Abstract
Background
Obesity is a risk factor for colorectal cancer (CRC).
Objective
The objective of this article is to investigate whether anthropometric measures reflecting visceral obesity are better predictors of CRC than body mass index (BMI).
Methods
Data were analysed from the Malmö Diet and Cancer study in Sweden, comprising 16,669 women and 10,805 men (median age 56.6 and 59.1 years) followed for a median 21.5 years. Diagnoses of CRC were identified using Swedish national registers. Cox regression was used to test the associations of BMI, waist circumference (WC), waist-hip ratio, waist-to-height ratio, waist-to-hip-to-height ratio, A Body Shape Index (ABSI) and percentage body fat with the development of CRC adjusted for age, alcohol consumption, smoking, education and physical activity in men and women.
Results
None of the measures were significantly associated with an increased risk for CRC in women. WC was the strongest predictor of colon cancer (CC) in men and the only measure that was independent of BMI. ABSI was the only measure significantly associated with the risk of rectal cancer in men.
Conclusions
Visceral obesity, best expressed as WC, is a risk factor for CC in men but a poor predictive marker for CRC in women.
Keywords: Anthropometric measures, A Body Shape Index, body mass index, colon cancer, colorectal cancer, colorectal cancer risk, predictive value, rectal cancer, waist circumference, waist-to-height-ratio
Key summary
Summarise the established knowledge on this subject:
Visceral obesity is a risk factor for colorectal cancer.
The anthropometric measure body mass index (BMI) is a poor discriminator between body fat and muscle, and a poor measure of visceral obesity.
What are the significant and/or new findings of this study?
Of the anthropometric measures tested, waist circumference is independent of BMI the strongest risk factor for colon cancer but not for rectal cancer in men.
Anthropometric measures did not predict colon cancer or rectal cancer in women at 22 years' follow-up.
The predictive capabilities of anthropometric measures for rectal cancer in women decreased with longer follow-up time, whereas they remained stable in men.
Introduction
Colorectal cancer (CRC) is the fourth most common cancer in terms of incidence and the third most common cause of cancer-related deaths worldwide in men and women, respectively.1 In the United States, the incidence of colon cancer (CC) among adults younger than 50 years has increased, with an even greater rise in the incidence of rectal cancer (RC).2 Recent changes in lifestyle-related factors, such as excess weight, sedentary lifestyles and unhealthy diet patterns have been proposed as mechanisms underlying the increased incidence.2 Obesity, especially visceral obesity, is a general risk factor for the development of cancer, including CRC.3 Visceral adipose tissue is an active endocrine organ, which produces pro-inflammatory factors, such as cytokines, and has a secretory profile different from that of subcutaneous adipose tissue.4 For example, a decrease in the levels of adiponectin, a peptide hormone mainly secreted by adipocytes, may be an important mechanism underlying the increased risk of CRC in obesity.5 Although body mass index (BMI) has long been used as a measure of obesity, it does not distinguish between body fat and muscular tissue or between visceral and non-visceral adiposity.6 Therefore, other anthropometric measures that better reflect visceral adiposity may be superior predictors of CRC, and the predictive value of these measures might differ for CC and RC. Previous research provided evidence suggesting that the predictive value of obesity for CRC differed for men and women, with childhood obesity seemingly more important for women and weight gain in adulthood apparently more relevant for men.7
Our aim was to investigate which anthropometric measure was the best predictor of CRC, CC and RC in adult men and women after a 22-year follow-up. We hypothesised that measures that better reflected visceral adiposity would be superior to BMI in predicting the development of CRC. In addition, we hypothesised that the anthropometric measures with the best predictive capabilities would differ according to cancer type (CC and RC) and sex. As an association between waist circumference and future CC in men and RC in women has been found in this cohort using a 14-year follow-up time and, with a slightly different aim and analysis strategy,8 we test a 14-year follow-up time in addition to the full available follow-up time.
Material and methods
Participants and procedure
The Malmö Diet and Cancer cohort is previously described in detail.8–10 This longitudinal study included all adult inhabitants of the city of Malmö, and baseline data collection was performed during baseline examinations between 1991 and 1996. In short, 28,098 individuals (women born between 1923 and 1950, n = 17,035; men born between 1923 and 1945, n = 11,063) attended a baseline examination by trained nurses at a screening centre, which included assessment of anthropometric measures. All the participants also completed a self-administered questionnaire including baseline data on smoking, alcohol consumption, educational level and physical activity. All participants provided informed consent.
All Swedish citizens are assigned a unique personal identity number,11 which allows for linkage between study cohorts and follow-up data from nationwide registers. Follow-up data were acquired from the Swedish Cancer Register and the Swedish Cause of Death register until 31 December 2016 for the purpose of this study. The Swedish Cancer Register was established in 1958 and gathers information about malignant disorders. It is estimated to contain data on approximately 96% of all cancer diagnoses.12 The Swedish Cause of Death register contains data on all deaths in Sweden since 1952.13
The regional ethics board in Lund approved the study (LU 51/90).
