Abstract
Lymphocytes and myeloid cells (monocyte/macrophages) have important roles in multiple types of diseases characterized by unresolved inflammation. The relatively recent appreciation of obesity, insulin resistance and type 2 diabetes (T2D) as chronic inflammatory diseases has stimulated interest in understanding the role of immune cells in metabolic imbalance. Myeloid cells regulate inflammation through cytokine production and the adipose tissue remodeling that accompanies hyper-nutrition, thus are critical players in metabolic homeostasis. More recently, multiple studies have indicated a role for T cells in obesity-associated inflammation and insulin resistance in model organisms, with parallel work indicating that pro-inflammatory changes in T cells also associate with human T2D. Furthermore, the expansion of T cells with similar antigen-binding sites in obesity and T2D indicates these diseases share characteristics previously attributed to inflammatory autoimmune disorders. Parallel pro-inflammatory changes in the B-cell compartment of T2D patients have also been identified. Taken together, these studies indicate that in addition to accepted pro-inflammatory roles of myeloid cells in T2D, pro-inflammatory skewing of both major lymphocyte subsets has an important role in T2D disease pathogenesis. Basic immunological principles suggest that alterations in lymphocyte function in obesity and T2D patients are an integral part of a feed-forward pro-inflammatory loop involving additional cell types. Importantly, the pro-inflammatory loop almost inevitably includes adipocytes, known to respond to pro-inflammatory, pro-diabetogenic cytokines originating from the myeloid and lymphoid compartments. We propose a model for inflammation in T2D that functionally links lymphocyte, myeloid and adipocyte contributions, and importantly proposes that tools for B-cell ablation or regulation of T-cell subset balance may have a place in the endocrinologist’s limited arsenal.
Keywords: T cell, B cells, monocytes, cytokines, chronic inflammation, type 2 diabetes
Introduction: an overview of type 2 diabetes (T2D) as a chronic inflammatory disease
Chronic inflammation in T2D is thought to initiate as an inflammatory response to altered lipolysis and remodeling/necrosis of expanding adipose tissue compartments.1,2 Altered lipolysis in response to over nutrition and rapidly expanding adipose tissue results in elevation of generally pro-inflammatory saturated fatty acids, which serve as ligands for cell surface Toll-like receptors (TLRs).3,4 TLRs and other surface receptors activate the signaling cascades that culminate in, among other events, nuclear factor-κB mobilization, thus inflammation (reviewed in ref. 5). Changes in lipid metabolism and the abundance of TLR ligands in the expanding adipose tissue likely trigger a temporal cascade of adipose infiltration first by neutrophils, followed in order by B cells, T cells, then finally monocyte/macrophages.1,6–8 This temporal cascade indicates that adipose inflammation fundamentally differs from classical inflammation, in which the initial neutrophil wave is followed first by macrophage then lymphocyte infiltration. Blocking the monocyte recruitment factor monocyte chemoattractant protein 1 either pharmacologically or genetically prevents macrophage adipose infiltration and protects against insulin resistance,9,10 presumably relatively late in the time course of immune cell infiltration after neutrophil and lymphocte numbers have peaked. However, the presence and source of chemokines that induce the earliest immunological event (neutrophil infiltration6) and set the stage for initiating adipose inflammation has not been reported. Regardless, a physiologically critical outcome of these biochemical processes is elevated pro-inflammatory cytokine production by cells of both the innate and adaptive immune systems.
The role of cytokines in T2D
Cytokines secreted by multiple immune and nonimmune cell types are dominant regulators of the pathological inflammation that characterizes and promotes T2D. Cytokines originating from the adipose compartment, designated adipokines, have both pro- and anti-inflammatory roles in obesity and T2D. The two adipokines linked most strongly to insulin resistance and T2D are adiponectin and leptin.11 Adiponectin is generally anti-inflammatory, as exemplified by its ability to decrease macrophage growth and function, and nuclear factor-κB activation12,13 In contrast, leptin has proinflammatory activities, including the promotion of pro-inflammatory T-cell subsets.14 Adipocytes, and to a greater extent, immune system cells are also potent sources of strongly diabetogenic cytokines such as interleukin (IL)-1β and tumor necrosis factor (TNF)-α. Clinical studies have demonstrated that pharmacological agents that block IL-1β or TNF-α activity decrease inflammation and perhaps insulin resistance in T2D patients, at least under some protocols.15–19
The role of non-adipocyte-specific cytokines in T2D etiology is most definitively demonstrated by murine studies showing, for example, that TNF-α-null mice are protected against obesity-associated insulin resistance.20 Similarly, treatment with a competitive inhibitor of IL-1β or an IL-1β-blocking antibody strongly protected mice from diet-induced insulin resistance,21,22 akin to the more modestly decreased inflammation and proinsulin:insulin ratio in T2D patients treated with the competitive inhibitor.15,17 However, the role of the generally proinflammatory cytokine IL-6, which lies downstream of the IL-1β-triggered inflammatory cascade23 is counterintuitive. Despite many studies that predict IL-6 would promote inflammation in T2D, IL-6-null mice spontaneously develop insulin resistance.24 Overall, only some anti-cytokine treatments trigger expected responses in rodent diet-induced obesity (DIO), raising questions as to how best design and test new anti-cytokine therapies for efficacy in promoting metabolic health.
To advance practical application of DIO mouse results in continued tests with anti-cytokine therapies for insulin resistance, it is important to consider the multiple clinical differences between human and murine responses to therapies for insulin resistance/T2D. We have recapitulated studies from innumerable investigators showing that for male C57BL/6 mice, the standard for DIO experiments, higher body weight appears to correlate quite closely with degree of insulin resistance as measured by glucose or insulin tolerance tests (D. Markham, unpublished). The association between body mass index and insulin resistance in humans is more variable. There are several possible clinical considerations to take into account when interpreting this seemingly mundane difference between human and mouse, which may also apply to expectations for murine vs human response to anti-cytokine drugs as therapies for insulin resistance. First, genetic variation among human subjects could explain the discrepancy between the efficacies of anti-cytokine therapy in the various human clinical trials, compared with the more consistent results in animal model studies. Importantly, averaged data from the outbred/variable human populations19 may not reflect significant effects documented in studies on highly selected cohorts. The genetic issue is absent in standard DIO models. Second, murine models for T2D recapitulate short-term diet-induced obesity, whereas most obesity in humans is a result of many years or even decades of over nutrition. Based on the impressive results from anti-cytokine intervention relatively early during the disease course in rodent DIO,22,25 early intervention with anti-cytokine therapies in obese patients could increase treatment efficacy (as measured by insulin sensitivity) over results obtained in long-term T2D patients. Third, dramatic results from mouse DIO studies indicate that the quantitatively modest metabolic results of anti-cytokine trials in humans may be improved by additional insights into, for example, pharmacokinetics of cytokine-blocking reagents. Finally, an unpopular notion in the field is the possibility that processes involved in the development of rodent insulin resistance could fundamentally differ from human T2D. Although strong evidence supports the appropriateness of murine diet studies as a valid model for the development of multiple characteristics of a T2D-like disease, the inevitable differences between species support the critical need for long-term longitudinal projects (and funding) to follow overweight individuals as many develop obesity and insulin resistance/loss of islet function over time.
The role of monocytes/macrophages in T2D
Many cell types produce inflammatory cytokines,26–28 but multiple lines of evidence show hematopoietic cells are major determinants of the inflammation that links obesity with insulin resistance and T2D. Cytokine-producing hematopoietic cells are necessary and sufficient for obese mice to become insulin resistant, at least under some conditions.29 Additional studies have specifically implicated myeloid cell cytokines in insulin resistance in mice,30 which is further supported by demonstrations that monocytes from T2D patients secrete elevated levels of key pro-inflammatory cytokines such as IL-1β and TNF-α.31 Peripheral blood monocytes from T2D patients are also constitutively activated,32 most likely due (at least in part) to the elevated levels of TLR ligands circulating in these patients.32–36 Monocytes from T2D patients have been shown to hyper-produce multiple pro-inflammatory cytokines in response to TLR ligand, most commonly TLR4.31,37–40 According to conventional logic, hyper-responsiveness to TLR ligands in the TLR ligand-rich milieu of T2D is unexpected. Instead, continuous TLR engagement by endogenous TLR4 ligands would be expected to result in an ameliorated response to a second ligand dose, via a well-understood phenomenon termed TLR/endotoxin tolerance.41,42 Regardless of the expected results, overwhelming evidence in mice demonstrated that TLR4 function and downstream cytokine production is required for the development of insulin resistance.43,44
Recent work implicating TLR4 and TLR4-triggered pathways in the development of insulin resistance has reached diverse conclusions: either TLR4 does or does not have a critical role in DIO-induced metabolic imbalance, despite evidence of TLR4 as a generally pro-inflammatory innate immune receptor.44–48 The unexpected discrepancy in results is likely explained by differences in the model systems used to test the role of TLR4 in insulin resistance. The studies that indicated a critical role for TLR4 in insulin resistance used the C3H/HeJ mouse, which has a natural TLR4 mutation that inactivates TLR4 on all cells.44,45 In this model, the vast majority of the TLR4 protein is intact, leaving the potential that non-traditional functions of the protein remain (at least hypothetically). In contrast, the studies that concluded TLR4 has no pivotal role in insulin resistance used bone marrow transplantation to create a ‘macrophage-specific’ TLR4 knockout in a lethally irradiated agouti mouse.48 In reality, this mouse is more accurately described as an ‘immune cell’ rather than ‘macrophage-specific’ TLR4 knockout. Furthermore, interpretation is confounded by the agouti background. Agouti mice have naturally occurring mutation that inactivates the neurotransmitter alpha melanocyte-stimulating hormone and are obese. Overall, the field generally believes, based on the whole animal mutant TLR4 studies and the role of TLR4 as a pro-inflammatory receptor, that TLR4 probably has a critical role in the development of insulin resistance, although the specific cell type involved requires additional investigation.
