Summary
Nppa is a cardiac hormone which plays critical roles in regulating salt–water balance. Its expression is restricted to the atria of the healthy post-natal heart. During heart development, spatio-temporal expression of Nppa is dynamically changed within the heart and becomes restricted to the atria upon birth. In contrast to its atrial specific expression after birth, Nppa is re-expressed in the adult ventricles in response to cardiac hypertrophy. To study cardiac chamber specification during development and pathological cardiac remodeling during heart disease, we generated a novel Nppa reporter mouse line by knocking-in a tagBFP reporter cassette into 3′-UTR of the Nppa gene without disrupting the endogenous gene. Our results demonstrated dynamic tagBFP expression in the developing heart, recapitulating the spatiotemporal expression pattern of endogenous Nppa. We also found that Nppa-tagBFP is induced in the ventricle during pathological remodeling. Taken together, Nppa-tagBFP reporter knock-in mouse model described in this article will serve as a valuable tool to study cardiac chamber specification during development as well as pathological cardiac remodeling.
Keywords: atrium, cardiomyocyte, heart, Nppa, ventricle
1 |. INTRODUCTION
Chamber formation and specification during heart development is a complex process requiring several morphological transitions (Buckingham, Meilhac, & Zaffran, 2005; Evans, Yelon, Conlon, & Kirby, 2010; Jensen, Wang, Christoffels, & Moorman, 2013; Olson, 2006). This complex process is initiated by the extension of the cardiac progenitor population, adopting a crescent shape (cardiac crescent). By moving toward ventrally, the cardiac progenitors start to form a linear heart tube. The linear heart tube undergoes elongation and rightward looping to form a spiral shape of the heart. As a result of looping, the inflow tract, future atrium, and outflow tract are located above the developing ventricles. Following subsequent further spiraling of the heart tube and septation, the four-chambered heart is formed. Each cardiac chamber has its distinct morphological and functional properties and gene expression patterns.
Nppa (natriuretic peptide A) is a peptide hormone primarily expressed in the heart. It plays roles in renal sodium and water excretion and vasodilation. Nppa is one of the cardiac genes whose expression during heart development has been most extensively studied, at least in part, due to its unique spatiotemporal expression pattern. Nppa mRNA expression first appears in the developing mouse heart at E8.0–8.5 (Christoffels et al., 2000). Nppa mRNA expression is specifically observed at the ventral portion of linear heart tube which develops into the ventricles. Within the looped embryonic heart at E9.0–9.5, Nppa mRNA expresses in the left and right ventricles, but more strongly in the left ventricle than right ventricle. At the same time, Nppa mRNA starts to weakly express in the atrial appendages. However, Nppa mRNA expression is not detected in atrioventricular canal, inflow tract, and outflow tract. At E9.5–E10.0, left dominant ventricular expression and atrial expression of Nppa mRNA persist in the four-chambered embryonic heart. At E11.0, Nppa mRNA expression in the right ventricle decreases to undetectable level, whereas it is still expressed in the left ventricle. Both the left and right atria uniformly express Nppa mRNA at this stage. Around the time of birth, left ventricular expression of Nppa mRNA is downregulated to undetectable level, and Nppa mRNA expression becomes restricted to the atria in mice (Bloch, Seidman, Naftilan, Fallon, & Seidman, 1986; Seidman, Schmidt, & Seidman, 1991). However, Nppa which becomes silent in the ventricle after birth is re-activated in the ventricle in response to pathological stress as a part of fetal gene activation program (Chien, Knowlton, Zhu, & Chien, 1991). Because of its dynamic and unique spatiotemporal expression pattern during heart development and disease, the Nppa gene has been considered as an important marker for studying chamber formation and specification of the heart, as well as molecular mechanisms of cardiac hypertrophy and heart failure.
To study atrial development and chamber specification, several transgenic mouse lines harboring rat Nppa promoter fragments (i.e., 638 bp, 2.4 kb, and 3 kb) coupled with a reporter gene have been generated (Habets et al., 2002; Knowlton et al., 1995; Seidman et al., 1991). The Nppa reporter activity was observed in the forming ventricles and atria during pre-natal heart development and then was restricted to the atria around the time of birth. However, Nppa promoter driven reporter gene expression does not precisely recapitulate the dynamic spatiotemporal expression pattern of endogenous Nppa. First, the previous transgenic Nppa reporter activity was observed in the inflow tract, where endogenous Nppa expression is absent (Habets et al., 2002). Second, compared to endogenous Nppa expression, the expression of LacZ in developing left ventricle was less extensive and homogeneous (Habets et al., 2002). Third, the reporter gene continued to express in the ventricle after birth (at P1) (Knowlton et al., 1995). Lastly, the reporter activity was not induced during pressure-overload ventricular stress, which re-activates endogenous Nppa expression (Knowlton et al., 1995). To more precisely mimic endogenous Nppa expression pattern, a larger Nppa cis-regulatory sequences (from −3.2 kb to +3.7 kb relative to the transcription start site) of mouse was used to drive Cre recombinase expression (de Lange, Moorman, & Christoffels, 2003). Although the reporter expression was atrial specific, there were some discrepancies between endogenous Nppa and the reporter expression pattern. The reporter expression was inhomogeneous, likely due to incomplete Cre recombination. In addition, the reporter expression was still detected in the inflow tract. Taken together, Nppa transgenic reporter mouse lines that have been generated do not precisely recapitulate endogenous Nppa expression during heart development and disease. It suggests that Nppa regulatory sequences used for generating the transgenic mice do not contain all the sequences which are necessary to precisely control dynamic and complex Nppa expression during heart development. Moreover, transgenic reporter mouse lines provide variable reporter gene copy numbers due to nonspecific integration of a reporter gene into the target genome, thereby often presenting non-biological expression levels of a reporter gene.