Variables
Anthropometric measures
Anthropometric measures BMI, waist circumference (WC), waist-hip ratio (WHR), waist-to-height ratio (WHtR: WC divided by height), waist-to-hip-to-height ratio (WHHR: WHR divided by height), A Body Shape Index (ABSI: WC/((BMI(2/3) × height(1/2))14 and body fat percentage (BFP, BIA-103 single-frequency analyser, JRL Systems, Detroit, MI, USA)15 was assessed as previously described in detail.16,17
Covariates
Smoking status was defined as being a current smoker, former smoker or never smoker. Alcohol consumption during the last 30 days18 was quantified in grams per day. Higher education was defined as secondary or further secondary education. Leisure-time physical activity scores were based on the number of minutes expended in 18 different activities, multiplied by an activity coefficient and classified as low, moderate or high.19
CRC
The Swedish Cancer Register was searched for CRC diagnoses, with the diagnoses based on the International Classification of Diseases seventh edition (ICD-7) or ninth edition (ICD-9). CRC was defined as an adenocarcinoma located in the colon or rectum. Anal and appendiceal cancers were excluded. The locations of the cancer were also classified using ICD codes. A cancer in the rectum or in the rectosigmoid junction was classified as RC. CRC that exhibited histology not concordant with an adenocarcinoma was excluded based on Systematized Nomenclature of Medicine codes 2 or 3. Cases with CRC of unknown location were included only in the analysis using CRC as outcome.
Statistics
The different anthropometric measures were tested as predictors of CRC, CC and RC using Cox-regression models, and the main analysis strategy described below has previously been used to investigate anthropometric measures as predictors of health outcomes in this cohort.16,17
The follow-up time started at recruitment in the beginning of 1991 and stopped 31 December 2016 or at the time of the first diagnosis of CRC, death or emigration. The data were censored for deaths from causes other than CRC during the follow-up.
To compare the different anthropometric measures, they are presented as standardised measures, and hazard ratios (HRs) reflect changes per standard deviation (SD) increment. HRs for non-standardised BMI, WHR and WC values (for which limits for overweight/obesity are established by the World Health Organisation20) are also presented to increase readability. These cut-offs are also used in the Kaplan–Meier analyses.
The main analyses included one crude model (Model 1) and one adjusted model (Model 2) for each anthropometric measure. A third model (Model 3), which controlled for BMI, was fitted in cases for which an anthropometric measure other than BMI was significantly associated with the outcome variable. Covariates in the multivariable analysis included age, smoking status, alcohol consumption, higher education and physical activity and all analyses were stratified by sex16,17 since anthropometric measures as well as age of onset of CRC, and possibly risk factors for CRC, differ between men and women.7
Harrell's C-statistics were used to identify the strongest predictor of CRC. A score of 1.0 indicates perfect predictive capacity, whereas a score of 0.5 indicates no predictive capacity.21 Likelihood ratio tests were used to evaluate whether the models including each anthropometric measure predicted CRC, CC and RC significantly better than a model including all covariates in the adjusted model. In addition, to ensure that the assumption about linearity in the associations are not violated, a univariable cubic spline regression model with three degrees of freedom and an alpha level of 0.05 was calculated per measure.22
Furthermore, the interaction between overweight status (BMI dichotomised </≥25 kg/m2) and the strongest predictor for CRC was analysed to test whether overweight status moderated the predictive capabilities of anthropometric measures in terms of the development of CRC. Furthermore, the interaction effect between the following variables were analysed: the effect of smoking status and overweight status on the development of CRC, and the effect of smoking status and the strongest predictor for CRC on the development of CRC.
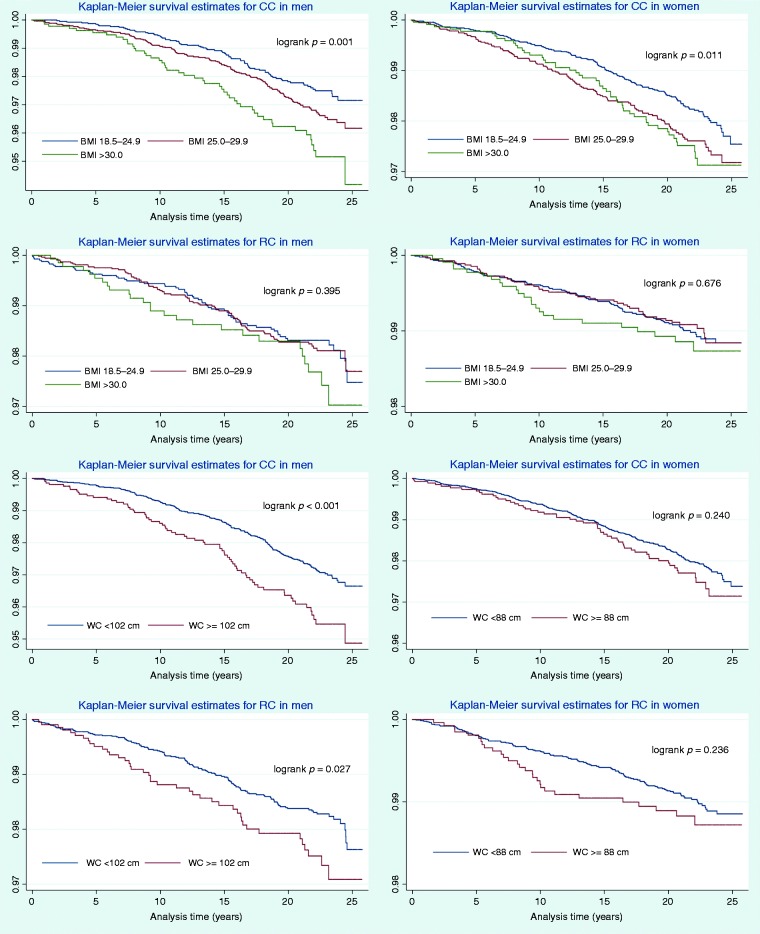
Kaplan–Meier survival analysis was performed to determine the association of BMI and WC with the development of CRC, stratified by sex and CRC location. A log-rank test was performed for each stratum. For the Kaplan–Meier analysis, BMI was categorised into three categories (normal weight ≥18.5–25 kg/m2, overweight ≥25–30 kg/m2 and obesity ≥30 kg/m2). The underweight category (BMI < 18.5 kg/m2) was excluded from the analysis, as the numbers were very small. WC was dichotomised using a cut-off of </≥88 cm for women and </≥102 cm for men and WHR was dichotomised using a cut-off of </≥0.85 for women and </≥0.9 for men.20
We repeated the main analysis (Model 2) with the same timeframe (end of follow-up 31 December 2009) and anthropometric measures (BMI, WC, BFP and WHR) as used by Brändstedt et al.8
The effect of the interaction between the body measure with the best predictive ability (continuous variable) and overweight status (stratified by BMI > or <25 kg/m2) on the development of CC was investigated to delineate whether the predictive capabilities of body measures were moderated by overweight status based on BMI.
