Abstract
Background
Data from trials of vedolizumab for inflammatory bowel disease and from real-world studies suggest an exposure-response relationship, such that vedolizumab trough levels may predict clinical and endoscopic outcomes.
Objective
The purpose of this study was to evaluate in a prospective observational study the utility of an early vedolizumab trough level assay for predicting the first-year vedolizumab therapy outcome.
Methods
This prospective observational study included consecutive inflammatory bowel disease patients. We measured vedolizumab trough levels and anti-vedolizumab antibodies at weeks 6 and 14. Clinical outcome was assessed at weeks 6, 14, 22 and 54. The primary endpoint was the correlation between early vedolizumab trough levels and vedolizumab persistence over the first year of treatment, defined as the maintenance of vedolizumab therapy due to sustained clinical benefit.
Results
We included 101 patients initiating vedolizumab. A cut-off vedolizumab trough level of 16.55 µg/ml at week 14 predicted vedolizumab persistence within the first year of therapy, with 73.3% sensitivity and 59.4% specificity (p = 0.0009). Week 14 vedolizumab trough level was significantly higher in patients with clinical remission at weeks 14, 22 and 54; and in patients achieving mucosal healing within 54 weeks.
Conclusion
High vedolizumab trough level at week 14 was associated with a higher probability of maintaining vedolizumab therapy over the first year due to sustained clinical benefit.
Keywords: Vedolizumab, therapeutic drug monitoring, Crohn’s disease, ulcerative colitis
Key summary
Established knowledge on this subject
The role of therapeutic drug monitoring (TDM) in vedolizumab (VDZ) therapy remains controversial.
New findings of this study
A VDZ trough level (VTL) at week 14 higher than 16.55 µg/ml predicted treatment persistence over the first year. Week 14 VTL was higher in patients with clinical remission at weeks 14, 22 and 54 and in patients achieving mucosal healing.
Introduction
Vedolizumab (VDZ) is a humanised monoclonal antibody able to bind α4β7 integrin expressed on circulating lymphocytes, selectively preventing their migration into the gut mucosa.1 The Food and Drug Administration and the European Medicines Agency have approved VDZ for moderate-to-severe Crohn’s disease (CD) and ulcerative colitis (UC) patients who do not respond or are intolerant to conventional therapy or tumour necrosis factor alpha (TNFα) antagonists.2,3 Its approval was based on the results of the phase 3 study of vedolizumab in patients with moderate to severe ulcerative colitis and Crohn's disease (GEMINI) programmes, which included ∼1500 patients who received VDZ therapy with follow-up of up to five years.4–8 Long-term GEMINI efficacy data showed that patients who initially responded to VDZ treatment had a large probability of maintaining benefit over time.7–10 Although therapeutic drug monitoring (TDM) has been widely validated and is routinely applied in referral centres for anti TNF-α drugs (especially in cases of loss of response),11,12 its role in VDZ therapy remains controversial. The GEMINI studies revealed a relationship between VDZ exposure and response, with higher VDZ concentration quartiles at week 6 being associated with increased rates of clinical response and remission at week 6, among both UC and CD patients.4,5,13 Moreover, UC patients with higher VDZ concentration quartiles at week 6 were more likely to achieve clinical remission at weeks 14 and 52.14 Real-world emerging data suggest that the assay of early VDZ trough levels (VTLs) could predict response, mucosal healing, and need for dose optimization.15–20
In clinical practice, therapeutic success can be appropriately gauged by persistence, which reflects several variables, including effectiveness, safety and tolerability although it may be influenced by the availability of additional lines of medication.21 In the present study, we investigated the utility of an early VTL assay for predicting the first-year VDZ therapy outcome and identifying patients at higher risk of VDZ therapy failure.
Materials and methods
Patient population and study design
We conducted an observational, prospective, cohort study at two Italian referral inflammatory bowel disease (IBD) centres. The study included consecutive IBD patients starting VDZ between August 2016–December 2017. Inclusion criteria were: being >18 years old, having confirmed diagnosis of UC or CD, moderately to severely active disease and being non-responder or intolerant to conventional therapies including anti-TNF, being naïve to VDZ or other anti-integrin drugs, receiving at least four VDZ infusions and signing the informed consent. Exclusion criteria were pregnancy, lactation, previous total colectomy in UC, and contra-indications to VDZ use as specified in the VDZ prescribing information.3 Serum samples were collected at weeks 6 and 14 and assayed by ELISA (Theradiag, Marne-la-Vallée, France). The limits of detection ranged from 2–60 µg/ml for VTL, and from 35–500 ng/ml for anti-vedolizumab antibodies (AVA). Throughout the study, the clinicians were blinded to the VTL and AVA measurements.
All patients received a 300 mg infusion of VDZ at weeks 0, 2 and 6 (induction phase), and then every eight weeks thereafter (maintenance phase). Patients who were non-responders at week 6 were given an additional 300 mg VDZ infusion at week 10, at their physician’s discretion. After week 14, shortening of the interval to one infusion every four weeks was performed in cases of continuous incomplete response or loss of response. All concomitant therapies were allowed, except for other biological therapies.
