Abstract
Background
Budesonide has been proven to be an effective treatment for microscopic colitis (MC). However, the two current commercially available preparations are released in the ileum. Beclomethasone dipropionate (Clipper®) is a synthetic corticosteroid with topical colonic release.
Objective
This study aimed to explore whether an open-label treatment with beclomethasone dipropionate is an effective treatment for MC.
Methods
Prospectively collected data of 30 patients from six centres were retrospectively analysed. All patients had a confirmed diagnosis of idiopathic MC (lymphocytic and collagenous colitis) and were symptomatic (i.e. ≥ 21 loose stools over a seven-day period). Treatment consisted of 10 mg beclomethasone daily for four weeks, followed by 5 mg daily for another four weeks. The primary end point was the proportion of patients in remission (i.e. a mean of < 3 stools/day and a mean of <1 watery stool per day) after an eight-week treatment period. Secondary end points were the proportion of patients responding to therapy at weeks 4 and 8, remission at weeks 4 and 12 and relapse at week 12. Reported adverse events were collected.
Results
Overall, at week 8, remission was achieved in 70%, and 77% of patients were responding to treatment. After four weeks of treatment, 80% were responding, and 67% were in remission. Four weeks after stopping treatment, 60% were still in remission.
Conclusion
This open-label study suggests that an eight-week course of beclomethasone could be a promising and relatively safe treatment for MC. A randomised controlled study is warranted.
Keywords: IBD, microscopic colitis, lymphocytic colitis, collagenous colitis, beclomethasone dipropionate, budesonide
Introduction
Microscopic colitis (MC) is a type of inflammatory bowel disease (IBD) causing chronic, watery diarrhoea and sometimes abdominal pain, distension and faecal incontinence.1–5 Unlike the better known forms of IBD, Crohn's disease and ulcerative colitis, it is a benign, non-progressive disease without complications or risk of malignancy. MC consists of two entities, lymphocytic colitis (LC) and collagenous colitis (CC), although overlap forms can occur. The diagnosis should be suspected in middle-aged patients with chronic diarrhoea and normal-appearing mucosa at (ileo)colonoscopy, and is confirmed by characteristic histopathological changes in colonic biopsies (inflammatory mononuclear infiltration of the lamina propria with intraepithelial lymphocytes for LC, and occurrence of a sub-epithelial collagenous band > 10 µm in thickness in CC).6
The pathophysiology has not yet been determined. Different hypotheses exist on genetic predisposition, immune dysregulation and autoimmunity (possible correlation with coeliac disease, rheumatoid arthritis, hypo- and hyperthyroidism), bile acid malabsorption, infection (campylobacter, yersinia, clostridium) and drug-induced MC (proton pump inhibitors, non-steroidal anti-inflammatory drugs, selective serotonin reuptake inhibitors, beta-blockers, statins, bisphosphonates and ticlopidine, mainly in LC). There might also be a connection with lifestyle factors such as caffeine, alcohol and dairy.4,6,7
Current first-line treatment is topically acting synthetic glucocorticosteroids.4,8 Most evidence is generated for oral budesonide in controlled ileal release preparations for both LC and CC, including two meta-analyses.3,8–11 Budesonide 6–9 mg orally daily for eight weeks shows short-term efficacy in 60–80% of patients but with a high recurrence rate when stopped. Longer-term treatment does not lower this recurrence rate.1,2,4,9–11 One small trial with Budesonide with controlled release in the colon achieves similar results.12 Other alternatives include bismuth subsalicylate (though there is some concern about central-nerve toxicity), mesalazine, cholestyramine and purely symptomatic therapy with anti-diarrheal agents and probiotics. Systemic corticosteroids are used in severe cases of MC, although long-term use should be avoided.3,4
Beclomethasone dipropionate (Clipper®) is a synthetic topically acting corticosteroid with topical release in the colon. Given its galenic formulation, it is a potentially better treatment than the commercially available budesonide formulations that are targeted for topical release mainly in the terminal ileum. Although beclomethasone is mentioned in literature reviews as a potential treatment, there are no systematic reports on its effect in this indication. Promising results were obtained with beclomethasone in the treatment of ulcerative colitis, suggesting that this might also be a possible treatment for MC.13–17
The aim of this study was to analyse treatment results of open-label use of beclomethasone dipropionate retrospectively in CC and LC. If the results of this exploratory analysis were encouraging, a controlled interventional study could be a next step. We hereby report the first series of patients with MC treated with open-label beclomethasone.
