Abstract
Introduction:
Patients with germline TP53 pathogenic variants (Li–Fraumeni syndrome [LFS]) are at extremely high lifetime risk of developing cancer. Recent data suggest that tumor surveillance for patients with LFS may improve survival through early cancer detection. The objective of this study was to assess the cost-effectiveness of a cancer surveillance strategy for patients with LFS compared with those whose tumors present clinically.
Methods:
A Markov decision analytic model was developed from a third-party payer perspective to estimate cost-effectiveness of routine cancer surveillance over a patient’s lifetime. The model consisted of four possible health states: no cancer, cancer, post-cancer survivorship, and death. Model outcomes were costs (2015 United States Dollars [USD]), effectiveness (life years [LY] gained), and incremental cost-effectiveness ratio (ICER; change in cost/LY gained). One-way sensitivity analyses and probabilistic sensitivity analyses examined parameter uncertainty.
Results:
The model showed a mean cost of $46 496 and $117 102 and yielded 23 and 27 LY for the nonsurveillance and surveillance strategies, respectively. The ICER for early cancer surveillance versus no surveillance was $17 125 per additional LY gained. At the commonly accepted willingness to pay threshold of $100 000/life-year gained, surveillance had a 98% probability of being the most cost-effective strategy for early cancer detection in this high-risk population.
Conclusions:
Presymptomatic cancer surveillance is cost-effective for patients with germline pathogenic variants in TP53. Lack of insurance coverage or reimbursement in this population may have significant consequences and leads to undetected cancers presenting in later stages of disease with worse clinical outcomes.
Keywords: cancer surveillance, cost-effectiveness analysis, familial cancer, ICER, Li–Fraumeni syndrome
1 |. INTRODUCTION
In 1969, Li and Fraumeni postulated the existence of a familial cancer syndrome based on their observation of a notable clustering of sarcomas, breast cancer, and other early-onset tumors.1 Eventually coined Li–Fraumeni syndrome (LFS), the high risk for such a broad range of cancers was explained when LFS was found to be associated with germline pathogenic variants in the key tumor suppressor gene TP53.2,3 Pathogenic variants in TP53 are inherited in an autosomal dominant manner. However, de novo mutations account for up to 20% of cases.4 The use of genetic testing to identify families with TP53 pathogenic variants has greatly broadened the spectrum of cancers associated with LFS beyond the “core” tumors initially described, namely, sarcomas, breast cancers, brain tumors, leukemia and adrenocortical carcinoma. Lung, gastrointestinal, thyroid, ovarian, skin, and other cancers have also been reported in mutation carriers, and present at ages earlier than in the general population.5,6
The overall cancer risks in individuals with LFS are substantial: 19%, 41%, and 73% for men and 12%, 84%, and 100% for women by ages 16, 45, and 85, respectively.7 A 2010 analysis of 105 Dutch families with LFS found a 4-fold relative risk for developing cancer in these families compared with the population rate (95% confidence interval [CI], 3.1–4.8).6 The risk for additional primary cancers is increased, with those diagnosed with cancer in childhood (ages 0–19) having the greatest risk for a second primary (RR, 83.0; 95% CI, 36.9–187.6) and those diagnosed with their first cancer after age 20 having a 9- to 10-fold increase in risk.8
These high cancer risks suggest a need for screening and/or prevention strategies that enable early cancer diagnosis and therefore decrease morbidity and mortality. However, these approaches must be both clinically effective to impact patient outcomes and justify genetic testing, and, ideally, cost-effective to support implementation on a large scale. There has been significant success in the area of early tumor surveillance for patients with BRCA1/2-associated hereditary breast/ovarian cancer and for Lynch syndrome. However, routine genetic testing and development of screening strategies for LFS have lagged behind, likely due to the greater challenges of screening for a heterogeneous array of tumors over a wide age range and the relative rarity of this syndrome.
A recent prospective analysis of LFS patients, 59 of whom had multimodal prescribed surveillance and 30 who declined surveillance, found 5-year survival in those diagnosed with cancer through screening to be 88.8% compared with 59.6% in the patients diagnosed with cancer on the basis of clinical presentation.9 This “Toronto surveillance protocol,” described by Villani et al, is very rigorous and demonstrates a significant survival benefit; however, the economic value of the screening protocol has not been evaluated. The National Comprehensive Cancer Network has endorsed some components of this protocol (Table 1).10 One of the main obstacles underlying widespread adoption of this protocol in the United States is the potential cost and lack of insurance reimbursement.9
TABLE 1.
