Abstract
Background
A recent prospective randomised controlled trial (‘STING’) showed superiority of over-the-scope clips compared to standard treatment in recurrent peptic ulcer bleeding. Cost-effectiveness studies on haemostasis with over-the-scope clips have not been reported so far.
Objective
The aim of this study was to investigate whether the higher efficacy of the over-the-scope clips treatment outweighs the higher costs of the device compared to standard clips.
Methods
For the analysis, the study population of the STING trial was used. Costs for the hospital stay in total as well as treatment-related costs were obtained. The average cost-effectiveness ratio, representing the mean costs per designated outcome, and the incremental cost-effectiveness ratio, expressing the additional costs of a new treatment strategy per difference in outcome were calculated. The designated outcome was defined as successful haemostasis without rebleeding within seven days, which was the primary endpoint of the STING trial. Average cost-effectiveness ratio and incremental cost-effectiveness ratio were calculated for total costs of the hospital stay as well as the haemostasis treatment alone. The cost-effectiveness analysis is taken from the perspective of the care provider.
Results: Total costs and treatment-related costs per patient were 13,007.07 € in the standard group vs 12,808.56 € in the over-the-scope clip group (p = 0.812) and 2084.98 € vs 1984.71 € respectively (p = 0.663). The difference was not statistically significant. Total costs per successful haemostasis (average cost-effectiveness ratio) were 30,677.05 € vs 15,104.43 € and 4917.41 € vs 2340.46 € for the haemostasis treatment. The additional costs per successful haemostasis with over-the-scope clip treatment (incremental cost-effectiveness ratio) is –468.18 € for the whole treatment and –236.49€ for the haemostasis treatment.
Conclusions
Over-the-scope clip treatment is cost-effective in recurrent peptic ulcer bleeding.
Keywords: Over-the-scope clips, cost-effectiveness analysis, average cost-effectiveness ratio, incremental cost-effectiveness ratio, peptic ulcer bleeding
Key summary
Established knowledge on this subject
About 10% of patients with peptic ulcer bleeding (PUB) suffer from a recurrence with a significant increase in morbidity and mortality.
Over-the-scope clips (OSTCs) have shown clinical superiority to standard clips to treat recurrent bleeding.
Costs per unit of OSTCs compared with standard clips are markedly higher.
Cost-effectiveness analyses in OTSC treatment do not exist so far.
Significant new findings of this study
Mean costs of OTSC treatment per successful haemostasis (average cost-effectiveness ratio (ACER)) are about half of the costs of the standard treatment.
Despite the higher retail price, OTSC treatment does not even produce additional costs by achieving an additional haemostasis in direct comparison to standard clips (incremental cost-effectiveness ratio (ICER)).
This finding is consistent both regarding total costs as well as treatment-related costs.
Introduction
Despite advances in medical and interventional therapy, the incidence of peptic ulcer bleeding (PUB) is still high with about 20–50 per 100,000 persons worldwide and mortality rates of 8.9–22%.1,2 Hence, PUB represents an enormous medical but also economic challenge.
The vast majority of cases can be managed endoscopically, but recurrent bleeding occurs in 10% of patients.3 For recurrent bleeding, success rates of endoscopic management decline to 75% and – in cases of failed endoscopic haemostasis – patients are often referred to angiographic or surgical salvage therapy.4,5 Surgical therapy is associated with high complication rates, prolonged hospital stay and increased mortality (14–29%).5–7 Over-the-scope clips (OTSC®; Ovesco Endoscopy, Tübingen, Germany) have been shown to be superior to standard endoscopic therapy for recurrent ulcer bleeding in a recent randomised controlled trial (RCT; ‘STING’).8 However, the costs of the device are higher compared to standard endoscopic devices such as through the scope clips. In order to investigate whether the higher efficacy outweighs the higher costs of the device, we performed a cost-effectiveness analysis.
