Abstract
Simple Summary
The improvement of porcine meat quality is one of the most challenging tasks in pig breeding. The identification and utilization of major genes and variations affecting meat quality traits has become a research hotspot in molecular breeding of pigs. In this study, we analyzed the characteristics of porcine Prospero-related homeobox 1 (Prox1) gene to lay a solid foundation for further functional study. The expression pattern of Prox1, as well as the correlation and association analysis of Prox1 with meat quality traits, indicates that Prox1 can be targeted to improve porcine meat quality traits.
Abstract
Prox1 is involved in muscle fiber conversion, adult-onset obesity, and type 2 diabetes. However, information regarding porcine Prox1 and its relationship with meat quality traits is still unknown. In this study, we characterized the full-length cDNA and proximal promoter of two transcript variants of porcine Prox1. Moreover, Prox1 was expressed abundantly in the skeletal muscle and its expression was higher in the soleus muscle than that in the biceps femoris muscle. Its expression pattern in the high and low meat color (redness) value a* groups was similar to that of myoglobin and MyHC I, but opposed to that of MyHC IIB. Importantly, there was a significant positive correlation between Prox1 expression and meat color (redness) value a* (r = 0.3845, p = 0.0394), and a significant negative correlation between Prox1 expression and drip loss (r = −0.4204, p = 0.0232), as well as the ratio of MyHC IIB to MyHC I expression (r = −0.3871, p = 0.0380). In addition, we found that the polymorphisms of three closely linked SNPs in Prox1 promoter 1 were significantly associated with pH24h in a pig population. Taken together, our data provide valuable insights into the characteristics of porcine Prox1 and indicate that Prox1 is a promising candidate gene affecting meat quality traits.
Keywords: pig, meat quality, Prox1, variation, association analysis
1. Introduction
Prospero-related homeobox 1 (Prox1) encodes a homeobox transcription factor in vertebrates. Previous studies have demonstrated that Prox1 is essential for the embryonic development of the liver [1], lymphatic system organs [2,3], pancreas [4], lens [5], and retina [6]. Moreover, adult-onset obesity caused by lymphatic vascular defects was observed in Prox1 haploinsufficient mice [2]. Prox1 also plays an important role in regulating hepatic triglycerides levels by mediating the HDAC3-HNF4α pathway [7], indicating that Prox1 is critical for adipogenesis and fat storage. Recent studies have shown that Prox1 plays a pivotal role in heart development [8] and skeletal muscle fiber conversion [9,10,11]. In our previous study, we detected differentially expressed genes (DEGs) between the classic red muscle (soleus muscle) and white muscle (biceps femoris muscle) with various muscle fibers, and identified Prox1 as a major DEG [12]. These studies suggest that Prox1 can be a potential candidate for improving growth and development of pigs, as well as their meat quality after slaughter.
Vertebrate Prox1 is homologous to the Drosophila homeobox protein prospero, which was discovered in 1991 as a key determinant of cell fate in the Drosophila central nervous system [13]. Human PROX1 and mouse Prox1 were identified in 1993 [14] and 1996 [15], respectively. The homeo-prospero domain is highly conserved among vertebrates [16]. However, the complete sequence for most species has not been reported yet. While the partial predicted sequence of porcine Prox1 is available in the National Center for Biotechnology Information (NCBI) database, the full-length cDNA and promoter sequences have not been reported yet. Furthermore, information regarding the role of Prox1 in the growth and development of pigs is still lacking.
To investigate the characteristics of porcine Prox1 and its relationship with meat quality traits, we performed cloning, expression pattern, and promoter activity analysis of porcine Prox1. We also performed correlation analysis between Prox1 expression and meat quality traits, and association analysis of the polymorphisms of SNPs g. −930 bp, g. −1421 bp, and g. −1573 bp in Prox1 with meat quality traits. In this study, we cloned the full-length cDNA of two transcript variants of porcine Prox1, and observed that the coding DNA sequence (CDS) of porcine Prox1 is conserved with that of Prox1 in other vertebrates. Moreover, we found that porcine Prox1 was abundantly expressed in the liver, heart, and skeletal muscle, and preferentially expressed in slow muscle, characterized by stronger oxidative metabolism capacity when compared with fast muscle with higher glycolytic metabolism capacity. Furthermore, we successfully cloned porcine Prox1 proximal promoter sequences of two transcript variants and confirmed the core regulatory regions. In addition, we observed that Prox1 expression was significantly correlated to meat color (redness) value a*, drip loss, and the ratio of MyHC IIB to MyHC I expression levels. Finally, we identified 18 variations in proximal promoter 1 of Prox1, and found that the polymorphisms of three closely linked SNPs were significantly associated with pH24h. Taken together, our data provide useful information for an in-depth study of porcine Prox1 as a candidate gene to improve the growth and development of pigs, as well as their meat quality.
2. Materials and Methods
2.1. Ethics Committee
All procedures involving pigs were performed in compliance with the guideline for the care and use of experimental animals, established by the Ministry of Agriculture of China. All experiments were approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University (SYXK2011-0036).
2.2. Animals and Sample Collection
Tissues from the heart, liver, spleen, kidney, fat, and muscles were collected from three adult Duroc × Meishan pigs, as described by Li et al. [12], and tissues from the heart, liver, spleen, lung, kidney, stomach, and muscles were collected from three 70-day fetuses of a pregnant Landrace female crossed with a pure Landrace boar for expression analysis. The longissimus dorsi muscles at different developmental stages were collected from three fetuses of Landrace pregnant females crossed with a pure Landrace boar at 70 and 110 days post-conception (dpc), and from three pigs at two postnatal periods (1 and 70 days after birth). In addition, 32 longissimus dorsi muscles with different meat color (redness) values (a*) from a population of 279 commercial hybrid pigs (Pietrain (P) × Duroc (D)) × (Landrace (L) × Yorkshire (Y)) were collected to determine the relationship between Prox1 expression and skeletal muscle fiber type. All the pigs were stunned by electricity and slaughtered humanely in a standardized commercial processing plant (Jiangsu Sushi Group, Jiangsu, China).
The 279 commercial hybrid pigs provided by Guangdong Wen’s Foodstuffs Group Co. Ltd. (Huaian, China) were also used for traits association analysis. The experimental pigs were raised under standard conditions and fed ad libitum with free access to water. Ear tissues for genetic diversity analysis were collected from 133 pigs using ear clips, belonging to seven breeds: three Western lean-type breeds (22 Landrace, 22 Yorkshire, and 9 Duroc pigs), three Chinese indigenous breeds (21 Erhualian, 20 Meishan, and 20 Mi pigs), and a cultivated breed (20 Suhuai pigs with 75% Yorkshire and 25% Huai heritage).
2.3. RNA Extraction
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA integrity was checked by electrophoresis on a 1% agarose gel, and the RNA concentration and purity were determined using NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA).