Despite the molecular inconsistencies in describing exactly how myeloid lineage cells contribute to insulin resistance and T2D, the fact remains that macrophages aggressively infiltrate the expanding adipose tissue in response to over nutrition in both models of insulin resistance and in obesity patients.1,2 Timing of macrophage infiltration/cytokine production relative to lymphocyte infiltration/cytokine production in the expanding adipose tissue is controversial, probably due to slight variations in protocols used for the various analyses.7,49–53 Regardless, both scenarios emphasize the likely importance of monocyte/lymphocyte cross talk through cytokine production alone or perhaps in combination with cell-cell interactions in organisms responding to high-fat diet (HFD). In vitro studies have also identified the likely presence of a paracrine pro-inflammatory loop between monocyte/macrophage and adipocytes, the cell type most traditionally associated with metabolic balance. This loop involves elevated release of inflammatory cytokines from monocytes that activate adipocytes, plus elevated levels of saturated fatty acids from obese adipose tissue that further activate macrophages.54
Despite the lingering ‘chicken or egg’ issue of macrophage/lymphocyte infiltration into expanding adipose tissue, and initiation of disease by adipocytes or immune system cells, the literature consistently supports the conclusion that over nutrition results in fundamental changes in monocyte and macrophage function, especially in the balance of cytokine production. In addition to elevated cytokine responses by circulating monocytes,31,55 tissue macrophages in obese and T2D patients take on a classically activated or ‘M1’ macrophage phenotype, and become a major source of pro-inflammatory cytokines.56 The increase of Ml macrophages in adipose tissue during diet-induced expansion and concomitantly-increased adipocyte diameter is followed by a remodeling event, characterized by further immune cell infiltration/expansion, an overall decrease in adipocyte size and shift in macrophage phenotype to the ‘M2’ (alternatively activated) subset.1 Careful time course analyses show expression of both pro-inflammatory (TNF-α, IL-6 and monocyte chemoattractant protein 1) and anti-inflammatory (lL-10) cytokines peak coincident with the maximum expression of activated macrophage-associated genes such as F4/80. Interestingly, these peaks occur slightly after adipocytes reach maximum size and expression of the pan-macrophage gene CD11c is highest (12 weeks post-HFD).1 These data suggest that macrophage phenotype changes from M1 to M2 following macrophage infiltration into the expanding adipose tissue. This change presumably aids in remodeling and adipocyte downsizing. Although initial observations suggested that alternatively activated M2 macrophages, which promote tissue remodeling and control inflammation via IL-10 production, resolve adipose inflammation following diet-mediated expansion, more comprehensive analyses suggest that both M1 and M2 macrophages influence adipose physiology throughout time course of disease.57 Taken together, these findings strongly support the conclusion that cytokine balance, instead of a simple M1/M2 paradigm, determines the adipose response to over nutrition. The ‘cytokine balance hypothesis’ raises the possibility that other potent sources of cytokines, such as T cells and B cells, significantly contribute to disease pathogenesis in obesity and T2D. The contribution of altered IL-10 production by immune cells from T2D patients is discussed further below.
The role of neutrophils in T2D
Neutrophils infiltrate the expanding adipose tissue in early responses to HFD, suggesting that neutrophils have a role in adipose tissue inflammation and/or insulin resistance. Neutrophil infiltration may be expedited due to the ability of neutrophils to adhere to adipocytes, and by demonstrations that this adherence is increased if neutrophils are activated.6 T2D furthermore associates with changes in neutrophil function including impaired bacterial phagocytosis and killing.58 These defects may be related to reduced neutrophil degranulation as measured with plasma elastase levels.59 A few studies also identified elevated respiratory burst function in neutrophils from T2D patients. Although none of these studies directly test the role of neutrophils in metabolic imbalance, increased oxidative stress from diabetic patient neutrophils likely contributes to oral complications of diabetes.60 The effects of T2D on innate immune responses are probably due to diabetes-associated metabolic syndrome rather than hyperglycemia itself, given that acute elevation of blood glucose in human subjects using glucose-clamp techniques did not result in altered neutrophil migration, phagocytosis or oxidative burst capacity.61 Overall, a strong link between neutrophil function and T2D etiology or pathogenesis remains to be demonstrated.62–65
Lymphocytes promote inflammation in T2D through multiple mechanisms
Lymphocytes can contribute to local (adipose tissue) and systemic inflammation in T2D patients by multiple mechanisms. Inflammation is thought to initiate in the visceral adipose tissue when neutrophils, followed closely by B cells, then T cells, infiltrate the expanding adipose tissue in response to HFD in mice. In these experiments, B cell numbers peaked at 3 weeks following initiation of HFD, then fell as T cells infiltrated.7 These data are consistent with the possibility that lymphocytes are activated by products of altered lipolysis in expanding adipose tissue, then exit adipose tissue to recirculate throughout the body. Alternatively, lymphocytes could be activated by early adipose-infiltrating neutrophils,6 then leave the adipose tissue and recirculate. The second mechanism by which lymphocytes contribute to systemic inflammation in T2D is direct secretion of cytokines into circulation, irrespective of the site of lymphocyte activation. B cells, in particular, may respond to elevated endotoxemia characteristic of T2D patients33,66 as indicated by demonstration of unexpected functions of TLRs on B cells from T2D donors.67 The ability of lymphocytes to distribute cytokines locally and systemically, along with their ability to recirculate, strongly implicates lymphocytes alongside myeloid cells in the net outcome of obesity-mediated inflammation.
Pro-inflammatory T cells in T2D
A role for T cells in T2D was perhaps predictable, given that T cells are implicated in the pathogenesis of multiple inflammatory diseases, and specific T-cell subsets have demonstrated roles in such diseases.68–72 For example, CD4+ cells are a major T-cell subset implicated in the pathogenesis of multiple sclerosis, rheumatoid arthritis and psoriasis, which are characterized by elevated numbers of pro-inflammatory cytokine-positive CD4+ T cells in blood and diseased tissues.73 Many recent studies have specifically implicated IL-17-producing CD4+ T cells, Th17 cells, in pathogenic inflammation.74,75 IL-17 activates inflammation through the widely expressed IL-17 receptor. Upon engagement with IL-17, the IL-17 receptor triggers nuclear factor-κB thus cytokine production by monocytes, fibroblast, stromal, epithelial and endothelial cells.76–78 IL-17 also induces mobilization, recruitment and activation of granulocytes via induction of granulocyte colony-stimulating factor.79 A second subset of CD4+ T cells, Th1 cells, are the dominant source of interferon-γ (IFN-γ), a second pro-inflammatory cytokine implicated in inflammatory disease.49,74 IFNγ-producing cells have been specifically implicated in inflammatory bowel disease, lupus nephritis, multiple sclerosis and a collagen-induced arthritis model of rheumatoid arthritis. In each of these diseases IFNγ-activated macrophages are present at the sites of inflammation.80 Overall, these diseases indicate an important role for T cells in the induction and exacerbation of multiple chronic inflammatory diseases, at least in part through their ability to trigger nuclear factor-κB target genes.