Results
At baseline, the cohort consisted of 28,098 individuals. Of these, 155 individuals were excluded because of a previous diagnosis of CRC. After exclusion of 439 individuals with missing data on the anthropometric measures or covariates, 27,504 individuals with complete data remained; 16,699 (60.7%) were women, and 10,805 (39.3%) were men. The mean age was 58.1 (range 44.5–73.6; SD 7.6) years and 88.1% were born in Sweden. The mean BMI was 25.7 (range 13.9–50.0; SD 3.9) kg/m2, and almost half (46.6%) the women and 62.3% of the men had a BMI >25 kg/m2. Table 1 presents descriptive baseline data on those who did and did not develop CRC, stratified by sex.
Table 1.
Descriptive data for women and men, respectively. For each sex, data on those who developed CRC, those who did not develop CRC and for the total population are given. Continuous variables are presented as medians with IQRs, and dichotomous or categorical variables are presented with numbers and percentages.
| Descriptive data for women | ||||||
|---|---|---|---|---|---|---|
| CRC, n = 486 |
No CRC, n = 16,213 |
All women, n = 16,699 |
||||
| Median/n | IQR/% | Median/n | IQR/% | Median/n | IQR/% | |
| Age (years) | 61.7 | 54.8–65.6 | 56.5 | 49.9–63.6 | 56.6 | 50.0–63.7 |
| BMI (kg/m2) | 25.1 | 23.2–27.9 | 24.7 | 22.4–27.6 | 24.7 | 22.5–27.6 |
| Waist (cm) | 77 | 72–84 | 76 | 70–83 | 76 | 70–83 |
| Hip (cm) | 98 | 92–104 | 97 | 91–103 | 97 | 91–103 |
| Waist-hip ratio (cm/cm) | .79 | .76–.83 | .79 | .76–.82 | .79 | .76–.83 |
| WHtR (cm/cm) | .47 | .44–.52 | .46 | .43–.51 | .46 | .43–.51 |
| WHHR (cm/cm2) | .48 | .46–.51 | .48 | .46–.51 | .48 | .46–.51 |
| ABSI (value) | .070 | .068–.074 | .070 | .068–.073 | .070 | .068–.073 |
| Body fat percentage (%) | 32 | 28–34 | 31 | 27–34 | 31 | 28–34 |
| Alcohol (g per day) | 5.0 | .8–10.5 | 5.4 | .8–11.5 | 5.4 | .8–11.5 |
| Smoking | ||||||
| Current | 120 | 24.7 | 4564 | 28.2 | 4684 | 28.0 |
| Past | 150 | 30.9 | 4482 | 27.6 | 4632 | 27.7 |
| Never | 216 | 44.4 | 7166 | 44.2 | 7383 | 44.2 |
| Higher education | 94 | 19.3 | 3803 | 23.5 | 3897 | 23.3 |
| Physical activity | ||||||
| Low | 105 | 21.6 | 3233 | 20.0 | 3339 | 20.0 |
| Medium | 282 | 58.0 | 9732 | 60.0 | 10,014 | 60.0 |
| High | 99 | 20.4 | 3247 | 20.0 | 3346 | 20.0 |
| Death during follow-up | 243 | 50.0 | 4501 | 27.8 | 4744 | 28.4 |
| Descriptive data for men | ||||||
| CRC, n = 451 |
No CRC, n = 10,354 | All men, n = 10,805 | ||||
|
|
Median/n |
IQR/% |
Median/n |
IQR/% |
Median/n |
IQR/% |
| Age (years) | 60.9 | 54.8–65.2 | 59.0 | 52.9–64.6 | 59.1 | 53.0–64.7 |
| BMI (kg/m2) | 26.5 | 24.1–28.7 | 25.9 | 23.9–28.1 | 26.0 | 23.9–28.3 |
| Waist (cm) | 95 | 88–102 | 93 | 87–99 | 93 | 87–100 |
| Hip (cm) | 100 | 96–104 | 99 | 95–103 | 99 | 95–103 |
| Waist-hip ratio (cm/cm) | .95 | .91–.99 | .94 | .90–.98 | .94 | .90–.98 |
| WHtR (cm/cm) | .54 | .50–.58 | .53 | .49–.56 | .53 | .49–.57 |
| WHHR (cm/cm2) | .54 | .51–.57 | .53 | .51–.56 | .53 | .51–.56 |
| ABSI (value) | .081 | .078–.083 | .080 | .078–.082 | .080 | .078–.082 |
| Body fat percentage (%) | 21 | 18–24 | 20 | 17–24 | 20 | 17–24 |
| Alcohol (g per day) | 12.6 | 5.1–23.3 | 11.4 | 3.7–22.2 | 11.5 | 3.8–22.3 |
| Smoking | ||||||
| Current | 105 | 23.3 | 2988 | 28.9 | 3094 | 28.6 |
| Past | 232 | 51.4 | 4440 | 42.9 | 4672 | 43.2 |
| Never | 114 | 25.3 | 2921 | 28.2 | 3039 | 28.1 |
| Higher education | 95 | 21.1 | 2347 | 22.7 | 3444 | 22.6 |
| Physical activity | ||||||
| Low | 89 | 19.7 | 2070 | 20.0 | 2160 | 20.0 |
| Medium | 268 | 59.4 | 6214 | 60.0 | 6484 | 60.0 |
| High | 94 | 20.8 | 2065 | 20.0 | 2161 | 20.0 |
| Death during follow-up | 270 | 59.9 | 4522 | 43.7 | 4792 | 44.3 |
ABSI: A Body Shape Index; BMI: body mass index; CRC: colorectal cancer; IQR: interquartile range, WC: waist circumference; WHHR: waist-to-hip-to-height ratio; WHtR: waist-to-height ratio.
