Abstract
Background:
We investigated the effect of thioctic acid (TA) on kidney function, oxidative stress, and inflammatory status in serum and kidney homogenates of a rat subjected to ischemia–reperfusion injury (IRI).
Materials and Methods:
Thirty male Wistar rats were randomly divided into three equal groups: sham, IR, and IR + TA in 50 mg/kg once-daily intraperitoneal injection for 2 weeks, before IR induction. The levels of urea and creatinine (Cr) in the serum of rats were measured. Malondialdehyde and nitric oxide (NO) as stress oxidative markers; tumor necrosis factor-α, interleukin-6, and myeloperoxidase as inflammatory markers, as well as activities of superoxide dismutase, glutathione peroxidase and catalase, and glutathione (GSH) level in both serum and kidney homogenates were determined.
Results:
Cr and urea increased in serum of IR group. Furthermore, levels of oxidative stress and inflammatory markers in serum and kidney homogenates of the cited group were higher than the sham group. TA not only decreased the levels of Cr, urea, oxidative stress, and inflammation but also elevated the level of GSH and activities of antioxidant enzymes (P < 0.001).
Conclusions:
The findings showed that TA protected IR rat against kidney dysfunction and IRI due to reinforcing endogenous antioxidant and subtracting of inflammatory markers.
Keywords: Antioxidant enzymes, inflammation, renal ischemia–reperfusion, oxidative stress, thioctic acid
Introduction
Kidney is susceptible to ischemia–reperfusion injury (IRI) during a number of events including hypotension, sepsis, surgical protocols such as partial nephrectomy and cardiac bypass surgery, as well as during kidney transplantation.[1,2] IRI is a capital cause of kidney dysfunction often following acute kidney failure, leading to high mortality among patients in intensive care who require dialysis.[3] Besides, renal IRI can contribute to delayed restoration of renal function and acute rejection after transplantation.[4]
Clinical outcomes of IRI or ischemic acute kidney injury (AKI) depend on the intensity of the injury and range from slight alternations in renal function to an assignment for dialysis or transplantation. Blood flow in the process of reperfusion phase can yield oxygen free radicals, which cause lipid peroxidation as a cardinal pathway of free radical tissue injuries.[5] Thus, generation of free radicals accelerates renal tissue injury through peroxidation of membrane lipids and oxidative damage of proteins and DNA participation in apoptosis and cell death.[6] Furthermore, downregulation of the antioxidant enzyme system such as glutathione peroxidase (GPX), catalase (CAT), and superoxide dismutase (SOD) could be liable for the pathophysiology of renal injury and IRI.[7] The potential pathological mechanisms of IRI include inflammation, oxidative stress, and apoptosis, which eventually lead to cell death. For this reason, multifunctional molecules with antioxidative, anti-inflammatory, and antiapoptotic properties are ideal protective agents against the outcomes of IR.[8] Furthermore, antioxidant therapy can protect against oxidative damage prompted by IR.[9] The guarding property of antioxidants may be linked to the capacity of antioxidant compounds to normalize primitive intracellular events connected to the movement of oxidative damage.[10]
Thioctic acid (TA), an endogenous short chain fatty acid, is a cofactor for multiple mitochondrial dehydrogenase enzymes. TA is suggested as a perfect antioxidant in fat, water-soluble, a reactive oxygen species (ROS) scavenger, a chelate of metal, and regenerates endogenous natural antioxidants and gene expression.[11] This study aimed to examine the effect of TA preconditioning on kidney function as well as oxidative stress and inflammatory markers in serum and kidney homogenates in IR rat.
Materials and Methods
Materials
All materials were in analytical grade and purchased from Sigma or Merck Chemical Companies.
Experimental group
Thirty male Sprague-Dawley rats (8 weeks) weighing 200 ± 15 g were kept under constant laboratory conditions (24 ± 1°C, 50 ± 10 humidity) and a 12-h light–dark cycle with ad libitum access to standard rodent chow and water in the animal laboratory of Razi Herbal Medicines Research Center. Furthermore, the rats were randomly divided into three equal groups (10 rats in each group) that were named as follows: (1) sham-operated group (sham group), wherein rats were treated only with separating the bilateral renal arteries and veins; (2) renal IR group (I/R group), wherein the rats were treated with ischemia by clamping the bilateral renal arteries and veins for 45 min and subsequent 24-h reperfusion;[12] and (3) renal I/R + TA group (TA group), wherein the rats were pretreated with TA. TA, 50 mg/kg, was administered to rats by daily intraperitoneal (i.p.) injection for 2 weeks before induction of renal IR; 24 hours after reperfusion rats were anesthetized with an i.p. injection of ketamine–xylosine (90 + 10 mg/kg body mass), a blood sample was collected from their heart and transferred into test tubes. Serum samples were prepared by 15 min of centrifugation of blood at 5000 ×g and were stored at −70°C for measurements. The right kidney was rapidly removed and homogenized for biochemical analysis. All experimental procedures were approved by the Ethics Committee for Animal Care and Use at the Lorestan Medical Sciences University (No. LUMS2015-1902).
