Abstract
Vitamin D insufficiency has become a common health problem worldwide, particularly among pregnant women and young children. Therefore, we sought to identify environmental, dietary, and genetic determinants of serum 25(OH)-vitamin D (25(OH)D) levels during pregnancy and early childhood. 25(OH)D was measured in women at 24-weeks of gestation (n = 738) and one-week postpartum (n = 284) in the population-based Danish COPSAC2010 mother–child cohort; and in cord blood (n = 257) and age 4 years (n = 298) in children from the at-risk COPSAC2000 mother–child cohort. Environmental, dietary, and genetic variables were tested for association with 25(OH)D using linear regression analyses. After adjusting for season of blood sampling, determinants of lower 25(OH)D levels during pregnancy in the women were higher pre-pregnancy BMI, lower age at birth, lower genetic vitamin D score, lower dietary vitamin D intake, and lower social circumstances. In children, the determinants were lower maternal age at birth, higher pre-pregnancy BMI, lower genetic vitamin D score, older siblings, exposure to tobacco smoking, and female sex. Genetics was an important determinant at all time points, alone explaining 2%–11% of the variance in 25(OH)D. Important determinants of circulating 25(OH)D levels during pregnancy and early childhood include environmental factors, diet, and to a large extent genetics.
Keywords: MeSH, 25(OH)-vitamin D, vitamin D, determinants, genetics, environmental factors, pregnancy, children
1. Introduction
Humans get vitamin D from exposure to sunlight, diet, and dietary supplements. Dietary sources of vitamin D include oily fish, egg yolk, and fortified foods including milk, cheese, orange juice, infant milk formula, etc. [1]. Vitamin D deficiency affects bone mineralization and can lead to rickets, increased fracture risk, and osteoporosis [2], but has also been shown to associate with several non-communicable diseases including childhood asthma [3], diabetes [4], hypertension [5], cardiovascular events [6,7], obesity [8], cancer [9,10,11,12,13,14], and multiple sclerosis [15].
Circulating 25(OH)-vitamin D (25(OH)D) levels vary among humans, and according to the majority of single-center studies, vitamin D deficiency (defined as 25(OH)D of less than 50 nmol/L (20 ng/mL) [16,17,18]) is widespread in children, pregnant women, and breastfeeding mothers [19,20,21,22]. It has been estimated that vitamin D insufficiency, defined as 25(OH)D between 50 and 75 nmol/L (20–30 ng/mL) [16,17,18], affects as many as one-half of otherwise healthy adults in developed countries and that one billion people worldwide have vitamin D deficiency or insufficiency [1].
Both dietary and environmental factors are speculated to play a role in the development of low 25(OH)D [1,14,20], and we have previously shown that season of birth affected cord blood 25(OH)D [23]. Further, the high heritability of vitamin D insufficiency suggests that genetic determinants may also be important, and genome-wide association studies have replicated four genetic markers influencing 25(OH)D levels, including GC (encoding vitamin D binding protein), CYP2R1 (encoding a C-25 hydroxylase converting vitamin D3 to an active receptor ligand), as well as DHCR7 and NADSYN1 (both involved in cholesterol synthesis) [24,25,26,27]. The aim of this study was to identify environmental, dietary, and genetic determinants of vitamin D deficiency during pregnancy in the population-based Copenhagen Prospective Studies on Asthma in Childhood (COPSAC2010) mother–child cohort and during early childhood in the at-risk COPSAC2000 cohort.
2. Methods
2.1. Ethics
The Copenhagen Ethics Committee (HKF 01-289/96; H-B-2008-093) and The Danish Data Protection Agency (2008-41-1754; 2015-41-3696) approved the study. Oral and written informed consent was obtained from all parents at enrolment.
2.2. Study Populations
COPSAC2000 is a prospective at-risk birth cohort study of 411 children born to mothers with a history of asthma during 1998–2001 [28,29,30]. The children were enrolled at age 1 month and subsequently attended 6-monthly visits to the COPSAC research unit until age 3 and yearly thereafter until age 7.
COPSAC2010 is a prospective population-based mother–child cohort of 743 mothers and their 700 children recruited during 2009–2010 [31,32,33]. The women were included during pregnancy week 24. Exclusion criteria were gestational age above week 26, intake of vitamin D exceeding 600 IU/d, or any endocrine/heart/kidney disorders. Of the included women, 623 were enrolled in a double-blind randomized clinical trial of 2400 IU/d cholecalciferol D3 supplementation or placebo (Camette, Denmark A/S) from pregnancy week 24 to one week postpartum. All participants were instructed to continue the recommended supplementation of 400 IU/d cholecalciferol D3.
2.3. 25(OH)D Measurements
In COPSAC2000, serum 25(OH)D levels were measured at birth in cord blood and at 4 years of age. Cord blood was collected by needle puncture from the umbilical cord vein by midwives and subsequently sent to the COPSAC research unit. At age 4, a blood sample was collected from a peripheral vein at the COPSAC research unit. In COPSAC2010, maternal 25(OH)D levels were measured at week 24 of pregnancy and one week postpartum in blood samples collected at the COPSAC research unit. All the blood samples were centrifuged for 10 min at 4300 rpm to separate serum and subsequently frozen at −80°C until analysis, which was done by isotope dilution liquid chromatography-tandem mass spectrometry [34,35] at the Dept. of Clinical Biochemistry, Aarhus University Hospital, Denmark. Further details are given in the Supplementary Materials.
2.4. Determinants
Anthropometry included child BMI at birth and age 4 years and maternal pre-pregnancy BMI. Length at birth was measured using an infantometer (Kiddimeter; Raven Equipment Ltd., Dunmow, Essex, England). Height at 4 years and maternal height was measured using a stadiometer (Harpenden; Holtain Ltd., Crymych, Dyfed, Wales).
Environmental determinants included maternal age at birth; season of birth; gestational age at birth; smoking during third pregnancy trimester (yes/no); maternal asthma status during third trimester (better, unchanged, worse) for COPSAC2000 and maternal asthma (yes/no) for COPSAC2010; mode of delivery (natural/Caesarean section); and social circumstances based on household income, maternal age, and maternal educational level. Postnatal exposures included season of blood sampling (winter (December–February), spring (March–May), summer (June–August), and fall (September–November)), older siblings (yes/no), and environmental tobacco exposure measured objectively as hair nicotine level (ng/mg) at age 1 year [36]. Race and gender were included for all participants.
Diet in COPSAC2010 included maternal mid-pregnancy dietary intake, obtained prospectively during a 4-week period by validated comprehensive food frequency questionnaires allowing for estimation of daily vitamin D intake [37]. The child’s dietary habit in COPSAC2000 was only available in the first year of life, and therefore not used in the analyses.
