This matched cohort study evaluates the association between switching from a non–high deductible health plan to a high-deductible health plan and discontinuation of antihyperglycemic medication among adults with type 2 diabetes.
Key Points
Question
What is the association between switching from a non–high-deductible to a high-deductible health plan and discontinuation of antihyperglycemic medication?
Findings
In this matched cohort study of 2980 adults with type 2 diabetes who were taking at least 1 antihyperglycemic medication, there was no difference in unadjusted discontinuation rates between groups with and without a high-deductible health plan. However, a greater proportion of patients with high-deductible health plans did not refill branded medications.
Meaning
Among patients using branded medications, switching to a high-deductible health plan may be associated with discontinuation of medication; however, this study’s results suggest no association between discontinuation of antihyperglycemic medications and switching to an HDHP overall.
Abstract
Importance
High-deductible health plans (HDHPs) are a common cost-savings option for employers but may lead to underuse of necessary treatments because beneficiaries bear the full cost of health care, including medications, until a deductible is met.
Objectives
To evaluate the association between switching from a non-HDHP to an HDHP and discontinuation of antihyperglycemic medication and to assess whether the association differs in patients using branded vs generic antihyperglycemic medications.
Design, Setting, and Participants
This retrospective matched cohort study used administrative claims from MarketScan databases to identify commercially insured adult patients with type 2 diabetes who used at least 1 antihyperglycemic medication in 2013. Patients in the HDHP cohort (n = 1490) were matched by propensity scores to a non-HDPH control cohort (n = 1490). Data were collected and analyzed from January 1, 2013, through December 31, 2014.
Exposures
Switching from a non-HDHP in 2013 to a full replacement HDHP in 2014 (no non-HDHP option offered) vs staying on a non-HDHP.
Main Outcomes and Measures
Difference-in-differences models estimated discontinuation of branded and generic antihyperglycemic medications.
Results
Among the 2980 patients included in the analysis (1932 men [64.8%]; mean [SD] age, HDHP cohort: 52.6 [6.9] years; non-HDHP cohort: 52.7 [7.3] years), no difference between the HDHP and non-HDHP cohorts was found in unadjusted follow-up discontinuation rates for all antihyperglycemic medications (255 [22.7%] vs 255 [23.3%]; P = .72); however, among patients using branded medication, a significantly greater proportion of patients in the HDHP group did not refill branded medications (81 of 396 [20.5%] vs 61 of 437 [14.0%]; P = .009). Difference-in-differences models were not statistically significant.
Conclusions and Relevance
These findings suggest switching to an HDHP is associated with discontinuation specifically of branded medications. Unintended health consequences may result and should be considered by employers making health care benefit decisions.
Introduction
In 2017, 28% of US adults with employer-sponsored insurance were enrolled in high-deductible health plans (HDHPs) requiring a general deductible of at least $1000 for single coverage or $2000 for family coverage.1 Among US individuals enrolled in single-only coverage through HealthCare.gov, 44% faced a general deductible of $1000 or more.2 High cost-sharing of this variety has been associated with financial stress,3 worse disease control,4 increases in hospitalizations,5,6 and exacerbation of health disparities.7
The potential for high out-of-pocket costs to adversely affect disease control for the 30.3 million US individuals with type 2 diabetes is especially concerning.8 Use of antihyperglycemic medications, along with certain key services, is critical for successful management.9 Multiple medications may be needed to achieve and sustain healthy glucose levels.10 The failure to achieve and maintain adequate disease control is responsible for more than 450 000 emergency department visits, 168 000 hospitalizations for diabetic ketoacidosis, and 108 000 lower extremity amputations each year.8 Type 2 diabetes also greatly increases the risks of cardiovascular and kidney disease.8 For these reasons, controlling type 2 diabetes is “one of the most important public health challenges of the twenty-first century.”11(p1)
A recent systematic review by Agarwal and colleagues12 assessed the association between enrollment in HDHPs and clinical and economic outcomes. Consistent with the findings of the RAND Health Insurance Experiment,13 Agarwal et al12 concluded that HDHPs can achieve savings by reducing the use of appropriate and inappropriate care alike. Their review found that 7 of 12 included studies found significant reductions in preventive care, with 5 of 13 included studies reporting a decrease in adherence to medication regimens. Among those examining any association between HDHP enrollment and the use of antihyperglycemic medications specifically, 1 study14 found no significant association; a second study15 found significant decreases in the number of prescriptions, the proportion of days covered, and adherence after 1 year but no significant changes after 2 years; and a third study16 reported mixed findings across measures of use.
