auditory cortex. Hear Res 181, 1–7. [DOI] [PubMed] [Google Scholar]
- Ouda L, Profant O & Syka J (2015). Age‐related changes in the central auditory system. Cell Tissue Res 361, 337–358. [DOI] [PubMed] [Google Scholar]
- Palombi PS & Caspary DM (1996). Responses of young and aged Fischer 344 rat inferior colliculus neurons to binaural tonal stimuli. Hear Res 100, 59–67. [DOI] [PubMed] [Google Scholar]
- Parras GG, Nieto‐Diego J, Carbajal GV, Valdes‐Baizabal C, Escera C & Malmierca MS (2017). Neurons along the auditory pathway exhibit a hierarchical organization of prediction error. Nat Commun 8, 2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Lai J & Bartlett EL (2016). Age‐related changes in processing simultaneous amplitude modulated sounds assessed using envelope following responses. J Assoc Res Otolaryngol 17, 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos W & Watson C (1998). The Rat Brain in Stereotaxic Coordinates. Academic Press, San Diego, CA. [Google Scholar]
- Peelle JE, Troiani V, Wingfield A & Grossman M (2010). Neural processing during older adults' comprehension of spoken sentences: age differences in resource allocation and connectivity. Cereb Cortex 20, 773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE & Wingfield A (2016). The Neural Consequences of Age‐Related Hearing Loss. Trends Neurosci 39, 486–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichora‐Fuller MK, Alain C & Schneider BA (2017). Older Adults at the Cocktail Party In The Auditory System at the Cocktail Party, ed. Middlebrooks JC, Simon JZ, Popper AN. & Fay RR, pp. 227–259. Springer International Publishing, Cham. [Google Scholar]
- Pichora‐Fuller MK, Kramer SE, Eckert MA, Edwards B, Hornsby BW, Humes LE, Lemke U, Lunner T, Matthen M, Mackersie CL, Naylor G, Phillips NA, Richter M, Rudner M, Sommers MS, Tremblay KL & Wingfield A (2016). Hearing impairment and cognitive energy: the Framework for Understanding Effortful Listening (FUEL). Ear Hear 37 (Suppl 1), 5S–27S. [DOI] [PubMed] [Google Scholar]
- Pichora‐Fuller MK & Schneider BA (1992). The effect of interaural delay of the masker on masking‐level differences in young and old adults. J Acoust Soc Am 91, 2129–2135. [DOI] [PubMed] [Google Scholar]
- Pichora‐Fuller MK, Schneider BA & Daneman M (1995). How young and old adults listen to and remember speech in noise. J Acoust Soc Am 97, 593–608. [DOI] [PubMed] [Google Scholar]
- Pichora‐Fuller MK, Schneider BA, Macdonald E, Pass HE & Brown S (2007). Temporal jitter disrupts speech intelligibility: a simulation of auditory aging. Hear Res 223, 114–121. [DOI] [PubMed] [Google Scholar]
- Pollack I & Pickett JM (1963). The intelligibility of excerpts from conversation. Lang Speech 6, 165–171. [Google Scholar]
- Presacco A, Simon JZ & Anderson S (2016a). Effect of informational content of noise on speech representation in the aging midbrain and cortex. J Neurophysiol 116, 2356–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presacco A, Simon JZ & Anderson S (2016b). Evidence of degraded representation of speech in noise, in the aging midbrain and cortex. J Neurophysiol 116, 2346–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabang CF & Bartlett EL (2011). A computational model of cellular mechanisms of temporal coding in the medial geniculate body (MGB). PLoS ONE 6, e29375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RP & Ballard DH (1999). Predictive coding in the visual cortex: a functional interpretation of some extra‐classical receptive‐field effects. Nat Neurosci 2, 79–87. [DOI] [PubMed] [Google Scholar]