Genetics included genome-wide genotyping data of the mothers and children in both cohorts. In COPSAC2000, a vitamin D genetic score was composed of GC, CYP2R1, DHCR7, and NADSYN1 genotype data, where the score is the number of effect alleles previously associated with an increase in 25(OH)D [24,25,26]. In COPSAC2010, the vitamin D genetic score was composed of two GC single nucleotide polymorphisms (SNPs) genotypes (rs4588 and rs7041) as the other SNPs used in COPSAC2000 were not available in this cohort.
2.5. Statistics
Baseline characteristics are presented as median and interquartile range (IQR), mean and standard deviation (SD), or as number and percentage. Prior to analyses, the levels of 25(OH)D were adjusted for season by using a cosinus–sinus model (25(OH)D = sin (2 * π * day of blood sample/365.25) + cos (2 * π * day of blood sample/365.25)) as we wanted to examine determinants of 25(OH)D apart from the season of blood sampling. Thereafter, univariate linear regression models were used for crude analysis of the associations between the risk factors and 25(OH)D levels. Subsequently, multiple forward regression analyses were used as a variable selection method. The combination of variables that resulted in the lowest Akaike information criterion (AIC) determined the best model fit. Race was not included in the forward regression analysis, since genetic data were only obtained from Caucasians. Finally, multivariate regression analyses of all variables were performed. All statistical analyses were performed with RStudio version 3.3.0., considering a p-value < 0.05 as significant.
3. Results
3.1. Pregnant Women from COPSAC2010
A blood sample was available for 25(OH)D analysis at pregnancy week 24 in 738 (99%) of the 743 enrolled women and in 284 women at one week postpartum, who were randomized to placebo. The median (IQR) 25(OH)D level in the study group was 74.7 nmol/L (57–92) at pregnancy week 24 and 71 nmol/L (48–92) one week postpartum. At week 24 and one week postpartum, 14% and 22% of the women had deficient vitamin D levels (<50 nmol/L), respectively. Sufficient levels (>75 nmol/L) were measured in 52% of the women at week 24 and in 45% at one week postpartum. Baseline characteristics of the women are shown in Online Table S1.
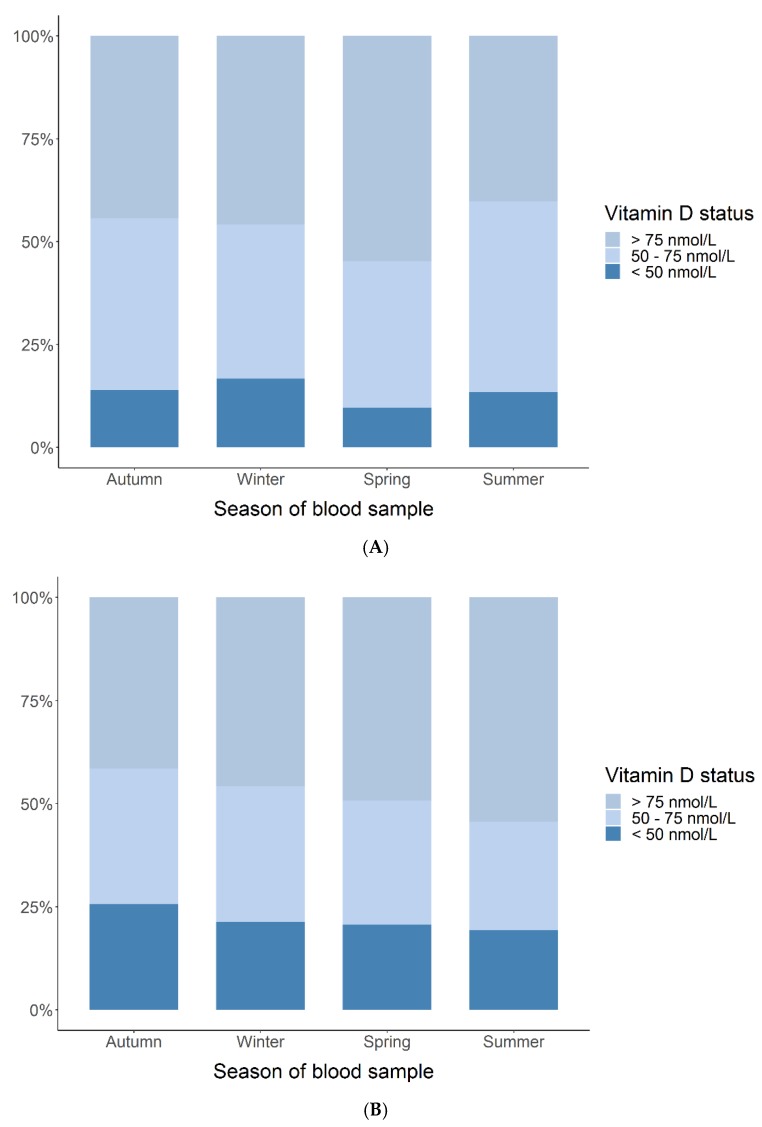
In Figure 1, maternal 25(OH)D levels at gestational week 24 and one week postpartum are depicted, stratified for season of blood sampling and categorized into sufficient (>75 nmol/L), insufficient (50–75 nmol/L), and deficient (<50 nmol/L). At week 24, 13% of the women had deficient 25(OH)D levels during summer compared to 17%, 10%, and 14% during winter, spring, and autumn, respectively. Sufficient levels were measured in approximately half of the women (40%–55%), with a slight preponderance during spring (55%). At one week postpartum, slightly more women (19%) had deficient 25(OH)D during summer compared to 21%, 21%, and 26% during winter, spring, and autumn, respectively. Sufficient levels were measured in 41%–54% of the women.
Figure 1.
Maternal 25(OH)-vitamin D (25(OH)D) status (deficient (<50 nmol/L), insufficient (50–75 nmol/L), sufficient (>75 nmol/L)) at week 24 (A) and one week postpartum (B) stratified for season of blood sampling.
The absolute 25(OH)D levels varied significantly with season. For week 24 when using winter as reference, 25(OH)D was 19.3 nmol/L higher during summer (95% CI [14.2; 24.4], p < 0.001) and 10.7 nmol/L higher during autumn (95% CI [6.0; 15.4], p < 0.001). For one week postpartum, 25(OH)D was 27.2 nmol/L higher during summer vs. winter (95% CI [17.3; 37.2], p < 0.001), but no significant differences were found for the rest of the seasons vs. winter. The 25(OH)D levels in week 24 and one week postpartum were associated both in the unadjusted and season-adjusted analyses: 0.54 (95% CI [0.41; 0.67], p < 0.001) and 0.68 (95% CI [0.56; 0.80], p < 0.001), respectively. We found no difference between the 25(OH)D levels at week 24 and one week postpartum: mean difference unadjusted was −3.2 nmol/L (95% CI [−6.9; 0.4]) and adjusted was 0.4 nmol/L (95% CI [−2.7; 3.5]) (Figure 2).
Figure 2.
Correlation between maternal 25(OH)D level measured at week 24 and one week postpartum in nmol/L unadjusted (A) and adjusted for season of blood sampling (B).
