Abstract
Small heat shock proteins (sHSPs) comprise numerous proteins with diverse structure and function. As molecular chaperones, they play essential roles in various biological processes, especially under thermal stresses. In this study, we identified three sHSP-encoding genes, LtHSP19.5, LtHSP20.8 and LtHSP21.7b from Liriomyza trifolii, an important insect pest of ornamental and vegetable crops worldwide. Putative proteins encoded by these genes all contain a conserved α-crystallin domain that is typical of the sHSP family. Their expression patterns during temperature stresses and at different insect development stages were studied by reverse-transcription quantitative PCR (RT-qPCR). In addition, the expression patterns were compared with those of LtHSP21.3 and LtHSP21.7, two previously published sHSPs. When pupae were exposed to temperatures ranging from −20 to 45 °C for 1 h, all LtsHSPs were strongly induced by either heat or cold stresses, but the magnitude was lower under the low temperature range than high temperatures. Developmentally regulated differential expression was also detected, with pupae and prepupae featuring the highest expression of sHSPs. Results suggest that LtsHSPs play a role in the development of the invasive leaf miner fly and may facilitate insect adaptation to climate change.
Keywords: Liriomyza trifolii, small heat shock protein, development stage, thermal stress, expression pattern
1. Introduction
Liriomyza trifolii (Burgess) (Diptera: Agromyzidae) is an economically important and highly polyphagous pest in fields and greenhouses [1]. Both larvae and adults damage crop plants. The larvae tunnels in the leaves, whereas female adults puncture the leaf tissues for oviposition [2,3,4]. Originating from America, it has rapidly spread throughout the world [5]. In mainland China, L. trifolii was first recorded in Guangdong in 2005 [6], and now it has distributed to more than ten provinces [7]. L. trifolii, L. sativae and L. huidobrensis are the most important vegetable leaf mining pests in China [8,9,10]. L. sativae is the dominant species and has been detected throughout the country, whereas L. huidobrensis only occurs in regions of relatively high altitude [11,12]. L. trifolii mostly occurs in southeast coastal regions, but recently has been identified in certain northern provinces as well [7,13]. Temperature, particularly low temperature, appears to be the most important environmental factor that affects the distribution of Liriomyza species [8]. Some studies have been carried out to explore the response of Liriomyza species to temperature change and temperature-mediated interspecific competition [8,14,15,16].
Heat shock proteins (HSPs), including HSP90, HSP70, HSP60, HSP40 and small HSPs (sHSPs), are categorized based on molecular weight and sequence information [17,18,19,20]. In response to various stress factors, such as extreme temperatures, drought, osmotic stresses, heavy metals, oxidation, and UV radiation, organisms commonly synthesize HSPs [21,22,23,24]. Over-expression of HSPs has been commonly used as an indicator of insect tolerance to temperature stress [19,25]. Small HSPs have the greatest variation in structure and function among molecular chaperones, with molecular weight ranging from 12 to 43 kDa and a conserved α-crystallin domain [23]. They assist in the correct folding of nascent proteins, and prevent functional proteins from denaturation and aggregation induced by stresses [19,26,27,28]. sHSPs play an important role in increasing heat tolerance and protect organisms from thermal injury [29]. For instance, in the rice stem borer, Chilo suppressalis, CsHSP19.8 and CsHSP24.3 are both induced by high and low temperature extremes [30,31]. Similarly, expression of multiple sHSP genes in Choristoneura fumiferana, Frankliniella occidentalis, and Bactrocera dorsalis is significantly upregulated under thermal stresses [32,33,34]. sHSPs are also developmentally regulated in many insects [35]. sHSPs in Drosophila melanogaster are highly expressed during male gametogenesis [36] and embryonic development [37]. Some sHSPs in Bombyx mori, B. dorsalis and Spodoptera litura are induced by 20-hydroxyecdysone exposure, implying their involvement in metamorphosis [34,38,39,40].
sHSPs in closely related L. sativae and L. huidobrensis are induced by high and low temperature stresses [41]. In L. sativae, the expression of sHSPs during development has been investigated and the transcription of LsHSP19.5, LsHSP20.8, and LsHSP21.7 peaks during the pupal stage [42]. In L. trifolii, the expression profile of LtHSP21.3 has been shown to be similar to those of homologous genes in congeneric species under different temperature treatments [13]. When used to evaluate the stability of reference genes, expression of LtHSP21.7 is shown to be induced by temperature stresses and is varied at different developmental stages [43].
In this study, to explore the diversity of structure and potential functions of sHSPs in L. trifolii, we identified three new sHSP cDNAs and analyzed their transcript profiles during temperature stresses and at different developmental stages. We compared their expression patterns with those of LtHSP21.3 and LtHSP21.7, which have already been published. Our results provide new insight into physiological responses of L. trifolii under climate change that potentially influences the distribution of leaf miner flies.
2. Materials and Methods
2.1. Preparation of Insects of Different Developmental Stages
L. trifolii, originally collected from Yangzhou, China, was reared in the laboratory for more than 3 years at 26 ± 1 °C with a 16:8 h light:dark cycle [44]. Larvae and adults were reared on bean plants (Vigna unguiculata) and the leaves with tunnels were collected for pupation. The 3rd instar larvae, prepupae, 2-day- and 10-day-old pupae, and male and female adults were subjected to gene expression analysis. Three biological replicates were collected (n = 30).
2.2. Temperature Treatments
Two-day-old pupae (n = 30) were collected and placed into a water bath (DC-3010, Ningbo, China) and exposed for 1 h at low temperatures of −20, −17.5, –15, −12.5, −10, −7.5, −5, −2.5, 0, and 2.5 °C, and high temperatures of 27.5, 30, 32.5, 35, 37.5, 40, 42.5, and 45 °C. Pupae used for control were maintained at 25 °C. After exposure to various temperatures, pupae were incubated at 25 °C for 1 h, followed by instant freezing in liquid nitrogen, and storage at −80 °C. Each treatment was repeated four times.