Comparison of cancer surveillance protocol by Villani et al with NCCNa guidelines by cancer type
| Cancer Type | Villani et al protocol9 | NCCN10 |
|---|---|---|
| ACC |
|
|
| Brain tumor |
|
|
| Soft tissue and osteosarcomas |
|
|
| Breast cancer |
|
|
| Colorectal |
|
|
| Melanoma |
|
|
| Leukemia |
|
|
| Other |
|
|
National Comprehensive Cancer Network.
The objective of this study was to assess the cost-effectiveness of the “Toronto surveillance protocol” for patients with LFS from the perspective of a US third-party healthcare payer.
2 |. METHODS
2.1 |. Model description and analysis
We developed a Markov decision analytic model that followed patients diagnosed with LFS over their lifetime.11 We compared the cumulative costs and life years (LY) gained for patients who received a surveillance strategy with those who did not. The incremental monetary value required to gain one LY from surveillance on a population level, also called incremental cost-effectiveness ratios (ICERs), was calculated based on the resulting costs and LY. As has been recommended and used in previous clinical cancer prevention cost-effectiveness analysis literature, we used a willingness-to-pay (WTP) threshold of $100 000.12,13
2.2 |. Model structure/health states
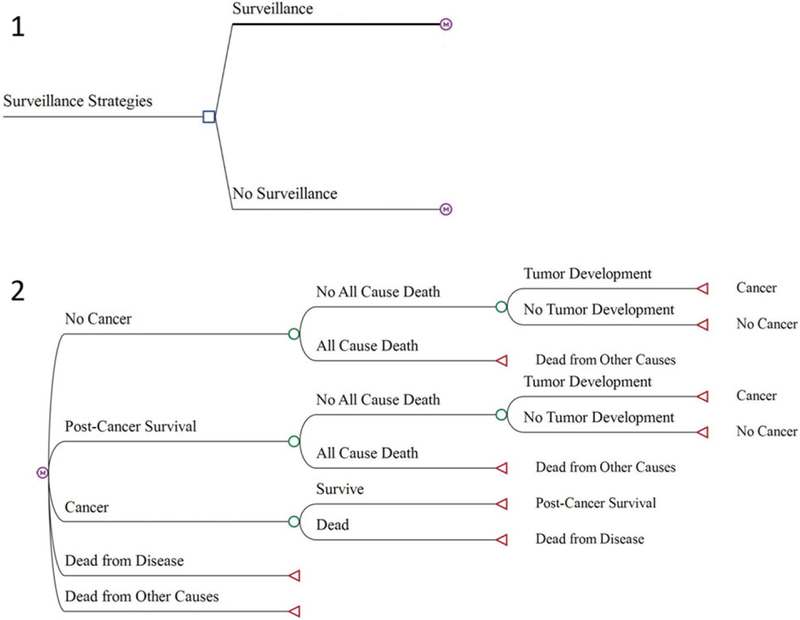
We compared two clinical management strategies (Figure 1): the Toronto surveillance protocol versus no surveillance. Patients entered the model at birth and were assumed to start in the “no cancer” health state. After one year, a patient can either stay in a “no cancer” health state, develop a tumor and transition to a “cancer” health state, or die from noncancer-related causes. Those who developed tumors could survive and proceed to a “post-cancer survivorship” health state, or die from cancer. For patients who enter a “post-cancer survivorship” health state, subsequent primary tumor development could occur or not. Post-cancer survivors who do not develop a subsequent primary tumor can either return to “no cancer” health state or die from noncancer-related causes. All health states transition occurred at one-year intervals. We constructed the model using TreeAge Pro 2017 (TreeAge Software, Williamstown, MA).
FIGURE 1.
Decision analytic model structure. Notes: 1. The blue square represents the decision node or the point at which a surveillance strategy is chosen. 2. The purple “M” circles represent Markov nodes after which a patient transitions between health states (e.g., no cancer, post-cancer survival, cancer, dead from disease, and dead from other causes) each cycle. The green circles represent chance nodes after which a probability is assigned to each event. The red triangles at the end of each branch represent terminal nodes, which indicate which state patients will transition to in the next cycle
2.3 |. Model parameters
Table 2 lists a summary of parameters used to build this model (i.e., transition probabilities and costs).
TABLE 2.