3.2. Environmental, Dietary, and Genetic Determinants of Maternal 25(OH)D Levels
Univariate analyses of week 24 samples showed that 25(OH)D levels were positively correlated to Caucasian origin, increasing maternal age at birth, both vitamin D SNPs, and increasing dietary intake, and negatively correlated to increasing maternal pre-pregnancy BMI. At one week postpartum, 25(OH)D levels were positively correlated to higher social circumstances, both vitamin D SNPs, and negatively correlated to maternal pre-pregnancy BMI. We did not find any other significant correlations with maternal 25(OH)D levels (Table 1).
Table 1.
Univariate analyses of determinants for maternal season-adjusted 25(OH)D levels during pregnancy.
| Week 24 Gestation β-Coefficient (nmol/L) |
95% CI | p-Value | One Week Postpartum β-Coefficient (nmol/L) |
5% CI | p-Value | |
|---|---|---|---|---|---|---|
| Caucasian vs. non-Caucasian | 11.71 | [3.00; 20.4] | 0.008 | 15.55 | [−1.09; 32.2] | 0.07 |
| Maternal BMI, kg/m² | −0.74 | [−1.17; −0.30] | 0.001 | −1.09 | [−1.92; −0.26] | 0.01 |
| Gestational age, weeks | - | - | - | −1.40 | [−3.60; 0.80] | 0.21 |
| Caesarian section vs. vaginal delivery | - | - | - | 0.01 | [−8.61; 8.63] | 1.00 |
| Maternal age at birth, years | 0.41 | [0.02; 0.80] | 0.038 | 0.41 | [−0.41; 1.22] | 0.33 |
| SNP rs4588 | 7.58 | [4.81; 10.4] | <0.001 | 12.49 | [6.76; 18.2] | <0.001 |
| SNP rs7041 | 4.74 | [2.26; 7.23] | <0.001 | 7.83 | [2.90; 12.8] | 0.002 |
| Maternal asthma, yes vs. no | −2.93 | [−6.96; 1.10] | 0.15 | −0.19 | [−8.11; 7.73] | 0.96 |
| Maternal smoking in third trimester, yes vs. no | −1.06 | [−10.6; 8.50] | 0.83 | −11.78 | [−27.9; 4.32] | 0.15 |
| Social circumstances, PCA score | 1.21 | [−0.57; 2.98] | 0.18 | 4.59 | [0.89; 8.29] | 0.015 |
| Dietary vitamin D intake, μg/day | 1.16 | [0.47; 1.84] | 0.001 | 0.88 | [−0.60; 2.35] | 0.24 |
To examine the most important determinants of 25(OH)D levels, a multivariate forward regression analysis was made. The following variables were included in the analysis of week 24: maternal pre-pregnancy BMI, age at birth, smoking in third trimester, maternal asthma, social circumstances, diet, and the vitamin D SNPs (rs4588 and rs7041). The same variables were used for the one week postpartum analysis, but gestational age at birth and Caesarian section were also included. The final models included maternal pre-pregnancy BMI, age at birth, diet, and rs4588 for week 24, explaining 7% of the variance in 25(OH)D levels (R2 = 0.07, p < 0.001), and maternal pre-pregnancy BMI, social circumstances, and both vitamin D SNPs for one week postpartum, explaining 10.3% of the variance in 25(OH)D levels (R2 = 0.103, p < 0.001) (Table 2).
Table 2.
Results from final multivariate forward regression analyses of maternal season-adjusted 25(OH)D levels.
| Week 24 Gestation β-Coefficient (nmol/L) |
95% CI | p-Value | One Week Postpartum β-Coefficient (nmol/L) |
95% CI | p-Value | |
|---|---|---|---|---|---|---|
| Maternal BMI, kg/m² | −0.70 | [−1.20; −0.25] | 0.002 | −1.05 | [−1.50; −0.15] | 0.02 |
| Maternal age at birth, years | 0.40 | [−0.08; 0.87] | 0.10 | - | - | - |
| rs4588 | 8.30 | [5.00; 11.60] | <0.001 | 7.50 | [1.10; 18.50] | 0.12 |
| rs7041 | - | - | - | 5.80 | [−4.80; 10.10] | 0.15 |
| Social circumstances, PCA score | - | - | - | 3.70 | [0.60; 8.29] | 0.09 |
| Dietary vitamin D intake, μg/day | 1.03 | [0.31; 1.76] | 0.005 | - | - | - |
To further explore the effect of the different determinants on 25(OH)D levels, a multivariate regression analysis was performed. At week 24 and one week postpartum, the environmental determinants explained 2.3% (R2 = 0.023, p = 0.002) and 1.3% (R2 = 0.013, p = 0.2) of the variance in 25(OH)D, genetics explained 3.5% (R2 = 0.029, p < 0.001) and 5.4% (R2 = 0.054, p < 0.001), and diet explained 1.6% (R2 = 0.016, p = 0.001) and 0.2% (R2 = 0.002, p = 0.24), respectively.
3.3. Children from COPSAC2000
Cord blood was available for 25(OH)D analysis in 257 (63%) of the 411 children and 298 (73%) had 25(OH)D measured at age 4 years. The median (IQR) 25(OH)D cord blood level was 40.9 nmol/L (28–55) and age 4 years was 75 nmol/L (59–91). In cord blood, 67% of the children had deficient vitamin D levels and only 8% had sufficient levels. At age 4 years, 14% had deficient and 50% had sufficient levels. Baseline characteristics of the children are given in Online Table S2.
