Abstract
Background
Budesonide-MMX has an established role in the management of relapsing mild-to-moderate ulcerative colitis. Data regarding effectiveness and tolerability in real-life clinical practice are limited.
Aim
The aim of this study was to assess the use of budesonide-MMX in ulcerative colitis, as well as short-term effectiveness and tolerability in real-life practice.
Methods
We conducted a retrospective study of adult patients with mild-to-moderate ulcerative colitis treated with budesonide-MMX at four tertiary inflammatory bowel disease centres in Italy from June 2016 to February 2018. Demographic and clinical features of patients, the use of budesonide-MMX, disease course and concomitant therapy were recorded. The primary outcome assessed was clinical remission at 2 months.
Results
A total of 82 patients with active mild-to-moderate ulcerative colitis were included in the study with a mean age of 45.9 years and a median partial Mayo Score of 4 (interquartile range 3–5). A total of 41 patients were male. Overall, 36 had extensive colitis, 38 left-sided colitis and eight proctitis. Treatments at the time of inclusion included 10 patients receiving biologic therapy, seven azathioprine and 54 mesalazine or salazopyrin. The main reasons for the addition of budesonide-MMX were clinical relapse (47.5%) or inadequate response to current therapy (39.0%). In total, 50% of patients achieved clinical remission, whereas 9.8% had clinical improvement. No response was noted in 40.2% of subjects. Using multivariate binary logistic regression, a moderate degree of activity was the main independent predictor of non-response. Eight significant adverse effects were reported in six patients with three discontinuing treatment.
Conclusion
In real-life clinical practice, budesonide-MMX is commonly used in combination with other therapies, both for acute disease flares and for partial response to therapy.
Keywords: Budesonide MMX, ulcerative colitis, real life
Key summary
Budesonide-MMX is recommended for the induction of remission in patients with mild-to-moderate ulcerative colitis where 5-ASA treatment is not sufficient.
Data on the efficacy and tolerability come mainly from randomized controlled trials, whereas data from real-life clinical practice are lacking.
In real-life clinical practice, budesonide-MMX is commonly used in combination with other therapies, both for disease flares and for partial response to therapy.
The effectiveness and safety profile of budesonide-MMX in real-life clinical practice appears consistent with previously published prospective trials.
Moderate disease activity and the need for immunosuppressant or biologic therapies appear to be the main predictors of non-response to budesonide-MMX.
Introduction
The management of ulcerative colitis (UC) is generally guided by the extent of the disease and the degree of activity.1
Conventional corticosteroids have well-established efficacy for the induction of clinical remission, but have numerous short- and long-term side effects that limit their use. Thus, corticosteroid formulations with low bioavailability were developed to mitigate systemic effects (Budesonide and beclomethasone). Recently, a new formulation of budesonide incorporating a multi-matrix technology (budesonide-MMX) to facilitate colonic delivery has been approved for the treatment of UC. There is a paucity of comparative studies between conventional corticosteroids and budesonide-MMX.2 In randomized clinical trials, budesonide-MMX has been shown to be safe and efficacious for the treatment of mild-to-moderate UC and is comparable with mesalazine in induction and maintenance of remission. In addition, it is superior to placebo in patients with UC intolerant and/or refractory to mesalazine.3–7 Further studies are needed to assess the real-life effectiveness of budesonide-MMX in clinical practice and determine its position as a therapeutic agent.
This retrospective study examines the use of budesonide-MMX in real-life clinical practice, as well as the short-term effectiveness and tolerability.
Patients and methods
Study design
We performed a multicentre retrospective cohort study of patients with UC treated with budesonide-MMX at four tertiary inflammatory bowel disease (IBD) referral centres (Fatebenefratelli-Sacco Hospital Milan; Policlinico San Donato, Milan; Legnano Hospital and Rho Hospital) from June 2016 to February 2018.
Inclusion criteria included a clearly established diagnosis of ulcerative colitis (>6 months), age >18 years and at least one prescription for budesonide-MMX. Patients were excluded if they had a previous colectomy, failed to take budesonide-MMX as prescribed or discontinued the therapy early for reasons unrelated to disease or adverse effects, started azathioprine or a biologic therapy within the prior 2 months, or were lost to follow-up where outcome data could not be assessed.