2.3. RNA Isolation and Cloning Experiments
Total RNA was extracted using the SV Total RNA Isolation System (Promega, Fitchburg, WI, USA) and treated with DNase I to eliminate DNA contamination, following the manufacturer’s protocol. Integrity and purity of RNA was determined by agarose gel electrophoresis and spectrophotometry (Eppendorf Bio Photometer plus, Hamburg, Germany). First-strand cDNA was synthesized from 1 μg of total RNA using the First Strand cDNA Synthesis Kit (Fermentas, Ontario, Canada). Three putative sHSP genes, selected based on the analysis of our unpublished transcriptome data, were PCR amplified with gene-specific primers (Table S1). Touchdown PCR conditions were as follows: 94 °C for 3 min, 19 cycles of 94 °C for 30 s, 65–45 °C (annealing temperature decreased by 1 °C /cycle, from 65 °C to a “touchdown” 45 °C) for 30 s, 72 °C for 1 min, and then 25 cycles of 94 °C for 30 s, 45 °C (annealing temperature) for 30 s, and 72 °C for 1 min, followed by extension at 72 °C for 10 min. 5’ and 3’ rapid amplification of cDNA ends (5’ and 3’ RACE) were performed to obtain full-length cDNAs using a SMART RACE cDNA Amplification Kit (Clontech, CA, USA) according to the manufacturer’s instructions. LA Taq DNA Polymerase (Takara, Japan) was used for the PCR amplification and PCR parameters were as follows: 94 °C for 3 min, 35 cycles of 94 °C for 30 s, 68 °C for 30 s, and 72 °C for 3 min, followed by extension at 72 °C for 10 min. After obtaining the sequence information of the three sHSP genes, specific primers were designed to amplify the full-length of cDNAs. Gene-specific primers are shown in Table S1. The full-length cDNAs were purified using a gel extraction kit (Axygen, New York, NY, USA), cloned into gam-T Easy Vector (Promega, Fitchburg, WI, USA) and subjected to sequencing.
2.4. Reverse Transcription Quantitative PCR
Total RNA (0.5 μg) was reverse-transcribed into first-strand cDNA using the Bio-Rad iScript™ cDNA Synthesis Kit (Bio-Rad, CA, USA). The RT-qPCR reactions were performed using a CFX96 Real-Time PCR System (Bio-Rad Laboratories, Berkeley, CA, USA) in 20 μL reaction volume, as previously described [13]. The relative quantifications of LtsHSPs were assessed using the 2−ΔΔCt method [45] and ACTIN was used as a reference gene, because it is commonly used and the most optimal reference gene in L. trifolii under different experimental conditions [43]. Each sample was assessed in triplicate (technical replicates).
2.5. Sequence Alignment of sHSPs and Data Analysis
Full-length cDNA sequences of the three LtsHSPs were used as queries to search for other homologous sHSPs using the BLAST programs (http://www.ncbi.nlm.gov/BLAST/). Sequence alignments were conducted using Clustal X software [46], and the open reading frames (ORFs) were identified with ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder/). The deduced amino acid sequences of sHSPs were analyzed by ExPASy (Swiss Institute of Bioinformatics, Switzerland). The phylogenetic relationship of sHSPs was generated by MEGA 6.0 [47], using a neighbor joining (NJ) method based on the Poisson correction model with a bootstrap value of 1000. The protein structure of sHSP genes was predicted by the SWISS-MODEL (https://www.swissmodel.expasy.org/).
Data were analyzed with one-way ANOVA, followed by Tukey’s multiple comparison and analysis with SPSS v. 16.0 (SPSS, Chicago, IL, USA). For ANOVA, data were transformed for homogeneity of variance tests. Differences were considered statistically significant when P < 0.05.