- Richardson BD, Hancock KE & Caspary DM (2013a). Stimulus‐specific adaptation in auditory thalamus of young and aged awake rats. J Neurophysiol 110, 1892–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BD, Ling LL, Uteshev VV & Caspary DM (2013b). Reduced GABA(A) receptor‐mediated tonic inhibition in aged rat auditory thalamus. J Neurosci 33, 1218–1227a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivenez M, Darwin CJ & Guillaume A (2006). Processing unattended speech. J Acoust Soc Am 119, 4027–4040. [DOI] [PubMed] [Google Scholar]
- Rogers CS & Wingfield A (2015). Stimulus‐independent semantic bias misdirects word recognition in older adults. J Acoust Soc Am 138, EL26–EL30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouiller EM & Welker E (1991). Morphology of corticothalamic terminals arising from the auditory cortex of the rat: a Phaseolus vulgaris‐leucoagglutinin (PHA‐L) tracing study. Hear Res 56, 179–190. [DOI] [PubMed] [Google Scholar]
- Rybalko N, Suta D, Nwabueze‐Ogbo F & Syka J (2006). Effect of auditory cortex lesions on the discrimination of frequency‐modulated tones in rats. Eur J Neurosci 23, 1614–1622. [DOI] [PubMed] [Google Scholar]
- Schofield BR & Hurley L (2018). Circuits for modulation of auditory function In The Mammalian Auditory Pathways, pp. 235–267. Springer, New York, NY. [Google Scholar]
- Schuknecht HF & Gacek MR (1993). Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol 102, 1–16. [DOI] [PubMed] [Google Scholar]
- Schwartz ZP & David SV (2018). Focal suppression of distractor sounds by selective attention in auditory cortex. Cereb Cortex 28, 323–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC & Kujawa SG (2013). Age‐related cochlear synaptopathy: an early‐onset contributor to auditory functional decline. J Neurosci 33, 13686–13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, Wygonski J & Ekelid M (1995). Speech recognition with primarily temporal cues. Science 270, 303–304. [DOI] [PubMed] [Google Scholar]
- Sheldon S, Pichora‐Fuller MK & Schneider BA (2008). Priming and sentence context support listening to noise‐vocoded speech by younger and older adults. J Acoust Soc Am 123, 489–499. [DOI] [PubMed] [Google Scholar]
- Shinn‐Cunningham BG & Wang D (2008). Influences of auditory object formation on phonemic restoration. J Acoust Soc Am 123, 295–301. [DOI] [PubMed] [Google Scholar]
- Skoe E, Chandrasekaran B, Spitzer ER, Wong PC & Kraus N (2014). Human brainstem plasticity: the interaction of stimulus probability and auditory learning. Neurobiology of learning and memory 109, 82–93. [DOI] [PubMed] [Google Scholar]
- Sohoglu E & Chait M (2016). Detecting and representing predictable structure during auditory scene analysis. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohoglu E, Peelle JE, Carlyon RP & Davis MH (2014). Top‐down influences of written text on perceived clarity of degraded speech. J Exp Psychol Hum Percept Perform 40, 186–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottile SY, Hackett TA, Cai R, Ling L, Llano DA & Caspary DM (2017a). Presynaptic neuronal nicotinic receptors differentially shape select inputs to auditory thalamus and are negatively impacted by aging. J Neurosci 37, 11377–11389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottile SY, Ling L, Cox BC & Caspary DM (2017b). Impact of ageing on postsynaptic neuronal nicotinic neurotransmission in auditory thalamus. J Physiol 595, 5375–5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spongr VP, Flood DG, Frisina RD & Salvi RJ (1997). Quantitative measures of hair cell loss in CBA and C57BL/6 mice throughout their life spans. J Acoust Soc Am 101, 3546–3553. [DOI] [PubMed] [Google Scholar]
- Srinivasan NK & Zahorik P (2014). Enhancement of speech intelligibility in reverberant rooms: role of amplitude envelope and temporal fine structure. J Acoust Soc Am 135, EL239–EL245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbings KA, Lesicko AM & Llano DA (2014). The auditory corticocollicular system: molecular and circuit‐level considerations. Hear Res 314, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange W (1989). Dynamic specification of coarticulated vowels spoken in sentence context. J Acoust Soc Am 85, 2135–2153. [DOI] [PubMed] [Google Scholar]