2.4. Cloning of Full-Length cDNA and Proximal Promoter
The mRNA and genomic DNA from Erhualian pigs were used to clone full-length cDNA and the proximal promoter of Prox1. The predicted partial sequence of porcine Prox1 mRNA (NM_001128490.1) was downloaded from the National Center for Biotechnology Information (NCBI) database. Three pairs of gene-specific primers (Table S1) were designed to amplify the partial sequence of porcine Prox1 mRNA, while the 5′-end and 3′-end sequences were amplified using the SMARTer® RACE 5′/3′ Kit (Clontech Laboratories, Inc., Mountain View, CA, USA), according to the user manual. The amplified fragments were cloned into the pMD19-T vector (TaKaRa Biotechnology, Dalian, China) and sequenced by Tsingke Company (Nanjing, China). Next, the full-length cDNA sequence was assembled using the SeqMan software (DNASTAR, INC., Madison, WI, USA). Subsequently, the predicted proximal promoter sequence of porcine Prox1 was determined by performing a comparative analysis between the porcine Prox1 full-length cDNA and porcine reference genome sequence (Sscrofa10.2). Five pairs of gene-specific primers were designed to amplify the proximal promoter sequences of porcine Prox1. The primer sequences used in the amplification of the porcine Prox1 full-length cDNA and the proximal promoter sequences are shown in Table S1.
2.5. Sequence Analysis
The nucleotide and protein sequences for various species downloaded from the NCBI database are as follows: human (Homo sapiens, variant 1: NM_001270616.1 and NP_001257545.1; variant 2: NM_002763.4 and NP_002754.2), monkey (Macaca mulatta, NM_001260873.1 and NP_001247802.1), mouse (Mus musculus, variant 1: NM_008937.3 and NP_032963.1; variant 2: NM_001360827.1 and NP_001347756.1), and cattle (Bos taurus, NM_001193232.1 and NP_001180161.1). The open reading frames (ORFs) for the porcine Prox1 gene were predicted using NCBI’s online ORFfinder tool (https://www.ncbi.nlm.nih.gov/orffinder/). Multiple sequence alignment was performed using the DNAMAN software. The potential binding sites of putative transcriptional factors were predicted using the online software JASPAR version 2016 (http://jaspar2016.genereg.net/).
2.6. Gene Expression and Correlation Analysis
The first strand cDNA was synthesized using the PrimerScript real-time polymerase chain reaction (RT-PCR) kit (TaKaRa Biotechnology, Dalian, China) for expression analysis. Quantitative real time PCR (qRT-PCR) primer pairs (Table S1) were designed using the online Primer 3 software. The gene expression pattern was determined using the AceQ qPCR SYBR Green Master Mix (Vazyme, Nanjing, China). All PCR reactions were performed in triplicate for each sample. The relative expression levels of porcine Prox1, myoglobin, MyHC I, MyHC IIB, and MyHC IIX were quantified using the 2−△△ct value method, and normalized with the porcine housekeeping gene HPRT.
2.7. Promoter Activity Analysis
Based on the two transcript variants of porcine Prox1, we constructed five luciferase reporter vectors containing truncated Prox1 promoter fragments from promoters 1 and 2. Briefly, SacI or KpnI and HindIII restriction enzyme sites were added to the 5′-and 3′-end of the five pairs of gene-specific primers to amplify the proximal promoter fragments of porcine Prox1 (Table S1). The promoter 1 fragments were inserted into the pGL3-bascic vector (Promega, Madison, WI), and the recombinant constructs were named pGL3-basic-P11 (+318/+122), pGL3-basic-P12 (+318/−192), pGL3-basic-P13 (+318/−682), pGL3-basic-P14 (+318/−1182), and pGL3-basic-P15 (+318/−1987). The promoter 2 fragments were inserted into the pGL3-bascic vector (Promega, Madison, WI), and the recombinant constructs were named pGL3-basic-P21 (+318/+122), pGL3-basic-P22 (+318/−192), pGL3-basic-P23 (+318/−682), pGL3-basic-P24 (+318/−1182), and pGL3-basic-P25 (+318/−1987). The sites of promoter fragments were relative to the transcription start site (+1) of Prox1. Subsequently, PK15 and 293T cells were cultured in 24-well plates and the recombinant constructs were transfected using Lipofectamine 3000 (Thermo Fisher Scientific, MA, USA). The activities of the promoter fragments were analyzed after 24 h transfection using the Dual-Luciferase® Reporter Assay System (Promega, Madison, WI, USA) on a Glomax® 20/20 luminometer platform (Promega, Madison, WI, USA). Each dual-luciferase reporter assay was repeated five times. Firefly luciferase activity was normalized to Renilla luciferase activity.
2.8. Identification of Variations
Variations in the Prox1 coding region were detected using cloning and sequencing methods. DNA samples were collected from seven breeds (three pigs from each breed), including three Western lean-type breeds (Landrace, Yorkshire, and Duroc), three Chinese indigenous breeds (Erhualian, Meishan, and Mi), and a cultivated breed (Suhuai). Prox1 variation sites were confirmed by sequence alignment analysis using the DNAMAN software (Lynnon Biosoft, San Ramon, CA, USA). Variations in the proximal promoter 1 region were identified using targeted sequencing methods, as described previously by Wu et al. [17]. DNA samples derived from seven breeds, including three Western lean-type breeds (22 Landrace, 22 Yorkshire, and 9 Duroc), three Chinese indigenous breeds (21 Erhualian, 20 Meishan, and 20 Mi), a cultivated breed (20 Suhuai), and 66 ((P) × (D)) × ((L) × (Y)) commercial pigs were used for the amplification of target sequences. The amplification primers are also shown in Table S1. The positions of variations were defined relative to the transcription start site (+1) of porcine Prox1. Genotype and allelic frequencies were calculated manually.
2.9. Traits and Genotyping
A total of 21 production traits in 279 commercial hybrid pigs were measured or recorded. The experimental pigs were slaughtered humanely in a standardized commercial processing plant (Jiangsu Sushi Group, Jiangsu, China) at an average age of 176 days in 11 random batches. Both left and right side carcass weights of each pig were measured together using the measurement tools in the slaughter system, and the backfat thickness of the last rib was measured using a digital Vernier caliper. The pH values were measured using an Hanna Instruments (HI) 9125 portable pH meter (Hanna, Thornleigh, NSW, Australia). Cooking loss was measured according to the international and national standard protocols. Shear force was measured using a C-LM3 digital tenderness instrument according to the manufacturer’s instructions. Meat color values lightness (L*), redness (a*), and yellowness (b*) were determined using a portable Minolta colorimeter (CR-10, Minolta, Japan). Drip loss was measured using the bag method. Intramuscular fat content (IMF) was measured using the Soxhlet extraction method. Muscle glucose (MG), glycogen (G), and lactate content were determined using the glucose, glycogen, and lactate test kits (Jiancheng, Nanjing, China), respectively. Glucose-6-phosphate (G6P) levels were determined using the Glucose-6-Phosphate Assay Kit (Sigma, Spruce Street, St. Louis, MO, USA). Glycolytic potential (GP) = 2 × ((glycogen) + (glucose) + (glucose-6-phosphate)) + (lactate).
Based on sequencing results of the target region, we selected SNPs SNV00011 (g. −930 bp), SNV00014 (g. −1421 bp), and SNV00018 (g. −1573 bp) in the proximal promoter 1 of Prox1, and conducted genotyping in 279 commercial hybrid pigs, which consisted of 66 previously sequenced commercial pigs ((P) × (D)) × ((L) × (Y)), using the PCR restriction fragment length polymorphism (PCR-RFLP) method. The amplification primers for PCR-RFLP and the corresponding restriction enzymes are shown in Table S1.