Median follow-up time was 21.5 years (interquartile range: 17.8–23.2, range 0–25.8), equal to 538,732 person-years. During follow-up, 9536 (34.7 %) individuals died, and 225 (0.8%) individuals emigrated. In total, 937 (3.4%) (women, n = 486; men, n = 451) members of the study cohort developed CRC. Of these, 590 (63.0 %) had CC, 319 women and 271 men; 328 (35.0 %), 157 women and 171 men, had RC; and 19 (2.0 %) individuals had CRC of unknown location.
Anthropometric measures as predictors of CRC
Table 2 presents crude and adjusted standardised HRs, C-statistics and likelihood ratios for the studied anthropometric measures for CRC, CC and RC, stratified by sex. In women, none of the studied anthropometric measures were significantly associated with the development of CC or RC after adjustments for covariates. In men, all the studied anthropometric measures other than WHHR were significantly associated with CRC in the adjusted model. The results were similar for CC. BMI, WC and WHtR had the strongest predictive value for CC, but the difference in C-statistics between them was small (<0.01), and WC was the only measure that predicted the development of CC independently of BMI (Table 2). Only ABSI showed a significant, albeit weak association with RC in men. No evidence against linearity in the associations was found in the cubic regression spline models (data not shown). Table 3 presents non-standardised HRs, representing a one-unit change for BMI and WC, in addition to HRs for predefined categories for BMI, WC and WHR. Figure 1 shows the Kaplan–Meier survival curves for the different categories of BMI and WC stratified by sex and cancer location.
Table 2.
Cox regression models for the risk of colorectal cancer for one standard deviation increase of each anthropometric measure. Model 1 shows the crude HR, representing the univariate analyses. Model 2 shows the multivariate analyses, where HRs are adjusted for age, alcohol, smoking, higher education and physical activity.
| Colorectal cancer | |||||||||||
|
n, women = 486 | |||||||||||
| Model 1 |
Model 2 | Model 3 | |||||||||
| Women n = 16,699 |
Crude HR |
95% CI |
p
|
Adjusted HR |
95% CI |
p
|
C-statistics |
p LR test |
|
|
|
| Null | .6653 | ||||||||||
| BMI | 1.15 | 1.06–1.25 | .001 | 1.08 | .99–1.18 | .084 | .6670 | .088 | |||
| WC | 1.17 | 1.08–1.28 | <.001 | 1.08 | .99–1.18 | .074 | .6668 | .077 | |||
| WHR | 1.09 | 1.00–1.19 | .061 | 1.05 | .96–1.14 | .31 | .6666 | .32 | |||
| WHHR | 1.12 | 1.02–1.22 | .015 | 1.02 | .93–1.12 | .66 | .6658 | .66 | |||
| WHtR | 1.19 | 1.10–1.30 | <.001 | 1.07 | .98–1.18 | .12 | .6667 | .13 | |||
| ABSI | 1.11 | 1.02–1.22 | .018 | 1.01 | .92–1.11 | .79 | .6653 | .79 | |||
| BFP | 1.20 | 1.09–1.31 | <.001 | 1.08 | .98–1.19 | .12 | .6662 | .12 | |||
|
n, Men = 451 | |||||||||||
| Model 1 |
Model 2 | Model 3 | |||||||||
| MEN n = 10,805 |
Crude HR |
95% CI |
p
|
Adjusted HR |
95% CI |
p
|
C-statistics |
p LR test |
Adjusted HR, including BMI |
95% CI |
p
|
| Null | .6238 | ||||||||||
| BMI | 1.18 | 1.07–1.29 | <.001 | 1.14 | 1.01–1.23 | .006 | .6281 | .007 | – | – | – |
| WC | 1.24 | 1.14–1.36 | <.001 | 1.20 | 1.09–1.32 | <.001 | .6350 | <.001 | 1.33 | 1.10–1.61 | .003 |
| WHR | 1.14 | 1.04–1.25 | .005 | 1.13 | 1.03–1.25 | .010 | .6304 | .011 | 1.07 | .95–1.21 | .23 |
| WHHR | 1.10 | 1.01–1.21 | .037 | 1.06 | .97–1.17 | .20 | .6257 | .20 | – | – | – |
| WHtR | 1.24 | 1.13–1.35 | <.001 | 1.17 | 1.06–1.29 | .001 | .6321 | .003 | 1.20 | .98–1.49 | .075 |
| ABSI | 1.21 | 1.10–1.33 | <.001 | 1.15 | 1.04–1.26 | .004 | .6336 | .004 | 1.13 | 1.03–1.25 | .011 |
| BFP | 1.14 | 1.04–1.24 | .007 | 1.11 | 1.01–1.21 | .035 | .6274 | .037 | 1.04 | .93–1.17 | .50 |