Several recent studies utilizing a mouse model of DIO demonstrate important roles for multiple T-cell subsets in adipose-associated inflammation. Subcutaneous and visceral adipose tissue in DIO mice have increased numbers of CD4+ and CD8+ T cells.52,81 CD4+ and CD8+ T cells are also elevated in obese human omental and abdominal adipose tissue, which indicates parallel changes in T-cell infiltration and inflammation in human adipose tissue.82 Although these reports suggest a role for T cells in adipose inflammation, there is controversy among studies as to which T-cell subset (CD4+, CD8+) has dominant roles, or whether T cells in general necessarily have an important role in inducing inflammation in obesity and T2D. Studies focused on pro-inflammatory functions of CD8+ T cells in DIO show these cells infiltrate adipose tissue and produce chemotactic cytokines such as monocyte chemotactic proteins 1 and 3. Such chemokines induce macrophage activation and recruitment into the adipose tissue.52 Thus CD8T cells function, in part, by directing myeloid cell trafficking. Furthermore, genetic depletion of CD8+ cells can decrease inflammation and improve insulin resistance to further demonstrate the importance of CD8+ T cells in adipose-associated inflammation.52 Overall, these studies support the concept that T cells promote T2D through pro-inflammatory activities, some of which involve other immune system members.
Multiple in depth studies on pro-inflammatory CD4+ T cells showed that these cells likely bolster CD8+ T cell-mediated inflammation in obesity and insulin resistance. CD4+ T cells from DIO mice produced elevated levels of IFN-γ and IL-17 (although CD4+ IL-17+ cells were only significantly elevated in subcutaneous adipose tissue).81 A companion study also showed the T2D-linked cytokine IL-6 increased the number of IL-17-producing cells in the spleen of obese mice, supporting the possibility that Th17s contribute to T2D-associated inflammation and insulin resistance.83 These data are consistent with our demonstration that the percentage of Th17 cells is elevated in blood of T2D patients.74 Interestingly, Th17 percentages correlated with human body mass index, a measure of adiposity, in T2D patients but not in metabolically healthy obese subjects.74 These findings suggest a unique relationship between Th17 cells and metabolic imbalance that is not absent in metabolically healthy individuals. Importantly, T cell IL-17 production correlates with T2D severity (as measured by HbA1c), further highlighting a likely relationship between the Th17 T cell population and metabolic imbalance.
Th17 cells may also have unappreciated indirect roles in the transition from euglycemic obesity to insulin resistance due to the plieotropic functions of IL-17. IL-17 can induce production of IL-8, a strong neutrophil chemoattractant,84 thus regulates immune cell infiltration into the expanding adipose tissue in response to HFD. However, the importance of Th17-mediated neutrophil recruitment, presumably following the initial (3–7 days post-HFD) neutrophil recruitment to adipose tissue before T-cell infiltration,6 is unknown.
The processes perpetuating the loss of metabolic regulation in only a subset of obese individuals are not understood, but focus on human inflammatory T cells has yielded some insight into this clinically critical question. Our detailed analysis of IL-17 showed that although stimulated T cells from both obese/non-diabetic (ND) and obese/T2D patients activate the IL-17 gene to similar levels, T cells from obese T2D donors have significantly elevated IL-17 protein production compared with T cells from obese/ND donors. These data indicate that T cells from ND donors have an additional layer of regulation that prevents excessive IL-17 protein production. Elevated IL-17 production despite structurally similar Th17 promoter structures, combined with a correlation between Th17 cells and body mass index in obese/T2D but not in obese/insulin-sensitive subjects, bring up the intriguing possibility that loss of specific layers of mRNA regulation in the immune system promotes the transition from insulin sensitive to insulin resistant. For example, temporal regulation of activating transcription factors, as we published for IL-1β,85 could shorten a normally sustained signal, or could alter the recruitment of repressive factors. It is also possible that the post-transcriptional mechanisms common to many cytokine mRNAs may yield more moderate levels of T-cell IL-17 in insulin sensitive vs. T2D individuals. Although our data only address the role of Th17 cells, similar mechanisms may apply to other cell types, leading to a global dysregulation in pro-inflammatory cytokine production. In addition, the contribution of coding or non-coding polymorphisms in many immunologically-active genes86 is likely important in human studies. We propose that further studies investigating potential roles of inflammatory T cells (and other immune cells), will assist clinicians in preventing the transition from obese/insulin sensitive to obese/T2D.
The role of regulatory T cells in T2D
Several studies expanded the demonstration that T-cell subset imbalance promotes inflammation thus insulin resistance in DIO mice. As outlined above, pro-inflammatory Th1 and Th17 cells increase in blood and adipose tissue of obese/insulin-resistant mice and humans. Importantly, elevated pro-inflammatory T-cell subsets are reinforced by the natural depletion of CD4+ Foxp3+ regulatory T cells (Tregs) in adipose tissue from insulin-resistant mice52,87 and in blood from T2D patients.74 Importantly, ex vivo or in vivo expanded Tregs protect against inflammation and insulin resistance87,88 at least in mice. These data indicate Tregs overall inhibit T2D and promote metabolic health. Further evidence supporting this conclusion is that Tregs can reverse the Th1-mediated pathology,81 again indicating the importance of T-cell compartment balance in the regulation of adipose tissue homeostasis. Although mouse studies indicated that Th2 cells can also counter insulin resistance in DIO,81 our studies on human T cells from T2D patients identified similar IL-4 levels in supernatants of T cells from patients and ND donors,74 questioning the relevance of Th2 function to disease. Overall, studies in both mice and humans identify T-cell subset imbalances that either promote or associate, respectively, with insulin resistance.
The overall role of T cells in obesity and T2D remains controversial
Not all studies have supported the expected conclusion that Th1 and Th17 cells would promote insulin resistance and macrophage infiltration, while Th2 and Tregs cells would counter inflammation, hence disease. Contrary to the studies mentioned above, some DIO mouse model studies have shown increased proportions of CD3+ T cells in adipose tissue but in the absence of a significant increase in CD4+ or CD8+ T cells.49 This work, however, showed IFNγ, RANTES and IL-12p40 secretion were elevated after 20 weeks of HFD. In these studies, IL-4 levels were also decreased starting at 4 weeks and the decrease continued throughout the remaining time frame of the study (20 weeks). Due to the presence of dramatically increased numbers of CD11c+ cells before CD3+ cell infiltration in the adipose tissue, the authors concluded that CD11c+ adipose-tissue macrophage recruitment is T-cell-enrichment independent.49 This conclusion directly contradicts work demonstrating CD8+ T-cells-induced macrophage recruitment to adipose tissue52 and that lymphocytes infiltrate expanding adipose tissue before macrophage infiltration.7 Additional contradictions on the role of T cells in HFD also emerge in a comparison between work demonstrating no change in the percentage of CD4+ cells in adipose tissue from DIO mice,52 and several reports demonstrating an increase in CD4+ T-cell subsets in expanded adipose tissue.81,82 Although these studies use a similar model of DIO, differences in feeding protocols, length of HFD, or calculation methods used for flow cytometric results could induce variability between these studies.
Roles for B cells in T2D inflammation
Antibodies have roles in many autoimmune inflammatory diseases, including lupus and type 1 diabetes. However, the lack of definitive evidence for an autoimmune component of T2D has limited interest in defining a role for antibodies in T2D, despite identification of, for example, anti-IL-6 or anti-GAD antibodies in patients with a pre-disposition or diagnosis of T2D.89,90 Recent findings support the idea that autoantibodies to important cellular components, such as G-coupled receptors and Rho GTPases, may associate with vascular complications common in T2D.91–93 However, the interest in classifying T2D as an auto-immune disease with a B-cell/auto-antibody component has been recently ignited by work demonstrating signatures of antigen-driven T-cell expansion in mouse models of obesity and T2D,81,82,87,94 presumably due to an elevated/inappropriate immune response to some internal antigen that activates T cells during initiation of disease and/or in response to disease. The putative antigen may be produced by apoptosing adipocytes in obese adipose tissue.95 Alternatively, antigen-dependent T-cell expansion could be a response to an unidentified infectious agent that has a role in T2D etiology or ongoing pathogenesis. Recent work has shown islets of T2D patients contain viral capsid protein,96 thus fueling the possibility that an infectious agent has a role in insulin resistance and concomitant oligoclonal T-cell expansion.