3. Results
3.1. Cloning, Characterization and Phylogenetic Analysis of Three sHSP Genes from Liriomyza trifolii
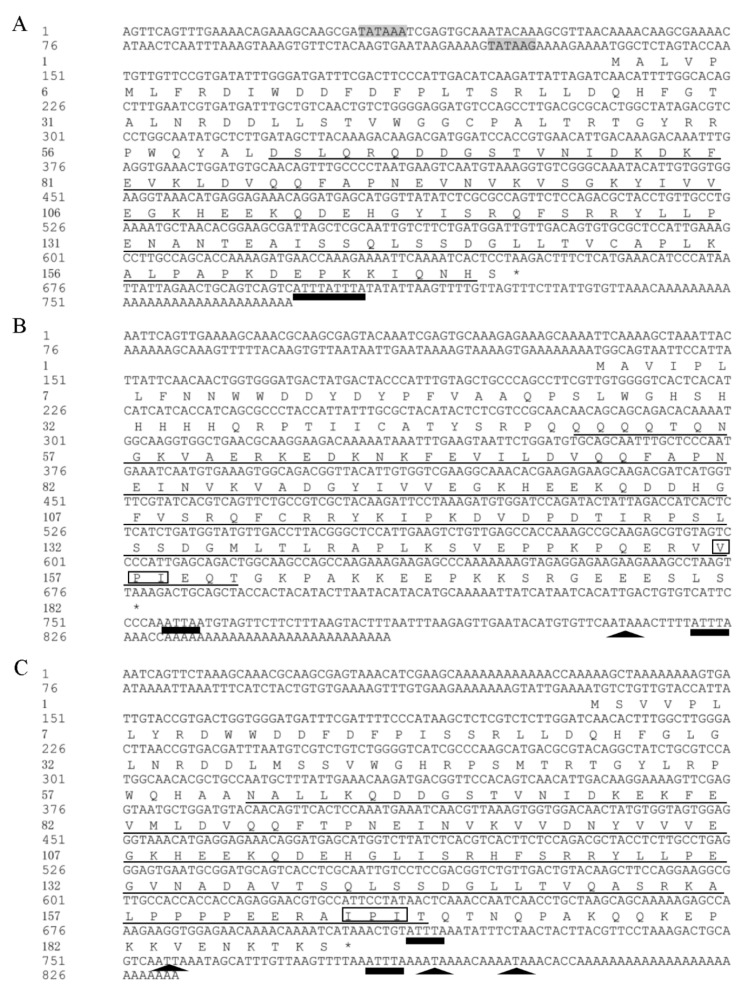
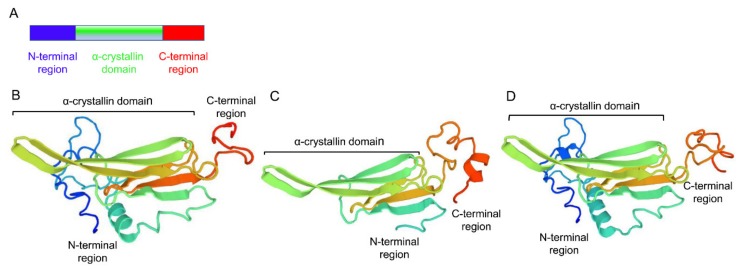
Three sHSPs, namely LtHSP19.5, LtHSP20.8, and LtHSP21.7b (GenBank accession nos. MG195951, MG195952, and MG195953, respectively), were cloned from L. trifolii. The ORFs were 516, 546, and 573 bp long, encoding proteins with 171, 181, and 190 amino acids, molecular weights of 19.46, 20.89, and 21.72 kDa, and isoelectric points of 5.56, 6.71, and 6.07, respectively, as seen in Table 1. Their deduced amino acid sequences contained a typical α-crystalline domain. LtHSP20.8 and LtHSP21.7b contained V/P/I and I/P/I motifs near the C-terminus, respectively, as seen in Figure 1. The three-dimensional (3D) structures of sHSP genes were predicted by the SWISS-MODEL website using the human αB-crystallin (PDB 2yjdA) as the best template, and it shares 43.23, 54.05 and 44.17% sequence identity with LtHSP19.5, LtHSP20.8, LtHSP21.7b, respectively, as seen in Figure 2. The deduced 3D structures of sHSPs share typical features of the sHSP family.
Table 1.
The Characteristics of Five sHSPs in L. trifolii.
| Name of Genes | Molecular Weight | Isoelectric Point | Developmental Stages (Highest Expression) | High Temperature (Tmax) | Low Temperature (Tmax) |
|---|---|---|---|---|---|
| Lt HSP19.5 | 19.46 kDa | 5.56 | prepupae | 40 °C | −17.5 °C |
| Lt HSP20.8 | 20.89 kDa | 6.71 | two-day-old pupae | 40 °C | −17.5 °C |
| Lt HSP21.7b | 21.72 kDa | 6.07 | two-day-old pupae | 40 °C | −17.5 °C |
| Lt HSP21.3 | 21.23 kDa * | 6.38 * | prepupae | 40 °C * | −17.5 °C * |
| Lt HSP21.7 | 21.66 kDa * | 7.03 * | prepupae * | 42.5 °C | −17.5 °C |
* Data of gene characteristics for HSP21.3 and HSP21.7 were obtained from Chang et al. (2017a, 2017b).
Figure 1.
Nucleotide sequences for L. trifolii sHSP cDNAs and their predicted amino acid sequences. Nucleotide numbering starts with the adenine in the first methionine codon of the putative open reading frame. The highly conserved region, α-crystallin domain, is underlined. The asterisk indicates the translational termination codon. The putative polyadenylation signal is black triangles, the AT-rich element is black rectangles, the V/P/I motif is boxed and the TA-rich regions are indicated by shading. sHSP = small heat shock proteins.
Figure 2.
Structure of sHSPs. (A) The domain organization of sHSPs: N-terminal region (blue), α-crystallin domain (turquoise), and the C-terminal region (red); (B–D) protein tertiary structure of LtHSP19.5 (B); LtHSP20.8 (C); and LtHSP21.7b (D).
Two TA-rich regions (TATA) were identified in the 5’UTRs of LtHSP19.5, but not in LtHSP20.8, and LtHSP21.7b. The 3’UTRs of these sHSPs also contain two typical motifs. AT-rich elements (ATTTA) were found in 3’UTRs of all three sHSPs, whereas polyadenylation signal elements (AATAAA or ATTAAA) were only found in 3’UTRs of LtHSP20.8 and LtHSP21.7b, as seen in Figure 1.
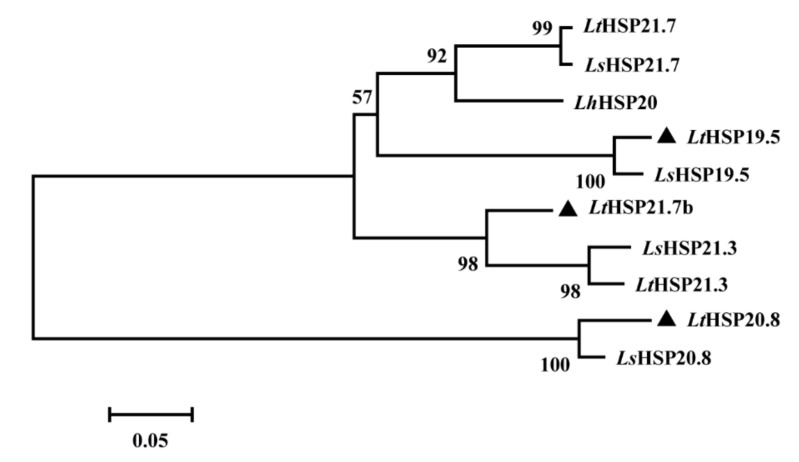
To examine the phylogenetic relationships between various sHSPs, the phylogenetic tree was generated using ten full-length sHSP family members, selected from three congener Liriomyza species using the neighbor-joining method. LtHSP20.8 was grouped with LsHSP20.8 in a separate branch, and LtHSP19.5 and LtHSP21.7b were clustered into a larger group that contained several other orthologs genes, as seen in Figure 3.
Figure 3.