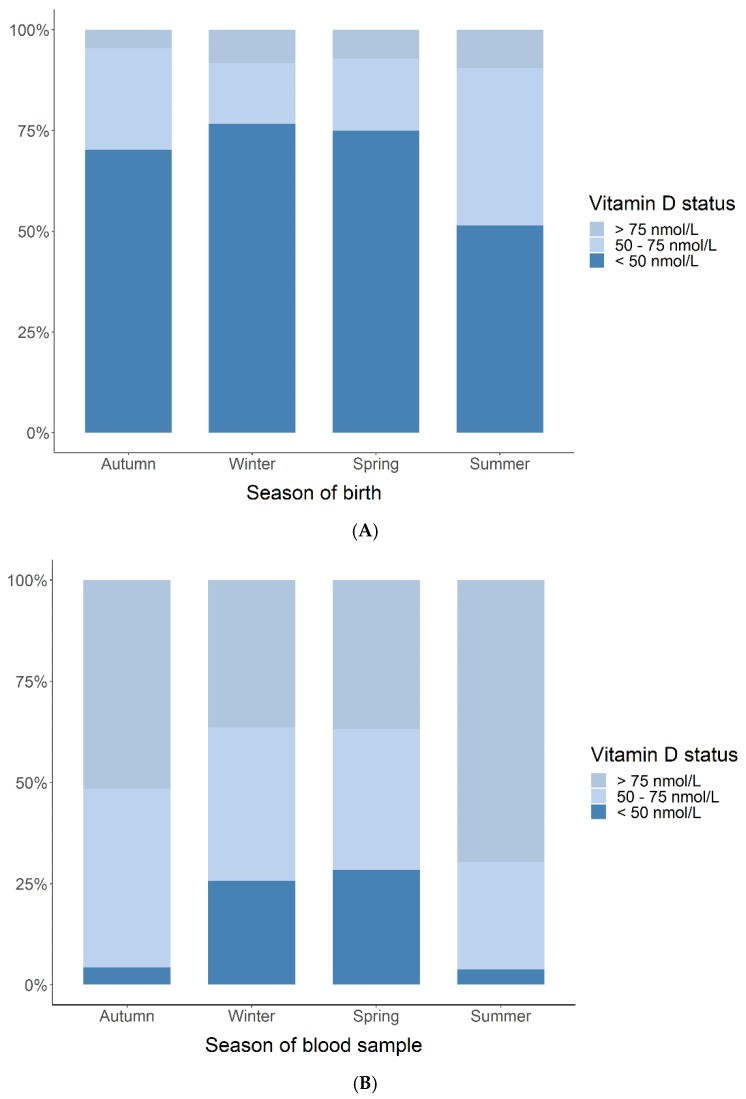
Fewer infants with deficient 25(OH)D cord blood levels were born during summer (51%) compared to 77%, 75%, and 70% born during winter, spring, and autumn (Figure 3). Only 4%–9% had sufficient levels at all seasons, with a slight preponderance of children born during summer (9%). At age 4 years, fewer children (3%) had deficient 25(OH)D during summer compared to 17%, 17%, and 4% during winter, spring, and autumn. Sufficient levels were measured in 36%–70% of the children.
Figure 3.
Child 25(OH)D status (deficient (<50 nmol/L), insufficient (50–75 nmol/L), sufficient (>75 nmol/L)) measured in cord blood (A) and at 4 years (B) stratified for season of blood sampling.
When using winter as reference, cord blood levels of 25(OH)D were 12.3 nmol/L higher in children born during summer (95% CI [5.5; 19.3], p < 0.001). For age 4 years, the levels measured during summer and autumn were 19.1 nmol/L (95% CI [11.1; 27.1], p < 0.001) and 12.4 nmol/L (95% CI [4.8; 20.1], p = 0.002) higher, respectively. No other seasonal differences were observed (data not shown). The 25(OH)D levels in cord blood and by age 4 years were associated both in the unadjusted and season-adjusted analyses: 0.18 (95% CI [0.02; 0.34], p = 0.03) and 0.25 (95% CI [0.08; 0.42], p = 0.004), respectively. We found an increase in the 25(OH)D levels from cord blood to age 4 years: mean difference unadjusted: 35.5 nmol/L (95% CI [31.7; 39.4]) and adjusted: 34.7 nmol/L (95% CI [31.0; 38.4]) (Figure 4).
Figure 4.
Correlation between cord blood and age 4 years 25(OH)D level in nmol/L unadjusted (A) and adjusted for season of blood sampling (B).
3.4. Environmental, Dietary, and Genetic Determinants of Child 25(OH)D Levels
In the univariate analyses, cord blood 25(OH)D level was positively correlated to maternal age at birth, the vitamin D genetic score, and negatively correlated to smoking during the third trimester. It was borderline positively correlated to Caucasian origin and social circumstances. At age 4, 25(OH)D was positively correlated to the vitamin D genetic score, male gender, and negatively correlated to tobacco exposure (Table 3).
Table 3.
Univariate analyses of determinants for cord blood and age 4 years season-adjusted 25(OH)D levels.
| Cord Blood β-Coefficient (nmol/L) |
95% CI | p-Value | 4 Years β-Coefficient (nmol/L) |
95% CI | p-Value | |
|---|---|---|---|---|---|---|
| Male | −0.47 | [−5.38; 4.43] | 0.85 | 6.39 | [1.06; 11.7] | 0.019 |
| Caucasian | 13.5 | [−0.51; 27.5] | 0.059 | 4.72 | [−10.2;19.6] | 0.53 |
| BMI, kg/m² | −0.19 | [−2.08; 1.69] | 0.84 | −1.58 | [−3.92; 0.75] | 0.18 |
| Gestational age, weeks | 0.61 | [−0.99; 2.21] | 0.46 | - | - | - |
| Caesarian section | −0.18 | [−6.36; 6.01] | 0.96 | - | - | - |
| Maternal age at birth, years | 0.73 | [0.16; 1.30] | 0.012 | - | - | - |
| Genetic score | 2.00 | [0.58; 3.44] | 0.006 | 4.45 | [2.90; 6.01] | <0.001 |
| Older siblings at birth | −4.01 | [−8.95; 0.94] | 0.11 | - | - | - |
| Maternal smoking in third trimester | −8.04 | [−14.7; −1.43] | 0.017 | - | - | - |
| Nicotine in hair, ng/mg | - | - | - | −0.03 | [−0.05; 0.00] | 0.021 |
| Asthma status in third trimester | ||||||
| Better | 1.86 | [−3.81; 7.53] | 0.52 | - | - | - |
| Unchanged | Ref. | |||||
| Worse | −1.47 | [−7.90; 4.95] | 0.65 | - | - | - |
| Social circumstances, PCA score | 2.59 | [−0.04; 5.21] | 0.053 | 1.54 | [−1.30; 4.37] | 0.29 |
A forward multivariate regression analysis for cord blood 25(OH)D yielded a final model including maternal age at birth, smoking during third trimester, older siblings, and the vitamin D genetic score, explaining 9.6% (R2 = 0.096, p < 0.001) of the variance in 25(OH)D. Particularly, having older siblings resulted in 8.7 nmol/L lower levels (p = 0.001) and smoking during the third trimester resulted in 6.5 nmol/L lower levels (p = 0.07) (Table 4). The final forward regression model for 4 years included the vitamin D genetic score, child BMI, and gender, explaining 10.8% (R2 = 0.108, p < 0.001) of the variance. Male gender resulted in 6.7 nmol/L higher 25(OH)D (p = 0.01).
Table 4.
Results from final multivariate forward regression analyses of child season-adjusted 25(OH)D levels.
| Cord Blood β-Coefficient (nmol/L) |
95% CI | p-Value | 4 Years β-Coefficient (nmol/L) |
95% CI | p-Value | |
|---|---|---|---|---|---|---|
| Maternal age at birth, years | 0.9 | [0.26; 1.54] | 0.006 | - | - | - |
| Genetic score | 2.07 | [0.7; 3.46] | 0.003 | 4.00 | [2.46; 5.56] | <0.001 |
| Older siblings at birth | −8.71 | [−14.2; −3.24] | 0.001 | - | - | - |
| Maternal smoking in third trimester | −6.50 | [−13.6; 0.52] | 0.07 | - | - | - |
| Child BMI, kg/m2 | - | - | - | −1.78 | [−4.03; 0.46] | 0.12 |
| Male gender | - | - | - | 6.7 | [1.54; 11.93] | 0.01 |
The environmental factors explained 5.2% (R2 = 0.052, p = 0.016) of the variance in 25(OH)D levels and genetics 2.9% (R2 = 0.029, p = 0.006). At 4 years, the environmental factors explained 1.8% (R2 = 0.018, p = 0.105) and genetics 10.4% (R2 = 0.104, p < 0.001).
