Abstract
Background
Cortiment®MMX® (budesonide MMX®) is currently approved for the induction of remission in mild-to-moderate ulcerative colitis (UC) patients when 5-ASA treatment is not sufficient. Data in real-life settings are lacking.
Methods
This was a multicentre observational prospective cohort study conducted in Europe and Canada. Effectiveness, safety, and tolerability of Cortiment®MMX® in a real-life setting of patients treated for mild-to-moderate UC was investigated. Patients were prescribed Cortiment®MMX® in accordance with the Summary of the Product Characteristics (SmPC).
The primary endpoint was the clinical benefit of Cortiment® MMX® in routine practice (improvement ≥ 3 points in the clinical sub-scores of the Ulcerative Colitis Disease Activity Index, UCDAI).
Results
Data from 326 patients with mild-to-moderate UC were analysed for the primary endpoint. Clinical benefit was achieved in 60.1% (196/326) of patients at the end of Cortiment®MMX® treatment. Clinical remission (UCDAI clinical sub-score ≤ 1), full symptoms resolution (rectal bleeding (RB) = 0 and stool frequency (SF) = 0) and symptoms resolution (RB = 0 + SF ≤ 1) at the end of the Cortiment®MMX® treatment were achieved in 51.8%, 45.1% and 63.2% of patients, respectively. The median time to symptoms resolution was 30 days (range 29.0–36.0 days). Fifty patients (14.3%) had to discontinue Cortiment®MMX® due to adverse events; 17.5% of patients (n = 61) reported at least one adverse event related to the study drug.
Conclusions
This was the first time that a large cohort study was conducted with Cortiment®MMX® in a real-life setting. It demonstrated that Cortiment®MMX® is effective, safe and well tolerated in about 60% of UC patients.
Keywords: Ulcerative colitis, inflammatory bowel disease, budesonide, MMX, 5-ASA, mesalamine
Key summary
Established knowledge on this subject
Budesonide MMX® is approved for the treatment of active mild-to-moderate ulcerative colitis;
Budesonide MMX® represents a valid and safer alternative to systemic steroids in patients with ulcerative colitis who failed 5-ASA treatment;
There are no large-scale real-life data showing the best therapeutic approach in patients with indication to treatment with budesonide MMX® (monotherapy vs. combination with 5-ASA).
What are the significant and/or new findings of this study?
Our real-life data confirm the effectiveness and safety of budesonide MMX® in patients with mild-to-moderate active ulcerative colitis in terms of clinical benefits and quality of life.
Our data suggest that adding budesonide MMX® to 5-ASA treatment might be the best therapeutic approach to induce clinical remission compared to budesonide MMX® monotherapy.
Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) that involves the colon and the rectum, with continuous inflammation, erosions and/or ulcers affecting the colonic mucosa.1,4 Usually, the course of the disease is characterised by flares of active disease with diarrhoea, rectal bleeding (RB) and rectal urgency, alternating with periods of remission.2 The main therapeutic goals to treat active flares are the control of symptoms, normalisation of bowel movements, disappearance of bleeding and rectal urgency, combined with the normalisation of inflammatory biomarkers, such as C-reactive protein and/or faecal calprotectin (FC), and the disappearance of mucosal lesions.3,4
Systemic corticosteroids, such as prednisolone or equivalents, are effective therapies in active UC of any severity, but their use is limited by their adverse effects.5 In order to minimise the side effects of systemic steroids, budesonide, a locally acting corticosteroid with low bioavailability and little systemic absorption, represents a valid alternative in IBD patients.6,7 However, the pH-dependent release mechanism of budesonide may limit effectiveness along the colonic mucosa.8 In order to address this unmet need, a colonic release system (MMX®, Multi-Matrix System) that provides targeted drug delivery to the entire colon has been used successfully in conjunction with both mesalazine and budesonide to allow a continuous and uniform release of the active principles along the colon.9–13
In the phase 2 trial, the first data in patients with left-sided UC showed that 47.1% of patients treated with budesonide MMX® 9 mg once daily achieved clinical remission compared to 33.0% of placebo-treated patients.14 More recently, the CORE-II trial, a phase 3, randomised, double-blind, double-dummy, placebo-controlled, parallel-group trial was conducted on 512 patients with active mild-to-moderate UC, mostly in European countries.9 Patients were randomised 1:1:1:1 to receive budesonide MMX® 9 mg/day, budesonide MMX® 6 mg/day, controlled ileal-release budesonide 9 mg/day once daily), or a placebo for 8 weeks.9 Combined clinical and endoscopic remission at week 8 was achieved in 17.4% of patients treated with budesonide MMX® 9 mg compared to 4.5% of patients in the placebo group (p = 0.0047).9 As a secondary study objective, more patients achieved histological healing (16.5% vs. 6.7%, p = 0.036) and complete symptoms resolution (23.9% vs. 11.2%, p = 0.022) when treated with budesonide MMX® 9 mg compared with the placebo.9 Similar results were found in the CORE-I trial conducted in the United States.15 In the pooled analysis, budesonide MMX® safety and tolerability was comparable to the placebo in terms of adverse events (AEs) and mean cortisol serum levels.16
Currently, budesonide MMX® 9 mg/day is approved for the induction of remission in patients with mild-to-moderate UC where 5-ASA treatment is not sufficient. The aim of this study was to evaluate the effectiveness and tolerability of budesonide MMX® (Cortiment®MMX®) in mild-to-moderate UC in a real-life setting.
Methods
This was a multicentre observational prospective cohort study conducted in IBD referral centres in Poland, Italy, Germany, the United Kingdom, the Netherlands, Sweden, Ireland and Canada. The main aim of this study was to collect and analyse data on the effectiveness, safety and tolerability of Cortiment®MMX® in a real-life setting of patients treated for mild-to-moderate UC.
Inclusion and exclusion criteria
Eligible patients were adult (aged ≥ 18 years), of either gender, who had been prescribed Cortiment®MMX® for the treatment of mild-to-moderate active UC within a 5-day window prior to being included in the study. Exclusion criteria were severe active/fulminant UC, use of concomitant antibiotics or systemic corticosteroids for the current flare, history of total/sub-total colectomy, known hypersensitivity to the active substance or to any of the excipients.