2.10. Statistical Analysis
The statistically significant difference for gene expression was performed using the unpaired sample t test and one-way analysis of variance (ANOVA) in SPSS 20.0. Here, p < 0.05 was considered to be statistically significant and the data are presented as the mean ± SEM (n = 3). Statistically significant differences for meat color (redness) value a* were assessed using the unpaired sample t test in SPSS 20.0. Here, p < 0.05 was considered to be statistically significant and data are presented as the mean ± SEM (n = 17 and 15). Correlation analysis between the expression of Prox1 and myoglobin, MyHC I, MyHC IIB, MyHC IIX, and phenotype traits was assessed in SPSS 20.0. Statistically significant differences for promoter activity were assessed using ANOVA with Duncan’s multiple range tests in SPSS 20.0. Here, p < 0.05 was considered to be statistically significant and data are presented as the mean ± SEM (n = 5). The association between the polymorphisms of the three selected SNPs (g. −930 bp, g. −1421 bp, g. −1573 bp) and carcass and meat quality traits was assessed using the general linear model (GLM) procedure in SAS version 8.0 (SAS Institute, Cary, NC, USA). The genotype, sex, and batch were used as fixed effects in the model, with carcass weight as a covariate.
3. Results and Discussion
3.1. Characteristics of Porcine Prox1 Sequences
We performed 5′ and 3′ rapid amplification of cDNA ends-PCR (RACE-PCR) to obtain the full-length cDNA of porcine Prox1. Two kinds of alternative splicing forms were found in the 5′-end sequence of Prox1. All Prox1 cDNA fragments (except the 5′-end sequence fragments) were amplified and sequenced; however, only one form was identified. Therefore, we deduced that at least two putative transcript variants exist in porcine Prox1. The first and major variant (transcript variant 1) contained five exons and four introns, and encoded 737 amino acids. The 3683 bp full-length cDNA of transcript variant 1 (GenBank Accession No: MK704404) contained a 2214 bp ORF flanked by a 484 bp 5′-untranslated region (5′ UTR) and 985 bp 3′ UTR. The second variant (transcript variant 2) contained four exons and three introns because the transcription start site (TSS) was present at the first intron of transcript variant 1. The 4236 bp full-length cDNA of transcript variant 2 (GenBank Accession No: MK704405) contained a 1037 bp 5′ UTR and 985 bp 3′ UTR.
The transcript variant 1 was identified as the major form because the sequencing data revealed a few clones of transcript variant 2 clones in sequencing data. The two transcript variants of human PROX1 and mouse Prox1 are deposited in the NCBI database and the nucleotide sequences of the coding region of both transcript variants are identical. Therefore, the coding region sequence of the porcine transcript variant 2 may be similar to transcript variant 1. Moreover, we performed multiple sequence alignment of the Prox1 coding region nucleotide sequences, as well as protein sequences for different species, including human, mouse, monkey, and cattle. The coding region nucleotide sequences showed 96.58% identity among all species, while the protein sequences were 99.19% identical (Figure S1 and Figure S2). These indicate that Prox1 is highly conserved among vertebrates, and might exhibit similar function in different species. In addition, we performed transcript factor prediction for Prox1 promoter 1 using the online software JASPAR (http://jaspar.genereg.net). A total of 434 non-redundant potential transcript factors were found in porcine Prox1 promoter 1 (Table S2).
3.2. Expression Patterns of Porcine Prox1
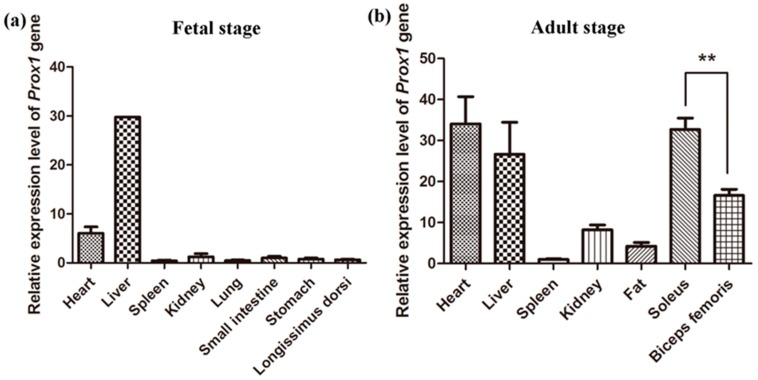
We determined the expression patterns of Prox1 in various tissues of adult pigs and 70-day fetuses using real-time PCR. Our results showed that porcine Prox1 is abundantly expressed in the heart and liver of both adult pigs and fetuses. However, Prox1 expression is low in the lung, spleen, kidney, stomach, and fat tissues (Figure 1a,b).
Figure 1.
Expression pattern of porcine Prox1. (a) Expression pattern of porcine Prox1 in fetal tissues. (b) Expression pattern of porcine Prox1 in adult tissues. Real-time polymerase chain reaction (PCR) was used to determine the expression levels of Prox1 genes. All PCR reactions were performed in triplicate for each sample. Relative expression levels were calculated using the 2−△△ct value method and normalized with the porcine housekeeping gene HPRT. Data are presented as the mean ± Standard Error of Mean (SEM) (n = 3). Statistical analysis was performed using the unpaired sample t test in SPSS 20.0. Note: ** indicates p < 0.01.
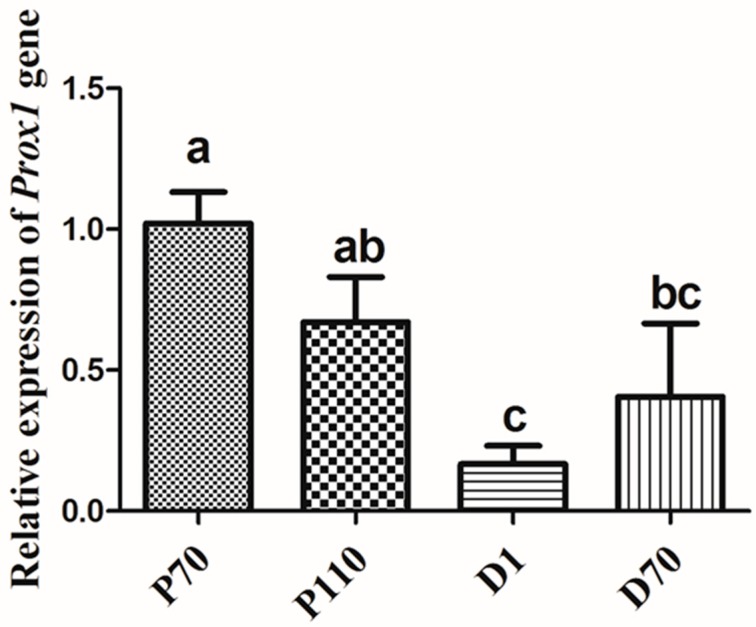
The high expression of porcine Prox1 in the liver and heart of adult pigs and fetuses suggests its importance in the development of embryonic heart [8] and liver [1]. Prox1 also has been shown to play a role in the development of the murine lymphatic system [2,3], retina [5,6], pancreas [4], and skeletal muscle [9]. Notably, Prox1 expression was lower in skeletal muscle when compared with that in the heart and liver at the fetal stage, but was highly expressed in skeletal muscle as well as heart and liver at the adult stage (Figure 1b), suggesting that Prox1 is critical for skeletal muscle, heart, and liver development in adult pigs. In humans, PROX1 is involved in the hepatic maturation of human-induced pluripotent stem-cell-derived hepatocyte-like cells (iPS-HLCs) and can promote hepatocellular carcinoma progression via the Wnt/β-catenin pathway [18,19]. Moreover, Prox1 was reported to play a critical role in heart growth and development [8], which is consistent with its high expression in porcine heart tissue. We also determined porcine Prox1 expression in the skeletal muscle at different developmental stages before and after birth. Prox1 expression was relatively higher before birth (Figure 2), although the possibility of its increased expression is not excluded at later developmental stages.