4. Discussion
4.1. Primary Findings
In both pregnant women from COPSAC2010 and children from COPSAC2000, we found a high prevalence of vitamin D deficiency and insufficiency despite the fact that all Danish pregnant women and children up to 2 years of age are advised to take a vitamin D supplement of 400 IU/d [38]. Less than half of the women had sufficient levels at pregnancy week 24 and one week postpartum, just 7% of the children had sufficient cord blood levels, and less than half had sufficient levels at age 4 years. The best determinants of season-adjusted 25(OH)D levels in pregnant women included pre-pregnancy BMI, social circumstances, age at birth, diet, and vitamin D genetics, whereas the best determinants in young children were maternal age at birth, older siblings, smoke exposure, child BMI, gender, and vitamin D genetics, with genetics alone explaining 2%–11% of the variance in 25(OH)D levels in the cohorts. These findings suggest that environment, diet, and genetics all influence 25(OH)D levels in pregnant women and young children, which is important for vitamin D supplementation strategies.
4.2. Strengths and Limitations
The main strength of this study is the single-center set-up with thorough longitudinal clinical phenotyping and data collection in both the COPSAC2000 and the COPSAC2010 cohort. All assessments were solely performed by the COPSAC research pediatricians, who ensured consistency in procedures, definitions of conditions, and data capture methods, and thereby limited inter-observer variation.
Another strength is the objective assessment of 25(OH)D levels by state-of-the-art LC-MS two times during pregnancy, in cord blood, and at age 4 years. Even though cord blood 25(OH)D level primarily reflects exposure during the third trimester and may be contaminated by maternal blood, it is considered a more accurate measure of fetal exposure compared to calculations from questionnaires on maternal diet, since only 10% of vitamin D is obtained through diet [39].
The comprehensive assessment of environmental exposure, diet, and genetics is another strength providing many relevant 25(OH)D determinants for analysis, but we lack information on certain variables, including the use of sunscreen or protective clothing, outdoor physical activity, and diet information in COPSAC2000. Regarding skin pigmentation, 96%–97% in the study groups were of Caucasian origin and we do not expect this to be of great influence; therefore, analyses were not adjusted for ethnicity. We observed that season had a major influence on 25(OH)D levels, but since it reflects the time of blood sampling rather than a long-term determinant of 25(OH)D level we adjusted all the risk-factor analyses for this. Regarding genetics, we chose a simple and clinically applicable approach using four genes where we had available genotype information, which have been replicated across genome-wide association studies to contribute to vitamin D levels. However, there are likely to be far more gene variants associated both with increased and decreased vitamin D, but these were not available in our study.
The population-based COPSAC2010 cohort provides findings generalizable to other populations, and it would have been interesting to analyze 25(OH)D levels in the children at age 4 years to compare them to the general population. Unfortunately, we only measured 25(OH)D levels in the children in the COPSAC2000 cohort who were born to asthmatic mothers and thus not directly comparable to the general population. However, this should not affect the ability to analyze determinants of 25(OH)D levels within the cohort, and some of the findings are in line with a similar study of unselected children [40].
4.3. Interpretation
We found a high prevalence of vitamin D deficiency and insufficiency in both mothers and children. After adjusting for season of blood sampling, significant determinants of lower 25(OH)D levels during pregnancy were higher pre-pregnancy BMI, lower age at birth, lower social circumstances, lower dietary intake of vitamin D, and lower genetic vitamin D score. In the children, the determinants were lower maternal age at birth, older siblings, exposure to tobacco smoking, higher pre-pregnancy BMI, female gender, and lower genetic score. Genetics alone contributed to 2%–11% of the variance in 25(OH)D levels in the pregnant women and young children.
Our finding of a high prevalence of vitamin D deficiency is in line with other studies of Danish pregnant women [39] and children aged 3 months to 6 years [40]. Season of blood sampling played a major role, as we found that 25% fewer children had deficient cord blood levels if they were born during summer compared to winter and spring, which aligns with cord blood results reported from an Australian prospective birth cohort study [41]. In the COPSAC2000 cohort, the season variation (summer vs. winter) of 25(OH)D levels in children was greater at age 4 years than in cord blood measurements, which is probably due to a greater variation of outside activity among children compared with mothers’ activity in the third trimester reflected in the cord blood levels. In the COPSAC2010 cohort, the seasonal variation of being vitamin D deficient between week 24 and one week post-partum was less profound, and this could be due to increased skin coverage and less outdoor activity in late pregnancy.
We found that 25(OH)D levels increased during childhood from birth until age 4, which is in line with results from another birth cohort study [42], but opposite to findings from a study of healthy children observing a decrease in 25(OH)D from 3 months to 6 years [40]. An increase in vitamin D levels could be explained by different adherence to supplementation strategies or the fact that we measured 25(OH)D in cord blood, where levels are generally lower than later in childhood. Exposure to sunlight during outdoor activities is presumably the main environmental driver of the increase from birth till age 4 years, but our data also suggest that dietary and lifestyle habits associated with BMI may be of importance.
In our study, we observed associated but stable 25(OH)D levels from week 24 to one week postpartum. Previous longitudinal studies have reported both a decrease in 25(OH)D concentrations throughout pregnancy and in the period after delivery [43,44] and an increase through the first, second, and third trimesters [45]. We speculate that our findings could be a result of increased adherence to the recommended supplementation guidelines, as the mothers were enrolled in a clinical trial.
We focused on vitamin D status during pregnancy and early childhood, whereas many previous studies have tried to estimate determinants of long-term vitamin D status in adulthood [46,47,48,49,50]. That may be more relevant as a model for assessing the risk of developing chronic diseases such as cancers, inflammatory diseases, and osteoporosis, while our model is a better predictor of vitamin D deficiency in shorter vulnerable periods where personalized vitamin D supplementation strategies would be particularly beneficial.
We observed higher 25(OH)D in children of male sex and in children and women with lower BMI. This could be explained by males and subjects with lower BMI being more outside and more physically active, but also by the fact that vitamin D is fat-soluble a therefore deposited in fat tissue in girls and overweight persons. Tobacco exposure was also associated with lower child 25(OH)D level, which could be caused by other lifestyle factors found more prevalent among smokers, such as less physical activity and nutritional deficiencies. A more direct effect of tobacco exposure could include an increased hepatic metabolism of 25(OH)D, as smokers have been shown to have enhanced hepatic degradation of estrogen [51]. In both the pregnant women and in the children, we found higher 25(OH)D levels with higher maternal age at birth, higher social circumstances, and in persons of Caucasian origin, which is in line with several previous studies [40,46,47,52,53]. The association with higher maternal age and higher social circumstances may once again be due to lifestyle factors, including higher dietary intake of vitamin D containing foods and higher adherence to the recommended daily vitamin D supplement.