Materials and methods
Patient cohort
For the cost-effectiveness-analysis, we analysed the patient cohort of the STING trial. This study was an open, randomised controlled, multicentre study comparing OTSC vs standard endoscopic therapy in recurrent peptic ulcer bleeding.8 On 15 January 2013 the study was approved by the ethical board. Written, informed consent was obtained from each patient included in the study. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution's human research committee. In total, 66 patients had either been assigned to retreatment with standard methods or with OTSCs. The combined primary endpoint was persistent or recurrent bleeding within seven days. Secondary endpoints were mortality, need for salvage surgical treatment, number of blood samples transfused, duration of the hospital stay and complications of the treatment. Further bleeding had occurred in 19 patients (57.6%) in the standard therapy group and in five patients (15.2%) in the OTSC group (p < 0.001), respectively. Ten patients with further bleeding in the standard therapy group had received successful OTSC therapy as rescue treatment. No significant differences in secondary outcomes were observed. A flowchart of the STING study summarising main results is provided in the Supplementary Material.
Cost calculation
Within the study, detailed information about number of endoscopic procedures, used endoscopic haemostatic devices and drugs (clips, injection needle, coagulation probe, fibrine glue, epinephrine), duration of hospital stay and blood transfusion had been recorded. This data was used for cost calculation. Total costs of treatment were calculated for each patient in both study arms. Total costs were defined as treatment-related costs plus the costs of the total hospital stay. Treatment-related costs were calculated as the sum of used endoscopic haemostatic devices, general costs for esophagogastroduodenoscopy (EGD) and costs for transfused blood units. For endoscopic haemostatic devices and medicines (clips, injection needles, coagulation probes, fibrin glue), we calculated using the official retail prices with the exception of epinephrine injection (this was calculated with the price of the pharmacy of the principal investigating hospital). In cases where the exact clip type was unidentifiable, a ‘mixed type’ with the mean cost of all used clips was calculated. The price per millilitre of epinephrine was then calculated for all patients of the study. The costs of an esophagogastroduodenoscopy (including staff-related costs, without biopsy) were derived from a nation-wide analysis of the German Society for Digestive and Metabolic Diseases (DGVS).9 The costs of the hospital stay were calculated as the costs for intensive care unit plus for the stay on the ward. For the analysis the results of the DRG (Diagnosis-related group) report 2017 and of a cost analysis of ICU (intensive care unit) were used.10 Costs were adjusted to the year 2017 and they relate to the German healthcare system.
Statistical analyses and cost-effectiveness calculation
Continuous variables are reported as means with the corresponding standard deviations whereas categorial variables are expressed as frequencies and percentages unless stated otherwise. For continuous variables, differences were determined using Wilcoxon-Mann-Whitney and Kruskal-Wallis tests as there was no Gaussian distribution of the data confirmed by the Kolmogorov-Smirnov test. X2 tests or Fisher exact tests were used for categorial variables. Values of p < 0.05 were considered significant. For assessment of cost-effectiveness, the average cost-effectiveness ratio (ACER) and the incremental cost-effectiveness ratio (ICER) were calculated. The average cost-effectiveness ratio expresses the mean costs for the investigated outcome.11 In our study, the ACER describes the mean costs per successful haemostasis in both treatment arms.
The ACER is calculated with the following computational formula:
ICER expresses the additional costs of a treatment alternative for the improvement in investigated outcome.12 In our study, these are the incremental costs for the OTSC treatment to achieve a successful haemostasis.
The ICER is calculated with the following computational formula:
Mean costs were total costs of the respective group divided by the number of patients in each group. Effectiveness was defined as clinical success. The primary endpoint of the STING study was ‘further bleeding’. Hence, clinical success was defined as absence of further bleeding. The computational formula was therefore:
As stated above, calculation of the effectiveness was performed in the same manner as for the ACER. Both ICER and ACER were calculated for the total costs and the haemostasis-related costs.
Statistical analyses were performed with SPSS (version 24.0, IBM, New York, USA) and GraphPad Prism (version 6, GraphPad Software, San Diego, California, USA).