One explanation for these inconsistent findings could be the failure to distinguish between the use of generic and branded antihyperglycemic agents among these 3 studies.14,15,16 Enrollment in HDHPs may have different effects for those patients for whom a branded option is the indicated treatment, such as those for whom generic agents are contraindicated or who fail to meet their clinical goals. The present study hypothesized that generic medications for management of type 2 diabetes—almost always less expensive than their branded counterparts—would be less susceptible to cost-related underuse than branded medications after HDHP enrollment. A more nuanced understanding of the association between HDHP enrollment and medication use could support efforts to reduce the risks of cost-related medication underuse through innovative benefit designs and could help identify patients at risk of cost-related underuse.
Methods
This retrospective matched cohort study was based on US administrative claims data contained in the IBM MarketScan research databases.17 All database records are statistically deidentified and certified to be fully compliant with US patient confidentiality requirements set forth in the Health Insurance Portability and Accountability Act of 1996 (HIPAA). Guidelines from the US Department of Health and Human Services Office for Human Research Protections indicate that institutional review board oversight is not necessary since this study did not involve intervention or interaction with patients and that the database contains only deidentified data in compliance with HIPAA. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
The MarketScan Commercial Claims and Encounters database contains the inpatient, outpatient, and outpatient prescription drug experience of approximately 120.3 million employees and their dependents covered under a variety of fee-for-service and managed care health plans, including large medical institutions, point-of-service plans, indemnity plans, and health maintenance organizations, from January 1, 1995, through December 31, 2014. As of 2013, the database included 35 million covered lives. The MarketScan Commercial Claims and Encounters database provides detailed cost, use, and outcomes data for health care services performed in inpatient and outpatient settings. The medical claims are linked to outpatient prescription drug claims and person-level enrollment data through the use of unique enrollee identifiers. The MarketScan Benefit Plan Design database was also used. This database contains detailed information on the benefit plan characteristics for a subset of the health plans represented in the commercial database. Information is abstracted from summary plan description booklets and includes financial provisions, descriptions of health service benefits, managed care features, and health coverage types. Patients for whom benefit design data was linked with health care claims data were considered for the study.
The study population consisted of patients enrolled in HDHPs and non-HDHPs during the study period. The HDHPs were defined as health plans with minimum patient out-of-pocket deductible amounts of $1250 for single members and $2500 for families, keeping in line with thresholds set by the Internal Revenue Service for 2013 and 2014.18 Health plans that did not require patient out-of-pocket deductibles and health plans with out-of-pocket deductibles less than the Internal Revenue Service thresholds were considered to be non-HDHPs. Owing to the nature of HDHPs, patients who are given the option to switch into an HDHP and elect to switch may be more likely to be healthy and to have less use of health care resources and health-related expenditure. Therefore, to avoid self-selection bias, patients identified as enrolled in an HDHP were selected from employers whose employees’ only coverage option was to switch from a non-HDHP to an HDHP during the study period. Full replacement, the term for the nonoptional switch from a non-HDHP to an HDHP, was assessed on the employer level through a manual review of health plan options. The study period of January 1, 2013, through December 31, 2014, was selected because it coincides with when the available data set provided the most comprehensive picture of plan designs that met our full replacement criteria.
A cohort of exposed patients with evidence of full replacement enrollment into an HDHP on January 1, 2014, after being enrolled in a non-HDHP benefit design during calendar year 2013 was identified. A control group of patients who remained in a non-HDHP on January 1, 2014, was also identified.
Patients were required to have at least 1 inpatient or at least 2 nondiagnostic outpatient medical claims for type 2 diabetes (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] diagnosis code 250.x0 or 250.x2) at any time from January 1, 2013, through December 31, 2014. Patients also had to have at least 12 months of continuous enrollment in medical and pharmacy benefits from a non-HDHP from January 1 through December 31, 2013 (baseline period), and at least 12 months of continuous enrollment in medical and pharmacy benefits from January 1 through December 31, 2014 (follow-up period), for a total of 24 months of continuous enrollment. Further, patients were required to be 18 years or older as of January 1, 2013, and to have at least 1 outpatient pharmacy claim for an antihyperglycemic agent other than insulin during the baseline period. Patients with at least 1 nondiagnostic inpatient or outpatient medical claim with a diagnosis code for type 1 diabetes (ICD-9-CM code 250.x1 or 250.x3) or gestational diabetes (ICD-9-CM code 648.8x) in any position during the baseline period or the follow-up period were excluded from the study.
