Abstract
Tea, Camellia sinensis, which belongs to the family Theaceae, is a shrub or evergreen tree up to 16 m in height. Green tea is very popular because of its marked health benefits comprising its anticancer, anti-oxidant, and antimicrobial activities, as well as its effectiveness in reducing body weight. Additionally, it was recognized by Chinese people as an effective traditional drink required for the prophylaxis against many health ailments. This is due to the complex chemical composition of green tea, which comprises different classes of chemical compounds, such as polyphenols, alkaloids, proteins, minerals, vitamins, amino acids, and others. The beneficial health effects of green tea ultimately led to its great consumption and increase its liability to be adulterated by either low-quality or non-green tea products with concomitant decrease in activity. Thus, in this review, green tea was selected to highlight its health benefits and phytoconstituents, as well as recent approaches for its quality-control monitoring that guarantee its incorporation in many pharmaceutical industries. More research is needed to find out other more biological activities, active constituents, and other simple and cheap techniques for its quality assurance that ascertain the prevention of its adulteration.
Keywords: biological activity, Camellia sinensis, phytochemistry, quality control
1. Introduction
Natural products were widely employed for many centuries in home remedies, as well as in over-the-counter drug products and as raw materials for the manufacturing of various pharmaceuticals, cosmeceuticals, and nutraceuticals, both in developed as well as developing countries [1,2]. They provide a cost-effective natural solution for many ailments and possess nearly the same effectiveness as synthetic pharmaceuticals. They act via a multi-systemic effect but with relatively lower hazardous adverse effects in comparison to synthetic ones [3,4].
However, natural products are facing many challenges concerning their usage, exemplified by the difficulties related to the acquisition of sufficient pure amounts, particularly for those plants that grow in small quantities or at distant locations. Besides, a lot of problems are sparked regarding the standardization, as well as the determination, of a specific dose in a manner appropriate to the intended use. It is worthy to highlight that although natural remedies are relatively safe [5,6], the majority of lethal events reported in relation to herbal products’ consumption are mainly attributable to their poor quality that eventually hindered their usage as raw materials in many pharmaceutical industries [7].
Establishing internationally recognized guidelines for assessing the quality of natural products appeared to be mandatory. Sensory, macroscopic, and microscopic examinations are the first essential steps for establishing the identity and the degree of purity of herbal materials in regard to authentic/genuine samples of the material in question. Many analytical tools are employed in quality control, including both spectroscopic and chromatographic methods [8].
Tea, Camellia sinensis (L.) Kuntze or Thea sinensis F. Theaceae, is a shrub or evergreen tree up to 16 m tall [9]. Its leaves are alternate, exstipulate, lanceolate to obovate, up to 30 cm long, 2–5 cm broad, pubescent, sometimes becoming glabrous, serrate, acute or acuminate. Fresh leaves are often picked from late March and early April to July every year [10]. Green tea represents about 20% of the dried tea which is manufactured annually and is mainly consumed in Asian countries, such as Japan, owing to its relative safety and cheaper price in comparison to other beverages [11]. This was concomitantly reflected by the annual increase in its production, which is estimated to be 6.4 percent. It is noteworthy to mention that China, India, and Sri Lanka are the biggest tea-producing countries, respectively, as reported by the 22nd Tea session held by FAO-Intergovernmental Group. Kenya and Sri Lanka constitute the two major tea-exporting countries, respectively.
The chemical composition of green tea is highly complex, owing to its possessing different classes of chemical compounds, including polyphenols, alkaloids, proteins, minerals, vitamins, and amino acids. Besides, it is greatly popular because of its marked health benefits comprising its anticancer [12], anti-oxidant [13], anti-hypercholesterolemic [14], and antimicrobial activities [15], in addition to its effectiveness in reducing body weight [16]. Herein, due to the beneficial health effects of green tea that ultimately lead to its great consumption, this paper highlights its health benefits and phytoconstituents, as well as the recent approaches for its quality-control monitoring that guarantee its incorporation in many pharmaceutical industries.
2. Health Benefits and Biological Activities of Green Tea (C. sinensis)
Green tea is well-known for its marked health benefits, and, additionally, it was recognized by Chinese people as an effective traditional drink required for the prophylaxis against many health ailments [17]. Most of its crucial health benefits and biological activities are discussed below and summarized in the supplementary data Table S1.
2.1. Antioxidant and Hepatoprotective Activity
In traditional medicines, green tea is considered a reference drug for many plant species with respect to its anti-oxidant potency [18]. This activity is mainly attributed to its polyphenols that are represented by its flavanols, which can be readily oxidized to the corresponding o-quinones, and thus function as hydrogen acceptors, as well as hydrogen donors [19]. The interaction with reactive oxygen species could be accomplished by the polyphenolic compounds existing in green tea via possessing different degrees of free radical scavenging properties, particularly toward oxygen-free radicals [20] and to some extent toward nitrogen (NO) species’ production inhibition [21]. The linkage of flavan-3-ol to gallic acid (GA) and the O-trihydroxy structure in the B ring are important criteria for the O2− and NO scavenging activity of tea polyphenol [22]. Besides, green tea polyphenols possessing ortho-dihydroxyl functional groups, exemplified by epi-catechin and epi-catechin gallate, are good antioxidants that act in synergism with endogenous α-tocopherol [23].
The mechanistic potency for the anti-oxidant activity of green tea polyphenols (GTPs) was shown in different studies. GTPs act in different ways to suppress the active oxygen species generated from phenolic metabolites of benzene [24] and protect some treated oils from oxidation [25]. Other examples include increasing the excretion of carcinogenic products formed endogenously [26] and causing a marked enhancement in the salivary antioxidants in smokers, in both the short- and long term [27].
Green tea can effectively inhibit induced dose-dependent oxidative DNA damage and cell proliferation in the liver, as well as hepatotoxicity [28]. It induces the activity of some hepatic phase II enzymes, such as hepatic glutathione S-transferase (GST) [29], correcting the imbalances of the anti-oxidative system [30,31] and counteracting the induced lowered glutathione [32] in the liver. Epi-gallo catechin gallate (EGCG) and (−)-epigallocatechin-3-(3”-Omethyl) gallate are potent hepatoprotective agents, as they suppress cytotoxin-induced cell death, such as suppression of induced morphological change and induced cell death in a dose-dependent manner [33,34].
Additionally, high amounts of total phenolic compounds and caffeine prevents fat storage in the liver in high-fat diet cases [35]. Green tea is a source of highly important hexogen antioxidants that cannot be synthesized by our body and counterattack free radicals produced during metabolism of xenobiotics in the liver [36].
Moreover, green tea leaves can cause a significant reduction in elevated serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), hepatic protein carbonyls, and ROS, and thus prevent the induced liver damage [13]. Dietary green tea extract treatment reduces liver injury during nonalcoholic steatohepatitis by decreasing the pro-inflammatory signaling [37]. In addition, the extract has a substantial therapeutic effect against alcohol-induced hepatic mitochondrial DNA damage [38].
2.2. Anticancer and Anti-Mutagenic Activity
Green tea polyphenolics GTPs are considered to be dietary chemopreventive compounds due to their potent effect on apoptosis and cell cycle progression inhibition [39]. It acts through different mechanisms to promote the function of P-glycoprotein-mediated efflux, reducing the cellular exposure to xenobiotics [40], and through the induction of the microsomal detoxification enzyme [41]. Green tea behaves as a potent anticancer, owing to its polyphenolics, which are effective against various chemically induced carcinogens, in addition to their pronounced effect on tumor suppression [42].
Many studies showed that green tea catechins inhibit matrix metalloproteinases (MMPs), which play an important role in tumor invasion and metastasis [43]. Epi-gallo catechin gallate (EGCG) is effective against cancer [44] via the prohibition of cell cycle progression [45]. It inhibits fatty-acid synthase (FAS) [46], modulates protease activity during endothelial morphogenesis [47], and inhibits the vascular endothelial growth factor (VEGF) family [12], so it prohibits angiogenesis, which is a crucial step in the growth and metastasis of cancers [48]. Additionally, the cytotoxic activity of EGCG is also due to its auto-oxidation products, which also possess equivalent cytotoxic activities to EGCG [49]. Moreover, methylxanthines and polysaccharide inhibit tumor metastasis [50].
Green tea exerts a significant anticarcinogenic effect against many different types of cancer. Oral or topical application of green tea polyphenols possesses a considerable potential to antagonize the tumorigenic effects of some polycyclic aromatic hydrocarbons on skin [51]. Regarding photo-carcinogenesis, oral administration of green tea inhibits the formation of skin lesions produced by UVB light in a dose-dependent manner and prolongs the time of tumor appearance and decreases their number [52]. However, topical application of GTPs or EGCG protects against UVB-induced local and systemic immune suppression and skin cancer induction [53]. Also, it was found that green tea consumption reduces UVB-induced skin redness and causes significantly fewer UVA+B light-induced skin papillomas and tumors, and thus diminishes the incidence of skin cancer [54]. Moreover, many studies reported that green tea can be used in the treatment of malignant melanoma [55]. Green tea treatment was also found to decrease the induced fore-stomach, duodenal, large intestine, and colon tumors, and it can protect against the metastatic process in gastric cancer cells and strongly inhibit the growth of hepatocellular carcinoma cells [56,57,58]. Furthermore, green tea exerted an antagonistic effect on induced micronuclei and apoptosis in colonic crypt cells [59]. Moreover, it inhibits the hyperoxidation of membrane phospholipids, which reflect the degree of DNA damage and carcinogenic alteration [60], and it causes less metachronous colorectal adenomas incidences in patients who have undergone the complete removal of colorectal adenomas [61].
Regarding lung cancer, green tea infusion revealed a notable efficacy in the alleviation of lung cancer via EGCG, which reduces the mean number of lung tumors, causes a decline in the multiplicity of lung adenomas, and counteracts metastasis with concomitant inhibition of induced lung tumorigenesis, acting synergistically with other cancer preventive agents [62,63]. Furthermore, EGCG also inhibits lung cancer stem cells [64] and stimulates apoptotic induction in human lung cancer cells [65].
Concerning leukemia, crude catechins exemplified by (+)-gallocatechin, EGC, EC, EGCG, and ECG have a suppressive effect on erythroblastic leukemia cells in a dose-dependent manner [66]. EGCG inhibits the growth of human leukemic cell lines without causing side effects and in a specific way [67]. Furthermore, EGCG exhibits antileukemic activity by inducing the differentiation of the leukemia cells, suppressing their proliferation [68] and inducing apoptosis of B-cell and T-cell chronic lymphocytic leukemia in a dose-dependent manner, without affecting healthy B-and T-cells [69]. In addition, green tea intake has a small protective effect against acute myeloid leukemia [70]. Moreover, breast cancer could be ameliorated via the daily consumption of green tea catechins. It could effectively reduce mortality triggered from induced mammary carcinogenesis, in addition to reducing the average sizes of tumors [71]. Polyphenon E, which contains about 58.4% EGCG, was noticed to exert a mild inhibitory effect on the early promotion stage [72]; however, catechins may act in the post-initiation stage, but not in a dose-dependent manner, in addition to reducing recurrences [73,74]. Recently, it was found that green tea drinkers have no excess risk for epithelial ovarian cancer [75]. Furthermore, green tea has proved to have antiproliferative effects against human prostate cancer cells [76] and can lead to cell growth inhibition in some prostate cancer cells [77] showing potent inhibition of 5α-reductase enzyme, which may be involved in the development of benign prostatic hyperplasia, hirsutism, and prostate cancer [78,79]. GTPs also strongly inhibit the growth of renal cell carcinoma cell lines in a concentration-dependent manner [80]. It is worthy to highlight that green tea catechins possessing the gallate group act as anticancer agents on glioblastoma cells and can prevent the inflammatory processes associated with aggressive glioma tumor growth [81,82]. Nowadays, green tea serves as a diet-derived immune-modulatory chemopreventive agent; as many of its contained flavonoids stimulate natural killer (NK) cell activity, so it may be used in the treatment and prevention of malignant diseases [83].
Meanwhile, aqueous extract of green tea has a marked antimutagenic activity against different major classes of dietary and occupational carcinogens [84]. This activity may be attributed to different mechanisms, such as improving the fidelity of DNA replication [85], affecting carcinogen metabolism [86], direct interaction between the reactive genotoxic species of the various promutagens, and nucleophilic tea components. In addition, it causes a potent inhibition of the cytochrome P450-dependent bioactivation of the promutagens [87], acting intracellularly as a blocking or suppressive agent [88], and potent dose-dependent antigenotoxic activity [89]. Besides the antimutagenic activity of green tea, it has anticlastogenic effects [90].
2.3. Antimicrobial and Antiviral Activity
Many studies reported the antimicrobial activity of green tea. Green tea is effective against Staphylococcus epidermidis, Staphylococcus aureus, and Vibrio cholerae O1, owing to the bactericidal catechins that primarily cause defection in the bacterial membranes [91]. Green tea is also effective against various bacteria that cause dental caries, such as Escherichia coli, Streptococcus salivarius, and Streptococcus mutans [92]. EGCG and gallocatechin gallate (GCG) markedly inhibit the secretion of extracellular Vero toxins from enterohemorrhagic Escherichia coli cells into the culture supernatant fluid [93]. Raw extract of green tea, especially gallocatechin gallate (GCG), is able to suppress 1-deoxy-d-xylulose 5-phosphate reductoisomerase activity, which is an antimicrobial target [94]. Furthermore, it can also reduce the lethality of ricin toxin [95], but it has a poor activity against Babesia divergens that infect cattle [96].
EGCG can inhibit the activity of Salmonella typhimurium type III, and thus reduce the bacterial invasion into host cells [97]. Green tea extract is bactericidal against Gram positive bacteria and bacteriostatic against Gram negative ones, with less antifungal activity against Aspergillus niger and Penicillium chrysogenum [98].
In addition, tea polyphenols have the ability to inhibit the development and growth of bacterial spores, as in case of Bacillus stearothermophilus and Clostridium thermoaceticum, due to their ability to decrease the heat resistance of these bacterial spores when added at high temperature [99]. However, chlorogenic acid induces the apoptotic markers through excessive potassium efflux and an apoptotic volume reduction, which induces cytosolic calcium uptake and cell cycle arrest in Candida albicans, in addition to its ability to induce caspase activation and DNA fragmentation [100].
Tea exhibits antiviral activity against human viruses and serves as a diet-derived immune-modulatory chemopreventive agent, as many of its contained flavonoids stimulate NK cell activity, so it may be used in the treatment and prevention of viral diseases [83]. Recently, it was found that EGCG is capable of inhibiting the Brazilian strain of Zika virus entry [101]. Additionally, topical application of EGCG can be used to prevent the sexual transmission of HIV, as it disaggregates existing amyloid fibrils termed semen-derived enhancers of viral infection fibers and inhibits the formation of new ones [102].
2.4. Anti-Schisosomiasis and Antiparasitic Activity
Green tea could be able to protect liver cells in mice after being infected with Schistosoma mansoni, and thus decreases cellular necrosis and regenerates total protein and glycogen levels partially. This could be achieved through suppression of the oxidative stress, owing to its scavenging properties toward free radicles [103]. Moreover, green tea has an antiparasitic activity as it noncompetitively inhibits the activity of Toxocara canis [104].
2.5. Cardioprotective Activity
Green tea could improve the risk factors for heart disease [105], as it significantly reduces total cholesterol, low density lipoprotein (LDL) cholesterol, and blood pressure [106]. It also improves microvascular function and skin oxygen tension in both older and younger populations [107]. Nonfermented Chinese green tea is considered to be an ideal beverage to prevent the incidence of coronary heart disease. Its consumption, together with its catechin-rich fractions, lowers the risk of coronary heart diseases through delaying atherogenesis by significantly preventing endothelial cell induced LDL oxidation and foam cell formation [108]. The inhibition of advanced glycation end products formation in collagen represents another important mechanism for the protective effects of green tea catechins against cardiovascular diseases [109].
The consumption of green tea decreases the risk of myocardial infarction (MI) in a dose-dependent manner up to ≥4 cups/day [110], as it reduces cardiac hypertrophy, improves systolic and diastolic dysfunction, restores the antioxidant enzyme activity, and stimulates the glucose pathway and mitochondrial function with reduced apoptosis after MI [111]. High dietary intake of green tea may be useful in the reduction and prevention of cardiac injury following ischemia [112].
The flavonoids that exist in green tea perform its cardio-protective effect by improving the reserve in coronary flow velocity [113]. EGCG is able to reduce both arsenic- and doxorubicin-induced cardiotoxicity [114]. It reduces the inflammation and preserves the cardiac function, with lowered mortality rate [115]. Concerning EC, it may be involved in treating cardiac arrhythmia [116]. Meanwhile, EC supplementation has a cardioprotective effect without changing blood pressure, arterial stiffness, or the blood lipid profile [117].
Additionally, green tea can be used as anti-hypercholesterolemic agent by acting in several ways, such as enhancing hepatic excretion of cholesterol or inhibiting absorption of cholesterol in the alimentary tract [118]. This is accompanied by increasing fecal bile acids and cholesterol excretion, resulting in the lowering of the plasma cholesterol [119] and the lowering of LDL oxidation by increasing cellular antioxidant status or inhibiting oxidizing enzyme activities in the arterial wall [14]. Other possible mechanisms include diminishing the levels of α-ketoglutarate and pyruvate dehydrogenases enzymes, which are vital in the cholesterol biosynthesis [120] or inhibition of the rate limiting enzyme of cholesterol biogenesis, squalene epoxidase (SE) [121]. Green tea also causes a prolongation in LDL oxidation lag time by flavonoids [122], inhibition of serum triglyceride elevation [123], and prevention of fat storage in the liver, lowering blood lipids and increasing fecal excretion of triglycerides [35].
Fresh green tea fermented under nitrogen gas produces γ-aminobutyric acid (GABA)-rich tea, which was proved to prevent the occurrence of hypertension [124]. Theanine exerted a significant decline in blood pressure following a dose-dependent manner [125]. EGCG and EGC were found to inhibit dopa decarboxylase enzymes in a concentration- and time-dependent manner, which is a known target for drugs used in hypertension [126]. EGCG that was chronically infused in the hypothalamic paraventricular nucleus (PVN) attenuates hypertension by chronic inhibition of ROS, in addition to regulating the balance of neurotransmitters, as well as cytokines, in the PVN [127]. Green tea extract was found to prevent high angiotensin II dose-induced hypertension and the accompanied organ damage by preventing or scavenging superoxide anion generation [128]. Decaffeinated green tea extract also reduces the metabolic syndrome through reduction of the formation of ROS, which results in lowered blood pressure [129].
Regarding green tea anti-thrombotic activity, it was found that unprocessed tea extracts can significantly reduce the levels of thromboxane-B2 and then eliminate the aggregation of platelets to produce microthrombi, while processed ones are unable to form any inhibition, significantly owing to the presence of a heat-labile compound [120]. Possibly, green tea has a fibrinolytic effect [105]. In addition, the catechins inhibit induced platelet aggregation in vitro in a dose-dependent manner, without changing the coagulation parameters [130]. The tea can also be used to prevent red blood cell hemolysis [131]. Different green tea extracts have different degrees of inhibition of dehydration of stored sickle cells, and this inhibitory activity increases by increasing the number of hydroxyl groups [132].
2.6. Antidiabetic and Anti-Obesity Activity
Green tea is used for the amelioration of diabetes and its complications, acting as a renoprotective in diabetes mellitus, in addition to the ability of EGCG to inhibit angiogenesis that is involved in many diseases, such as diabetic retinopathy [48,133]. Furthermore, EGCG can help in controlling hyperglycemia and alleviating diabetes complications via reducing plasma glucose, insulin level, and liver and kidney weight [134]. Recently, it was found that EGCG is useful for resensitizing insulin-resistant muscle [135].