In the forward regression analysis, smoke exposure and older siblings were the variables with the greatest effect on cord blood 25(OH)D when looking at the estimates. Correspondingly, at age 4 years being male had the most influence on 25(OH)D levels. However, when looking at all the environmental factors they only accounted for 2%–5% of the variance in 25(OH)D levels, with the greatest effect on cord blood levels. This may be due to our adjustment for seasons prior to the risk-factor analysis or due to other unknown determinants lacking in our model (e.g., outdoor physical activity).
Regarding heritability, both the pregnant women and young children had significantly higher 25(OH)D levels with higher vitamin D genetic score, that is, increasing number of wildtype alleles involved in the vitamin D metabolism, explaining in itself 2%–11% of the variance in 25(OH)D. In the forward regression analysis, genetics was included in all the final models from both pregnant women and children and was the variable with greatest influence on 25(OH)D in the pregnant women, when looking at the estimates. Undoubtedly, the genetic regulation of the vitamin D pathway and levels (i.e., both increased and decreased levels) is very complex and presumably not yet completely understood. We chose a simple and clinically applicable approach using four genes where we had available genotype information, but there are likely to be far more gene variants associated with both increased and decreased vitamin D [54]. In a future study, it would be interesting to explore a wider range of gene variants, gene expression data, as well as gene–gene and gene–environment interactions contributing to trajectories of vitamin D levels during pregnancy and early childhood.
In the pregnant women, diet was significantly associated with 25(OH) levels at gestational week 24, where the food frequency questionnaire was fulfilled. Diet was not a determinant of 25(OH)D at one week postpartum, which aligns with one previous study [53] but is in contrast to most other studies [46,49,50,52], and could be explained by changing dietary habits in pregnancy. Diet was included in the week-24 forward regression model but explained <2% of the variance in 25(OH)D.
Overall, our regression models only explained 7%–11% of the variance in 25(OH)D status in pregnant women and young children, which is lower than the 21%–40% reported in previous studies of pregnant women, adolescents, and adults [47,48,52]. This is probably because we adjusted the 25(OH)D levels for season of blood sampling to minimize this as a confounder for other risk factors, particularly when assessing determinants of 25(OH)D at several time points in the same subject [55].
5. Conclusions
We found a high prevalence of vitamin D deficiency during pregnancy and in early childhood, and that important determinants include environmental factors, diet, and vitamin D genetics. These findings may be used to identify at-risk individuals providing a more personalized approach to vitamin D supplementation advice for pregnant women and young children.
Acknowledgments
We express our deepest gratitude to the children and families of the COPSAC2000 and COPSAC2010 cohort study for all their support and commitment. We acknowledge and appreciate the unique efforts of the COPSAC research team.
Abbreviations
| COPSAC | Copenhagen Prospective Study on Asthma in Childhood |
| 25(OH)D | 25-hydroxyvitamin D |
| SNP | single nucleotide polymorphism |
| IQR | interquartile range |
| CI | confidence interval |
| SD | standard deviation |
| AIC | Akaike information criterion |
Supplementary Materials
The following are available online at https://www.mdpi.com/2227-9067/6/10/116/s1, Table S1: Baseline characteristics of pregnant women in COPSAC2010, Table S2: Baseline characteristics of the children in COPSAC2000.
Author Contributions
Conceptualization, H.B and B.L.C.; Methodology, H.B. and B.L.C.; Software, S.N. and B.L.C; Validation, S.N., H.B. and B.L.C.; Formal Analysis, S.N. and B.L.C.; Investigation, H.B., A.-M.M.S., C.V., B.L.C; Resources, H.B.; Data Curation, H.B.; Writing—Original Draft Preparation, A.-M.M.S. and C.V.; Writing—Review & Editing, N.B., J.S., K.B., H.B. and B.L.C.; Visualization, B.L.C., A.-M.M.S. and C.V.; Supervision, H.B. and B.L.C.; Project Administration, H.B., K.B., J.S. and B.L.C.; Funding Acquisition, H.B., K.B., J.S. and B.L.S.
Funding
All funding received by COPSAC is listed on www.copsac.com. The Lundbeck Foundation (Grant no R16-A1694); The Ministry of Health (Grant no 903516); Danish Council for Strategic Research (Grant no 0603-00280B) and The Capital Region Research Foundation have provided core support to the COPSAC research center.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Holick M.F. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Holick M.F. Resurrection of vitamin D deficiency and rickets. J. Clin. Investig. 2006;116:2062–2072. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng H., Xun P., Pike K., Wills A.K., Chawes B.L., Bisgaard H., Cai W., Wan Y., He K. In utero exposure to 25-hydroxyvitamin D and risk of childhood asthma, wheeze, and respiratory tract infections: A meta-analysis of birth cohort studies. J. Allergy Clin. Immunol. 2017;139:1508–1517. doi: 10.1016/j.jaci.2016.06.065. [DOI] [PubMed] [Google Scholar]
- 4.Hyppönen E., Läärä E., Reunanen A., Järvelin M.-R., Virtanen S.M. Intake of vitamin D and risk of type 1 diabetes: A birth-cohort study. Lancet. 2001;358:1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 5.Krause R., Bühring M., Hopfenmüller W., Holick M.F., Sharma A.M. Ultraviolet B and blood pressure. Lancet. 1998;352:709–710. doi: 10.1016/S0140-6736(05)60827-6. [DOI] [PubMed] [Google Scholar]
- 6.Dobnig H., Pilz S., Scharnagl H., Renner W., Seelhorst U., Wellnitz B., Kinkeldei J., Boehm B.O., Weihrauch G., Maerz W. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch. Intern. Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 7.Ginde A.A., Scragg R., Schwartz R.S., Camargo C.A. Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older U.S. adults. J. Am. Geriatr. Soc. 2009;57:1595–1603. doi: 10.1111/j.1532-5415.2009.02359.x. [DOI] [PubMed] [Google Scholar]
- 8.Wortsman J., Matsuoka L.Y., Chen T.C., Lu Z., Holick M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 9.Garland C.F., Comstock G.W., Garland F.C., Helsing K.J., Shaw E.K., Gorham E.D. Serum 25-hydroxyvitamin D and colon cancer: Eight-year prospective study. Lancet. 1989;2:1176–1178. doi: 10.1016/S0140-6736(89)91789-3. [DOI] [PubMed] [Google Scholar]
- 10.Gorham E.D., Garland C.F., Garland F.C., Grant W.B., Mohr S.B., Lipkin M., Newmark H.L., Giovannucci E., Wei M., Holick M.F. Vitamin D and prevention of colorectal cancer. J. Steroid Biochem. Mol. Biol. 2005;97:179–194. doi: 10.1016/j.jsbmb.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Hanchette C.L., Schwartz G.G. Geographic patterns of prostate cancer mortality. Evidence for a protective effect of ultraviolet radiation. Cancer. 1992;70:2861–2869. doi: 10.1002/1097-0142(19921215)70:12<2861::AID-CNCR2820701224>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 12.Grant W.B. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer. 2002;94:1867–1875. doi: 10.1002/cncr.10427. [DOI] [PubMed] [Google Scholar]