Data collection
Demographic and clinical characteristics were collected including age, sex, duration of UC, disease extent (proctitis, left-sided colitis, extensive colitis), relevant comorbidities (assessed using the Charlson Comorbidity Index), prior use of immunosuppressant or biologic agents, and current therapies (mesalazine, salazopyrin, immunosuppressants and biologic agents). Reasons for using budesonide-MMX were recorded and categorized as: mild-to-moderate disease relapse, partial or incomplete response to prior therapy, adverse effects to prior therapy, or as an alternative to systemic corticosteroids. Clinical activity was determined by the partial Mayo Score, whereas biochemical activity was assessed using C-reactive protein at the time of budesonide-MMX initiation. Regular clinic visits typically occur every 6 months, or earlier if needed in the case of any adverse event or worsening disease. Thus, follow-up data were generally available within 6 months of a prescription for budesonide-MMX. Endoscopic activity was assessed within 2 weeks prior to the start of budesonide-MMX therapy and at the time of the next endoscopic evaluation. However, due to the retrospective design of this ‘real-life’ study, data on the endoscopic subscore of the Mayo score are not provided.
Disease monitoring
Budesonide-MMX is approved for treatment courses up to 8 weeks long and thus we assessed the response to therapy at 2 months. In patients who continued therapy for longer, further effectiveness and safety data were also collected. In addition to the clinical data recorded at 2 months, it was also collected at a visit between 2 and 6 months and was confirmed at a later date by phone interview.
Adherence to budesonide-MMX therapy was confirmed retrospectively by assessing the number of days the patient took the therapy. The reasons for treatment discontinuation (lack of response, partial response and adverse effects), changes in concomitant therapy after starting budesonide-MMX (both systemic and topical) and all potential side effects related to budesonide-MMX use were recorded.
Outcomes
The primary outcome was clinical remission at the end of 2 months of budesonide-MMX therapy, defined as a partial Mayo Clinic Score of 0–1 with a rectal bleeding sub-score = 0. Clinical response has been defined as reduction of the partial Mayo score ≥3 points and ≥30% compared with baseline.8 Secondary outcomes included compliance to the treatment, prevalence of side effects and identification of factors predictive of clinical remission.
Statistical analysis
Descriptive statistics (mean or median, with standard deviation or range) were calculated according to the parameter distribution. Because this was a preliminary descriptive study, an estimated sample size was not determined. The demographic and clinical parameters for responders and non-responders to budesonide-MMX was assessed using the Chi-square test with Yates correction and the student's t test or Mann-Whitney U test, where appropriate. Parameters with missing values were not considered in the analysis. Univariate and multivariate binary logistic regression was performed to examine independent predictors of complete response to budesonide-MMX treatment. A p value <0.05 was considered significant.
Results
From June 2016 to February 2018, 89 patients with UC at four IBD referral centres received prescriptions for budesonide-MMX therapy (53 at Fatebenefratelli-Sacco Hospital Milan, 11 at Policlinico San Donato, Milan, 10 at Legnano Hospital and 15 at Rho Hospital). However, three patients did not start treatment, two patients received concomitant initiation of adalimumab and azathioprine and two were lost to follow-up and excluded from the study (Figure 1). The baseline characteristics of the 82 patients included in the study are provided in Table 1.
Figure 1.
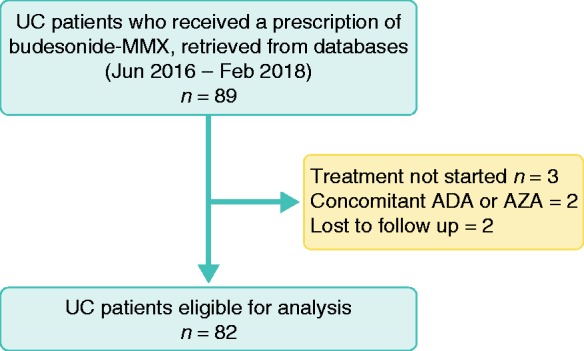
Flow chart of the study population.
Table 1.