The following data were collected from the medical records: gender, age, IBD type and duration, IBD location according to the Montreal classification,22 body mass index (BMI), smoking habit, clinical and endoscopic activity, concomitant use of steroids and/or immunosuppressants, and previous exposure to anti-TNFα therapy. We also recorded the date of the last infusion of anti-TNF-α, which was categorised as either > or <12 weeks after the first VDZ infusion. Baseline and follow-up clinical activity were measured using the Harvey Bradshaw Index (HBI)23 for CD, and the Partial Mayo Score (PMS)24 for UC. Baseline endoscopy was executed within three months before starting VDZ, and follow-up endoscopy within 54 weeks after starting VDZ. Baseline and follow-up endoscopies were performed at the discretion of the treating physician. We also recorded the baseline serum albumin and C-reactive protein (CRP) values at baseline and at weeks 14, 22, and 54 (normal range: 34–48 g/l and <5 mg/l, respectively). All recorded procedures were performed as in the usual clinical routine.
Ethical considerations
The protocol was approved by the local Ethics Committee (Fondazione Policlinico Universitario A. Gemelli, 21 July 2016) and all enrolled patients gave written informed consent. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the human research committee of Fondazione Policlinico Universitario A. Gemelli.
Outcomes and definitions
The primary aim was to explore the correlation between early VTLs (weeks 6 and 14) and VDZ persistence over the first year of treatment for sustained clinical benefit for IBD. We defined clinical benefit as a significant improvement in symptoms according to the treating physician’s opinion, in order to maintain treatment, without steroids, new prescription of immunosuppressants or surgery.
Secondary outcomes were the correlations of early TL with clinical remission at weeks 14, 22, and 54, with clinical remission at weeks 22 and 54 plus CRP < 5 mg/l, with mucosal healing (MH) within the first year and with CRP, serum albumin, and BMI. In CD patients, clinical remission was defined as an HBI of ≤4, without steroid treatment.23 In UC patients, clinical remission was defined as a PMS of ≤2 with no subscore of >1, without steroid treatment.24 Endoscopic remission was defined as the absence of any deep ulcerations for CD, as a Rutgeerts’score25 <2 for operated CD, or as an endoscopic Mayo subscore of ≤1 for UC.24
Statistical analysis
Continuous data were described as median and interquartile range (IQR), or mean and 95% confidence interval (CI), as appropriate. Discrete data were described as percentages. Baseline characteristics, clinical and endoscopic outcomes, and VTL assessment were compared using the Mann-Whitney test, t test, χ2 and Fisher’s exact tests, as applicable. Correlations were explored using Spearman’s rank test. We established the VTL cut-off level that predicted the outcomes using receiver-operating characteristic (ROC) curve analysis. Treatment persistency curves were obtained using Kaplan-Meier survival curves and the log-rank test. A two-sided p value of <0.05 was considered statistically significant. Analyses were performed using MedCalc Statistical Software, 9.2.1.0 (MedCalc software bvba, Ostende, Belgium).
Results
Study population and patient outcomes
We included 101 IBD patients (42 CD and 59 UC). Table 1 summarises the clinical and demographic characteristics of the whole population.
Table 1.
Baseline patients’ characteristics.
| Overall (n = 101) | UC (n = 59) | CD (n = 42) | p | |
|---|---|---|---|---|
| Female – n (%) | 38 (37.6) | 20 (33.9) | 18 (42.9) | 0.36 |
| Mean age – years (IQR) | 47.9 (45–50.8) | 47.4 (43.5–51.2) | 48.7 (44–53.4) | 0.65 |
| Smokers – n (%) | 13 (13) | 5 (8.5) | 8 (19) | 0.12 |
| Montreal – n (%) | ||||
| A1 | 2 (4.7) | |||
| A2 | 27 (64.3) | |||
| A3 | 13 (31) | |||
| L1 | 13 (30.9) | |||
| L2 | 6 (14.3) | |||
| L3 | 22 (52.4) | |||
| L4 | 1 (2.3) | |||
| B1 | 23 (54.8) | |||
| B2 | 13 (30.9) | |||
| B3 | 6 (14.3) | |||
| P | 6 (14.3) | |||
| E1 | 1 (1.7) | |||
| E2 | 20 (33.9) | |||
| E3 | 38 (64.4) | |||
| Previous surgery – n (%) | 27 (26.7) | 0 | 27 (64.3) | |
| Previous exposure to anti-TNFα – n (%) | 83 (82.2) | 48 (81.4) | 35 (83.3) | 0.8 |
| 1 – n (%) | 39 (38.6) | 27 (45.8) | 12 (28.6) | 0.08 |
| 2 – n (%) | 40 (39.6) | 18 (30.5) | 22 (52.4) | 0.02 |
| 3 – n (%) | 4 (4) | 3 (5.1) | 1 (2.3) | 0.49 |
| Concomitant immunosuppressants-n (%) | 8 (7.9) | 5 (8.5) | 3 (7.1) | 0.8 |
| Steroid use at baseline | 50 (49.5) | 38 (64) | 12 (28.5) | 0.0005 |
| Median BMI – (IQR) | 22.9 (20.9–24.8) | 23.6 (21.1–25.7) | 22.8 (16.8–24.1) | 0.53 |
| Median CRP – mg/l (IQR) | 8 (2.1–13.4) | 5.4 (1–12.8) | 9.4 (4–14) | 0.34 |
| Median serum albumin – g/l (IQR) | 3.8 (3.5–4.2) | 3.9 (3.5–4.2) | 3.7 (3.5–4.1) | 0.8 |
| Median PMS, (IQR) | – | 6 (6–7) | – | |
| Median HBI, (IQR) | – | – | 8 (8–10) | |
anti-TNFα: anti-tumour necrosis factor alpha; BMI: body mass index; CD: Crohn's disease; CRP: C-reactive protein; HBI: Harvey Bradshaw Index; IQR: interquartile range; PMS: Partial Mayo score; UC: ulcerative colitis.