Neighbor-joining phylogenetic tree of Liriomyza sHSPs. Those three new L. trifolii sHSPs of this study are labeled with triangles. Numbers on the branches are the bootstrap values obtained from 1000 replicates (only bootstrap values >50 are shown). Abbreviations, species, and accession numbers include LtHSP21.7 (L. trifolii, KY558641); LsHSP21.7 (L. sativae, DQ452372); LhHSP20 (L. huidobrensis, DQ452370); LtHSP19.5 (L. trifolii, MG195951); LsHSP19.5 (L. sativae, DQ452373); LtHSP21.7b (L. trifolii, MG195953); LsHSP21.3 (L. sativae, DQ452371); LtHSP21.3 (L. trifolii, KY231145); LtHSP20.8 (L. trifolii, MG195952); and LsHSP20.8 (L. sativae, DQ452374).
3.2. Expression of Three LtsHSPs in Response to Temperature Treatments
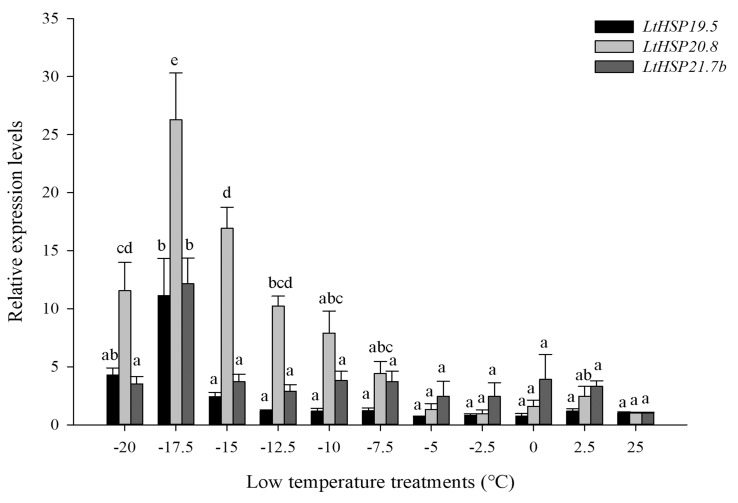
The relative mRNA levels of the three sHSPs were observed at different temperature stresses. In cold stress, compared with the control group at 25 °C, the expression level of three LtsHSPs were significantly increased after low temperature treatment (LtHSP19.5: F10, 33 = 23.514, P < 0.001; LtHSP20.8: F10, 33 = 29.116, P < 0.001; and LtHSP21.7b: F10, 33 = 4.245, P < 0.05). Gene expression peaked at –17.5 °C, which were 11.12-, 26.27- and 12.16-fold increase relative to the control, as seen in Figure 4.
Figure 4.
Relative expression levels of LtHSP19.5, LtHSP20.8, and LtHSP21.7b under low temperature treatments. The relative level of HSP expression represented the fold increase as compared with the expression in controls. The data were denoted as mean ± SE. One-way analysis of variance (ANOVA) was used to analyze the relative expression levels of three sHSPs under low temperature treatments. For the ANOVA, data were tested for homogeneity of variances and normality. Different lowercase letters indicate significant differences among different temperature treatments. Tukey’s multiple range test was used for pairwise comparison for mean separation (P < 0.05).
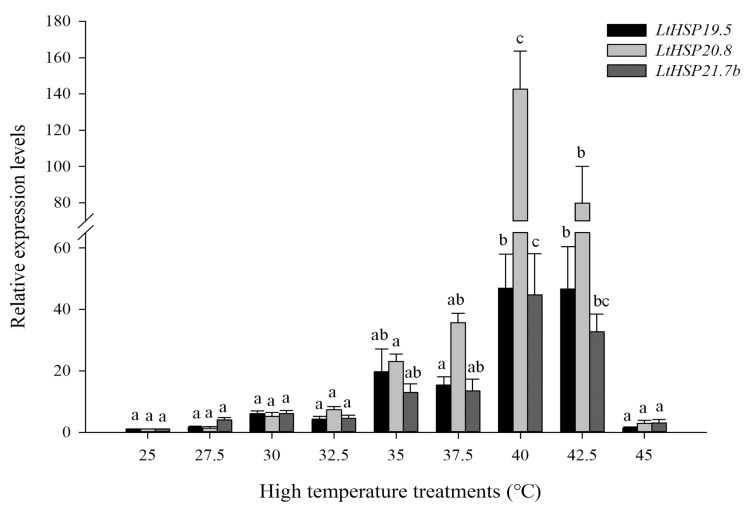
In heat stress, compared with the control group at 25 °C, expression of LtsHSPs were also significantly increased (LtHSP19.5: F8, 27 = 31.087, P < 0.001; LtHSP20.8: F8, 27 = 51.124, P < 0.001; and LtHSP21.7b: F8, 27 = 26.267, P < 0.001). The highest expression of LtsHSPs occurred at 40 °C, which were 46.93-, 142.56-, and 44.71-fold higher than that of control, respectively, as seen in Figure 5.
Figure 5.
Relative expression levels of LtHSP19.5, LtHSP20.8, and LtHSP21.7b under high temperature treatments. The relative level of HSP expression represented the fold increase as compared with the expression in controls. The data were denoted as mean ± SE. One-way analysis of variance (ANOVA) was used to analyze the relative expression levels of three sHSPs under high temperature treatments. For the ANOVA, data were tested for homogeneity of variances and normality. Different lowercase letters indicate significant differences among different temperature treatments. Tukey’s multiple range test was used for pairwise comparison for mean separation (P < 0.05).
3.3. Expression of Three LtsHSPs at the Developmental Stages
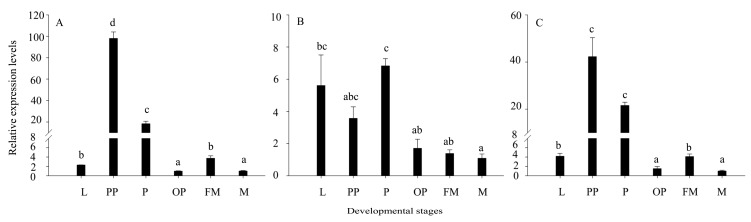
We investigated the mRNA level of three sHSPs through the developmental stage of L. trifolii, including 3rd instar larvae, prepupae, 2-day- and 10-day-old pupae, and male and female adults. All three LtsHSPs showed expression variations throughout the developmental stages. The expression of LtHSP19.5 and LtHSP21.7b peaked at prepupae, which were significantly up-regulated by 98.10- and 42.27-fold relative to the control-male adults (LtHSP19.5: F5, 12 = 338.747, P < 0.001; LtHSP21.7b: F5,12 = 54.757, P < 0.001). Expression of LtHSP20.8 peaked at 2-day-old pupae (up-regulated by 6.83-fold; F5, 12 = 6.640, P < 0.05) as seen in Figure 6.