Parameter inputs and sensitivity analysis ranges
| Variable | Base-case estimate | Low | High | PSA distribution | Source |
|---|---|---|---|---|---|
| Age-specific risk of tumor development | |||||
| Age 0–15 | 0.15 | 0.08 | 0.27 | Beta | Chompret et al |
| Age 16–45 | 0.54 | 0.37 | 0.71 | Beta | Chompret et al |
| Age 46+ | 0.68 | 0.14 | 0.97 | Beta | Chompret et al |
| Probability of survival | |||||
| Surveillance | 0.84 | 0.65 | 1.00 | Beta | Villani et al |
| No surveillance | 0.49 | 0.36 | 0.64 | Beta | Villani et al |
| Probability of early-stage cancer | |||||
| Surveillance | 0.78 | 0.22 | 1.0 | Beta | Buys et al/Jarvinen et al/Oluwole et al |
| No surveillance | 0.53 | 0.22 | 0.89 | Beta | Buys et al/Jarvinen et al/Hofvind et al |
| Discounting | |||||
| Effectiveness | 0.03 | 0.01 | 0.05 | Normal | |
| Cost | 0.03 | 0.01 | 0.05 | Normal | |
| Cost of surveillance | |||||
| Age 0–17 | $2279 | $1140 | $3419 | Gamma | Villani et al/CMS |
| Age 18–30 | $3465 | $1733 | $5198 | Gamma | Villani et al/CMS |
| Age 31+ | $2316 | $1158 | $3474 | Gamma | Villani et al/CMS |
| Cost of cancer treatment | |||||
| Early-stage (surveillance group) | |||||
| Age 0–17 | $71 872 | $35 936 | $143 745 | Gamma | Mariotto et al |
| Age 18+ | $46 209 | $23 105 | $92 418 | Gamma | Mariotto et al |
| Late-stage (nonsurveillance group) | |||||
| Age 0–17 | $149 680 | $74 840 | $299 360 | Gamma | Mariotto et al |
| Age 18+ | $120 369 | $60 184 | $240 738 | Gamma | Mariotto et al |
| Cost of cancer survivorship | |||||
| Pediatric | $7493 | $3746 | $14 986 | Gamma | Mariotto et al |
| Adult | $4053 | $2016 | $8105 | Gamma | Mariotto et al |
2.3.1 |. Patients and surveillance
The surveillance strategy and effectiveness estimates were obtained from the results of the study by Villani et al describing the updated outcomes from the Toronto surveillance protocol.9 In the surveillance strategy, patients were assumed to be screened from birth to death. We assumed 100% compliance to the protocol by patients based on the assumption that reducing financial barriers through third-party payer reimbursement would allow for 100% compliance. Patients in the non-surveillance strategy were assumed to have regular care following population cancer screening guidelines.
2.3.2 |. Clinical outcomes
Probabilities of tumor development were obtained from the published literature by Chompret et al, who estimated age-specific rates of tumor development for patients with LFS.7 These rates were converted to annual age-specific probabilities. We assumed the definition of a lifetime to be 65 years for the LFS age-specific tumor development probabilities, but allowed the final probability of tumor development to extend until death, whichever occurred later.
Mortality probabilities for patients with tumor development were based on survival data for patients from the surveillance and non-surveillance arms in the Villani et al study.9 All-cause mortality estimates were derived from life tables that excluded breast neoplasms.14 LY were discounted at a 3% annual rate.15
2.3.3 |. Costs
We conducted our analysis from a US third-party payer’s perspective; thus, we included only direct medical costs in the model. Costs for the components of the surveillance strategies were obtained from the Centers for Medicare and Medicaid Services (CMS) Medicare Reimbursement Fee Schedule.16 Cost of surveillance was incurred each year while the patient was in the no-cancer health state and was adjusted to reflect the age-specific costs of screening.
The costs of two phases of cancer care, initial (referring to the first 12 months of cancer treatment after diagnosis) and cancer death (referring to up to the last 12 months of life after cancer diagnosis), obtained by Mariotto et al were used as proxies for early- and late-stage cancers, respectively.17 The cost of cancer treatment was weighted based upon the frequency of LFS-related cancers obtained from the International Agency for Research on Cancer (IARC) TP53 Database (http://p53.iarc.fr/; accessed April 18, 2016), which is an international database that collects data on TP53-related cancers. Cancer categories obtained from IARC for which there was no equivalent in the Mariotto et al study were placed into the “Other” category.
Furthermore, we weighted the cost of early- and late-stage cancers for each surveillance strategy based on the published literature from other surveillance strategies in different populations.18–21 We applied a 3% annual discount for costs for the duration of the model.15 We adjusted all cost estimates to 2015 USD using the healthcare component of the Personal Consumption Expenditures Index.