Materials and methods
From January 2017 until October 2017, patients with CC or LC from six participating centres in Belgium were screened and invited to participate in this open-label exploratory observational trial. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethical committees of all the participating centres (16 October 2017). Informed consent was obtained from all patients. The collected data were blinded at the site before being sent to the organising centre for analysis. There, the data were analysed by two independent physicians (T.D. and E.J.) who had not been in contact with any of the participating patients. All results are presented based on an intention to treat analysis.
In order to qualify for this analysis, patients had to be aged ≥18 years with a confirmed histopathological diagnosis of active CC or LC (according to generally accepted criteria)1 with a disease activity index of ≥21 over a seven-day period (i.e. an average of ≥ 3 stools per day of which ≥1 stool was watery).18
Exclusion criteria were concomitant IBD, ongoing treatment for MC (including steroids, topical steroids, anti-diarrheal agents), corticosteroid treatment of any type in the last four weeks, clinical evidence of uncontrolled coeliac disease (patients not following their gluten-free diet and/or positive coeliac serology), pregnancy and lactation, or any other reason for (chronic) diarrhoea. If drug-induced CC or LC was suspected, the putative drug had to have been stopped at least two weeks before screening. In order to allow for a uniform analysis, the following guidance was given.
Before starting beclomethasone, the treating physician was asked to fill in a questionnaire about each patient's baseline complaints, background and vital signs. Patients were asked to keep a diary for seven days prior to starting beclomethasone. Treatment consisted of 10 mg beclomethasone (two tablets taken together in the morning) daily for four weeks, followed by 5 mg (one tablet taken in the morning) daily for another four weeks. Compliance was recorded (defined as drug adherence of >80% of the prescribed dose). Patient follow-up was scheduled after four and eight weeks of treatment for clinical re-evaluation. Patients were asked to keep a symptom and Bristol stool scale diary one week prior to the follow-up visits. At week 12, four weeks after stopping treatment, patients were contacted by telephone for a questionnaire.
The primary end point of this study was the proportion of patients in remission (defined as a mean of <3 stools/day and a mean of <1 watery stool per day) after an eight-week treatment period. Secondary end points were the proportion of patients responding to therapy (response defined as a 50% reduction of stool frequency) at weeks 4 and 8, the proportion of patients in remission at weeks 4 and 12 and the proportion of patients with relapse at week 12. Reported adverse events were collected.18
Results
A total of 30 patients (23 female; median age 69.5 years; range 42–89 years) were included from six hospitals. Of these 30 patients, 20 had LC (15 female; median age 68.5 years; range 42–89 years) and 10 had CC (8 female; median age 70 years; range 56–86; Table 1). At baseline, the mean number of stools per day was 6.8 (range 3–30). Other main symptoms included faecal incontinence (n = 16), abdominal pain (n = 16), abdominal distension (n = 15) and weight loss (n = 5). All patients were started on 10 mg beclomethasone daily. At week 8, two patients had prematurely stopped treatment (one for constipation and one due to an accidental hip fracture). Two patients were excluded from the per protocol analysis (one due to the start of another treatment during the study and the other being the aforementioned patient who broke her hip and who dropped out before sufficient data could be collected). Patients who completed the study had a compliance rate of 100%.
Table 1.