Based on the clinician’s judgement, 35 patients (34.6%, 27 CD and eight UC) received a supplementary dose of VDZ at week 10, and 53 patients (52.5%, 30 CD and 23 UC) received VDZ infusions at a shortened four-week interval after week 14. At weeks 14 and 22, respectively, 32.7% and 35.7% of patients showed clinical remission without steroids, and 17.8% and 22.8% of patients showed clinical remission plus normal CRP. Median CRP values dropped from 8 mg/l (IQR, 5.3–9.9) at baseline to 3.15 mg/l (IQR, 1.5–6.6) at 54 weeks of treatment (p = 0.0016). At week 54, 65 patients (64.4%) continued to receive VDZ treatment, of whom 41 patients (63%) were in clinical remission without steroids and 22 patients (21.8%) were in clinical remission and had normal CRP. VDZ persistence was not significantly different among CD and UC patients (73% and 57%, respectively, p = 0.09). CD and UC patients had significantly different remission rates only at week 54 (52.4% CD and 32.2% UC; p = 0.04) but did not significantly differ with regards to the rates of patients in remission at week 54 with normal CRP (Supplementary Material Table 1).
Among the 89 patients who had baseline endoscopy data available, 58 underwent follow-up endoscopy after a median of 52 weeks. MH was achieved in 39% of these 58 patients (41% UC and 37% CD; p = 0.75). However, the percentage of patients achieving MH calculated according to non-responder imputation (NRI) was 25.8% (39% UC and 21.8% CD, p = 0.52). Compared to patients receiving the standard dose, patients who received treatment optimization were more likely to achieve MH (67% vs 39%; p = 0.037, as observed).
Correlation between early VTL and patient outcome
In the overall population, median VTL was 28.3 µg/ml (IQR, 16.9–39.8) at week 6, and 18.4 µg/ml (IQR, 11.8–25) at week 14. VTL at week 14 did not significantly differ between patients who did and did not receive an additional dose at week 10 (20.3 µg/ml vs 17.9 µg/ml; p = 0.06). Compared to UC patients, CD patients had a significantly higher VTL at week 6 (31.8 vs 27.1; p = 0.04) and week 14 (20.6 vs 17.5; p = 0.01). AVA were detected in five patients (5%, four CD and one UC) at week 6, and in three patients (3.2%, all UC) at week 14, and were not correlated with clinical outcomes. Only two of the eight patients with detectable AVA had a low VTL. Concomitant immunosuppressant therapy did not impact VTL or AVA at week 6 and at week 14 (data not shown).
Analysis of all included patients revealed that a VTL cut-off of >16.55 µg/ml at week 14 predicted VDZ persistence within the first year of therapy with a sensitivity of 73.3% and a specificity of 59.4% (p = 0.0009). The area under the receiver-operating curve (AUROC) was 0.686 (Figure 1). Kaplan-Meier survival analysis confirmed that a VTL of >16 µg/ml at week 14 was associated with VDZ persistence up to 54 weeks (hazard ratio (HR) = 2.4; log-rank test: p = 0.009; Figure 2). We also performed a separate survival analysis for VDZ persistence in the two subpopulations of UC and CD, and the trend was maintained although it was only marginally significant for UC (HR 2.18; log-rank test: p = 0.047) and non-significant for CD (HR 2.39; log-rank test: p = 0.16) (Supplementary Material Figure 1(a) and (b)). We found no significant correlation between VTL at week 6 and VDZ persistence.
Figure 1.