2.4 |. Sensitivity analyses
We conducted one-way sensitivity analyses (OWSA) at WTP thresholds of $50 000/LY and $100 000/LY to examine uncertainty in the estimates for probabilities and costs. As few confidence intervals were reported for any of the probabilities and costs used in the model, we calculated 95% confidence intervals with the continuity corrected Wilson’s score interval for survival and early- versus late-stage cancer probabilities.22 We also calculated a 95% confidence interval for costs approximately equal to four times the standard error.22–24
We also performed probabilistic sensitivity analysis (PSA). This allowed us to examine the combined uncertainty of the model parameters. For the PSA, we used beta distributions for probabilities and gamma distributions for costs.25
2.4.1 |. Scenario analysis
To explore the impact of surveillance on the overdiagnosis of tumors, we ran the model under a scenario in which the tumor detection for the surveillance group is increased, but all other parameters remained the same. We increased the probability of tumor development based on the results from Bleyer et al, who estimated that 31% of breast cancer detected by surveillance was overdiagnosed (i.e., “excess incidence”).26
2.5 |. Model validation
We adapted the model to a microsimulation of 1000 hypothetical patients, which enabled us to capture the individual history of patients, to determine the proportion of patients experiencing a tumor diagnosis with each strategy. We compared these results with data available in the IARC TP53 Database. We also examined the proportion of patients diagnosed with at least one tumor versus patients without tumor development by age 30 and over a lifetime.
Because this study involved secondary analyses of publicly available, deidentified data, no institutional review board approval was sought.
3 |. RESULTS
3.1 |. Base-case results
The model showed a mean cost of $46 496 and $117 103 and yielded 23 and 27 LY for the nonsurveillance and surveillance strategies, respectively (see Supporting Information Table S1). The ICER for surveillance versus nonsurveillance was $17 125 per additional LY gained.
3.2 |. Sensitivity analyses
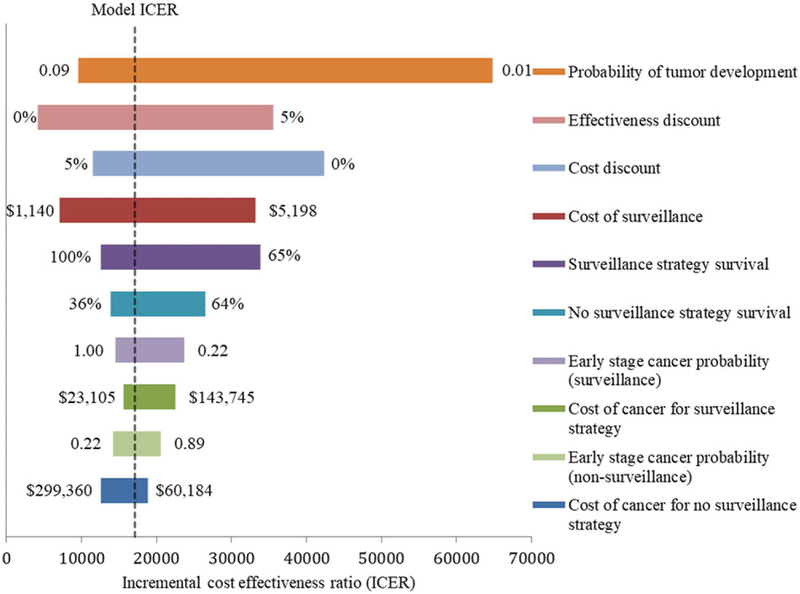
The OWSA showed the model was most sensitive to probability of tumor development, the effectiveness discount applied, the cost discount applied, the surveillance cost, and the probability of survival for the surveillance strategy (Figure 2). The surveillance strategy remained cost-effective in all cases at our WTP threshold; however, at a WTP threshold of $50 000/LY, surveillance is no longer cost-effective when the probability of tumor development decreases below 0.0066 per year.
FIGURE 2.
One-way sensitivity analysis tornado diagram depicting changes to the incremental cost-effectiveness ratio of surveillance vs no surveillance over plausible values in the model parameters. Notes: The tornado diagram shows the changes in the ICER across the plausible values for each of the 10 variables listed. Each horizontal bar represents a variable in the model. The values on each end of the bars represent the extreme of values, with other values modeled in between. The x-axis shows the ICER values of surveillance vs no surveillance. The vertical dotted line shows the base-case model ICER of $17 125
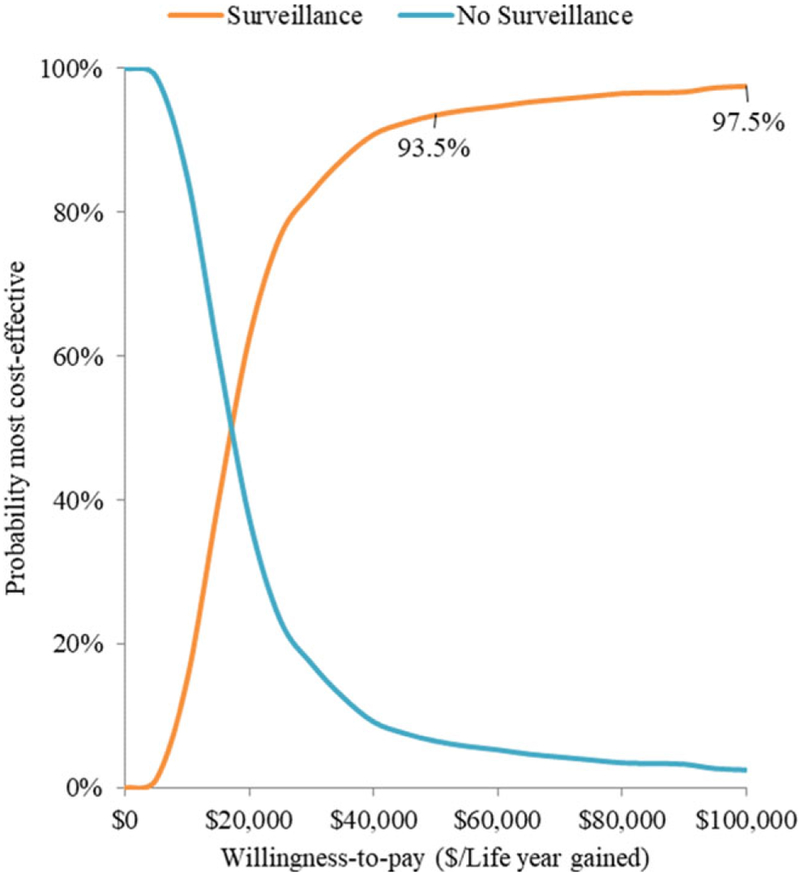
The PSA found a mean cost of $46 418 and $117 006 and yielded 23 and 27 LY for the nonsurveillance and surveillance strategies, respectively (see Supporting Information Figure S1). The ICER for surveillance versus nonsurveillance was $16 548 per additional LY gained. Above WTP thresholds of $50 000/LY and $100 000/LY, surveillance had a 94% and 98% probability of being cost-effective, respectively (Figure 3).
FIGURE 3.
Probabilistic sensitivity analysis cost-effectiveness acceptability curve (CEAC). Notes: This graph shows the probabilities of each strategy being the most cost-effective across a range of WTP thresholds. The curves are based on 1000 second-order Monte Carlo simulations. Surveillance is shown to have a higher probability of being cost-effective at WTP thresholds of $50,000/LY and $100,000/LY