Figure 2.
Expression pattern of porcine Prox1 at different developmental stages. Longissimus dorsi muscles were collected from three fetuses of Landrace pregnant females at 70 days (P70) and 110 days (P110) post conception (dpc), and from three pigs on day 1 (D1) and at 70 days (D70) after birth. Real-time PCR was performed to determine Prox1 expression levels. All PCR reactions were performed in triplicate for each sample. The relative expression levels were calculated using the 2−△△ct value method and normalized with porcine housekeeping gene HPRT. Statistical analysis was performed using one-way ANOVA with Duncan’s multiple range tests. Data are presented as the mean ± SEM (n = 3). Different letters above the bars indicate significant differences. Note: p < 0.05.
Several recent studies have indicated that Prox1 is essential for skeletal muscle growth and development, and can regulate muscle fiber conversion by interacting with the nuclear factor of activated T cells (NFAT) and Notch pathways [9,11]. Our results showed that porcine Prox1 expression in the soleus muscle (red muscle) is higher than that in the biceps femoris muscle (white muscle) (Figure 1b), which also suggests that porcine Prox1 plays an important role in skeletal muscle fiber conversion. Moreover, Prox1 is preferentially expressed in slow muscle, characterized by stronger oxidative metabolism capacity in zebrafish [20] and mice [10]. In addition, Prox1 expression is significantly increased in fast muscle gene Sox6 knockout muscles, suggesting that Prox1 is critical for the conversion of slow to fast muscle fibers [10].
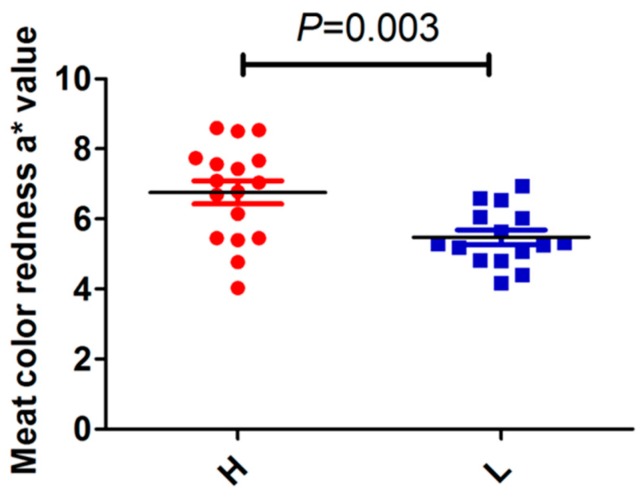
To demonstrate the relationship between porcine Prox1 expression and skeletal muscle fiber type, we randomly selected 32 longissimus dorsi muscles from a population of 279 commercial hybrid pigs ((P) ×(D)) × ((L) × (Y)) and determined the expression patterns of porcine Prox1, myoglobin, MyHC I, MyHC IIB, and MyHC IIX. The samples were divided into two groups according the ratio of MyHC IIB to MyHC I expression level; a ratio of > 2.0 was defined as high meat color a* group (H) and a ratio < 2.0 was defined as low meat color a* group (L). Our results indicated that a ratio of 2.0 can be used as the threshold value to distinguish the meat color (redness) value (a*) measured 24 h after slaughter (Figure 3), which also suggests that it is possible to predict meat color (redness) using the expression pattern of porcine Prox1.
Figure 3.
Comparison of meat color (redness) value a* between high and low groups. Thirty-two longissimus dorsi muscles were divided into two groups according the ratio of MyHC IIB to MyHC I expression (threshold value = 2.0). The ratios of > 2.0 and < 2.0 were defined as high meat color a* group (H) and low meat color a* group (L), respectively. Statistical analysis between the H and L groups was performed using the unpaired sample t test method in SPSS 20.0. Data are presented as the mean ± SEM (n = 17 for the H group and n = 15 for the L group).
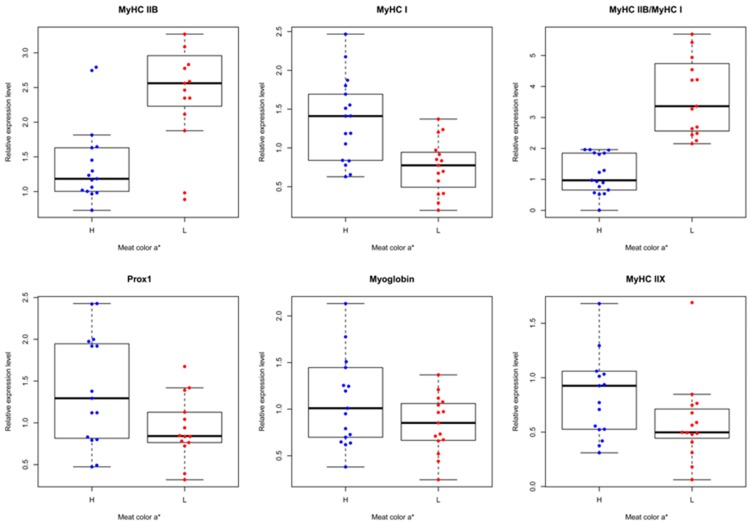
Our results showed that the expression pattern of porcine Prox1 in the H and L groups was similar to that of myoglobin and MyHC I, but opposed to that of MyHC IIB (Figure 4), indicating that porcine Prox1 may be closely related to skeletal muscle fiber diversity. These results further confirm the importance of Prox1 in skeletal muscle fiber conversion [9,11].
Figure 4.
Comparison of porcine Prox1 expression to that of skeletal muscle-fiber-related genes. Thirty-two longissimus dorsi muscles from a population of 279 commercial hybrid pigs were randomly selected, and the expression patterns of porcine Prox1 and skeletal muscle-fiber-related genes (including myoglobin, MyHC I, MyHC IIB, and MyHC IIX) were determined using real-time PCR. All PCR reactions were performed in triplicate for each sample. The relative expression levels were calculated using the 2−△△ct value method and normalized with the porcine housekeeping gene HPRT. The samples were divided into two groups according the ratio of MyHC IIB to MyHC I expression level, which was set at 2.0.