Results
Mean number of used conventional clips per patient in the standard group was 2.09 versus 0.09 in the OTSC group (p < 0.001). In detail, the following clips were used: Resolution (Boston Scientific, Marlborough, USA) (mean amount 0.51 vs 0), Instinct (Cook Medical, Bloomington, Indiana, USA) (0.48 vs 0.09), Olympus Quick Clip (Olympus, Hamburg, Germany) (0.72 vs 0), Olympus EZ Clips (Olympus, Hamburg, Germany) (0.12 vs 0) and Mixed type (0.24 vs 0). The detailed amount and costs of each product used are also shown in Table 1. Mean number of OTSCs used in both groups were 0.52 and 1.18 in the OTSC group (p < 0.001). In most cases, the 12/6 OTSC was used (mean 0.48 vs 1.09). The mean amount of epinephrine (7.84 ml vs 6.87 ml; p = 0.20), the number of injection needles (0.81 vs 0.6; p = 0.15) and the total amount of fibrin (5 ml vs 9 ml; p = 0.47) and coagulation probe (1 vs 0) were not significantly different in both groups. The mean number of blood units transfused in both groups (5.24 vs 4.9) were also not significantly different (p = 0.39). Furthermore, the mean number of esophagogastroduodenoscopies were 3.33 in the standard group and 2.78 in the OTSC group. This difference was not significantly different with a p-value of 0.234. Duration of the EGD in the situation of recurrent or persistent bleeding was 27 ± 9 min in the standard group and 32 ± 12 min in the OTSC group (p = 0.055). The mean duration on the ward was 18 ± 13 days and 18 ± 18 days in both groups. The difference was not statistically significant (p = 0.672). The mean days on the ICU did also not differ significantly (4.7 ± 9.4 and 4.6 ± 6.9, p = 0.396).
Table 1.
Overview on hospital- and treatment-related costs per each cost item and treatment time. Values are shown as costs per patient and for the whole treatment group. A value of p < 0.05 is considered being significant. Over-the-scope clip (OTSC) 11 and OTSC 12 represent the 11 mm and 12 mm versions of the clip.
| Standard arm |
OTSC arm |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Type of costs (mean values) | Unit (n) | Cost per unit (EUR) | Costs per patient (EUR) | Total group | Unit (n) | Cost per unit (EUR) | Costs per patient (EUR) | Total group | p-Value |
| 1. Hospital costs | |||||||||
| 1.1 Ward (d) | 18 | 276.69 | 4980.42 | 164,353.86 | 18 | 276.69 | 4980.42 | 164,353.86 | 0.672 |
| 1.2 ICU (d) | 4.7 | 1265 | 5945.50 | 196,201.5 | 4.6 | 1265 | 5819.00 | 192,027.00 | 0.396 |
| 2. Haemostasis treatment | |||||||||
| 2.1 Clips | |||||||||
| 2.1.1 OTSC 11 | 0.03 | 269 | 8.07 | 266.31 | 0.09 | 269 | 24.21 | 798.93 | 0.306 |
| 2.1.2 OTSC 12 | 0.48 | 369 | 177.12 | 5844.96 | 1.09 | 369 | 402.21 | 13,272.93 | <0,001 |
| 2.1.3 BSC resolution | 0.51 | 95 | 48.45 | 1598.85 | 0 | 95 | -00 | -00 | 0.003 |
| 2.1.4 Olympus QuickClip | 0.72 | 80 | 57.60 | 1900.80 | 0 | 80 | -00 | -00 | 0.001 |
| 2.1.5 Olympus EZ Clip+Applicator | 0.12 | 65.97 | 7.92 | 261.24 | 0 | 65.97 | -00 | -00 | 0.317 |
| 2.1.6 Cook Instinct | 0.48 | 72 | 34.56 | 1140.48 | 0.09 | 72 | 6.48 | 213.84 | 0.002 |
| 2.1.7 Mixed type | 0.24 | 37.24 | 8.94 | 294.94 | 0 | 37.24 | -00 | -00 | 0.003 |
| 2.2. Coagulation probe/Gold probe | 0.03 | 189 | 5.67 | 187.11 | 0 | 189 | -00 | -00 | 0.317 |
| 2.3. Injection | -00 | ||||||||
| 2.3.1 Injection needle | 0.81 | 7.58 | 6.14 | 202.61 | 0.6 | 7.58 | 4.55 | 150.08 | 0.149 |
| 2.3.2 Epinephrine (ml) | 7.84 | 0.49 | 3.84 | 126.77 | 6.87 | 0.49 | 3.37 | 111.09 | 0.2 |
| 2.3.3 Fibrine glue (2 ml) Tisseel | 0.09 | 133.56 | 12.02 | 396.67 | 0.12 | 133.56 | 16.03 | 528.90 | 0.069 |
| 2.3.4 Fibrin glue (4 ml) Tisseel | 0.15 | 261.77 | 39.27 | 1295.76 | 0 | 261.77 | -00 | -00 | 0.079 |
| 2.4 Blood units | 5.24 | 176 | 922.24 | 30,433.92 | 4.9 | 176 | 862.40 | 28,459.20 | 0.386 |
| 2.5 EGD (without haemostasis material) | 3.33 | 230.56 | 767.76 | 25,336.24 | 2.78 | 230.56 | 640.96 | 21,151.57 | 0.234 |
| 2.6. Mean duration of ‘rebleeding’ EGD (min) | 27 | 32 | 0.055 | ||||||
EGD: esophagogastroduodensocopy; ICU: intensive care unit.
