Abstract
Friedreich’s ataxia is a progressive disease affecting multiple organs that is caused by systemic insufficiency of the mitochondrial protein frataxin. Current therapeutic strategies aim to elevate frataxin levels or alleviate consequences of frataxin deficiency. Recent, significant advances within the FRDA therapeutic pipeline are bringing patients closer to a cure.
Keywords: Friedreich’s ataxia, oligonucleotide, frataxin, gene therapy, oxidative stress, mitochondria
Friedreich’s ataxia is a multifaceted disease
Friedreich’s ataxia (FRDA) is an autosomal recessive disorder manifesting with progressive ataxia, loss of tendon reflexes, difficulty with speech articulation (dysarthria), and often accompanied by hypertrophic cardiomyopathy, diabetes and skeletal abnormalities. It is the most common inherited ataxia with a prevalence of ~ 1 – 2/50,000 and typical onset during adolescence. FRDA is caused by reduced expression of the mitochondrial protein frataxin (FXN) with more than 96 % of patients bearing homozygous expansions of GAA trinucleotide repeats in intron 1 of the FXN gene [1]. Unaffected alleles contain up to ~ 35 GAA repeats while disease alleles most frequently span 600 – 900 GAA units. Carriers of one mutated FXN allele are asymptomatic and comprise 1 – 2 % of the population. Expanded GAA repeats trigger local chromatin changes resulting in transcriptional repression of the gene. FXN mRNA levels in patient samples are reduced by 70 – 95%. The lengths of the GAA expansions determine the extent of FXN downregulation and inversely correlate with age of onset, but positively correlate with disease severity and progression. From a therapeutic point of view, all FRDA patients produce a limited amount of functional frataxin.
Frataxin participates in iron-sulfur cluster (ISC) biosynthesis in the mitochondria and many of the overt FRDA phenotypes result from disturbed intracellular iron homeostasis as well as deficient activities of proteins containing ISC cofactors, such as mitochondrial respiratory complexes, Krebs cycle proteins, DNA repair and replication proteins. The ubiquitous functions of ISCs as prosthetic groups result in pleiotropic and multi-organ manifestation of FRDA. There is currently no cure for FRDA, however tremendous efforts by FRDA clinicians and researchers, alongside patients and advocacy groups are driving the field closer to this goal (Box 1, Figure I). Recent reviews comprehensively describe current therapeutic advancements for FRDA [2–4]. Herein, we introduce several therapeutic prospects for FRDA that were selected from the many promising approaches currently in various stages of developmenti. The strategies chosen for discussion either highlight major efforts in the field to increase FXN levels or efforts to alleviate one of the most cited downstream consequences of frataxin deficiency, oxidative stress.
Increasing FXN expression at the gene level
Low expression of an otherwise functional frataxin protein is a molecular hallmark of FRDA. Efforts to increase FXN mRNA and/or protein levels are thought to be the most straight-forward therapeutic approaches. Furthermore, FRDA carriers remain asymptomatic despite expressing approximately half the level of frataxin as unaffected individuals, indicating that even a relatively small increase of FXN expression in FRDA patients should be therapeutic or even curative. Initial efforts were focused on reversing the chromatin changes that occur at the FXN locus harboring expanded GAAs, specifically with histone acetylation and DNA methylation in mind. Now, genome editing [5], oligonucleotide (ODN)-based approaches [6], and synthetic transcription factors (e.g. Syn-TEF1) [7] (Box 1, Figure I) are emerging as techniques that can expand opportunities for therapeutic intervention at the gene level for this disease. Each of these strategies is intended to alleviate the transcription block imposed by the expanded GAA repeats or by the non-canonical DNA-DNA / DNA-RNA hybrid (R-loop) formations adopted by these repeat sequences. Whether it be by removing the expanded GAA tract entirely (genome editing), or by preventing R-loop formation (GAA-specific ODNs),or enhancing transcription through the FXN locus via selective targeting of elongation factors (GAA-targeted Syn-TEF1), these approaches take direct aim at the molecular defect that causes FRDA. The path to the clinic for genome editing and artificial transcription factors is long, but recent successes using ODNs to treat spinal muscular atrophy (SMA) and Duchenne muscular dystrophy (DMD) [8] suggest that soon we will be able to develop a specific ODN approach to increase frataxin levels in FRDA patients.