Demographics and baseline characteristics.
| LC (N = 20; 65%) | CC (N = 10; 32%) | Total population (N = 30) | |
|---|---|---|---|
| Age | 68.5 (42–89) | 72 (56–86) | 69.5 (42–89) |
| Sex | |||
| Female | 15 (75%) | 8 (80%) | 23 (77%) |
| Male | 5 (25%) | 2 (20%) | 7 (23%) |
| Symptoms | |||
| Mean number of stools a day (range) | 6.8 (3–30) | 6.7 (3–15) | 6.8 (3–30) |
| Of which diarrhoea (range) | 6.8 (3–30) | 6.5 (3–15) | 6.7 (3–30) |
| Other symptoms | |||
| Faecal incontinence | 10 (50%) | 6 (60%) | 16 (53%) |
| Abdominal pain | 9 (45%) | 7 (70%) | 16 (53%) |
| Abdominal distension | 9 (45%) | 5 (50%) | 14 (47%) |
| Number of previous episodes | |||
| 0 (first episode) | 16 (80%) | 7 (70%) | 23 (76%) |
| 1 | 4 (20%) | 2 (20%) | 6 (20%) |
| 2 | 1 | 1 (10%) | 1 (3%) |
| If previous episode, previous treatments | |||
| None (except anti-diarrhoeal medication) | 4 | ||
| Budesonide | 5 | ||
| Beclomethasone | 2 | ||
LC: lymphocytic colitis; CC: collagenous colitis.
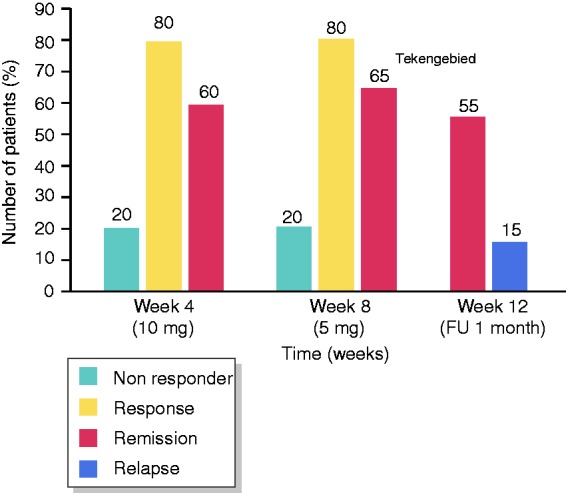
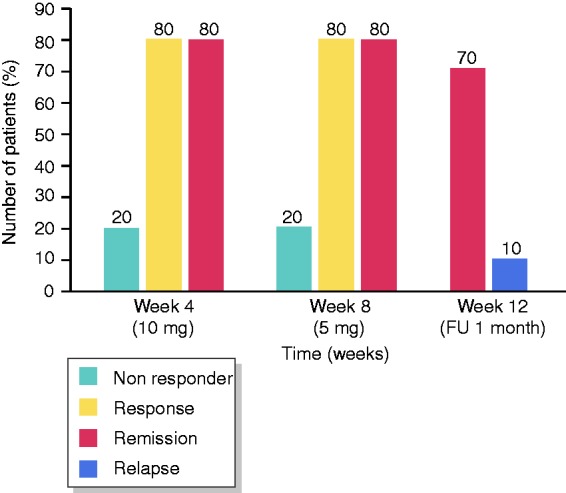
At week 8, 70% (21/30) of patients (LC 65%, CC 80%) were in remission, and 77% (23/30) of patients (LC 75%, CC 80%) were responding to treatment. The mean number of stools a day decreased from 6.8 (range 3–30, all of them diarrhoea) at baseline to 1.6 (range 1–5) with 0.66 diarrhoea (range 0–5; Figure 1). After four weeks of treatment with 10 mg beclomethasone, 80% (24/30) of patients (LC 80%, CC 80%) were responsive, and 67% (20/30) of patients (LC 60%, CC 80%) were in remission. Four weeks after stopping treatment, 60% (18/30) of patients (LC 55%, CC 70%) were in remission (Figures 2–4).
Figure 3.
Results (lymphocytic colitis).
Figure 1.
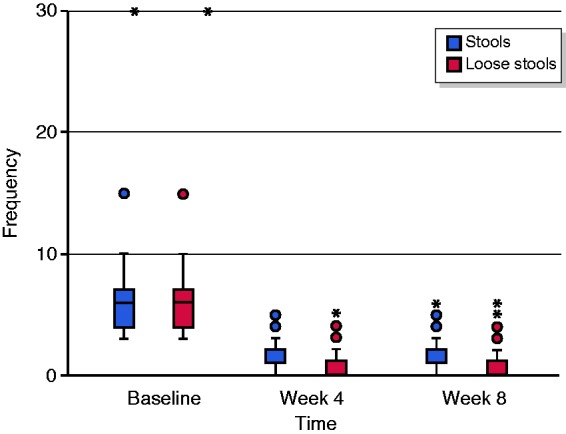
Evolution of daily stools and diarrhoea.