- 13.The Association of Solar Ultraviolet B (UVB) with Reducing Risk of Cancer: Multifactorial Ecologic Analysis of Geographic Variation in Age-Adjusted Cancer Mortality Rates. [(accessed on 2 July 2019)]; Available online: https://www.ncbi.nlm.nih.gov/pubmed/?term=The+association+of+solar+ultraviolet+B+(UVB)+with+reducing+risk+of+cancer%3A+multifactorial+ecologic+analysis+of+geographic+variation+in+age-adjusted+cancer+mortality+rates. [PubMed]
- 14.Giovannucci E., Liu Y., Rimm E.B., Hollis B.W., Fuchs C.S., Stampfer M.J., Willett W.C. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J. Natl. Cancer Inst. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 15.Munger K.L., Zhang S.M., O’Reilly E., Hernán M.A., Olek M.J., Willett W.C., Ascherio A. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60–65. doi: 10.1212/01.WNL.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 16.Dawson-Hughes B., Heaney R.P., Holick M.F., Lips P., Meunier P.J., Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 17.Thomas M.K., Lloyd-Jones D.M., Thadhani R.I., Shaw A.C., Deraska D.J., Kitch B.T., Vamvakas E.C., Dick I.M., Prince R.L., Finkelstein J.S. Hypovitaminosis D in medical inpatients. N. Engl. J. Med. 1998;338:777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 18.Gallagher J.C., Sai A.J. Vitamin D Insufficiency, Deficiency, and Bone Health. J. Clin. Endocrinol. Metab. 2010;95:2630–2633. doi: 10.1210/jc.2010-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mansbach J.M., Ginde A.A., Camargo C.A. Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: Do children need more vitamin D? Pediatrics. 2009;124:1404–1410. doi: 10.1542/peds.2008-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bodnar L.M., Simhan H.N., Powers R.W., Frank M.P., Cooperstein E., Roberts J.M. High Prevalence of Vitamin D Insufficiency in Black and White Pregnant Women Residing in the Northern United States and Their Neonates. J. Nutr. 2007;137:447–452. doi: 10.1093/jn/137.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rovner A.J., O’Brien K.O. Hypovitaminosis D among healthy children in the United States: A review of the current evidence. Arch Pediatr. Adolesc. Med. 2008;162:513–519. doi: 10.1001/archpedi.162.6.513. [DOI] [PubMed] [Google Scholar]
- 22.Lee J.M., Smith J.R., Philipp B.L., Chen T.C., Mathieu J., Holick M.F. Vitamin D deficiency in a healthy group of mothers and newborn infants. Clin. Pediatr. (Phila.) 2007;46:42–44. doi: 10.1177/0009922806289311. [DOI] [PubMed] [Google Scholar]
- 23.Chawes B.L., Bønnelykke K., Jensen P.F., Schoos A.-M.M., Heickendorff L., Bisgaard H. Cord blood 25(OH)-vitamin D deficiency and childhood asthma, allergy and eczema: The COPSAC 2000 birth cohort study. PLoS ONE. 2014;9:e99856. doi: 10.1371/journal.pone.0099856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn J., Yu K., Stolzenberg-Solomon R., Simon K.C., McCullough M.L., Gallicchio L., Jacobs E.J., Ascherio A., Helzlsouer K., Jacobs K.B., et al. Genome-wide association study of circulating vitamin D levels. Hum. Mol. Genet. 2010;19:2739–2745. doi: 10.1093/hmg/ddq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang T.J., Zhang F., Richards J.B., Kestenbaum B., van Meurs J.B., Berry D., Kiel D.P., Streeten E.A., Ohlsson C., Koller D.L., et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet. 2010;376:180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carpenter T.O., Zhang J.H., Parra E., Ellis B.K., Simpson C., Lee W.M., Balko J., Fu L., Wong B.Y.-L., Cole D.E.C. Vitamin D binding protein is a key determinant of 25-hydroxyvitamin D levels in infants and toddlers. J. Bone Miner. Res. 2013;28:213–221. doi: 10.1002/jbmr.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navas-Nazario A., Li F.Y., Shabanova V., Weiss P., Cole D.E.C., Carpenter T.O., Bazzy-Asaad A. Effect of vitamin D-binding protein genotype on the development of asthma in children. Ann. Allergy Asthma Immunol. 2014;112:519–524. doi: 10.1016/j.anai.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bisgaard H. The Copenhagen Prospective Study on Asthma in Childhood (COPSAC): Design, rationale, and baseline data from a longitudinal birth cohort study. Ann. Allergy Asthma Immunol. 2004;93:381–389. doi: 10.1016/S1081-1206(10)61398-1. [DOI] [PubMed] [Google Scholar]
- 29.Bisgaard H., Hermansen M.N., Loland L., Halkjaer L.B., Buchvald F. Intermittent inhaled corticosteroids in infants with episodic wheezing. N. Engl. J. Med. 2006;354:1998–2005. doi: 10.1056/NEJMoa054692. [DOI] [PubMed] [Google Scholar]
- 30.Bisgaard H., Hermansen M.N., Buchvald F., Loland L., Halkjaer L.B., Bønnelykke K., Brasholt M., Heltberg A., Vissing N.H., Thorsen S.V., et al. Childhood asthma after bacterial colonization of the airway in neonates. N. Engl. J. Med. 2007;357:1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 31.Bisgaard H., Vissing N.H., Carson C.G., Bischoff A.L., Følsgaard N.V., Kreiner-Møller E., Chawes B.L.K., Stokholm J., Pedersen L., Bjarnadóttir E., et al. Deep phenotyping of the unselected COPSAC 2010 birth cohort study. Clin. Exp. Allergy. 2013;43:1384–1394. doi: 10.1111/cea.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chawes B.L., Bønnelykke K., Stokholm J., Vissing N.H., Bjarnadóttir E., Schoos A.-M.M., Wolsk H.M., Pedersen T.M., Vinding R.K., Thorsteinsdóttir S., et al. Effect of Vitamin D3 Supplementation During Pregnancy on Risk of Persistent Wheeze in the Offspring: A Randomized Clinical Trial. Jama. 2016;315:353–361. doi: 10.1001/jama.2015.18318. [DOI] [PubMed] [Google Scholar]
- 33.Bisgaard H., Stokholm J., Chawes B.L., Vissing N.H., Bjarnadóttir E., Schoos A.-M.M., Wolsk H.M., Pedersen T.M., Vinding R.K., Thorsteinsdóttir S., et al. Fish Oil-Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. N. Engl. J. Med. 2016;375:2530–2539. doi: 10.1056/NEJMoa1503734. [DOI] [PubMed] [Google Scholar]
- 34.Højskov C.S., Heickendorff L., Møller H.J. High-throughput liquid-liquid extraction and LCMSMS assay for determination of circulating 25 (OH) vitamin D3 and D2 in the routine clinical laboratory. Clin. Chim. Acta. 2010;411:114–116. doi: 10.1016/j.cca.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Maunsell Z., Wright D.J., Rainbow S.J. Routine Isotope-Dilution Liquid Chromatography-Tandem Mass Spectrometry Assay for Simultaneous Measurement of the 25-Hydroxy Metabolites of Vitamins D2 and D3. Clin. Chem. 2005;51:1683–1690. doi: 10.1373/clinchem.2005.052936. [DOI] [PubMed] [Google Scholar]
- 36.Sørensen M., Bisgaard H., Stage M., Loft S. Biomarkers of exposure to environmental tobacco smoke in infants. Biomarkers. 2007;12:38–46. doi: 10.1080/13547500600943148. [DOI] [PubMed] [Google Scholar]
- 37.Olsen S.F., Mikkelsen T.B., Knudsen V.K., Orozova-Bekkevold I., Halldórsson T.I., Strøm M., Østerdal M.L. Data collected on maternal dietary exposures in the Danish National Birth Cohort. Paediatr. Perinat. Epidemiol. 2007;21:76–86. doi: 10.1111/j.1365-3016.2007.00777.x. [DOI] [PubMed] [Google Scholar]
- 38.Sundhedsstyrelsen. [(accessed on 21 August 2019)]; Available online: https://www.sst.dk/da.