For haemostasis treatment mean costs of the conventional clips per patient were significantly different with 159.05 € ± 148.75 in the standard group vs 6.55€ ± 37.60 in the OTSC group (p < 0.001). Vice versa costs of the OTSCs per patient in both groups were 187.06 € ± 206.80 and 427.00 € ± 220.29 (p < 0.001). Mean costs per patient for EGD, transfused blood units, epinephrine, coagulation probe were not significantly different.
The mean costs per patient for the stay on the ward are 4980.42 € ± 3748.10 for the standard group and 4997.19 € ± 5064.23 for the OTSC group (p = 0.672). The mean costs per patient for the ICU are 5941.67 € ± 11,910.85 in the standard group vs 5826.67 ± 8689.34 in the OTSC group and is also not statistically significant (p = 0.396).
Total costs were 429,233 € in the standard group and 422,683 € in the OTSC group resulting in mean costs per patient of 13,007.07 € ± 15,242.34 and 12,808.56 € ±13,079.50, respectively (p = 0.812). Treatment-related costs account for 68,804 € vs 65,495 €. This results in mean costs per patient of 2084.98 € ± 1167.25 in the standard group and 1984.71 € ± 1219.96 in the OTSC group (p = 0.663). Results are also shown in Table 2.
Table 2.
Total costs and treatment-related costs.
| Standard treatment n = 33 | OTSC n = 33 | p-Value | |
|---|---|---|---|
| Total costs | |||
| Total costs of the respective group | 429,233 | 422,683 | |
| Mean costs per patient | 13,007.07 ± 15,242.34 | 12,808.56 ± 13,079.50 | 0.812 |
| Haemostasis-related costs | |||
| Costs of the respective group | 68,804 | 65,495 | |
| Mean costs per patient | 2084.98 ± 1167.25 | 1984.71 ± 1219.96 | 0.663 |
Mean costs are shown with standard deviation. Costs are expressed in Euros.
With the results of the STING trial, efficacy of standard treatment was calculated as 42.4% and of OTSC treatment 84.8%.
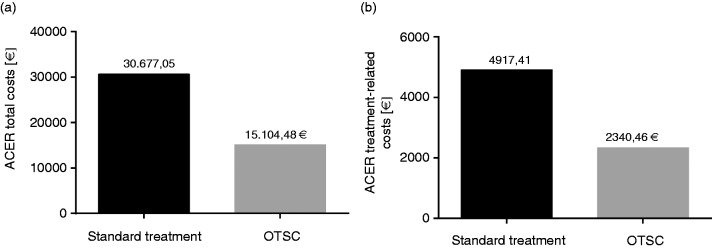
The ACER per successful haemostasis in the standard group is 30,677.05 €. and is 15,104.48 € in the OTSC group. The ACER per successful haemostasis regarding only treatment-related costs are 4917.41 € in the standard group and 2340.46 € in the OTSC group. See also Table 3 and Figure 1.
Table 3.