| Colon cancer |
|||||||||||
|
n, Women = 319 |
|||||||||||
| Model 1 |
Model 2 | ||||||||||
| Women n = 16,669 |
Crude HR |
95% CI |
p
|
HR |
95% CI |
p
|
C-statistics |
p LR test |
|
|
|
| Null | .6861 | ||||||||||
| BMI | 1.17 | 1.05–1.29 | .003 | 1.07 | .96–1.20 | .187 | .6874 | .19 | |||
| WC | 1.17 | 1.05–1.30 | .003 | 1.06 | .95–1.19 | .288 | .6868 | .29 | |||
| WHR | 1.07 | .96–1.20 | .21 | 1.03 | .92–1.15 | .600 | .6887 | .60 | |||
| WHHR | 1.11 | 1.00–1.24 | .059 | 1.00 | .90–1.12 | .937 | .6861 | .94 | |||
| WHtR | 1.19 | 1.08–1.32 | .001 | 1.05 | .94–1.17 | .395 | .6866 | .40 | |||
| ABSI | 1.09 | .97–1.21 | .14 | .98 | .87–1.09 | .682 | .6882 | .68 | |||
| BFP | 1.19 | 1.06–1.33 | .003 | 1.05 | .93–1.18 | .444 | .6865 | .44 | |||
|
n, Men = 271 | |||||||||||
| Model 1 |
Model 2 | Model 3 | |||||||||
| Men n = 10,805 |
Crude HR |
95% CI |
p
|
HR |
95% CI |
p
|
C-statistics |
p LR test |
Adjusted HR, including BMI |
95% CI |
p |
| Null | .6350 | ||||||||||
|
n, Men = 271 | |||||||||||
| Model 1 |
Model 2 | Model 3 | |||||||||
| Men n = 10,805 |
Crude HR |
95% CI |
p
|
HR |
95% CI |
p
|
C-statistics |
p LR test |
Adjusted HR, including BMI |
95% CI |
p |
| BMI | 1.21 | 1.08–1.36 | .001 | 1.20 | 1.06–1.34 | .004 | .6443 | .005 | – | – | |
| WC | 1.28 | 1.14–1.44 | <.001 | 1.25 | 1.11–1.41 | <.001 | .6534 | <.001 | 1.36 | 1.06–1.74 | .014 |
| WHR | 1.15 | 1.02–1.30 | .022 | 1.17 | 1.03–1.32 | .015 | .6457 | .016 | 1.07 | .92–1.26 | .34 |
| WHHR | 1.09 | .96–1.22 | .18 | 1.06 | .94–1.20 | .37 | .6370 | .37 | – | – | – |
| WHtR | 1.25 | 1.11–1.40 | <.001 | 1.20 | 1.06–1.35 | .004 | .6469 | .004 | 1.12 | .85–1.47 | .41 |
| ABSI | 1.20 | 1.06–1.35 | .004 | 1.14 | 1.00–1.28 | .042 | .6439 | .042 | 1.11 | .98–1.26 | .091 |
| BFP | 1.14 | 1.02–1.29 | .026 | 1.12 | 1.00–1.2 | .052 | .6400 | .055 | 1.03 | .89–1.19 | .69 |
| Rectal cancer n, Women = 157 | |||||||||||
| Model 1 |
Model 2 | ||||||||||
| Women n = 16,669 |
Crude HR |
95% CI |
p
|
HR |
95% CI |
p
|
C-statistics |
p LR test |
|
|
|
| Null | .6303 | ||||||||||
| BMI | 1.13 | .97–1.31 | .12 | 1.08 | .93–1.27 | .305 | .6314 | .31 | |||
| WC | 1.20 | 1.04–1.39 | .013 | 1.15 | .98–1.34 | .079 | .6337 | .085 | |||
| WHR | 1.14 | .98–1.33 | .079 | 1.11 | .95–1.29 | .192 | .6320 | .20 | |||
| WHHR | 1.14 | .98–1.33 | .098 | 1.06 | .91–1.25 | .439 | .6310 | .44 | |||
| WHtR | 1.21 | 1.04–1.39 | .012 | 1.13 | .97–1.32 | .119 | .6330 | .13 | |||
| ABSI | 1.21 | 1.03–1.40 | .015 | 1.13 | .96–1.32 | .133 | .6368 | .14 | |||
| BFP | 1.21 | 1.03–1.42 | .022 | 1.13 | .96–1.35 | .137 | .6328 | .14 | |||
|
n, Men = 171 | |||||||||||
| Model 1 |
Model 2 | Model 3 | |||||||||
| Men n = 10,805 |
Crude HR |
95% CI |
p
|
HR |
95% CI |
p
|
C-statistics |
p LR test |
Adjusted HR, including BMI |
95% CI |
p
|
| Null | .6118 | ||||||||||
| BMI | 1.11 | .95–1.29 | .18 | 1.05 | .90–1.23 | .54 | .6130 | .54 | – | – | – |
| WC | 1.18 | 1.01–1.36 | .031 | 1.11 | .95–1.30 | .18 | .6159 | .18 | – | – | – |
| WHR | 1.13 | .97–1.31 | .12 | 1.09 | .93–1.27 | .30 | .6142 | .31 | – | – | – |
| WHHR | 1.13 | .97–1.31 | .11 | 1.07 | .91–1.25 | .40 | .6139 | .40 | – | – | – |
| WHtR | 1.20 | 1.03–1.39 | .017 | 1.11 | .95–1.30 | .18 | .6168 | .18 | – | – | – |
| ABSI | 1.23 | 1.06–1.44 | .007 | 1.17 | 1.00–1.36 | .048 | .6217 | .049 | 1.16 | 1.00–1.36 | 0.055 |
| BFP | 1.08 | .93–1.26 | .32 | 1.03 | .88–1.20 | .67 | .6123 | .69 | – | – | – |
ABSI: A Body Shape Index; BFP: body fat percentage; BMI: body mass index; CI: confidence interval; HR: hazard ratio; WC: waist circumference; WHHR: waist-to-hip-to-height ratio; WHR: waist-hip ratio, WHtR: waist-to-height ratio.