Demographic and clinical features of study population.
| Male (%) | 41 (50.0) |
| Age, mean (SD), yr | 45.9 (19.1) |
| Disease duration, median (IQR), yr, n (%) | 5 (2–13) |
| >6 | 29 (35.4) |
| 4–6 | 21 (25.6) |
| ≤3 | 32 (39.0) |
| Disease location, n (%) | |
| Proctitis | 8 (9.8) |
| Left-sided | 38 (46.3) |
| Extensive colitis | 36 (43.9) |
| Partial Mayo score, median (IQR) | 4 (3-5) |
| Remission (partial Mayo score < 2) n (%) | 1 (1.2) |
| Mild activity (partial Mayo score 2–4) n (%) | 53 (64.6) |
| Moderate activity (partial Mayo score 5–7) n (%) | 28 (34.2) |
| Increased C-reactive proteina, n (%) | 10 (11.0) |
| Charlson Index, median (IQR) | 0 (0–2) |
| Primary concomitant medications, n (%) | |
| Infliximab | 1 (1.2) |
| Adalimumab | 3 (3.7) |
| Golimumab | 3 (3.7) |
| Vedolizumab | 3 (3.7) |
| Azathioprine | 7 (8.5) |
| Salazopyrin | 2 (2.4) |
| Mesalazine | 44 (53.7) |
| Mesalazine MMX | 10 (12.2) |
| Escherichia coli Nissle | 1 (1.2) |
| Beclometasone dipropionate | 1 (1.2) |
| No oral or systemic therapy | 7 (8.5) |
| Previous anti TNF agents or azathioprine, n (%) | 6 (7.1) |
| Topical therapy, n (%) | |
| Mesalazine | 42 (51.2) |
| Beclometasone Mesalazine + beclometasone | 8 (9.8) 3 (3.7) |
| Use for budesonide-MMX therapy, n (%) | |
| Clinical relapse | 39 (47.5) |
| Partial or not satisfactory response to previous therapy | 32 (39.0) |
| Side effects of the previous therapy | 3 (3.7) |
| Alternative use to systemic corticosteroids | 8 (9.8) |
Normal value < 10 ml/L.
IQR: interquartile range.
Study population
The study population had a mean age of 45.9 years (SD 19.1, range 18–87) and a median disease duration of 5 years (interquartile range (IQR) 2–10.5). In total, 41 (50%) patients were male, 36 patients (43.9%) had extensive colitis, 38 (46.3%) left-sided colitis and eight (9.8%) proctitis. Overall, 47 patients had no relevant comorbidities, whereas 35 had at least one other illness, with a median Charlson index score of 3 (IQR 1–4). The median partial Mayo score at the time of Budesonide-MMX treatment was 4 (IQR 3–5, range 0–7; mean 4.13, SD 1.39) and 10 patients (11%) had an increased C-reactive protein. Only 18 patients underwent colonoscopy within 2 weeks before starting budesonide-MMX (one had severe disease activity, 12 had moderate activity and five mild activity), therefore, endoscopic activity was not further evaluated in this study.
At the time of budesonide-MMX initiation, 75 patients were using another medication for UC, 10 were receiving biologics (three in combination with mesalazine and one with azathioprine), seven were treated with azathioprine (one also with mesalazine), 58 were treated with mesalazine or salazopyrin and four patients were on topical therapy (5-ASA or beclomethasone suppositories) alone. In total, 42 (51.2%) patients were also using topical mesalazine, eight (9.8%) were using topical beclometasone and three (3.7%) were using both. All patients remained on topical therapy during treatment with Budesonide-MMX.
The baseline therapy remained unchanged after budesonide-MMX treatment for 77 patients, whereas two patients switched from mesalazine with mesalazine-MMX, one switched from mesalazine to Escherichia coli Nissle and two discontinued either mesalazine-MMX or beclometasone.
Budesonide-MMX use
All patients received a dose of budesonide-MMX 9 mg daily. The primary use for the addition of budesonide-MMX was due to a clinical relapse (47.5%) or partial response to the prior therapy (39.0%). Budesonide-MMX was prescribed for one (1.2%) patient in clinical remission (intolerant of previous treatment), for 53 (64.6%) patients with mild disease activity (partial Mayo score 2–4) and for 28 (34.1%) patients with moderate disease activity (partial Mayo score 5–7). In total, 22 (26.8%) patients (likely with steroid-refractory or steroid-dependent UC) had been (five) or were being (17) treated with biologics or azathioprine.