In this study, we present a new Nppa reporter mouse line by knocking-in a tagBFP reporter cassette into 3′-UTR of the Nppa gene by homologous recombination. This new Nppa reporter mouse line generated by a specific knock-in approach eliminates the possibility of uncontrolled reporter expression often found in transgenic reporter mice. As compared to previously generated multiple transgenic mouse lines based on cis-regulatory sequences of the Nppa gene, our new Nppa-tagBFP reporter knock-in mouse line faithfully recapitulates dynamic and complex spatiotemporal expression pattern of Nppa during heart development and diseases. The heterozygous or homozygous mice carrying the knock-in allele are healthy and fertile. Therefore, Nppa-tagBFP reporter knock-in mouse line that we described here will be a valuable new tool for studying chamber formation and specification during heart development as well as fetal gene reactivation during heart diseases.
2 |. RESULTS AND DISCUSSION
2.1 |. Generation of Nppa-tagBFP reporter knock-in mice
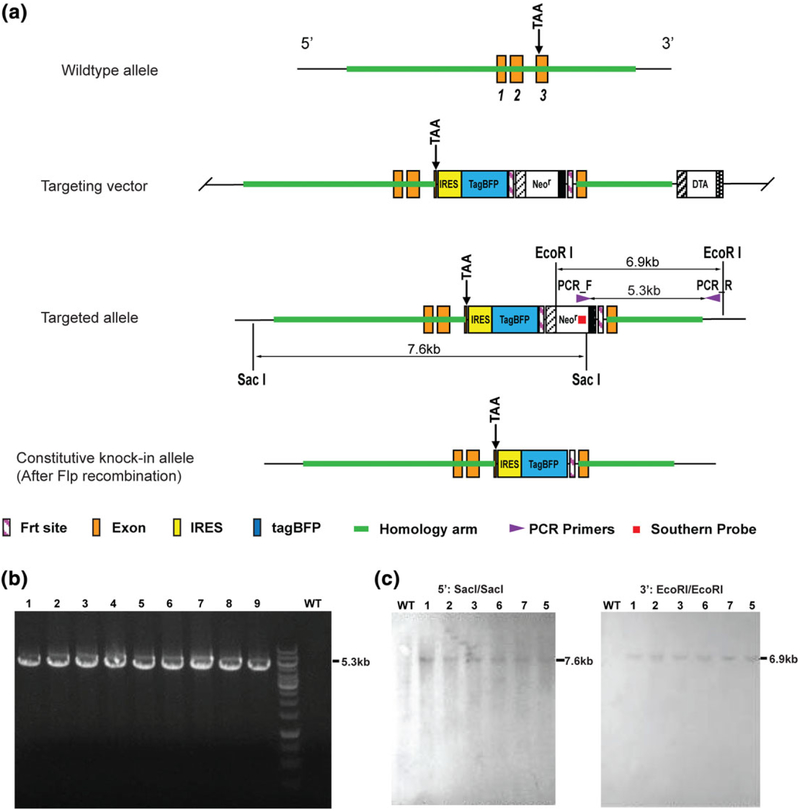
To generate Nppa-tagBFP reporter knock-in mouse line, we inserted IRES-tagBFP-Neomycin resistance (NeoR) gene cassette into the 3′-UTR immediately following the second exon of the Nppa gene. We designed a targeting construct to contain an IRES-tagBFP-FRT-NeoR-FRT knock-in cassette flanked by homology arms for homologous recombination as illustrated in Figure 1a. NeoR was flanked by two FRT sites for FLP (flippase)-FRT (flippase recognition target) recombination. The linearized targeting construct was electroporated into embryonic stem cells (ESCs) isolated from C57BL/6 mice. The targeted ESC clones were treated with Neomycin and expanded. Using Neomycin resistant ESC clones, polymerase chain reaction (PCR) was performed to verify homologous recombination at 3′ by amplifying 5.3 kb PCR product (Figure 1b). We further tested homologous recombination of the targeted ESC clones using Southern blotting (Figure 1c). The correctly targeted ESC clones confirmed by Southern blotting were injected into blastocysts of C57BL/6 mice to generate mouse chimeras. By cross breeding the chimeras with FLP transgenic mice, NeoR cassette was removed to create the Nppa-IRES-tagBFP knock-in allele (Figure 1a). No abnormal viability or fertility was observed in the generated heterozygous or homozygous Nppa-tagBFP reporter knock-in mice.
FIGURE 1.