P values for the likelihood ratio (LR) tests showing if the anthropometric measure significantly improved a Cox regression model using only covariates are also presented (null model). Model 3 is analysed only for men, where HRs are adjusted for BMI in addition to the covariates in Model 2. Colon cancer and rectal cancer are modelled in the same manner.
Table 3.
Cox regression models for the risk of CRC, CC and RC using established cut-offs for WHR, WC and BMI.
| Colorectal cancer | ||||||
|---|---|---|---|---|---|---|
| Women (n = 486) |
Men (n = 451) |
|||||
| Parameter | HR | 95% CI | p | HR | 95% CI | p |
| BMI | ||||||
| Continuous | 1.02 | 1.00–1.04 | .084 | 1.04 | 1.01–1.07 | .006 |
| Categorised | ||||||
| <18.5 | 1.32 | .65–2.69 | .435 | 1.20 | .30–4.88 | .792 |
| 18.5–24.9 | (Ref) | (Ref) | ||||
| 25–29.9 | 1.08 | .89–1.36 | .418 | 1.10 | .89–1.36 | .383 |
| >30 | 1.12 | .86–1.47 | .400 | 3.13 | 1.11–1.98 | .002 |
| WHR | ||||||
| Categorised | ||||||
| Below cut-off | (Ref) | (Ref) | ||||
| Above cut-off | 1.00 | .78–1.30 | .971 | 1.42 | 1.18–1.72 | <.001 |
| WC | ||||||
| Continuous | 1.01 | 1.00–1.02 | .073 | 1.02 | 1.01–1.03 | <.001 |
| Above cut-off | 1.01 | .80–1.29 | .886 | 1.45 | 1.17–1.80 | .001 |
| Colon cancer | ||||||
| Women (n = 319) |
Men (n = 271) | |||||
| Parameter |
HR |
95% CI |
p
|
HR |
95% CI |
p
|
| BMI | ||||||
| Continuous | 1.02 | .99–1.04 | .186 | 1.05 | 1.02–1.09 | .004 |
| Categorised | ||||||
| <18.5 | 1.90 | .89–4.08 | .098 | 1.08 | .15–7.78 | .938 |
| 18.5–24.9 | (Ref) | (Ref) | ||||
| 25–29.9 | 1.18 | .92–1.50 | .198 | 1.25 | .95–1.65 | .111 |
| >30 | 1.18 | .85–1.64 | .322 | 1.80 | 1.25–2.59 | .001 |
| WHR | ||||||
| Categorised | ||||||
| Below cut-off | (Ref) | (Ref) | ||||
| Above cut-off | .82 | .59–1.15 | .248 | 1.43 | 1.12–1.82 | .004 |
| WC | ||||||
| Continuous | 1.01 | 1.00–1.02 | .286 | 1.02 | 1.01–1.04 | <.001 |
| Above cut-off | .97 | .72–1.31 | .854 | 1.49 | 1.13–1.96 | .005 |
| Rectal cancer | ||||||
| Women (n = 157) |
Men (n = 171) | |||||
| Parameter |
HR |
95% CI |
p
|
HR |
95% CI |
p
|
| BMI | ||||||
| Continuous | 1.01 | .98–1.06 | .305 | 1.01 | .97–1.06 | .538 |
| Categorised | ||||||
| <18.5 | .45 | .06–3.24 | .428 | 1.46 | .20–10.56 | .708 |
| 18.5–24.9 | (ref) | (Ref) | ||||
| 25–29.9 | .87 | .61–1.24 | .441 | .92 | .67–1.30 | .669 |
| >30 | 1.07 | .67–1.69 | .788 | 1.16 | .72–1.84 | .544 |
| WHR | ||||||
| Categorised | ||||||
| Below cut-off | (Ref) | (Ref) | ||||
| Above cut-off | 1.39 | .92–2.01 | .115 | 1.36 | 1.01–1.85 | .046 |
| WC | ||||||
| Continuous | 1.01 | 1.00–1.03 | .079 | 1.01 | 1.00–1.03 | .177 |
| Above cut-off | 1.15 | .76–1.73 | .512 | 1.33 | .93–1.89 | .114 |
BMI: body mass index; CC: colon cancer; CI: confidence interval; HR: hazard ratio; RC: rectal cancer; WC: waist circumference; WHR: waist-hip ratio.
BMI is presented as a continuous parameter per kg/m2 and categorised according to the World Health Organisation (WHO). The cut-off for WHR defined as 0.85 for women and 0.90 for men, comparing below and above cut-off. WC is presented both a continuous parameter and as above by the WHO-defined cut-off, 88 cm for women and 102 cm for men. HRs are adjusted for age, alcohol, smoking, higher education and physical activity.
Figure 1.
Kaplan–Meier survival analysis for categorised BMI (normal weight, overweight and obesity) and WC (dichotomised), stratified by sex and by location of the CRC. Log-rank test is shown for each stratum. Ranges for the Y-axis differ between graphs to optimise resolution. Without adjusting for other covariates, obesity assessed as BMI is a significant risk factor for CC in men and women alike. Likewise, in men WC ≥102 cm is a significant risk factor both for RC and CC. BMI: body mass index; CC: colorectal cancer; CRC: colorectal cancer; RC: rectal cancer; WC: waist circumference.