Figure 6.
Relative expression levels of LtHSP19.5, LtHSP20.8, and LtHSP21.7b in different developmental stages of L. trifolii. The relative level of HSP expression represented the fold increase as compared with the expression in controls. (A) Relative expression levels of LtHSP19.5; (B) relative expression levels of LtHSP20.8; and (C) relative expression levels of LtHSP21.7b. The data were denoted as mean ± SE. One-way analysis of variance (ANOVA) was used to analyze the relative expression levels of three sHSPs in different developmental stages. For the ANOVA, data were tested for homogeneity of variances and normality. Different lowercase letters indicate significant differences among different developmental stages. Tukey’s multiple range test was used for pairwise comparison for mean separation (P < 0.05). Abbreviations: FM = females adult; M = males adult; L = third instar larvae; PP = prepupae; P = two-day-old pupae; and OP = ten-day-old pupae.
3.4. Comparative Characteristics and Expression Pattern of Five LtsHSPs
We have previously characterized the expression pattern of LtHSP21.3 under temperature stresses [13]. In this study, for comparison, the expression pattern of LtHSP21.3 was also investigated at different developmental stages. As seen in Figure S1, the highest expression stage of LtHSP21.3 occurred at 2-day-old pupal stages (F5, 12 = 13.935, P < 0.001), similar to that of LtHSP20.8, but different from other sHSPs in L. trifolii.
Previously, the expression pattern of LtHSP21.7 was studied under different experimental conditions for reference gene selection [43]. In this study, for comparison, the expression pattern of LtHSP21.7 was also investigated at different temperature stresses (low temperatures: –20, −17.5, −15, −12.5, −10, −7.5, −5, −2.5, 0 and 2.5 °C; high temperature: 27.5, 30, 32.5, 35, 37.5, 40, 42.5 and 45 °C). The highest expression temperatures of LtHSP21.7 was at −17.5 °C for low temperature stress (F10, 33 = 16.577, P < 0.001) as seen in Figure S1, and at 42.5 ℃ for high temperature stress (F8, 27 = 33.221, P < 0.001) also seen in Figure S1. Expression fold changes of LtHSP21.7 under temperature stress were significantly lower than those of the other sHSPs in L. trifolii. The sequence characteristics of the five LtsHSPs were detailed in Table 1.
4. Discussion
In this study, three new sHSP-encoding genes were cloned from L. trifolii. Sequence analysis shows that all three predicted protein sequences contain the characteristic α-crystalline domain. In addition, LtHSP20.8 and LtHSP21.7b also contained V/IXI/V motif. The propensity of the IPI/V motif to form multiple inter-subunit interactions may contribute to the diversity in structure and function seen in the α-crystallin [27]. Moreover, the 3’UTRs of LtsHSPs contain several other typical motifs, such as the poly adenylation signal (AATAAA or ATTAAA) [48] and the AT-rich element (ATTTA), which have been shown to afford greater mRNA stability and to contribute to the maintenance and re-establishment of basal levels of gene expression [42,49,50,51]. The number of TA-rich regions in 5’UTR of those three LtsHSPs were different; only two TA-rich regions were found in the 5’UTR of LtHSP19.5, but it was lacking in LtHSP20.8 and LtHSP21.7b. The difference in the number of these elements have also been found in HSPs of other insect species [41], and the number of these elements may be related to the expression pattern of HSPs [52,53,54]. The phylogenetic analysis showed that LtHSP19.5 and LtHSP20.8 clustered with representatives of their orthologs, but LtHSP21.7b was clustered with HSP21.3. This clustering pattern was also recorded in previous studies [23,55], which suggested that the evolution of sHSP may be complex. However, the available sHSP data are limited, and the systematic illustration of the evolution of sHSPs is expected to be achieved through the genome-wide analysis in future.
In this study, three new LtsHSPs and two previously identified LtsHSPs are all significantly up-regulated by low and high temperature treatments. The same expression patterns have also been observed in most previously recorded responses of sHSPs [30,56,57,58]. The sensitivity to temperature stresses of five LtsHSPs was different but the temperatures of the maximal (Tmax) expression were comparable. The same highest expression temperature range suggests similar functionality under temperature stresses, except for LtHSP21.7 at high temperatures. In this study, the response level of three sHSPs to different temperature stresses was higher than that of two published sHSPs (LtHSP21.3 and LtHSP21.7), which was reflected by a higher gene expression fold. The five LtsHSPs varied in terms of temperature sensitivity and suggest a synergistic effect of different sHSP family members with respect to thermal tolerance, which is consistent with recent research of LtHSP70s [59]. Several sHSPs play a role in temperature stress together, and similar expression patterns were also found in Chilo suppressalis and Bemisia tabaci [31,60]. At the same time, it is worth noting that, like in cases of other HSPs, expression level of LtHSPs induced by high temperatures is higher than that induced by low temperatures. In addition, other mechanisms for confronting with the stress may play a role in resisting extreme low temperature stress, such as antioxidation and supercooling phenomenon.