Average cost-effectiveness-ration (ACER) and incremental cost-effectiveness ratio (ICER) Costs are expressed in Euros.
| Standard treatment | OTSC | |
|---|---|---|
| Effect | 0.424 | 0.850 |
| Total costs | ||
| ACER | 30,677.05 | 15,104.48 |
| ICER | −468.18 | |
| Haemostasis-related costs | ||
| ACER | 4917.41 | 2340.46 |
| ICER | −236.49 | |
Figure 1.
Average cost-effectiveness ratio (ACER) for (a) the total costs and for (b) the haemostasis-related costs are shown in both treatment arms. Values are expressed in Euros. OTSC: over-the-scope clip.
The ICER, meaning the additional costs per successful haemostasis with OTSC treatment are calculated with –468.18 € and –236.49 € for the haemostasis-related costs. See also Table 3 and Figure 2.
Figure 2.
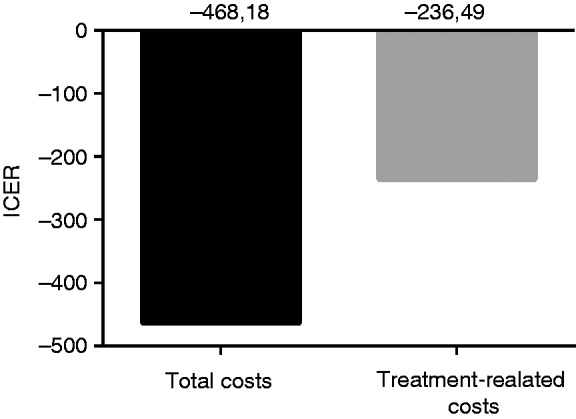
Incremental cost-effectiveness ratio (ICER) for the over-the-scope clip (OTSC) treatment in relation to the whole treatment and the haemostasis-related treatment are shown. Values are expressed in Euros.
Discussion
In recent years, technical innovations have continuously improved patient care but can also be drivers of increased healthcare costs. Therefore, there is a growing need to assess efficacy of new methods and devices relative to their costs.13 To our knowledge, this is the first cost-effectiveness analysis (CEA) for haemostasis with OTSCs among few CEAs presented for interventional endoscopy in general or haemostasis in particular. Our results demonstrate that OTSC therapy for recurrent PUB is cost-effective in comparison to standard endoscopic therapy.
In our study, both treatment-related and total costs in both groups did not differ significantly. This is surprising as the costs of an OTSC can be up to 10 times higher than the costs of a ‘conventional’ clip. However, most important drivers of total therapy costs are not haemostatic devices, but rather general costs of hospital stay. This was also shown in a study of Barkun and colleagues, which compared costs and effectiveness in preventing recurrent PUB by applying a Doppler-probe guided haemostasis approach.14 In our patient cohort, duration of total hospital and ICU stay was not significantly different in both groups: costs of hospital stay in the standard therapy (ST) and OTSC group were 360,429 € and 357,188 € compared to costs of haemostasis treatment of 68,804 € and 65,495 €. Second, due to the lower efficacy of conventional clips in the STING trial, a significant number of patients in the ST group were allocated to rescue OTSC treatment. The costs of OTSC rescue were therefore added to the costs of standard treatment. However, successful OTSC rescue prevented further treatment and resulted in similar rates of angiographic/surgical treatment in both groups. The number of EGDs performed was also not significantly different in both groups because all OTSCs in the standard group were placed immediately after failure of standard therapy and resulted in durable haemostasis. Treatment time of the OTSC group was slightly longer (mean procedural time 27 ± 9 min vs 31 ± 12 min). Although this difference nearly reached statistical significance (p = 0.055), it does not seem clinically relevant and is therefore not likely to affect costs.
Nonetheless, even under the condition of the crossover design, OTSC therapy showed cost-effectiveness for haemostasis as well as for total treatment costs. Therefore, it seems reasonable to assume that, with added costs of the subsequent therapy, the cost-effectiveness ratio would even be worse in the standard group without the cross-over design. Our results are in line with Imperiale et al.,15 who showed that cost-effectiveness in secondary haemostasis is linked to the success rate of the second intervention.