Frataxin replacement by gene therapy
Besides targeting the genomic locus, gene therapy is another promising approach to improve symptoms caused by low FXN expression. Administration of adeno-associated virus (AAV) carrying human FXN prior to and post-development of cardiac or neurological dysfunction in two different FRDA conditional mouse models led to either inhibition of or complete reversal of their severe conditions, respectively [9, 10]. These studies demonstrate the potential for gene therapy to effectively treat FRDA patients at all stages of the disease course. Efforts to advance FXN gene therapy from laboratory testing to a viable treatment option for FRDA patients have been undertaken in several academic institutions and biotechnology/pharmaceutical companies and are progressing at a rapid pace. Clinical trials for AAV gene therapy are currently underway for other neurodegenerative diseases, including SMA and Parkinson’s disease, and are setting important precedents for the first FRDA gene therapy trial [11].
The development of synthetic lipid nanoparticles as a viral-free system to deliver FXN mRNA was recently reported as a gene therapy approach that has the potential to overcome some of the immunological limitations associated with viral delivery systems. When administered into the spinal cord of adult mice (via intrathecal injection), these particles penetrated the blood-brain barrier and human frataxin protein was detectable in dorsal root ganglia [12]. It will be critical to determine if a therapeutic benefit accompanies ectopic expression of frataxin in vivo, and if so, establish an appropriate dosage and administration regimen for this approach.
Targeting impaired mitochondrial function and increased oxidative stress in FRDA
Reduced antioxidant capacity has been recurrently observed in numerous cell and animal models of FRDA. Downregulation of the transcription factor NF-E2-related factor (NRF2), which regulates the expression of antioxidant response element (ARE) target genes, is thought to underlie this defective antioxidant response [13]. NRF2 levels are tightly regulated by ubiquitin-mediated degradation, therefore one strategy to mitigate oxidative stress in FRDA is to stabilize NRF2 by blocking this pathway. The drug omaveloxolone (RTA 408) inhibits NRF2 ubiquitination and protects neuronal cells isolated from FRDA animal models or skin fibroblasts from FRDA patients from applied oxidative stressors [14]. A Phase II study is currently recruiting adults and children with FRDA to evaluate the safety, pharmacodynamics, and efficacy of omaveloxolone (Clinical Trial Number: NCT02255435).
Another antioxidant being investigated as a treatment for FRDA is acetyl-l-carnitine (ALCAR). ALCAR is a derivative of the intracellular fatty acid transporter, l-carnitine. Reported outcomes from two independent one-year trials indicated that ALCAR was well-tolerated by study participants, however conclusions regarding improvements to neurological function ranged from modest to no effect (reviewed in [15]). Given the ease of administration (oral capsule) and tolerability, perhaps longer-term trials designed with a larger participant pool are warranted to test the efficacy of ALCAR in FRDA patients.
A therapeutic intervention to counteract downstream effects of oxidative stress in FRDA is by the introduction of deuterated polyunsaturated fatty acid (dPUFAs). Reduced levels of frataxin in the mitochondria of FRDA cells causes an imbalance in iron metabolism, and free iron can initiate and propagate oxidation of membrane lipids in an environment of elevated oxidative stress [16, 17]. Reactive aldehydes that are produced from peroxidized mitochondrial inner membrane lipids adduct to enzymes and other biologically active molecules and inhibit their function [18]. dPUFAs are refractory to peroxidation, therefore their incorporation into mitochondrial membranes could mitigate the effects of oxidative stress in FRDA cells and improve overall mitochondrial fitness [16, 17]. Deuterated ethyl linoleate (RT001) is a dPUFA that was recently found to be both safe and tolerable in a Phase I/II double-blind, placebo-controlled clinical trial in adults with FRDA (Clinical Trial Number: NCT02445794) [19]. Results of cardiopulmonary exercise testing from this first study also indicated a clinical benefit, offering support for additional longer and larger-scale trials to test the efficacy of RT001 in alleviating symptoms associated with FRDA.