In total, 25 patients did not complete the 8-week course of therapy with budesonide-MMX (Figure 2). Of these, six (24%) achieved the primary endpoint of clinical remission at 2 months, whereas most non-responders stopped treatment within 4 weeks (Figure 2).
Figure 2.
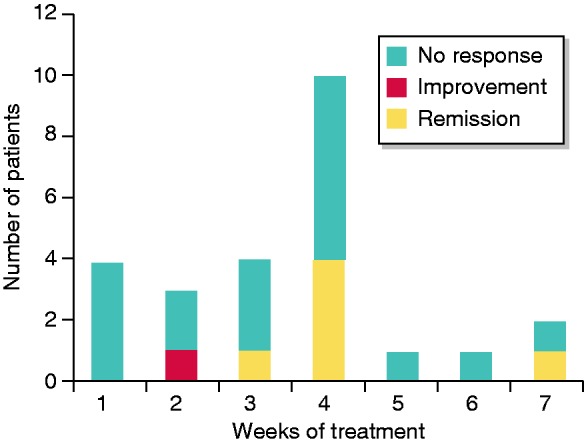
Duration of treatment with Budesonide MMX and outcome at 2 months in patients who did not complete 8 weeks of treatment.
Budesonide-MMX effectiveness
Clinical remission was achieved in 41 out of 82 patients (50%) and clinical response in two patients (2.4%), whereas no response was reported in 39 patients (47.6%).
Those that achieved clinical remission had a greater prevalence, although not statistically significant, of proctitis and left-sided colitis, compared with extensive colitis (65.9% vs 34.1%, p = 0.119) and were not receiving biologic or immunosuppressant therapy (90.2% vs 9.8%, p = 0.029). In addition, those with mild disease activity were more likely to reach clinical remission versus those with moderate disease activity (78.0% vs 21.9%, p = 0.036).
Univariate and multivariate binary logistic regression found that concomitant therapy with biologics and immunosuppressants, as well as degree of disease activity were independent predictors of non-response (Table 2). Furthermore, clinical remission was achieved in 75% (18/24) of patients with mild disease, limited to left-sided colitis or proctitis, who had never been treated with a biologic or immunosuppressant medication. In contrast, eight patients with moderate disease and either prior or current use of a biologic or immunosuppressant did not achieve clinical remission. Age, sex, comorbidity, duration of UC, use of topical therapy and the use for budesonide-MMX were not associated with achieving the primary outcome.
Table 3.
Side effects and outcome.
| Patient n | Side effect(s) | Outcome at 2 months | Duration of treatment |
|---|---|---|---|
| 1 | Mild face hirsutism | Clinical response | 20 weeks |
| 2 | Constipation and tenesmus | No response | 2 weeks |
| 3 | Hypertensive crisis | Clinical response | 3 weeks |
| 4 | Iatrogenic Cushing's syndrome | Clinical response | 4 weeks |
| 5 | Headache | Clinical response | 8 weeks |
| 6 | Acne and insomnia | Clinical response | 8 weeks |
Table 2.
Factors predicting complete remission.
| Factor | Univariate (OR (95% CI)) | Multivariate (OR (95% CI)) |
|---|---|---|
| Disease extension (Proctitis/left-sided colitis vs. extensive colitis) | 2.23 (0.92–5.44) | –- |
| Concomitant treatment with biologics or immunosuppressors (no vs pervious/active treatment) | 0.23 (0.069–0.79) | 0.35 (0.095–1.28) |
| Global activity evaluation at baseline (mild vs. moderate) | 5.9 (1.17–19.8) | 4.6 (1.13–16.0) |
CI: confidence interval; OR: odds ratio.
Side effects and tolerability
Eight significant adverse effects were reported in six patients (facial hirsutism, constipation and tenesmus, hypertensive crisis, iatrogenic Cushing's syndrome, headache, acne and insomnia). Treatment was discontinued in three of these patients.
In total, 58 (70.7%) patients completed at least 8 weeks of treatment, with eight patients continuing beyond 8 weeks for a median duration of 18.5 weeks (IQR 13–20). However, 25 patients (30.5%) discontinued treatment < 8 weeks after a median of 4 weeks (IQR 2–4) due to persistent symptoms or lack of symptomatic improvement in 19 patients, clinical remission in four patients and significant side effects in two patients (hypertensive crisis, iatrogenic Cushing's syndrome).