Generation of Nppa-tagBFP reporter knock-in mouse line. (a) Schematic illustration of the gene targeting strategy. IRES-tagBFP-FRT-NeoR-FRT cassette was inserted into 3′-UTR of the Nppa gene locus. The NeoR gene cassette is flanked by two FRT sites. By homologous recombination, the mice carrying IRES-tagBFP-FRT-NeoR-FRT cassette (targeted allele) were generated. By cross breeding the chimera mice with FLP transgenic mice, Nppa-tagBFP knock-in mice carrying constitutive knock-in allele were generated by deleting NeoR cassette. (b) PCR screening for the targeted ESCs using the indicated PCR primers in (a). Nine expanded clones demonstrated expected size of PCR band (5.3 kb). A wild type (WT) clone did not show the band. (c) Southern blot analyses to confirm the correctly targeted ESC clones. Using six (1, 2, 3, 5, 6, and 7) of nine PCR positive ESC clones, restrictive digestions with the indicated enzymes and hybridizations with the indicated southern probe in (a) were performed. Targeted ESCs for Nppa-tagBFP knock-in displayed 7.6 kb (with SacI) and 6.9 kb (with EcoRI) bands, whereas WT ESCs did not show any band. The southern blot results confirmed that all six expanded clones (1, 2, 3, 5, 6, and 7) were correctly targeted
2.2 |. Characterization of Nppa-tagBFP knock-in reporter expression during heart development
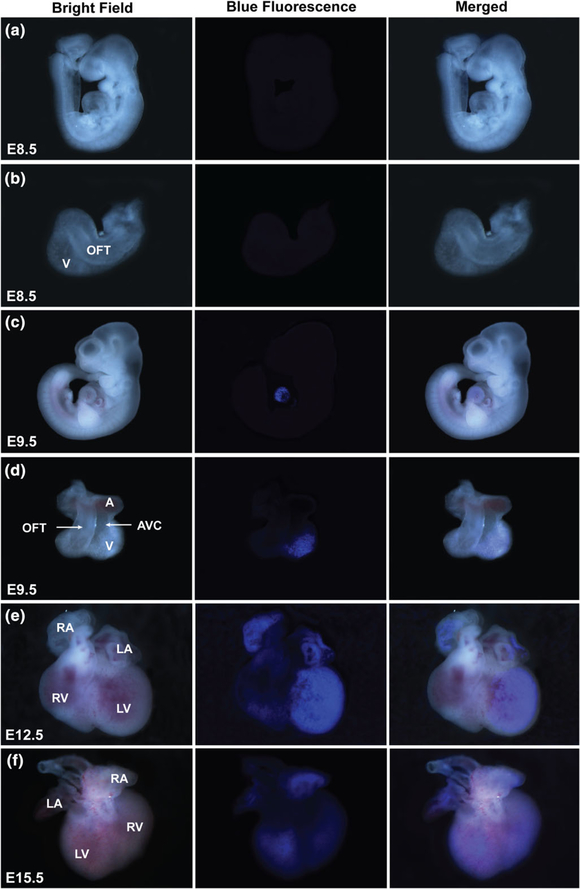
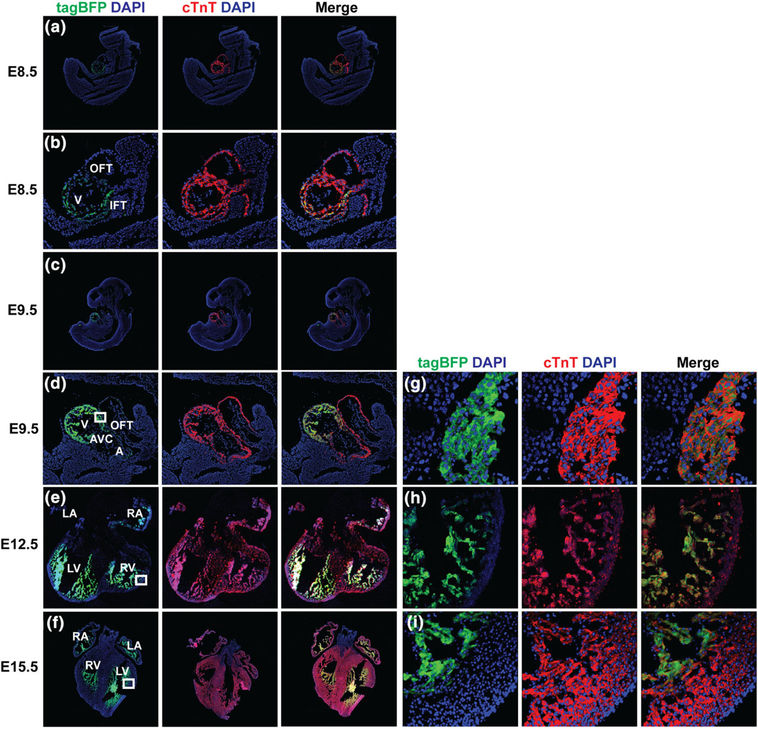
We examined the Nppa-tagBFP knock-in reporter expression during heart development by fluorescence imaging of whole mount of embryonic hearts and immunostaining of frozen embryonic heart sections (Figures 2 and 3). We found that Nppa-tagBFP fluorescence was first noticeable on whole mount heart at E9.5 without immunostaining (Figure 2a–d). tagBFP knock-in reporter expression was restricted to the ventricle of E9.5 embryo (Figure 2c,d). It was not observed in the out flow tract (OFT), atrioventricular canal (AVC), or atria. At E12.5, tagBFP fluorescence was observed in both atria and ventricles (Figure 2e). Its expression in the left ventricle was stronger than the right ventricle. At E15.5, similar bi-atrial and left dominant ventricular expression of Nppa-tagBFP persisted (Figure 2f). However, ventricular expression of Nppa-tagBFP knock-in reporter significantly decreased at E15.5, compared to E12.5. Although Nppa-tagBFP reporter expression was not obvious in the whole mount of E8.5 embryo and heart (Figure 2a,b), we were able to detect Nppa-tagBFP expression specifically in the ventricle at E8.5 using immunostaining (Figure 3a,b). This result suggests that Nppa-tagBFP reporter protein expression is relatively weak at E8.5. Using immunostaining, we confirmed that Nppa-tagBFP reporter expression was confined to the ventricle of developing hearts at E9.5 (Figure 3c,d) and found in all four chambers at E12.5 and E15.5 (Figure 3e,f). More extensive left ventricular expression than right ventricle was observed at E12.5 and E15.5. Nearly all ventricular cardiomyocytes expressing cardiac troponin T (cTnT) at the entire ventricular wall of E9.5 expressed Nppa-tagBFP reporter (Figure 3g). In contrast, at E12.5 and E15.5, only the ventricular cardiomyocytes at the trabecular layer expressed Nppa-tagBFP reporter (Figure 3h,i). Taken together, Nppa-tagBFP knock-in reporter protein expression during heart development precisely mimics endogenous Nppa mRNA expression as previously described (Christoffels et al., 2000), in terms of its sequential appearance in specific anatomical locations within the heart. However, the timing of Nppa-tagBFP knock-in reporter protein expression may be slightly delayed compared to endogenous Nppa mRNA expression previously reported. It was shown that Nppa mRNA first appears in the developing ventricle at E8.0–E8.5 and starts to weakly express in the forming atrium at E9.0–E9.5 (Christoffels et al., 2000).