The administration of GTPs significantly increases glucose tolerance and reduces induced elevated serum glucose levels [136]. Floratheasaponins A, B, and C in the flower buds of green tea exhibit potent inhibitory effects against sucrose-induced serum glucose elevation [137]. Green tea water-soluble polysaccharide fraction has an inhibitory effect against α-amylase, which leads to decreased blood glucose levels [138], along with galloyl moiety in catechin, which increases the inhibition of pancreatic α-amylase, due to enhanced association with the enzyme active site [139].
Regarding obesity, green tea extract is a well-tolerated natural product for the management of obesity by reducing fat digestion through marked inhibition of digestive lipases, such as gastric and pancreatic lipases, especially by saponins, which lead to altered lipid emulsification in gastric or duodenal media [140]. In addition, it stimulates thermogenesis/energy expenditure and fat oxidation mainly by EGCG and caffeine [16], without significant differences in either plasma cholesterol or blood pressure [141]. Furthermore, chronic usage of short-time decoction of green tea decreases perirenal and epididymal adipose tissues and weight gains in high-fat diet cases [35].
Recent studies confirm that a mixture of green tea catechins and caffeine has a beneficial effect on body-weight management through sustained energy expenditure, fat oxidation, and preservation of fat-free body mass [142]. Polyphenols such as EGCG can increase lipolysis, in addition to possessing anti-adipogenic effects through being a fatty-acid synthase (FAS), which is a possible therapeutic target for appetite and weight control [143,144]. Polyphenols have “exercise mimetic” properties through exercise-inducible pathways [16].
2.7. Gastrointestinal Tract Problems Relieving Activity
The purified green tea EC can cause both endothelium-dependent and -independent mesenteric arteries relaxation [145]. However, EGCG preserves the aortic thickness and regenerates elastin content, due to its anti-inflammatory effect, so could prevent abdominal aortic aneurysm [146]. Furthermore, high green tea consumption is associated with a reduction in the risk of precancerous chronic atrophic gastritis [147].
In addition, floratheasaponins A, B, and C in flower buds exhibit potent inhibitory effects against ethanol- and indomethacin-induced gastric mucosal lesions, so green tea seems to have a gastro-protective effect [137]. However, daily intake of green tea is able to alter the growth and composition of the intestinal flora and modulate the genesis of potentially harmful agents like Clostridium difficile and Clostridium perfringens, due to the effect of its components, such as vitamin C and polyphenols [148]. Green tea has recently proved to modulate the fecal microbiome and thus endogenous metabolites [149].
2.8. Neuroprotective Activity
Green tea extract has a protective effect on the ischemia/reperfusion-induced brain injury and behavior deficit. It also reduces the number of ischemia/reperfusion-induced apoptotic neuronal cells [150]. GTPs, EGCG, ECG, EGC, and EC are able to protect synaptosomes from induced lipid peroxidation damage [151]. EGCG alone has a protective effect against stress-induced neural injuries [152]. EGCG was shown to be easily absorbed from the digestive tract and penetrate the brain, reaching levels similar to those found in lung, liver, kidney, and others [153]. It has a neuroprotective effect against neuronal damage following transient global ischemia in the gerbils acting by different mechanisms as angiogenesis in the early stage of ischemic stroke promoting [154,155]. Regarding neurotoxicity, l-theanine has a protective effect against cadmium-induced neurotoxicity by reducing brain cadmium levels and oxidative damage, which lead to neurodegenerative diseases [156].
In addition, GTPs can be considered therapeutic agents to alter brain aging processes by serving as neuroprotective agents in major neurodegenerative disorders, such as Parkinson’s disease and Alzheimer’s disease [157,158]. In addition, green tea catechin intake may be useful in the improvement of the morphologic and functional changes that occur naturally in the accelerated senile brains [159]. EGCG and EGC were found to be inhibitors of dopa decarboxylase enzyme in a concentration- and time-dependent manner [126]. Green tea containing high levels of EGCG prevents the loss of tyrosine hydroxylase (TH)-positive cells in the substantia nigra [160]. Both tea and EGCG, when used alone or with Parkinson’s disease’s inducers, can decrease the neuronal nitric oxide synthase (nNOS) expressions in the substantia nigra, which provides a neuroprotective effect [161].
Regarding Alzheimer’s disease, EGCG may be beneficial for its prevention, as it has protective effects against amyloid ß-induced hippocampal neuronal apoptosis [162] in which neuronal loss is accompanied by the deposition of amyloid ß protein in senile plaques [163]. EGCG regulates the growth and survival of astrocytes without being cytotoxic. Green tea catechins possess an inhibitory effect against β-secretase, which is known as one of the most important amyloid precursor protein cleaving enzymes in Alzheimer’s disease [164]. EC reduces amyloid-β-induced β-secretase-1 expression and thus inhibits the most toxic amyloid-β aggregates [165].
The theanine existing in green tea enhances the memory and learning ability, owing to its significant effect on the release or reduction of memory- and learning-linked neurotransmitters, such as dopamine and serotonin [125,166]. Polyphenols also have a potential role in the prevention/treatment of dementia [167]. It was proved that combined ingestion of EGC and GA increases the learning ability in a better way than EGCG, which reaches the brain parenchyma at a very low concentration [168]. Additionally, theanine may affect emotions by interacting with neurotransmitters in the brain, due to a modulation of synaptic transmission [42]. Moreover, drinking theanine-rich and low-caffeine green tea has anti-stress effects, as theanine, EGC, and arginine together can counteract the effect of caffeine and EGCG on psychosocial stress-induced adrenal hypertrophy [169]. Theanine also produces a marked relaxation effect due to its rapid absorption and transportation to the brain in 30 min, without suffering any metabolic degradation [125].
2.9. Anti-Inflammatory, Analgesic, Antipyretic, and Anti-Allergic Activity
Green tea is considered to be a potent anti-inflammatory and antipyretic agent [170,171]. EGCG reduces nitric oxide production via its gallate structure [172]. It inhibits the endothelial cell growth which leads to the inhibition of angiogenesis that is involved in many diseases, such as chronic inflammation [48]. The non-polyphenolic fraction of green tea represented by Pheophytin a and b exerts suppressive activities against the activation of human polymorphonuclear neutrophils (PMNs) associated with inflammatory reactions in a dose-dependent manner [173]. Moreover, green tea extract/tablets showed a high efficacy in controlling pain, particularly in patients suffering from osteoarthritis as well as [174].
Regarding the anti-allergic activity of green tea, polyphenols are considered to be the major inhibitory components of hot-water-soluble extracts of green tea against histamine release from mast cells [175]. In addition, green tea has an anti-allergic effect due to leaf saponins [176] and floratheasaponins from flower buds that possess anti-allergic activities, as well [177]. It was reported that methylated EGCG blocks the production of two compounds in the body mainly involved in producing an allergic response, histamine and immunoglobulin E (IgE), and works by triggering and sustaining allergic reactions. It is worthy to mention that green tea can activate heat-generating mechanisms in the body such that a fall in core body temperature can be lowered upon cold exposure [178].
2.10. Skeletomuscular System Relieving Activity
EGCG has direct vasodilator action in skeletal muscle and increasing muscle microvascular blood flow [179]. It can induce myogenic differentiation [180] and increase the recovery of muscle mass and function [181]. Moreover, EGCG induces apoptotic cell death of osteoclasts in a dose-dependent manner with no effect on osteoblasts [182]. Appropriate concentrations of EGCG have an anti-inflammatory effect in the treatment of collagen membranes and thus can be used in bone regeneration [183]. It was also found that green tea has a protective effect against induced bone and hyaline cartilage alteration [184]. Furthermore, EGCG enhances osteoprotegerin synthesis, which is secreted from osteoblasts, and suppresses osteoclastic bone resorption, especially in the elderly [185]. Meanwhile, EGCG has the potential to inhibit the development of arthritis, as pretreatment with EGCG inhibits the release of induced lactate dehydrogenase in human chondrocytes of osteoarthritis cartilage [186]. Green tea extract can also be used as an adjunctive treatment, because it can control the pain and improve the knee-joint physical function in adults with osteoarthritis [187].
Green tea can be useful as a medicament for treatment of infected root canals, as Japanese green tea extracts have antibacterial and bactericidal effects against some facultative anaerobes and obligative anaerobes [188]. It also has a high amount of fluoride, which may help strengthen teeth and bones and reduce tooth decay [189]. Besides fluoride, green tea has a high concentration of polysaccharides, such as pectin, which enhances the inhibition activity against Streptococcus mutans, thus preventing dental caries [190]. Green tea is also effective against other various bacteria that cause dental caries, such as Escherichia coli and Streptococcus salivarius [92]. The combination of green tea and miswak (Salvadora persica L.) extracts exhibits synergistic antiplaque properties [191]. Additionally, green tea can be used to prevent periodontal diseases, due to its matrix metalloproteinases (MMPs) inhibitory activity in a dose-dependent manner [192], with particular reduction in their secretion in gingival fibroblasts [193].
2.11. Miscellaneous Activity
Moreover, green tea experienced a lot of pronounced activities in relieving skin damage and promoting wound healing. Oral or topical treatment of GTPs prevents damages such as ultraviolet (UV)-induced sunburn response, immuno-suppression, and photo-aging [194], such as dermal extracellular damage [195] and DNA damage [196]. Treating the skin with green tea extracts inhibits induced erythema response in a dose-dependent manner, especially when treated with EGCG and ECG [197], due to the ability of EGCG to inhibit UVB-induced oxidative stress [198]. In addition, green tea ethanol extract is effective in the healing process of surgical wounds, as it decreases the healing duration [199]. Recently, it was found that tannase-converted green tea extract can be used in cosmetics as a skin anti-wrinkling or depigmenting agent [200], as tannase-derived bioconversion, which occurs on catechins compositions in green tea, was found to be useful in improving the antioxidant and radicals scavenging activities [201].
It is notable that anti-amyloidogenic activity has been attributed to oxidized EGCG comparable to the intact molecule, as it has a disruptive effect on preformed fibrils more than the native form [202]. Green tea revealed a high potency in the prohibition of proMMP-9 and MMP-9 activities, which play an important role in the development of pulmonary hypertension [203]; meanwhile, l-theanine proved efficacy in the alleviation of oxidative stress-induced airway inflammation in asthma [204]. Recent studies showed that dietary supplementation enriched with green tea promotes the antioxidant defense system in plasma and thus provides protection against oxidative damage induced by both short-term muscular endurance test and long-term strength training [205]. Additionally, the tea extract can prolong the time to exhaustion [206] and improve vascular function and physical performance in healthy individuals [207].
Green tea extract has a protective effect against induced reproductive toxicities and reduced testicular androgen receptors [208]. In addition, green tea improves induced damage of the reproductive system; this is probably due to its high catechins content [209], as catechins have a quenching effect on the reactive oxygen species that lead to oxidative stress in male and female reproduction systems and cause infertility [210]. EGCG increases the total efficiency of fertilization through improving the in vitro penetration rate of the sperms [211]. Meanwhile, oral administration of polyphenone-60 (P-60), green tea extract catechins, can stimulate goiter and reduce weights of the body, testis, and prostate gland, along with elevation in plasma thyroid stimulating hormone (TSH), luteinizing hormone (LH), and testosterone levels and reduction in tri-iodothyronine (T3) and thyroxine (T4). P-60, as a whole, and some of its constituents, can inhibit human placental aromatase activity which then can be used as preventive agents against benign prostatic hypertrophy (BPH) [212].
Regarding the effect of green tea extract on eyes, it has potential cataracto-static ability and can delay the progression of lens opacity, as it decreases smoking-induced frequencies of micronuclei in peripheral-blood lymphocytes [213]. It also counteracts the oxidative insult caused by cigarette smoke that causes oxidative damage to the constituent molecules and consequent lenticular opacity [214]. GTPs can bind to the cleft between the domains of human γB-crystallin protein, which is then protected from UV induced-oxidative stress [215].
Green tea may have selective protective effects within the body, especially on the kidney [216]. Green tea tannins, especially EGCG and ECG, cause a dose-dependent decrease in the progression of renal failure [217]. Recently, it was found that green tea extract can prevent the induced lipid peroxidation and antioxidant depletion in the kidney [218].
3. Effect of Green Tea Administration on the Bioavailability of Other Drugs
Saponins from flower buds have a stimulating effect on gastrointestinal transit and prohibiting effects versus pancreatic lipase [219], while green tea polyphenols, EGCG, ECG, and catechin gallate (CG), inhibit the binding and efflux of drugs by P-glycoprotein [220], with EGCG being the most potent inhibitor, since it remains in the intestine, whereas EGC and EC are rapidly absorbed [221].
First, neither caffeine nor flavonoids were likely to be responsible for the increase in CYP4A1, as well as expression [222]. Then, it was found that flavanols are not responsible for the effects of tea on the cytochrome P450 system, but caffeine could be responsible for the increase in CYP1A2 [223]. Controversially, EGCG was found to be able to inhibit CYP1A transcription but in a less effective manner than whole green tea extracts [224]. It causes a decrease in the level of intestinal bacteria of Clostridium species, which leads to a decrease in the drug-metabolizing enzyme cytochrome P450 3A (CYP3A) expression level and activity [225]. Recently, it was found that oligomerized EGCG can improve pharmacokinetic parameters in oral drug delivery systems [226].
4. Phytoconstituents of Green Tea (C. sinensis)
Green tea’s chemical composition is highly complex, owing to its richness with different classes of chemical compounds, which are described below.
4.1. Polyphenols
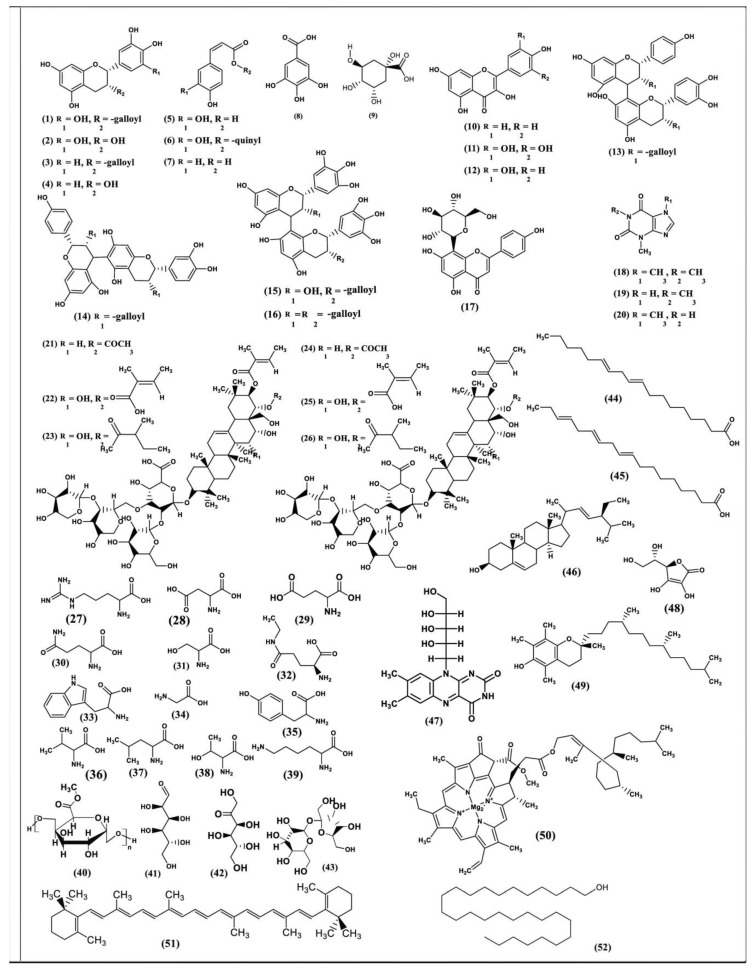
These represent the most important group of green tea leaf components, and thus they are regarded as the popular dietary source of polyphenols that accounts for 50–70% of tea water extract [227]. Basically, these polyphenols are particularly flavonoids that are considered biosynthetically produced in considerable amounts ranging from 0.5% to 1.5%, with more than 4000 varieties [228]. The major abundant flavonoids in green tea comprises catechins (flavan-3-ols), which represent about 30–40% of its dry weight [229]. Four major catechins predominate in green tea: (−)-epigallocatechin-3-gallate (EGCG) (1), epigallocatechin (EGC) (2), epicatechin-3-gallate (ECG) (3), and epicatechin (EC) (4). The former accounts for 59% of the total catechins and about 10% of dry weight [230]. However, the three latter catechins represent approximately 19%, 13.6%, and 6.4% of the total catechins, respectively [231]. These different structures are very important for their pharmacological usage [20].
Additionally, tannins are the second major polyphenols present in tea products, and they are responsible for the astringency of green tea [232]; that is why green tea is considered to be a selected food-grade plant extract which is also called ‘commercial tannins’ [233]. In addition, phenolic acids comprising caffeic acid (5), chlorogenic acid (6), coumaric acid (7), gallic acid (GA) (8), and its quinic acid ester (9), as well as flavanols represented mainly by kaempferol (10), myricetin (11), and quercetin (12), are also found [234]. Additionally, proanthocyanidins like epiafzelechingallate-(4β→8)-epicatechingallate (EAG-4β→8-ECG) (13), epiafzelechingallate-(4β→6)-epicatechingallate (EAG-4β→6-ECG) (14) [235], epigallocatechin-(4β→8)-epigallocatechingallate (EGC-4β→8-EGCG) (15), and epigallocatechingallate-(4β→8)-epigallocatechingallate (EGCG-4β→8-EGCG) (16) [236], as well as and traces of flavones [237], like vitexin pigment (17) [238], are present.
4.2. Xanthine Bases/Purine Alkaloids
They are represented mainly by caffeine (18), which is considered to be the second major constituent of the dry leaf [239]; meanwhile, its two metabolites, theophylline (19) and theobromine (20), are also present but in smaller amounts.
4.3. Triterpenoid Saponins
They are represented by floratheasaponin A, B, C, D, E, and F (21–26) [137], which exist at high concentrations in seeds and flowers [240].
4.4. Amino Acids
Amino acids constitute about 1–4% of dry weight and are represented by arginine (27), aspartic acid (28), glutamic acid (29), glutamine (30), and serine (31), as well as theanine (32) or 5-Nethylglutamine, which account for more than 90% of the whole amino acids present in the leaves of C. sinensis [241]. It is worthy to mention that theanine is the major amino acid that exists in the largest amounts, comprising about 1–2% of the dry weight of the green tea leaf [242], and thus it is considered the third major constituent of dry leaf [239]. Furthermore, it is recognized to be the only amino acid that exists solely in tea plants to which the flavor, as well as the exotic taste of green tea, is attributed [125]. Tryptophan (33), glycine (34), tyrosine (35), valine (36), leucine (37), threonine (38), and lysine (39) are also found.
4.5. Minerals and Trace Elements
They include Al, Ca, Cr, Co, Cu, F, Fe, K, Mg, Mo, Mn, Na, Ni, P, Se, Sr, and Zn, which constitute about 5% of dry weight [243].
4.6. Miscellaneous Compounds
These include proteins, which constitute about 15–20% of dry weight, whose enzymes represent an important fraction (e.g., carotenoid cleavage enzymes). Moreover, carbohydrates, such as pectin (40), glucose (41), fructose (42), and sucrose (43), represent about 5–7% of dry weight. Additionally, lipids are found and represented by linoleic and α-linolenic acids (44 and 45), as well as sterols such as stigmasterol (46). Green tea is also considered a source of vitamins, such as vitamin B (47), vitamin C (48), and vitamin E (49). Chlorophyll (50) and carotenoids (51) are important tea pigments that play a crucial role in enhancing the quality of green tea [244]. Moreover, volatiles, such as aldehydes, alcohols, esters, lactones, hydrocarbons, and terpenoids, are mainly responsible for the formation of tea aroma [245]. However, policosanol (52), which is composed of a group of health-promoting bioactive long-chain aliphatic alcohols, is exceptionally rich in green tea [246].