Patient age, sex, region of residence (Northeast, North Central, South, West, or unknown), health plan type (HDHP vs non-HDHP), total health care expenditures, and whether or not a patient’s employer made any contribution to a health reimbursement or health savings account were captured during the baseline period. Clinical characteristics, including the Deyo-Charlson Comorbidity Index (a proxy measure for comorbidity burden)19; the count of antihyperglycemic medications; and whether a patient was a new vs experienced user of antihyperglycemic medication, were measured. New vs experienced users were identified according to whether the first prescription fill date occurred in the first 90 days of the 2013 calendar year (January 1 through March 31, 2013) or in days 91 through 365 (April 1 through December 31, 2013).
The primary outcomes of interest were refill and, separately, discontinuation of antihyperglycemic medication. In this study, refill of medication was defined as evidence of having 1 or more prescriptions for any antihyperglycemic medication during the follow-up period after at least 1 initial medication prescription in the baseline period. Medication refills (or lack thereof) were measured among the following 3 mutually exclusive groups: use of all medications, only branded medications, and only generic medications, with use initiated during the baseline period among all patients. Branded medications included all medications for which a branded form was available regardless of the availability of a generic medication with the same active ingredient. Discontinuation was defined as the failure to fill a prescription for any antihyperglycemic medication within 60 days of exhausting the supply of the previous prescription. To account for patients overstocking their medications, in cases of overlapping days’ supply between 2 prescriptions with the same generic ingredient(s), the start date of the subsequent prescription was adjusted to be the day after the prior fill had ended, regardless of the fill date. Discontinuation was measured among the following 3 mutually exclusive groups: all medications, only branded medications, and only generic medications, with use initiated during the baseline period for all patients. Discontinuation was measured in the baseline and follow-up periods. During the follow-up period, discontinuation rates were only measured among patients who did not discontinue therapy during the baseline period. All study variables were measured based on inpatient medical, outpatient medical, and outpatient pharmaceutical claims data using ICD-9-CM diagnosis and procedure codes, Current Procedural Terminology codes, Healthcare Common Procedure Coding System codes, and National Drug Codes as appropriate.
Statistical Analysis
Propensity scores were estimated using a logistic regression model. The dependent variable was a binary indicator for membership in the full replacement HDHP cohort.20 A vector of independent variables consisted of the following patient demographic, insurance, and clinical characteristics as described above: age, sex, region of residence, family size, Deyo-Charlson Comorbidity Index score, total number of antihyperglycemic medications used, baseline antihyperglycemic medication adherence, new vs prevalent use of antihyperglycemic medication during baseline, total medical expenditures during baseline exceeding $2500, and whether or not a patient’s employer made any contribution to a health savings or a health reimbursement account during the follow-up period.
Once the propensity score was estimated, patients in the HDHP group were matched to patients in a non-HDHP group at a 1:1 ratio using the nearest-neighbor technique, enforcing a caliper of 0.25 times the SD of the propensity score.21 The discriminative accuracy of the propensity score model was evaluated using the area under the receiver operating characteristic curve, also known as the C statistic. The balance achieved by the propensity score matching was assessed by comparing the prematching and postmatching distributions of the independent variables included in the propensity score model via the standardized difference, a measure of balance that is not sensitive to study sample sizes and therefore less susceptible than t tests and χ2 tests to type I error in the presence of large samples or type II error in the presence of small samples.22
For refill and discontinuation measures, bivariate descriptive statistics were used to test for statistically significant differences between the HDHP and non-HDHP cohorts. We used χ2 tests to test for differences in categorical variables and unpaired t tests with unequal variances and 2-tailed hypothesis or analysis of variance for differences in continuous variables. P ≤ .05 was selected a priori as the maximum P value for which differences were considered statistically significant.
This study used a difference-in-differences method to assess the association between HDHP benefit plan design and discontinuation. Discontinuation (gap ≥60 days) was assessed during the baseline and follow-up periods. Baseline and follow-up discontinuation were compared in the HDHP and non-HDHP cohorts, and baseline discontinuation was compared with follow-up period discontinuation within each cohort (eg, baseline vs follow-up). For each difference-in-differences estimation, the change in discontinuation for the HDHP cohort was compared with their propensity score–matched non-HDHP controls. Second, discontinuation (no refills) during the follow-up period was compared between HDHP and matched non-HDHP cohorts. Data were analyzed from January 1, 2013, through December 31, 2014. All analyses were conducted using SAS, version 9.4 (SAS Institute Inc).