FIGURE 2.
Nppa-tagBFP knock-in reporter expression during heart development. Whole mount embryos (a and c) and embryonic hearts (b, d–f) demonstrate Nppa-tagBFP reporter expression within the heart at the indicated developmental stages. A: atrium; V: ventricle; OFT: out flow tract; AVC: atrioventricular canal; LV: left ventricle; RV: right ventricle; LA: left atrium: RA; right atrium. The same expression patterns were confirmed with at least three litters of mouse embryos at each time point
FIGURE 3.
Characterization of Nppa-tagBFP knock-in reporter expression during heart development using immunostaining. Frozen sections of the whole embryos and/or hearts at E9.5, E12.5, and E15.5 were processed. The frozen sections were immunostained for a cardiomyocyte marker, cardiac troponin-T (cTnT), and tagBFP. Immunostained frozen sections of whole embryo at E9.5 (a) showed tagBFP expression in the ventricle. Magnified view of heart area at E9.5 demonstrated ventricular specific tagBFP expression (b). Embryonic heart sections at E12.5 (e) and E15.5 (f) showed tagBFP expression in all four chambers. The ventricular walls indicated by the white inlets at E9.5, E12.5, and E15.5 were magnified (g–i). A: atrium; V: ventricle; IFT: inflow tract; OFT: out flow tract; AVC: atrioventricular canal; LV: left ventricle; RV: right ventricle; LA: left atrium: RA; right atrium
2.3 |. Characterization of Nppa-tagBFP knock-in reporter expression in post-natal hearts
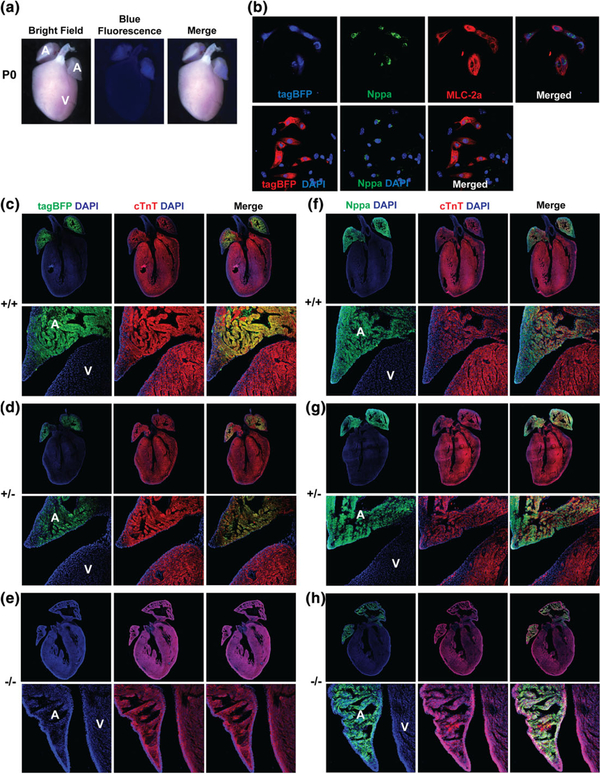
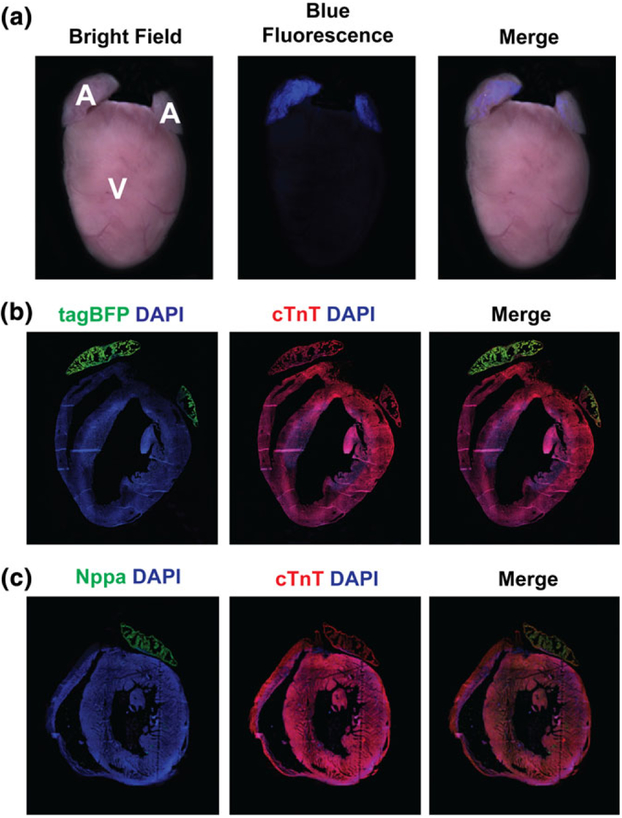
Upon birth, Nppa-tagBFP knock-in reporter exclusively expressed in the atria of the heart without its ventricular expression (Figure 4a). To precisely demonstrate a cell type which expresses Nppa-tagBFP knock-in reporter in the atria, we isolated neonatal atrial cardiomyocytes at P0 and performed immunostaining for endogenous Nppa and MLC-2a (atrial marker). We found that atrial cardiomyocytes expressing Nppa and MLC-2a co-express Nppa-tagBFP knock-in reporter (Figure 4b). Next, we examined if knocking-in tagBFP into the endogenous locus of Nppa alters the endogenous expression of Nppa by comparing endogenous Nppa expression in a wild type littermate with the heterozygous or homozygous Nppa-tagBFP knock-in reporter mouse heart. Each distinct genotype of mouse heart (i.e., homozygote, heterozygote, and wild type) demonstrated a different level of Nppa-tagBFP reporter expression (stronger expression in the homozygous heart, weaker expression in the heterozygous heart, and no expression in the wild type heart) (Figure 4c–e). However, we did not observe any significant difference in endogenous Nppa protein expression in the heterozygous or homozygous Nppa-tagBFP knock-in reporter mouse heart, as opposed to a wild type mouse heart (Figure 4f–h). As expected, atrial specific Nppa-tagBFP expression persisted in the adult heart (Figure 5). Although Nppa-tagBFP reporter strongly expressed in both atria, there was no tagBFP expression in either of the ventricles.
FIGURE 4.