Figure 2.
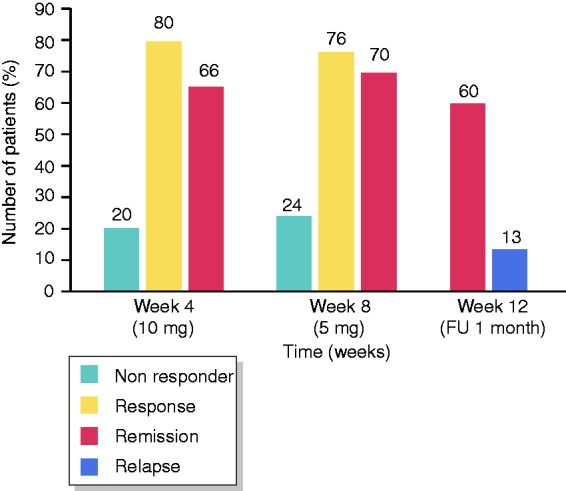
Results.
Figure 4.
Results (collagenous colitis).
Out of 30 patients, seven had been treated earlier for MC. Five episodes were treated with budesonide, two with beclomethasone and two with a combination of agents (one with budesonide and levofloxacine and one with budesonide and beclomethasone). Of the five patients who received prior treatment with budesonide during another flare, three reported a subjective better and/or faster response with beclomethasone and one reported a similar response, and for the remaining patient, this information was missing.
A total of 16 patients reported 22 adverse events: constipation (n = 6) facial flushing (n = 2), myalgia (n = 3), hyperglycaemia (n = 1), hyperactivity (n = 1), vaginal discharge (n = 1), headache (n = 1), hoarseness (n = 1), loss of appetite (n = 1), lower urinary tract symptoms (n = 2), anal mucosal prolapse (n = 1), oral candidiasis (n = 1) and insomnia (n = 1). All adverse events were graded as mild and transient, except for one case of muscle cramps and one case of constipation (leading to discontinuation). Two adverse events (hyperglycaemia in a patient with diabetes and myalgia) were still ongoing at the end of the study.
Discussion
This open-label exploratory analysis confirms that beclomethasone dipropionate seems to be a promising treatment for MC, with 21/30 (70%) patients being in remission at the end of treatment and 23/30 (77%) being responders. However, three patients were no longer in remission one month after withdrawal from therapy.
MC is a relatively rare condition that is, given its nature and patient population, usually not concentrated in academic or tertiary referral hospitals. Therefore, all studies are small. We studied both LC and CC. Although our study population was too small to reach statistical significance, sub-analysis shows a trend that treatment with beclomethasone dipropionate provides a more sustainable remission in CC than in LC. The high compliance rate illustrates that treatment is feasible and relatively well tolerated in everyday life. Of the reported adverse events, all can be considered minor or expected, yet the observed events can suggest some systemic effects. All but one of the side effects were mild; most side effects were transient. Therefore, overall, this treatment can be considered relatively safe.
Since this was the first time beclomethasone dipropionate was studied in patients with MC, we opted to use a similar definition for remission and an approximately same duration of treatment as used in some previous studies with other drugs, making our findings more easily comparable. Only one other prospective trial studied the effect of beclomethasone dipropionate in patients with LC (in comparison to mesalazine). They reported slightly higher rates of clinical remission after eight weeks (84% in patients taking 10 mg beclomethasone dipropionate daily, 83% in patients taking 5 mg daily).19 Other previous studies were performed with Budesonide; the achieved remission and response rates are comparable to ours.3,4,9,20 However, as stated before, exact comparison to all of the previous studies is difficult, since some studies used different definitions of response and/or remission.3,4 Nonetheless, our findings indicate that beclomethasone dipropionate could be a valid alternative to current treatment regimens.