We observed similar results to Brändstedt et al.8 in the analysis employing the 14-year follow-up. In women, no association was found between anthropometric measures and CC risk in the present study with the shorter follow-up period. However, statistically significant associations were seen between WC and BFP and the development of RC, similar to the study of Brändstedt and colleagues,8 were found using the shorter timeframe. Among men, WC, BMI, WHR and BFP were significantly associated with an increased risk of CC but not RC, similar to the results from the full 22-year follow-up time used in the present study.
Overweight status and association between anthropometric markers and risk of CRC and CC
Among the anthropometric measures, WC best predicted CRC and CC but not RC in men. Thus, we tested whether the predictive capabilities of WC were moderated by overweight status based on BMI. Overweight status did not moderate the association between WC and CC in men (p for the interaction = 0.19). Similarly, smoking did not moderate the association between WC or overweight status and future CC (p for the interaction between smoking and overweight status = 0.96 and p for the interaction between smoking and WC = 0.73).
Discussion
Several studies indicate that obesity, particularly visceral obesity, is a risk factor for CRC in men.23,24 For women the results of studies have differed, with some research observing an association between anthropometric measures and risk of CRC25 but other research observing no such association.23 Here, we found that visceral obesity was a strong risk factor for CC but not RC in men, and that anthropometric measures did not reliably predict future CRC in women. Previous studies have reported discordant findings on the association between anthropometric measures and risk of RC both in men and women.23,24 Studies on CRC prediction often differ in terms of the age ranges, follow-up times and variables used for adjustment. Thus, it can be difficult to compare the results. For example, a recent Norwegian study, in which ethnicity is very similar to that of our population,24 reported that WC predicted RC in women. One reason for the discrepancies between studies may be disease processes relating to menopause. In the present study we had, based on age, almost exclusively peri- and post-menopausal women, whereas in the Norwegian study, the menopausal status of the population was likely mixed. The failure of anthropometric measures to predict cancer in women after extended follow-up may also be explained by women's weights being more prone to fluctuations over time as compared with those of men. Previous research found that weight in childhood is a good predictor in women, while weight change is a better predictor in men.7 In the present cohort, anthropometric measures were assessed only at baseline, and influence of changes in measures over time on risk for CRC could not be tested.
In the present study, smoking was a risk factor for CRC and RC both in men and women (data not shown), but there was no interaction between smoking and anthropometric measures in terms of the risk of CRC. This finding is in contrast to that of a large Australian population-based study that found a prominent interaction between smoking status and anthropometric measures and future risk for cancer.26
In the present study, WC was associated with a 25% increased hazard per SD for CC in men and adjusting for BMI strengthened this association, likely due to BMI being a poor discriminator between muscle and adipose tissue in men.27 The inclusion of height-related measures did not improve the predictive value of WC. There is an ongoing discussion about how to target high-risk patients for CRC screening. Based on the results of the present study, we suggest that WC may be considered instead of BMI for identifying men with an increased risk of CC. However, none of the anthropometric measures included in the present study proved useful in identifying future risk of CC in women or RC in women and in men.
Strengths and limitations
The main advantages of the present study include the large and representative sample of the general population, the long follow-up period (median 21.5 years) and the very low level of loss to follow-up (0.8%). Furthermore, trained nurses sampled the anthropometric markers, thereby removing the risk of under-reporting common with self-reports of weight measures.28,29 The outcome variables were extracted from validated nationwide registers containing high-quality data. In addition, detailed registry data on tumour histology and location enabled us to exclude tumours with histology not consistent with adenocarcinoma and tumours located in regions other than the colon and rectum.
The limitations include the health status of the cohort, with the health status of the individuals who consented to take part in the study general better than that of those who declined to participate.30 This may have led to an underestimation of the effect size. The cohort was representative of the general population in terms of socio-demographic factors, such as the proportion of immigrants. Although the cohort was mainly composed of Swedish-born participants, the genetic diversity in Sweden is as large as that in the United Kingdom, and the genetic profile of inhabitants in Southern Sweden is similar to that of inhabitants in Northern Europe overall.31 Another limitation was the absence of data on family history of CRC, an important risk factor for CRC. Furthermore, we did not have data on hormone-replacement therapy in women; there are studies indicating that hormone-replacement therapy in women may mask the association between overweight and the risk of CRC.23,32
Conclusion
Anthropometric measures did not predict CC or RC in women after 22 years' follow-up. WC was the best predictor of CC in men and the only predictor that was independent of BMI, whereas anthropometric measures were poor predictors of RC in men.
Acknowledgements
Author contributions include the following: Study conception and design: AA, HH and AMF. Acquisition of data: KÖ. Statistical analysis: AA, HH and AMF. Analysis and interpretation of data: AA and AMF. Drafting of the manuscript: AA and AMF. Critical revision: AA, HH, FS, KÖ, ACC, PTS and AMF. All authors approved the final version of the article, including the authorship list.
Declaration of conflicting interests
None declared.
Ethics approval
The regional ethics board in Lund approved this study (LU 51/90).