Discussion
Data from real-life in medicine may be different from that of clinical trials and sometimes the management of diseases and compliance of patients do not follow the clinical guidelines.
The results of this real-life study are in keeping with these assumptions.
In this study we found the adherence and compliance rates to budesonide-MMX, not previously reported, was rather unsatisfactory. Three patients did not initiate therapy and 25% of patients, despite being in clinical remission, discontinued treatment before completing an 8-week course (between 2 and 7 weeks). However, these findings, specifically the rate of patients that did not start the prescribed therapy, are consistent with adherence rates of mesalazine in similar clinical conditions9 and in keeping with studies assessing nonadherence to medical therapy in UC patients using budesonide for acute disease flares.10, 11 The exact reasons remain unknown, but may be because of spontaneous or quick improvement of symptoms early in the disease course, particularly in patients with mild disease, where flares may be occasional or only ‘symptomatic’ and not always correlated with a true relapse.
In fact, most patients (80%) started the treatment without any endoscopic assessment and only on account of symptoms and signs suggestive of clinical relapse or incomplete response to prior therapy. There are several reasons for this, including the reluctance of patients to undergo repeated endoscopic exams, need to promptly start the therapy and confidence of safe and effective treatment. In this regard it is worth nothing that 44% of patients had extensive colitis, 34% had moderate disease activity (partial Mayo score 5–7) and 27% had steroid-refractory or steroid-dependent UC, with most patients receiving biologics or azathioprine. The use of budesonide-MMX for these clinical conditions has not been fully evaluated by the previously published prospective clinical trials. This seems to suggest that budesonide-MMX therapy was, and could be, considered a prompt temporary adjunct therapy to concurrent oral and/or topical therapies (which remained unchanged during budesonide treatment) to stop recurrence in an early phase, or to overcome a possible loss of efficacy of a long-term treatment.
In particular, most patients received a combination therapy with mesalazine and budesonide-MMX. These patients seemed to have better outcomes compared with the previous prospective studies, where budesonide-MMX was used as monotherapy with clinical efficacy not exceeding 20%.5–7 In our study, 50% of patients achieved clinical remission and 10% showed clinical improvement without remission. These results seem to be superior to those that emerged in the previous study by Rubin et al.,3 which included a population very similar in treatment (mesalamine with budesonide-MMX); this could be explained by the fact that in this latter study, patients with evidence of limited distal proctitis were excluded. Our study also demonstrates the variable, heterogenous and somewhat irrational use of budesonide-MMX in real-life settings compared with use in clinical trials, including extensive colitis and even in steroid-dependent patients receiving biologic therapy. Overall, the effectiveness of budesonide-MMX in achieving complete remission was greater in patients with proctitis and left-sided colitis than those with extensive colitis, as shown in previous randomised controlled trials (RCTs), as well as among patients naive to biologics or immunosuppressive agents. These findings are expected, as UC patients treated with corticosteroid or immunosuppressant therapy are likely to have more severe disease.
Univariate analysis found that concomitant treatment with biologics and immunosuppressants and degree of global activity were independent predictors of non-response, but only the latter was confirmed by multivariate binary logistic regression analyses. In addition, clinical remission was achieved in 75% of patients with mild disease limited to left-sided colitis or proctitis, who were not treated with biologics or immunosuppressants. None of the eight patients with moderate colitis and previous or current use of biologics or immunosuppressive agents reached clinical remission or improvement. Thus, these results demonstrate that in carefully selected patients in real-world practice, budesonide-MMX may provide excellent results with more than 75% of patients reaching clinical remission.
Patients that achieved a complete and stable response to budesonide-MMX were allowed to continue treatment, in most cases with satisfactory results. However, data regarding long-term treatment with budesonide-MMX are not available.
Overall, budesonide-MMX was well tolerated. The adverse effect rate was low and patients who developed adverse effects generally did not require discontinuation of treatment. These results are comparable with those from prospective trials.