Results
A total of 125 032 patients were identified in the MarketScan Benefit Plan Design and Commercial Claims and Encounters research databases, with enrollment in an HDHP benefit design in the calendar year 2014. Of these, 99 006 had at least 12 months of continuous enrollment in medical and pharmacy benefits in a non-HDHP from January 1 through December 31, 2013, with evidence of full replacement enrollment (nonoptional switch) into an HDHP on January 1, 2014. Of these, 1496 were 18 years or older, had a diagnosis of type 2 diabetes, and had at least 1 outpatient pharmacy claim for an antihyperglycemic medication (other than insulin) from January 1, 2013, through December 31, 2014. A total of 1 772 022 patients without evidence of enrollment in an HDHP as of January 1, 2014, after enrollment in a non-HDHP in 2013 were also identified. After the application of all study eligibility criteria to the sample of patients without full replacement in an HDHP plan, including the requirement for continuous eligibility for the full 24-month study period, a total of 21 623 patients remained in the non-HDHP cohort. After matching, 1490 patients from each cohort were included in the study for a total of 2980 participants (1932 men [64.8%] and 1048 women [35.2%]).
The distribution of demographic and clinical characteristics is shown in Table 1. The mean (SD) age of the study population was 52.6 (6.9) years in the HDHP cohort and 52.7 (7.3) years in the non-HDHP cohort, with the largest proportion of patients (670 [45.0%] in the HDHP cohort and 675 [45.3%] in the non-HDHP cohort) aged 55 to 64 years. The study sample included a higher proportion of male patients (961 [64.5%] in the HDHP and 971 [65.2%] in the non-HDHP cohorts) compared with female patients (529 [35.5%] in the HDHP and 519 [34.8%] in the non-HDHP cohorts), and most patients resided in the North Central region (924 [62.0%] in the HDHP and 941 [63.2%] in the non-HDHP cohorts). More patients in the non-HDHP cohort had mean baseline health care expenditures at or exceeding $2500 compared with those in the HDHP cohort (1104 [74.1%] vs 1088 [73.0%]). Patients in both cohorts had similar Deyo-Charlson Comorbidity Index scores (mean [SD], 1.4 [1.0] vs 1.4 [1.2]).
Table 1. Demographic and Clinical Characteristics.
| Characteristic | Patient Cohort | Standardized Difference, % | |
|---|---|---|---|
| HDHP (n = 1490) | Non-HDHP (n = 1490) | ||
| Age, mean (SD), y | 52.6 (6.9) | 52.7 (7.3) | 1.60 |
| Sex, No. (%) | |||
| Male | 961 (64.5) | 971 (65.2) | 1.40 |
| Female | 529 (35.5) | 519 (34.8) | 1.40 |
| Family size, mean (SD), No. of members | 2.8 (1.3) | 2.9 (1.3) | 7.00 |
| Region, No. (%) | |||
| Northeast | 82 (5.5) | 100 (6.7) | 5.10 |
| North Central | 924 (62.0) | 941 (63.2) | 2.40 |
| South | 442 (29.7) | 401 (26.9) | 6.10 |
| West | 42 (2.8) | 40 (2.7) | 0.90 |
| Unknown | 0 | 8 (0.5) | 10.40 |
| Baseline health care expenditure ≥$2500, No. (%) | 1088 (73.0) | 1104 (74.1) | 2.40 |
| Employer made annual contribution to employee HSA during 2014, No. (%) | 532 (35.7) | 529 (35.5) | 0.50 |
| Deyo-Charlson Comorbidity Index, mean (SD)a | 1.4 (1.0) | 1.4 (1.2) | 1.40 |
| Count of antihyperglycemic medication subclasses during baseline, mean (SD), No. | 1.5 (0.7) | 1.5 (0.7) | 3.20 |
| Early treatment, No. (%) | 1135 (76.2) | 1143 (76.7) | 1.30 |
Abbreviations: HDHP, high-deductible health plan; HSA, health savings account.
Calculated as a proxy measure for comorbidity burden and measured as mean (SD).