Study endpoints and outcome measures
Primary endpoint
The primary endpoint of this study was to assess the clinical benefit of Cortiment®MMX® in routine practice, defined as the percentage of patients achieving improvement ≥3 points in the clinical sub-scores of the UCDAI (Ulcerative Colitis Disease Activity Index) at the end of Cortiment®MMX® induction treatment.17
Secondary endpoints
Secondary effectiveness endpoints assessed at the end of Cortiment®MMX® induction treatment were:
the percentage of patients with clinical remission defined as UCDAI clinical sub-score ≤ 1;
the percentage of patients with symptoms resolution (RB = 0 + stool frequency (SF) ≤ 1, urgency = 0);
the percentage of patients with full symptoms resolution (RB = 0 + SF = 0, urgency = 0);
time to symptoms resolution (RB = 0, SF ≤ 1, urgency = 0);
the change in health-related quality of life (Short Inflammatory Bowel Disease Questionnaire, SIBDQ)18,19;
the change in health economic parameters (Work Productivity and Activity Impairment, WPAI)20;
the percentage of patients with FC within normal range (when FC tests were performed);
the percentage of patients with endoscopic healing (UCDAI endoscopic sub-score = 0); and
the percentage of patient in remission (UCDAI endoscopic sub-score ≤ 1) (when endoscopies performed).
Additional study endpoints
As additional study endpoints, tolerability of Cortiment®MMX® in a real-life setting (AEs) and adverse drug reactions (ADRs) were assessed from 2 weeks up to 6 months after the last Cortiment®MMX® dose for prolonged tapering off. Moreover, to determine how Cortiment®MMX® is prescribed and used by gastroenterologists in routine clinical practice, the study evaluated the percentage of patients given Cortiment®MMX® as monotherapy or as an add-on therapy to 5-ASA and the relative time frames, rates of Cortiment®MMX® treatment discontinuation/end of treatment (rate, reason, time frame, tapering off), and follow-up UC treatments (5-ASA, corticosteroids, immunosuppressants or biologics) were evaluated.
Safety
For safety analyses, AEs and concomitant diseases were recorded, by assigned preferred terms (PTs) and categorisation into the Primary System Organ Class according to the MedDRA thesaurus version 20.1. Patients reporting more than one AE or concomitant disease with the same PTs were counted once. The AEs were reported as per national safety reporting requirements.
Study time points
Effectiveness was evaluated at the end of Cortiment®MMX® induction treatment. The tolerability and use of Cortiment®MMX® were assessed from 2 weeks up to a maximum of 6 months (in particular circumstances) after the last Cortiment®MMX® dose.
Study groups
The primary endpoint, clinical benefit, (improvement ≥ 3 points in the UCDAI clinical sub-scores at end of treatment) was compared between three cohorts of patients. The cohorts were defined as:
Cortiment®MMX® added to 5-ASA at least 14 days after increased/optimised 5-ASA dose for the treatment of flare (late add-on) (Cohort 1);
Cortiment®MMX® added to 5-ASA within 14 days since 5-ASA increased/optimised for the treatment of flare or without 5-ASA dose modification (early add-on) (Cohort 2); and
Cortiment®MMX® as monotherapy for the treatment of flare (mono) (Cohort 3).
Statistical analyses
Descriptive statistics were used to analyse the study data. Continuous variables were summarised using the number of patients, mean, standard deviation, minimum, first quartile, median, third quartile and maximum. For categorical variables, data were summarised by the number and percentage of patients in each category; 95% confidence intervals (CIs) were provided to evaluate the level of precision of the calculated estimates.
Incidence percentages were calculated as follows: number of patients with one or more occurrences of the item divided by the number of patients in the analysis set, times 100.
Missing data were not imputed. When summarising categorical variables, in case of any missing responses, these were shown as a separate category. For continuous variables, the number of non-missing observations was displayed.
All discontinuations after enrolment were summarised by time of, and reason for, discontinuation.
Tests were carried out with a two-sided significance level of 0.05. CIs were set at 95%.
The association between the explanatory clinical variables and the outcome (response) variable was tested in univariable analyses with the chi-square test. Then, all selected variables (using p < 0.20 as selection criterion) were included in the multivariable logistic regression analysis, and a backwards stepwise selection process was followed.23 Odds ratios with 95% CIs derived from the final multivariable logistic regression model are presented.
No formal hypothesis was tested in this observational, prospective cohort study. All enrolled patients who received at least one dose of Cortiment®MMX® and met the inclusion or exclusion criteria were included in the analyses.
The estimated sample size was 350 patients.23
In order to perform a comparison among the three cohorts, patients were matched to adjust for the effects of covariates. Matching was based on the propensity score method. The following covariates were considered for the calculation of the propensity score: gender (male, female), age (18–34; 35–59; 60 + years), number of UC flares in the last year, baseline extension of disease, baseline endoscopic sub-score, baseline score for stool frequency during the last 3 days, baseline score for RB during the last 3 days, baseline response for urgency episodes during the last week, baseline UC-related extra-intestinal symptoms during the last week, Physician's Global Assessment at baseline. The propensity score was calculated by means of a multivariable logistic regression analysis. The absolute difference among cohorts’ patients’ matched propensity scores was set to 0.01.
Ethical considerations
This study was conducted according to the 1975 Declaration of Helsinki and the European Medicines Agency Guidelines for Good Clinical Practice. All ethical review boards from each participating centre approved this study. This study was registered on clinicaltrials.gov (ClinicalTrials.gov Identifier: NCT02586259). Written, informed consent was obtained from each patient included in the study prior to the start of any study procedure.
Results
Study population
A total of 378 patients from eight countries were screened (Poland, Italy, Germany, the United Kingdom, the Netherlands, Sweden, Ireland and Canada); 349 evaluable patients, who had been prescribed Cortiment®MMX® for the treatment of mild-to-moderate active UC, within a 5-day window, were included in the study. The major protocol deviations consisted of no Cortiment®MMX® treatment and inclusion/exclusion criteria not satisfied. Baseline characteristics are summarised in Table 1. Of these 349 patients, 23 were lost at follow-up after Visit 1 and therefore were excluded from the primary endpoint analysis.
Table 1.