Our study has limitations intrinsic to observational retrospective research. For example, recall bias cannot be excluded, we did not have endoscopic and biochemical – including faecal calprotectin – data at baseline and at the end of treatment and data collection was performed between 2–4 months. But this study is reflective of real-life clinical practice. We attempted to mitigate these limitations, involving only four centres close to our hospital, to have a tighter control on data collection and to include all patients who have had been provided with a prescription of the drug. Another limitation is the lack of quality of life and patient satisfaction data, which are not collected routinely.
To our knowledge, this is the first study exploring the effectiveness of budesonide-MMX in a real-life setting demonstrating that it is frequently used in combination with other medications and providing outcome data on the effectiveness and safety.
In conclusion, our results show that in real-life clinical practice, budesonide-MMX is frequently used in combination with mesalazine for both disease flares of UC and incomplete response to conventional therapy. It seems to be safe and effective, especially for mild disease activity and in patients not receiving immunosuppressives or biologics, with higher rates than in reported prospective trials. Our study confirms the best results are seen in patients who fit the 2017 UC European Crohn's and Colitis Organisation recommendations.
Acknowledgements
The authors thank Michale Stewart for editing and language revision of this manuscript.
Authorship statement
GM performed the research, collected and analysed the data, designed the research study and wrote the paper; SL, SG, CB, MB, LP, NM, SC, PM, AC, AM collected and analysed the data; AD analysed the data and wrote the paper; RC and SA contributed to writing the paper.
Declaration of conflicting interests
GM is Speaker honoraria, Abbvie, Alfa Sigma, Janssen-Cilag; Advisory Board/Consultant fee, Janssen-Cilag, Allergan, Novartis, Takeda, THD. AS has received consulting and/or advisory board fees and/or research support from AbbVie, MSD, Ferring, MSD, Janssen, Takeda, Mundipharma, Zambon, Sofar, Recordati.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The protocol was approved by the local Ethics Committee.
Informed consent
The data collection was performed after obtaining the patient’s informed consent.
References
- 1.Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: Current Management. J Crohns Colitis 2017; 11: 769–784. [DOI] [PubMed] [Google Scholar]
- 2.Bezzio C, Festa S, Zerboni G, et al. A safety evaluation of budesonide MMX for the treatment of ulcerative colitis. Expert Opin Drug Saf 2018; 17(4): 437–444. [DOI] [PubMed] [Google Scholar]
- 3.Rubin DT, Cohen RD, Sandborn WJ, et al. Budesonide multi-matrix is efficacious for mesalamine-refractory, mild to moderate ulcerative colitis: A randomized, placebo-controlled trial. J Crohns Colitis 2017; 11: 785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherlock ME, MacDonald JK, Griffiths AM, et al. Oral budesonide for induction of remission in ulcerative colitis. Cochrane Database Syst Rev 2015; 26(10): CD007698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandborn WJ, Danese S, D'Haens G, et al. Induction of clinical and colonoscopic remission of mild-to-moderate ulcerative colitis with budesonide MMX 9 mg: pooled analysis of two phase 3 studies. Aliment Pharmacol Ther. 2015; 41(5): 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandborn WJ, Travis S, Moro L, et al. Once-daily budesonide MMX® extended-release tablets induce remission in patients with mild to moderate ulcerative colitis: results from the CORE I study. Gastroenterol 2012; 143(5): 1218-26.e1–2. [DOI] [PubMed] [Google Scholar]
- 7.Travis SP, Danese S, Kupcinskas L, et al. Once-daily budesonide MMX in active, mild-to-moderate ulcerative colitis: results from the randomised CORE II study. Gut 2014; 63(3): 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis JD, Chuai S, Nessel L, et al. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis 2008; 14: 1660–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lachaine J, Yen L, Beauchemin C, et al. Medication adherence and persistence in the treatment of Canadian ulcerative colitis patients: analyses with the RAMQ database. BMC Gastroenterol 2013; 30(13): 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bezzio C, Fascì-Spurio F, Viganò C, et al. The problem of adherence to therapy in ulcerative colitis and the potential utility of multi-matrix system (MMX) technology. Expert Rev Gastroenterol Hepatol 2017; 11: 33–41. [DOI] [PubMed] [Google Scholar]
- 11.Severs M, Mangen MJ, Fidder HH, et al. Clinical predictors of future nonadherence in inflammatory bowel disease. Inflamm Bowel Dis 2017; 23(9): 1568–1576. [DOI] [PubMed] [Google Scholar]