Refill rates are presented in Table 2. When examining all antihyperglycemic medications, the difference between patients in the HDHP and non-HDHP cohorts who did not refill their baseline antihyperglycemic medication was not significant (103 [6.9%] vs 97 [6.5%]; P = .80), nor was the discontinuation rate during the follow-up period (255 [22.7%] vs 255 [23.3%]; P = .72). However, when examining branded medications, a significantly higher proportion of patients who switched to an HDHP did not refill their branded antihyperglycemic medications during follow-up compared with patients in the non-HDHP cohort (81 of 396 [20.5%] vs 61 of 437 [14.0%]; P = .009).
Table 2. Refill and Discontinuation Rates During Follow-up.
| Antihyperglycemic Medication Group | Patient Cohort, No./Total No. (%) | P Valuea | |
|---|---|---|---|
| HDHP | Non-HDHP | ||
| All | |||
| No refills during follow-up period | 103/1490 (6.9) | 97/1490 (6.5) | .80 |
| Discontinuation | |||
| Baseline period | 365/1490 (24.5) | 396/1490 (26.6) | .19 |
| Follow-up period | 255/1125 (22.7) | 255/1094 (23.3) | .72 |
| Branded | |||
| No refills | 81/396 (20.5) | 61/437 (14.0) | .009 |
| Discontinuation | |||
| Baseline period | 105/396 (26.5) | 129/437 (29.5) | .34 |
| Follow-up period | 86/291 (29.6) | 87/308 (28.2) | .72 |
| Generic | |||
| No refills during follow-up period | 117/1405 (8.3) | 125/1367 (9.1) | .45 |
| Discontinuation occurred during | |||
| Baseline period | 377/1405 (26.8) | 396/1367 (29.0) | .21 |
| Follow-up period | 239/1028 (23.2) | 229/971 (23.6) | .86 |
Abbreviation: HDHP, high-deductible health plan.
Differences in categorical variables were calculated using χ2 tests; differences in continuous variables, an unpaired t test or analysis of variance with unequal variances and 2-tailed hypothesis.
The unadjusted patterns suggested a similar proportion of patients in the HDHP cohort discontinued therapy at any time during the study period compared with patients in the non-HDHP cohort (620 [41.6%] vs 651 [43.7%]; P = .25). The difference in the proportion of patients in the HDHP (191 [48.2%]) and non-HDHP (216 [49.4%]) cohorts who discontinued their branded antihyperglycemic medications during the study was not significant (P = .22). In addition, the proportion of patients with at least 1 generic medication who discontinued treatment did not significantly differ between the HDHP and non-HDHP cohorts (616 [43.8%] vs 625 [45.7%]; P = .32).
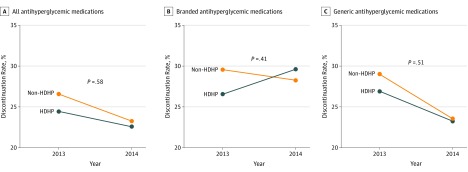
Difference-in-differences models suggested no statistically significant difference in all discontinuation of antihyperglycemic medication between the HDHP and non-HDHP cohorts (Figure, panel A). As shown in panels B and C of the Figure, among generic and branded antihyperglycemic medications, the change from baseline to follow-up discontinuation rate increased 1.81 and 4.24 percentage points for patients in the HDHP group using generic and branded antihyperglycemic medications, respectively, compared with the change for patients in the non-HDHP cohort, but the results were not statistically significant in either group.
Figure. Difference-in-Differences Comparison of Discontinuation Rates for Antihyperglycemic Medications.
A, Difference in all medications from 2013 to 2014 was −3.3% for the non–high-deductible health plan (HDHP) cohort (orange line); −1.8% for the HDHP cohort (blue line); and 1.5% for the difference-in-differences estimated comparison. B, Difference in branded medications from 2013 to 2014 was −1.2% for the non–HDHP cohort (orange line); 3.1% for the HDHP cohort (blue line); and 4.3% for the difference-in-differences estimated comparison. C, Difference in generic medications from 2013 to 2014 was −5.4% for the non–HDHP cohort (orange line); −3.5% for the HDHP cohort (blue line); and 1.9% for the difference-in-differences estimated comparison.