The limitations of our study include the small sample size, open-label nature and retrospective analysis (although from prospectively collected data). The number of participants was too small to draw any statistically significant conclusions, and the usual bias that can be expected from retrospective studies cannot be excluded. Although the proposed treatment regimen in our study design was not mandatory, all patients and clinicians adhered to the suggested plan except for one who changed his diet concomitantly with the treatment regimen.
In previous studies with other pharmacological agents (in LC), the median time to relapse is approximately two months after cessation of therapy, and almost all of the relapses in patients on maintenance therapy occur during the first two months. The duration of follow-up in our study might be too short to evaluate the long-term effect.13,20–22 A previous trial of beclomethasone dipropionate in LC provided a longer follow-up of 12 months (10 months after cessation of beclomethasone dipropionate) that showed a cumulative relapse rate of approximately 30%.19
In our study, no dose finding was done. Therefore, other dosing regimens could potentially improve response and remission rates in both the short and long term.
Conclusion
This exploratory open-label study suggests that an eight-week course of beclomethasone could be a promising and relatively safe treatment for MC. A randomised placebo-controlled study is warranted to demonstrate its effect. Alternatively, a randomised controlled trial with an active control including budesonide can be considered.
Acknowledgements
This project would not have been possible without the extensive efforts of each of the participating centres with regard to patient selection, inclusion and follow-up. We would like to thank Koen Thorrez, Joris Arts, Katrien Dejaegher, François D’Heygere, Annelies Holvoet, Bart van Besien, Luc Harlet, Harald Peeters, Wouter Vanmoerkercke, Filip Baert and Ann D’Hondt. We would also like to thank all the study coordinators at the participating centres – Patsy Vlieghe, Sofie Himpe, Sophie Claeys, Marijke Ghekiere and Evelien Rijsman – for patient inclusion and critical revision of the manuscript. In particular, we would like to thank Filip Baert and Ann D’Hondt for the opportunity, the support and guidance during this project and the final revision of the manuscript.
Part of these data was also presented at ECCO 2018 (DOP035 Beclomethasone dipropionaat is effective for microscopic colitis: results of an open-label multicentre study. J Crohns Colitis 2018;12:S055. DOI: 10.1093/ecco-jcc/jjx180.072) as a digital oral presentation and as the winning abstract at the Belgian Week of Gastroenterology in Antwerp 2018 (available at: https://bwge-abstracts.be/BIRD.html).
Declaration of conflicting interests
None declared.
Ethics approval
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethical committees of all the participating centres.
Funding
This research was funded with an unrestricted grant by Chiesi Belgium.
Informed consent
Informed consent was obtained from all patients.
References
- 1.Okamoto R, Negi M, Tomii S, et al. Diagnosis and treatment of microscopic colitis. Clin J Gastroenterol 2016; 9: 169–174. [DOI] [PubMed] [Google Scholar]
- 2.Baert F, Wouters K, D'Haens G, et al. Lymphocytic colitis: a distinct clinical entity? A clinicopathological confrontation of lymphocytic and collagenous colitis. Gut 1999; 45: 375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baert F, Schmit A, D'Haens G, et al. Budesonide in collagenous colitis: a double-blind placebo-controlled trial with histologic follow-up. Gastroenterology 2002; 122: 20–25. [DOI] [PubMed] [Google Scholar]
- 4.Simondi D, Pellicano R, Reggiani S, et al. A retrospective study on a cohort of patients with lymphocytic colitis. Rev Esp Enferm Dig 2010; 102: 381–384. [DOI] [PubMed] [Google Scholar]