3.3. Correlation Analysis of Prox1 Expression with Meat Quality Traits
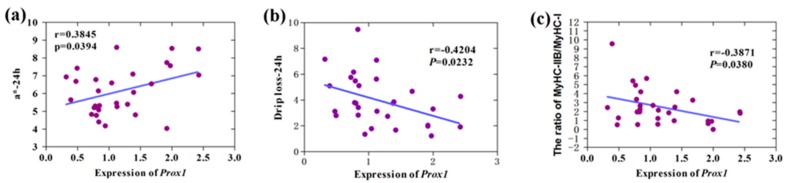
Skeletal muscles composed of different fibers exhibit different physiological and metabolic properties, including strength, twitch speed, and endurance, and oxidative and glycolytic capacity [21]. After slaughter, muscles composed of different fibers are subjected to various changes during the conversion of muscle to meat. Therefore, the type of skeletal muscle fiber definitely affects meat quality traits, such as pH, meat color, and drip loss [22,23]. Our results showed that Prox1 expression is significantly positively correlated to meat color (redness) value a* at 24 h postmortem (r = 0.3845, p = 0.0394), but significantly negatively correlated to drip loss at 24 h postmortem (r = −0.4204, p = 0.0232) (Figure 5).
Figure 5.
Correlation between porcine Prox1 expression and meat quality traits. The expression of porcine Prox1 was compared with that of myoglobin, MyHC I, MyHC IIB, and MyHC IIX in Figure 4, and the phenotype data of 32 commercial hybrid pigs are shown in Table 2. (a) Correlation between Prox1 expression and meat color (redness) value a* at 24 h postmortem. (b) Correlation between Prox1 expression and drip loss at 24 h postmortem. (c) Correlation between Prox1 expression and the ratio of MyHC IIB to MyHC I expression levels.
A significant correlation was also observed between Prox1 expression and the ratio of MyHC IIB to MyHC I expression level (r = −0.3871, p = 0.0380). It is known that skeletal muscles with different muscle fibers, such as biceps femoris and soleus muscles in the pigs, show obvious differences in the meat color [12], and exhibit different glycolytic capacities, accounting for muscle pH decline postmortem. Moreover, the change of pH is closely related to drip loss. Notably, the correlation analysis of meat quality traits in the population of 279 commercial hybrid pigs used in this study indicated that meat color is significantly related to drip loss. Therefore, our data suggest that Prox1 improves the meat quality by regulating skeletal muscle fiber conversion.
3.4. Identification of Core Promoter Region
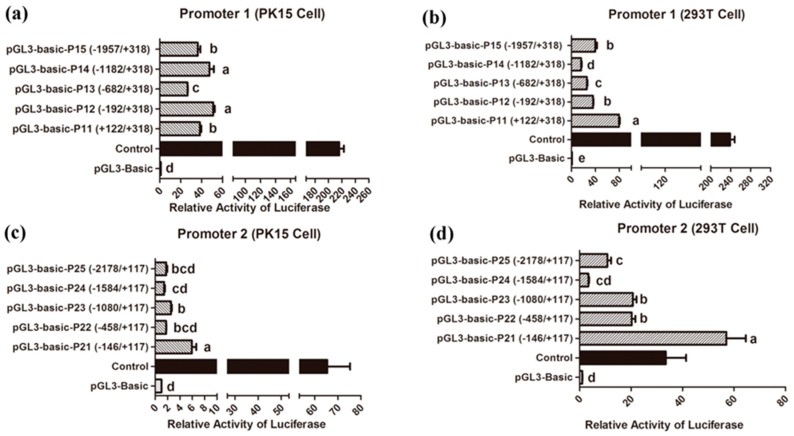
We cloned the two proximal promoters of the porcine Prox1 transcript variants and identified the promoter active region using the Dual-Luciferase® Reporter Assay System. The promoter activity was analyzed in PK15 and 293T cells. As shown in Figure 6a,b, the luciferase activity of all promoter 1 fragments was significantly higher than that of the pGL3-basic negative control, although the promoter activity differed among the different fragments in PK15 and 293T cells. The most active regions of promoter 1 were located between +318 bp and −192 bp (pGL3-basic-P11) in PK15 cells and between +318 bp and +122 bp (pGL3-basic-P11) in 293T cells. Moreover, based on the luciferase activity results of different promoter fragments in 293T cells, it can be deduced that some negative elements may exist in the regions from −1182 to −682 bp, from −682 to −192 bp, and from −192 to +122 bp. However, the region from −1957 to −1182 bp may contain potential positive elements. Similarly, negative elements may exist in the region from −682 to −192bp in PK15 cells. These results suggest that the regulatory mechanisms of promoter 1 may vary among different cells. In addition, the luciferase activity of all promoter 2 fragments showed that there were large differences between the 293T cells and PK15 cells; the promoter activity in the 293T cells was stronger than that in the PK15 cells (Figure 6c,d). Of note, the most active region of promoter 2 was consistently located between −146 bp and +117 bp (pGL3-basic-P21), both in 293T and PK15 cells. However, differences in the luciferase activity were still observed, suggesting that it is a core regulatory region for promoter 2 activity.
Figure 6.
Promoter activity analysis for the two transcript variants of porcine Prox1. Promoter activity analysis for Prox1 transcript variant 1 (Promoter 1) in PK15 cells (a) and 293T cells (b). Promoter activity analysis for Prox1 transcript variant 2 (Promoter 2) in PK15 cells (c) and 293T cells (d). The left side of the x-axis represents a series of truncated 5′-flanking sequences of Prox1 that were fused to the firefly luciferase reporter gene in the pGL3-basic vector, and their positions were defined relative to the transcription start site (+1) of porcine Prox1. The right side of the x-axis indicates the relative luciferase activity of promoter fragments. Here, pGL3-basic and -control were the negative and positive control vectors, respectively. The pRL-TK vector encoding Renilla luciferase was co-transfected as the internal reference. Promoter activity was defined by the normalization of firefly luciferase activity to Renilla luciferase activity, and the value of pGL3-basic negative vector was defined as 1. Data are presented as the mean ± SEM (n = 5). Statistical analysis was performed in SPSS 20.0 using one-way ANOVA test with Duncan’s multiple range tests. Data are presented as the mean ± SEM (n = 5). Different letters above the bars indicate significant differences. Note: p < 0.05.
3.5. Genetic Diversity of Sequence Variations
No variation was detected in the coding region of porcine Prox1. However, 18 variations in the Prox1 proximal promoter 1 region were identified using targeted sequencing methods. The detailed information regarding each variation, including the location of gene body, variation type, and reference genome position, are shown in Table 1.
Table 1.
Variations identified in the porcine Prox1 promoter 1.
| Gene Localization | Polymorphism | Chromosome | Reference Genome Position 1 |
|---|---|---|---|
| g. +123 | G/A | 9 | 142477946 |
| g. −268 | T/G | 9 | 142478335 |
| g. −414 | C/T | 9 | 142478482 |
| g. −427 | C/A | 9 | 142478495 |
| g. −502 | G/A | 9 | 142478570 |
| g. −516 | A/C | 9 | 142478584 |
| g. −759 | C/T | 9 | 142478827 |
| g. −780 | AGA/- | 9 | 142478846-142478848 |
| g. −848 | -/A | 9 | 142478913-142478913 |
| g. −924 | T/C | 9 | 142478992 |
| g. −930 | C/A | 9 | 142478998 |
| g. −1143 | G/T | 9 | 142479211 |
| g. −1179 | C/G | 9 | 142479247 |
| g. −1421 | A/G | 9 | 142479489 |
| g. −1448 | AC/- | 9 | 142479515-142479516 |
| g. −1452 | ACACAC/- | 9 | 142479515-142479520 |
| g. −1454 | ACACACAC/- | 9 | 142479515-142479522 |
| g. −1573 | C/G | 9 | 142479641 |
1Sus scrofa 10.2.