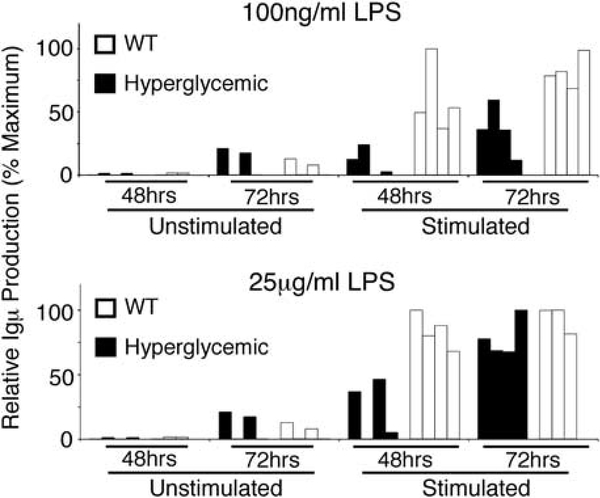
Although antigen-driven T-cell expansion does not definitely indicate that T2D has an auto-immune component, a selectively expanded T cell repertoire does support the existence of a T-cell target antigen that is either endogenous, such as antigens identified by anti-DNA antibody tests, or exogenous, such as bacterial antigens from the gut flora or invading viruses. Regardless, the hint of antigen-specific T-cell expansions begs the question of the existence of an auto-reactive B-cell expansion in T2D. This analysis has not been reported, but our preliminary work indicates that B-cell immunoglobulin production is indeed altered by hyperglycemia, one common outcome of uncontrolled T2D. Hyperglycemia, even in the absence of other components of the metabolic syndrome, can temporally alter B-cell responses. Stimulation of total spleen cells from Akita mice, a model of hyperglycemia due to insulin misfolding,97 results in delayed Igμ production (Figure 1). This difference was especially obvious under more modest stimulation conditions (top panel). Taken together with the likely antigen-dependent expansion of T cells in DIO mice,81,87 our work suggests that re-visiting the idea of T2D as an auto-immune disease with alterations in antibody production/function might reveal new paradigms in understanding disease pathogenesis.
Figure 1.
Hyperglycemia alters B-cell immunoglobulin secretion. Splenic B cells from the Akita mouse, a model of hyperglycemia, were purified by magnetic-bead-mediated negative selection, then incubated with E. coli LPS as a stimulus in moderate (top panel) or high (bottom panel) concentrations. IgM secretion was measured by ELISA and plotted as a function of maximal IgM concentration measured in the assay. Unstimulated cell supernatants are shown as a control (leftmost bars in both panels). IgM secretion 48–72 h post-LPS are shown from four independent determinations from C57BL/6 (wild-type; white bars) or Akita (on a C57BL/6 background; black bars). Note that IgM production/secretion is delayed in hyperglycemic vs wild-type samples.
In contrast to the strong association between altered myeloid or T-cell function and T2D, antibody-dependent or -independent roles for B cells in T2D have been addressed in very few studies. Recent studies in DIO mice demonstrated that B cells infiltrate the adipose tissue in an early response to DIO, followed by T cells, then macrophages,7 although as discussed above, this order is controversial.49 RAG-null mice (lacking both B and T lymphocytes) have an elevated number of macrophages in the adipose tissue late in DIO compared with the numbers in lymphocyte-sufficient controls, indicating cellular infiltration into adipose tissue is a highly regulated process perhaps orchestrated by early B-cell infiltration.7 Furthermore, RAG-null mice responded to DIO by increased weight gain, accumulation of more epididymal fat (a leading measure of altered lipid metabolism) and larger diameter adipocytes (a second measure of changes in lipid metabolism) compared with RAG-sufficient mice.81 As discussed above, follow-up analyses in these reports showed T cells have non-redundant roles in insulin resistance.52,81,87 However, the function of B cells and regulatory B cells (Bregs; refs.98–103) in these mice was not reported.
B cells are demonstrated sources of cytokines both in healthy individuals and those with chronic inflammatory disease, with IL-10 identified as the B-cell cytokine most commonly implicated in unresolved inflammation. IL-10 is usually considered anti-inflammatory, although it has pro-inflammatory functions in some circumstances (that is, upregulation of surface TLR2; ref.104). IL-10-producing human B cells arise upon stimulation through surface Ig alone or in combination with CD40,105 or upon TLR-mediated stimulation.106 Studies in mice have identified IL-10-producing B cells as a separate B-cell subpopulation, designated as B10 cells, or as a more inclusive subset designated as regulatory B cells/Bregs.98,100,101,107–109 Evidence for a human equivalent of Bregs was first uncovered in a population of transitional B cells that might protect against inflammatory disease in humans.110 Additional studies have confirmed the existence of anti-inflammatory human B-cell populations, although the markers associated with this subset remain controversial.102,103 Breg cells may be identical to the CD27—IL-10-producing B cells that repopulate multiple sclerosis patients following B-cell depletion,111 or the B cells that repopulate B-cell-depleted lupus and rheumatoid arthritis patients.110,112–114
Our recently published work suggested that lack of B-cell IL-10 in T2D patients may compound elevated pro-inflammatory cytokine production by cells in the myeloid and T-cell compartments of the same individuals. B cells from T2D patients, in contrast to B cells from ND donors, fail to secrete IL-10 in response to stimulation through TLR2, TLR4 or TLR9.67 Thus the altered IL-10 levels highlighted by genetic studies in T2D patients,115 may originate, at least in part, from lack of B-cell IL-10, making B-cell deficiencies physiologically dominant in inflammation as demonstrated for other chronic diseases. Interestingly, monocytes from T2D and ND donors produce similar levels of IL-10, at least in initial response to activating stimuli (Jagannathan-Bogdan et al., unpublished observations). These data indicate that monocytes fail to compensate for loss of B-cell IL-10 in T2D. Although neither IL-10 nor B-cell IL-10 has been definitively linked to T2D etiology or pathogenesis, the role of B-cell IL-10 in other inflammatory diseases, such as experimental autoimmune encephalomyelitis (EAE)/multiple sclerosis and arthritis, suggests that lack of IL-10 is a strong candidate mechanism for elevated inflammation in T2D.111,116,117 A likely regulatory role for IL-10 in T2D is further supported by the demonstration that low IL-10 production by human peripheral blood mononuclear cells positively associates with the incidence of metabolic syndrome and T2D, at least in geriatric individuals.115 Because IL-10-producing Bregs can moderate inflammatory disease by blocking pro-inflammatory Th1 differentiation (in arthritis and EAE, the latter a model for multiple sclerosis116,117), our identification of elevated Th1 function in T2D patients raises the possibility that the Bregs similarly hold Th1 cells at bay to maintain metabolic homeostasis.
B cells as sources of pro-inflammatory cytokines in T2D
In addition to anti-inflammatory IL-10, B cells are important sources of additional cytokines that exhibit generally pro-inflammatory biological functions.105,111,118–120 IL-6 is a second prototypic B-cell cytokine implicated in inflammatory diseases, although it may have an unexpected protective role in T2D, as outlined above. IL-6 is a multi-functional cytokine, and polymorphisms in IL-6 have been implicated in the transition from glucose intolerance to T2D.86 In addition to its inflammatory actions, elevated serum IL-6 probably has roles in glucose metabolism through skeletal muscle stimulation.121,122 Our data indicate that B cells from T2D patients produce ND amounts of IL-6,67 suggesting that B-cell IL-6 may have an insignificant role in T2D, leaving the possibility of changes in other sources of IL-6 uninvestigated.
We have also identified IL-8 as an unexpected pro-inflammatory chemokine produced by B cells from T2D patients, although the role of IL-8 in T2D has not been rigorously addressed. The general increase in IL-8 secretion in B cells from multiple classes of inflammatory disease patients in response to TLR ligands (reviewed in ref.123), demonstrates that TLR-mediated B-cell IL-8 secretion is a shared feature of B cells from inflammatory disease patients regardless of the downstream effects of IL-8.
A comprehensive model for the role of immune system cells in T2D
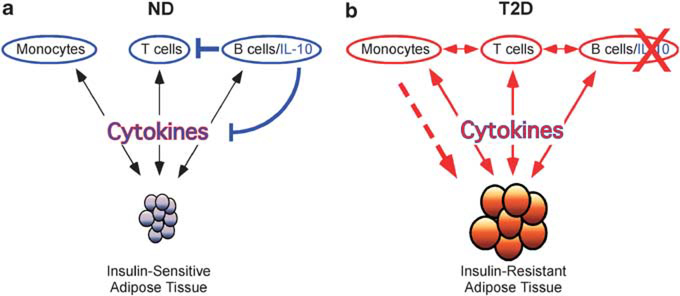
Multiple pieces of evidence indicate a potential pro-inflammatory feed-forward loop between adipocytes and immune system cells in obesity and T2D. One potential working model describing these interactions is shown in Figure 2. In ND individuals, cytokines produced by monocytes, T cells and B cells affect adipose tissue and vise versa in a non-pathogenic homeostasis (Figure 2a). These cells and cytokines are further regulated by B-cell IL-10, as indicated by the blue lines. With increasing obesity, circulating free fatty acids increase.34 These free fatty acids can activate monocytes and B cells through TLR4, inducing inflammatory cytokine production.35 (Figure 2b, red downward pointing arrows). Increasing obesity also induces necrosis of adipocytes leading to recruitment of monocytes into the adipose tissue (Figure 2b dotted line), where they become resident macrophages and secrete inflammatory cytokines.7,124 This pro-inflammatory status is reinforced systemically via FFA-mediated activation of immune cells, thus propagating chronic inflammation and insulin resistance, both of which are hallmarks of T2D.