Discussion
This study showed that the association between HDHP enrollment and antihyperglycemic medication refills and subsequent discontinuation is mixed. Although no statistically significant differences in rates of discontinuation of antihyperglycemic treatment were found, the findings suggest a tendency toward switching to less costly treatment options. These varied results are consistent with the literature on the association between HDHP enrollment and medication use. The findings of the difference-in-differences analysis and simple comparison between patients in HDHP and non-HDHP benefit designs are consistent with the results of 5 high-quality investigations, whereas 8 distinct high-quality investigations have not reported significant reductions in adherence.12 This study suggests that enrollment in an HDHP may disrupt delivery of care for patients with type 2 diabetes, especially those for whom branded options offer optimal disease management. Two implications of these findings are apparent.
First, incorporating a value-based insurance design (V-BID) in the structuring of formularies could reduce the risk of cost-related underuse for these medications. A V-BID entails aligning patients’ out-of-pocket cost-sharing with the value of the underlying service—that is, lowering cost-sharing for high-value services and/or increasing cost-sharing for low-value services. Such an approach encourages the use of high-value care while maintaining or strengthening incentives to avoid low-value spending.23 A V-BID incorporates clinical nuance, “recognizing that the clinical benefit of a specific service or therapy depends on who receives it, who provides it, and where and when in the course of disease, the service or therapy is provided.”24(pE1)
Concretely, V-BID in type 2 diabetes treatment would call for plan designs that favor the use of low-cost generic medications when possible—consistent with the design of most current drug formularies—while ensuring that cost-sharing is low when a higher-cost, branded medication is clinically indicated. For example, scenarios arise when management fails to achieve clinical goals solely with generic antihyperglycemic medications. Precision benefit designs that alter cost-sharing based on a specific individual’s clinical course could serve to reward patients who follow recommended treatment paths.25,26,27,28
Until recently, plan sponsors offering health savings account–qualified HDHPs were constrained in their ability to pursue this strategy. In July 2019, the US Department of the Treasury released Notice 2019-45, a guidance allowing health savings account–qualified HDHP plans the flexibility to cover specified medications and services used to treat chronic diseases before meeting the plan deductible. The list of allowed services explicitly includes insulin and other agents to lower glucose levels. As a result, plan sponsors now have the flexibility to adopt V-BID principles that would enhance access and affordability of clinically indicated therapies for type 2 diabetes while maintaining strict fidelity to Internal Revenue Service guidelines.24
Second, study findings can also inform care management efforts. Enrollment in an HDHP can be discerned through administrative data that ought to be available to plans and health care professionals alike. Recognizing that enrollment has been associated with a 4% increase in medication discontinuation rates as shown by the difference-in-differences analysis and a 7% increase in the simple HDHP vs non-HDHP comparison, physicians and their teams should prioritize outreach to HDHP-enrolled patients currently prescribed branded therapy. Education regarding the availability of patient assistance programs, such as copayment cards and financial assistance charities, could be offered.29
Care improvement efforts of this sort should prioritize outreach to lower-income patients with type 2 diabetes because evidence suggests that the association between HDHP enrollment and access to high-value type 2 diabetes care varies across socioeconomic lines. A 2018 study by Wharam and colleagues30 reported that patients from low-income neighborhoods required to switch from low-deductible health plans to HDHPs experienced a 24% increase in high-severity emergency department expenditures and a 27% increase in high-severity inpatient days in association with the switch. Changes in the broader cohort (ie, including higher-income enrollees) were attenuated.30 A 2017 report by Wharam and colleagues31 on the same natural experiment found similar increases in acute emergency department visits for complications of type 2 diabetes among low-income enrollees, as well as reductions in outpatient visits for complications of type 2 diabetes in this population. Changes were again concentrated in the low-income groups.31 For these reasons, future research on discontinuation and adherence should seek to stratify patients by household income. Future research should also distinguish between branded and generic medications in assessing the association between HDHP enrollment and adherence because study results are suggestive of differing associations across levels of required out-of-pocket expenditures. In addition, more research is needed to gauge the association between HDHP enrollment and intermediate outcomes, such as hemoglobin A1c levels. According to Agarwal et al,12 receipt of screening measurements of hemoglobin A1c levels has been investigated, but the hemoglobin A1c levels have not been assessed. Finally, research is needed to assess the implications of HDHP enrollment for long-term health care costs for individuals with type 2 diabetes and among patients younger than 18 years.