3.3 |. Scenario analysis
The scenario analysis, which increased the probability of tumor detection for the surveillance strategy, found a mean cost of $46 496 and $131 277 and yielded 23 and 26 LY for the nonsurveillance and surveillance strategies, respectively. The corresponding ICER was $26 322 per additional LY gained. In an OWSA under this scenario, surveillance was no longer cost-effective (i.e., ICER > $100 000/LY or dominated) when the survival of patients in the surveillance strategy decreased below approximately 0.69. Under a WTP threshold of $50 000/LY, surveillance was no longer cost-effective under the following conditions: effectiveness discounting is greater than 4.8%, probability of survival in the surveillance groups is below 0.74, cost discounting is less than 0.8%, or the probability of survival in the nonsurveillance group is greater than 0.64. No other significant changes were found.
3.4 |. Model validation
The model predicted that by age 30, 41.2% and 36.7% of patients will have experienced at least one tumor, 5.7% and 9.3% will have experienced two tumors, and 0.6% and 1.3% of patients will have experienced three tumors for the nonsurveillance and surveillance groups, respectively. This combines for a total of 47.5% in both groups, closely resembling the cumulative risk of tumor development in patients with LFS of approximately 50% by age 30 indicated in the literature.4,27
The model also predicted a tumor frequency distribution for the nonsurveillance group that closely mirrors that of the IARC TP53 Database (see Supporting Information Table S2).
4 |. DISCUSSION
Patients with LFS have a high risk of multiple primary tumors. This analysis reiterates that the Toronto surveillance protocol provides a significant survival benefit with a gain of four LYs. We also found that this surveillance strategy is cost-effective at our WTP threshold. These results underscore the effectiveness of this surveillance protocol and may serve to inform third-party payer organizations trying to make reimbursement decisions on surveillance-screening strategies.
Our findings are corroborated by similar analyses conducted in high cancer risk populations. Joergensen et al reported that screening patients genetically predisposed to pancreatic cancer may add between five and seven LY and found screening to be cost-effective with an ICER between $31 722/LY and $42 128/LY.28 Similarly, Olsen et al found that surveillance for families at high and moderate risk of hereditary nonpolyposis colorectal cancer was cost-effective, adding one LY for under €2400 (approximately $3261 [2004 USD]).29
Third-party payer reimbursement can impact patient care either through the care being provided or via the willingness of the patient to pay for the care. It is well known that reduced reimbursements can be a disincentive for providers to perform procedures and may not be entirely concordant with good clinical practice.30 Reduced reimbursement may also adversely impact the utilization of preventive services by patients due to cost-sharing barriers.31 Conversely, higher reimbursement has been associated with increases in cancer screening and earlier detection of cancer.32,33
The Patient Protection and Affordable Care Act (ACA) mandated that payers cover certain cancer-prevention services. Although this and the expansion of Medicaid have significantly provided greater access to patients in need of cancer screening, many coverage gaps remain.34,35 Thus, some patients, such as those with LFS, continue to face financial obstacles to clinically important cancer screening.9,36 Moody, in his editorial to JAMA in 1903, emphasized the importance of disease prevention, declaring that no subject “can be more important to the welfare of humanity.”37 Clinical and reimbursement decision makers need to carefully consider the added costs and benefits to the implementation of clinical surveillance programs and the impact these decisions, including the corresponding cost-sharing requirements, have on patient care and outcomes.