Attention has been paid to the role of HSPs in the regulation of insect development [35]. It seemed that all three new LtsHSPs and two previous LtsHSPs could be expressed in all developmental stages in L. trifolii. However, all of the five sHSPs’ expression levels were significantly different in the developmental stages, and the expression fold of LtHSP19.5, LtHSP21.3, and LtHSP21.7 [43] in prepupae was significantly different from that in control, while LtHSP20.8 and LtHSP21.7b reached a peak in the pupal stage. For prepupae, the mature larvae just leave the leaves for pupation, so it may lead to more sensitivity to the environment temperature. Meanwhile, the expression level increased significantly during the transition from larvae to pupae, which is the same as the one observed in LtHSP70s [59]. This suggests that metamorphosis itself can serve as a factor to induce the expression of HSPs. The metamorphosis has large physical changes and HSPs, as important molecular chaperones, are involved in processes such as assembling, removing, folding, and refolding of different kinds of proteins [19]. Thus, the change of protein structure may lead to a relatively high expression level of HSPs [42].
5. Conclusions
In summary, genes encoding sHSPs of L. trifolii contained several typical conserved domain and motifs. Those LtsHSPs could be significantly induced by temperature stresses and expressed during different stages of insect development. This study provides further insights into physiological responses of L. trifolii under climate change, and the mechanisms of distribution of leaf miner flies in response to temperature.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/10/10/775/s1, Figure S1: Supplementary relative expression levels of LtHSP21.3 and LtHSP21.7 at different developmental stages or under temperature treatments. The relative level of HSP expression represented the fold increase as compared with the expression in controls. (A) Relative expression levels of LtHSP21.3 in different developmental stages; (B) Relative expression levels of LtHSP21.7 under low temperatures; and (C) Relative expression levels of LtHSP21.7 under high temperatures. The data were denoted as mean ± SE. One-way analysis of variance (ANOVA) was used to analyze the relative expression levels of three sHSPs in different developmental stages and under temperature treatments. For the ANOVA, data were tested for homogeneity of variances and normality. Different lowercase letters indicate significant differences among different temperature treatments. Tukey’s multiple range test was used for pairwise comparison for mean separation (P < 0.05). Abbreviations: FM = females adult; M: males adult; L: third instar larvae; PP: prepupae; P: two-day-old pupae; OP: ten-day-old pupae, Table S1: Primers used in the cDNA cloning and real-time quantitative PCR.
Author Contributions
Y.-W.C., M.-X.L. and Y.-Z.D. conceived and designed experiments. Y.-W.C., and X.-X.Z. conducted experiments. Y.-W.C., and M.-X.L. analyzed the data. Y.-W.C. and K.Z.-S. wrote the manuscript. All authors assisted in the critical follow up of the work, read and approved the manuscript.
Funding
This research was funded by the Modern Agriculture Industrial Technology System Program of Jiangsu (SXGC (2017) 218), the Jiangsu Science & Technology Support Program (BE2014410), the postgraduate Research and Practice Innovation Program of Jiangsu Province (KYCX18_2374), and the Yangzhou University International Academic Exchange Fund.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Spencer K.A. Agromyzidae (Diptera) of Economic Importance. The Hague Publishers; Bath, UK: 1973. pp. 19–28. (9: Series Entomologica). [Google Scholar]
- 2.Johnson M.W., Welter S.C., Toscano N.C., Tingi P., Trumble J.T. Reduction of tomato leaflet photosynthesis rates by mining activity of Liriomyza sativae (Diptera: Agromyzidae) J. Econ. Entomol. 1983;76:1061–1063. doi: 10.1093/jee/76.5.1061. [DOI] [Google Scholar]
- 3.Parrella M.P., Jones V.P., Youngman R.R., Lebeck L.M. Effect of leaf mining and leaf stippling of Liriomyza spp. on photosynthetic rates of chrysanthemum. Ann. Entomol. Soc. A. 1985;78:90–93. doi: 10.1093/aesa/78.1.90. [DOI] [Google Scholar]
- 4.Reitz S.R., Kund G.S., Carson W.G., Phillips P.A., Trumble J.T. Economics of reducing insecticide use on celery through low-input pest management strategies. Agric. Ecosyst. Environ. 1999;73:185–197. doi: 10.1016/S0167-8809(99)00016-X. [DOI] [Google Scholar]
- 5.Scheffer S.J., Lewis M.L. Mitochondrial phylogeography of the vegetable pest Liriomyza trifolii (Diptera: Agromyzidae): Diverged clades and invasive populations. Ann. Entomol. Soc. Am. 2006;99:991–998. doi: 10.1603/0013-8746(2006)99[991:MPOTVP]2.0.CO;2. [DOI] [Google Scholar]
- 6.Wang Z.G., Guan W., Chen D.H. Preliminary report of the Liriomyza trifolii in Zhongshan area. Plant Quarantine. 2007;21:19–20. [Google Scholar]
- 7.Gao Y.L., Reitz S., Xing Z.L., Ferguson S., Lei Z.R. A decade of a leafminer invasion in China: Lessons learned. Pest Manag. Sci. 2017;73:1775–1779. doi: 10.1002/ps.4591. [DOI] [PubMed] [Google Scholar]
- 8.Kang L., Chen B., Wei J.N., Liu T.X. Roles of thermal adaptation and chemical ecology in Liriomyza distribution and control. Annu. Rev. Entomol. 2009;54:127–145. doi: 10.1146/annurev.ento.54.110807.090507. [DOI] [PubMed] [Google Scholar]
- 9.Yang F., Cao J.M., Du Y.Z. Survey and molecular identification of Liriomyza trifolii in Jiangsu, China. Plant Prot. 2010;36:108–111. [Google Scholar]
- 10.Xiang J.C., Lei Z.R., Wang H.H. Interspecific competition among three invasive Liriomyza species. Acta Ecol. Sin. 2012;32:1616–1622. doi: 10.5846/stxb201101140077. [DOI] [Google Scholar]