Figure 2.
Loss of immunological homeostasis in T2D. (a) Monocytes, T cells and B cells produce cytokines (downward-pointing black arrows), which are actively regulated by B-cell IL-10 (blue line). Adipose tissue also produces inflammatory mediators (upward-pointing black arrows) that are subject to B-cell IL-10 regulation. Homeostasis between adipose tissue and the immune system is maintained by balance among functionally opposed cytokines and results in the maintenance of insulin sensitivity, indicated by small blue adipocytes. (b) Immune system cells are activated (indicated in red) by diet-induced changes in ligands such as free fatty acids (FFAs), which stimulate monocytes and B cells via Toll-like receptors. Activated monocytes and B cells produce pro-inflammatory cytokines (red downward-pointing arrows) that influence adipose tissue function, resulting in increased inflammatory adipokine secretion (red upward-pointing arrows). Increasing necrosis in the expanding adipose tissue can also promote monocyte migration and subsequent macrophage differentiation in adipose tissue (red dotted arrow). Increasing obesity also directly affects adipose tissue by inducing adipocytes to produce inflammatory mediators (red upward-pointing arrows), which feed forward on inflammatory immune system cells. Moderation of inflammation by IL-10 is decreased owing to loss of B-cell IL-10 production (red ‘X’ over IL-10) in T2D patients.
Over nutrition and obesity can also induce adipose tissue to secrete pro-inflammatory cytokines such as IL-6 and IL-1β125,126 (Figure 2b, red upwards pointing arrows). These two cytokines are known to induce pro-inflammatory Th17 differentiation.126–129 Increased secretion of IL-6, along with IL-1β and (perhaps surprisingly) TGFβ theoretically leads to systemic inflammation and a pro-Th17 skewing milieu, which in turn results in elevated levels of IL-17. T2D patients also have elevated levels of serum IL-12,130,131 a cytokine that promotes Th1 differentiation and elevated IFNγ production.132–138 Importantly, adipocytes are also sensitive targets of pro-inflammatory T-cell cytokines (Figure 2b, red downward pointing arrow). Adipocytes express significant levels of the IL-17 receptors IL-17RA and IL-17RC, and respond to IL-17 by secreting IL-6, which may reinforce the IL-6 produced under hyper-caloric conditions.139 Similarly, adipocytes respond to IFNγ by attenuating JAK/STAT activation hence insulin signaling,140 which leads to insulin resistance. Overall, these data indicate a pro-inflammatory feed-forward loop between adipose tissue and T cells potentially inducing a ‘snowball effect’ consequently transitioning an obese insulin-sensitive individual to an obese insulin-resistant T2D patient.
Elevated pro-inflammatory cytokine production is primarily thought to be produced by activated macrophages in T2D patients.34 Recent studies also indicate cross-talk between monocytes and T cells, in which monocyte-T cell interactions induce elevated levels of IL-17 (Figure 2b, horizontal arrows74). Whether these T cells have a low activation threshold due to the pro-inflammatory milieu found in T2D patients, or if hyper-activated macrophages alone can induce elevated IL-17 production from these T cells is still unknown. Preliminary data from our group suggest B cells inhibit elevated IL-17 production, perhaps via IL-10 (Figure 2a, horizontal blue line). Taken together, these data indicate two levels of immune deregulation in T2D patients that may influence the pro-inflammatory feed-forward loop: (1) hyper-activation of monocytes/macrophages and T cells; and (2) loss of anti-inflammatory action of B-cell IL-10, which likely lead to an exacerbated pro-inflammatory milieu and in turn, insulin resistance. Additional work on the specific roles of immune cells in exacerbating chronic inflammation (perhaps via adipocytes) is essential towards furthering our understanding of the complex interplay between these two seemingly unrelated systems in T2D.
The promise of lymphocyte depletion as a therapy for T2D
Due to the well-established role for T cells in inflammatory diseases, the development of anti-CD3 therapies has proceeded in earnest, and the results have been noteworthy. In the NOD mouse (a model of type 1 diabetes/T1D), anti-CD3 antibody administration delayed the onset of T1D. As treatment was retracted, the disease re-emerged.141 The initial delay of disease onset depended on monoclonal ‘induced’ Treg expansion into niches that are normally Treg-low.141 Furthermore Treg expansion depended on IL-2 and IL-7Ra, but TGFβ was dispensable.141 Another study showed that human peripheral blood mononuclear cells stimulated with humanized anti-CD3 antibodies produced lower amounts of IFNγ and increased amounts of IL-10, compared with results from stimulation with the murine anti-CD3 antibody.142 These humanized antibodies also induced expression of Foxp3, indicating development of ‘natural’ Tregs or expansion of induced Tregs, although the function of these cells was not tested.142 Apart from the mouse studies, human clinical trials using monoclonal anti-CD3 antibodies have also yielded promise in treating T1D. Anti-CD3 antibody therapy in T1D patients for 12–18 months maintained or improved insulin production, and improved glycosylated hemoglobin levels while decreasing insulin dosage needs. However, the mechanism of action was not clear.142,143 Thus far, anti-CD3 therapies have been most rigorously tested in autoimmune T1D, but the emerging appreciation of the autoimmune component of T2D and our data indicating changes in the Treg compartment in T2D patients demand the field to consider appropriateness of anti-CD3 as a treatment. Proof of concept studies showed anti-CD3 therapy in DIO mice improved fasting glucose and insulin levels, and greatly improved glucose tolerance and insulin sensitivity.81 Further testing of these anti-CD3 antibodies would indicate probable efficacy for increasing the Treg:Th1/17 ratio in vivo to control the chronic inflammatory component (thus insulin resistance) in T2D. A similar argument for considering B-cell ablation as a treatment for T2D has been previously described.123
Although the very idea of lymphocyte ablation raises fears of life-threatening consequences of immunosuppressive therapy, demonstrated success and high safety profiles of CD20-mediated B cell depletion in rheumatic disease and multiple sclerosis treatment144–146 raise the possibility that lymphocyte depletion does not lead to wide-spread devastation of immune protection. By its nature, anti-CD20 therapy removes only a subset of B lineage cells, leaving the plasma cell compartment largely intact to supply a broad antibody repertoire in adults. Perhaps the development of anti-lymphocyte therapies, with selective action on pathogenic subsets or with the ability to promote protective Treg function, will increase the number of available options for T2D treatment.
Future directions
Immune cell-mediated inflammation has been implicated in both the etiology and ongoing pathogenesis of insulin resistance and T2D. Although macrophages are important sources of diabetogenic cytokines and adipose remodeling activity, recent work from multiple laboratories has demonstrated definitive roles for lymphocytes in T2D. Multiple T-cell subsets and T-cell cytokines have critical roles in the regulation of insulin resistance. The importance of T cells in animal models of T2D is echoed by changes in the T-cell compartment in T2D patients. In contrast, strong evidence supporting a pro-inflammatory role for B cells in obesity and insulin resistance has not been reported, although characterization of differences in B-cell function between T2D and ND donors strongly suggests they too may contribute to the overall inflammatory environment through decreased production of IL-10. Immunological studies have also raised the possibility of a previously unappreciated auto-immune component of T2D. Although the treatment arsenal available to clinicians to avoid long-term devastating consequences of obesity and T2D remain limited, developments to potentially counter these effects with immunomodulatory drugs are being reported at an ever-accelerating pace. More rigorous demonstrations of roles for immune cells, and specifically lymphocytes, in human T2D would allow endocrinologists to take advantage of FDA-approved immunomodulatory drugs (for example, rituxan) in the short term, and to be first in line to test efficacy of new immune system-targeted drugs currently in non-metabolic disease trials. Such a fundamental shift in treatment options may offer new hope toward curbing the T2D epidemic before doomsday predictions of incidence and cost become a reality.