Conclusions and future perspectives
As indicated in the discussion above, a number of diverse approaches are being pursued as treatment options for FRDA. Defining robust and reliable biomarkers of the disease is a critical task that will undoubtedly facilitate therapy development. For example, diagnostic and prognostic biomarkers can serve as indicators to determine if a patient is an appropriate candidate for a particular treatment. Moreover, once qualified, a biomarker is a powerful addition to clinical trial design as an outcome or surrogate outcome measure. However, identifying biomarkers and defining a context of use is challenging for rare and heterogeneous conditions like FRDA. Efforts to discover and validate various categories of FRDA biomarkers are underway and include global gene and protein expression profiling, metabolic profiling, and/or frataxin quantitation in patient tissues and biospecimens, and longitudinal symptom assessments. Data collected from FRDA natural history studies in both the United States (Clinical Trial Number: NCT03090789) and Europe (Clinical Trial Number: NCT02069509) is also supporting the biomarker discovery effort.
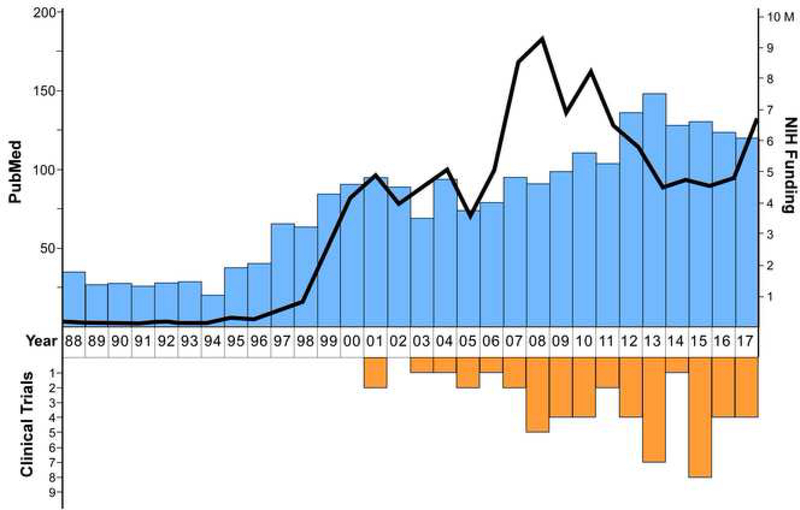
Since discovery of the pathogenic GAA repeat expansion in FXN in 1996 [1], research efforts dedicated to finding therapies or a cure for FRDA have intensified and remain strong (Figure 1). Ideally, treatments designed to target the underlying pathophysiology in FRDA could help to manage disease progression and onset of symptoms while therapeutic strategies aimed at disease prevention or reversal, such as gene therapy, ODN and/or gene editing, are developed for human trials. Moreover, combination therapy strategies should be considered for targeting affected tissues that differ amongst individuals living with FRDA.
Figure 1. A 30-year perspective of FRDA research.
Blue bars depict the number of publications appearing in PubMed per year from 1988 – 2017. The data was obtained by entering the search criteria “Friedreich’s ataxia” OR “Friedreich ataxia” OR “frataxin” in PubMed. The black line depicts the dollar amount (in millions) corresponding to grants funded by the National Institutes of Health (NIH) per year from 1988 – 2017 as identified by entering the search terms “Friedreich’s ataxia” OR “Friedreich ataxia” OR “frataxin” in the NIH RePORT search engineii. The orange bars show the number of clinical trials initiated per year from 1988 – 2017 as identified on ClinicalTrials.goviii using the search term “Friedreich’s ataxia”.
Box 1.