Our analysis demonstrated that cost-effectiveness of cancer surveillance for patients with LFS is sensitive to the estimates of a few parameters, including the probability of tumor development and the probability of survival in the surveillance strategy. If cancer surveillance does indeed detect a greater frequency of tumors that are treated as compared with what would normally be treated (i.e., unrecognized overdiagnosis of nonmalignant lesions leading to possible overtreatment38), this could render the strategy less cost-effective. However, recent analyses among patients with LFS have found that overdiagnosis and unnecessary treatment of indolent lesions to be uncommon.36,39–41 Additional estimates of tumor development and patient survival using real-world data are needed to determine the likelihood of this occurring.
The model we developed has a number of strengths. We were able to compare the results of our model with data available from the IARC database and found that the distribution of tumor frequency was roughly equivalent for those not undergoing surveillance and the patients in the database. We also found that our model closely matched the published literature on the frequency of at least one tumor by age 30 in individuals with LFS. Finally, our model was robust to the variations of the parameters in the OWSA and the PSA at our WTP threshold, despite varying the parameters over their respective ranges.
Our model also has some limitations. The cancer costs used in this analysis were limited to those estimated for adults by Mariotto et al.17 These costs do not reflect the true treatment cost of early-stage versus late-stage tumors at presentation; rather, they represent the phase of care.17 This likely overestimates the true cost of early stage versus late stage and distorts pediatric versus adult cancer care costs. Overestimation of early-stage cancer treatment renders our results conservative as the surveillance strategy increases treatment of early-stage cancer. Economic analyses are needed to examine the true cost of cancer treatment—including survivorship—among patients with LFS.
The estimates for the cost of surveillance we used were based on CMS costs. This may largely underestimate the true cost of surveillance for LFS patients as CMS reimbursements are lower than private insurance.42 Furthermore, cost of surveillance was found to largely impact the model in the OWSA; thus, costs above what we estimated may impact the cost-effectiveness of this surveillance strategy. For example, an OWSA demonstrated that annual surveillance costs surpassing $7809 and $15 582 render cancer surveillance no longer cost-effective at the WTP thresholds of $50 000/LY and $100 000/LY, respectively. Although these costs are well above what we estimated, real-world cost analyses are needed to refine this parameter.
We based our survival probabilities on the results from one study, Villani et al.9 Although this study provides a longitudinal outlook, it is currently unknown how LFS survival may change over a lifetime. Survival estimates were shown in this analysis to have a large impact on the cost-effectiveness of surveillance. In a post hoc subgroup analysis using age-specific survival estimates from Villani et al, we compared the cost-effectiveness of the surveillance program in children and adults separately. The surveillance program appeared to be more cost-effective for adults than for children (see Supporting Information Figures S2 and S3). However, these estimates were based on few observations and are very susceptible to small changes.
In this cost-effectiveness analysis, we assumed 100% patient compliance with the protocol. This may not be realistic as many patients, particularly those in the United States, encounter reimbursement barriers. This is evident in the results of the Villani study, which indicated that Canadian patients, covered by a publicly funded healthcare system, were 100% compliant with the protocol, whereas patients in the United States were not as compliant, presumably at least in part due to lack of insurance coverage.9
The effectiveness measurement used in this study was LY. This provides an important but limited insight into the cost-effectiveness of surveillance of LFS patients. Cancer screening, along with cancer treatment and post-cancer survivorship, affects the quality of life of a patient and often incurs indirect costs through loss of wages and caretaker involvement. Alternatively, cancer screening may lessen indirect costs of intensive cancer treatment through early-stage tumor management. Moreover, one of the primary benefits of prevention programs is the provision of a healthier life, which may translate into greater productivity.31 Therefore, examining the cost-effectiveness of surveillance may be more appropriate from a societal perspective with an effectiveness measure of quality-adjusted life years (QALYs). Unfortunately, little has been done to explore health utilities specific to LFS patients. LFS patients are a unique population; thus, utility extrapolation from other populations may not be appropriate.