Most of the phytoconstituents isolated from green tea (C. sinensis) are represented in Figure 1.
Figure 1.
Most of the phytoconstituents isolated from green tea (C. sinensis).
5. Recent Approaches for Monitoring the Quality of Green Tea (C. sinensis)
Many approaches were adopted for assessing the quality control of green tea, owing to the great consumption of green tea worldwide and the high variability in its quality that ultimately influences its biological activities, as well as its cost. In addition, green tea is highly susceptible to adulteration with various non-green-tea materials, and this necessitates its strict quality-control monitoring. The various techniques that were previously adopted for monitoring the quality of green tea are summarized below.
5.1. Morphological Aspects
Green tea processed from fresh leaves picked at an early stage is considered to possess a high quality that consequently reduces with time [10]. The amount of pubescence on leaf epidermis is an important morphological marker for the quality of green tea, as the tender tea was also found to have more pubescence than old one [32]. Tea with plenty of pubescence has a better taste depending on the profiling of tea leaf pubescence metabolites [247].
Moreover, tea leaves should not be stored for more than two months at room temperature, since they become oxidized. Bioactive-ingredients extraction from green tea through ultrahigh pressure extraction (UPE) technique is characterized by being less time-consuming, producing higher extraction yields, consuming less solvent, and possessing higher purity with minimal heat generation, which may lead to thermal degradation of bioactive components. Its micromechanism is the disruption of cellular organelle which enhances the diffusion and mass transfer [248].
5.2. Physical Quality Parameters
5.2.1. Aroma and Flavor
The number of volatile constituents existing in green tea and the percentages in which they exist greatly influence its aroma and flavor, which are undoubtedly considered determinant factors when judging its quality [249,250]. Green tea aroma is severely affected by any unfavorable conditions that take place during manufacture or/and storage; thus, it should be monitored via objective, simple, easy, and rapid methods, like an odorant sensor [251]. Electronic nose (E-nose) is a fast, reliable, and robust technology which is easy-to-use for the evaluation of tea aroma and cost-efficient. It is able to handle continuous real-time controlling of odor at certain places in the field, over hours, days, weeks, or even months [252,253].
E-nose is widely used for qualitative purposes, such as distinguishing different types of tea, defining multiple brands, and classifying samples obtained by various processing methods [254]. It is noteworthy to mention that little research exists that describes the use of E-nose for evaluating the variable quality grades of green tea [253]. Chemometric tools make it possible to use the E-nose technique in the field of quality control of green tea, as it was found that combining the E-nose technique with the Support Vector Machine (SVM) classification tool can be used to develop a discrimination model for green tea’s quality and identifying tea grades [255]. In addition, E-nose, possessing an array of six metal oxide semiconductor sensors, was also used to obtain olfactive fingerprints of the volatile compounds in the infusions headspace. This is concomitantly associated with another chemometric tool such as Linear Discriminant Analysis (LDA) of the resulted data that can be used as a model for fast characterization of green teas subjected to long-term storage. Additionally, classifying unknown samples into aged and fresh samples was also made possible by the utilization of the previously described E-nose [256].
Regarding its flavor, it was found that rated acceptance of green tea is decreased as bitterness increases [257]. Taste of tea infusions is crucial for both judging tea quality, as well as acting as a reference in tea classification [258]. The taste quality of green tea infusions prepared using purified water is higher than those prepared using mineral water or tap water, while decreasing the pH value in the case of mineral water partially improves the taste quality [259].
Expert tasters who are well qualified do flavor-quality evaluations by using their own experience to describe multiple quality levels of tea infusions that are in some cases very tedious to be comprehended by consumers [10]. However, in most cases examining flavor manually using human tasting is not fully accurate, and sensitivity may be reduced by time, owing to prolonged exposure [249]. Besides, the results may be greatly altered by many factors, including emotional ones, feelings of exhaustion, and infection attacks [260]. Alternatively, electronic tongue (E-tongue), which is also termed artificial tongue or taste sensor, was used since the 2000s and is effectively capable of classifying different tea categories and evaluating their quality levels according to their content of amino acids, catechins, caffeine, and polyphenols [10,258].
E-tongue coupled with pattern recognition using Artificial Neural Network (ANN) can be used to build an identification model that would be able to identify tea-grade level. The results showed high precision and considerable accuracy, in addition to reliability, but, unfortunately, they consume a lot of time, making them destructive and unsuitable for online monitoring [258].
5.2.2. Color, Grain Shape, and Size
They can be accessed via organoleptic methods exemplified by human sensory panel, as well as analytical instruments, but these methods consume time, are tedious, have a high cost, and reveal a high variability. Thus, the technology of computer vision was adopted in an effort to combat the previously mentioned drawbacks. This technology aims to duplicate the human vision via electronic perception, which is an automatic process that causes no destruction and is cost-effective. It can be used for the differentiation of tea brands, classes, and colors, as well as in accordance to their grades and discrimination of tea grains under different illumination conditions [261]. Image information of green tea is able to evaluate its sensory quality, which can be predicted by the leaf temperature and moisture content measured during production [262].
Back propagation multilayer perceptron (BP-MLP) and radial basis function (RBF) neural networks, along with image information technology, can effectively predict and evaluate the sensory quality of needled green tea, a kind of premium-quality green tea popular in China with an elegant appearance, as changes in leaf temperature and moisture content during green tea processing can affect the content of tea quality ingredients [263] and thus affect the sensory quality of the product [262].
5.3. Chemical Markers
5.3.1. Catechins
The catechins index (CI) can be used to estimate the quality of green tea owing to the closeness between the quality of green tea and its main catechins contents [10]. The catechin concentration of green tea infusions prepared using purified water is higher than those prepared using mineral water or tap water, as using high-conductivity water reduces the efficiency of catechins extraction, and high pH influences their stability, so reducing the pH of mineral water partially increases the concentration of catechins in the infusions [259]. Recently, a carbon paste working electrode modified by electropolymerizing methylene blue was used for detecting and quantifying catechin concentrations in green tea [264]. In addition, the combined analytical approach using UV-Vis spectrophotometry, matrix-assisted laser desorption/ionization-time-of-flight-mass spectrometry (MALDI-TOF-MS), and inductively coupled plasma-mass spectrometry (ICP-MS) is suitable for the profiling and quality control of green tea via using the polyphenolic fraction (e.g., galloylated flavonoids) as the authentication marker [233].
5.3.2. Caffeine
Additionally, caffeine constitutes a determinant factor in assessing the quality level of green tea. Official green tea was supposed to contain at least 2% of caffeine calculated for the dried substance, as specified by the French Pharmacopoeia [265].
5.3.3. Minerals
Great differences in the mineral content (Al, Ca, Mg, and Mn) in green tea from different origins were observed [266]. However, no clear variations were detected between green and black teas regarding the levels of Al, Ba, Ca, Cu, Fe, K, Mg, Mn, Na, Sr, Ti, and Zn [267]. However, a great variation was observed concerning the levels of fluoride and aluminum in various tea varieties, as green tea has lesser Al and F concentrations than black tea [268,269].
Additionally, Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES) is used to determine the metal content in different tea samples, and is thus used as a method for its quality control. This was applied in a study done in Saudi Arabia on 17 black tea samples and could effectively differentiate between them. The lowest concentration of Pb was found in Abu Jabal tea; meanwhile, the highest concentration was detected in Manasul tea. The highest Cd level was found in Al-Diafa tea; the Cd level is greatly used to differentiate between black and green samples [270].
A physical fractionation of the previously mentioned minerals in green tea infusion was done and used to classify the infusions of different green teas, using chemometric techniques, such as PCA and LDA [271]. However, default transition rates for certain metals, including aluminum, arsenic, cadmium, copper, lead, and mercury, should exist in green tea samples (C. sinensis) from different origins, leaf grades, and manufacturing techniques in the dry product and in the infusion, to avoid overestimation of exposure levels upon consumption [272].
5.3.4. Amino Acids
High-grade teas are found to be richer in amino acids in comparison to low-grade ones [273]. Hence, the amount of amino acids in the green tea infusions, especially theanine, which is highly related to the quality evaluation of green tea, should be carefully monitored. Theanine can be measured by a simple, fast, and very convenient method like an enzyme sensor, which consists of l-amino acid oxidase and an oxygen electrode [241]. Meanwhile, a turn-on fluorescent sensor, based on Hg2+ coordination conjugated polymer, is able to recover cysteine in several green tea drinks [274].
5.3.5. Carotene
It was also found that all quality parameters of tea are enhanced by the increase in endogenous carotene content, which is highly stable in tea due to the existence of antioxidants such as polyphenols and catechins [244].
5.4. Antioxidant Capacity and Determination of Antioxidant Constituents in Green Tea
The total antioxidant capacity of a green tea extract is an important quality criterion. It can be evaluated via a decolonization assay like the Trolox equivalent antioxidant capacity (TEAC) method, in which the antioxidant capacity is expressed as the concentration of a Trolox solution with equal antioxidant capacity [275]. The antioxidant capacity of green tea infusions prepared using purified water is higher than those prepared using mineral water or tap water [259]. The quantification of the relative antioxidant activities of tea leaves can be made through a direct method like Electron Paramagnetic Resonance (EPR) spectroscopy [276], while Electron Spin Resonance Spectroscopy (ESR) was used directly to measure the level of free radicals, as a marker of early events in oxidation of low-fat dried products such as potato flakes, which can be protected by natural antioxidant green tea extracts [277].
ESR and peroxalate chemiluminescence detection system were also used to determine the total catechin levels in green tea through the identification of H2O2 generated by catechin group, (+)-catechin (CC), EGCG, ECG, and gallic acid, in basic solution [278]. Recently, a zinc oxide fetched lossy mode resonance-based fiber optic biosensor can be used for the determination of H2O2 production by green tea that is rich in polyphenol [279], and a highly sensitive voltammetric sensor based on a carbon paste electrode with a CuFe2O4 nanoparticle can be used for the assay of H2O2 in green tea [280].
Furthermore, the identification and quantification of catechins, gallic acid, and caffeine in green tea extracts was achieved using a rapid reversed-phase HPLC-MS method adopting a gradient elution system with EGCG and EGC as indices of the antioxidant quality of tea extracts [281]. Besides that, five major catechins (EGCG, EGC, ECG, EC, and C) and caffeine in green tea leaves were assessed simultaneously by HPLC, which was effectively used to determine the quality level of green tea concomitantly, with a new chemical pattern recognition tool, Support Vector Classification (SVC), to develop an identification model [10]. Moreover, ultrahigh performance liquid chromatography (UPLC) coupled with UV was adopted for the rapid quantification of green tea catechins, as well as caffeine [282]. UPLC coupled with triple-quadrupole tandem mass spectrometry (HPLC–MS–MS) in multiple-reaction monitoring (MRM) mode was also used to analyze catechins present in green tea simultaneously [283].
Tea quality assessment using an electro-analytical technique based on a flow injection analysis coupled with dual electrochemical detector system was used for the highly selective simultaneous detection of polyphenolic functional groups in catechins, especially in EGCG [284]. A modified electrode with high selectivity to catechol can be used to determine polyphenol [285], while gold nanoparticles decorated on porous aromatic framework can be used to directly determine quercetin in green tea samples [286].
NIR spectroscopy is a spectroscopic method that uses the near-infrared region of the electromagnetic spectrum, ranging from 780 to 2500 nm. It was recently adopted as an advanced method for assessing the quality of green tea [261]. It is worthy to highlight that NIR spectroscopy needs reference measurements to construct the calibration model by chemometric tools, so analyses of large amounts of similar samples can be made in a few minutes, and even in a few seconds, if the most recent instruments are used. NIR spectroscopy, combined with an appropriate preprocessing method like Standard Normal Variate (SNV), followed by derivation of the spectra in addition to Partial Least Square (PLS) regression, was proved to be an effective method for the prediction of certain quality parameters of green tea, including caffeine content and total antioxidant capacity from the NIR spectra of the entire tea leaves, the total antioxidant capacity from the ground tea, and the EGCG content, but not the EC content, which may be due to its low concentration in the tea [265]. NIR can be used together with PLS algorithm for simultaneous measurement of the main four catechins (EGCG, EGC, ECG, and EC) contents in green tea [287].
However, because of the highly complex composition of green tea, the approach of measuring certain parameters is neither appropriate nor sufficient and could be replaced by the information originated from the entire characteristic profile, which is called a “fingerprint” [288].
A fingerprint can be obtained by spectroscopic and/or chromatographic techniques [288], such as HPLC, GC, and CE. Chromatograms can give qualitative and quantitative information of the existing compounds that can be quickly retrieved, if required [289]. Fingerprint chromatograms are developed and compared to that of a standardized extract to achieve authentication, identification, and quality control of the green tea, and, in combination with TEAC assay, it permits a good evaluation of the quality of a tea extract [275].
Nowadays, the multi-sensors technique represents a new analytical tool that was recently adopted and showed high effectiveness in discrimination, classification, and quality control, along with many advantages, such as being a rapid, low-cost analytical tool relative to HPLC and GC [290].
A visual evaluation cannot discriminate between lots of profiles; therefore, mathematical data-handling techniques are recommended [288]. The influence of the experimental variability, including instrumental repeatability, precision, and the variation in sample preparation, together with data preprocessing methods on the ability of the multivariate data analysis methods to discriminate between samples (as well as a new algorithm based on linear regression that was used in the assessment of the quality of green tea extracts) all need to be taken into account [291]. Multivariate analysis was done using by UPLC–quadrupole time-of-flight mass spectrometry (UPLC–Q-TOF/MS) for metabolomics profiling and thus acts as a model for its quality control [292].
5.5. Determination of Green Tea Contaminants
Nowadays, there is a great interest in tea safety, because contaminants negatively deteriorate human and animal health, as well as cause lots of damage to the environment, resulting in great economic losses. ELISA and immunochemical methods are particularly suitable for ensuring tea safety, owing to their rapidity, simplicity, sensitivity, and low price. The directly suspended droplet micro-extraction method has a high enrichment factor, excellent selectivity, good linearity, and reasonable recovery. Recently, it was found that a hybrid ZnO/g-C3N4 fiber can be used for simultaneous determination of nine pesticide residues in green tea [293].
In addition, an automated multi-plug filtration cleanup medial prefrontal cortex method on modified quick, easy, cheap, effective, rugged, and safe extracts is used to determine pesticide residues in green tea [294]. These include hymexazol and fungicide residue in foods like green tea that could be coupled with liquid chromatography tandem–mass spectrometry [295]. Meanwhile, GC–MS can be used to quantify polycyclic aromatic hydrocarbons as a toxic contaminant in green tea [296]. Using the attenuated total reflection-FTIR (ATR-FTIR) spectroscopic technique in combination with chemometrics techniques, such as HCA and PCA techniques, for the detection of sibutramine in green tea can be achieved [297].
However, the combined analytical approach using UV-Vis spectrophotometry, matrix-assisted laser desorption/ionization-time-of-flight-mass spectrometry (MALDI-TOF-MS), and inductively coupled plasma-mass spectrometry (ICP-MS) are suitable for measuring the content of toxic elements, such as Cu, Sr, As, Co, Cr, Fe, Zn, Li, Ba, and Pb, relative to their permitted and recommended limits [233]. Moreover, an automated sequential injection (SI) with second order light scattering (SOS) detection can be used to determine GABA quantity in green tea samples [298]. So many kinds of famous green teas can be identified effectively using the “turn-off” model of quantum dots combined with chemometrics [299].
6. Current Trends in Green Tea in Researches and Future Recommendations
Green tea represents about 20% of the dried tea which is manufactured annually and is mainly consumed in Asian countries, such as Japan, owing to its relative safety and cheaper price in comparison to other beverages. Aside from that, it is well-known for its health benefits comprising its anticancer, anti-oxidant, and antimicrobial activities, effectiveness in reducing body weight, and possession of different classes of active constituents, represented by polyphenols, xanthine bases/purine alkaloids, triterpenoid saponins, amino acids, minerals, and trace elements. The beneficial health effects of green tea ultimately lead to its massive consumption and increase its liability to be adulterated by either low-quality or non-green-tea products with a concomitant decrease in activity.
Additionally, a lot of varieties of tea exist depending upon the different processing techniques represented by green tea, yellow tea, black tea, and white tea, as well as oolong tea. Consequently, the metabolic profile of tea, as well as its biological activity, would drastically change according to the multiple variations between different varieties, as well as due to environmental effects, processing procedure, and the mode of preparation, which ultimately reflect on its quality. Hence, more research aiming to develop advanced, high-quality products to satisfy the diverse demand is needed. This could be achieved via enhancing the efficient use of tea resources, improving both the research and the development level of the different varieties of tea.
New derivatives of teas with a higher quality and more secondary metabolites should be targeted that can be utilized in the form of tea food, tea drinks, tea crafts, tea health products, and tea cosmetics. Undoubtedly, this will require a special focus on improving the quality of these products through the use of simple and available technical methods. Concomitantly, promotion of tea culture should be promoted using various events, like Global Tea Day, that ultimately improve consumers’ recognition of high-quality tea all over the globe. Besides, this will ensure the consumer knowledge about the origins, flavors, and history of the tea they are drinking so that the consumer can act as a driving force for the factories to improve their quality via exploring more advanced but simple techniques in monitoring tea quality and preventing its adulteration worldwide. Moreover, more attention should be given to young consumers and researchers, who are becoming more aware of the beneficial effects of drinking high-quality tea in contrast to drinking adulterated low-quality types. Thus, modern investigations, as well as research, should be conducted to meet their demand with more efforts to be given to find out other active constituents and simple and cheap techniques for its quality assurance that ascertain the prevention of its adulteration and the ease of its incorporation in pharmaceutical industries. Many tea products should be carefully considered; for instance, kombucha tea, which is a type of sugared tea obtained by fermentation by using two types of bacteria, namely acetic acid bacteria and yeast (tea fungus), is known worldwide owing to its massive beneficial and refreshing potentials. It was found to be effective in the prohibition of many types of cancer, in addition to the alleviation of cardiovascular disorders, enhancement of liver functions, and probing of the immune system; thus, special consideration should be given to kombucha tea [300].
7. Conclusions
Green tea represents about 20% of the dried tea which is manufactured annually and is mainly consumed in Asian countries, such as Japan, owing to its relative safety and cheaper price comparable to other beverages. Besides, it is greatly familiar by its marked health benefits comprising its anticancer, anti-oxidant, antimicrobial activities, effectiveness in reducing body weight, and possession of different classes of active constituents, represented by polyphenols, xanthine bases/purine alkaloids, triterpenoid saponins, amino acids, minerals, and trace elements. The beneficial health effects of green tea ultimately lead to its massive consumption and increase its liability to be adulterated by either low-quality or non-green tea products with concomitant decrease in activity. More studies need to be conducted to find out other biological activates, active constituents, and other simple and cheap techniques for its quality assurance that ascertain the prevention of its adulteration and the ease of its incorporation in the products of pharmaceutical industries.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3921/8/10/455/s1. Table S1: Summary of the major biological activities and mechanism of action of different parts, extracts as well as compounds obtained from green tea (Camellia sinensis).