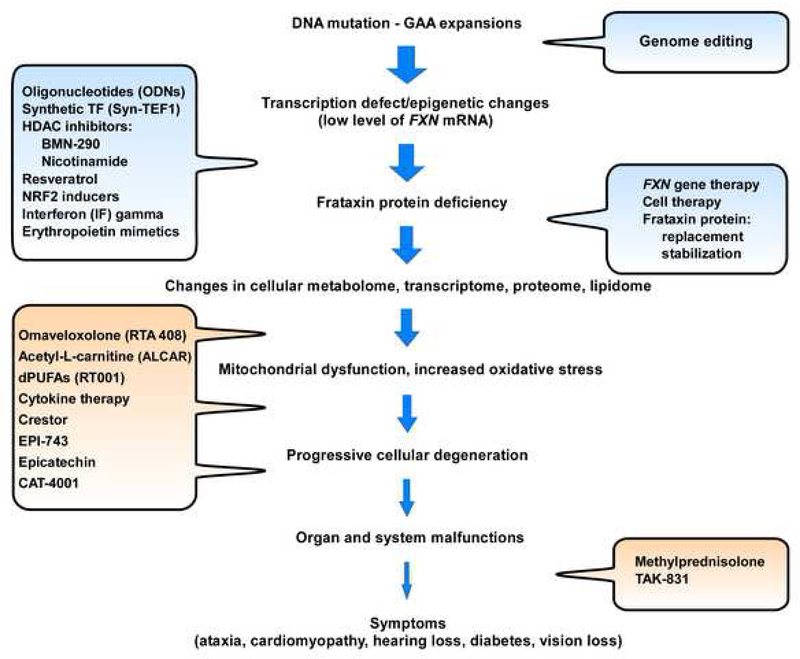
While selected therapeutic approaches from Figure I are discussed in the main text, short descriptions of the remaining strategies are provided below. Several recent publications comprehensively review these therapeutic approaches for FRDA [2–4] in details.
Histone deacetylase inhibitors (HDACi) – are small molecules that target repressive chromatin modifying enzymes. Both BMN-290 and nicotinamide (vitamin B3) are being pursued with the goal of upregulating FXN transcription.
Resveratrol – is a natural compound with antioxidant properties demonstrated to stimulate frataxin expression in vitro in FRDA cells and in mouse models. The exact mechanism of this upregulation has not been elucidated.
NRF2 inducers – are compounds that stimulate expression of the transcription factor NRF2. Because expression of frataxin and NRF2 are directly correlated, NRF2 inducers have been shown to increase expression of FXN mRNA. The transcriptional interplay between NRF2 and FXN is not fully understood.
Interferon (IF) gamma – is a cytokine involved in the immune response shown to stimulate FXN transcription and/or increase FXN mRNA stability in vitro in patient cells, in animal models and in initial clinical trials.
Erythropoietin mimetics – are small molecule agonists of the erythropoietin receptor shown to increase FXN mRNA and protein levels via a yet undefined mechanism.
Cell therapy – refers to bone marrow as well as hematopoietic stem and progenitor cell transplants that were demonstrated to improve several phenotypic abnormalities in FRDA mouse models.
Frataxin protein replacement – refers to synthetic frataxin proteins or peptides capable of localizing to mitochondria via specific organelle targeting sequences.
Frataxin protein stabilization – refers to small molecules designed to interfere with natural frataxin protein degradation pathways resulting in increased intracellular levels of this protein.
Cytokine therapy – refers to granulocyte-colony stimulating factor (G-CSF) and stem cell factor (SCF) treatments that showed beneficial effects toward FRDA pathology in an FRDA mouse model, including: increased FXN mRNA and frataxin protein, improved neurophysiological function, increased neural stem cell numbers and reduced inflammation.
Crestor – is a drug targeting the ApoA-1 protein, which is a component of high-density lipoprotein (HDL) cholesterol. The level of ApoA-1 is lower in FRDA patients compared to controls, however, the significance and potential consequences of the ApoA-1 decrease in FRDA is currently unknown.
EPI-743 – is an antioxidant molecule that targets NADPH quinone oxidoreductase 1 (NQO1) to improve cellular energy metabolism in patients with FRDA.
Epicatechin – is a flavanol shown to stimulate mitochondria biogenesis and improve metabolism in patients with FRDA.
CAT-4001 – is a small molecule that activates NRF2 and inhibits NF-kB developed as a potential treatment for FRDA.
Methylprednisolone – is a steroid aimed to treat the secondary inflammatory response detected in FRDA.
TAK-831 – is an inhibitor of D-amino-acid oxidase (DAAO) that increases levels of D-serine to improve motor coordination and alleviate neurological symptoms of FRDA.
Box 1, Figure I. Multi-level therapeutic strategies for FRDA.
The flow diagram illustrates many phases underlying disease manifestation in FRDA, highlighting multiple and diverse opportunities for therapeutic intervention (text bubbles). Strategies aimed to increase FXN/frataxin levels are shown in blue, while approaches aimed at improving consequences of frataxin deficiency are shown in orange. The clinical development stage for these strategies can be found on the Friedreich’s Ataxia Research Alliance websitei.