Comparing the absolute costs calculated in our study with the literature is not possible as no similar study investigating costs in recurrent PUB has been published so far. There is only one RCT investigating endoscopic re-therapy versus surgery in recurrent PUB. In this study, the amount of haemostatic devices (e.g. number of clips used etc.) is not reported. The reported median length of hospital stay of 10 (range 2–111) days is similar to the one reported in the STING trial, while the length of the ICU treatment and number of blood units transfused are markedly higher.5 However, it has to be noted that the study by Lau et al. was performed in 1999 with other standards of care on the ICU and other thresholds for blood transfusions.
The ACER expresses the costs per successful haemostasis. Regarding total costs, the ACER was calculated as 30,677.05 € for the standard group and 15,104.48 € for the OTSC group. In other words, the costs of OTSC treatment (overall hospital costs) per successful haemostasis are about half of the costs of the standard treatment. This effect was also consistent when comparing only haemostasis-related costs in both groups. A general principle in CEA is the calculation of the incremental costs of a new method or treatment causes per outcome. The calculated ICERs in our study show clearly that OTSC treatment in this indication does not even lead to higher costs per successful haemostasis. This finding is consistent both regarding total costs as well as treatment-related costs. When analysing the calculation of ICER and ACER, the crucial difference in both groups is the different rate of effectiveness in both treatment arms. The question whether this difference is representative for the investigated clinical setting is extensively discussed in the STING study and therefore not part of this discussion.8
Comparing our results with other CEAs is difficult as this is the first one for this indication. In most analyses, a decision tree model is created to compare different outcome scenarios. Subsequently, each treatment path is filled with probabilities of occurrence. Afterwards, the costs per predefined outcome are calculated. A potential bias of this approach is that the data for the probabilities of occurrence – which influence the costs most – are taken totally15,16 or at least in part17 from different studies. For our analysis, we decided not to use this approach for the following reasons: entering outcome data of the STING trial into a decision tree model would have resulted in very low sample sizes for each treatment path with unreliable probabilities of occurrence. On the other hand, extracting outcomes out of published literature would not be reliable either, as sufficient data on subsequent salvage treatment (angiographic or surgical) after failed use of OTSCs is scarce. Extracting data from similar studies would result in a very heterogenous study population with the risk of uncontrolled confounders. A decision tree model can theoretically be repeated over different time horizons (e.g. 3, 10 and 20 years). However, this would not be feasible for our analysis. In the STING trial, follow-up was done over a maximum of 30 days and currently long-term data on outcomes of recurrent PUB after OTSC treatment is not available.
Similar to the study by Barkun and colleagues,14 we derived our data from a recent randomised controlled study. The patient population of the STING trial is a solid basis for a cost analysis. The patient characteristics were well-balanced in both groups and there was a very detailed prospective documentation of endoscopic procedures, medical therapy and hospital/ICU stay. We believe that this approach reflects the clinical situation more precisely than a decision tree model, which is mainly based on assumptions. In our view, further prospective studies investigating different treatment scenarios and long-term outcome are necessary before a reliable decision tree analysis can be performed.
In most CEAs, the costs per quality-adjusted life years (QALYs) are calculated and taken for healthcare decisions. While QALYs are suitable for chronic diseases,18 there are limitations for acute diseases with usually complete recovery after a short period of time with markedly decreased quality of life.19 This is why most of the recently published CEAs14–17 calculated costs per defined outcome as primary endpoint as we did.
We did not include indirect costs (e.g. referring to administration, cost of finance, facility and capital equipment maintenance, depreciation and amortization) in our analysis. However, since the duration of hospital stay did not significantly differ between the groups and usually complete convalescence after successful haemostasis is achieved, indirect hospital costs are also very likely not to be significantly different in both treatment groups. Cashflow discounting using a varied interest rate or benefit discounting were also not taken into account but are likely not to have a major impact on CEA outcomes.
Sensitivity analyses generally help us to understand if small changes in variables of data already result in changes of outcomes. This approach addresses variables, which are affected by relevant uncertainty, based on assumptions made. In many health economic studies, assumptions need to be taken to fill in simulations. As explained above, our CEA did not use this approach, but was based on factual variables and outcome data, established by the STING trial. Costs of goods were not assumed but rather reflected the true costs encountered by the study sites, which were compiled in a micro-costing approach. Thus, sensitivity analysis was not found applicable and therefore was not performed.