Acknowledgements
This work was supported by NIH R01 AI54611, NIH R21 DK089270 and a Research Grant from the American Diabetes Association. The authors thank Susan Fried, Gerald Denis, Jongsoon Lee, Marty Obin, and Marie McDonnell for valuable conversations related to this work.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Strissel KJ, Stancheva Z, Miyoshi H, Perfield II JW, DeFuria J, Jick Z et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 2007; 56: 2910–2918. [DOI] [PubMed] [Google Scholar]
- 2.Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M et al. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol 2008; 28: 1654–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Senn JJ. Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J Biol Chem 2006; 281: 26865–26875. [DOI] [PubMed] [Google Scholar]
- 4.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006; 116: 3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowie A, O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal generators for pro-inflammatory interleukins and microbial products. J Leukoc Biol 2000; 67: 508–514. [DOI] [PubMed] [Google Scholar]
- 6.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res 2008; 49: 1894–1903. [DOI] [PubMed] [Google Scholar]
- 7.Duffaut C, Galitzky J, Lafontan M, Bouloumie A. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem Biophys Res Commun 2009; 384: 482–485. [DOI] [PubMed] [Google Scholar]
- 8.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112: 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 2006; 116: 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 2006; 116: 1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fantuzzi G Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol 2005; 115: 911–919; quiz 920. [DOI] [PubMed] [Google Scholar]
- 12.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood 2000; 96: 1723–1732. [PubMed] [Google Scholar]
- 13.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation 2000; 102: 1296–1301. [DOI] [PubMed] [Google Scholar]
- 14.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 1998; 394: 897–901. [DOI] [PubMed] [Google Scholar]
- 15.Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 2007; 356: 1517–1526. [DOI] [PubMed] [Google Scholar]
- 16.Berchtold LA, Larsen CM, Vaag A, Faulenbach M, Workman CT, Kruhoffer M et al. IL-1 receptor antagonism and muscle gene expression in patients with type 2 diabetes. Eur Cytokine Netw 2009; 20: 81–87. [DOI] [PubMed] [Google Scholar]
- 17.Larsen CM, Faulenbach M, Vaag A, Ehses JA, Donath MY, Mandrup-Poulsen T. Sustained effects of interleukin-1-receptor antagonist treatment in type 2 diabetes mellitus. Diabetes Care 2009; 32: 1663–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yazdani-Biuki B, Stelzl H, Brezinschek HP, Hermann J, Mueller T, Krippl P et al. Improvement of insulin sensitivity in insulin resistant subjects during prolonged treatment with the anti-TNF-alpha antibody infliximab. Eur J Clin Invest 2004; 34: 641–642. [DOI] [PubMed] [Google Scholar]
- 19.Ofei F, Hurel S, Newkirk J, Sopwith M, Taylor R. Effects of an engineered human anti-TNF-alpha antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes 1996; 45: 881–885. [DOI] [PubMed] [Google Scholar]
- 20.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 1997; 389: 610–614. [DOI] [PubMed] [Google Scholar]
- 21.Sauter NS, Schulthess FT, Galasso R, Castellani LW, Maedler K. The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology 2008; 149: 2208–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osborn O, Brownell SE, Sanchez-Alavez M, Salomon D, Gram H, Bartfai T. Treatment with an interleukin 1 beta antibody improves glycemic control in diet-induced obesity. Cytokine 2008; 44: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jura J, Wegrzyn P, Korostynski M, Guzik K, Oczko-Wojciechowska M, Jarzab M et al. Identification of interleukin-1 and interleukin-6-responsive genes in human monocyte-derived macrophages using microarrays. Biochim Biophys Acta 2008; 1779: 383–389. [DOI] [PubMed] [Google Scholar]
- 24.Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 2002; 8: 75–79. [DOI] [PubMed] [Google Scholar]
- 25.Ehses JA, Lacraz G, Giroix MH, Schmidlin F, Coulaud J, Kassis N et al. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc Natl Acad Sci USA 2009; 106: 13998–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 2005; 11: 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993; 259: 87–91. [DOI] [PubMed] [Google Scholar]
- 28.Laharrague P, Fontanilles AM, Tkaczuk J, Corberand JX, Penicaud L, Casteilla L. Inflammatory/haematopoietic cytokine production by human bone marrow adipocytes. Eur Cytokine Netw 2000; 11: 634–639. [PubMed] [Google Scholar]
- 29.Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab 2007; 6: 386–397. [DOI] [PubMed] [Google Scholar]
- 30.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 2005; 11: 191–198. [DOI] [PubMed] [Google Scholar]
- 31.Giulietti A, Stoffels K, Decallonne B, Overbergh L, Mathieu C. Monocytic expression behavior of cytokines in diabetic patients upon inflammatory stimulation. Ann N Y Acad Sci 2004; 1037: 74–78. [DOI] [PubMed] [Google Scholar]
- 32.Dasu MR, Devaraj S, Park S, Jialal I. Increased toll-like receptor activation and TLR ligands in recently diagnosed type 2 diabetes subjects. Diabetes Care 2010; 33: 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Creely SJ, McTernan PG, Kusminski CM, Fisher M, Da Silva NF, Khanolkar M et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab 2007; 292: E740–E747. [DOI] [PubMed] [Google Scholar]
- 34.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963; 1: 785–789. [DOI] [PubMed] [Google Scholar]
- 35.Fraze E, Donner CC, Swislocki AL, Chiou YA, Chen YD, Reaven GM. Ambient plasma free fatty acid concentrations in noninsulin-dependent diabetes mellitus: evidence for insulin resistance. J Clin Endocrinol Metab 1985; 61: 807–811. [DOI] [PubMed] [Google Scholar]
- 36.Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes 1988; 37: 1020–1024. [DOI] [PubMed] [Google Scholar]
- 37.Salvi GE, Beck JD, Offenbacher S. PGE2, IL-1 beta, and TNF-alpha responses in diabetics as modifiers of periodontal disease expression. Ann Periodontol 1998; 3: 40–50. [DOI] [PubMed] [Google Scholar]
- 38.Wen Y, Gu J, Li SL, Reddy MA, Natarajan R, Nadler JL. Elevated glucose and diabetes promote interleukin-12 cytokine gene expression in mouse macrophages. Endocrinology 2006; 147: 2518–2525. [DOI] [PubMed] [Google Scholar]
- 39.Stephens JW, Hurel SJ, Lowe GD, Rumley A, Humphries SE. Association between plasma IL-6, the IL6 −174G>C gene variant and the metabolic syndrome in type 2 diabetes mellitus. Mol Genet Metab 2006; 90: 422–428. [DOI] [PubMed] [Google Scholar]
- 40.Foss-Freitas MC, Foss NT, Donadi EA, Foss MC. In vitro TNF-alpha and IL-6 production by adherent peripheral blood mononuclear cells obtained from type 1 and type 2 diabetic patients evaluated according to the metabolic control. Ann N Y Acad Sci 2006; 1079: 177–180. [DOI] [PubMed] [Google Scholar]
- 41.Virca GD, Kim SY, Glaser KB, Ulevitch RJ. Lipopolysaccharide induces hyporesponsiveness to its own action in RAW 264.7 cells. J Biol Chem 1989; 264: 21951–21956. [PubMed] [Google Scholar]
- 42.Yoza B, LaRue K, McCall C. Molecular mechanisms responsible for endotoxin tolerance. Prog Clin Biol Res 1998; 397: 209–215. [PubMed] [Google Scholar]
- 43.Poggi M, Bastelica D, Gual P, Iglesias MA, Gremeaux T, Knauf C et al. C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia 2007; 50: 1267–1276. [DOI] [PubMed] [Google Scholar]
- 44.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 2007; 56: 1986–1998. [DOI] [PubMed] [Google Scholar]
- 45.Poggi M, Bastelica D, Gual P, Iglesias MA, Gremeaux T, Knauf C et al. C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia 2007; 50: 1267–1276. [DOI] [PubMed] [Google Scholar]
- 46.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J et al. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res 2007; 100: 1589–1596. [DOI] [PubMed] [Google Scholar]
- 47.Hosoi T, Yokoyama S, Matsuo S, Akira S, Ozawa K. Myeloid differentiation factor 88 (MyD88)-deficiency increases risk of diabetes in mice. PLoS One 2010; 5: e12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coenen KR, Gruen ML, Lee-Young RS, Puglisi MJ, Wasserman DH, Hasty AH. Impact of macrophage toll-like receptor 4 deficiency on macrophage infiltration into adipose tissue and the artery wall in mice. Diabetologia 2009; 52: 318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strissel KJ, Defuria J, Shaul ME, Bennett G, Greenberg AS, Obin MS. T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity (Silver Spring) 2010; 18: 1918–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation 2007; 115: 1029–1038. [DOI] [PubMed] [Google Scholar]