Acknowledgements
We acknowledge the work of our colleagues contributing to the FRDA treatment pipeline that was not discussed in this article due to space limitations. The work in the Napierala laboratory is supported by grants from the National Institute of Neurological Disorders and Stroke, National Institutes of Health (R01NS081366 and R21 NS101145 to M Napierala and R03 NS099953 to J S Napierala), the Friedreich’s Ataxia Research Alliance, Muscular Dystrophy Association (MDA418838), National Ataxia Foundation, American Academy of Neurology, and by a sponsored research agreement with CRISPR Therapeutics. M. Napierala is a UAB Pittman Scholar.
Disclosures
M. Napierala is in part supported by CRISPR Therapeutics. The sponsor had no role or any influence over the content of this manuscript.
Footnotes
RESOURCES
REFERENCES
- 1.Campuzano V. et al. (1996) Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271 (5254), 1423–7. [DOI] [PubMed] [Google Scholar]
- 2.Strawser C. et al. (2017) Pharmacological therapeutics in Friedreich ataxia: the present state. Expert Rev Neurother 17 (9), 895–907. [DOI] [PubMed] [Google Scholar]
- 3.Indelicato E. and Bosch S. (2018) Emerging therapeutics for the treatment of Friedreich’s ataxia. Expert Opinion on Orphan Drugs 6 (1), 57–67. [Google Scholar]
- 4.Tai G. et al. (2018) Progress in the treatment of Friedreich ataxia. Neurol Neurochir Pol 52 (2), 129–139. [DOI] [PubMed] [Google Scholar]
- 5.Li Y. et al. (2015) Excision of Expanded GAA Repeats Alleviates the Molecular Phenotype of Friedreich’s Ataxia. Mol Ther 23 (6), 1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L. et al. (2016) Activating frataxin expression by repeat-targeted nucleic acids. Nat Commun 7, 10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erwin GS et al. (2017) Synthetic transcription elongation factors license transcription across repressive chromatin. Science 358 (6370), 1617–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen X. and Corey DR (2018) Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res 46 (4), 1584–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perdomini M. et al. (2014) Prevention and reversal of severe mitochondrial cardiomyopathy by gene therapy in a mouse model of Friedreich’s ataxia. Nat Med 20 (5), 542–7. [DOI] [PubMed] [Google Scholar]
- 10.Piguet F. et al. (2018) Rapid and Complete Reversal of Sensory Ataxia by Gene Therapy in a Novel Model of Friedreich Ataxia. Mol Ther 26 (8), 1940–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deverman BE et al. (2018) Gene therapy for neurological disorders: progress and prospects. Nat Rev Drug Discov 17 (9), 641–659. [DOI] [PubMed] [Google Scholar]
- 12.Nabhan JF et al. (2016) Intrathecal delivery of frataxin mRNA encapsulated in lipid nanoparticles to dorsal root ganglia as a potential therapeutic for Friedreich’s ataxia. Sci Rep 6, 20019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shan Y. et al. (2013) Frataxin deficiency leads to defects in expression of antioxidants and Nrf2 expression in dorsal root ganglia of the Friedreich’s ataxia YG8R mouse model. Antioxid Redox Signal 19 (13), 1481–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abeti R. et al. (2018) Novel Nrf2-Inducer Prevents Mitochondrial Defects and Oxidative Stress in Friedreich’s Ataxia Models. Front Cell Neurosci 12, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aranca TV et al. (2016) Emerging therapies in Friedreich’s ataxia. Neurodegener Dis Manag 6 (1), 49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cotticelli MG et al. (2013) Insights into the role of oxidative stress in the pathology of Friedreich ataxia using peroxidation resistant polyunsaturated fatty acids. Redox Biol 1, 398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abeti R. et al. (2016) ‘Mitochondrial energy imbalance and lipid peroxidation cause cell death in Friedreich’s ataxia’. Cell Death Dis 7, e2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fritz KS and Petersen DR (2013) An overview of the chemistry and biology of reactive aldehydes. Free Radic Biol Med 59, 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zesiewicz T. et al. (2018) Randomized, clinical trial of RT001: Early signals of efficacy in Friedreich’s ataxia. Mov Disord 33 (6), 1000–1005. [DOI] [PubMed] [Google Scholar]