To understand the genetic diversity of these variations, we conducted genotype and allele frequency analyses in three Western lean-type breeds (Landrace, Yorkshire, and Duroc), three Chinese indigenous breeds (Erhualian, Meishan, and Mi), a cultivated breed (Suhuai), and 66 commercial pigs ((P) × (D)) × ((L) × (Y)). The genotypes and allele frequencies of all variations are shown in Table S3. We found that SNV00003, SNV00005, SNV00009, SNV00010, SNV00011, SNV00014, SNV00015, and SNV00018 variations showed extensive polymorphisms among different pig breeds. Notably, we found the SNV00010 and SNV00011 variations are completely linked in all tested pig breeds. In humans, several SNPs in Prox1 have been shown to significantly correlate with type 2 diabetes, and the underling mechanism is thought to be mediated by affecting glucose or lipid metabolism [24,25,26,27]. However, the correlation between the polymorphisms of specific SNPs in porcine Prox1 and porcine production traits, especially growth, carcass, and meat quality traits, remain unclear. Hence, we subsequently performed trait association analysis.
3.6. Trait Association Analysis
We found the polymorphisms of three variations, namely, SNV00011 (g. −930 bp), SNV00014 (g. −1421 bp), and SNV00018 (g. −1573 bp), were obviously different among the Western lean-type and Chinese indigenous breeds, and they were highly linked. We further performed genotyping in the 279 commercial hybrid pigs using the PCR-RFLP method and a trait association analysis. The genotypic gel electrophoresis results are shown in Figure S3. The trait phenotype with the mean values, SD, and coefficient of variation (CV) are shown in Table 2.
Table 2.
Phenotype information for experimental pig population.
| Trait 1 | N 2 | Mean ± SD 3 | CV% 4 |
|---|---|---|---|
| Age (d) | 279 | 176.92 ± 4.14 | 2.34 |
| CW (kg) | 279 | 82.50 ± 11.10 | 13.46 |
| BF (mm) | 279 | 1.77 ± 0.48 | 27.13 |
| pH45min | 279 | 6.22 ± 0.30 | 4.78 |
| pH24h | 279 | 5.58 ± 0.18 | 3.28 |
| IMF (%) | 279 | 2.64 ± 0.91 | 34.55 |
| L*45min | 129 | 45.37 ± 2.29 | 5.04 |
| a*45min | 129 | 3.85 ± 1.500 | 38.92 |
| b*45min | 129 | 7.67 ± 0.93 | 12.14 |
| L* 24h | 279 | 51.49 ± 3.00 | 5.81 |
| a*24h | 279 | 6.32 ± 1.15 | 18.14 |
| b*24h | 279 | 5.88 ± 1.26 | 21.42 |
| DL24h (%) | 279 | 1.75 ± 0.97 | 55.31 |
| DL48h (%) | 279 | 3.89 ± 1.54 | 39.66 |
| CL (%) | 279 | 30.88 ± 3.48 | 11.26 |
| SF (N) | 279 | 47.23 ± 12.58 | 26.65 |
| MG (μmol/g) | 279 | 5.28 ± 1.71 | 32.44 |
| G (μmol/g) | 279 | 3.33 ± 0.84 | 25.23 |
| G6P (μmol/g) | 279 | 1.48 ± 0.19 | 12.69 |
| LA (μmol/g) | 279 | 130.82 ± 23.41 | 17.90 |
| GP (μmol/g) | 279 | 150.03 ± 26.27 | 17.50 |
1 Traits: Age, days after slaughter; CW, carcass weight; BF, backfat; pH45min, pH value at 45 min postmortem; pH24h, pH value at 24 h postmortem; IMF, intramuscular fat content; L*, lightness at 45 min or 24 h postmortem; a*, redness at 45 min or 24 h postmortem; b*, yellowness at 45 min or 24 h postmortem; DL24h, drip loss measured at 24 h postmortem; DL48h, drip loss measured at 48 h postmortem; CL, cooking loss; SF, shear force; MG, muscle glycogen; G, glucose; G6P, glucose-6-phosphate; LA, lactic acid; GP, glycolytic potential. 2 N = number of pigs. 3 SD = standard deviation. 4 CV = coefficient of variation.
The data showed that the coefficient of variation (CV) of backfat, intramuscular fat content (IMF), color a*, color b*, drip loss, shear force, and muscle glycogen exceeded 20%, indicating a large variation in phenotypic trait values existed, which provided a good basis for trait association analysis in the ((P) × (D)) × ((L) × (Y)) commercial pig population. The genotype results (Table S3) showed that three SNPs are highly linked in the ((P) × (D)) × ((L) × (Y)) commercial pig population. As the number with AA genotype at g. −930 bp site, GG genotype at g. −1421 bp site, and GG genotype at g. −1573 bp site was limited (n = 8), we performed an association analysis for the pigs with the other two genotypes at each SNP. The results (Table 3) indicated that the polymorphisms of the three SNPs are significantly associated with pH24h (p = 0.022). Th results indicate that pH decline is the major factor causing drip loss, and pH value will be stable at 24 h or longer. So, drip loss measured at 24 h and 48 h was commonly used for meat quality assessment after slaughter. Notably, the DL24h among various genotypes showed suggesting differences (p = 0.070), and the homozygous genotype pigs with higher pH24h correspondingly showed lower DL24h and DL48h, suggesting that allele C at g. −930 bp, allele A at g. −1421 bp, and allele C at g. −1573 bp are favorable alleles for better meat quality.
Table 3.
Association analysis of SNPs polymorphisms with porcine production traits.
| Traits | Genotype (g. −930 bp) |
Genotype (g. −1421 bp) |
Genotype (g. −1573 bp) |
Number | Least Squares Means (LSM) ± SE |
p Value |
|---|---|---|---|---|---|---|
| pH24h | CA | AG | CG | 87 | 5.55 ± 0.02 | 0.022 |
| CC | AA | CC | 184 | 5.60 ± 0.01 | ||
| Drip loss24h (%) | CA | AG | CG | 87 | 1.95 ± 0.12 | 0.070 |
| CC | AA | CC | 184 | 1.71 ± 0.09 | ||
| Drip loss48h (%) | CA | AG | CG | 87 | 5.04 ± 0.33 | 0.193 |
| CC | AA | CC | 184 | 4.78 ± 0.31 |
The pH value is a critical parameter for evaluating meat quality. Low pH denatures muscle proteins and reduces protein solubility, thereby affecting meat quality [28,29,30,31,32]. For example, pale, soft, and exudative (PSE) meat is produced by a rapid decrease in the pH value after slaughter, and dark, firm, and dry (DFD) meat is generated by a high ultimate pH value [33]. The pH value is directly influenced by glycolysis, in which glycogen is converted to lactate and H+. Recent studies have shown that Prox1 is significantly correlated with type 2 diabetes, and the underlying mechanism is mediated by affecting glucose metabolism [24,25,26,27,34,35]. Importantly, Prox1 was demonstrated to regulate the muscle fiber conversion, and the loss of Prox1 promotes conversion of slow to fast muscle fibers [9,20]. Skeletal muscles with various muscle fibers display different properties; slow muscle fibers (type I and type IIa) exhibit high oxidative metabolism capacity, while fast muscle fibers (type IIx and type IIb) exhibit high glycolytic metabolism capacity [21,36]. Therefore, the association of porcine Prox1 with pork pH value may be mediated by the regulation of skeletal muscle fiber type and glucose metabolism. Moreover, pH value is closely related to other meat quality traits, such as drip loss [37,38]. Our results obtained from the phenotype data in pig populations used in this study also indicated that pH45min and pH24h both exhibit a strong negative effect on DL24h and DL48h (data not shown), which is consistent with the three SNPs polymorphisms of porcine Prox1, suggesting association with DL24h. Generally, these results are consistent with the conclusion that the expression of Prox1 is significantly correlated to meat color (redness) value a* and drip loss at 24 h postmortem (Figure 5).