Author Contributions
M.M.A., collection of data and preliminary writing of the manuscript; F.S.Y., complete data collection and organizing and complete writing of the manuscript; H.A.G., A.E.A., M.M.A.-A., and M.L.A., revised the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Fathy S., Emam M., Agwa S.A., Zahra F.A., Youssef F., Sami R. The antiproliferative effect of Origanum majorana on human hepatocarcinoma cell line: Suppression of NF-kB. Cell. Mol. Biol. 2016;62:80–84. [PubMed] [Google Scholar]
- 2.Ashour M.L., Youssef F.S., Gad H.A., El-Readi M.Z., Bouzabata A., Abuzeid R.M., Sobeh M., Wink M. Evidence for the anti-inflammatory activity of Bupleurum marginatum (Apiaceae) extracts using in vitro and in vivo experiments supported by virtual screening. J. Pharm. Pharmacol. 2018;70:952–963. doi: 10.1111/jphp.12904. [DOI] [PubMed] [Google Scholar]
- 3.Janibekov A.A., Youssef F.S., Ashour M.L., Mamadalieva N.Z. New flavonoid glycosides from two Astragalus species (Fabaceae) and validation of their antihyperglycaemic activity using molecular modelling and in vitro studies. Ind. Crop. Prod. 2018;118:142–148. doi: 10.1016/j.indcrop.2018.03.034. [DOI] [Google Scholar]
- 4.Thabet A.A., Youssef F.S., El-Shazly M., El-Beshbishy H.A., Singab A.N.B. Validation of the antihyperglycaemic and hepatoprotective activity of the flavonoid rich fraction of Brachychiton rupestris using in vivo experimental models and molecular modelling. Food Chem. Toxicol. 2018;114:302–310. doi: 10.1016/j.fct.2018.02.054. [DOI] [PubMed] [Google Scholar]
- 5.Youssef F.S., Labib R.M., Eldahshan O.A., Singab A.N. Synergistic hepatoprotective and antioxidant effect of Artichoke, Fig, Mulberry Herbal mixture on HepG2 Cells and their metabolic profiling Using NMR coupled with chemometrics. Chem. Biodivers. 2017;14:e1700206. doi: 10.1002/cbdv.201700206. [DOI] [PubMed] [Google Scholar]
- 6.Talaat A.N., Ebada S.S., Labib R.M., Esmat A., Youssef F.S., Singab A.N.B. Verification of the anti-inflammatory activity of the polyphenolic-rich fraction of Araucaria bidwillii Hook. using phytohaemagglutinin-stimulated human peripheral blood mononuclear cells and virtual screening. J. Ethnopharmacol. 2018;226:44–47. doi: 10.1016/j.jep.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 7.Couturier F.J., Colemont L.J., Fierens H., Verhoeven V.M. Toxic hepatitis due to a food supplement: “Natural” is no synonym for “harmless”. Clin. Res. Hepatol. Gastroenterol. 2016;40:e38–e43. doi: 10.1016/j.clinre.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Aboulwafa M.M., Youssef F.S., Gad H.A., Sarker S.D., Nahar L., Al-Azizi M.M., Ashour M.L. Authentication and discrimination of green tea samples using UV-Visible, FTIR and HPLC techniques coupled with chemometrics analysis. J. Pharm. Biomed. Anal. 2018;164:653–658. doi: 10.1016/j.jpba.2018.11.036. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara L., Montesano D., Senatore A. The distribution of minerals and flavonoids in the tea plant (Camellia sinensis) Il Farmaco. 2001;56:397–401. doi: 10.1016/S0014-827X(01)01104-1. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q., Guo Z., Zhao J. Identification of green tea’s (Camellia sinensis L.) quality level according to measurement of main catechins and caffeine contents by HPLC and support vector classification pattern recognition. J. Pharm. Biomed. Anal. 2008;48:1321–1325. doi: 10.1016/j.jpba.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Graham H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992;21:334–350. doi: 10.1016/0091-7435(92)90041-F. [DOI] [PubMed] [Google Scholar]
- 12.Rashidi B., Malekzadeh M., Goodarzi M., Masoudifar A., Mirzaei H. Green tea and its anti-angiogenesis effects. Biomed. Pharmacother. 2017;89:949–956. doi: 10.1016/j.biopha.2017.01.161. [DOI] [PubMed] [Google Scholar]
- 13.Jayashree G.V., Krupashree K., Rachitha P., Khanum F. Patulin induced oxidative stress mediated apoptotic damage in mice, and its modulation by Green tea leaves (GTL) J. Clin. Exp. Hepatol. 2017;7:127–134. doi: 10.1016/j.jceh.2017.01.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afzalpour M.E., Ghasemi E., Zarban A. Effects of 10 weeks of high intensity interval training and green tea supplementation on serum levels of Sirtuin-1 and peroxisome proliferator-activated receptor gamma co-activator 1-alpha in overweight women. Sci. Sports. 2017;32:82–90. doi: 10.1016/j.scispo.2016.09.004. [DOI] [Google Scholar]
- 15.Nibir Y.M., Sumit A.F., Akhand A.A., Ahsan N., Hossain M.S. Comparative assessment of total polyphenols, antioxidant and antimicrobial activity of different tea varieties of Bangladesh. Asian Pac. J. Trop. Biomed. 2017;7:352–357. doi: 10.1016/j.apjtb.2017.01.005. [DOI] [Google Scholar]
- 16.Levy Y., Narotzki B., Reznick A.Z. Green tea, weight loss and physical activity. Clin. Nutr. 2017;36:315. doi: 10.1016/j.clnu.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Samali A., Rukaiyatu A., Mustapha K. Qualitative and quantitative evaluation of some herbal teas commonly consumed in Nigeria. Afr. J. Pharm. Pharmacol. 2012;6:384–388. doi: 10.5897/AJPP11.658. [DOI] [Google Scholar]
- 18.McCune L.M., Johns T. Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus used by the Indigenous Peoples of the North American boreal forest. J. Ethnopharmacol. 2002;82:197–205. doi: 10.1016/S0378-8741(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 19.Barroso M.F., Ramalhosa M.J., Alves R.C., Dias A., Soares C.M.D., Oliva-Teles M.T., Delerue-Matos C. Total antioxidant capacity of plant infusions: Assessment using electrochemical DNA-based biosensor and spectrophotometric methods. Food Cont. 2016;68:153–161. doi: 10.1016/j.foodcont.2016.03.029. [DOI] [Google Scholar]
- 20.Zhao B., Guo Q., Xin W. Free radical scavenging by green tea polyphenols. Methods Enzymol. 2001;335:217. doi: 10.1016/s0076-6879(01)35245-x. [DOI] [PubMed] [Google Scholar]
- 21.Sarkar A., Bhaduri A. Black tea is a powerful chemopreventor of reactive oxygen and nitrogen species: Comparison with its individual catechin constituents and green tea. Biochem. Biophys. Res. Commun. 2001;284:173–178. doi: 10.1006/bbrc.2001.4944. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa T., Yokozawa T. Direct scavenging of nitric oxide and superoxide by green tea. Food Chem. Toxicol. 2002;40:1745–1750. doi: 10.1016/S0278-6915(02)00169-2. [DOI] [PubMed] [Google Scholar]
- 23.Cai Y.-J., Ma L.-P., Hou L.-F., Zhou B., Yang L., Liu Z.-L. Antioxidant effects of green tea polyphenols on free radical initiated peroxidation of rat liver microsomes. Chem. Phys. Lipids. 2002;120:109–117. doi: 10.1016/S0009-3084(02)00110-X. [DOI] [PubMed] [Google Scholar]
- 24.Khan S.G., Katiyar S.K., Agarwal R., Mukhtar H. Enhancement of antioxidant and phase II enzymes by oral feeding of green tea polyphenols in drinking water to SKH-1 hairless mice: Possible role in cancer chemoprevention. Cancer Res. 1992;52:4050–4052. [PubMed] [Google Scholar]
- 25.Wanasundara U.N., Shahidi F. Antioxidant and pro-oxidant activity of green tea extracts in marine oils. Food Chem. 1998;63:335–342. doi: 10.1016/S0308-8146(98)00025-9. [DOI] [Google Scholar]
- 26.Rashidinejad A., Birch E.J., Hindmarsh J., Everett D.W. Molecular interactions between green tea catechins and cheese fat studied by solid-state nuclear magnetic resonance spectroscopy. Food Chem. 2017;215:228–234. doi: 10.1016/j.foodchem.2016.07.179. [DOI] [PubMed] [Google Scholar]
- 27.Azimi S., Mansouri Z., Bakhtiari S., Tennant M., Kruger E., Rajabibazl M., Daraei A. Does green tea consumption improve the salivary antioxidant status of smokers? Arch. Oral Biol. 2017;78:1–5. doi: 10.1016/j.archoralbio.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Hasegawa R., Chujo T., Sai-Kato K., Umemura T., Tanimura A., Kurokawa Y. Preventive effects of green tea against liver oxidative DNA damage and hepatotoxicity in rats treated with 2-nitropropane. Food Chem. Toxicol. 1995;33:961–970. doi: 10.1016/0278-6915(95)00064-9. [DOI] [PubMed] [Google Scholar]
- 29.Bu-Abbas A., Clifford M., Walker R., Ioannides C. Contribution of caffeine and flavanols in the induction of hepatic phase II activities by green tea. Food Chem. Toxicol. 1998;36:617–621. doi: 10.1016/S0278-6915(98)00019-2. [DOI] [PubMed] [Google Scholar]
- 30.Lee S.F., Liang Y.C., Lin J.K. Inhibition of 1, 2, 4-benzenetriol-generated active oxygen species and induction of phase II enzymes by green tea polyphenols. Chem. Biol. Int. 1995;98:283–301. doi: 10.1016/0009-2797(95)03652-0. [DOI] [PubMed] [Google Scholar]
- 31.Kim E., Lee M., Kim S.S., Kim J.H., Jeon Y.K., Kim B.H., Kim I.J., Kim Y.K. Green tea but not coffee consumption is inversely associated with metabolic syndrome; An epidemiological study in Korean adults. Diabetes Res. Clin. Practice. 2016;120:S85. doi: 10.1016/S0168-8227(16)31119-6. [DOI] [Google Scholar]
- 32.Chen J., Wang P., Xia Y., Xu M., Pei S. Genetic diversity and differentiation of Camellia sinensis L. (cultivated tea) and its wild relatives in Yunnan province of China, revealed by morphology, biochemistry and allozyme studies. Genet. Resour. Crop Evol. 2005;52:41–52. doi: 10.1007/s10722-005-0285-1. [DOI] [Google Scholar]
- 33.Sobeh M., Mahmoud M.F., Petruk G., Rezq S., Ashour M.L., Youssef F.S., El-Shazly A.M., Monti D.M., Abdel-Naim A.B., Wink M. Syzygium aqueum: A polyphenol-rich leaf extract exhibits antioxidant, hepatoprotective, pain-killing and anti-inflammatory activities in animal models. Front. Pharmacol. 2018;9:556. doi: 10.3389/fphar.2018.00566. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Sobeh M., Youssef F.S., Esmat A., Petruk G., El-Khatib A.H., Monti D.M., Ashour M.L., Wink M. High resolution UPLC-MS/MS profiling of polyphenolics in the methanol extract of Syzygium samarangense leaves and its hepatoprotective activity in rats with CCl4-induced hepatic damage. Food Chem. Toxicol. 2018;113:145–153. doi: 10.1016/j.fct.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 35.Hamdaoui M.H., Snoussi C., Dhaouadi K., Fattouch S., Ducroc R., Le Gall M., Bado A. Tea decoctions prevent body weight gain in rats fed high-fat diet; black tea being more efficient than green tea. J. Nutr. Intermed. Metab. 2016;6:33–40. doi: 10.1016/j.jnim.2016.07.002. [DOI] [Google Scholar]
- 36.Casas-Grajales S., Muriel P. Liver Pathophysiology. Academic Press; Boston, MA, USA: 2017. Chapter 43—The Liver, Oxidative Stress, and Antioxidants; pp. 583–604. [Google Scholar]
- 37.Li J., Sapper T.N., Mah E., Moller M.V., Kim J.B., Chitchumroonchokchai C., McDonald J.D., Bruno R.S. Green tea extract treatment reduces NFκB activation in mice with diet-induced nonalcoholic steatohepatitis by lowering TNFR1 and TLR4 expression and ligand availability. J. Nutr. Biochem. 2017;41:34–41. doi: 10.1016/j.jnutbio.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Reddyvari H., Govatati S., Matha S.K., Korla S.V., Malempati S., Pasupuleti S.R., Bhanoori M., Nallanchakravarthula V. Therapeutic effect of green tea extract on alcohol induced hepatic mitochondrial DNA damage in albino wistar rats. J. Adv. Res. 2017;8:289–295. doi: 10.1016/j.jare.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmad N., Cheng P., Mukhtar H. Cell cycle dysregulation by green tea polyphenol epigallocatechin-3-gallate. Biochem. Biophys. Res. Commun. 2000;275:328–334. doi: 10.1006/bbrc.2000.3297. [DOI] [PubMed] [Google Scholar]
- 40.Wang E.-J., Barecki-Roach M., Johnson W.W. Elevation of P-glycoprotein function by a catechin in green tea. Biochem. Biophys. Res. Commun. 2002;297:412–418. doi: 10.1016/S0006-291X(02)02219-2. [DOI] [PubMed] [Google Scholar]
- 41.Embola C., Sohn O., Fiala E., Weisburger J. Induction of UDP-glucuronosyltransferase 1 (UDP-GT1) gene complex by green tea in male F344 rats. Food Chem. Toxicol. 2002;40:841–844. doi: 10.1016/S0278-6915(02)00022-4. [DOI] [PubMed] [Google Scholar]
- 42.Pan C.-Y., Kao Y.-H., Fox A.P. Enhancement of inward Ca 2+ currents in bovine chromaffin cells by green tea polyphenol extracts. Neurochem. Int. 2002;40:131–137. doi: 10.1016/S0197-0186(01)00083-3. [DOI] [PubMed] [Google Scholar]
- 43.Jha S., Kanaujia S.P., Limaye A.M. Direct inhibition of matrix metalloproteinase-2 (MMP-2) by (−)-epigallocatechin-3-gallate: A possible role for the fibronectin type II repeats. Gene. 2016;593:126–130. doi: 10.1016/j.gene.2016.07.061. [DOI] [PubMed] [Google Scholar]
- 44.Chen Z.P., Schell J.B., Ho C.-T., Chen K.Y. Green tea epigallocatechin gallate shows a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts. Cancer Lett. 1998;129:173–179. doi: 10.1016/S0304-3835(98)00108-6. [DOI] [PubMed] [Google Scholar]
- 45.Ahmad N., Adhami V.M., Gupta S., Cheng P., Mukhtar H. Role of the retinoblastoma (PRB)–E2F/DP pathway in cancer chemopreventive effects of green tea polyphenol epigallocatechin-3-gallate. Arch. Biochem. Biophys. 2002;398:125–131. doi: 10.1006/abbi.2001.2704. [DOI] [PubMed] [Google Scholar]
- 46.Wang X., Tian W. Green tea epigallocatechin gallate: A natural inhibitor of fatty-acid synthase. Biochem. Biophys. Res. Commun. 2001;288:1200–1206. doi: 10.1006/bbrc.2001.5923. [DOI] [PubMed] [Google Scholar]
- 47.Morbidelli L. Polyphenol-based nutraceuticals for the control of angiogenesis: Analysis of the critical issues for human use. Pharmacol. Res. 2016;111:384–393. doi: 10.1016/j.phrs.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Cao Y., Cao R., Bråkenhielm E. Antiangiogenic mechanisms of diet-derived polyphenols. J. Nutr. Biochem. 2002;13:380–390. doi: 10.1016/S0955-2863(02)00204-8. [DOI] [PubMed] [Google Scholar]
- 49.Wei Y., Chen P., Ling T., Wang Y., Dong R., Zhang C., Zhang L., Han M., Wang D., Wan X., et al. Certain (−)-epigallocatechin-3-gallate (EGCG) auto-oxidation products (EAOPs) retain the cytotoxic activities of EGCG. Food Chem. 2016;204:218–226. doi: 10.1016/j.foodchem.2016.02.134. [DOI] [PubMed] [Google Scholar]
- 50.Park H.-R., Hwang D., Suh H.-J., Yu K.-W., Kim T.Y., Shin K.-S. Antitumor and antimetastatic activities of rhamnogalacturonan-II-type polysaccharide isolated from mature leaves of green tea via activation of macrophages and natural killer cells. Int. J. Biol. Macromol. 2017;99:179–186. doi: 10.1016/j.ijbiomac.2017.02.043. [DOI] [PubMed] [Google Scholar]
- 51.Khan W., Wang Z., Athar M., Bickers D., Mukhtar H. Inhibition of the skin tumorigenicity of (k)-7P, 8wdihydroxy-90, IOa-epoxy-7, 8, 9, 10-tetrahydrobenzo [a] pyrene by tannic acid, green tea polyphenols and quercetin in Sencar mice. Cancer Lett. 1988;42:7–12. doi: 10.1016/0304-3835(88)90232-7. [DOI] [PubMed] [Google Scholar]
- 52.Conney A.H., Wang Z.-Y., Huang M.-T., Ho C.-T., Yang C.S. Inhibitory effect of green tea on tumorigenesis by chemicals and ultraviolet light. Prev. Med. 1992;21:361–369. doi: 10.1016/0091-7435(92)90043-H. [DOI] [PubMed] [Google Scholar]
- 53.Katiyar S.K., Perez A., Mukhtar H. Green tea polyphenol treatment to human skin prevents formation of ultraviolet light B-induced pyrimidine dimers in DNA. Clin. Cancer Res. 2000;6:3864–3869. [PubMed] [Google Scholar]
- 54.Record I.R., Dreosti I.E. Protection by black tea and green tea against UVB and UVA+ B induced skin cancer in hairless mice. Mut. Res. Fundam. Mol. Mech. Mutagenes. 1998;422:191–199. doi: 10.1016/S0027-5107(98)00192-4. [DOI] [PubMed] [Google Scholar]
- 55.Liao B., Ying H., Yu C., Fan Z., Zhang W., Shi J., Ying H., Ravichandran N., Xu Y., Yin J., et al. (−)-Epigallocatechin gallate (EGCG)-nanoethosomes as a transdermal delivery system for docetaxel to treat implanted human melanoma cell tumors in mice. Int. J. Pharm. 2016;512:22–31. doi: 10.1016/j.ijpharm.2016.08.038. [DOI] [PubMed] [Google Scholar]
- 56.Uesato S., Kitagawa Y., Kamishimoto M., Kumagai A., Hori H., Nagasawa H. Inhibition of green tea catechins against the growth of cancerous human colon and hepatic epithelial cells. Cancer Lett. 2001;170:41–44. doi: 10.1016/S0304-3835(01)00571-7. [DOI] [PubMed] [Google Scholar]
- 57.Yang F., Oz H.S., Barve S., De Villiers W.J., McClain C.J., Varilek G.W. The green tea polyphenol (−)-epigallocatechin-3-gallate blocks nuclear factor-κB activation by inhibiting IκB kinase activity in the intestinal epithelial cell line IEC-6. Mol. Pharmacol. 2001;60:528–533. [PubMed] [Google Scholar]
- 58.Arcone R., Palma M., Pagliara V., Graziani G., Masullo M., Nardone G. Green tea polyphenols affect invasiveness of human gastric MKN-28 cells by inhibition of LPS or TNF-α induced Matrix Metalloproteinase-9/2. Biochim. Open. 2016;3:56–63. doi: 10.1016/j.biopen.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zao J., Zhu Q., Cheng S. The antagonistic action effects of green tea on micronuclei and apoptosis induced by 1, 2-dimethylhydrazine (1, 2 DMH) in colonic crypt cells of mice. Acta Nutr. Sin. 1992;14:251–259. [Google Scholar]