Baseline characteristics of the study population.
| Variable | Cohort 1 (n = 59) | Cohort 2 (n = 260) | Cohort 3 (n = 30) | Total (N = 349) |
|---|---|---|---|---|
| Gender (N (%)) | ||||
| Females | 36 (61.0%) | 132 (50.8%) | 15 (50.0%) | 183 (52.4%) |
| Age (years, median) | 45.0 | 39.0 | 42.5 | 40.0 |
| Smoking status (N (%)) | ||||
| Current smoker | 2 (3.4%) | 12 (4.6%) | 0 (0%) | 14 (4.0%) |
| Former Smoker | 17 (28.8%) | 40(15.4%) | 8 (26.7%) | 65 (18.6%) |
| Non-smoker | 40 (67.8%) | 208 (80.0%) | 22 (73.3%) | 270 (77.4%) |
| Maximal extension of UC in the past (N (%)) | ||||
| Proctitis | 6 (10.2%) | 24 (9.2%) | 1 (3.3%) | 31 (8.9%) |
| Proctosigmoiditis | 7 (11.9%) | 34 (13.1%) | 6 (20.0%) | 47 (13.5%) |
| Left-sided Colitis | 30 (50.8%) | 104 (40.0%) | 8 (26.7%) | 142 (40.7%) |
| Pancolitis | 12 (20.3%) | 87 (33.5%) | 13 (43.3%) | 112 (32.1%) |
| Unknown | 4 (6.8%) | 11 (4.2%) | 2 (6.7%) | 17 (4.9%) |
| Median UCDAI score | 5.0 | 5.0 | 4.0 | 5.0 |
| Mean UCDAI score (SD) | 5.0 (1.7) | 5.2 (1.5) | 4.3 (1.8) | 5.0 (1.7) |
| Last oral corticosteroid (N (%)) | ||||
| None | 38 (64.4%) | 165 (63.5%) | 21 (70.0%) | 224 (64.2%) |
| Prednisone or prednisolone | 18 (30.5%) | 74 (28.5%) | 9 (30.0%) | 101 (28.9%) |
| Budesonide MMX® | 1 (1.7%) | 7 (2.7%) | 1 (0%) | 8 (2.3%) |
| Beclomethasone Betamethasone | 0 (0%) 0 (0%) | 6 (2.3%) 0 (0%) | 0 (0%) 0 (0%) | 6 (1.7%) 1 (0%) |
| Budesonide | 0 (0%) | 2 (0.8%) | 0 (0%) | 2 (0.6%) |
| Other | 2 (3.4%) | 6 (2.3%) | 0 (0%) | 8 (2.3%) |
| History of immunosuppressants (N (%)) | ||||
| Yes | 13 (22.0%) | 77 (29.6%) | 8 (26.7%) | 98 (28.1%) |
| No | 46 (78.0%) | 176 (67.7%) | 20 (66.7%) | 242 (69.3%) |
| Unknown | 0 (0%) | 7 (2.7%) | 2 (6.7%) | 9 (2.6%) |
| History of biologics (N (%)) | ||||
| Yes | 2 (3.4%) | 29 (11.2%) | 5 (16.7%) | 36 (10.3%) |
| No | 56 (94.9%) | 224 (86.2%) | 24 (80.0%) | 304 (87.1%) |
| Unknown | 1 (1.7%) | 7 (2.7%) | 1 (3.3%) | 9 (2.6%) |
UC: ulcerative colitis; UCDAI: Ulcerative Colitis Disease Activity Index; SD: standard deviation
Effectiveness on symptoms
In the entire study population, 196 patients (60.1%) achieved clinical benefit (reduction of ≥ 3 points in the UCDAI clinical sub-score) at the end of treatment (64.3%, 62.1%, and 33.3% in cohorts 1, 2 and 3 respectively), with a median drop of 3.0 UCDAI points (p = 0.0096) (Table 2). At the end of treatment, the median UCDAI score dropped from 5.0 to 1.0 in the overall cohort (5.0 to 1.0 in cohorts 1 and 2, 4.0 to 2.0 in Cohort 3) compared to baseline.
Table 2.
Clinical effectiveness endpoints at the end of treatment.
| Cohort 1 (n = 59) | Cohort 2 (n = 260) | Cohort 3 (n = 30) | Total (N = 349) | ||
|---|---|---|---|---|---|
| Clinical benefit (≥3 point reduction UCDAI clinical sub-score) | N (%) | 36 (64.3%) | 151 (62.1%) | 9 (33.3%) | 196 (60.1%) |
| [95% CI] | [50.4–76.6] | [55.7–68.3] | [16.5–54.0] | [54.6–65.5] | |
| Clinical remission (UCDAI clinical sub-score ≤ 1) | N (%) | 32 (57.1%) | 128 (52.7%) | 9 (33.3%) | 169 (51.8%) |
| [95% CI] | [43.2–70.3] | [46.2–59.1] | [16.5–54.0] | [46.3–57.4] | |
| Symptoms resolution (RB = 0 + SF ≤ 1 and no urgency) | N (%) | 40 (71.4%) | 153 (63.0%) | 13 (48.1%) | 206 (63.2%) |
| [95% CI] | [57.8–82.7] | [56.6–69.0] | [28.7–68.1] | [57.7–68.4] | |
| Full symptoms resolution (RB = 0 + SF = 0 and no urgency) | N (%) | 29 (51.8%) | 110 (45.3%) | 8 (29.6%) | 147 (45.1%) |
| [95% CI] | [38.0–65.3] | [38.9–51.8] | [13.8–50.2] | [39.6–50.7] | |
| UC status | N (%) | ||||
| No response/relapse | 22 (12.2%) | 11 (15.9%) | 3 (14.3%) | 36 (13.3%) | |
| Partial response | 54 (29.8%) | 19 (27.5%) | 4 (19.0%) | 77 (28.4%) | |
| Complete response | 105 (58.0%) | 39 (56.5%) | 14 (66.7%) | 158 (58.3%) |
UCDAI = Ulcerative Colitis Disease Activity Index
Clinical remission, defined as UCDAI clinical sub-score ≤ 1, was achieved in 169 (51.8%) patients in the overall evaluable set (57.1%, 52.7% and 33.3% patients in cohorts 1, 2 and 3 respectively) (Table 2).
Full resolution of symptoms at the end of the Cortiment®MMX® treatment, defined as the absence of rectal bleeding (RB = 0), normalisation of bowel habit (SF = 0) and no urgency was achieved in 147 patients (45.1%) (51.8%, 45.3% and 29.6% in cohorts 1, 2 and 3 respectively). Symptoms resolution (RB = 0, SF ≤ 1 and no urgency) was achieved in 206 (63.2%) patients from the entire study population (71.4%, 63.0%, and 48.1% in the three cohorts, respectively). The median time to symptoms resolution was 30 days (range 29.0–36.0 days) (Table 2).
After propensity score matching, the overall difference was statistically significant for clinical benefit (p = 0.0101), clinical remission (p = 0.0216) and symptoms resolution (p = 0.0366), whereas it was not statistically significant for full symptoms resolution (p = 0.0752).