Comparing cost-effectiveness of OTSCs with other endoscopic approaches for treatment of recurrent PUB is difficult. Application of Hemospray (Cook Medical) has been described to be feasible for refractory upper GI bleeding. Reported rebleeding rates in retrospective studies range around 25%20,21 when used in secondary haemostasis, which is significantly higher compared to the rebleeding rate of OTSCs in the STING study. Furthermore, the cost of the device is higher than the cost of an OTSC. It is therefore likely that OTSC treatment in the investigated indication would be more cost-effective compared to Hemospray. A study by Barkun et al. showed cost-effectiveness for Hemospray for first-line therapy of non-variceal upper gastro-intestinal tract bleeding. However, the study included different indications and is therefore not directly comparable to our analysis.22
Our study has several limitations. First, this study only provides information about second-line therapy after failed endoscopic haemostasis. Prospective RCTs on OTSC haemostasis as first-line treatment are ongoing but results are still not available. Second, our analysis is specific to the German healthcare system and maybe therefore not be fully comparable to different healthcare systems in the world. On the other hand, with the detailed analysis of every single step of the treatment in the STING cohort, we can provide a matrix where costs can be transferred into different healthcare systems. Third, we could not take into account survival and long-term outcome. Recurrent PUB has been shown to be associated with impaired survival. However, in the STING study a difference in mortality was not shown. As discussed above, this might be due to the design of the trial (small sample size, crossover design). We also do not have any other robust evidence on the impact of OTSC treatment on survival or long-term outcome in these patients. Hence, we were not able to include those factors in our analysis.
In conclusion, endoscopic haemostasis with OTSCs in recurrent PUB is cost-effective. Further studies will follow to investigate cost-effectiveness in first-line OTSC treatment.
Supplemental Material
Supplemental material, UEG871754 Supplemental Material for Over-the-scope clips are cost-effective in recurrent peptic ulcer bleeding by Armin Kuellmer, Juliane Behn, Benjamin Meier, Andreas Wannhoff, Dominik Bettinger, Robert Thimme, Karel Caca and Arthur Schmidt in United European Gastroenterology Journal
Acknowledgements
Study data derives from the STING study. This study was IRB approved and informed consent on the analysis was obtained by each patient.
Declaration of conflicting interests
A Kuellmer: none; J Behn: none; B Meier: none; A Wannhoff: none; D Bettinger: none; K Caca has received lecture fees and study grants from Ovesco Endoscopy; R Thimme: none; A Schmidt has received lecture fees and study grants from Ovesco Endoscopy.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Ethics approval
Not applicable.
Informed consent
Not applicable.
Supplemental material
Supplemental material for this article is available online.
References
- 1.van Leerdam ME. Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol 2008; 22: 209–224. [DOI] [PubMed] [Google Scholar]
- 2.Hearnshaw SA, Lowe D, Logan RFA, et al. Acute upper gastrointestinal bleeding in the UK: Patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut 2011; 60: 1327–1335. [DOI] [PubMed] [Google Scholar]
- 3.Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol 2012; 107: 345–360. [DOI] [PubMed] [Google Scholar]
- 4.Elmunzer BJ, Young SD, Inadomi JM, et al. Systematic review of the predictors of recurrent hemorrhage after endoscopic hemostatic therapy for bleeding peptic ulcers. Am J Gastroenterol 2008; 103: 2625–2632. [DOI] [PubMed] [Google Scholar]
- 5.Lau JY, Sung JJ, Lam YH, et al. Endoscopic retreatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med 1999; 340: 751–756. . [DOI] [PubMed] [Google Scholar]