Cancer surveillance as outlined in the Toronto surveillance protocol of individuals diagnosed with LFS is cost-effective at our WTP threshold. The results herein support the institution of cancer screening reimbursement for patients with LFS. Additional analyses using real-world data from large national or international LFS-based databases are needed to provide further evidence to support surveillance reimbursement.
Supplementary Material
Funding information
This work was supported in part by the Program in Personalized Health in the Center for Clinical and Translational Science (CCTS) at the University of Utah funded by the National Center for Advancing Translational Sciences of the NIH under award UL1TR002538, and also the Genetic Counseling Shared Resource at Huntsman Cancer Institute funded by the National Cancer Institute under award P30CA042014. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Further support was also provided by Soccer For Hope and the Li-Fraumeni Syndrome Association.
Abbreviations:
- ACA
Patient Protection and Affordable Care Act
- CI
confidence interval
- CMS
Centers for Medicare and Medicaid Services
- IARC
International Agency for Research on Cancer
- ICER
incremental cost-effectiveness ratio
- LFS
Li–Fraumeni syndrome
- LY
life-year
- OWSA
one-way sensitivity analysis
- PSA
probabilistic sensitivity analysis
- QALY
quality-adjusted life year
- US
United States
- USD
United States Dollar
- WTP
willingness to pay
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Previous Presentations
This work has been presented at the 2017 International Society of Pharmacoeconomics and Outcomes Research Annual Conference in Boston, Massachusetts.
REFERENCES
- 1.Li FP. Soft-tissue sarcomas, breast cancer, and other neoplasms. A familial syndrome. Ann Intern Med. 1969;71:747–752. [DOI] [PubMed] [Google Scholar]
- 2.Malkin D, Li FP, Strong LC, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250:1233–1238. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava S, Zou ZQ, Pirollo K, Blattner W, Chang EH. Germ-line transmission of a mutated p53 gene in a cancer-prone family with Li-Fraumeni syndrome. Nature. 1990;348:747–749. [DOI] [PubMed] [Google Scholar]
- 4.Schneider K, Zelley K, Nichols KE, Garber J. Li-Fraumeni syndrome In: Pagon RA, Adam MP, Ardinger HH, eds. GeneReviews. Seattle, WA: University of Washington; 1999;1993–2019. [Google Scholar]
- 5.Gonzalez KD, Noltner KA, Buzin CH, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol. 2009;27:1250–1256. [DOI] [PubMed] [Google Scholar]
- 6.Ruijs MW, Verhoef S, Rookus MA, et al. TP53 germline mutation testing in 180 families suspected of Li-Fraumeni syndrome: mutation detection rate and relative frequency of cancers in different familial phenotypes. J Med Genet. 2010;47:421–428. [DOI] [PubMed] [Google Scholar]
- 7.Chompret A, Brugieres L, Ronsin M, et al. P53 germline mutations in childhood cancers and cancer risk for carrier individuals. Br J Cancer. 2000;82:1932–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hisada M, Garber JE, Fung CY, Fraumeni JF Jr, Li FP. Multiple primary cancers in families with Li-Fraumeni syndrome. J Natl Cancer Inst. 1998;90:606–611. [DOI] [PubMed] [Google Scholar]
- 9.Villani A, Shore A, Wasserman JD, et al. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: 11 year follow-up of a prospective observational study. Lancet Oncol. 2016;17:1295–1305. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. NCCN Guidelines. 2017; https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#detection. Accessed July 6, 2017.
- 11.Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics. 1998;13:397–409. [DOI] [PubMed] [Google Scholar]
- 12.Hunt TL, Luce BR, Page MJ, Pokrzywinski R. Willingness to pay for cancer prevention. Pharmacoeconomics. 2009;27:299–312. [DOI] [PubMed] [Google Scholar]
- 13.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–797. [DOI] [PubMed] [Google Scholar]
- 14.Arias E, Heron M, Tejada-Vera B. United States life tables eliminating certain causes of death, 1999–2001. Natl Vital Stat Rep. 2013;61:1–128. [PubMed] [Google Scholar]
- 15.Macones GA, Goldie SJ, Peipert JF. Cost-effectiveness analysis: an introductory guide for clinicians. Obstet Gynecol Surv. 1999;54:663–672. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Medicare and Medicaid Services. Medicare physician fee schedule, CPT code book and Medicare outpatient prospective payment system. 2016; https://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed February 19, 2016.