- Tang X, Zhu X, Ding B, Walton JP, Frisina RD & Su J (2014). Age‐related hearing loss: GABA, nicotinic acetylcholine and NMDA receptor expression changes in spiral ganglion neurons of the mouse. Neuroscience 259, 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlow W (1957). An auditory figure‐ground effect. Am J Psychol 70, 653–654. [PubMed] [Google Scholar]
- Tremblay KL, Inoue K, McClannahan K & Ross B (2010). Repeated stimulus exposure alters the way sound is encoded in the human brain. PLoS ONE 5, e10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay KL, Piskosz M & Souza P (2002). Aging alters the neural representation of speech cues. Neuroreport 13, 1865–1870. [DOI] [PubMed] [Google Scholar]
- Ulanovsky N, Las L & Nelken I (2003). Processing of low‐probability sounds by cortical neurons. Nat Neurosci 6, 391–398. [DOI] [PubMed] [Google Scholar]
- Vaden KI, Jr. , Kuchinsky SE, Ahlstrom JB, Teubner‐Rhodes SE, Dubno JR & Eckert MA (2016). Cingulo‐opercular function during word recognition in noise for older adults with hearing loss. Exp Aging Res 42, 67–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Behrens W, Bauerle P, Kossl M & Gaese BH (2009). Correlating stimulus‐specific adaptation of cortical neurons and local field potentials in the awake rat. J Neurosci 29, 13837–13849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Turner JG, Ling L, Parrish JL, Hughes LF & Caspary DM (2009). Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience 164, 747–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lu T, Bendor D & Bartlett E (2008). Neural coding of temporal information in auditory thalamus and cortex. Neuroscience 157, 484–494. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang J, Zou J, Luo H & Ding N (2019). Prior knowledge guides speech segregation in human auditory cortex. Cereb Cortex 29, 1561–1571. [DOI] [PubMed] [Google Scholar]
- Warren RM (1970). Perceptual restoration of missing speech sounds. Science 167, 392–393. [DOI] [PubMed] [Google Scholar]
- Wetzel W, Ohl FW & Scheich H (2008). Global versus local processing of frequency‐modulated tones in gerbils: an animal model of lateralized auditory cortex functions. Proc Natl Acad Sci U S A 105, 6753–6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott JF, Parham K & Hunter KP (1991). Comparison of the auditory sensitivity of neurons in the cochlear nucleus and inferior colliculus of young and aging C57BL/6J and CBA/J mice. Hear Res 53, 78–94. [DOI] [PubMed] [Google Scholar]
- Winer JA, Diehl JJ & Larue DT (2001). Projections of auditory cortex to the medial geniculate body of the cat. J Comp Neurol 430, 27–55. [PubMed] [Google Scholar]
- Winer JA, Miller LM, Lee CC & Schreiner CE (2005). Auditory thalamocortical transformation: structure and function. Trends Neurosci 28, 255–263. [DOI] [PubMed] [Google Scholar]
- Wingfield A, Alexander AH & Cavigelli S (1994). Does memory constrain utilization of top‐down information in spoken word recognition? Evidence from normal aging. Lang Speech 37, 221–235. [DOI] [PubMed] [Google Scholar]
- Yin P, Johnson JS, O'Connor KN & Sutter ML (2011). Coding of amplitude modulation in primary auditory cortex. J Neurophysiol 105, 582–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun Rui Y, He J, Zhai YY, Sun ZH & Yu X (2018). Frequency‐dependent stimulus‐specific adaptation and regularity sensitivity in the rat auditory thalamus. Neuroscience 392, 13–24. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Suga N & Yan J (1997). Corticofugal modulation of frequency processing in bat auditory system. Nature 387, 900–903. [DOI] [PubMed] [Google Scholar]