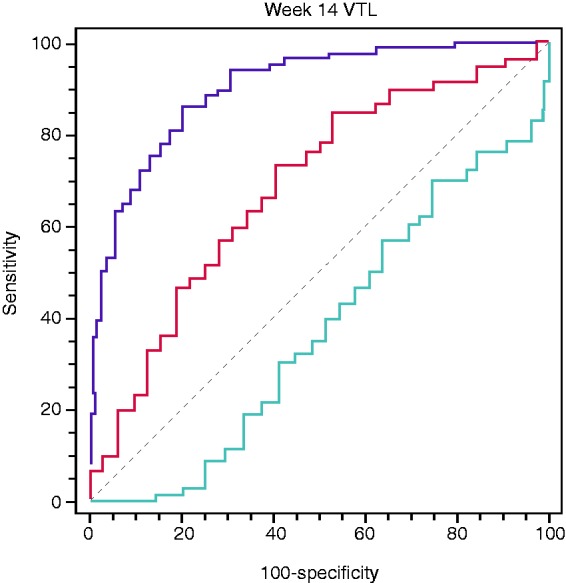
Receiver-operating characteristic (ROC) curve analysis of week 14 vedolizumab trough level (VTL) and vedolizumab treatment persistence within the first year, cut-off >16.6 µg/ml, area under the receiver-operating curve (AUROC) 0.686, 95% confidence interval (CI) 0.581–0.779, sensitivity 73.3%, specificity 59.4%, p = 0.0009.
Figure 2.
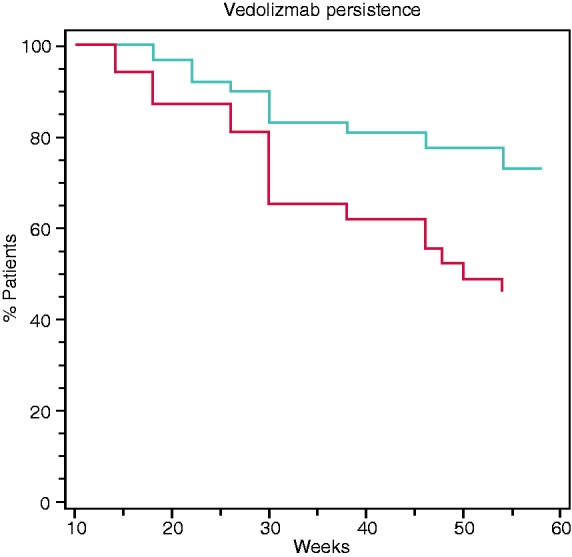
Kaplan-Meier survival analysis of vedolizumab treatment persistence up to 54 weeks according to week 14 vedolizumab trough level (VTL) (blue line: VTL > 16 µg/ml, red line: VTL ≤ 16 µg/ml). Hazard ratio (HR) = 2.4, 95% confidence interval (CI) 1.3–5.9, log-rank test: p = 0.009.
Table 2 reports the results of assays at week 6. VTL at week 6 was significantly higher among patients who were in remission at week 14 (p = 0.02) and at week 22 (p = 0.01) compared to those who were not. Among UC patients, VTL at week 6 maintained a similar correlation with clinical remission at weeks 14 and 22 (p = 0.005; Supplementary Material Table 2). Among CD patients, higher VTL at week 6 was associated with MH (p = 0.0059; Supplementary Material Table 3).
Table 2.
Association between median week 6 vedolizumab trough level (VTL) and clinical and endoscopic outcomes for all patients.a
| Median W6 VTL, µg/ml | IQR | Median W6 VTL, µg/ml | IQR | p | ||
|---|---|---|---|---|---|---|
| W14 remitters | 32.5 | 24.4–48 | W14 non-remitters | 24.9 | 15.8–38.3 | 0.02 |
| W14 remitters plus normal CRP | 40.5 | 27.9–54.1 | W14 non-remitters and/or without normal CRP | 26.4 | 15.8–38.4 | 0.004 |
| W22 remitters | 32.4 | 23.4–49 | W22 non-remitters | 23.9 | 15.5–37.4 | 0.01 |
| W22 remitters plus normal CRP | 32.2 | 22.6–51.7 | W22 non-remitters and/or without normal CRP | 25.4 | 15.8–37.6 | 0.06 |
| W54 remitters | 29.8 | 20.2–50.7 | W54 non-remitters | 27.06 | 21.9–35.2 | 0.36 |
| W54 remitters plus normal CRP | 32.4 | 25.4–53.8 | W54 non-remitters and/or without normal CRP | 26.7 | 18.8–40.8 | 0.08 |
| MH at W54 | 29.8 | 16.8–47.9 | No MH at W54 | 26.7 | 15.8–34.8 | 0.25 |
CRP: C-reactive protein; IQR: interquartile range; MH: mucosal healing.
aMH data are referred to patients having both baseline and follow-up endoscopy.
ROC curve analysis revealed that within the whole IBD population, a VTL of >29.9 µg/ml at week 6 was indicative of remission at week 14 (AUROC, 0.641; p = 0.02), and a VTL of >29.3 µg/ml at week 6 was predictive of remission at week 22 (AUROC, 0.652; p = 0.01) (Supplementary Material Figure 2(a) and (b)). VTL values at week 14 were significantly higher in patients who showed clinical remission at weeks 14, 22 and 54 (p = 0.0001, <0.0001 and 0.008); in patients who showed clinical remission plus normal CRP at weeks 14, 22 and 54 (p = 0.006, 0.004 and 0.03); and in patients who achieved mucosal healing (p = 0.0008) (Table 3). Similar observations were made when analysing each clinical outcome among UC patients; however, among patients with CD, VTL at week 14 was higher only in patients with mucosal healing (Supplementary Material Tables 4 and 5).