- 60.Matsumoto H., Yamane T., Inagake M., Nakatani H., Iwata Y., Takahashi T., Nishimura H., Nishino H., Nakagawa K., Miyazawa T. Inhibition of mucosal lipid hyperoxidation by green tea extract in 1, 2-dimethylhydrazine-induced rat colonic carcinogenesis. Cancer Lett. 1996;104:205–209. doi: 10.1016/0304-3835(96)04248-6. [DOI] [PubMed] [Google Scholar]
- 61.Shin C.M., Lee D.H., Seo A.Y., Lee H.J., Kim S.B., Son W.-C., Kim Y.K., Lee S.J., Park S.-H., Kim N., et al. Green tea extracts for the prevention of metachronous colorectal polyps among patients who underwent endoscopic removal of colorectal adenomas: A randomized clinical trial. Clin. Nutr. 2017;37:452–458. doi: 10.1016/j.clnu.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 62.Xu Y., Ho C.-T., Amin S.G., Han C., Chung F.-L. Inhibition of tobacco-specific nitrosamine-induced lung tumorigenesis in A/J mice by green tea and its major polyphenol as antioxidants. Cancer Res. 1992;52:3875–3879. [PubMed] [Google Scholar]
- 63.Cao J., Xu Y., Chen J., Klaunig J.E. Chemopreventive effects of green and black tea on pulmonary and hepatic carcinogenesis. Toxicol. Sci. 1996;29:244–250. doi: 10.1093/toxsci/29.2.244. [DOI] [PubMed] [Google Scholar]
- 64.Zhu J., Jiang Y., Yang X., Wang S., Xie C., Li X., Li Y., Chen Y., Wang X., Meng Y., et al. Wnt/β-catenin pathway mediates (−)-Epigallocatechin-3-gallate (EGCG) inhibition of lung cancer stem cells. Biochem. Biophys. Res. Commun. 2017;482:15–21. doi: 10.1016/j.bbrc.2016.11.038. [DOI] [PubMed] [Google Scholar]
- 65.Oya Y., Mondal A., Rawangkan A., Umsumarng S., Iida K., Watanabe T., Kanno M., Suzuki K., Li Z., Kagechika H., et al. Down-regulation of histone deacetylase 4, −5 and −6 as a mechanism of synergistic enhancement of apoptosis in human lung cancer cells treated with the combination of a synthetic retinoid, Am80 and green tea catechin. J. Nutr. Biochem. 2017;42:7–16. doi: 10.1016/j.jnutbio.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 66.Fujie K., Aoki T., Ito Y., Maeda S. Sister-chromatid exchanges induced by trihalomethanes in rat erythroblastic cells and their suppression by crude catechin extracted from green tea. Mut. Res. Genet. Toxicol. 1993;300:241–246. doi: 10.1016/0165-1218(93)90056-J. [DOI] [PubMed] [Google Scholar]
- 67.Otsuka T., Ogo T., Eto T., Asano Y., Suganuma M., Niho Y. Growth inhibition of leukemic cells by (−)-epigallocatechin gallate, the main constituent of green tea. Life Sci. 1998;63:1397–1403. doi: 10.1016/S0024-3205(98)00406-8. [DOI] [PubMed] [Google Scholar]
- 68.Lung H.L., Ip W.K., Wong C.K., Mak N.K., Chen Z.Y., Leung K.N. Anti-proliferative and differentiation-inducing activities of the green tea catechin epigallocatechin-3-gallate (EGCG) on the human eosinophilic leukemia EoL-1 cell line. Life Sci. 2002;72:257–268. doi: 10.1016/S0024-3205(02)02236-1. [DOI] [PubMed] [Google Scholar]
- 69.Cornwall S., Cull G., Joske D., Ghassemifar R. Green tea polyphenol “epigallocatechin-3-gallate”, differentially induces apoptosis in CLL B-and T-Cells but not in healthy B-and T-Cells in a dose dependant manner. Leuk. Res. 2016;51:56–61. doi: 10.1016/j.leukres.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 70.Parodi S., Merlo D.F., Stagnaro E. Coffee and tea consumption and risk of leukaemia in an adult population: A reanalysis of the Italian multicentre case-control study. Cancer Epidemiol. 2017;47:81–87. doi: 10.1016/j.canep.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 71.Hirose M., Hoshiya T., Akagi K., Futakuchi M., Ito N. Inhibition of mammary gland carcinogenesis by green tea catechins and other naturally occurring antioxidants in female Sprague-Dawley rats pretreated with 7, 12-dimethylbenz [a] anthracene. Cancer Lett. 1994;83:149–156. doi: 10.1016/0304-3835(94)90312-3. [DOI] [PubMed] [Google Scholar]
- 72.Hirose M., Mizoguchi Y., Yaono M., Tanaka H., Yamaguchi T., Shirai T. Effects of green tea catechins on the progression or late promotion stage of mammary gland carcinogenesis in female Sprague-Dawley rats pretreated with 7, 12-dimethylbenz (a) anthracene. Cancer Lett. 1997;112:141–147. doi: 10.1016/S0304-3835(96)04560-0. [DOI] [PubMed] [Google Scholar]
- 73.Tanaka H., Hirose M., Kawabe M., Sano M., Takesada Y., Hagiwara A., Shirai T. Post-initiation inhibitory effects of green tea catechins on 7, 12-dimethylbenz [a] anthracene-induced mammary gland carcinogenesis in female Sprague–Dawley rats. Cancer Lett. 1997;116:47–52. doi: 10.1016/S0304-3835(97)04749-6. [DOI] [PubMed] [Google Scholar]
- 74.Li W., He N., Tian L., Shi X., Yang X. Inhibitory effects of polyphenol-enriched extract from Ziyang tea against human breast cancer MCF-7 cells through reactive oxygen species-dependent mitochondria molecular mechanism. J. Food Drug Anal. 2016;24:527–538. doi: 10.1016/j.jfda.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leung A.C.Y., Cook L.S., Swenerton K., Gilks B., Gallagher R.P., Magliocco A., Steed H., Köbel M., Nation J., Brooks-Wilson A., et al. Tea, coffee, and caffeinated beverage consumption and risk of epithelial ovarian cancers. Cancer Epidemiol. 2016;45:119–125. doi: 10.1016/j.canep.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 76.Gupta S., Ahmad N., Nieminen A.-L., Mukhtar H. Growth inhibition, cell-cycle dysregulation, and induction of apoptosis by green tea constituent (−)-epigallocatechin-3-gallate in androgen-sensitive and androgen-insensitive human prostate carcinoma cells. Toxicol. Appl. Pharmacol. 2000;164:82–90. doi: 10.1006/taap.1999.8885. [DOI] [PubMed] [Google Scholar]
- 77.Wang S.I., Mukhtar H. Gene expression profile in human prostate LNCaP cancer cells by (−) epigallocatechin-3-gallate. Cancer Lett. 2002;182:43–51. doi: 10.1016/S0304-3835(02)00065-4. [DOI] [PubMed] [Google Scholar]
- 78.Hiipakka R.A., Zhang H.-Z., Dai W., Dai Q., Liao S. Structure–activity relationships for inhibition of human 5α-reductases by polyphenols. Biochem. Pharmacol. 2002;63:1165–1176. doi: 10.1016/S0006-2952(02)00848-1. [DOI] [PubMed] [Google Scholar]
- 79.Sawada N. Risk and preventive factors for prostate cancer in Japan: The Japan public health center-based prospective (JPHC) study. J. Epidemiol. 2017;27:2–7. doi: 10.1016/j.je.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carvalho M., Jerónimo C., Valentão P., Andrade P.B., Silva B.M. Green tea: A promising anticancer agent for renal cell carcinoma. Food Chem. 2010;122:49–54. doi: 10.1016/j.foodchem.2010.02.014. [DOI] [Google Scholar]
- 81.Annabi B., Lachambre M.-P., Bousquet-Gagnon N., Pagé M., Gingras D., Béliveau R. Green tea polyphenol (−)-epigallocatechin 3-gallate inhibits MMP-2 secretion and MT1-MMP-driven migration in glioblastoma cells. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2002;1542:209–220. doi: 10.1016/S0167-4889(01)00187-2. [DOI] [PubMed] [Google Scholar]
- 82.Mazzio E.A., Bauer D., Mendonca P., Taka E., Soliman K.F.A. Natural product HTP screening for attenuation of cytokine-induced neutrophil chemo attractants (CINCs) and NO2—in LPS/IFNγ activated glioma cells. J. Neuroimmunol. 2017;302:10–19. doi: 10.1016/j.jneuroim.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burkard M., Leischner C., Lauer U.M., Busch C., Venturelli S., Frank J. Dietary flavonoids and modulation of natural killer cells: Implications in malignant and viral diseases. J. Nutr. Biochem. 2017;46:1–12. doi: 10.1016/j.jnutbio.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 84.Edenharder R., Sager J.W., Glatt H., Muckel E., Platt K.L. Protection by beverages, fruits, vegetables, herbs, and flavonoids against genotoxicity of 2-acetylaminofluorene and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in metabolically competent V79 cells. Mut. Res. Genet. Toxicol. Environ. Mut. 2002;521:57–72. doi: 10.1016/S1383-5718(02)00212-7. [DOI] [PubMed] [Google Scholar]
- 85.Kada T., Kaneko K., Matsuzaki S., Matsuzaki T., Hora Y. Detection and chemical identification of natural bio-antimutagens: A case of the green tea factor. Mut. Res. Fundam. Mol. Mech. Mutagenes. 1985;150:127–132. doi: 10.1016/0027-5107(85)90109-5. [DOI] [PubMed] [Google Scholar]
- 86.Wang Z.Y., Cheng S.J., Zhou Z.C., Athar M., Khan W.A., Bickers D.R., Mukhtar H. Antimutagenic activity of green tea polyphenols. Mut. Res. Genet. Toxicol. 1989;223:273–285. doi: 10.1016/0165-1218(89)90120-1. [DOI] [PubMed] [Google Scholar]
- 87.Bu-Abbas A., Clifford M., Walker R., Ioannides C. Marked antimutagenic potential of aqueous green tea extracts: Mechanism of action. Mutagenesis. 1994;9:325–331. doi: 10.1093/mutage/9.4.325. [DOI] [PubMed] [Google Scholar]
- 88.Kuroda Y. Bio-antimutagenic activity of green tea catechins in cultured Chinese hamster V79 cells. Mut. Res. Environ. Mut. Relat. Subj. 1996;361:179–186. doi: 10.1016/S0165-1161(96)00039-8. [DOI] [PubMed] [Google Scholar]
- 89.Okai Y., Higashi-Okai K. Potent suppressing activity of the non-polyphenolic fraction of green tea (Camellia sinensis) against genotoxin-induced umu C gene expression in Salmonella typhimurium (TA 1535/pSK 1002)–association with pheophytins a and b. Cancer Lett. 1997;120:117–123. doi: 10.1016/S0304-3835(97)00294-2. [DOI] [PubMed] [Google Scholar]
- 90.Gupta S., Saha B., Giri A.K. Comparative antimutagenic and anticlastogenic effects of green tea and black tea: A review. Mut. Res. Rev. Mut. Res. 2002;512:37–65. doi: 10.1016/S1383-5742(02)00024-8. [DOI] [PubMed] [Google Scholar]
- 91.Ikigai H., Nakae T., Hara Y., Shimamura T. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta (BBA) Biomembr. 1993;1147:132–136. doi: 10.1016/0005-2736(93)90323-R. [DOI] [PubMed] [Google Scholar]
- 92.Rasheed A., Haider M. Antibacterial activity of Camellia sinensis extracts against dental caries. Arch. Pharm. Res. 1998;21:348–352. doi: 10.1007/BF02975300. [DOI] [PubMed] [Google Scholar]
- 93.Sugita-Konishi Y., Hara-Kudo Y., Amano F., Okubo T., Aoi N., Iwaki M., Kumagai S. Epigallocatechin gallate and gallocatechin gallate in green tea catechins inhibit extracellular release of Vero toxin from enterohemorrhagic Escherichia coli O157: H7. Biochim. Biophys. Acta (BBA) Gen. Subj. 1999;1472:42–50. doi: 10.1016/S0304-4165(99)00102-6. [DOI] [PubMed] [Google Scholar]
- 94.Hui X., Liu H., Tian F.-L., Li F.-F., Li H., Gao W.-Y. Inhibition of green tea and the catechins against 1-deoxy-d-xylulose 5-phosphate reductoisomerase, the key enzyme of the MEP terpenoid biosynthetic pathway. Fitoterapia. 2016;113:80–84. doi: 10.1016/j.fitote.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 95.Dyer P.D.R., Kotha A.K., Gollings A.S., Shorter S.A., Shepherd T.R., Pettit M.W., Alexander B.D., Getti G.T.M., El-Daher S., Baillie L., et al. An in vitro evaluation of epigallocatechin gallate (eGCG) as a biocompatible inhibitor of ricin toxin. Biochim. Biophys. Acta (BBA) Gen. Subj. 2016;1860:1541–1550. doi: 10.1016/j.bbagen.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 96.Rizk M.A., El-Sayed S.A.E.-S., AbouLaila M., Tuvshintulga B., Yokoyama N., Igarashi I. Large-scale drug screening against Babesia divergens parasite using a fluorescence-based high-throughput screening assay. Vet. Parasitol. 2016;227:93–97. doi: 10.1016/j.vetpar.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 97.Tsou L.K., Yount J.S., Hang H.C. Epigallocatechin-3-gallate inhibits bacterial virulence and invasion of host cells. Bioorg. Med. Chem. 2017;25:2883–2887. doi: 10.1016/j.bmc.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Piva G., Fracassetti D., Tirelli A., Mascheroni E., Musatti A., Inglese P., Piergiovanni L., Rollini M. Evaluation of the antioxidant/antimicrobial performance of Posidonia oceanica in comparison with three commercial natural extracts and as a treatment on fresh-cut peaches (Prunus persica Batsch) Postharvest Biol. Technol. 2017;124:54–61. doi: 10.1016/j.postharvbio.2016.10.001. [DOI] [Google Scholar]
- 99.Sakanaka S., Juneja L.R., Taniguchi M. Antimicrobial effects of green tea polyphenols on thermophilic spore-forming bacteria. J. Biosci. Bioeng. 2000;90:81–85. doi: 10.1016/S1389-1723(00)80038-9. [DOI] [PubMed] [Google Scholar]
- 100.Yun J., Lee D.G. Role of potassium channels in chlorogenic acid-induced apoptotic volume decrease and cell cycle arrest in Candida albicans. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017;1861:585–592. doi: 10.1016/j.bbagen.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 101.Carneiro B.M., Batista M.N., Braga A.C.S., Nogueira M.L., Rahal P. The green tea molecule EGCG inhibits Zika virus entry. Virology. 2016;496:215–218. doi: 10.1016/j.virol.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 102.Hartjen P., Frerk S., Hauber I., Matzat V., Thomssen A., Holstermann B., Hohenberg H., Schulze W., zur Wiesch J.S., van Lunzen J. Assessment of the range of the HIV-1 infectivity enhancing effect of individual human semen specimen and the range of inhibition by EGCG. AIDS Res. Ther. 2012;9:2. doi: 10.1186/1742-6405-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bin Dajem S.M., Shati A.A., Adly M.A., Ahmed O.M., Ibrahim E.H., Mostafa O.M.S. Green tea (Camellia sinesis) ameliorates female Schistosoma mansoni-induced changes in the liver of Balb/C mice. Saudi J. Biol. Sci. 2011;18:361–368. doi: 10.1016/j.sjbs.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wickramasinghe S., Yatawara L., Nagataki M., Agatsuma T. Arginine kinase in Toxocara canis: Exon–intron organization, functional analysis of site-directed mutants and evaluation of putative enzyme inhibitors. Asian Pac. J. Trop. Med. 2016;9:995–1001. doi: 10.1016/j.apjtm.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 105.Vinson J.A., Dabbagh Y.A. Effect of green and black tea supplementation on lipids, lipid oxidation and fibrinogen in the hamster: Mechanisms for the epidemiological benefits of tea drinking. FEBS Lett. 1998;433:44–46. doi: 10.1016/S0014-5793(98)00880-1. [DOI] [PubMed] [Google Scholar]
- 106.Hartley L., Flowers N., Holmes J., Clarke A., Stranges S., Hooper L., Rees K. Green and Black Tea for the Primary Prevention of Cardiovascular Disease. The Cochrane Library; Bethesda, MD, USA: 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wasilewski R., Ubara E.O., Klonizakis M. Assessing the effects of a short-term green tea intervention in skin microvascular function and oxygen tension in older and younger adults. Microvasc. Res. 2016;107:65–71. doi: 10.1016/j.mvr.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 108.Yang T., Koo M. Inhibitory effect of Chinese green tea on endothelial cell-induced LDL oxidation. Atherosclerosis. 2000;148:67–73. doi: 10.1016/S0021-9150(99)00239-7. [DOI] [PubMed] [Google Scholar]
- 109.Song D.U., Do Jung Y., Chay K.O., Chung M.A., Lee K.H., Yang S.Y., Shin B.A., Ahn B.W. Effect of drinking green tea on age-associated accumulation of Maillard-type fluorescence and carbonyl groups in rat aortic and skin collagen. Arch. Biochem. Biophys. 2002;397:424–429. doi: 10.1006/abbi.2001.2695. [DOI] [PubMed] [Google Scholar]
- 110.Pang J., Zhang Z., Zheng T.-z., Bassig B.A., Mao C., Liu X., Zhu Y., Shi K., Ge J., Yang Y.-j. Green tea consumption and risk of cardiovascular and ischemic related diseases: A meta-analysis. Int. J. Cardiol. 2016;202:967–974. doi: 10.1016/j.ijcard.2014.12.176. [DOI] [PubMed] [Google Scholar]
- 111.Lustosa B.B., Polegato B., Minicucci M., Rafacho B., Santos P.P., Fernandes A.A., Okoshi K., Batista D., Modesto P., Gonçalves A., et al. Green tea (Cammellia sinensis) attenuates ventricular remodeling after experimental myocardial infarction. Int. J. Cardiol. 2016;225:147–153. doi: 10.1016/j.ijcard.2016.09.092. [DOI] [PubMed] [Google Scholar]
- 112.Bordoni A., Hrelia S., Angeloni C., Giordano E., Guarnieri C., Caldarera C.M., Biagi P.L. Green tea protection of hypoxia/reoxygenation injury in cultured cardiac cells. J. Nutr. Biochem. 2002;13:103–111. doi: 10.1016/S0955-2863(01)00203-0. [DOI] [PubMed] [Google Scholar]
- 113.Cheng T.O. All teas are not created equal: The Chinese green tea and cardiovascular health. Int. J. Cardiol. 2006;108:301–308. doi: 10.1016/j.ijcard.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 114.Sun T.-L., Liu Z., Qi Z.-J., Huang Y.-P., Gao X.-Q., Zhang Y.-Y. (−)-Epigallocatechin-3-gallate (EGCG) attenuates arsenic-induced cardiotoxicity in rats. Food Chem. Toxicol. 2016;93:102–110. doi: 10.1016/j.fct.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 115.Oyama J., Shiraki A., Nishikido T., Maeda T., Komoda H., Shimizu T., Makino N., Node K. EGCG, a green tea catechin, attenuates the progression of heart failure induced by the heart/muscle-specific deletion of MnSOD in mice. Int. J. Cardiol. 2017;69:417–427. doi: 10.1016/j.jjcc.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 116.Ale-Agha N., Stahl W., Sies H. (−)-Epicatechin effects in rat liver epithelial cells: Stimulation of gap junctional communication and counteraction of its loss due to the tumor promoter 12-O-tetradecanoylphorbol-13-acetate. Biochem. Pharmacol. 2002;63:2145–2149. doi: 10.1016/S0006-2952(02)01021-3. [DOI] [PubMed] [Google Scholar]