Effectiveness on objective signs of inflammation
Data about objective measures of inflammation, such as FC and endoscopy, at baseline and at the end of induction were available for 14 and 32 patients, respectively. Due to the small number of patients who underwent dosing of FC at both baseline and end of Cortiment®MMX® induction treatment, no clear tendency can be drawn. Mucosal healing (defined as a Mayo endoscopic sub-score equal to 0) was achieved in 5/32 patients (15.6%) at the end of treatment, whereas endoscopic remission (Mayo endoscopic sub-score ≤ 1) was achieved in 16/32 patients (50.0%).
Patient-reported outcomes
The median increase in the SIBDQ was 10.0 points for the whole cohort compared to baseline assessment (p < 0.001). Improvement in quality of life was observed in all three cohorts (median SIBDQ increase of 11.0, 14.0 and 7.0 respectively) with a significant improvement in quality of life (p < 0.001) only in cohorts 1 and 2 (Figure 1). Additionally, patient satisfaction of Cortiment®MMX® treatment was high (Visual Analogue Scale (VAS) scale: rating of 7- to 10/10) in 61.3% of patients, with similar rates in the three cohorts (71.3%, 60.4% and 60.4% respectively).
Figure 1.
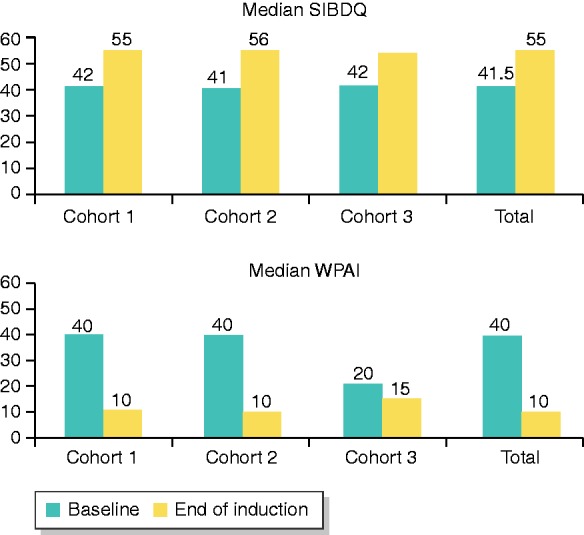
Median improvement of quality of life (SIBDQ scores) and decrease of work impairment scores (WPAI) compared to baseline among the study cohorts and in the overall populations.
Regarding work productivity, the mean percent work time missed due to ill health, the mean percent impairment while working due to ill health and the mean percent overall work impairment due to ill health were all reduced in the whole population by 8.9%, 21.3% and 23.7% respectively (p < 0.0001 in both the paired t-test and the non-parametric signed rank sum test for all variables, Figure 1).
Predictors of clinical benefit
Baseline RB (p < 0.0001), Physician's Global Assessment (p < 0.0001), compliance to Cortiment®MMX® treatment during induction phase (p = 0.0002), country (p < 0.0001), age (p = 0.0469), smoking history (p = 0.0351), baseline urgency episodes (p = 0.0012), cohort (p = 0.0117), number of UC flares in the last year (p = 0.0205), and number of oral 5-ASA induction courses in the last year (p = 0.0013) were selected for analysis in the multivariable model. In the multivariable analysis with logistic regression, baseline RB (streaks/obvious/blood alone vs. none), Physician's Global Assessment (moderate/severe vs. normal/mild), compliance to Cortiment®MMX® treatment (≥80% vs. <80%) and country (Poland vs. other countries) were independently associated with a higher probability of achieving clinical benefit from budesonide MMX® (Table 3).
Table 3.
Final multivariable logistic regression model for predictors of clinical benefit in the study cohort.
| Variable | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| Presence of baseline rectal bleeding | 3.443 | 1.786–6.637 | <0.001 |
| Physician's Global Assessment | 2.642 | 1.547–4.512 | <0.001 |
| Compliance to Cortiment®MMX® treatment during induction phase > = 80% | 2.693 | 1.459–4.971 | 0.001 |
| Country: Poland | 2.382 | 1.393–4.071 | 0.001 |
95% CI = 95% confidence interval
Safety
In the overall population, 24.1% (n = 84) of patients reported at least one AE. Sixty patients (17.5%) reported at least one AE related to the study drug; 50 patients (14.3%) had to discontinue budesonide MMX® due to AEs; in two patients (0.6%) ADRs were recorded as serious and two (0.6%) as severe. No deaths were reported during the study period. The most common AEs reported were drug ineffectiveness for 12% of patients (n = 43), product use issues for 5% of patients (n = 16), and gastrointestinal disorders for 3% of patients (n = 9). All other AEs were reported in <1.0% of patients (Table 4).
Table 4.
Patients with adverse events.
| Variable (N (%)) | Cohort 1 (n = 59) | Cohort 2 (n = 260) | Cohort 3 (n = 30) | Total (n = 349) |
|---|---|---|---|---|
| Number of patients with at least one AE | 9 (15.3%) | 68 (26.2%) | 7 (23.3%) | 84 (24.1%) |
| Number of deaths | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Number of patients with at least one serious AE | 0 (0.0%) | 7 (2.7%) | 0 (0.0%) | 7 (2.0%) |
| Number of patients with at least one AE leading to discontinuation | 6 (10.2%) | 40 (15.4%) | 4 (13.3%) | 50 (14.3%) |
| Number of patients with at least one severe AE | 0 (0.0%) | 6 (2.3%) | 0 (0.0%) | 6 (1.7%) |
| Number of patients with at least one ADR | 9 (15.3%) | 46 (17.7%) | 6 (20.0%) | 61 (17.5%) |
| Number of patients with at least one serious ADR | 0 (0.0%) | 2 (0.8%) | 0 (0.0%) | 2 (0.6%) |
| Number of patients with at least one severe ADR | 0 (0.0%) | 2 (0.8%) | 0 (0.0%) | 2 (0.6%) |
AE = adverse event; ADR = adverse drug reaction.
Prescribing patterns
The study showed that Cortiment®MMX® was mainly prescribed as an add-on therapy to 5-ASA (91.4% vs. 8.6% as monotherapy). In this patient population, 5-ASA dose was optimised before Cortiment®MMX® induction treatment in 41.0% of patients (143). The 5-ASA dose optimisation was performed within 14 days for 24.1% (84) of patients with a median time of 0.0 days (95% CI [29.0, 53.0]), and after a minimum of 14 days for 16.9% (59) of patients with a median time of 43 days (95% CI [0.0, 1.0]).