- 11.Wen J.Z., Lei Z.R., Wang Y. Survey of Liriomyza huidobrensis in Yunnan Province and Guizhou Province, China. Plant Prot. 1998;24:18–20. [Google Scholar]
- 12.Wen J.Z., Wang Y., Lei Z.R. New record of Liriomyza sativae Blanchard (Diptera: Agromyzidae) from China. Entomotaxonomia. 1996;18:311–312. [Google Scholar]
- 13.Chang Y.W., Chen J.Y., Lu M.X., Gao Y., Tian Z.H., Gong W.R., Dong C.S., Du Y.Z. Cloning and expression of genes encoding heat shock proteins in Liriomyza trifolii and comparison with two congener leafminer species. PLoS ONE. 2017;12:e0181355. doi: 10.1371/journal.pone.0181355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reitz S.R., Trumble J.T. Interspecific and intraspecific differences in two Liriomyza leafminer species in California. Entomol. Exp. Appl. 2002;102:101–113. doi: 10.1046/j.1570-7458.2002.00930.x. [DOI] [Google Scholar]
- 15.Abe Y., Tokumaru S. Displacement in two invasive species of leafminer fly in different localities. Biol. Invasions. 2008;10:951–953. doi: 10.1007/s10530-007-9173-2. [DOI] [Google Scholar]
- 16.Wang H.H., Reitz S.R., Xiang J.C., Smagghe G., Lei Z.R. Does Temperature-Mediated Reproductive Success Drive the Direction of Species Displacement in Two Invasive Species of Leafminer Fly? PLoS ONE. 2014;9:e98761. doi: 10.1371/journal.pone.0098761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindquist S., Craig E.A. The heat-shock proteins. Annu. Rev. Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 18.Moseley P.L. Heat shock proteins and heat adaptation of the whole organism. J. Appl. Physiol. 1997;83:1413–1417. doi: 10.1152/jappl.1997.83.5.1413. [DOI] [PubMed] [Google Scholar]
- 19.Feder M.E., Hofmann G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- 20.Sørensen J.G., Kristensen T.N., Loeschcke V. The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 2003;6:1025–1037. doi: 10.1046/j.1461-0248.2003.00528.x. [DOI] [Google Scholar]
- 21.Horwitz J. α-Crystalline can function as a molecular chaperone. Proc. Natl. Acad. Sci. USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gusev N.B., Bogatcheva N.V., Marston S.B. Structure and properties of the small heat shock proteins (shsps) and their interaction with cytoskeleton proteins. Biochemistry (Mosc.) 2002;67:511–519. doi: 10.1023/A:1015549725819. [DOI] [PubMed] [Google Scholar]
- 23.Franck E., Madsen O., van-Rheede T., Ricard G., Huynen M.A., de-Jong W.W. Evolutionary diversity of vertebrate small heat shock proteins. J. Mol. Evol. 2004;59:792–805. doi: 10.1007/s00239-004-0013-z. [DOI] [PubMed] [Google Scholar]
- 24.Haslbeck M., Franzmann T., Weinfurtner D., Buchner J. Some like it hot: The structure and function of small heat-shock proteins. Nat. Struct. Mol. Biol. 2005;12:842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- 25.Hu J.T., Chen B., Li Z.H. Thermal plasticity is related to the hardening response of heat shock protein expression in two Bactrocera fruit flies. J. Insect. Physiol. 2014;67:105–113. doi: 10.1016/j.jinsphys.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Johnston J.A., Ward C.L., Kopito R.R. Aggresomes: A cellular response to misfolded proteins. J. Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basha E., O’Neill H., Vierling E. Small heat shock proteins and α-crystallins: Dynamic proteins with flexible functions. Trends Biochem. Sci. 2012;37:106–117. doi: 10.1016/j.tibs.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King A.M., Macrae T.H. Insect heat shock proteins during stress and diapause. Annu. Rev. Entomol. 2015;60:59–75. doi: 10.1146/annurev-ento-011613-162107. [DOI] [PubMed] [Google Scholar]
- 29.Gehring W.J., Wehner R. Heat shock protein synthesis and thermotolerance in Cataglyphis, an ant from the Sahara Desert. Proc. Natl. Acad. Sci. USA. 1995;92:2994–2998. doi: 10.1073/pnas.92.7.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu M.X., Hua J., Cui Y.D., Du Y.Z. Five small heat shock protein genes from Chilo suppressalis: Characteristics of gene, genomic organization, structural analysis, and transcription profiles. Cell Stress Chaperon. 2014;19:91–104. doi: 10.1007/s12192-013-0437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan D.D., Lu M.X., Li Q.Y., Du Y.Z. Characteristics and expression of genes encoding two small heat shock protein genes lacking introns from Chilo suppressalis. Cell Stress Chaperon. 2017;23:1–10. doi: 10.1007/s12192-017-0823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H.H., Rreitz S., Wang L.X., Wang S.Y., Xue L.I., Lei Z.R. The mRNA expression proifles of five heat shock protein genes from Frankliniella occidentalis at different stages and their responses to temperatures and insecticides. J. Integr. Agric. 2014;13:2196–2210. doi: 10.1016/S2095-3119(13)60680-2. [DOI] [Google Scholar]
- 33.Quan G.X., Duan J., Ladd T., Krell P.J. Identification and expression analysis of multiple small heat shock protein genes in spruce budworm, Choristoneura fumiferana, (L.) Cell Stress Chaperon. 2017;23:141–154. doi: 10.1007/s12192-017-0832-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dou W., Tian Y., Liu H., Shi Y., Smagghe G., Wang J.J. Characteristics of six small heat shock protein genes from Bactrocera dorsalis: Diverse expression under conditions of thermal stress and normal growth. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2017;213:8–16. doi: 10.1016/j.cbpb.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Rinehart J.P., Li A., Yocum G.D., Robich R.M., Hayward S.A., Denlinger D.L. Up-regulation of heat shock proteins is essential for cold survival during insect diapause. Proc. Natl. Acad. Sci. USA. 2007;104:11130–11137. doi: 10.1073/pnas.0703538104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joanisse D.R., Michaud S., Inaguma Y., Tanguay R.M. Small heat shock proteins of Drosophila: Developmental expression and functions. J. Biosci. 1998;23:369–376. doi: 10.1007/BF02936130. [DOI] [Google Scholar]