- 117.Dower J.I., Geleijnse J.M., Gijsbers L., Zock P.L., Kromhout D., Hollman P.C. Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: A randomized, double-blind, placebo-controlled, crossover trial. Am. J. Clin. Nutr. 2015;101:914–921. doi: 10.3945/ajcn.114.098590. [DOI] [PubMed] [Google Scholar]
- 118.Keiichiro M., Mayumi F., Hara Y. Effect of green tea catechins on plasma cholesterol level in cholesterol-fed rats. J. Nutr. Sci. Vitaminol. 1986;32:613–622. doi: 10.3177/jnsv.32.613. [DOI] [PubMed] [Google Scholar]
- 119.Yang T.T., Koo M.W. Chinese green tea lowers cholesterol level through an increase in fecal lipid excretion. Life Sci. 1999;66:411–423. doi: 10.1016/S0024-3205(99)00607-4. [DOI] [PubMed] [Google Scholar]
- 120.Ali M., Afzal M., Gubler C., Burka J.F. A potent thromboxane formation inhibitor in green tea leaves. Prostaglandins Leukot. Essent. Fat. Acids. 1990;40:281–283. doi: 10.1016/0952-3278(90)90050-U. [DOI] [PubMed] [Google Scholar]
- 121.Abe I., Seki T., Umehara K., Miyase T., Noguchi H., Sakakibara J., Ono T. Green tea polyphenols: Novel and potent inhibitors of squalene epoxidase. Biochem. Biophys. Res. Commun. 2000;268:767–771. doi: 10.1006/bbrc.2000.2217. [DOI] [PubMed] [Google Scholar]
- 122.Kasaoka S., Hase K., Morita T., Kiriyama S. Green tea flavonoids inhibit the LDL oxidation in osteogenic disordered rats fed a marginal ascorbic acid in diet. J. Nutr. Biochem. 2002;13:96–102. doi: 10.1016/S0955-2863(01)00202-9. [DOI] [PubMed] [Google Scholar]
- 123.Yoshikawa M., Morikawa T., Yamamoto K., Kato Y., Nagatomo A., Matsuda H. Floratheasaponins A−C, acylated oleanane-type triterpene oligoglycosides with anti-hyperlipidemic activities from flowers of the Tea plant (Camellia sinensis) J. Nat. Prod. 2005;68:1360–1365. doi: 10.1021/np0580614. [DOI] [PubMed] [Google Scholar]
- 124.Abe Y., Umemura S., Sugimoto K.-I., Hirawa N., Kato Y., Yokoyama N., Yokoyama T., Iwai J., Ishii M. Effect of green tea rich in γ-aminobutyric acid on blood pressure of Dahl salt-sensitive rats. Am. J. Hypertens. 1995;8:74–79. doi: 10.1016/0895-7061(94)00141-W. [DOI] [PubMed] [Google Scholar]
- 125.Juneja L.R., Chu D.-C., Okubo T., Nagato Y., Yokogoshi H. L-theanine—A unique amino acid of green tea and its relaxation effect in humans. Trends Food Sci. Technol. 1999;10:199–204. doi: 10.1016/S0924-2244(99)00044-8. [DOI] [Google Scholar]
- 126.Bertoldi M., Gonsalvi M., Voltattorni C.B. Green tea polyphenols: Novel irreversible inhibitors of dopa decarboxylase. Biochem. Biophys. Res. Commun. 2001;284:90–93. doi: 10.1006/bbrc.2001.4945. [DOI] [PubMed] [Google Scholar]
- 127.Yi Q.-Y., Li H.-B., Qi J., Yu X.-J., Huo C.-J., Li X., Bai J., Gao H.-L., Kou B., Liu K.-L., et al. Chronic infusion of epigallocatechin-3-O-gallate into the hypothalamic paraventricular nucleus attenuates hypertension and sympathoexcitation by restoring neurotransmitters and cytokines. Toxicol. Lett. 2016;262:105–113. doi: 10.1016/j.toxlet.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 128.Antonello M., Montemurro D., Bolognesi M., Di Pascoli M., Piva A., Grego F., Sticchi D., Giuliani L., Garbisa S., Rossi G.P. Prevention of hypertension, cardiovascular damage and endothelial dysfunction with green tea extracts. Am. J. Hypertens. 2007;20:1321–1328. doi: 10.1016/j.amjhyper.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 129.Ihm S.-H., Jang S.-W., Kim O.-R., Chang K., Oak M.-H., Lee J.-O., Lim D.-Y., Kim J.-H. Decaffeinated green tea extract improves hypertension and insulin resistance in a rat model of metabolic syndrome. Atherosclerosis. 2012;224:377–383. doi: 10.1016/j.atherosclerosis.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 130.Kang W.-S., Lim I.-H., Yuk D.-Y., Chung K.-H., Park J.-B., Yoo H.-S., Yun Y.-P. Antithrombotic activities of green tea catechins and (−)-epigallocatechin gallate. Thromb. Res. 1999;96:229–237. doi: 10.1016/S0049-3848(99)00104-8. [DOI] [PubMed] [Google Scholar]
- 131.Lau K.-M., He Z.-D., Dong H., Fung K.-P., But P.P.-H. Anti-oxidative, anti-inflammatory and hepato-protective effects of Ligustrum robustum. J. Ethnopharmacol. 2002;83:63–71. doi: 10.1016/S0378-8741(02)00192-7. [DOI] [PubMed] [Google Scholar]
- 132.Ohnishi S.T., Ohnishi T., Ogunmola G.B. Green tea extract and aged garlic extract inhibit anion transport and sickle cell dehydration in vitro. Blood Cells Mol. Dis. 2001;27:148–157. doi: 10.1006/bcmd.2000.0368. [DOI] [PubMed] [Google Scholar]
- 133.Peixoto E.B., Papadimitriou A., Teixeira D.A.T., Montemurro C., Duarte D.A., Silva K.C., Joazeiro P.P., Lopes de Faria J.M., Lopes de Faria J.B. Reduced LRP6 expression and increase in the interaction of GSK3β with p53 contribute to podocyte apoptosis in diabetes mellitus and are prevented by green tea. J. Nutr. Biochem. 2015;26:416–430. doi: 10.1016/j.jnutbio.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 134.Sampath C., Rashid M.R., Sang S., Ahmedna M. Green tea epigallocatechin 3-gallate alleviates hyperglycemia and reduces advanced glycation end products via nrf2 pathway in mice with high fat diet-induced obesity. Biomed. Pharmacother. 2017;87:73–81. doi: 10.1016/j.biopha.2016.12.082. [DOI] [PubMed] [Google Scholar]
- 135.Pournourmohammadi S., Grimaldi M., Stridh M.H., Lavallard V., Waagepetersen H.S., Wollheim C.B., Maechler P. Epigallocatechin-3-gallate (EGCG) activates AMPK through the inhibition of glutamate dehydrogenase in muscle and pancreatic ß-cells: A potential beneficial effect in the pre-diabetic state? Int. J. Biochem. Cell Biol. 2017;88:220–225. doi: 10.1016/j.biocel.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 136.Sabu M., Smitha K., Kuttan R. Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J. Ethnopharmacol. 2002;83:109–116. doi: 10.1016/s0378-8741(02)00217-9. [DOI] [PubMed] [Google Scholar]
- 137.Yoshikawa M., Wang T., Sugimoto S., Nakamura S., Nagatomo A., Matsuda H., Harima S. Functional saponins in tea flower (flower buds of Camellia sinensis): Gastroprotective and hypoglycemic effects of floratheasaponins and qualitative and quantitative analysis using HPLC. J. Pharm. Soc. Jpn. 2008;128:141–151. doi: 10.1248/yakushi.128.141. [DOI] [PubMed] [Google Scholar]
- 138.Han Q., Yu Q.-Y., Shi J., Xiong C.-Y., Ling Z.-J., He P.-M. Molecular characterization and hypoglycemic activity of a novel water-soluble polysaccharide from tea (Camellia sinensis) flower. Carbohydr. Polym. 2011;86:797–805. doi: 10.1016/j.carbpol.2011.05.039. [DOI] [Google Scholar]
- 139.Sun L., Warren F.J., Netzel G., Gidley M.J. 3 or 3′-Galloyl substitution plays an important role in association of catechins and theaflavins with porcine pancreatic α-amylase: The kinetics of inhibition of α-amylase by tea polyphenols. J. Funct. Foods. 2016;26:144–156. doi: 10.1016/j.jff.2016.07.012. [DOI] [Google Scholar]
- 140.Juhel C., Armand M., Pafumi Y., Rosier C., Vandermander J., Lairon D. Green tea extract (AR25®) inhibits lipolysis of triglycerides in gastric and duodenal medium in vitro. J. Nutr. Biochem. 2000;11:45–51. doi: 10.1016/S0955-2863(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 141.Diepvens K., Kovacs E., Vogels N., Westerterp-Plantenga M. Metabolic effects of green tea and of phases of weight loss. Physiol. Behav. 2006;87:185–191. doi: 10.1016/j.physbeh.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 142.Janssens P.L.H.R., Hursel R., Westerterp-Plantenga M.S. Nutraceuticals for body-weight management: The role of green tea catechins. Physiol. Behav. 2016;162:83–87. doi: 10.1016/j.physbeh.2016.01.044. [DOI] [PubMed] [Google Scholar]
- 143.Loftus T.M., Jaworsky D.E., Frehywot G.L., Townsend C.A., Ronnett G.V., Lane M.D., Kuhajda F.P. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science. 2000;288:2379–2381. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- 144.Wu M., Liu D., Zeng R., Xian T., Lu Y., Zeng G., Sun Z., Huang B., Huang Q. Epigallocatechin-3-gallate inhibits adipogenesis through down-regulation of PPARγ and FAS expression mediated by PI3K-AKT signaling in 3T3-L1 cells. Eur. J. Pharmacol. 2017;795:134–142. doi: 10.1016/j.ejphar.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 145.Huang Y., Chan N.W.K., Lau C.W., Yao X.Q., Chan F.L., Chen Z.Y. Involvement of endothelium/nitric oxide in vasorelaxation induced by purified green tea (−) epicatechin. Biochim. Biophys Acta (BBA) Gen. Subj. 1999;1427:322–328. doi: 10.1016/S0304-4165(99)00034-3. [DOI] [PubMed] [Google Scholar]
- 146.Setozaki S., Minakata K., Masumoto H., Hirao S., Yamazaki K., Kuwahara K., Ikeda T., Sakata R. Prevention of abdominal aortic aneurysm progression by oral administration of green tea polyphenol in a rat model. J. Vasc. Surg. 2016;65:1803–1812. doi: 10.1016/j.jvs.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 147.Shibata K., Moriyama M., Fukushima T., Kaetsu A., Miyazaki M., Une H. Green tea consumption and chronic atrophic gastritis: A cross-sectional study in a green tea production village. J. Epidemiol. 2000;10:310–316. doi: 10.2188/jea.10.310. [DOI] [PubMed] [Google Scholar]
- 148.Ahn Y.-J., Kawamura T., Kim M., Yamamoto T., Mitsuoka T. Tea polyphenols: Selective growth inhibitors of Clostridium spp. Agric. Biol. Chem. 1991;55:1425–1426. doi: 10.1080/00021369.1991.10870770. [DOI] [Google Scholar]
- 149.Jung E.S., Park H.M., Hyun S.M., Shon J.-C., Liu K.-H., Hwang J.S., Lee C.H. Modulation of gut microbiome by green tea diet and its correlation with metabolic changes. Drug Metab. Pharmacokinet. 2017;32:S62. doi: 10.1016/j.dmpk.2016.10.248. [DOI] [Google Scholar]
- 150.Hong J.T., Ryu S.R., Kim H.J., Lee J.K., Lee S.H., Kim D.B., Yun Y.P., Ryu J.H., Lee B.M., Kim P.Y. Neuroprotective effect of green tea extract in experimental ischemia-reperfusion brain injury. Brain Res. Bull. 2000;53:743–749. doi: 10.1016/S0361-9230(00)00348-8. [DOI] [PubMed] [Google Scholar]
- 151.Guo Q., Zhao B., Li M., Shen S., Xin W. Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim. Biophys. Acta (BBA) Lipid Lipid Metab. 1996;1304:210–222. doi: 10.1016/S0005-2760(96)00122-1. [DOI] [PubMed] [Google Scholar]
- 152.Zhao X., Liu F., Jin H., Li R., Wang Y., Zhang W., Wang H., Chen W. Involvement of PKCα and ERK1/2 signaling pathways in EGCG’s protection against stress-induced neural injuries in Wistar rats. Neuroscience. 2017;346:226–237. doi: 10.1016/j.neuroscience.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Suganuma M., Okabe S., Oniyama M., Tada Y., Ito H., Fujiki H. Wide distribution of [3H](-)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis. 1998;19:1771–1776. doi: 10.1093/carcin/19.10.1771. [DOI] [PubMed] [Google Scholar]
- 154.Nagai K., Jiang M.H., Hada J., Nagata T., Yajima Y., Yamamoto S., Nishizaki T. (−)-Epigallocatechin gallate protects against NO stress-induced neuronal damage after ischemia by acting as an anti-oxidant. Brain Res. 2002;956:319–322. doi: 10.1016/S0006-8993(02)03564-3. [DOI] [PubMed] [Google Scholar]
- 155.Bai Q., Lyu Z., Yang X., Pan Z., Lou J., Dong T. Epigallocatechin-3-gallate promotes angiogenesis via up-regulation of Nfr2 signaling pathway in a mouse model of ischemic stroke. Behav. Brain Res. 2017;321:79–86. doi: 10.1016/j.bbr.2016.12.037. [DOI] [PubMed] [Google Scholar]
- 156.Ben P., Zhang Z., Zhu Y., Xiong A., Gao Y., Mu J., Yin Z., Luo L. l-Theanine attenuates cadmium-induced neurotoxicity through the inhibition of oxidative damage and tau hyperphosphorylation. Neurotoxicology. 2016;57:95–103. doi: 10.1016/j.neuro.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 157.Levites Y., Youdim M.B.H., Maor G., Mandel S. Attenuation of 6-hydroxydopamine (6-OHDA)-induced nuclear factor-kappaB (NF-κB) activation and cell death by tea extracts in neuronal cultures1. Biochem. Pharmacol. 2002;63:21–29. doi: 10.1016/S0006-2952(01)00813-9. [DOI] [PubMed] [Google Scholar]
- 158.Weinreb O., Mandel S., Amit T., Youdim M.B. Neurological mechanisms of green tea polyphenols in Alzheimer’s and Parkinson’s diseases. J. Nutr. Biochem. 2004;15:506–516. doi: 10.1016/j.jnutbio.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 159.Unno K., Takabayashi F., Kishido T., Oku N. Suppressive effect of green tea catechins on morphologic and functional regression of the brain in aged mice with accelerated senescence (SAMP10) Exp. Gerontol. 2004;39:1027–1034. doi: 10.1016/j.exger.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 160.McConnell J.S., McConnell R., Hossner L. Ultraviolet spectra of acetic acid, glycine, and glyphosate. Proc. Ark. Acad. Sci. 1993;47:73–76. [Google Scholar]
- 161.Choi J.-Y., Park C.-S., Kim D.-J., Cho M.-H., Jin B.-K., Pie J.-E., Chung W.-G. Prevention of nitric oxide-mediated 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced Parkinson’s disease in mice by tea phenolic epigallocatechin 3-gallate. Neurotoxicology. 2002;23:367–374. doi: 10.1016/S0161-813X(02)00079-7. [DOI] [PubMed] [Google Scholar]
- 162.Choi Y.-T., Jung C.-H., Lee S.-R., Bae J.-H., Baek W.-K., Suh M.-H., Park J., Park C.-W., Suh S.-I. The green tea polyphenol (−)-epigallocatechin gallate attenuates β-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sci. 2001;70:603–614. doi: 10.1016/S0024-3205(01)01438-2. [DOI] [PubMed] [Google Scholar]
- 163.Selkoe D.J. Deciphering Alzheimer’s disease: The amyloid precursor protein yields new clues. Science. 1990;248:1058–1060. doi: 10.1126/science.2111582. [DOI] [PubMed] [Google Scholar]
- 164.Jeon S.-Y., Bae K., Seong Y.-H., Song K.-S. Green tea catechins as a BACE1 (β-Secretase) inhibitor. Bioorg. Med. Chem. Lett. 2003;13:3905–3908. doi: 10.1016/j.bmcl.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 165.Geiser R.J., Chastain S.E., Moss M.A. Regulation of Bace1 Mrna Expression in Alzheimer’S Disease by Green Tea Catechins and Black Tea Theaflavins. Biophys. J. 2017;112:362a. doi: 10.1016/j.bpj.2016.11.1965. [DOI] [Google Scholar]
- 166.Kaur T., Pathak C.M., Pandhi P., Khanduja K.L. Effects of green tea extract on learning, memory, behavior and acetylcholinesterase activity in young and old male rats. Brain Cogn. 2008;67:25–30. doi: 10.1016/j.bandc.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 167.Molino S., Dossena M., Buonocore D., Ferrari F., Venturini L., Ricevuti G., Verri M. Polyphenols in dementia: From molecular basis to clinical trials. Life Sci. 2016;161:69–77. doi: 10.1016/j.lfs.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 168.Pervin M., Unno K., Nakagawa A., Takahashi Y., Iguchi K., Yamamoto H., Hoshino M., Hara A., Takagaki A., Nanjo F., et al. Blood brain barrier permeability of (−)-epigallocatechin gallate, its proliferation-enhancing activity of human neuroblastoma SH-SY5Y cells, and its preventive effect on age-related cognitive dysfunction in mice. Biochem. Biophys. Rep. 2017;9:180–186. doi: 10.1016/j.bbrep.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Unno K., Hara A., Nakagawa A., Iguchi K., Ohshio M., Morita A., Nakamura Y. Anti-stress effects of drinking green tea with lowered caffeine and enriched theanine, epigallocatechin and arginine on psychosocial stress induced adrenal hypertrophy in mice. Phytomedicine. 2016;23:1365–1374. doi: 10.1016/j.phymed.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 170.Wang Z.Y., Khan W.A., Bickers D.R., Mukhtar H. Protection against polycyclic aromatic hydrocarbon-induced skin tumor initiation in mice by green tea polyphenols. Carcinogenesis. 1989;10:411–415. doi: 10.1093/carcin/10.2.411. [DOI] [PubMed] [Google Scholar]
- 171.Yang F., De Villiers W.J., McClain C.J., Varilek G.W. Green tea polyphenols block endotoxin-induced tumor necrosis factor-production and lethality in a murine model. J. Nutr. 1998;128:2334–2340. doi: 10.1093/jn/128.12.2334. [DOI] [PubMed] [Google Scholar]
- 172.Chan M.M.-Y., Fong D., Ho C.-T., Huang H.-I. Inhibition of inducible nitric oxide synthase gene expression and enzyme activity by epigallocatechin gallate, a natural product from green tea. Biochem. Pharmacol. 1997;54:1281–1286. doi: 10.1016/S0006-2952(97)00504-2. [DOI] [PubMed] [Google Scholar]
- 173.Higashi-Okai K., Otani S., Okai Y. Potent suppressive activity of pheophytin a and b from the non-polyphenolic fraction of green tea (Camellia sinensis) against tumor promotion in mouse skin. Cancer Lett. 1998;129:223–228. doi: 10.1016/S0304-3835(98)00113-X. [DOI] [PubMed] [Google Scholar]
- 174.Ashraf A., Sadrneshin S., Mosavat S.H., Hashempur M.H. Efficacy of the green tea (Camellia sinensis) tablet in knee osteoarthritis. Eur. J. Integr. Med. 2016;8:10–11. doi: 10.1016/j.eujim.2016.08.027. [DOI] [Google Scholar]
- 175.Maeda Y. Inhibitory effects of tea extracts on histamine release from mast cells. J. Jpn. Food Hyg. 1980;30:295–299. doi: 10.3358/shokueishi.30.295. [DOI] [Google Scholar]
- 176.Akagi M., Fukuishi N., Tomoko K., SAGESAKA Y.M., Akagi R. Anti-allergic effect of tea-leaf saponin (TLS) from tea leaves (Camellia sinensis var. sinensis). Biol. Pharm. Bull. 1997;20:565–567. doi: 10.1248/bpb.20.565. [DOI] [PubMed] [Google Scholar]