Table 3.
Association between median week 14 vedolizumab trough level (VTL) and clinical and endoscopic outcomes for all patients.a
| Median W14 VTL, µg/ml | IQR | Median W14 VTL, µg/ml | IQR | p | ||
|---|---|---|---|---|---|---|
| W14 remitters | 22.3 | 18.3–31.4 | W14 non-remitters | 15.6 | 9–20.9 | 0.0001 |
| W14 remitters plus normal CRP | 20.6 | 18.6–34.7 | W14 non-remitters and/or without normal CRP | 17.2 | 10.2–23.2 | 0.006 |
| W22 remitters | 22.4 | 18.6–32.2 | W22 non-remitters | 14.2 | 9–20.9 | <0.0001 |
| W22 remitters plus normal CRP | 20.8 | 19–30.5 | W22 non-remitters and/or without normal CRP | 16.2 | 9.5–27.6 | 0.004 |
| W54 remitters | 20.9 | 16.9–31.4 | W54 non-remitters | 15.9 | 8.5–19.5 | 0.008 |
| W54 remitters plus normal CRP | 20 | 17.8–26.9 | W54 non-remitters and/or without normal CRP | 14.5 | 8–20.9 | 0.03 |
| MH at W54 | 20.4 | 16.9–25.9 | No MH at W54 | 11.9 | 7.3–17.4 | 0.0008 |
CRP: C-reactive protein; IQR: interquartile range; MH: mucosal healing.
MH data are referred to patients having both baseline and follow-up endoscopy.
We also performed ROC curve analysis with VTL at 14 weeks, and found that a cut-off of >16.4 µg/ml at week 14 was associated with clinical remission at week 14 (AUROC, 0.741; p = 0.0001), while a cut-off of >17.3 µg/ml was associated with clinical remission plus normal CRP at week 14 (AUROC, 0.712; p = 0.0049) (Supplementary Material Figure 3(a) and (b)). A VTL of >16.4 µg/ml at week 14 also predicted clinical remission at week 22 (AUROC, 0.772; p = 0.0001), and clinical remission plus normal CRP at week 22 (AUROC, 0.708; p = 0.0027) (Supplementary Material Figure 3(c) and (d)). Furthermore, a VTL cut-off of >18 µg/ml at week 14 predicted clinical remission at week 54 (AUROC, 0.744; p = 0.0003), and a VTL cut-off of >16.6 µg/ml at week 14 predicted clinical remission plus normal CRP at week 54 (AUROC, 0.690; p = 0.018), and mucosal healing (AUROC, 0.773; p = 0.0001) (Supplementary Material Figure 4(a)–(c)). We also examined the correlations of early VTL with CRP, serum albumin and BMI, and only found that week 14 VTL showed a significant correlation with albumin levels (Spearman’s rho = 0.204; p = 0.0107).
Quartile analysis
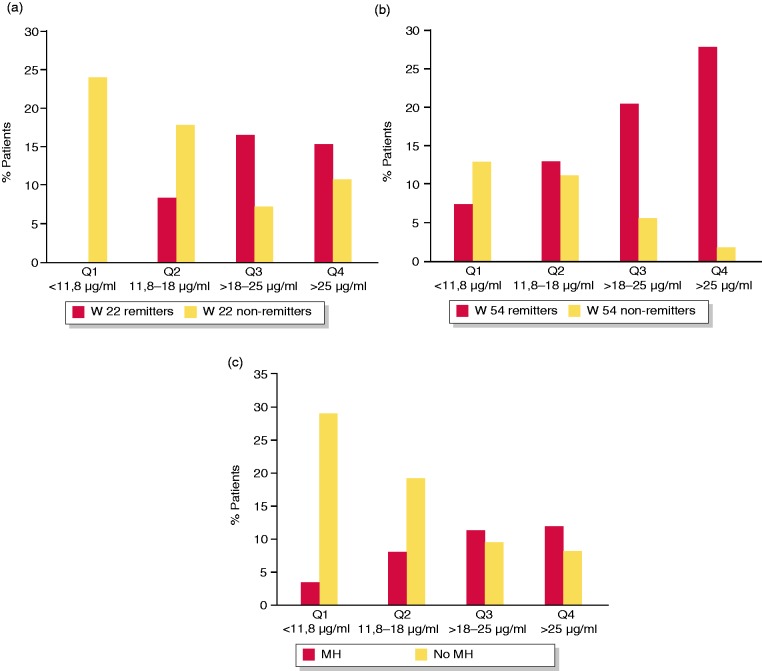
We also analysed the clinical outcomes according to quartiles of VTL. Remission rates at week 14 significantly differed between VTL quartiles 1 and 2 versus VTL quartiles 3 and 4 measured at week 6 (p < 0.019), and remission rates at week 22 significantly differed according to quartiles of VTL measured at week 6 (p = 0.04) (Supplementary Material Figure 5(a) and (b)). We also found that remission rates at week 22 (p < 0.001) and week 54 (p = 0.0076), and mucosal healing (p = 0.013) significantly differed according to quartiles of VTL measured at week 14 (Figure 3(a)–(c)).