At the end of Cortiment®MMX® induction treatment, Cortiment®MMX® treatment was stopped for the majority of patients (83.8%, n = 274) and was modified for tapering in 16.2% (53) of patients. The majority of patients with tapering were taking 9mg/day of Cortiment®MMX® every 2 days (77.4%, n = 41).
The median duration of Cortiment®MMX® treatment was 57.0 days. The mean (standard deviation, SD) time from the end of Cortiment®MMX® induction treatment to follow-up visit was 29.3 (24.8) days, and was performed in 270 (77.4%) patients. The most common UC treatment at follow-up was oral 5-ASA, which was taken by 230 patients (85.2% of patients participated in the follow-up assessment), whereas systemic corticosteroids were taken by 38 (14.1%) patients, biological drugs were taken by 21 (7.8%) patients and immunosuppressants were taken by 32 (11.9%) patients.
Discussion
Budesonide MMX® is currently approved in Europe for the treatment of active mild-to-moderate UC, for patients refractory or intolerant to mesalazine. Data from the CORE trials8,15,18 showed that one-third of patients receiving Cortiment®MMX® 9 mg/day for 8 weeks as monotherapy achieved clinical improvement (reduction of ≥3 points in UCDAI sub-score), and about one-quarter achieved full symptoms resolution (RB = 0 + SF = 0). However, data from real-life clinical practice were lacking.
Our cohort study included 349 patients who had been prescribed Cortiment®MMX® by their treating physician according to the local SmPC, and independently of their participation in the study. Of these patients, 23 were lost at follow-up before Visit 2, and were therefore excluded from the primary endpoint analysis. When Cortiment®MMX® was used as an add-on to 5-ASA therapy, we found that 64.3% (Cohort 1) and 62.1% (Cohort 2) of patients achieved clinical benefit (improvement ≥3 points in the UCDAI clinical sub-score) with no differences in the timing of administration (less or more than 14 days since 5-ASA was started). Moreover, in both add-on cohorts, 51.8% (Cohort 1) and 45.3% (Cohort 2) of patients achieved full symptoms resolution, defined as the absence of RB, normalisation of bowel habits and no urgency, compared to 29.6% receiving Cortiment®MMX® as monotherapy (Cohort 3). Absence of RB and normalisation of bowel habits are currently considered as the most valuable patient-reported outcomes in evaluating treatment success in UC.5 The time to symptoms resolution (RB = 0 + SF ≤ 1) ranged from 29 to 36 days. Outcomes related to a patient's daily life were improved significantly following Cortiment®MMX® treatment. Health-related quality of life measures (according to the SIBDQ) increased and WPAI scores reduced. Moreover, Cortiment®MMX® treatment satisfaction, as reported by the patients, was generally high. These data might suggest that there is greater benefit from being treated with Cortiment®MMX® in combination with 5-ASA compounds rather than as a monotherapy. Regarding the objective signs of inflammation, the number of patients where data were available both at baseline and end of treatment was small. Thus, no clear conclusion on the effect of Cortiment®MMX® treatment on endoscopic appearance and FC levels can be given.
Baseline RB, baseline Physician's Global Assessment, compliance with the treatment during induction ≥80% and being treated in Poland were factors associated with a higher probability of responding to budesonide MMX®.
Budesonide was well tolerated, with 24.0% of patients reporting at least one AE, compared to 56.5% of patients in the CORE pooled analysis. No major AEs that are usually seen with systemic steroids were observed, and the most common AEs related to drug inefficacy, gastrointestinal disorders or product use issues. These data confirm that budesonide MMX® acts locally with negligible systemic effects and can be considered a safe drug in the mild-to-moderate UC population.
Our study has some limitations. First, there was a different proportion of patients enrolled in the add-on cohorts, 1 and 2, compared to Cohort 3 (monotherapy). Patients were not stratified for strategy chosen, however, our cohort was large enough to capture significant differences among the cohorts and our data are not different from previous Randomized Controlled Trial (RCT) data,8,15,18 supporting the validity of our results. Second, the difference in response rates among countries (i.e. Poland vs. other countries) reflects the heterogeneity of the study population, which may have had an impact on these findings (Polish patients represent 51.2% of the population). However, this is expected in a real-life cohort, which usually enrols patients that are different from RCT study populations.8,15,18 Third, data on objective measures of inflammation are limited, as few patients underwent endoscopies and FC tests at both baseline and end of Cortiment®MMX® treatment.
However, this study has also several strengths. This is the largest real-life cohort study in mild-to-moderate UC patients treated with Cortiment®MMX®, who were mainly treated in third-level referral centres with experienced IBD clinicians. Our findings demonstrate good effectiveness in real-life practice and help to understand the best patient profile and the best strategies to adopt for the use of Cortiment®MMX® in daily practice. The cohort affiliation of patients also indicated that in about half of the cases, 5-ASA dose optimisation is not carried out according to mild-to-moderate UC treatment guidelines.6
In conclusion, in this large real-life UC cohort, Cortiment®MMX® was confirmed to be effective, safe and well tolerated.
Acknowledgments
Ferring and the authors would like to thank all study investigators and their teams for their contributions. (Participating sites and principal study investigators listing in the Appendix.) The principal investigators in this study were Ardizzone Sandro, Astegiano Marco, Augustyn Monika, Baluta Małgorzata, Barczyk Wojciech, Bendia Emanuele, Binkowska-Borgosz Izabela, Błaszczyńska Małgorzata, Bresso Francesca, Brymora Małgorzata, Castiglione Fabiana, Comberlato Michele, Cosintino Rocco, Costa Francesco, Detka-Kowalska Iga, Drobińska Anna, Dudkowiak Robert, Eberhardson Michael, Frączek Grzegorz, Geccherle Andrea, Gionchetti Paolo, Halfvarson Jonas, Hayee Bu, Hertervig Erik, Hjortswang Henrik, Janiak Maria, Jansen Jeroen, Jasiński Bolesław, Jastrzębska Marta, Jessen Petra, Jonas Maurycy, Kaczmarzyk Paweł, Kaniewska Magdalena, Kędzierska Lidia, Kempiński Radosław, Klincewicz Beata, Klugmann Tobias, Kosik-Warzyńska Romana, Krause Thomas, Krawczyk Marek, Krela-Kaźmierczak Iwona, Łapiński Janusz, Latos Wojciech, Łykowska-Szuber Liliana, Maciejewska Katarzyna, Majowski Jarosław, Malewski Waldemar, Małuch Piotr, Mamos Arkadiusz, Manerowski Marcin, Markowski Adam, Mazurek Tadusz, Miehlke Stefan, Mowat Craig, Mularczyk Aldona, Mulcahy Hugh, Nijhawan Pardep, Orlando Ambrogio, Orłowski Marcin, Pastorelli Luca, Penpicki Andrzej, Pilecka Dorota, Piotrowski Wojciech, Prasad Neeraj, Reddy Jagan, Rocca Rodolfo, Romatowski Jacek, Rydzewska Grażyna, Sangfelt Per, Schubert Stefan, Seenan John Paul, Selinger Christian, Singh Andrew, Strózik Agnieszka, Świątkowski Tomasz, Tomecki Roman, Walentek Tomasz, Waśko-Czopnik Dorota, West Rachel, Wierzbicka Katarzyna, Wiśniewska Jarosińśka Maria, Wiśniowski Marek, Wojnarowska Renata, Wójtowicz Henryk, Wontor-Buksińska Aleksandra, Worobiec Jacek and Wyszkowski Mariusz.