Nppa-tagBFP knock-in reporter expression in the neonatal heart. (a) Whole mount fluorescent image of Nppa-tagBFP knock-in reporter mice at P0. (b) Co-expression of Nppa-tagBFP reporter with endogenous Nppa and MLC-2a in neonatal atrial cardiomyocytes. Neonatal atrial cardiomyocytes were isolated from Nppa-tagBFP reporter knock-in mice. Isolated neonatal atrial cardiomyocytes were immunostained for MLC-2a and Nppa. tagBFP fluorescence was visualized without immunostaining. The frozen heart sections of homozygous and heterozygous Nppa-tagBFP knock-in mice, and wild type littermates at P0 were immunostained with tagBFP (c–e) or Nppa (f–h), and cTnT (c–h). The top panels show whole hearts demonstrating four chambers. The bottom panels are magnified views showing both the atrium and ventricle. A: atrium; V: ventricle
FIGURE 5.
Nppa-tagBFP knock-in reporter expression in the adult heart. (a) Whole mount fluorescent image of Nppa-tagBFP knock-in reporter mice at 6 weeks. (b, c) Immunofluorescent staining of frozen heart sections of 6 weeks old Nppa-tagBFP knock-in reporter mice with tagBFP or Nppa and cTnT. RA: right atrium; LA: left atrium; V: ventricle; RV: right ventricle; LV: left ventricle
2.4 |. Expression of Nppa-tagBFP knock-in reporter during heart diseases
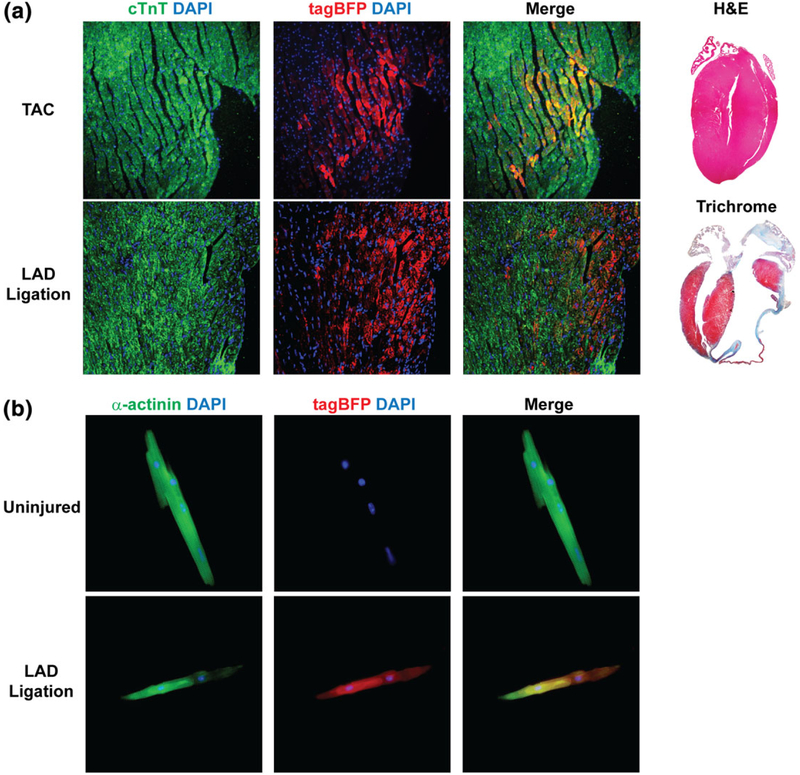
We examined whether Nppa-tagBFP knock-in reporter is re-expressed in the ventricle during pathological adverse heart remodeling processes. Pressure-overload cardiac hypertrophy or myocardial infarction was induced by thoracic aortic constriction (TAC) or left anterior descending coronary artery (LAD) ligation as described previously (Gao et al., 2010; Zhou et al., 2010). We found that a significant number of ventricular cardiomyocytes expressed Nppa-tagBFP reporter after TAC or LAD ligation (Figure 6a), whereas no Nppa-tagBFP expression was observed in the adult heart without cardiac injury (Figure 5). In addition, we isolated adult ventricular cardiomyocytes from uninjured and injured Nppa-tagBFP reporter mice (Figure 6b). Following immunostaining of ventricular cardiomyocytes, we showed specific expression of tagBFP reporter in the individual cardiomyocytes from the injured heart. However, we were not able to find tagBFP expression in the ventricular cardiomyocytes isolated from the uninjured Nppa-tagBFP reporter mice. These results indicate that Nppa-tagBFP knock-in reporter recapitulates induction of endogenous Nppa expression in response to reactivation of fetal gene program during heart diseases.
FIGURE 6.
Nppa-tagBFP knock-in reporter expression during heart diseases. (a) Immunofluorescent staining of frozen heart sections of Nppa-tagBFP knock-in reporter mice following injuries. Two months after TAC (top) or LAD ligation (bottom), heart sections of Nppa-tagBFP knock-in reporter mice were obtained from a mid-portion of septum (TAC) and a remote area of infarction (LAD ligation). Heart sections were processed and immunostained for tagBFP and cTnT. Nppa-tagBFP knock-in reporter expression in ventricular cardiomyocytes was shown. H&E (for TAC) and Trichrome (for LAD ligation) stainings were performed to demonstrate the extent of insult (right). (b) Immunofluorescent staining of isolated adult ventricular cardiomyocytes from Nppa-tagBFP knock-in reporter mice with or without injury. Three weeks after LAD ligation, ventricular cardiomyocytes were isolated from the mice using Langendorff perfusion and immunostained for tagBFP and α-actinin. Age-matched uninjured mice were used as a control