- 51.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 2008; 32: 451–463. [DOI] [PubMed] [Google Scholar]
- 52.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009; 15: 914–920. [DOI] [PubMed] [Google Scholar]
- 53.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003; 112: 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol 2005; 25: 2062–2068. [DOI] [PubMed] [Google Scholar]
- 55.Giulietti A, van Etten E, Overbergh L, Stoffels K, Bouillon R, Mathieu C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-Dihydroxyvitamin D(3) works as anti-inflammatory. Diabetes Res Clin Pract 2007; 77: 47–57. [DOI] [PubMed] [Google Scholar]
- 56.Mosser DM. The many faces of macrophage activation. J Leukoc Biol 2003; 73: 209–212. [DOI] [PubMed] [Google Scholar]
- 57.Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet-induced obesity in mice. Diabetes 2010; 59: 1171–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park S, Rich J, Hanses F, Lee JC. Defects in innate immunity predispose C57BL/6J-Leprdb/Leprdb mice to infection by Staphylococcus aureus. Infect Immun 2009; 77: 1008–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stegenga ME, van der Crabben SN, Blumer RM, Levi M, Meijers JC, Serlie MJ et al. Hyperglycemia enhances coagulation and reduces neutrophil degranulation, whereas hyperinsulinemia inhibits fibrinolysis during human endotoxemia. Blood 2008; 112: 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ayilavarapu S, Kantarci A, Fredman G, Turkoglu O, Omori K, Liu H et al. Diabetes-induced oxidative stress is mediated by Ca2+-independent phospholipase A2 in neutrophils. J Immunol 2010; 184: 1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stegenga ME, van der Crabben SN, Dessing MC, Pater JM, van den Pangaart PS, de Vos AF et al. Effect of acute hyperglycaemia and/or hyperinsulinaemia on proinflammatory gene expression, cytokine production and neutrophil function in humans. Diabet Med 2008; 25: 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hand WL, Hand DL, Vasquez Y. Increased polymorphonuclear leukocyte respiratory burst function in type 2 diabetes. Diabetes Res Clin Pract 2007; 76: 44–50. [DOI] [PubMed] [Google Scholar]
- 63.Gupta A, Tripathi AK, Tripathi RL, Madhu SV, Banerjee BD. Advanced glycosylated end products-mediated activation of polymorphonuclear neutrophils in diabetes mellitus and associated oxidative stress. Indian J Biochem Biophys 2007; 44: 373–378. [PubMed] [Google Scholar]
- 64.Omori K, Ohira T, Uchida Y, Ayilavarapu S, Batista EL Jr, Yagi M et al. Priming of neutrophil oxidative burst in diabetes requires preassembly of the NADPH oxidase. J Leukoc Biol 2008; 84: 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karima M, Kantarci A, Ohira T, Hasturk H, Jones VL, Nam BH et al. Enhanced superoxide release and elevated protein kinase C activity in neutrophils from diabetic patients: association with periodontitis. J Leukoc Biol 2005; 78: 862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Al-Attas OS, Al-Daghri NM, Al-Rubeaan KA, da Silva NF, Sabico SL, Kumar S et al. Changes in endotoxin levels in T2DM subjects on anti-diabetic therapies. Cardiovasc Diabetol 2009; 8: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jagannathan M, McDonnell M, Liang Y, Hasturk H, Hetzel J, Rubin D et al. Toll-like receptors regulate B cell cytokine production in patients with diabetes. Diabetologia 2010; 53: 1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med 2006; 203: 1685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Furuzawa-Carballeda J, Vargas-Rojas MI, Cabral AR. Autoimmune inflammation from the Th17 perspective. Autoimmun Rev 2007; 6: 169–175. [DOI] [PubMed] [Google Scholar]
- 70.Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol 2008; 9: 166–175. [DOI] [PubMed] [Google Scholar]
- 71.Galicia G, Kasran A, Uyttenhove C, De Swert K, Van Snick J, Ceuppens JL. ICOS deficiency results in exacerbated IL-17 mediated experimental autoimmune encephalomyelitis. J Clin Immunol 2009; 29: 426–433. [DOI] [PubMed] [Google Scholar]
- 72.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol 2006; 177: 566–573. [DOI] [PubMed] [Google Scholar]
- 73.Pene J, Chevalier S, Preisser L, Venereau E, Guilleux MH, Ghannam S et al. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol 2008; 180: 7423–7430. [DOI] [PubMed] [Google Scholar]
- 74.Jagannathan-Bogdan M, McDonnell ME, Shin H, Rehman Q, Hasturk H, Apovian CM et al. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J Immunol 2011; 186: 1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zuniga LA, Shen WJ, Joyce-Shaikh B, Pyatnova EA, Richards AG, Thom C et al. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol 2010; 185: 6947–6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev 2003; 14: 155–174. [DOI] [PubMed] [Google Scholar]
- 77.Schwandner R, Yamaguchi K, Cao Z. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J Exp Med 2000; 191: 1233–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology 2010; 129: 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity 2004; 21: 467–476. [DOI] [PubMed] [Google Scholar]
- 80.Mosser DM, Edwards JR Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8: 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med 2009; 15: 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol 2010; 185: 1836–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Winer S, Paltser G, Chan Y, Tsui H, Engleman E, Winer D et al. Obesity predisposes to Th17 bias. Eur J Immunol 2009; 39: 2629–2635. [DOI] [PubMed] [Google Scholar]
- 84.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med 1996; 183: 2593–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liang M, Zhang Y, McDevit M, Marecki S, Nikolajczyk B. The IL-1 beta gene is transcribed from a poised promoter architecture in monocytes. J Biol Chem 2006; 281: 9227–9237. [DOI] [PubMed] [Google Scholar]
- 86.Kubaszek A, Pihlajamaki J, Komarovski V, Lindi V, Lindstrom J, Eriksson J et al. Promoter polymorphisms of the TNF-alpha (G-308A) and IL-6 (C-174G) genes predict the conversion from impaired glucose tolerance to type 2 diabetes: the Finnish Diabetes Prevention Study. Diabetes 2003; 52: 1872–1876. [DOI] [PubMed] [Google Scholar]
- 87.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009; 15: 930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ilan Y, Maron R, Tukpah AM, Maioli TU, Murugaiyan G, Yang K et al. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci USA 2010; 107: 9765–9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lundgren VM, Isomaa B, Lyssenko V, Laurila E, Korhonen P, Groop LC et al. GAD antibody positivity predicts type 2 diabetes in an adult population. Diabetes 2010; 59: 416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fosgerau K, Galle P, Hansen T, Albrechtsen A, Rieper Cde L, Pedersen BK et al. Interleukin-6 autoantibodies are involved in the pathogenesis of a subset of type 2 diabetes. J Endocrinol 2010; 204: 265–273. [DOI] [PubMed] [Google Scholar]
- 91.Fredrikson GN, Anand DV, Hopkins D, Corder R, Alm R, Bengtsson E et al. Associations between autoantibodies against apolipoprotein B-100 peptides and vascular complications in patients with type 2 diabetes. Diabetologia 2009; 52: 1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zimering MB, Pan Z. Autoantibodies in type 2 diabetes induce stress fiber formation and apoptosis in endothelial cells. J Clin Endocrinol Metab 2009; 94: 2171–2177. [DOI] [PubMed] [Google Scholar]
- 93.Hempel P, Karczewski P, Kohnert KD, Raabe J, Lemke B, Kunze R et al. Sera from patients with type 2 diabetes contain agonistic autoantibodies against G protein-coupled receptors. Scand J Immunol 2009; 70: 159–160. [DOI] [PubMed] [Google Scholar]
- 94.Yang H, Youm YH, Vandanmagsar B, Rood J, Kumar KG, Butler AA et al. Obesity accelerates thymic aging. Blood 2009; 114: 3803–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alkhouri N, Gornicka A, Berk MP, Thapaliya S, Dixon LJ, Kashyap S et al. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J Biol Chem 2010; 285: 3428–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. The prevalence of enteroviral capsid protein vp1 immuno-staining in pancreatic islets in human type 1 diabetes. Diabetologia 2009; 52: 1143–1151. [DOI] [PubMed] [Google Scholar]
- 97.Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes 1997; 46: 887–894. [DOI] [PubMed] [Google Scholar]