- 37.Haass C.H., Klein U.L., Kloetzel P.M. Developmental expression of Drosophila melanogaster small heat-shock proteins. J. Cell Sci. 1990;96:413–418. doi: 10.1242/jcs.96.3.413. [DOI] [PubMed] [Google Scholar]
- 38.Saravanakumar R., Ponnuvel K.M., Qadri S.M.H. Expression of metabolic enzyme genes and heatshock protein genes during embryonic development in diapause and non-diapause egg of multivoltine silkworm Bombyx mori. Biologia. 2008;63:737–744. doi: 10.2478/s11756-008-0124-x. [DOI] [Google Scholar]
- 39.Shen Y., Gu J., Huang L.H., Zheng S.C., Liu L., Xu W.H., Feng Q.L., Kang L. Cloning and expression analysis of six small heat shock protein genes in the common cutworm, Spodoptera litura. J. Insect Physiol. 2011;57:908–914. doi: 10.1016/j.jinsphys.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 40.Gu J., Huang L.X., Shen Y., Huang L.H., Feng Q.L. Hsp70 and small Hsps are the major heat shock protein members involved in midgut metamorphosis in the common cutworm, Spodoptera litura. Insect Mol. Biol. 2012;21:534–543. doi: 10.1111/j.1365-2583.2012.01158.x. [DOI] [PubMed] [Google Scholar]
- 41.Huang L.H., Kang L. Cloning and interspecific altered expression of heat shock protein genes in two leafminer species in response to thermal stress. Insect Mol. Biol. 2007;16:491–500. doi: 10.1111/j.1365-2583.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 42.Huang L.H., Wang C.Z., Kang L. Cloning and expression of five heat shock protein genes in relation to cold hardening and development in the leafminer, Liriomyza sativa. J. Insect Physiol. 2009;55:279–285. doi: 10.1016/j.jinsphys.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Chang Y.W., Chen J.Y., Lu M.X., Gao Y., Tian Z.H., Gong W.R., Zhu W., Du Y.Z. Selection and validation of reference genes for quantitative real time PCR analysis under different experimental conditions in the leafminer Liriomyza trifolii (Diptera: Agromyzidae) PLoS ONE. 2017;12:e0181862. doi: 10.1371/journal.pone.0181862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen B., Kang L. Cold hardiness and supercoo ling capacity in the pea leafminer Liriomyza huidobrensis. Cryo Letters. 2002;23:173–182. [PubMed] [Google Scholar]
- 45.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 46.Thompson J.D., Gibson T.J., Plewniak F., Jeanmougin F., Higgins D.G. The Clustal-X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabaska J.E., Zhang M.Q. Detection of polyadenylation signals in human DNA sequences. Gene. 1999;231:77–86. doi: 10.1016/S0378-1119(99)00104-3. [DOI] [PubMed] [Google Scholar]
- 49.Lindquist S., Petersen R. Selective translation and degradation of heat-shock messenger RNAs in Drosophila. Enzyme. 1990;44:147–166. doi: 10.1159/000468754. [DOI] [PubMed] [Google Scholar]
- 50.Colgan D.F., Manley J.L. Mechanism and regulation of mRNA polyadenylation. Genes Dev. 1997;11:2755–2766. doi: 10.1101/gad.11.21.2755. [DOI] [PubMed] [Google Scholar]
- 51.Rubenstein R.C., Lyons B.M. Sodium 4-phenylbutyrate downregulates HSC70 expression by facilitating mRNA degradation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;281:L43–L51. doi: 10.1152/ajplung.2001.281.1.L43. [DOI] [PubMed] [Google Scholar]
- 52.Hunt C., Morimoto R.I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of Human hsp70. Proc. Natl. Acad. Sci. USA. 1985;82:6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu C.H., Madabusi L., Nishioka H., Emanuel P., Sypes M., Arkhipova I., Gilmour D.S. Analysis of core promoter sequences located downstream from the TATA element in the hsp70 promoter from Drosophila melanogaster. Mol. Cell. Biol. 2001;21:1593–1602. doi: 10.1128/MCB.21.5.1593-1602.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grace M.L., Chandrasekharan M.B., Hall T.C., Crowe A.J. Sequence and spacing of TATA box elements are critical for accurate initiation from the beta-phaseolin promoter. J. Biol. Chem. 2004;279:8102–8110. doi: 10.1074/jbc.M309376200. [DOI] [PubMed] [Google Scholar]
- 55.Martín-Folgar R., Fuente M., Morcillo G., Martínez-Guitarte J. Characterization of six small HSP genes from Chironomus riparius (Diptera, Chironomidae): Differential expression under conditions of normal growth and heat-induced stress. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015;188:76–86. doi: 10.1016/j.cbpa.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 56.Sun M., Lu M.X., Tang X.T., Du Y.Z. Characterization and Expression of Genes Encoding Three Small Heat Shock Proteins in Sesamia inferens (Lepidoptera: Noctuidae) Int. J. Mol. Sci. 2014;15:23196–23211. doi: 10.3390/ijms151223196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang X.T., Sun M., Lu M.X., Du Y.Z. Expression patterns of five heat shock proteins in Sesamia inferens, (Lepidoptera: Noctuidae) during heat stress. J. Asia-Pac. Entomol. 2015;18:529–533. doi: 10.1016/j.aspen.2015.07.005. [DOI] [Google Scholar]
- 58.Lu M.X., Li H.B., Zheng Y.T., Shi L., Du Y.Z. Identification, genomic organization and expression profiles of four heat shock protein genes in the western flower thrips, Frankliniella occidentalis. J. Therm. Biol. 2016;57:110–118. doi: 10.1016/j.jtherbio.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 59.Chang Y.W., Zhang X.X., Chen J.Y., Lu M.X., Gong W.R., Du Y.Z. Characterization of three heat shock protein 70 genes from Liriomyza trifolii and expression during thermal stress and insect development. B. Entomol. Res. 2019;109:150–159. doi: 10.1017/S0007485318000354. [DOI] [PubMed] [Google Scholar]
- 60.Bai J., Liu X.N., Lu M.X., Du Y.Z. Characterization of genes encoding small heat shock proteins from Bemisia tabaci and expression under thermal stress. PeerJ. 2019;7:e6992. doi: 10.7717/peerj.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.