- 177.Yoshikawa M., Nakamura S., Kato Y., Matsuhira K., Matsuda H. Medicinal Flowers. XIV. 1) New acylated oleanane-type triterpene oligoglycosides with antiallergic activity from flower buds of chinese tea plant (Camellia sinensis) Chem. Pharm. Bull. 2007;55:598–605. doi: 10.1248/cpb.55.598. [DOI] [PubMed] [Google Scholar]
- 178.Pandit C., Anilakumar K.R. Cold adaptive thermogenesis following consumption of certain pungent spice principles: A validation study. J. Therm. Biol. 2017;64:35–40. doi: 10.1016/j.jtherbio.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 179.Ng H.L.H., Premilovac D., Rattigan S., Richards S.M., Muniyappa R., Quon M.J., Keske M.A. Acute vascular and metabolic actions of the green tea polyphenol epigallocatechin 3-gallate in rat skeletal muscle. J. Nutr. Biochem. 2017;40:23–31. doi: 10.1016/j.jnutbio.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 180.Kim A.R., Kim K.M., Byun M.R., Hwang J.-H., Park J.I., Oh H.T., Jeong M.G., Hwang E.S., Hong J.-H. (−)-Epigallocatechin-3-gallate stimulates myogenic differentiation through TAZ activation. Biochem. Biophys. Res. Commun. 2017;29:378–384. doi: 10.1016/j.bbrc.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 181.Takahashi H., Suzuki Y., Mohamed J.S., Gotoh T., Pereira S.L., Alway S.E. Epigallocatechin-3-gallate increases autophagy signaling in resting and unloaded plantaris muscles but selectively suppresses autophagy protein abundance in reloaded muscles of aged rats. Exp. Gerontol. 2017;92:55–56. doi: 10.1016/j.exger.2017.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nakagawa H., Wachi M., Woo J.-T., Kato M., Kasai S., Takahashi F., Lee I.-S., Nagai K. Fenton reaction is primarily involved in a mechanism of (−)-epigallocatechin-3-gallate to induce osteoclastic cell death. Biochem. Biophys. Res. Commun. 2002;292:94–101. doi: 10.1006/bbrc.2002.6622. [DOI] [PubMed] [Google Scholar]
- 183.Chu C., Deng J., Xiang L., Wu Y., Wei X., Qu Y., Man Y. Evaluation of epigallocatechin-3-gallate (EGCG) cross-linked collagen membranes and concerns on osteoblasts. Mater. Sci. Eng. C. 2016;67:386–394. doi: 10.1016/j.msec.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 184.Tomaszewska E., Dobrowolski P., Winiarska-Mieczan A., Kwiecień M., Tomczyk A., Muszyński S., Radzki R. Alteration in bone geometric and mechanical properties, histomorphometrical parameters of trabecular bone, articular cartilage, and growth plate in adolescent rats after chronic co-exposure to cadmium and lead in the case of supplementation with green, black, red and white tea. Environ. Toxicol. Pharmacol. 2016;46:36–44. doi: 10.1016/j.etap.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 185.Kuroyanagi G., Tokuda H., Yamamoto N., Kainuma S., Fujita K., Ohguchi R., Kawabata T., Sakai G., Matsushima-Nishiwaki R., Harada A., et al. (−)-Epigallocatechin gallate synergistically potentiates prostaglandin E2-stimulated osteoprotegerin synthesis in osteoblasts. Prostaglandins Lipid Med. 2017;128–129:27–33. doi: 10.1016/j.prostaglandins.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 186.Ahmed S., Rahman A., Hasnain A., Lalonde M., Goldberg V.M., Haqqi T.M. Green tea polyphenol epigallocatechin-3-gallate inhibits the IL-1β-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes. Free Radic. Biol. Med. 2002;33:1097–1105. doi: 10.1016/S0891-5849(02)01004-3. [DOI] [PubMed] [Google Scholar]
- 187.Hashempur M.H., Sadrneshin S., Mosavat S.H., Ashraf A. Green tea (Camellia sinensis) for patients with knee osteoarthritis: A randomized open-label active-controlled clinical trial. Clin. Nutr. 2016;37:85–90. doi: 10.1016/j.clnu.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 188.Horiba N., Maekawa Y., Ito M., Matsumoto T., Nakamura H. A pilot study of Japanese green tea as a medicament: Antibacterial and bactericidal effects. J. Endod. 1991;17:122–124. doi: 10.1016/S0099-2399(06)81743-7. [DOI] [PubMed] [Google Scholar]
- 189.You S. Study on feasibility of Chinese green tea polyphenols (CTP) for preventing dental caries. Chin. J. Stomatol. 1993;28:197–199. [PubMed] [Google Scholar]
- 190.Kawarai T., Narisawa N., Yoneda S., Tsutsumi Y., Ishikawa J., Hoshino Y., Senpuku H. Inhibition of Streptococcus mutans biofilm formation using extracts from Assam tea compared to green tea. Arch. Oral Biol. 2016;68:73–82. doi: 10.1016/j.archoralbio.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 191.Abdulbaqi H.R., Himratul-Aznita W.H., Baharuddin N.A. Anti-plaque effect of a synergistic combination of green tea and Salvadora persica L. against primary colonizers of dental plaque. Arch. Oral Biol. 2016;70:117–124. doi: 10.1016/j.archoralbio.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 192.Kim-Park W.K., Allam E.S., Palasuk J., Kowolik M., Park K.K., Windsor L.J. Green tea catechin inhibits the activity and neutrophil release of Matrix Metalloproteinase-9. J. Tradit. Complement. Med. 2016;6:343–346. doi: 10.1016/j.jtcme.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Morin M.-P., Grenier D. Regulation of matrix metalloproteinase secretion by green tea catechins in a three-dimensional co-culture model of macrophages and gingival fibroblasts. Arch. Oral Biol. 2017;75:89–99. doi: 10.1016/j.archoralbio.2016.10.035. [DOI] [PubMed] [Google Scholar]
- 194.Hu S., Zhang X., Chen F., Wang M. Dietary polyphenols as photoprotective agents against UV radiation. J. Funct. Foods. 2017;30:108–118. doi: 10.1016/j.jff.2017.01.009. [DOI] [Google Scholar]
- 195.Jongil K., Jaesung H., Younki C., Yongku H., Youngjin J., Yang K.-H. Protective effects of green tea polyphenols on the ultraviolet-induced dermal extracellular damage. J. Dermatol. Sci. 1998;16:127. [Google Scholar]
- 196.Katiyar S.K., Bergamo B.M., Vyalil P.K., Elmets C.A. Green tea polyphenols: DNA photodamage and photoimmunology. J. Photochem. Photobiol. B Biol. 2001;65:109–114. doi: 10.1016/S1011-1344(01)00248-2. [DOI] [PubMed] [Google Scholar]
- 197.Elmets C.A., Singh D., Tubesing K., Matsui M., Katiyar S., Mukhtar H. Cutaneous photoprotection from ultraviolet injury by green tea polyphenols. J. Am. Acad. Dermatol. 2001;44:425–432. doi: 10.1067/mjd.2001.112919. [DOI] [PubMed] [Google Scholar]
- 198.Katiyar S.K., Afaq F., Azizuddin K., Mukhtar H. Inhibition of UVB-induced oxidative stress-mediated phosphorylation of mitogen-activated protein kinase signaling pathways in cultured human epidermal keratinocytes by green tea polyphenol (−)-epigallocatechin-3-gallate. Toxicol. Appl. Pharmacol. 2001;176:110–117. doi: 10.1006/taap.2001.9276. [DOI] [PubMed] [Google Scholar]
- 199.Asadi S.Y., Parsaei P., Karimi M., Ezzati S., Zamiri A., Mohammadizadeh F., Rafieian-kopaei M. Effect of green tea (Camellia sinensis) extract on healing process of surgical wounds in rat. Int. J. Surg. 2013;11:332–337. doi: 10.1016/j.ijsu.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 200.Hong Y.-H., Jung E.Y., Noh D.O., Suh H.J. Physiological effects of formulation containing tannase-converted green tea extract on skin care: Physical stability, collagenase, elastase, and tyrosinase activities. Int. Med. Res. 2014;3:25–33. doi: 10.1016/j.imr.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lu M.-J., Chen C. Enzymatic tannase treatment of green tea increases in vitro inhibitory activity against N-nitrosation of dimethylamine. Process Biochem. 2007;42:1285–1290. doi: 10.1016/j.procbio.2007.06.003. [DOI] [Google Scholar]
- 202.An T.-T., Feng S., Zeng C.-M. Oxidized epigallocatechin gallate inhibited lysozyme fibrillation more strongly than the native form. Redox Biol. 2017;11:315–321. doi: 10.1016/j.redox.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sarkar J., Nandy S.K., Chowdhury A., Chakraborti T., Chakraborti S. Inhibition of MMP-9 by green tea catechins and prediction of their interaction by molecular docking analysis. Biomed. Pharmacother. 2016;84:340–347. doi: 10.1016/j.biopha.2016.09.049. [DOI] [PubMed] [Google Scholar]
- 204.Hwang Y.P., Jin S.W., Choi J.H., Choi C.Y., Kim H.G., Kim S.J., Kim Y., Lee K.J., Chung Y.C., Jeong H.G. Inhibitory effects of l-theanine on airway inflammation in ovalbumin-induced allergic asthma. Food Chem. Toxicol. 2017;99:162–169. doi: 10.1016/j.fct.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 205.Jówko E., Sacharuk J., Balasińska B., Ostaszewski P., Charmas M., Charmas R. Green tea extract supplementation gives protection against exercise-induced oxidative damage in healthy men. Nutr. Res. 2011;31:813–821. doi: 10.1016/j.nutres.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 206.Mohamad Shalan N.A.A., Mustapha N.M., Mohamed S. Morinda citrifolia leaf enhanced performance by improving angiogenesis, mitochondrial biogenesis, antioxidant, anti-inflammatory & stress responses. Food Chem. 2016;212:443–452. doi: 10.1016/j.foodchem.2016.05.179. [DOI] [PubMed] [Google Scholar]
- 207.Sanguigni V., Manco M., Sorge R., Gnessi L., Francomano D. Natural antioxidant ice cream acutely reduces oxidative stress and improves vascular function and physical performance in healthy individuals. Nutrition. 2017;33:225–233. doi: 10.1016/j.nut.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 208.Abdelrazek H.M.A., Helmy S.A., Elsayed D.H., Ebaid H.M., Mohamed R.M. Ameliorating effects of green tea extract on cadmium induced reproductive injury in male Wistar rats with respect to androgen receptors and caspase-3. Reprod. Biol. 2016;16:300–308. doi: 10.1016/j.repbio.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 209.Zanchi M.M., Manfredini V., dos Santos Brum D., Vargas L.M., Spiazzi C.C., Soares M.B., Izaguirry A.P., Santos F.W. Green tea infusion improves cyclophosphamide-induced damage on male mice reproductive system. Toxicol. Rep. 2015;2:252–260. doi: 10.1016/j.toxrep.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Roychoudhury S., Agarwal A., Virk G., Cho C.-L. Potential role of green tea catechins in the management of oxidative stress-associated infertility. Reprod. BioMed. 2017;34:487–498. doi: 10.1016/j.rbmo.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 211.Gadani B., Bucci D., Spinaci M., Tamanini C., Galeati G. Resveratrol and Epigallocatechin-3-gallate addition to thawed boar sperm improves in vitro fertilization. Theriogenology. 2017;90:88–93. doi: 10.1016/j.theriogenology.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 212.Satoh K., Sakamoto Y., Ogata A., Nagai F., Mikuriya H., Numazawa M., Yamada K., Aoki N. Inhibition of aromatase activity by green tea extract catechins and their endocrinological effects of oral administration in rats. Food Chem. Toxicol. 2002;40:925–933. doi: 10.1016/S0278-6915(02)00066-2. [DOI] [PubMed] [Google Scholar]
- 213.Xue K.X., Wang S., Ma G.J., Zhou P., Wu P.Q., Zhang R.F., Xu Z., Chen W.S., Wang Y.Q. Micronucleus formation in peripheral-blood lymphocytes from smokers and the influence of alcohol-and tea-drinking habits. Int. J. Cancer. 1992;50:702–705. doi: 10.1002/ijc.2910500506. [DOI] [PubMed] [Google Scholar]
- 214.Shalini V.K., Luthra M., Srinivas L., Rao S.H., Basti S., Reddy M., Balasubramanian D. Oxidative damage to the eye lens caused by cigarette smoke and fuel smoke condensates. Ind. J. Biochem. Biophys. 1994;31:261–266. [PubMed] [Google Scholar]
- 215.Chaudhury S., Roy P., Dasgupta S. Green tea flavanols protect human γB-crystallin from oxidative photodamage. Biochimie. 2017;137:46–55. doi: 10.1016/j.biochi.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 216.Alessio H.M., Hagerman A.E., Romanello M., Carando S., Threlkeld M.S., Rogers J., Dimitrova Y., Muhammed S., Wiley R.L. Consumption of green tea protects rats from exercise-induced oxidative stress in kidney and liver. Nutr. Res. 2002;22:1177–1188. doi: 10.1016/S0271-5317(02)00421-9. [DOI] [Google Scholar]
- 217.Yokozawa T., Dong E., Oura H. Proof that green tea tannin suppresses the increase in the blood methylguanidine level associated with renal failure. Exp. Toxicol. Pathol. 1997;49:117–122. doi: 10.1016/S0940-2993(97)80079-6. [DOI] [PubMed] [Google Scholar]
- 218.Delwing-Dal Magro D., Roecker R., Junges G.M., Rodrigues A.F., Delwing-de Lima D., da Cruz J.G.P., Wyse A.T.S., Pitz H.S., Zeni A.L.B. Protective effect of green tea extract against proline-induced oxidative damage in the rat kidney. Biomed. Pharmacother. 2016;83:1422–1427. doi: 10.1016/j.biopha.2016.08.057. [DOI] [PubMed] [Google Scholar]
- 219.Yoshikawa M., Sugimoto S., Kato Y., Nakamura S., Wang T., Yamashita C., Matsuda H. Acylated oleanane-type triterpene saponins with acceleration of gastrointestinal transit and inhibitory effect on pancreatic lipase from flower buds of Chinese tea plant (Camellia sinensis) Chem. Biodivers. 2009;6:903–915. doi: 10.1002/cbdv.200800153. [DOI] [PubMed] [Google Scholar]
- 220.Jodoin J., Demeule M., Béliveau R. Inhibition of the multidrug resistance P-glycoprotein activity by green tea polyphenols. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2002;1542:149–159. doi: 10.1016/S0167-4889(01)00175-6. [DOI] [PubMed] [Google Scholar]
- 221.Chen L., Lee M.-J., Li H., Yang C.S. Absorption, distribution, and elimination of Tea polyphenols in Rats. Drug Metab. Dispos. 1997;25:1045–1050. [PubMed] [Google Scholar]
- 222.Bu-Abbas A., Clifford M., Walker R., Ioannides C. Selective induction of rat hepatic CYP1 and CYP4 proteins and of peroxisomal proliferation by green tea. Carcinogenesis. 1994;15:2575–2579. doi: 10.1093/carcin/15.11.2575. [DOI] [PubMed] [Google Scholar]
- 223.Bu-Abbas A., Clifford M.N., Walker R., Ioannides C. Modulation of hepatic cytochrome P450 activity and carcinogen bioactivation by black and decaffeinated black tea. Environ. Toxicol. Pharmacol. 1999;7:41–47. doi: 10.1016/S1382-6689(98)00049-0. [DOI] [PubMed] [Google Scholar]
- 224.Williams S.N., Shih H., Guenette D.K., Brackney W., Denison M.S., Pickwell G.V., Quattrochi L.C. Comparative studies on the effects of green tea extracts and individual tea catechins on human CYP1A gene expression. Chem. Biol. Int. 2000;128:211–229. doi: 10.1016/S0009-2797(00)00204-0. [DOI] [PubMed] [Google Scholar]
- 225.Ikarashi N., Ogawa S., Hirobe R., Kon R., Kusunoki Y., Yamashita M., Mizukami N., Kaneko M., Wakui N., Machida Y., et al. Epigallocatechin gallate induces a hepatospecific decrease in the CYP3A expression level by altering intestinal flora. Eur. J. Pharm. Sci. 2017;100:211–218. doi: 10.1016/j.ejps.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 226.Li W., Yalcin M., Lin Q., Ardawi M.-S.M., Mousa S.A. Self-assembly of green tea catechin derivatives in nanoparticles for oral lycopene delivery. J. Control. Release. 2017;248:117–124. doi: 10.1016/j.jconrel.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 227.Yao L.H., Jiang Y.M., Caffin N., D’Arcy B., Datta N., Liu X., Singanusong R., Xu Y. Phenolic compounds in tea from Australian supermarkets. Food Chem. 2006;96:614–620. doi: 10.1016/j.foodchem.2005.03.009. [DOI] [Google Scholar]
- 228.Vinson J.A., Dabbagh Y.A., Serry M.M., Jang J. Plant flavonoids, especially tea flavonols, are powerful antioxidants using an in vitro oxidation model for heart disease. J. Agric. Food Chem. 1995;43:2800–2802. doi: 10.1021/jf00059a005. [DOI] [Google Scholar]
- 229.Millin D.J., Crispin D.J., Swaine D. Nonvolatile components of black tea and their contribution to the character of the beverage. J. Agric. Food Chem. 1969;17:717–722. doi: 10.1021/jf60164a026. [DOI] [Google Scholar]
- 230.Wang Z.-Y., Huang M.-T., Ferraro T., Wong C.-Q., Lou Y.-R., Reuhl K., Iatropoulos M., Yang C.S., Conney A.H. Inhibitory effect of green tea in the drinking water on tumorigenesis by ultraviolet light and 12-O-tetradecanoylphorbol-13-acetate in the skin of SKH-1 mice. Cancer Res. 1992;52:1162–1170. [PubMed] [Google Scholar]
- 231.McKay D.L., Blumberg J.B. The role of tea in human health: An update. J. Am. Coll. Nutr. 2002;21:1–13. doi: 10.1080/07315724.2002.10719187. [DOI] [PubMed] [Google Scholar]
- 232.Okuda T., Mori K., Hatano T. Relationship of the structures of tannins to the binding activities with hemoglobin and methylene blue. Chem. Pharm. Bull. 1985;33:1424–1433. doi: 10.1248/cpb.33.1424. [DOI] [PubMed] [Google Scholar]
- 233.Ricci A., Parpinello G.P., Palma A.S., Teslić N., Brilli C., Pizzi A., Versari A. Analytical profiling of food-grade extracts from grape (Vitis vinifera sp.) seeds and skins, green tea (Camellia sinensis) leaves and Limousin oak (Quercus robur) heartwood using MALDI-TOF-MS, ICP-MS and spectrophotometric methods. J. Food Compos. Anal. 2017;59:95–104. doi: 10.1016/j.jfca.2017.01.014. [DOI] [Google Scholar]
- 234.Bhagwat S., Haytowitz D.B., Holden J.M. USDA Database for the Flavonoid Content of Selected Foods Release 3. US Department of Agriculture, ARS. [(accessed on 5 October 2012)];2011 Available online: http://www.nal.usda.gov/fnic/foodcomp/Data/Flav/flav.pdf.