Appendix:
Participating sites listing
| Country | Principal investigator | Institution name | City |
|---|---|---|---|
| Canada | Nijhawan, Pardep | Digestive Health Clinic | Richmond Hill |
| Reddy, Jagan | Sudbury Endoscopy Centre | Sudbury | |
| Singh, Andrew | PerCuro Clinical Research | Victoria | |
| Germany | Miehlke, Stefan | Magen-Darm-Zentrum, Facharztzentrum Eppendorf | Hamburg |
| Krause, Thomas | Gastroenterologie Opernstraße | Kassel | |
| Schubert, Stefan | Gastroenterologie am Bayerischen Platz | Berlin | |
| Klugmann, Tobias | Internistische Gemeinschaftspraxis | Leipzig | |
| Jessen, Petra | Gemeinschaftspraxis | Altenholz | |
| United Kingdom | Prasad, Neeraj | Royal Albert Edward Infirmary | Wigan |
| Hayee, Bu | Kings College Hospital | London | |
| Selinger, Christian | St. James's University Hospital | Leeds | |
| Seenan, John Paul | Queen Elizabeth University Hospital | Glasgow | |
| Mowat, Craig | Tayside University Hospitals NHS Trust, Ninewells Hospital and Medical School | Dundee | |
| Ireland | Mulcahy, Hugh | St. Vincent's University Hospital | Dublin |
| Italy | Gionchetti, Paolo | A.O.U. Policlinico S. Orsola Malpighi | Bologna |
| Pastorelli, Luca | Policlinico S. Donato | San Donato Milanese | |
| Astegiano, Marco | A.O.U. Città della Salute e della Scienza | Torino | |
| Rocca, Rodolfo | A.O. Ordine Mauriziano P.O. Umberto I | Torino | |
| Cosintino, Rocco | A.O. San Camillo Forlanini | Roma | |
| Orlando, Ambrogio | A.O. Ospedali Riuniti Villa Sofia - Cervello | Palermo | |
| Castiglione, Fabiana | A.O.U. Federico II | Napoli | |
| Geccherle, Andrea | A.O. Ospedale Sacro Cuore Don Calabria | Negrar | |
| Bendia, Emanuele | A.O.U. Ospedali Riuniti Umberto I, G.M. Lancisi, G. Salesi | Ancona | |
| Comberlato, Michele | A.S.A.A. Ospedale di Bolzano | Bolzano | |
| Costa, Francesco | A.O.U. Pisana Presidio di Cisanello | Pisa | |
| Ardizzone, Sandro | A.S.S.T. Fatebenefratelli Sacco | Milano | |
| Netherlands | Jansen, Jeroen | OLVG – Oost | Amsterdam |
| West, Rachel | Fransiscus Gasthuis | Rotterdam | |
| Poland | Wiśniewska Jarosińśka, Maria | Centrum Medyczne Św Rodziny Poradnia Gastrologiczna | Łódź |
| Brymora, Małgorzata | Poradnia Gastroenterologiczna-Szpital Uniwersytecki nr 2 | Bydgoszcz | |
| Manerowski, Marcin | Poradnia Chorób Jelitowych-Szpital Uniwersytecki nr 2 | Bydgoszcz | |
| Błaszczyńska, Małgorzata | SP ZOZ ZESPÓŁ SZPITALI MIEJSKICH | Chorzów | |
| Binkowska-Borgosz, Izabela | SPSK 1 PUM w Szczecinie | Szczecin | |
| Wontor-Buksińska, Aleksandra | Indywidualna Praktyka Gastrologiczna | Częstochowa | |
| Dudkowiak, Robert | Uniwersytecki Szpital Kliniczny | Wrocław | |
| Kędzierska, Lidia | SPSK 2 PUM w Szczecinie | Szczecin | |
| Kempiński, Radosław | Uniwersytecki Szpital Kliniczny Wrocław | Wrocław | |
| Malewski, Waldemar | NZOZ Termedica | Poznań | |
| Mamos, Arkadiusz | NZOZ Med-Gastr | Łódź | |
| Mazurek, Tadusz | Centrum Medyczne Medicor | Rzeszów | |
| Mularczyk, Aldona | Specjalistyczna Praktyka Lekarska | Katowice | |
| Pilecka, Dorota | SPWSZ Szczecin | Szczecin | |
| Piotrowski, Wojciech | Centrum Medyczne Poradnia Gastrologiczna | Łódź | |
| Walentek, Tomasz | Szpital Specjalistyczny 1 | Bytom | |
| Kosik-Warzyńska, Romana | SPWSZ w Szczecinie | Szczecin | |
| Wojnarowska, Renata | Klinika Gastroenterologii USK nr 1 | Łódź | |
| Markowski, Adam | Humana Media Omeda | Białystok | |
| Romatowski, Jacek | NZOZ Specjalistyczne Centrun Gastrologii GASTROMED | Białystok | |
| Tomecki, Roman | Przychodnia Fundacji Gastroenterologicznej | Warszawa | |
| Wierzbicka, Katarzyna | SPZOZ Szpital Bielański | Warszawa | |
| Wiśniowski, Marek | Centrum Medyczne Medyk | Rzeszów | |
| Baluta, Małgorzata | Centrum Medyczne Dąbrowa - Dąbrówka | Gdynia | |
| Detka-Kowalska, Iga | Oddział Gastroenterologiczny Szpital Specjalistyczny | Końskie | |
| Drobińska, Anna | Klinika Gastroenterologii | Gdańsk | |
| Frączek, Grzegorz | Centrum Medyczne Starówka | Sokołów Podlaski | |
| Janiak, Maria | Klinika Gastroenterologii I Hepatologii | Gdańsk | |
| Jasiński, Bolesław | Klinika Gastroenterologii i Hepatologii/Gabinet Prywatny | Wojkowice | |
| Jastrzębska, Marta | Oddział Gastroenterologiczny Szpital Specjalistyczny im. Św. Łukasza | Końskie | |
| Kaczmarzyk, Paweł | Szpital Kielecki św. Aleksandra Sp. z o.o. | Kielce | |
| Krawczyk, Marek | Szpital Miejski | Tychy | |
| Krela-Kaźmierczak, Iwona | Szpital Kliniczny UM | Poznań | |
| Latos, Wojciech | Gabinet | Gliwice | |