Limitations
This investigation has several limitations that were likely to affect our findings. First, patient-level variables, such as income and race, were not included in the data set. The MarketScan data set is compiled predominantly from large employers, and patients tend to be healthier than the overall population. This variable may be further affected by limiting the study to employers reporting benefit plan characteristics. Notably, the population examined in this study was drawn predominantly for patients early in the natural history of type 2 diabetes, and the findings may not be generalizable to all patients with the disease.
Previous research5,6,8 has demonstrated that the negative association between high out-of-pocket costs, such as high deductibles, and poor outcomes is most likely concentrated in populations who are financially vulnerable and have multiple chronic conditions. Also, data on patient payment assistance programs (ie, copayment cards) that are increasingly used by consumers to help afford their prescriptions when faced with high out-of-pocket costs were unobtainable. To remove patient preference from the insurance selection process, this analysis examined medication discontinuation among patients involuntarily switched to an HDHP; however, employers making this switch may be under greater financial stress, which biases the study outcomes.
Conclusions
The discontinuation of antihyperglycemic medications absent clinical justification is potentially dangerous. The findings of the present study, especially when viewed in light of previous research,12,31 provide evidence to suggest that HDHP enrollment is a potential risk factor for discontinuation of branded medications. Payers, policy makers, and health care professionals can and should implement aligned incentives to ensure patients have access to clinically indicated care.
References
- 1.Claxton G, Rae M, Long M, Damico A, Foster G, Whitmore H. Employer health benefits 2017. https://www.kff.org/health-costs/report/2017-employer-health-benefits-survey/. Published September 19, 2017. Accessed November 20, 2017.
- 2.Centers for Medicare & Medicaid Services . Data brief: 2016. median marketplace deductible $850, with seven health services covered before the deductible on average. https://www.cms.gov/newsroom/press-releases/median-marketplace-deductible-only-850. Published July 12, 2016. Accessed November 17, 2017.
- 3.Collins SR, Rasmussen PW, Beutel S, Doty MM. The problem of underinsurance and how rising deductibles will make it worse: findings from the Commonwealth Fund Biennial Health Insurance Survey, 2014. http://www.commonwealthfund.org/~/media/files/publications/issue-brief/2015/may/1817_collins_problem_of_underinsurance_ib.pdf. Published May 2015. Accessed July 30, 2017. [PubMed]
- 4.Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: associations with medication and medical utilization and spending and health. JAMA. 2007;298(1):-. doi: 10.1001/jama.298.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trivedi AN, Moloo H, Mor V. Increased ambulatory care copayments and hospitalizations among the elderly. N Engl J Med. 2010;362(4):320-328. doi: 10.1056/NEJMsa0904533 [DOI] [PubMed] [Google Scholar]
- 6.Chandra A, Gruber J, McKnight R. Patient cost-sharing and hospitalization offsets in the elderly. Am Econ Rev. 2010;100(1):193-213. doi: 10.1257/aer.100.1.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chernew M, Gibson TB, Yu-Isenberg K, Sokol MC, Rosen AB, Fendrick AM. Effects of increased patient cost sharing on socioeconomic disparities in health care. J Gen Intern Med. 2008;23(8):1131-1136. doi: 10.1007/s11606-008-0614-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control & Prevention . National diabetes statistics report, 2017; estimates of diabetes and its burden in the United States. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed November 17, 2017.
- 9.American Diabetes Association . Standards of medical care in diabetes—2017: summary of revisions. Diabetes Care. 2017;40(suppl 1):S4-S5. doi: 10.2337/dc17-S003 [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association . Pharmacologic approaches to glycemic treatment. Diabetes Care. 2017;40(suppl 1):S64-S74. doi: 10.2337/dc17-S011 [DOI] [PubMed] [Google Scholar]
- 11.Zimmet P, Alberti KG, Magliano DJ, Bennett PH. Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nat Rev Endocrinol. 2016;12(10):616-622. doi: 10.1038/nrendo.2016.105 [DOI] [PubMed] [Google Scholar]
- 12.Agarwal R, Mazurenko O, Menachemi N. High-deductible health plans reduce health care cost and utilization, including use of needed preventive services. Health Aff (Millwood). 2017;36(10):1762-1768. doi: 10.1377/hlthaff.2017.0610 [DOI] [PubMed] [Google Scholar]
- 13.Newhouse JP; Insurance Experiment Group . Free for All? Lessons From the RAND Health Insurance Experiment. Cambridge, MA: Harvard University Press; 1996. [Google Scholar]
- 14.Chen S, Levin RA, Gartner JA. Medication adherence and enrollment in a consumer-driven health plan. Am J Manag Care. 2010;16(2):e43-e50. [PubMed] [Google Scholar]
- 15.Fronstin P, Sepulveda M-J, Roebuck MC. Medication utilization and adherence in a health savings account–eligible plan. Am J Manag Care. 2013;19(12):e400-e407. [PubMed] [Google Scholar]
- 16.Reiss SK, Ross-Degnan D, Zhang F, Soumerai SB, Zaslavsky AM, Wharam JF. Effect of switching to a high-deductible health plan on use of chronic medications. Health Serv Res. 2011;46(5):1382-1401. doi: 10.1111/j.1475-6773.2011.01252.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.IBM Watson Health. White paper: IBM MarketScan research databases for health services researchers. https://www.ibm.com/downloads/cas/6KNYVVQ2. Accessed August 5, 2019.