In summary, we generated a new Nppa-tagBFP reporter knock-in mouse line and characterized spatiotemporal expression of Nppa-tagBFP knock-in reporter during heart development and disease. Although Nppa-tagBFP knock-in reporter expression was localized at both atria and ventricles during embryonic heart development, it became restricted to the atria after birth. However, its expression was re-induced in the ventricles in response to pathological cardiac stress. Our results indicate that Nppa-tagBFP knock-in reporter faithfully recapitulates the endogenous Nppa expression pattern during pre- and post-natal heart development. Because our targeting strategy did not disrupt the endogenous Nppa gene, the heterozygous or homozygous mice carrying the knock-in allele did not show any developmental defect. Therefore, this new mouse line will be an important genetic tool for studying heart development and heart disease processes. For example, Nppa-tagBFP reporter will provide an important marker not only for serial steps of chamber specification during heart development but also for cardiac subtype specification during directed differentiation of pluripotent stem cells to cardiomyocytes. During heart disease processes, the reporter can function as an indicator to visually demonstrate the extent of pathological remodeling of the heart.
3 |. METHODS
3.1 |. Animals
All animal procedures were performed with the approval of Vanderbilt University Medical Center. Nppa-tagBFP reporter knock-in mice were generated with the aid of Cyagen Biosciences Inc. (Santa Clara, CA). In brief, the targeting vector was generated by inserting IRES-tagBFP-FRT-NeoR-FRT flanked by 3 kb 5′homology arm and 4.5 kb 3′ homology arm. The linearized targeting vector was electroporated into ESCs isolated from C57BL/6 mice. Neomycin (G418) resistant clones were expanded and screened by PCR for homologous recombination. Homologous recombination demonstrated by PCR in the selected ESC clones was confirmed using Southern blotting. The primers used for PCR and southern blotting include: (1) PCR_F: GCTGACCGCTTCCTCGTGCTTTA, (2) PCR_R: GGGGTTTTACTAACTTCCTCTGTAAGAATC, (3) Southern_F: TCATCTCACCTTGCTCCTGC, and (4) Southern_R: AAGGCGATAGAAGGCGATGC. The correctly targeted ESC clones were expanded and then injected into C57BL/6 blastocysts to generate mouse chimeras. The chimera mice were cross-bred with FLP transgenic mice, obtained from Jackson Laboratory (Stock number 009086), to remove NeoR cassette. Nppa-tagBFP reporter knock-in mice will be available to the academic research community upon acceptance of the manuscript.