- 235.Lakenbrink C., Engelhardt U.H., Wray V. Identification of two novel proanthocyanidins in green tea. J. Agric. Food Chem. 1999;47:4621–4624. doi: 10.1021/jf9813081. [DOI] [PubMed] [Google Scholar]
- 236.Nonaka G., Kawahara O., Nishioka I. Tannins and related compounds. XV. A new class of dimeric flavan-3-ol gallates, theasinensins A and B, and proanthocyanidin gallates from green tea leaf. (1) Chem. Pharm. Bull. 1983;31:3906–3914. doi: 10.1248/cpb.31.3906. [DOI] [Google Scholar]
- 237.Crozier A., Jaganath I.B., Clifford M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009;26:1001–1043. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- 238.Sakamoto Y. Flavones in Green Tea. Agric. Biol. Chem. 1967;31:1029–1034. [Google Scholar]
- 239.Schmidt M., Schmitz H.-J., Baumgart A., Guedon D., Netsch M., Kreuter M.-H., Schmidlin C., Schrenk D. Toxicity of green tea extracts and their constituents in rat hepatocytes in primary culture. Food Chem. Toxicol. 2005;43:307–314. doi: 10.1016/j.fct.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 240.Man S., Gao W., Zhang Y., Huang L., Liu C. Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia. 2010;81:703–714. doi: 10.1016/j.fitote.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 241.Horie H., Fukatsu S., Mukai T., Goto T., Kawanaka M., Shimohara T. Quality evaluation on green tea. Sens. Actuators B Chem. 1993;13:451–454. doi: 10.1016/0925-4005(93)85424-9. [DOI] [Google Scholar]
- 242.Sadzuka Y., Sugiyama T., Miyagishima A., Nozawa Y., Hirota S. The effects of theanine, as a novel biochemical modulator, on the antitumor activity of adriamycin. Cancer Lett. 1996;105:203–209. doi: 10.1016/0304-3835(96)04282-6. [DOI] [PubMed] [Google Scholar]
- 243.Cabrera C., Artacho R., Giménez R. Beneficial effects of green tea—A review. J. Am. Coll. Nutr. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- 244.Ravichandran R. Carotenoid composition, distribution and degradation to flavour volatiles during black tea manufacture and the effect of carotenoid supplementation on tea quality and aroma. Food Chem. 2002;78:23–28. doi: 10.1016/S0308-8146(01)00303-X. [DOI] [Google Scholar]
- 245.Zhu Y., Shao C.-Y., Lv H.-P., Zhang Y., Dai W.-D., Guo L., Tan J.-F., Peng Q.-H., Lin Z. Enantiomeric and quantitative analysis of volatile terpenoids in different teas (Camellia sinensis) J. Chromatogr. A. 2017;1490:177–190. doi: 10.1016/j.chroma.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 246.Choi S.J., Park S.Y., Park J.S., Park S.-K., Jung M.Y. Contents and compositions of policosanols in green tea (Camellia sinensis) leaves. Food Chem. 2016;204:94–101. doi: 10.1016/j.foodchem.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 247.Zhu M., Li N., Zhao M., Yu W., Wu J.-L. Metabolomic profiling delineate taste qualities of tea leaf pubescence. Food Res. Int. 2017;94:36–44. doi: 10.1016/j.foodres.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 248.Yan L.-g., Xi J. Micro-mechanism analysis of ultrahigh pressure extraction from green tea leaves by numerical simulation. Sep. Purif. Technol. 2017;180:51–57. doi: 10.1016/j.seppur.2017.02.041. [DOI] [Google Scholar]
- 249.Wunsch D.C., Hasselmo M.E., Venayagamoorthy G.K. Advances in Neural Networks Research: IJCNN 2003. Elsevier; Amsterdam, The Netherlands: 2003. [Google Scholar]
- 250.Goschnick J., Koronczi I., Frietsch M., Kiselev I. Water pollution recognition with the electronic nose KAMINA. Sens. Actuators B Chem. 2005;106:182–186. doi: 10.1016/j.snb.2004.05.055. [DOI] [Google Scholar]
- 251.Horie H., Fukatsu S., Mukai T., Takeuchi A., Goto T. Quality evaluation of medium class green tea; Proceedings of the International Symposium on Tea Science; Shizuoka, Japan. 26–29 April 1991. [Google Scholar]
- 252.Yu H., Wang J. Discrimination of LongJing green-tea grade by electronic nose. Sens. Actuators B Chem. 2007;122:134–140. doi: 10.1016/j.snb.2006.05.019. [DOI] [Google Scholar]
- 253.Yu H., Wang J., Yao C., Zhang H., Yu Y. Quality grade identification of green tea using E-nose by CA and ANN. LWT Food Sci. Technol. 2008;41:1268–1273. doi: 10.1016/j.lwt.2007.08.018. [DOI] [Google Scholar]
- 254.Dutta R., Hines E., Gardner J., Kashwan K., Bhuyan M. Tea quality prediction using a tin oxide-based electronic nose: An artificial intelligence approach. Sens. Actuators B Chem. 2003;94:228–237. doi: 10.1016/S0925-4005(03)00367-8. [DOI] [Google Scholar]
- 255.Chen Q., Zhao J., Chen Z., Lin H., Zhao D.-A. Discrimination of green tea quality using the electronic nose technique and the human panel test, comparison of linear and nonlinear classification tools. Sens. Actuators B Chem. 2011;159:294–300. doi: 10.1016/j.snb.2011.07.009. [DOI] [Google Scholar]
- 256.Mirasoli M., Gotti R., Di Fusco M., Leoni A., Colliva C., Roda A. Electronic nose and chiral-capillary electrophoresis in evaluation of the quality changes in commercial green tea leaves during a long-term storage. Talanta. 2014;129:32–38. doi: 10.1016/j.talanta.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 257.Akella G., Henderson S., Drewnowski A. Genetic Sensitivity to 6-N-Propylthiouracil (prop) and Sensory Acceptance of Soy Products and Japanese Green Tea. J. Am. Diet. Assoc. 1997;97:A61. doi: 10.1016/S0002-8223(97)00526-9. [DOI] [PubMed] [Google Scholar]
- 258.Chen Q., Zhao J., Vittayapadung S. Identification of the green tea grade level using electronic tongue and pattern recognition. Food Res. Int. 2008;41:500–504. doi: 10.1016/j.foodres.2008.03.005. [DOI] [Google Scholar]
- 259.Xu Y.-Q., Zou C., Gao Y., Chen J.-X., Wang F., Chen G.-S., Yin J.-F. Effect of the type of brewing water on the chemical composition, sensory quality and antioxidant capacity of Chinese teas. Food Chem. 2016;236:142–151. doi: 10.1016/j.foodchem.2016.11.110. [DOI] [PubMed] [Google Scholar]
- 260.Panigrahi S., Balasubramanian S., Gu H., Logue C., Marchello M. Neural-network-integrated electronic nose system for identification of spoiled beef. LWT Food Sci. Technol. 2006;39:135–145. doi: 10.1016/j.lwt.2005.01.002. [DOI] [Google Scholar]
- 261.Chen Q., Zhang D., Pan W., Ouyang Q., Li H., Urmila K., Zhao J. Recent developments of green analytical techniques in analysis of tea’s quality and nutrition. Trends Food Sci. Technol. 2015;43:62–82. doi: 10.1016/j.tifs.2015.01.009. [DOI] [Google Scholar]
- 262.Zhu H., Ye Y., He H., Dong C. Evaluation of green tea sensory quality via process characteristics and image information. Food Bioprod. Process. 2017;102:116–122. doi: 10.1016/j.fbp.2016.12.004. [DOI] [Google Scholar]
- 263.Yin J., Xu Y., Yuan H., Yu S., Wei K., Chen J., Wang F., Wu R. Dynamic change of main biochemical components of premium green tea fresh leaves during spreading. J. Tea Sci. 2009;29:102–110. [Google Scholar]
- 264.Manasa G., Mascarenhas R.J., Satpati A.K., D’Souza O.J., Dhason A. Facile preparation of poly(methylene blue) modified carbon paste electrode for the detection and quantification of catechin. Mater. Sci. Eng. C. 2017;73:552–561. doi: 10.1016/j.msec.2016.12.114. [DOI] [PubMed] [Google Scholar]
- 265.Luypaert J., Zhang M., Massart D. Feasibility study for the use of near infrared spectroscopy in the qualitative and quantitative analysis of green tea, Camellia sinensis (L.) Anal. Chim. Acta. 2003;478:303–312. doi: 10.1016/S0003-2670(02)01509-X. [DOI] [Google Scholar]
- 266.Costa L.M., Gouveia S.T., Nobrega J.A. Comparison of heating extraction procedures for Al, Ca, Mg, and Mn in tea samples. Anal. Sci. 2002;18:313–318. doi: 10.2116/analsci.18.313. [DOI] [PubMed] [Google Scholar]
- 267.Fernández-Cáceres P.L., Martín M.J., Pablos F., González A.G. Differentiation of tea (Camellia sinensis) varieties and their geographical origin according to their metal content. J. Agric. Food Chem. 2001;49:4775–4779. doi: 10.1021/jf0106143. [DOI] [PubMed] [Google Scholar]
- 268.Shu W., Zhang Z., Lan C., Wong M. Fluoride and aluminium concentrations of tea plants and tea products from Sichuan Province, PR China. Chemosphere. 2003;52:1475–1482. doi: 10.1016/S0045-6535(03)00485-5. [DOI] [PubMed] [Google Scholar]
- 269.Fung K.F., Zhang Z.Q., Wong J.W.C., Wong M.H. Aluminium and fluoride concentrations of three tea varieties growing at lantau island, Hong Kong. Environ. Geochem. Health. 2003;25:219–232. doi: 10.1023/A:1023233226620. [DOI] [PubMed] [Google Scholar]
- 270.Ashraf W., Mian A.A. Levels of selected heavy metals in black tea varieties consumed in Saudi Arabia. Bull. Environ. Cont. Toxicol. 2008;81:101–104. doi: 10.1007/s00128-008-9402-0. [DOI] [PubMed] [Google Scholar]
- 271.Pohl P., Szymczycha-Madeja A., Stelmach E., Welna M. Multivariate data reduction and discrimination of black and green teas due to the physical fractionation pattern of selected metals determined in their infusions. Talanta. 2016;160:314–324. doi: 10.1016/j.talanta.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 272.Schulzki G., Nüßlein B., Sievers H. Transition rates of selected metals determined in various types of teas (Camellia sinensis L. Kuntze) and herbal/fruit infusions. Food Chem. 2017;215:22–30. doi: 10.1016/j.foodchem.2016.07.093. [DOI] [PubMed] [Google Scholar]
- 273.Nakagawa M. Chemical components and taste of green tea. Jpn. Agric. Res. Quart. 1975;9:156–160. [Google Scholar]
- 274.Mi H., Guan M., Liu J., Shan H., Fei Q., Huan Y., Feng G. Conjugated polymer with carboxylate groups-Hg2 + system as a turn-on fluorescence probe for label-free detection of cysteine-containing compounds. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017;176:168–173. doi: 10.1016/j.saa.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 275.Van Nederkassel A., Daszykowski M., Massart D., Vander Heyden Y. Prediction of total green tea antioxidant capacity from chromatograms by multivariate modeling. J. Chromatogr. A. 2005;1096:177–186. doi: 10.1016/j.chroma.2005.03.102. [DOI] [PubMed] [Google Scholar]
- 276.Morsy M., Khaled M. Novel EPR characterization of the antioxidant activity of tea leaves. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2002;58:1271–1277. doi: 10.1016/S1386-1425(01)00716-8. [DOI] [PubMed] [Google Scholar]
- 277.Nissen L.R., Huynh-Ba T., Petersen M.A., Bertelsen G., Skibsted L.H. Potential use of electron spin resonance spectroscopy for evaluating the oxidative status of potato flakes. Food Chem. 2002;79:387–394. doi: 10.1016/S0308-8146(02)00160-7. [DOI] [Google Scholar]
- 278.Arakawa H., Kanemitsu M., Tajima N., Maeda M. Chemiluminescence assay for catechin based on generation of hydrogen peroxide in basic solution. Anal. Chim. Acta. 2002;472:75–82. doi: 10.1016/S0003-2670(02)00982-0. [DOI] [Google Scholar]
- 279.Usha S.P., Shrivastav A.M., Gupta B.D. Silver nanoparticle noduled ZnO nanowedge fetched novel FO-LMR based H2O2 biosensor: A twin regime sensor for in-vivo applications and H2O2 generation analysis from polyphenolic daily devouring beverages. Sens. Actuators B Chem. 2017;241:129–145. doi: 10.1016/j.snb.2016.10.067. [DOI] [Google Scholar]
- 280.Benvidi A., Nafar M.T., Jahanbani S., Tezerjani M.D., Rezaeinasab M., Dalirnasab S. Developing an electrochemical sensor based on a carbon paste electrode modified with nano-composite of reduced graphene oxide and CuFe2O4 nanoparticles for determination of hydrogen peroxide. Mater. Sci. Eng. C. 2017;75:1435–1447. doi: 10.1016/j.msec.2017.03.062. [DOI] [PubMed] [Google Scholar]
- 281.Pelillo M., Biguzzi B., Bendini A., Gallina Toschi T., Vanzini M., Lercker G. Preliminary investigation into development of HPLC with UV and MS-electrospray detection for the analysis of tea catechins. Food Chem. 2002;78:369–374. doi: 10.1016/S0308-8146(02)00112-7. [DOI] [Google Scholar]
- 282.Naldi M., Fiori J., Gotti R., Périat A., Veuthey J.-L., Guillarme D., Andrisano V. UHPLC determination of catechins for the quality control of green tea. J. Pharm. Biomed. Anal. 2014;88:307–314. doi: 10.1016/j.jpba.2013.08.054. [DOI] [PubMed] [Google Scholar]
- 283.Tao W., Zhou Z., Zhao B., Wei T. Simultaneous determination of eight catechins and four theaflavins in green, black and oolong tea using new HPLC–MS–MS method. J. Pharm. Biomed. Anal. 2016;131:140–145. doi: 10.1016/j.jpba.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 284.Kumar A.S., Shanmugam R., Nellaiappan S., Thangaraj R. Tea quality assessment by analyzing key polyphenolic functional groups using flow injection analysis coupled with a dual electrochemical detector. Sens. Actuators B Chem. 2016;227:352–361. doi: 10.1016/j.snb.2015.12.072. [DOI] [Google Scholar]
- 285.Alessio P., Martin C.S., de Saja J.A., Rodriguez-Mendez M.L. Mimetic biosensors composed by layer-by-layer films of phospholipid, phthalocyanine and silver nanoparticles to polyphenol detection. Sens. Actuators B Chem. 2016;233:654–666. doi: 10.1016/j.snb.2016.04.139. [DOI] [Google Scholar]
- 286.Vilian A.T.E., Puthiaraj P., Kwak C.H., Choe S.R., Huh Y.S., Ahn W.-S., Han Y.-K. Electrochemical determination of quercetin based on porous aromatic frameworks supported Au nanoparticles. Electrochim. Acta. 2016;216:181–187. doi: 10.1016/j.electacta.2016.08.150. [DOI] [Google Scholar]
- 287.Chen Q., Zhao J., Lin H. Study on discrimination of Roast green tea (Camellia sinensis L.) according to geographical origin by FT-NIR spectroscopy and supervised pattern recognition. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009;72:845–850. doi: 10.1016/j.saa.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 288.Alaerts G., Van Erps J., Pieters S., Dumarey M., Van Nederkassel A., Goodarzi M., Smeyers-Verbeke J., Vander Heyden Y. Similarity analyses of chromatographic fingerprints as tools for identification and quality control of green tea. J. Chromatogr. B. 2012;910:61–70. doi: 10.1016/j.jchromb.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 289.Dumarey M., Van Nederkassel A., Deconinck E., Vander Heyden Y. Exploration of linear multivariate calibration techniques to predict the total antioxidant capacity of green tea from chromatographic fingerprints. J. Chromatogr. A. 2008;1192:81–88. doi: 10.1016/j.chroma.2008.03.052. [DOI] [PubMed] [Google Scholar]
- 290.Chen Q., Zhao J., Guo Z., Wang X. Determination of caffeine content and main catechins contents in green tea (Camellia sinensis L.) using taste sensor technique and multivariate calibration. J. Food Compos. Anal. 2010;23:353–358. doi: 10.1016/j.jfca.2009.12.010. [DOI] [Google Scholar]
- 291.Iorgulescu E., Voicu V.A., Sârbu C., Tache F., Albu F., Medvedovici A. Experimental variability and data pre-processing as factors affecting the discrimination power of some chemometric approaches (PCA, CA and a new algorithm based on linear regression) applied to (+/−)ESI/MS and RPLC/UV data: Application on green tea extracts. Talanta. 2016;155:133–144. doi: 10.1016/j.talanta.2016.04.042. [DOI] [PubMed] [Google Scholar]
- 292.Jing J., Shi Y., Zhang Q., Wang J., Ruan J. Prediction of Chinese green tea ranking by metabolite profiling using ultra-performance liquid chromatography–quadrupole time-of-flight mass spectrometry (UPLC–Q-TOF/MS) Food Chem. 2017;221:311–316. doi: 10.1016/j.foodchem.2016.10.068. [DOI] [PubMed] [Google Scholar]
- 293.Zhang N., Gao J., Huang C., Liu W., Tong P., Zhang L. In situ hydrothermal growth of ZnO/g-C3N4 nanoflowers coated solid-phase microextraction fibers coupled with GC-MS for determination of pesticides residues. Anal. Chim. Acta. 2016;934:122–131. doi: 10.1016/j.aca.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 294.Qin Y., Zhang J., Zhang Y., Li F., Han Y., Zou N., Xu H., Qian M., Pan C. Automated multi-plug filtration cleanup for liquid chromatographic–tandem mass spectrometric pesticide multi-residue analysis in representative crop commodities. J. Chromatogr. A. 2016;1462:19–26. doi: 10.1016/j.chroma.2016.07.073. [DOI] [PubMed] [Google Scholar]
- 295.Jiang Z., Li H., Cao X., Du P., Shao H., Jin F., Jin M., Wang J. Determination of hymexazol in 26 foods of plant origin by modified QuEChERS method and liquid chromatography tandem-mass spectrometry. Food Chem. 2017;228:411–419. doi: 10.1016/j.foodchem.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 296.Rolle F., Pennecchi F., Perini S., Sega M. Metrological traceability of Polycyclic Aromatic Hydrocarbons (PAHs) measurements in green tea and mate. Measurement. 2017;98:290–299. doi: 10.1016/j.measurement.2016.03.009. [DOI] [Google Scholar]
- 297.Cebi N., Yilmaz M.T., Sagdic O. A rapid ATR-FTIR spectroscopic method for detection of sibutramine adulteration in tea and coffee based on hierarchical cluster and principal component analyses. Food Chem. 2017;229:517–526. doi: 10.1016/j.foodchem.2017.02.072. [DOI] [PubMed] [Google Scholar]
- 298.Jinnarak A., Anantavichian P., Intanin A., Fungladda S., Choengchan N., Wilairat P., Nacapricha D., Teerasong S. Sequential injection for determination of gamma-aminobutyric acid based on its effect on second order light scattering of silver nanoparticles. J. Food Compos. Anal. 2016;51:69–75. doi: 10.1016/j.jfca.2016.06.013. [DOI] [Google Scholar]
- 299.Liu L., Fan Y., Fu H., Chen F., Ni C., Wang J., Yin Q., Mu Q., Yang T., She Y. “Turn-off” fluorescent sensor for highly sensitive and specific simultaneous recognition of 29 famous green teas based on quantum dots combined with chemometrics. Anal. Chim. Acta. 2017;963:119–128. doi: 10.1016/j.aca.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 300.Jayabalan R., Malbaša R.V., Lončar E.S., Vitas J.S., Sathishkumar M. A review on kombucha tea—Microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr. Rev. Food Sci. F. 2014;13:538–550. doi: 10.1111/1541-4337.12073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.