| Łykowska-Szuber, Liliana | Szpital Kliniczny z przychodnia im. H.Święcickiego UM | Poznań | |
| Małuch, Piotr | Specjalistyczna Praktyka | Dąbrowa Górnicza | |
| Penpicki, Andrzej | NZOZ GASTROMED | Białystok | |
| Świątkowski, Tomasz | Przychodnia Specjalistyczna | Kędzierzyn - Koźle | |
| Wójtowicz, Henryk | Przychodnia Sucholeska | Suchy Las | |
| Strózik, Agnieszka | Gabinet Internistyczny | Częstochowa | |
| Augustyn, Monika | Indywidualna Praktyka Lekarska | Kraków | |
| Barczyk, Wojciech | 105 Szpital Wojskowy SPZOZ Żary | Żary | |
| Jonas, Maurycy | Spółdzielnia Pracy Specjalistów Rentgenologów | Warszawa | |
| Łapiński, Janusz | Centrum Gastrologiczno Hepatologiczne | Wrocław | |
| Majowski, Jarosław | Centrum Medyczne Medita | Jelenia Góra | |
| Worobiec, Jacek | Specjalistyczna Poradnia Lekarska i Szpital CDT MEDICUS | Lubin | |
| Orłowski, Marcin | Przychodnia Lekarska Nowy Chełm | Gdańsk | |
| Waśko-Czopnik, Dorota | USK Wrocław | Wrocław | |
| Klincewicz, Beata | Szpital Kliniczny im. Karola Jonschera Uniwersytetu Medycznego im. Karola Marcinkowskiego w Poznaniu | Poznań | |
| Rydzewska, Grażyna | CSK MSW 1 | Warszawa | |
| Wyszkowski, Mariusz | Zespół Przychodni Specjalistycznych PRIMA | Warszawa | |
| Maciejewska, Katarzyna | CSK MSWiA | Warszawa | |
| Kaniewska, Magdalena | Szpital MSWiA | Warszawa | |
| Sweden | Bresso, Francesca | Karolinska University Hospital | Stockholm |
| Halfvarson, Jonas | Örebro University Hospital | Örebro | |
| Sangfelt, Per | Uppsala University Hospital | Uppsala | |
| Hjortswang, Henrik | Linköping University Hospital | Linköping | |
| Hertervig, Erik | Skåne University Hospital | Lund | |
| Eberhardson, Michael | Danderyds sjukhus | Stockholm |
Conflict of interest
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SD has served as a speaker, consultant and advisory board member for Schering-Plough, Abbott (AbbVie) Laboratories, Merck and Co, UCB Pharma, Ferring, Cellerix, Millenium Takeda, Nycomed, Pharmacosmos, Actelion, α Wasserman, Genentech, Grunenthal, Pfizer, Astra Zeneca, Novo Nordisk, Cosmo Pharmaceuticals, Vifor, and Johnson and Johnson. AH has lectured/been on advisory boards for Atlantic, BMS, Falk Pharma, MSD, AbbVie, Takeda, Hospira, Napp Pharmaceuticals, Ferring, Yakult, Pfizer, Janssen, Warner Chillcott, and Genentech. AD has served as a speaker and consultant for Ferring, Falk Pharma, Mundipharma, Hospira, Sandoz, Otsuka, AbbVie, Janssen, Takeda, MSD, Vifor, and Pharmacosmos. GF received consultancy fees from Ferring, MSD, AbbVie, Takeda, Janssen, Amgen, Sandoz, Samsung Bioepis, Celltrion. EL has received fees for: Educational Grant: fees for Educational activities paid by the Company, MSD, AbbVie; speaker fees: AbbVie, Ferring, MSD, Chiesi, Mitsubishi Pharma, Hospira, Janssen, Takeda; serving on an advisory board: AbbVie, Ferring, MSD, Takeda, Mitsubishi Pharma, Celltrion, Prometheus. BS is supported by FIRMAD and has served as advisor for Ferring. GD’H has served as advisor for AbbVie, Ablynx, Amakem, AM Pharma, Avaxia, Biogen, Bristol Meiers Squibb, Boerhinger Ingelheim, Celgene, Celltrion, Cosmo, Covidien, Ferring, DrFALK Pharma, Engene, Ferring, Galapagos, Gilead, Glaxo Smith Kline, Hospira, Johnson and Johnson, Medimetrics, Millenium/Takeda, Mitsubishi Pharma, Merck Sharp Dome, Mundipharma, Novonordisk, Pfizer, Prometheus laboratories/Nestle, Receptos, Robarts Clinical Trials, Salix, Sandoz, Setpoint, Shire, Teva, Tigenix, Tillotts, Topivert, Versant and Vifor, and received speaker fees from AbbVie, Ferring, Johnson and Johnson, MSD, Mundipharma, Norgine, Pfizer, Shire, Millenium/Takeda, Tillotts, and Vifor. ID has served as a speaker and/or consultant for Ferring, Falk Pharma, Rafa laboratories, AbbVie, Janssen, Takeda, Genentech, Pfizer, Protalix, Arena, Gilead, Celltrion, MSD, and Given Imaging. GR has served as a speaker and consultant for AbbVie, Ardeypharm, AstraZeneca, Boehringer, Celgene, Ferring, Falk Pharma, Genentech, Janssen, Novartis, Merck, MSD, Pfizer, Pharmabiome, Roche, Takeda, Tillots, UCB, Vital Solutions, and Vifor, Zeller.KP is an employee of Ferring. LPB has received consulting fees from Merck, AbbVie, Janssen, Genentech, Mitsubishi, Ferring, Norgine, Tillots, Vifor, Therakos, Pharmacosmos, Pilège, BMS, UCB Pharma, Hospira, Celltrion, Takeda, Biogaran, Boerhinger Ingelheim, Lilly, Pfizer, HAC-Pharma, Index Pharmaceuticals, Amgen, and Sandoz; and lecture fees from Merck, AbbVie, Takeda, Janssen, Takeda, Ferring, Norgine, Tillots, Vifor, Therakos, Mitsubishi, and HAC-Pharma.