3.2 |. Mouse surgeries
Permanent occlusion of the left anterior descending coronary artery was performed using 8 weeks old Nppa-tagBFP reporter knock-in mice as previously described (Gao et al., 2010). Briefly, a small left thoracotomy was performed, and the heart was temporarily displaced. A suture (6.0 silk) was placed 2 mm below the origin of the LAD. The heart was replaced immediately into the thoracic cavity. After evacuating air, the chest was closed. Transverse aortic constriction (TAC) was performed as previously described (Zhou et al., 2010). Following endotracheal intubation and thoracotomy through the second intercostal space, the transverse arch of the aorta was isolated between the right and left carotid arteries. The aortic arch was ligated along with 27-gauge blunted needle, and then, the needle was removed to generate a constriction of approximately 0.4 mm in diameter. Eight weeks later, mice were euthanized for frozen heart sections.
3.3 |. Whole mount epifluorescent microscopy and immunohistochemistry
Mouse embryos at E8.5, E9.5, E12.5, and E13.5, neonatal heart at P0, and adult hearts at 6 weeks were harvested and washed with ice-cold PBS. Epifluorescent images of whole embryos, embryonic hearts, neonatal hearts, or adult hearts were acquired using Nikon Eclipse 800. The mouse embryos or hearts were fixed with pre-chilled 4% paraformaldehyde (PFA) in PBS for 30 min, and then incubated in 30% sucrose in PBS overnight at 4°C. The fixed tissue samples were embedded in O.C.T compound and frozen in pre-chilled isopentane. The frozen samples were sectioned at 8 μm thickness. After 5–10 min air dry, the cryosection slides were washed with PBS three times, fixed with pre-chilled 4% PFA in PBS on ice for 20 min, followed by PBS washing three times. The fixed heart sections were permeabilized in 0.1% Triton X-100 in PBS for 20 minutes. Following PBS washing three times, the heart sections were blocked with M.O.M mouse IgG blocking reagent (Vector Labs) for 1 hr and 5% goat serum in M.O.M protein diluent (Vector Labs) for 30 min at room temperature. The heart sections were incubated with different combinations of primary antibodies including anti-cardiac troponin T (mouse monoclonal, Thermofisher, 1:400), anti-Nppa (Rabbit polyclonal, Abgent, 1:200), and anti-tagRFP (rabbit polyclonal, Evrogen, 1:400 dilution, for detecting tagBFP) for 1hr at room temperature. After washing with PBS three times, the heart sections were incubated with secondary antibodies (goat anti-mouse AlexaFluor 555 and goat anti-rabbit AlexaFluor 488, Thermofisher) for 1 hr at room temperature and mounted with Vectashield antifade mounting medium with DAPI (VectorLabs). Images were captured using Zeiss LSM 710 confocal microscope.
3.4 |. Isolation of neonatal atrial cardiomyocytes and immunocytochemistry
Neonatal atrial cardiomyocytes were isolated using the method described previously with minor modification (Nam et al., 2014). P0 hearts of Nppa-tagBFP reporter knock-in mice were dissected, washed in ice-cold PBS, and placed in Cold Balanced Solution (20 mM HEPES 7.6, 130 mM NaCl, 1 mM NaH2PO4, 4 mM glucose, and 3 mM KCl). The dissected atria were minced in a minimal volume ice-cold PBS and then collected into the Eppendorf tube. After the minced tissue was settled down to the bottom of the tube, the PBS solution was aspirated as much as possible. The minced atrial tissue was incubated in 0.25% trypsin with frequent agitation for 2 min in a 37°C water bath. After settling down atrial tissue for 1 min without agitation, the first fraction was collected by removing the supernatant to a fresh tube containing Culture Medium (DMEM, 20% FBS, 2 mM L-glutamine, and 3 mM sodium pyruvate). This cycle was repeated six times to collect a total of eight fractions that were pooled, passed through a 100 μm filter, and combined with additional Culture Medium. Cells were pelleted at 600g for four min at 4°C and re-suspended in warm Culture Medium before plating on Poly-D-Lysine/Laminin coated coverslip (BD Biosciences). Two days later, isolated neonatal atrial cardiomyocytes on the cover slips were fixed with 4% PFA for 15 min and permeabilized with permeabilization buffer (0.05% Triton-X in PBS) for 5 min three times at room temperature. Isolated neonatal atrial cardiomyocytes were incubated with blocking buffer (Universal blocking buffer, BiogeneX) for 45 min and then with primary antibodies against Nppa (mouse monoclonal, Abcam, 1:400) and MLC-2a (rabbit polyclonal, Protein tech, 1:200 dilution) for overnight at 4°C. Following washing three times for 5 min with permeabilization buffer, cells were incubated with secondary antibodies (goat anti-mouse AlexaFluor 488 and goat anti-rabbit AlexaFluor 555, Thermofisher) at room temperature for 1 hr. After another set of washing (5 min ×3 with permeabilization buffer), cells were mounted with Vectashield antifade mounting medium with DAPI (VectorLabs), and images were captured using Zeiss LSM 710 confocal microscope. tagBFP was visualized without immunostaining.