- 17.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oluwole SF, Ali AO, Adu A, et al. Impact of a cancer screening program on breast cancer stage at diagnosis in a medically underserved urban community. J Am Coll Surg. 2003;196:180–188. [DOI] [PubMed] [Google Scholar]
- 19.Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality: The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. Jama. 2011;305:2295–2303. [DOI] [PubMed] [Google Scholar]
- 20.Järvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829–834. [DOI] [PubMed] [Google Scholar]
- 21.Hofvind S, Lee CI, Elmore JG. Stage-specific breast cancer incidence rates among participants and non-participants of a population-based mammographic screening program. Breast Cancer Res Treat. 2012;135:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallis S. Binomial confidence intervals and contingency tests: mathematical fundamentals and the evaluation of alternative methods. J Quant Linguist. 2013;20:178–208. [Google Scholar]
- 23.Del Rio RA, Post AB, Singer ME. Cost-effectiveness of hematologic growth factors for anemia occurring during hepatitis C combination therapy. Hepatology. 2006;44:1598–1606. [DOI] [PubMed] [Google Scholar]
- 24.Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000;17:479–500. [DOI] [PubMed] [Google Scholar]
- 25.Briggs A. Probabilistic analysis of cost-effectiveness models: statistical representation of parameter uncertainty. Value Health. 2005;8:1–2. [DOI] [PubMed] [Google Scholar]
- 26.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998–2005. [DOI] [PubMed] [Google Scholar]
- 27.Lustbader ED, Williams WR, Bondy ML, Strom S, Strong LC. Segregation analysis of cancer in families of childhood soft-tissue-sarcoma patients. Am J Hum Genet. 1992;51:344–356. [PMC free article] [PubMed] [Google Scholar]
- 28.Joergensen MT, Gerdes AM, Sorensen J, Schaffalitzky de Muckadell O, Mortensen MB. Is screening for pancreatic cancer in high-risk groups cost-effective? – Experience from a Danish national screening program. Pancreatology. 2016;16:584–592. [DOI] [PubMed] [Google Scholar]
- 29.Olsen KR, Bojesen SE, Gerdes AM, Lindorff-Larsen K, Bernstein IT. Cost-effectiveness of surveillance programs for families at high and moderate risk of hereditary non-polyposis colorectal cancer. Int J Technol Assess Health Care. 2007;23:89–95. [DOI] [PubMed] [Google Scholar]
- 30.Gomella LG. Punishing physicians for PSA screening. Can J Urol. 2015;22:8042. [PubMed] [Google Scholar]
- 31.Dixon RB, Hertelendy AJ. Interrelation of preventive care benefits and shared costs under the Affordable Care Act (ACA). Int J Health Policy Manag. 2014;3:145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gross CP, Andersen MS, Krumholz HM, McAvay GJ, Proctor D, Tinetti ME. Relation between Medicare screening reimbursement and stage at diagnosis for older patients with colon cancer. Jama. 2006;296:2815–2822. [DOI] [PubMed] [Google Scholar]
- 33.Halpern MT, Romaire MA, Haber SG, Tangka FK, Sabatino SA, Howard DH. Impact of state-specific Medicaid reimbursement and eligibility policies on receipt of cancer screening. Cancer. 2014;120:3016–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soni A, Simon K, Cawley J, Sabik L. Effect of Medicaid expansions of 2014 on overall and early-stage cancer diagnoses. Am J Public Health. 2018;108:216–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabik LM, Adunlin G. The ACA and cancer screening and diagnosis. Cancer J. 2017;23:151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Neill AF, Voss SD, Jagannathan JP, et al. Screening with whole-body magnetic resonance imaging in pediatric subjects with Li-Fraumeni syndrome: a single institution pilot study. Pediatr blood cancer. 2018;65. [DOI] [PubMed] [Google Scholar]
- 37.Moody HA. The future of preventive medicine. J Am Med Assoc. 1903:1199–1201. [Google Scholar]
- 38.Esserman LJ, Thompson IM, Reid B, et al. Addressing overdiagnosis and overtreatment in cancer: a prescription for change. Lancet Oncol. 2014;15:e234–e242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paixao D, Guimaraes MD, de Andrade KC, Nobrega AF, Chojniak R, Achatz MI. Whole-body magnetic resonance imaging of Li-Fraumeni syndrome patients: observations from a two rounds screening of Brazilian patients. Cancer Imaging. 2018;18:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mai PL, Khincha PP, Loud JT, et al. Prevalence of cancer at baseline screening in the National Cancer Institute Li-Fraumeni Syndrome Cohort. JAMA Oncol. 2017;3:1640–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anupindi SA, Bedoya MA, Lindell RB, et al. Diagnostic performance of whole-body MRI as a tool for cancer screening in children with genetic cancer-predisposing conditions. Am J Roentgenol. 2015;205:400–408. [DOI] [PubMed] [Google Scholar]
- 42.Hsia RY, MacIsaac D, Palm E, Baker LC. Trends in charges and payments for nonhospitalized emergency department pediatric visits, 1996–2003. Acad Emerg Med. 2008;15:347–354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.