Figure 3.
(a) Rates of clinical remission at week 22 by quartiles (Qs) of vedolizumab trough level (VTL) at week 14 (p < 0.001); (b) rates of clinical remission at week 54 by Qs of VTL at week 14 (p = 0.0076); (c) rates of mucosal healing (MH) by Qs of VTL at week 14 (p = 0.013).
Discussion
We analysed the value of early VTL assessment for predicting first-year therapeutic success with VDZ in clinical practice. We observed a positive correlation between exposure and response, with patients having a VTL of >16.55 µg/ml at week 14 showing a higher probability of maintaining VDZ therapy at one year. To our knowledge, this is the first real-world study to identify a correlation between early VTL and treatment persistence at one year. VTL at 14 weeks was higher among patients who achieved clinical remission at weeks 14, 22 and 54, and those who achieved mucosal healing within the first 54 weeks. VTL at six weeks seemed to mainly predict clinical outcomes during the first six months, including clinical remission at weeks 14 and 22. Quartile analysis for VTL at weeks 6 and 14 confirmed the dose-response relationship.
These results suggest that early VTL assessment may be useful for achieving adequate levels during induction and for obtaining better clinical outcomes during the first year of treatment. These findings confirm prior data from the literature, and extend the clinical follow-up up to one year.15–18 In particular our results apparently outline a better correlation of VTL with clinical outcomes in UC patients as compared with CD, although this difference could be at least partly due to an insufficient number of patients for a separate analysis. A multicentre French study reported that a six-week VTL of <18.5 µg/ml was a good predictor of the need for dose-optimization (VTL every four weeks) within the first six months, highlighting the importance of adopting prompt optimization or a switch strategy in this patient group.15 Other studies have shown that week 6 VTL seems to predict MH and long-term sustained response on therapy.16,17 According to the summary of product characteristics of Entyvio, in non-responsive cases, therapy should not be continued beyond 10 weeks for UC patients or beyond 14 weeks for CD patients. However, CD patients could receive an additional infusion at week 10, and both UC and CD patients could benefit from shortening the interval between infusions to every four weeks after week 14.26
There is not enough evidence to support the use of a TDM-guided optimization strategy in non-responsive cases in clinical practice. However, as suggested by Dreesen et al., early VTL assessment could potentially help physicians to distinguish two categories of patients: those with sub-therapeutic levels, who might benefit more from dose optimization (high VDZ clearance/insufficient exposure); and those who did not respond despite therapeutic levels (insufficient time/mechanistic failure).18 The ongoing randomised vedolizumab intravenous dose optimization in ulcerative colitis (ENTERPRET) study (NCT030291439) should provide a better understanding of the VDZ exposure-response relationship, and more robust evidence regarding the appropriateness of adopting a TDM-guided decision-making strategy for VDZ efficacy (in terms of mucosal healing). In the ENTERPRET study, UC week 6 non-responders with sub-therapeutic VDZ levels at week 5 (cut-off of <50 µg/ml) are randomised to receive either VDZ standard or optimised treatment (300 or 600 mg every 4–8 weeks).
We found a correlation between VTL and serum albumin, but not between VTL and BMI. This might be related to the rather homogeneous distribution of BMI within our cohort (median BMI, 22.91; IQR, 20.9–24.8), not including undernourished or obese patients. In our study, the immunogenicity rate of VDZ was 8%, regardless of the concomitant immunosuppressant use, and had no significant impact on clinical outcomes, as previously reported.4–6,15–20
Our study has limitations: first, the calculated AUROCs for VTL predictive accuracy are modest, although significant, and should be confirmed by further studies. Second, it was an observational study, and VDZ therapy was managed in terms of dose optimization, regardless of the VTL and AVA results. Third, we included a mixed cohort of IBD patients. When the patients were stratified by disease, VTL was associated with all effectiveness outcomes only among UC patients. Indeed, most CD patients received an additional VDZ dose at week 10, regardless of their week 6 VTL, and CD patients displayed higher VTL values than UC patients at both weeks 6 and 14. Furthermore, our immunogenicity data could have been affected by the use of a drug-sensitive assay. Finally, we did not report endoscopic outcome data for the whole cohort, and did not use an endoscopic score to assess CD.
In conclusion, we showed that a high VTL at week 14 was associated with a higher probability of maintaining VDZ therapy over the first year for sustained clinical benefit. Moreover, VTL at weeks 6 and 14 seemed to predict clinical and endoscopic remissions within the first year. These findings underscore the utility of achieving an adequate VTL during VDZ induction, and support the potential use of early VTL assessment to identify patients who may benefit from continuation and/or optimization of VDZ therapy after induction. Future randomised controlled trials are needed to address the cost-effectiveness of adopting a TDM-guided optimization strategy for patients who do not respond or show a suboptimal response to VDZ.