- 5.Tong J, Zheng Q, Zhang C, et al. Incidence, prevalence, and temporal trends of microscopic colitis: a systematic review and meta-analysis. Am J Gastroenterol 2015; 110: 265–276. [DOI] [PubMed] [Google Scholar]
- 6.Tysk C, Bohr J, Nyhlin N, et al. Diagnosis and management of microscopic colitis. World J Gastroenterol 2008; 14: 7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernández-Bañares F, Esteve M, Espinós JC, et al. Drug consumption and the risk of microscopic colitis. Am J Gastroenterol 2007; 102: 324–330. [DOI] [PubMed] [Google Scholar]
- 8.Nunes T, Barreiro-de Acosta M, Marin-Jiménez I, et al. Oral locally active steroids in inflammatory bowel disease. J Crohns Colitis 2013; 7: 183–191. [DOI] [PubMed] [Google Scholar]
- 9.Chande N, Al Yatama N, Bhanji T, et al. Interventions for treating lymphocytic colitis. Cochrane Database Syst Rev 2017; 7: CD006096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feyen B, Wall GC, Finnerty EP, et al. Meta-analysis: budesonide treatment for collagenous colitis. Aliment Pharmacol Ther 2004; 20: 745–749. [DOI] [PubMed] [Google Scholar]
- 11.Stewart MJ, Seow CH, Storr MA. Prednisolone and budesonide for short- and long-term treatment of microscopic colitis: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2011; 9: 881–890. [DOI] [PubMed] [Google Scholar]
- 12.Kamboj AK, Cotter TG, Hicks SB, et al. Extended-release multimatrix budesonide for microscopic colitis. Inflamm Bowel Dis 2017; 23: E21–22. [DOI] [PubMed] [Google Scholar]
- 13.Miehlke S, Madisch A, Karimi D, et al. Budesonide is effective in treating lymphocytic colitis: a randomized double-blind placebo-controlled study. Gastroenterology 2009; 136: 2092–2100. [DOI] [PubMed] [Google Scholar]
- 14.Van Assche G, Manguso F, Zibellini M, et al. Oral prolonged release beclomethasone dipropionate and prednisone in the treatment of active ulcerative colitis: results from a double-blind, randomized, parallel group study. Am J Gastroenterol 2015; 110: 708–715. [DOI] [PubMed] [Google Scholar]
- 15.Rizzello F, Gionchetti P, D'Arienzo A, et al. Oral beclometasone dipropionate in the treatment of active ulcerative colitis: a double-blind placebo-controlled study. Aliment Pharmacol Ther 2002; 16: 1109–1116. [DOI] [PubMed] [Google Scholar]
- 16.Nunes T, Barreiro-de Acosta M, Nos P, et al. Usefulness of oral beclometasone dipropionate in the treatment of active ulcerative colitis in clinical practice: the RECLICU Study. J Crohns Colitis 2010; 4: 629–636. [DOI] [PubMed] [Google Scholar]
- 17.Campieri M, Adamo S, Valpiani D, et al. Oral beclometasone dipropionate in the treatment of extensive and left-sided active ulcerative colitis: a multicentre randomised study. Aliment Pharmacol Ther 2003; 17: 1471–1480. [DOI] [PubMed] [Google Scholar]
- 18.Hjortswang H, Tysk C, Bohr J, et al. Defining clinical criteria for clinical remission and disease activity in collagenous colitis. Inflamm Bowel Dis 2009; 15: 1875–1881. [DOI] [PubMed] [Google Scholar]
- 19.Latella G, Angelucci E, Tsolaki A, et al. Beclometasone dipropionate and mesalazine are equally effective in the treatment of lymphocytic colitis. Dig Liver Dis 2010; 42: S87. [Google Scholar]
- 20.Miehlke S, Madisch A, Bethke B, et al. Oral budesonide for maintenance treatment of collagenous colitis: a randomized, double-blind, placebo-controlled trial. Gastroenterology 2008; 135: 1510–1516. [DOI] [PubMed] [Google Scholar]
- 21.Papi C, Aratari A, Moretti A, et al. Oral beclomethasone dipropionate as an alternative to systemic steroids in mild to moderate ulcerative colitis not responding to aminosalicylates. Dig Dis Sci 2010; 55: 2002–2007. [DOI] [PubMed] [Google Scholar]
- 22.Miehlke S, Heymer P, Bethke B, et al. Budesonide treatment for collagenous colitis: a randomized, double-blind, placebo-controlled, multicenter trial. Gastroenterology 2002; 123: 978–984. [DOI] [PubMed] [Google Scholar]