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Informed consent
All participants in this study provided informed consent.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. J Natl Cancer Inst 2017, pp. 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tilg H, Moschen AR. Mechanisms behind the link between obesity and gastrointestinal cancers. Best Pract Res Clin Gastroenterol 2014; 28: 599–610. [DOI] [PubMed] [Google Scholar]
- 4.Kralova Lesna I, Kralova A, Cejkova S, et al. Characterisation and comparison of adipose tissue macrophages from human subcutaneous, visceral and perivascular adipose tissue. J Transl Med 2016; 14: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawashima K, Maeda K, Saigo C, et al. Adiponectin and intelectin-1: Important adipokine players in obesity-related colorectal carcinogenesis. Int J Mol Sci 2017, pp. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez MC, Correia MITD, Heymsfield SB. A requiem for BMI in the clinical setting. Curr Opin Clin Nutr Metab Care 2017; 20: 314–321. [DOI] [PubMed] [Google Scholar]
- 7.Kim H, Giovannucci EL. Sex differences in the association of obesity and colorectal cancer risk. Cancer Causes Control 2017; 28: 1–4. [DOI] [PubMed] [Google Scholar]
- 8.Brändstedt J, Wangefjord S, Nodin B, et al. Gender, anthropometric factors and risk of colorectal cancer with particular reference to tumour location and TNM stage: A cohort study. Biol Sex Differ 2012; 3: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berglund G, Elmstähl S, Janzon L, et al. The Malmö Diet and Cancer Study. Design and feasibility. J Intern Med 1993; 233: 45–51. [DOI] [PubMed] [Google Scholar]
- 10.Melander O, Newton-Cheh C, Almgren P, et al. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA 2009; 302: 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, et al. The Swedish personal identity number: Possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 2009; 24: 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barlow L, Westergren K, Holmberg L, et al. The completeness of the Swedish Cancer Register: A sample survey for year 1998. Acta Oncol 2009; 48: 27–33. [DOI] [PubMed] [Google Scholar]
- 13.Brooke HL, Talbäck M, Hörnblad J, et al. The Swedish Cause of Death Register. Eur J Epidemiol 2017; 32: 765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS One 2012; 7: e39504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calling S, Hedblad B, Engström G, et al. Effects of body fatness and physical activity on cardiovascular risk: Risk prediction using the bioelectrical impedance method. Scand J Public Health 2006; 34: 568–575. [DOI] [PubMed] [Google Scholar]
- 16.Andreasson A, Carlsson AC, Önnerhag K, et al. Waist/hip ratio better predicts development of severe liver disease within 20 years than body mass index: A population-based cohort study. Clin Gastroenterol Hepatol 2017; 15: 1294–1301.e2. [DOI] [PubMed] [Google Scholar]
- 17.Hagström H, Andreasson A, Carlsson AC, et al. Body composition measurements and risk of hematological malignancies: A population-based cohort study during 20 years of follow-up. PLoS One 2018; 13: e0202651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Göransson M, Hanson BS. How much can data on days with heavy drinking decrease the underestimation of true alcohol consumption? J Stud Alcohol 1994; 55: 695–700. [DOI] [PubMed] [Google Scholar]
- 19.Mattisson I, Wirfält E, Gullberg B, et al. Fat intake is more strongly associated with lifestyle factors than with socio-economic characteristics, regardless of energy adjustment approach. Eur J Clin Nutr 2001; 55: 452–461. [DOI] [PubMed] [Google Scholar]
- 20.Waist circumference and waist-hip ratio report of a WHO expert consultation. Geneva; 2008. https://apps.who.int/iris/handle/10665/44583 (accessed 10 April 2019).
- 21.Harrell FE, Jr, Lee KL, Califf RM, et al. Regression modelling strategies for improved prognostic prediction. Stat Med 1984; 3: 143–152. [DOI] [PubMed] [Google Scholar]
- 22.Royston PSW. Multivariable modeling with cubic regression splines: A principled approach. Stata J 2007; 7: 45–70. [Google Scholar]
- 23.Keimling M, Renehan AG, Behrens G, et al. Comparison of associations of body mass index, abdominal adiposity, and risk of colorectal cancer in a large prospective cohort study. Cancer Epidemiol Biomarkers Prev 2013; 22: 1383–1394. [DOI] [PubMed] [Google Scholar]
- 24.Lu Y, Ness-Jensen E, Martling A, et al. Anthropometry-based obesity phenotypes and risk of colorectal adenocarcinoma: A large prospective cohort study in Norway. Epidemiology 2016; 27: 423–432. [DOI] [PubMed] [Google Scholar]
- 25.Kabat GC, Xue X, Kamensky V, et al. Risk of breast, endometrial, colorectal, and renal cancers in postmenopausal women in association with a body shape index and other anthropometric measures. Cancer Causes Control 2015; 26: 219–229. [DOI] [PubMed] [Google Scholar]
- 26.Harding JL, Shaw JE, Anstey KJ, et al. Comparison of anthropometric measures as predictors of cancer incidence: A pooled collaborative analysis of 11 Australian cohorts. Int J Cancer 2015; 137: 1699–1708. [DOI] [PubMed] [Google Scholar]
- 27.Pasco JA, Nicholson GC, Brennan SL, et al. Prevalence of obesity and the relationship between the body mass index and body fat: Cross-sectional, population-based data. PLoS One 2012; 7: e29580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Visscher TL, Viet AL, Kroesbergen IH, et al. Underreporting of BMI in adults and its effect on obesity prevalence estimations in the period 1998 to 2001. Obesity (Silver Spring) 2006; 14: 2054–2063. [DOI] [PubMed] [Google Scholar]
- 29.Stommel M, Schoenborn CA. Accuracy and usefulness of BMI measures based on self-reported weight and height: Findings from the NHANES & NHIS 2001–2006. BMC Public Health 2009; 9: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manjer J, Carlsson S, Elmståhl S, et al. The Malmö Diet and Cancer Study: Representativity, cancer incidence and mortality in participants and non-participants. Eur J Cancer Prev 2001; 10: 489–499. [DOI] [PubMed] [Google Scholar]
- 31.Humphreys K, Grankvist A, Leu M, et al. The genetic structure of the Swedish population. PLoS One 2011; 6: e22547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freisling H, Arnold M, Soerjomataram I, et al. Comparison of general obesity and measures of body fat distribution in older adults in relation to cancer risk: Meta-analysis of individual participant data of seven prospective cohorts in Europe. Br J Cancer 2017; 116: 1486–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]