- 6.Camus M, Jensen DM, Kovacs TO, et al. Independent risk factors of 30-day outcomes in 1264 patients with peptic ulcer bleeding in the USA: large ulcers do worse. Aliment Pharmacol Ther 2016; 43: 1080–1089. [DOI] [PMC free article] [PubMed]
- 7.Nykänen T, Peltola E, Kylänpää L, et al. Bleeding gastric and duodenal ulcers: Case-control study comparing angioembolization and surgery. Scand J Gastroenterol 2017; 52: 523–530. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt A, Gölder S, Goetz M, et al. Over-the-scope clips are more effective than standard endoscopic therapy for patients with recurrent bleeding of peptic ulcers. Gastroenterology 2018; 155: 674–686.e6. [DOI] [PubMed] [Google Scholar]
- 9.Rathmayer M, Heinlein W, Reiß C, et al. Cost assessment for endoscopic procedures in the German diagnosis-related-group (DRG) system – 5 year cost data analysis of the German Society of Gastroenterology project. Z Gastroenterol 2017; 55: 1038–1051. [DOI] [PubMed]
- 10.Martin J, Neurohr C, Bauer M, et al. Kosten der intensivmedizinischen Versorgung in einem deutschen Krankenhaus. Anaesthesist 2008; 57: 505–512. [DOI] [PubMed] [Google Scholar]
- 11.Bangh H, Zhao H. Average cost-effectiveness ratio with censored data. J Biopharm Stat 2012; 22: 401–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutubessy RC, Baltussen RM, Torres-Edejer TT, et al. Generalised cost-effectiveness analysis: an aid to decision making in health. Appl Health Econ Health Policy 2002; 1: 89–95. [PubMed]
- 13.Cohen DJ, Reynolds MR. Interpreting the results of cost-effectiveness studies. J Am Coll Cardiol 2008; 52: 2119–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barkun AN, Adam V and Wong RCK. Use of Doppler Probe in Nonvariceal Upper-Gastrointestinal Bleeding Is Less Costly and More Effective Than Standard of Care. Clin Gastroenterol Hepatol 2019 Feb 14. pii: S1542-3565(19)30179-X. [DOI] [PubMed]
- 15.Imperiale TF, Kong N. Second-look endoscopy for bleeding peptic ulcer disease: A decision-effectiveness and cost-effectiveness analysis. J Clin Gastroenterol 2012; 46: e71–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YI, Barkun AN, Adam V, et al. Cost-effectiveness analysis comparing lumen-apposing metal stents with plastic stents in the management of pancreatic walled-off necrosis. Gastrointest Endosc 2018; 88: 267–276.e1. [DOI] [PubMed] [Google Scholar]
- 17.Bahin FF, Williams SJ, McLeod D, et al. Wide-field endoscopic mucosal resection versus endoscopic submucosal dissection for laterally spreading colorectal lesions: A cost-effectiveness analysis. Gut 2017; 67: 1965–1973. [DOI] [PubMed] [Google Scholar]
- 18.Areia M, Fuccio L, Hassan C, et al. Cost-utility analysis of colonoscopy or faecal immunochemical test for population-based organised colorectal cancer screening. United Eur Gastroenterol J 2019; 7: 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bala MV, Zarkin GA. Are QALYs an appropriate measure for valuing morbidity in acute diseases? Health Econ 2000; 9: 177–180. . [DOI] [PubMed] [Google Scholar]
- 20.Hussein M, Alzoubaidi D, López MF, et al. 550 outcomes on the use of hemospray in upper gastrointestinal bleeds secondary to peptic ulcers: Prospective multicentre international hemospray registry. Gastrointest Endosc 2019; 89: AB94–AB95. [Google Scholar]
- 21.Rodríguez de Santiago E, Burgos-Santamaría D, Pérez-Carazo L, et al. Hemostatic spray powder TC-325 for GI bleeding in a nationwide study: survival and predictors of failure via competing risks analysis. Gastrointest Endosc 2019. Jun 17; pii: S0016-5107(19)31953-4. . [DOI] [PubMed] [Google Scholar]
- 22.Barkun AN, Adam V, Lu Y, et al. Using hemospray improves the cost-effectiveness ratio in the management of upper gastrointestinal nonvariceal bleeding. J Clin Gastroenterol 2018; 52: 36–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, UEG871754 Supplemental Material for Over-the-scope clips are cost-effective in recurrent peptic ulcer bleeding by Armin Kuellmer, Juliane Behn, Benjamin Meier, Andreas Wannhoff, Dominik Bettinger, Robert Thimme, Karel Caca and Arthur Schmidt in United European Gastroenterology Journal