4. Conclusions
Prox1 is an essential gene for skeletal muscle development and muscle fiber conversion. Porcine Prox1 is a significantly DEG between the skeletal muscles with different muscle fibers. The present study firstly analyzed the characteristics of porcine Prox1, laying a solid foundation for future functional studies. The expression pattern of Prox1, as well as the correlation and association analysis of Prox1 with meat quality traits, indicates that Prox1 can be targeted to improve porcine meat quality traits. Overall, our data provide a basis for the application of Prox1 gene in pig genetics to improve meat quality traits.
Acknowledgments
We thank to the staff and technicians for collecting samples.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2615/9/10/744/s1. The sequences of the two transcript variants of porcine Prox1 were deposited to the NCBI GenBank database under accession numbers: MK704404 and MK704405. Table S1: Primers used in this study. Table S2: Prediction of transcript factors in porcine Prox1 promoter 1. Table S3: Genotypes and allele frequencies of variations in different pig breeds. Figure S1: Alignment of Prox1 CDS nucleotide sequences derived from different species. Figure S2: Alignment of Prox1 protein sequences of CDS derived from different species. Figure S3: Genotypic gel electrophoresis pattern using the PCR-RFLP method.
Author Contributions
Conceptualization, W.W.; investigation, C.D. and X.Z.; resources, Z.C., P.L., and H.L.; data curation, K.L., B.L., A.J., and R.L.; writing—original draft preparation, W.W.; writing—review and editing, W.W., C.D., X.Z., and P.L.; funding acquisition, W.W., Z.C., and H.L.
Funding
This research was funded by the National Key R&D Program of China (2017YFD0502002), Breeding and Reproduction in The Plateau Mountainous Region, Ministry of Education (Guizhou University, GYSD-K-2018-02), Program of Hainan Provincial Engineering Research Center for Livestock and Poultry Cultivation (XQ2016JSZX001), National Natural Science Foundation of China (31501920), and the National Major Project of Breeding for Transgenic Pigs (2016ZX08006001-003).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Sosa-Pineda B., Wigle J.T., Oliver G. Hepatocyte migration during liver development requires Prox1. Nat. Genet. 2000;25:254–255. doi: 10.1038/76996. [DOI] [PubMed] [Google Scholar]
- 2.Harvey N.L., Srinivasan R.S., Dillard M.E., Johnson N.C., Witte M.H., Boyd K., Sleeman M.W., Oliver G. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat. Genet. 2005;37:1072–1081. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- 3.Wigle J.T., Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/S0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 4.Wang J., Kilic G., Aydin M., Burke Z., Oliver G., Sosa-Pineda B. Prox1 activity controls pancreas morphogenesis and participates in the production of “secondary transition” pancreatic endocrine cells. Dev. Biol. 2005;286:182–194. doi: 10.1016/j.ydbio.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 5.Wigle J.T., Chowdhury K., Gruss P., Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nat. Genet. 1999;21:318–322. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]
- 6.Dyer M.A., Livesey F.J., Cepko C.L., Oliver G. Prox1 function controls progenitor cell proliferation and horizontal cell genesis in the mammalian retina. Nat. Genet. 2003;34:53–58. doi: 10.1038/ng1144. [DOI] [PubMed] [Google Scholar]
- 7.Armour S.M., Remsberg J.R., Damle M., Sidoli S., Ho W.Y., Li Z., Garcia B.A., Lazar M.A. An HDAC3-PROX1 corepressor module acts on HNF4 alpha to control hepatic triglycerides. Nat. Commun. 2017;8:549. doi: 10.1038/s41467-017-00772-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Risebro C.A., Risebro C.A., Searles R.G., Melville A.A., Ehler E., Jina N., Shah S., Pallas J., Hubank M., Dillard M., et al. Prox1 maintains muscle structure and growth in the developing heart. Development. 2009;136:495–505. doi: 10.1242/dev.030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petchey L.K., Risebro C.A., Vieira J.M., Roberts T., Bryson J.B., Greensmith L., Lythgoe M.F., Riley P.R. Loss of Prox1 in striated muscle causes slow to fast skeletal muscle fiber conversion and dilated cardiomyopathy. Proc. Natl. Acad. Sci. USA. 2014;111:9515–9520. doi: 10.1073/pnas.1406191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An C.I., Dong Y., Hagiwara N. Genome-wide mapping of Sox6 binding sites in skeletal muscle reveals both direct and indirect regulation of muscle terminal differentiation by Sox6. BMC Dev. Biol. 2011;11:59. doi: 10.1186/1471-213X-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kivela R., Salmela I., Nguyen Y.H., Petrova T.V., Koistinen H.A., Wiener Z., Alitalo K. The transcription factor Prox1 is essential for satellite cell differentiation and muscle fibre-type regulation. Nat. Commun. 2016;7:59. doi: 10.1038/ncomms13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li B.J., Dong C., Li P., Ren Z., Wang H., Yu F., Ning C., Liu K., Wei W., Huang R., et al. Identification of candidate genes associated with porcine meat color traits by genome-wide transcriptome analysis. Sci. Rep. 2016;6:35224. doi: 10.1038/srep35224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doe C.Q., Chu-LaGraff Q., Wright D.M., Scott M.P. The prospero gene specifies cell fates in the Drosophila central nervous system. Cell. 1991;65:451–464. doi: 10.1016/0092-8674(91)90463-9. [DOI] [PubMed] [Google Scholar]
- 14.Oliver G., Sosa-Pineda B., Geisendorf S., Spana E.P., Doe C.Q., Gruss P. Prox 1, a prospero-related homeobox gene expressed during mouse development. Mech. Dev. 1993;44:3–16. doi: 10.1016/0925-4773(93)90012-M. [DOI] [PubMed] [Google Scholar]
- 15.Zinovieva R.D., Duncan M.K., Johnson T.R., Torres R., Polymeropoulos M.H., Tomarev S.I. Structure and chromosomal localization of the human homeobox gene Prox 1. Genomics. 1996;35:517–522. doi: 10.1006/geno.1996.0392. [DOI] [PubMed] [Google Scholar]
- 16.Elsir T., Smits A., Lindström M.S., Nistér M. Transcription factor PROX1: Its role in development and cancer. Cancer Metastasis Rev. 2012;31:793–805. doi: 10.1007/s10555-012-9390-8. [DOI] [PubMed] [Google Scholar]