- 98.Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol 2006; 176: 705–710. [DOI] [PubMed] [Google Scholar]
- 99.Mizoguchi A, Mizoguchi E, Smith RN, Preffer FI, Bhan AK. Suppressive role of B cells in chronic colitis of T cell receptor alpha mutant mice. J Exp Med 1997; 186: 1749–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 2002; 16: 219–230. [DOI] [PubMed] [Google Scholar]
- 101.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 2008; 28: 639–650. [DOI] [PubMed] [Google Scholar]
- 102.Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 2011; 117: 530–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity 2010; 32: 129–140. [DOI] [PubMed] [Google Scholar]
- 104.Ganley-Leal LM, Liu X, Wetzler LM. Toll-like receptor 2-mediated human B cell differentiation. Clin Immunol 2006; 120: 272–284. [DOI] [PubMed] [Google Scholar]
- 105.Duddy ME, Alter A, Bar-Or A. Distinct profiles of human B cell effector cytokines: a role in immune regulation? J Immunol 2004; 172: 3422–3427. [DOI] [PubMed] [Google Scholar]
- 106.Jagannathan M, Hasturk H, Liang Y, Shin H, Hetzel JT, Kantarci A et al. TLR cross-talk specifically regulates cytokine production by B cells from chronic inflammatory disease patients. J Immunol 2009; 183: 7461–7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol 2009; 182: 7459–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol 2007; 178: 7868–7878. [DOI] [PubMed] [Google Scholar]
- 109.Tian J, Zekzer D, Hanssen L, Lu Y, Olcott A, Kaufman DL. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J Immunol 2001; 167: 1081–1089. [DOI] [PubMed] [Google Scholar]
- 110.Anolik JH, Looney RJ, Lund FE, Randall TD, Sanz I. Insights into the heterogeneity of human B cells: diverse functions, roles in autoimmunity, and use as therapeutic targets. Immunol Res 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol 2007; 178: 6092–6099. [DOI] [PubMed] [Google Scholar]
- 112.Roll P, Palanichamy A, Kneitz C, Dorner T, Tony HP. Regeneration of B cell subsets after transient B cell depletion using anti-CD20 antibodies in rheumatoid arthritis. Arthritis Rheum 2006; 54: 2377–2386. [DOI] [PubMed] [Google Scholar]
- 113.Anolik JH, Barnard J, Owen T, Zheng B, Kemshetti S, Looney RJ et al. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis Rheum 2007; 56: 3044–3056. [DOI] [PubMed] [Google Scholar]
- 114.Anolik JH, Friedberg JW, Zheng B, Barnard J, Owen T, Cushing E et al. B cell reconstitution after rituximab treatment of lymphoma recapitulates B cell ontogeny. Clin Immunol 2007; 122: 139–145. [DOI] [PubMed] [Google Scholar]
- 115.van Exel E, Gussekloo J, de Craen AJ, Frolich M, Bootsma-Van Der Wiel A, Westendorp RG. Low production capacity of interleukin-10 associates with the metabolic syndrome and type 2 diabetes: the Leiden 85-Plus Study. Diabetes 2002; 51: 1088–1092. [DOI] [PubMed] [Google Scholar]
- 116.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol 2002; 3: 944–950. [DOI] [PubMed] [Google Scholar]
- 117.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med 2003; 197: 489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Harris DP, Goodrich S, Mohrs K, Mohrs M, Lund FE. Cutting edge: the development of IL-4-producing B cells (B effector 2 cells) is controlled by IL-4, IL-4 receptor alpha, and Th2 cells. J Immunol 2005; 175: 7103–7107. [DOI] [PubMed] [Google Scholar]
- 119.Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol 2000; 1: 475–482. [DOI] [PubMed] [Google Scholar]
- 120.Durali D, de Goer de Herve MG, Giron-Michel J, Azzarone B, Delfraissy JF, Taoufik Y. In human B cells, IL-12 triggers a cascade of molecular events similar to Th1 commitment. Blood 2003; 102: 4084–4089. [DOI] [PubMed] [Google Scholar]
- 121.Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes 2005; 54 (Suppl 2): S114–S124. [DOI] [PubMed] [Google Scholar]
- 122.Pickup JC, Chusney GD, Thomas SM, Burt D. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci 2000; 67: 291–300. [DOI] [PubMed] [Google Scholar]
- 123.Nikolajczyk BS. B cells as under-appreciated mediators of non-auto-immune inflammatory disease. Cytokine 2010; 50: 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006; 444: 860–867. [DOI] [PubMed] [Google Scholar]
- 125.Andriankaja OM, Barros SP, Moss K, Panagakos FS, DeVizio W, Beck J et al. Levels of serum interleukin (IL)-6 and gingival crevicular fluid of IL-1beta and prostaglandin E(2) among non-smoking subjects with gingivitis and type 2 diabetes. J Periodontol 2009; 80: 307–316. [DOI] [PubMed] [Google Scholar]
- 126.Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm 2006; 74: 443–477. [DOI] [PubMed] [Google Scholar]
- 127.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol 2007; 8: 942–949. [DOI] [PubMed] [Google Scholar]
- 128.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature 2008; 454: 350–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007; 132: 2169–2180. [DOI] [PubMed] [Google Scholar]
- 130.Wegner M, Winiarska H, Bobkiewicz-Kozlowska T, Dworacka M. IL-12 serum levels in patients with type 2 diabetes treated with sulphonylureas. Cytokine 2008; 42: 312–316. [DOI] [PubMed] [Google Scholar]
- 131.Tsiavou A, Degiannis D, Hatziagelaki E, Koniavitou K, Raptis SA. Intracellular IFN-gamma production and IL-12 serum levels in latent autoimmune diabetes of adults (LADA) and in type 2 diabetes. J Interferon Cytokine Res 2004; 24: 381–387. [DOI] [PubMed] [Google Scholar]
- 132.Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G et al. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med 1993; 177: 1199–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu CY, Demeure C, Kiniwa M, Gately M, Delespesse G. IL-12 induces the production of IFN-gamma by neonatal human CD4T cells. J Immunol 1993; 151: 1938–1949. [PubMed] [Google Scholar]
- 134.Yssel H, Fasler S, de Vries JE, de Waal Malefyt R. IL-12 transiently induces IFN-gamma transcription and protein synthesis in human CD4+ allergen-specific Th2T cell clones. Int Immunol 1994; 6: 1091–1096. [DOI] [PubMed] [Google Scholar]
- 135.Puddu P, Fantuzzi L, Borghi P, Varano B, Rainaldi G, Guillemard E et al. IL-12 induces IFN-gamma expression and secretion in mouse peritoneal macrophages. J Immunol 1997; 159: 3490–3497. [PubMed] [Google Scholar]
- 136.Kim E, Kim SH, Kim S, Kim TS. The novel cytokine p43 induces IL-12 production in macrophages via NF-kappaB activation, leading to enhanced IFN-gamma production in CD4+ T cells. J Immunol 2006; 176: 256–264. [DOI] [PubMed] [Google Scholar]
- 137.Gately MK, Warrier RR, Honasoge S, Carvajal DM, Faherty DA, Connaughton SE et al. Administration of recombinant IL-12 to normal mice enhances cytolytic lymphocyte activity and induces production of IFN-gamma in vivo. Int Immunol 1994; 6: 157–167. [DOI] [PubMed] [Google Scholar]
- 138.Nastala CL, Edington HD, McKinney TG, Tahara H, Nalesnik MA, Brunda MJ et al. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol 1994; 153: 1697–1706. [PubMed] [Google Scholar]
- 139.Shin JH, Shin DW, Noh M. Interleukin-17A inhibits adipocyte differentiation in human mesenchymal stem cells and regulates pro-inflammatory responses in adipocytes. Biochem Pharmacol 2009; 77: 1835–1844. [DOI] [PubMed] [Google Scholar]
- 140.McGillicuddy FC, Chiquoine EH, Hinkle CC, Kim RJ, Shah R, Roche HM et al. Interferon gamma attenuates insulin signaling, lipid storage, and differentiation in human adipocytes via activation of the JAK/STAT pathway J Biol Chem 2009; 284: 31936–31944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nishio J, Feuerer M, Wong J, Mathis D, Benoist C. Anti-CD3 therapy permits regulatory T cells to surmount T cell receptor-specified peripheral niche constraints. J Exp Med 2010; 207: 1879–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Silva HM, Vieira PM, Costa PL, Pimentel BM, Moro AM, Kalil J et al. Novel humanized anti-CD3 antibodies induce a predominantly immunoregulatory profile in human peripheral blood mononuclear cells. Immunol Lett 2009; 125: 129–136. [DOI] [PubMed] [Google Scholar]
- 143.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 2002; 346: 1692–1698. [DOI] [PubMed] [Google Scholar]
- 144.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 2004; 350: 2572–2581. [DOI] [PubMed] [Google Scholar]
- 145.Ng KP, Cambridge G, Leandro MJ, Edwards JC, Ehrenstein M, Isenberg DA. B cell depletion therapy in systemic lupus erythematosus: long-term follow-up and predictors of response. Ann Rheum Dis 2007; 66: 1259–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 2008; 358: 676–688. [DOI] [PubMed] [Google Scholar]