3.5 |. Isolation of adult mouse cardiomyocytes
Adult mouse ventricular cardiomyocytes were isolated using Langendorff perfusion as we described previously (Zhang & Nam, 2018). Dissected hearts were cannulated through the aorta and sequentially perfused with following buffers: (1) perfusion buffer (NaCl 120.4 mM, KCl 14.7 mM, Na2HPO4 0.6 mM, Ka2HPO4 0.6 mM, MgSO4 1.2 mM, Na-HEPES 10 mM, NaHCO3 4.6 mM, Taurine 30 mM, BDM 10 mM, Glucose 5.5 mM, pH 7.0), (2) digestion buffer without CaCl2 (Collagenase II 2.4 mg/mL in perfusion buffer), and (3) digestion buffer with CaCl2 (Collagenase II 2.4 mg/mL and CaCl2 40 μM in perfusion buffer). Perfused hearts were removed from the Langendorff apparatus and mechanically dissociated and triturated in stopping buffer (CaCl2 11.7 μM in calf serum 2 mL plus perfusion buffer 18 mL). Dissociated cells were centrifuged at low speed. The pellet, mainly composed of ventricular cardiomyocytes, was fixed with 4% PFA for 15 min. After PBS washing, a fraction of ventricular cardiomyocytes were immunostained in a 1.5 mL tube using a similar method described above. After immunostaining, a drop of cardiomyocyte containing solution was placed on glass slide and mounted with a coverslip and Vectashield with DAPI (VectorLabs). Images were captured using Olympus IX81 epifluorescent microscope.
ACKNOWLEDGMENT
This work was supported by Gilead Sciences Research Scholars Program and NIH R03 HL140264 (Y-.J. N.)
Funding information
Center for Scientific Review, Grant/Award Number: NIH R03 HL140264; Gilead Sciences, Grant/Award Number: Gilead Sciences Research Scholars Program; NIH, Grant/Award Number: R03 HL140264; Gilead Sciences Research Scholars Program
Footnotes
CONFLICT OF INTEREST
The authors have no competing financial interests.
REFERENCES
- Bloch KD, Seidman JG, Naftilan JD, Fallon JT, & Seidman CE (1986). Neonatal atria and ventricles secrete atrial natriuretic factor via tissue-specific secretory pathways. Cell, 47(5), 695–702. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Meilhac S, & Zaffran S (2005). Building the mammalian heart from two sources of myocardial cells. Nature Reviews. Genetics, 6 (11), 826–835. 10.1038/nrg1710 [DOI] [PubMed] [Google Scholar]
- Chien KR, Knowlton KU, Zhu H, & Chien S (1991). Regulation of cardiac gene expression during myocardial growth and hypertrophy: Molecular studies of an adaptive physiologic response. The FASEB Journal, 5(15), 3037–3046. [DOI] [PubMed] [Google Scholar]
- Christoffels VM, Habets PE, Franco D, Campione M, de Jong F, Lamers WH, … Moorman AF (2000). Chamber formation and morphogenesis in the developing mammalian heart. Developmental Biology, 223(2), 266–278. 10.1006/dbio.2000.9753 [DOI] [PubMed] [Google Scholar]
- Evans SM, Yelon D, Conlon FL, & Kirby ML (2010). Myocardial lineage development. Circulation Research, 107(12), 1428–1444. 10.1161/CIRCRESAHA.110.227405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, … Koch WJ (2010). A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circulation Research, 107(12), 1445–1453. 10.1161/CIRCRESAHA.110.223925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habets PE, Moorman AF, Clout DE, van Roon MA, Lingbeek M, van Lohuizen M, … Christoffels VM (2002). Cooperative action of Tbx2 and Nkx2.5 inhibits ANF expression in the atrioventricular canal: Implications for cardiac chamber formation. Genes & Development, 16(10), 1234–1246. 10.1101/gad.222902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen B, Wang T, Christoffels VM, & Moorman AF (2013). Evolution and development of the building plan of the vertebrate heart. Biochimica et Biophysica Acta, 1833(4), 783–794. 10.1016/j.bbamcr.2012.10.004 [DOI] [PubMed] [Google Scholar]
- Knowlton KU, Rockman HA, Itani M, Vovan A, Seidman CE, & Chien KR (1995). Divergent pathways mediate the induction of ANF transgenes in neonatal and hypertrophic ventricular myocardium. The Journal of Clinical Investigation, 96(3), 1311–1318. 10.1172/JCI118166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange FJ, Moorman AF, & Christoffels VM (2003). Atrial cardiomyocyte-specific expression of Cre recombinase driven by an Nppa gene fragment. Genesis, 37(1), 1–4. 10.1002/gene.10220 [DOI] [PubMed] [Google Scholar]
- Nam YJ, Lubczyk C, Bhakta M, Zang T, Fernandez-Perez A, McAnally J, … Munshi NV (2014). Induction of diverse cardiac cell types by reprogramming fibroblasts with cardiac transcription factors. Development, 141(22), 4267–4278. 10.1242/dev.114025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EN (2006). Gene regulatory networks in the evolution and development of the heart. Science, 313(5795), 1922–1927. 10.1126/science.1132292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman CE, Schmidt EV, & Seidman JG (1991). Cis-dominance of rat atrial natriuretic factor gene regulatory sequences in transgenic mice. Canadian Journal of Physiology and Pharmacology, 69(10), 1486–1492. [DOI] [PubMed] [Google Scholar]
- Zhang Z, & Nam YJ (2018). Generation of MLC-2v-tdTomato knock-in reporter mouse line. Genesis, 56, e23256 10.1002/dvg.23256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Lal H, Chen X, Shang X, Song J, Li Y, … Force T (2010). GSK-3alpha directly regulates beta-adrenergic signaling and the response of the heart to hemodynamic stress in mice. The Journal of Clinical Investigation, 120(7), 2280–2291. 10.1172/JCI41407 [DOI] [PMC free article] [PubMed] [Google Scholar]