- 18.Internal Revenue Service . Health savings accounts and other tax-favored health plans. Washington, DC: International Revenue Service; 2016. Publication 969. https://www.irs.gov/publications/p969. Updated March 6, 2019. Accessed January 31, 2018.
- 19.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41-55. doi: 10.1093/biomet/70.1.41 [DOI] [Google Scholar]
- 21.Baser O. Too much ado about propensity score models? comparing methods of propensity score matching. Value Health. 2006;9(6):377-385. doi: 10.1111/j.1524-4733.2006.00130.x [DOI] [PubMed] [Google Scholar]
- 22.D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265-2281. doi: [DOI] [PubMed] [Google Scholar]
- 23.Chernew ME, Rosen AB, Fendrick AM. Value-based insurance design. Health Aff (Millwood). 2007;26(2):w195-w203. doi: 10.1377/hlthaff.26.2.w195 [DOI] [PubMed] [Google Scholar]
- 24.Fischer W. Additional preventive care benefits permitted to be provided by a high deductible health plan under § 223. Notice 2019-45. https://www.irs.gov/pub/irs-drop/n-19-45.pdf. Accessed August 27, 2019.
- 25.Chernew M, Gibson TB, Fendrick AM. Trends in patient cost sharing for clinical services used as quality indicators. J Gen Intern Med. 2010;25(3):243-248. doi: 10.1007/s11606-009-1219-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fendrick AM; Center for Value-Based Insurance Design Staff . Reward the good soldier: a dynamic approach to consumer cost-sharing for prescription drugs. http://www.ajmc.com/contributor/vbid-center/2016/09/reward-the-good-soldier-a-dynamic-approach-to-consumer-cost-sharing-for-prescription-drugs. Published September 26, 2016. Accessed March 8, 2017.
- 27.Fendrick AM, Buxbaum J, Westrich K. Supporting consumer access to specialty medications through value-based insurance design. http://vbidcenter.org/wp-content/uploads/2014/10/vbid-specialty-medications-npc2014-final-web.pdf. Accessed March 7, 2017.
- 28.Center for Value-Based Insurance Design . A “dynamic” approach to consumer cost-sharing for prescription drugs. http://vbidcenter.org/a-dynamic-approach-to-consumer-cost-sharing-for-prescription-drugs/. Published 2016. Accessed January 27, 2017.
- 29.Center for Value-Based Insurance Design . Precision patient assistance programs to enhance access to clinically indicated therapies: right drug, right time, right cost-share. http://vbidcenter.org/precision-patient-assistance-programs-to-enhance-access-to-clinically-indicated-therapies-right-drug-right-time-right-cost-share/. Published June 5, 2017 Accessed October 30, 2017.
- 30.Wharam JF, Zhang F, Eggleston EM, Lu CY, Soumerai SB, Ross-Degnan D. Effect of high-deductible insurance on high-acuity outcomes in diabetes: a Natural Experiment for Translation in Diabetes (NEXT-D) study. Diabetes Care. 2018;41(5):940-948. doi: 10.2337/dc17-1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wharam JF, Zhang F, Eggleston EM, Lu CY, Soumerai S, Ross-Degnan D. Diabetes outpatient care and acute complications before and after high-deductible insurance enrollment: a Natural Experiment for Translation in Diabetes (NEXT-D) study. JAMA Intern Med. 2017;177(3):358-368. doi: 10.1001/jamainternmed.2016.8411 [DOI] [PMC free article] [PubMed] [Google Scholar]