- 39.Sichert-Hellert W., Wenz G., Kersting M. Vitamin intakes from supplements and fortified food in German children and adolescents: Results from the DONALD study. J. Nutr. 2006;136:1329–1333. doi: 10.1093/jn/136.5.1329. [DOI] [PubMed] [Google Scholar]
- 40.Carpenter T.O., Herreros F., Zhang J.H., Ellis B.K., Simpson C., Torrealba-Fox E., Kim G.J., Savoye M., Held N.A., Cole D.E.C. Demographic, dietary, and biochemical determinants of vitamin D status in inner-city children. Am. J. Clin. Nutr. 2012;95:137–146. doi: 10.3945/ajcn.111.018721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones A.P., Palmer D., Zhang G., Prescott S.L. Cord blood 25-hydroxyvitamin D3 and allergic disease during infancy. Pediatrics. 2012;130:e1128–e1135. doi: 10.1542/peds.2012-1172. [DOI] [PubMed] [Google Scholar]
- 42.Wang G., Liu X., Bartell T.R., Pearson C., Cheng T.L., Wang X. Vitamin D Trajectories from Birth to Early Childhood and Elevated Systolic Blood Pressure During Childhood and Adolescence. Hypertension. 2019;74:421–430. doi: 10.1161/HYPERTENSIONAHA.119.13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J.Y., Lucey A.J., Horgan R., Kenny L.C., Kiely M. Impact of pregnancy on vitamin D status: A longitudinal study. Br. J. Nutr. 2014;112:1081–1087. doi: 10.1017/S0007114514001883. [DOI] [PubMed] [Google Scholar]
- 44.Narchi H., Kochiyil J., Zayed R., Abdulrazzak W., Agarwal M. Maternal vitamin D status throughout and after pregnancy. J Obstet. Gynaecol. 2010;30:137–142. doi: 10.3109/01443610903315652. [DOI] [PubMed] [Google Scholar]
- 45.Lawlor D.A., Wills A.K., Fraser A., Sayers A., Fraser W.D., Tobias J.H. Association of maternal vitamin D status during pregnancy with bone-mineral content in offspring: A prospective cohort study. Lancet. 2013;381:2176–2183. doi: 10.1016/S0140-6736(12)62203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan J., Jaceldo-Siegl K., Fraser G.E. Determinants of serum 25 hydroxyvitamin D levels in a nationwide cohort of blacks and non-Hispanic whites. Cancer Causes Control. 2010;21:501–511. doi: 10.1007/s10552-009-9481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bertrand K.A., Giovannucci E., Liu Y., Malspeis S., Eliassen A.H., Wu K., Holmes M.D., Laden F., Feskanich D. Determinants of plasma 25-hydroxyvitamin D and development of prediction models in three US cohorts. Br. J. Nutr. 2012;108:1889–1896. doi: 10.1017/S0007114511007409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Millen A.E., Wactawski-Wende J., Pettinger M., Melamed M.L., Tylavsky F.A., Liu S., Robbins J., LaCroix A.Z., LeBoff M.S., Jackson R.D. Predictors of serum 25-hydroxyvitamin D concentrations among postmenopausal women: The Women’s Health Initiative Calcium plus Vitamin D clinical trial. Am. J. Clin. Nutr. 2010;91:1324–1335. doi: 10.3945/ajcn.2009.28908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hill T.R., Cotter A.A., Mitchell S., Boreham C.A., Dubitzky W., Murray L., Strain J.J., Flynn A., Robson P.J., Wallace J.M.W., et al. Vitamin D status and its determinants in adolescents from the Northern Ireland Young Hearts 2000 cohort. Br. J. Nutr. 2008;99:1061–1067. doi: 10.1017/S0007114507842826. [DOI] [PubMed] [Google Scholar]
- 50.Hirani V., Mosdøl A., Mishra G. Predictors of 25-hydroxyvitamin D status among adults in two British national surveys. Br. J. Nutr. 2009;101:760–764. doi: 10.1017/S0007114508023416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jensen G.F., Meinecke B., Boesen J., Transbøl I. Does 1,25(OH)2D3 accelerate spinal bone loss? A controlled therapeutic trial in 70-year-old women. Clin. Orthop. Relat. Res. 1985:215–221. [PubMed] [Google Scholar]
- 52.Bjørn Jensen C., Thorne-Lyman A.L., Vadgård Hansen L., Strøm M., Odgaard Nielsen N., Cohen A., Olsen S.F. Development and validation of a vitamin D status prediction model in Danish pregnant women: A study of the Danish National Birth Cohort. PLoS ONE. 2013;8:e53059. doi: 10.1371/journal.pone.0053059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodriguez A., Santa Marina L., Jimenez A.M., Esplugues A., Ballester F., Espada M., Sunyer J., Morales E. Vitamin D Status in Pregnancy and Determinants in a Southern European Cohort Study. Paediatr. Perinat. Epidemiol. 2016;30:217–228. doi: 10.1111/ppe.12281. [DOI] [PubMed] [Google Scholar]
- 54.Jiang X., O’Reilly P.F., Aschard H., Hsu Y.-H., Richards J.B., Dupuis J., Ingelsson E., Karasik D., Pilz S., Berry D., et al. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nat. Commun. 2018;9:260. doi: 10.1038/s41467-017-02662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bruinse H.W., van den Berg H. Changes of some vitamin levels during and after normal pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 1995;61:31–37. doi: 10.1016/0028-2243(95)02150-Q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.