Supplemental Material
Supplemental material, Supplemental Material1 for Early vedolizumab trough levels predict treatment persistence over the first year in inflammatory bowel disease by Luisa Guidi, Daniela Pugliese, Tommaso Panici Tonucci, Lorenzo Bertani, Francesco Costa, Giuseppe Privitera, Barbara Tolusso, Clara Di Mario, Eleonora Albano, Gherardo Tapete, Elisa Gremese, Alfredo Papa, Antonio Gasbarrini, Gian Ludovico Rapaccini and Alessandro Armuzzi in United European Gastroenterology Journal
Supplemental Material
Supplemental material, Supplemental Material2 for Early vedolizumab trough levels predict treatment persistence over the first year in inflammatory bowel disease by Luisa Guidi, Daniela Pugliese, Tommaso Panici Tonucci, Lorenzo Bertani, Francesco Costa, Giuseppe Privitera, Barbara Tolusso, Clara Di Mario, Eleonora Albano, Gherardo Tapete, Elisa Gremese, Alfredo Papa, Antonio Gasbarrini, Gian Ludovico Rapaccini and Alessandro Armuzzi in United European Gastroenterology Journal
Declaration of conflicting interests
The authors declare the following conflicts of interest: Luisa Guidi: consultancies and/or speaker fees from: AbbVie, Janssen, MSD, Mundipharma, Takeda, Vifor Pharma, Zambon. Daniela Pugliese received speaker fees from AbbVie, MSD, Takeda and Janssen. Alessandro Armuzzi: consulting and/or advisory board fees from AbbVie, Allergan, Amgen, Biogen, Celgene, Celltrion, Ferring, Hospira, Janssen, Lilly, MSD, Mundipharma, Pfizer, Samsung Bioepis, Sofar and Takeda; lecture and/or speaker bureau fees from AbbVie, AstraZeneca, Chiesi, Ferring, Hospira, Janssen, MSD, Mitsubishi-Tanabe, Medtronic, Mundipharma, Nikkiso, Pfizer, Otsuka, Samsung Bioepis, Takeda, Tigenix and Zambon; and research grants from MSD and Takeda. Elisa Gremese has received fees for consultancy and/or lectures from: Abbvie, BristolMyers Squibb, Celgene, Eli Lilly, Janssen, MSD, Mundipharma, Novartis, Pfizer, Roche, Sandoz, Sanofi and UCB. All the other authors have no conflict of interest to declare.
Ethics approval
The study protocol was approved by the Ethics Committe of Fondazione Policlinico Universitario A. Gemelli IRCCS on July 21, 2016.
Funding
This work was supported by D1 2016 Università Cattolica del Sacro Cuore, Rome, Italy.
Informed consent
All enrolled patients have signed a written informed consent.
Supplemental material
Supplemental material for this article is available online.
References
- 1.Soler D, Chapman T, Yang LL, et al. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther 2009; 330: 864–875. [DOI] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration. Highlights of prescribing information – ENTYVIO (vedolizumab) 2018, https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125476s022lbl.pdf (2018, accessed 11 March 2019).
- 3.European Medicines Agency. Highlights of prescribing information – ENTYVIO (vedolizumab) 2018, https://www.ema.europa.eu/en/medicines/human/EPAR/entyvio (2018, accessed 11 March 2019).
- 4.Feagan BG, Rutgeerts P, Sands BE, et al. GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013; 369: 699–710. [DOI] [PubMed] [Google Scholar]
- 5.Sandborn WJ, Feagan BG, Rutgeerts P, et al. GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med 2013; 369: 711–721. [DOI] [PubMed] [Google Scholar]
- 6.Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn's disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014; 147: 618–627. [DOI] [PubMed] [Google Scholar]
- 7.Vermeire S, Loftus EV, Jr, Colombel JF, et al. Long-term efficacy of vedolizumab for Crohn's disease. J Crohns Colitis 2017; 11: 412–424. [DOI] [PubMed] [Google Scholar]
- 8.Loftus EV, Jr, Colombel JF, Feagan BG, et al. Long-term efficacy of vedolizumab for ulcerative colitis. J Crohns Colitis 2017; 11: 400–411. [DOI] [PubMed] [Google Scholar]
- 9.Loftus E, Jr, Colombel JF, Feagan B, et al. Long-term effectiveness and safety of vedolizumab in patients with ulcerative colitis: 5-Year cumulative exposure of GEMINI 1 completers rolling into the GEMINI open-label extension study. J Crohns Colitis 2017; 11: S182–S183. [Google Scholar]
- 10.Vermeire S, Loftus E, Jr, Colombel JF, et al. Long-term effectiveness and safety of vedolizumab in patients with Crohn's disease: 5-Year cumulative exposure of GEMINI 2 completers rolling into the GEMINI open-label extension study. J Crohns Colitis 2017; 11: S39–S39. [Google Scholar]