- 17.Wu W.J., Liu K.Q., Li B.J., Dong C., Zhang Z.K., Li P.H., Huang R.H., Wei W., Chen J., Liu H.L. Identification of an (AC)n microsatellite in the Six1 gene promoter and its effect on production traits in Pietrain x Duroc x Landrace x Yorkshire pigs. J. Anim. Sci. 2018;96:17–26. doi: 10.1093/jas/skx024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y., Ye X., Zhang J.B., Ouyang H., Shen Z., Wu Y., Wang W., Wu J., Tao S., Yang X. PROX1 promotes hepatocellular carcinoma proliferation and sorafenib resistance by enhancing beta-catenin expression and nuclear translocation. Oncogene. 2015;34:5524–5535. doi: 10.1038/onc.2015.7. [DOI] [PubMed] [Google Scholar]
- 19.Nakamori D., Takayama K., Nagamoto Y., Mitani S., Sakurai F., Tachibana M., Mizuguchi H. Hepatic maturation of human iPS cell-derived hepatocyte-like cells by ATF5, c/EBPalpha, and PROX1 transduction. Biochem. Biophys. Res. Commun. 2016;469:424–429. doi: 10.1016/j.bbrc.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Roy S., Wolff C., Ingham P.W. The u-boot mutation identifies a Hedgehog-regulated myogenic switch for fiber-type diversification in the zebrafish embryo. Genes Dev. 2001;15:1563–1576. doi: 10.1101/gad.195801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gundersen K. Excitation-transcription coupling in skeletal muscle: The molecular pathways of exercise. Biol. Rev. 2011;86:564–600. doi: 10.1111/j.1469-185X.2010.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryu Y.C., Kim B.C. The relationship between muscle fiber characteristics, postmortem metabolic rate, and meat quality of pig longissimus dorsi muscle. Meat Sci. 2005;71:351–357. doi: 10.1016/j.meatsci.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 23.Lee S.H., Joo S.T., Ryu Y.C. Skeletal muscle fiber type and myofibrillar proteins in relation to meat quality. Meat Sci. 2010;86:166–170. doi: 10.1016/j.meatsci.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 24.Scott L.J., Erdos M.R., Huyghe J.R., Welch R.P., Beck A.T., Wolford B.N., Chines P.S., Didion J.P., Narisu N., Stringham H.M., et al. The genetic regulatory signature of type 2 diabetes in human skeletal muscle. Nat. Commun. 2016;7:11764. doi: 10.1038/ncomms11764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamet P., Haloui M., Harvey F., Marois-Blanchet F.C., Sylvestre M.P., Tahir M.R., Simon P.H.G., Kanzki B.S., Raelson J., Long C., et al. PROX1 gene CC genotype as a major determinant of early onset of type 2 diabetes in slavic study participants from Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation study. J. Hypertens. 2017;35(Suppl. 1):S24–S32. doi: 10.1097/HJH.0000000000001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kretowski A., Adamska E., Maliszewska K., Wawrusiewicz-Kurylonek N., Citko A., Goscik J., Bauer W., Wilk J., Golonko A., Waszczeniuk M., et al. The rs340874 PROX1 type 2 diabetes mellitus risk variant is associated with visceral fat accumulation and alterations in postprandial glucose and lipid metabolism. Genes Nutr. 2015;10:4. doi: 10.1007/s12263-015-0454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lecompte S., Pasquetti G., Hermant X., Grenier-Boley B., Gonzalez-Gross M., De Henauw S., Molnar D., Stehle P., Béghin L., Moreno L.A., et al. Genetic and Molecular Insights In to the Role of PROX1 in Glucose Metabolism. Diabetes. 2013;62:1738–1745. doi: 10.2337/db12-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joo S.T., Kauffman R.G., Kim B.C., Park G.B. The relationship of sarcoplasmic and myofibrillar protein solubility to colour and water-holding capacity in porcine longissimus muscle. Meat Sci. 1999;52:291–297. doi: 10.1016/S0309-1740(99)00005-4. [DOI] [PubMed] [Google Scholar]
- 29.Van Laack R.L.J.M., Lane J.L. Denaturation of myofibrillar proteins from chicken as affected by pH, temperature, and adenosine triphosphate concentration. Poult. Sci. 2000;79:105–109. doi: 10.1093/ps/79.1.105. [DOI] [PubMed] [Google Scholar]
- 30.Choi Y.M., Lee S.H., Choe J.H., Rhee M.S., Lee S.K., Joo S.T., Kim B.C. Protein solubility is related to myosin isoforms, muscle fiber types, meat quality traits, and postmortem protein changes in porcine longissimus dorsi muscle. Livestig. Sci. 2010;131:148. doi: 10.1016/j.livsci.2010.03.014. [DOI] [Google Scholar]
- 31.Chan J.T.Y., Omana D.A., Betti M. Effect of ultimate pH and freezing on the biochemical properties of proteins in turkey breast meat. Food Chem. 2011;127:109–117. doi: 10.1016/j.foodchem.2010.12.095. [DOI] [Google Scholar]
- 32.Yin Y., Zhang W.G., Zhou G.H., Guo B. Comparison of protein degradation, protein oxidation, and mu-calpain activation between pale, soft, and exudative and red, firm, and nonexudative pork during postmortem aging. J. Anim. Sci. 2014;92:3745–3752. doi: 10.2527/jas.2014-7850. [DOI] [PubMed] [Google Scholar]
- 33.O’Neill D.J., Lynch P.B., Troy D.J., Buckley D.J., Kerry J.P. Influence of the time of year on the incidence of PSE and DFD in Irish pigmeat. Meat Sci. 2003;64:105–111. doi: 10.1016/S0309-1740(02)00116-X. [DOI] [PubMed] [Google Scholar]
- 34.Paul L., Walker E.M., Drosos Y., Cyphert H.A., Neale G., Stein R., South J., Grosveld G., Herrera P.L., Sosa-Pineda B. Lack of Prox1 Downregulation Disrupts the Expansion and Maturation of Postnatal Murine beta-Cells. Diabetes. 2016;65:687–698. doi: 10.2337/db15-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goto T., Elbahrawy A., Furuyama K., Horiguchi M., Hosokawa S., Aoyama Y., Tsuboi K., Sakikubo M., Hirata K., Masui T., et al. Liver-specific Prox1 inactivation causes hepatic injury and glucose intolerance in mice. FEBS Lett. 2017;591:624–635. doi: 10.1002/1873-3468.12570. [DOI] [PubMed] [Google Scholar]
- 36.Bassel-Duby R., Olson E.N. Signaling pathways in skeletal muscle remodeling. Annu. Rev. Biochem. 2006;75:19–37. doi: 10.1146/annurev.biochem.75.103004.142622. [DOI] [PubMed] [Google Scholar]
- 37.Bee G., Anderson A.L., Lonergan S.M., Huff-Lonergan E. Rate and extent of pH decline affect proteolysis of cytoskeletal proteins and water-holding capacity in pork. Meat Sci. 2007;76:359–365. doi: 10.1016/j.meatsci.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Bowker B., Zhuang H. Relationship between water-holding capacity and protein denaturation in broiler breast meat. Poult. Sci. 2015;94:1657–1664. doi: 10.3382/ps/pev120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.