Funding
Ferring Pharmaceuticals funded the study and participated in the study design, interpretation of data, review, and approval of the publication.
Ethics approval
The first Ethical Committee approval has been obtained on 22/12/2015 in Ethikkommission der Ärztekammer Hamburg, Germany. Then, the protocol was approved as follows: Ireland 25/10/2016 Ethics and Medical Research Committee The Netherlands 26/09/2016 ACWO OLVG (Notification only) Sweden 08/06/2016 Regionala etikprövningsnämnden i Stockholm Canada 05/12/2016 IRB Services Italia 13/12/2016 Comitato Etico Azienda Ospedaliera Universitaria Policlinico “Sant'Orsola – Malpighi Great Britain 20/07/2017 South East Scotland Research Ethics Committee Poland 18/03/2016 Komisja Bioetyczna Okregowej Izby Lekarskie (Notification only).
Informed consent
An informed consent form in the patients' native language was used. Two versions of the informed consent form were reviewed and approved by the local Ethical Committees, and used during the study (version 1.0, Sep 8th, 2015, and version 2.0, July 4th, 2017).
References
- 1.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med 2011; 365: 1713–1725. [DOI] [PubMed]
- 2.Solberg IC, Lygren I, Jahnsen J, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study). Scand J Gastroenterol 2009; 44: 431–440. [DOI] [PubMed] [Google Scholar]
- 3.Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015; 110: 1324–1338. [DOI] [PubMed] [Google Scholar]
- 4.Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis 2017; 11: 649–670. [DOI] [PubMed] [Google Scholar]
- 5.Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis 2017; 11: 769–784. [DOI] [PubMed] [Google Scholar]
- 6.Bonovas S, Nikolopoulos GK, Lytras T, et al. Comparative safety of systemic and low-bioavailability steroids in inflammatory bowel disease: systematic review and network meta-analysis. Br J Clin Pharmacol 2018; 84: 239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn's disease 2016. Part 1: diagnosis and medical management. J Crohns Colitis 2017; 11: 3–25. [DOI] [PubMed] [Google Scholar]
- 8.Travis SPL, Danese S, Kupcinskas L, et al. Once-daily budesonide MMX in active, mild-to-moderate ulcerative colitis: results from the randomised CORE II study. Gut 2014; 63(3): 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunner M, Ziegler S, Di Stefano AF, et al. Gastrointestinal transit, release and plasma pharmacokinetics of a new oral budesonide formulation. Br J Clin Pharmacol 2006; 61: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCormack PL, Robinson DM, Perry CM. Delayed-release Multi Matrix System (MMX) mesalazine: in ulcerative colitis. Drugs 2007; 67: 2635–2642. [DOI] [PubMed] [Google Scholar]
- 11.Nicholls A, Harris-Collazo R, Huang M, et al. Bioavailability profile of Uceris MMX extended-release tablets compared with Entocort EC capsules in healthy volunteers. Journal Int Med Res 2013; 41: 386–394. [DOI] [PubMed] [Google Scholar]
- 12.Prantera C, Viscido A, Biancone L, et al. A new oral delivery system for 5-ASA: preliminary clinical findings for MMx. Inflamm Bowel Dis 2005; 11: 421–427. [DOI] [PubMed] [Google Scholar]
- 13.Tenjarla S, Romasanta V, Zeijdner E, et al. Release of 5-aminosalicylate from an MMX mesalamine tablet during transit through a simulated gastrointestinal tract system. Adv Ther 2007; 24: 826–840. [DOI] [PubMed] [Google Scholar]
- 14.D'Haens GR, Kovacs A, Vergauwe P, et al. Clinical trial: preliminary efficacy and safety study of a new Budesonide-MMX(R) 9 mg extended-release tablets in patients with active left-sided ulcerative colitis. J Crohns Colitis 2010; 4: 153–160. [DOI] [PubMed] [Google Scholar]
- 15.Sandborn WJ, Travis S, Moro L, et al. Once-daily budesonide MMX(R) extended-release tablets induce remission in patients with mild to moderate ulcerative colitis: results from the CORE I study. Gastroenterology 2012; 143: 1218–1226. [DOI] [PubMed] [Google Scholar]
- 16.Lichtenstein GR, Travis S, Danese S, et al. Budesonide MMX for the induction of remission of mild to moderate ulcerative colitis: a pooled safety analysis. J Crohns Colitis 2015; 9: 738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma C, Panaccione R, Fedorak RN, et al. Heterogeneity in definitions of endpoints for clinical trials of ulcerative colitis: a systematic review for development of a core outcome set. Clin Gastroenterol Hepatol 2018; 16: 637–647. [DOI] [PubMed] [Google Scholar]
- 18.Irvine EJ, Zhou Q, Thompson AK The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn s Relapse Prevention Trial. Am J Gastroenterol 1996; 91(8): 1571–1578. [PubMed]
- 19.Han SW, Gregory W, Nylander D, et al. The SIBDQ: Further validation in ulcerative colitis patients. The American Journal of Gastroenterology 2000; 95(1): 145–151. [DOI] [PubMed]
- 20.Jowett SL, Seal CJ, Barton JR, et al. The short inflammatory bowel disease questionnaire is reliable and responsive to clinically important change in ulcerative colitis. The American Journal of Gastroenterology 2001; 96(10): 2921–2928. [DOI] [PubMed]
- 21.Reilly MC, Zbrozek AS, & Dukes E. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmaco Economics 1993; 4(5): 353–365. [DOI] [PubMed]
- 22.de Boer AG, van Lanschot JJ, Stalmeier PF, et al. Is a single-item visual analogue scale as valid, reliable and responsive as multi-item scales in measuring quality of life? Quality of Life Research 2004; 13(2): 311–320. [DOI] [PubMed]
- 23.Brokelman RBG, Haverkamp D, et al. The validation of the visual analogue scale for patient satisfaction after total hip arthroplasty. Eur Orthop Traumatol 2012; 3: 101–105. [DOI] [PMC free article] [PubMed]