- 11.Guidi L, Pugliese D, Panici Tonucci T, et al. Therapeutic drug monitoring is more cost-effective than a clinically-based approach in the management of loss of response to infliximab in inflammatory bowel disease: An observational multi-centre study. J Crohns Colitis 2018. May 31 (Epub ahead of print) DOI: 10.1093/ecco-jcc/jjy076. [DOI] [PubMed] [Google Scholar]
- 12.Mitrev N, Vande Casteele N, Seow CH, et al. IBD Sydney Organisation and the Australian Inflammatory Bowel Diseases Consensus Working Group. Review article: Consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment Pharmacol Ther 2017; 46: 1037–1053. [DOI] [PubMed] [Google Scholar]
- 13.Rosario M, French JL, Dirks NL, et al. Exposure-efficacy relationships for vedolizumab induction therapy in patients with ulcerative colitis or Crohn's disease. J Crohns Colitis 2017; 11: 921–929. [DOI] [PubMed] [Google Scholar]
- 14.Osterman MT, Rosario M, Lasch K, et al. Vedolizumab exposure levels and clinical outcomes in ulcerative colitis: Determining the potential for dose optimisation. Aliment Pharmacol Ther 2019; 49: 408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williet N, Boschetti G, Fovet M, et al. Association between low trough levels of vedolizumab during induction therapy for inflammatory bowel diseases and need for additional doses within 6 months. Clin Gastroenterol Hepatol 2017; 15: 1750–1757.e3. [DOI] [PubMed] [Google Scholar]
- 16.Yacoub W, Williet N, Pouillon L, et al. Early vedolizumab trough levels predict mucosal healing in inflammatory bowel disease: A multicentre prospective observational study. Aliment Pharmacol Ther 2018; 47: 906–912. [DOI] [PubMed] [Google Scholar]
- 17.Liefferinckx C, Minsart C, Cremer A, et al. Early vedolizumab trough levels at induction in inflammatory bowel disease patients with treatment failure during maintenance. Eur J Gastroenterol Hepatol 2019; 31: 478–485. [DOI] [PubMed] [Google Scholar]
- 18.Dreesen E, Verstockt B, Bian S, et al. Evidence to Support monitoring of vedolizumab trough concentrations in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2018; 16: 1937–1946.e8. [DOI] [PubMed] [Google Scholar]
- 19.Ungar B, Kopylov U, Yavzori M, et al. Association of vedolizumab level, anti-drug antibodies, and α4β7 occupancy with response in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2018; 16: 697–705.e7. [DOI] [PubMed] [Google Scholar]
- 20.Schulze H, Esters P, Hartmann F, et al. A prospective cohort study to assess the relevance of vedolizumab drug level monitoring in IBD patients. Scand J Gastroenterol 2018; 53: 670–676. [DOI] [PubMed] [Google Scholar]
- 21.Reenaers C, Cremer A, Dewit O, et al. Effectiveness and persistence of vedolizumab in patients with inflammatory bowel disease: Results from the Belgian REal-LIfe study with VEdolizumab (Be-RELIVE). J Crohns Colitis 2018; 12: S476–S477. [PubMed] [Google Scholar]
- 22.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005; 19: 5–36. [DOI] [PubMed] [Google Scholar]
- 23.Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet 1980; 1: 514–514. [DOI] [PubMed] [Google Scholar]
- 24.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalcylic acid therapy for mildly to moderately active ulcerative colitis. N Eng J Med 1987; 317: 1625–1629. [DOI] [PubMed] [Google Scholar]
- 25.Rutgeerts P, Geboes K, Vantrappen G, et al. Predictability of the postoperative course of Crohn's disease. Gastroenterology 1990; 99: 956–963. [DOI] [PubMed] [Google Scholar]
- 26.Takeda Pharma A/S. Entyvio (vedolizumab) summary of product characteristics 2016. Taastrup, Denmark: Takeda Pharma A/S 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental Material1 for Early vedolizumab trough levels predict treatment persistence over the first year in inflammatory bowel disease by Luisa Guidi, Daniela Pugliese, Tommaso Panici Tonucci, Lorenzo Bertani, Francesco Costa, Giuseppe Privitera, Barbara Tolusso, Clara Di Mario, Eleonora Albano, Gherardo Tapete, Elisa Gremese, Alfredo Papa, Antonio Gasbarrini, Gian Ludovico Rapaccini and Alessandro Armuzzi in United European Gastroenterology Journal
Supplemental material, Supplemental Material2 for Early vedolizumab trough levels predict treatment persistence over the first year in inflammatory bowel disease by Luisa Guidi, Daniela Pugliese, Tommaso Panici Tonucci, Lorenzo Bertani, Francesco Costa, Giuseppe Privitera, Barbara Tolusso, Clara Di Mario, Eleonora Albano, Gherardo Tapete, Elisa Gremese, Alfredo Papa, Antonio Gasbarrini, Gian Ludovico Rapaccini and Alessandro Armuzzi in United European Gastroenterology Journal