Biochemical analysis
Serum creatinine (Cr) and urea were determined as a marker of kidney function by kits (Pars Azmoon, Iran) according to its manufacturers. The level of malondialdehyde (MDA) was measured using thiobarbituric acid by the spectrophotometric method at 532 nm. Glutathione (GSH) was assayed in serum and renal homogenates based on the development of a relatively stable yellow color when 5,5′-dithiobis-2′-nitrobenzoic acid was added to sulfhydryl compounds. Furthermore, the reaction color was read at 412 nm. Nitric oxide (NO) metabolites (nitrate and nitrite, NOx) were measured with a commercial assay kit (Cayman Chemical Co., Ann Arbor, MI, USA) based on Griess reaction. Activities of superoxide dismutase (SOD). CAT, GPX, and myeloperoxidase (MPO) were assayed by continuous spectrophotometric rate determination. The results of activists of SOD, CAT, GPX, and MPO were expressed as unit/mg of protein. The levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were determined by an ELISA kit (Immunotech, France) in serum and kidney homogenates.
Statistical analysis
All data were expressed as mean ± standard deviation. Multiple analysis of variance (Tukey's) test was used to compare different variables used in all groups and Pearson's rank correlation coefficient was used to compare the relationship between MDA and other parameters using SPSS version 16. Statistical significance was defined as P < 0.05.
Results
Kidney function is disturbed following IRI as represented by a notable increase in serum urea and Cr of IR group when compared with sham group [Figure 1a and b]. The levels of the cited parameters of IR rats treated with TA significantly were lower than untreated IR rats (P < 0.05).
Figure 1.
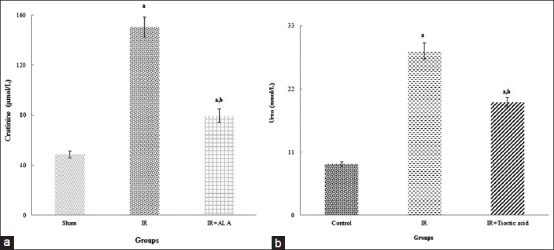
Levels of creatinine and urea in the serum of all groups (a and b, respectively). aIndicates significance of data comparing the sham group with other groups (P < 0.001). bIndicates significance of data comparing IR group with other groups (P < 0.001)
IRI induced oxidative stress and inflammation in both serum and kidney homogenates. TA exhibited an attenuating effect on oxidative stress. Thus, the level of GSH and activates of SOD, CAT, and GPX was higher in the treated group than the untreated group [Table 1]. Over and above, the levels of MDA and NO were lower in the treated group. Inflammatory markers, TNF-α, IL-6, and MPO significantly increased in IR group rather than the sham group [Table 2]. Inflammatory markers were lower in the treated group when compared with untreated group (P < 0.001). There was no difference in the activity of MPO in both serum and kidney homogenates between sham and IR-treated groups.
Table 1.
Effect of thioctic acid on oxidative stress markers, MDA, GSH, and NO levels in addition to antioxidant enzyme activities (SOD, CAT, and GSH peroxidase), in serum and renal homogenates of all groups
| Parameter | Sham | IR | IR + TA |
|---|---|---|---|
| MDA | |||
| Serum (nmol/L) | 5.10±0.23 | 170.29±9.71a,b | 99.36±5.48a,b |
| Kidney (nmol/mg protein) | 3.32±0.12 | 35.76±2.26a | 16.78±1.19a,b |
| NO | |||
| Serum (µmol/L) | 25.78±1.13 | 55.67±2.75a | 40.32±2.21a,b |
| Kidney (µmol/mg protein) | 0.51±0.04 | 0.83±0.08a | 0.66±0.06a,b |
| GSH | |||
| Serum (µmol/L) | 178.43±7.21 | 90.05±4.15a | 136.89±6.19a,b |
| Kidney (nmol/mg protein) | 43.88±3.21 | 25.34±1.15a | 35.89±2.87a,b |
| SOD | |||
| Serum (U/mg protein) | 145.35±8.01 | 80.74±4.22a | 110.87±6.12a,b |
| Kidney (U/mg protein) | 250.01±12.32 | 137.65±6.65a | 201.32±9.94a,b |
| CAT | |||
| Serum (U/mg protein) | 90.43±4.02 | 54.87±2.06a | 76.12±3.11a,b |
| Kidney (U/mg protein) | 105.33±5.24 | 60.65±2.82a | 83.03±3.91a,b |
| GPX | |||
| Serum (U/mg protein) | 70.35±2.41 | 36.74±1.22a | 58.67±2.54a,b |
| Kidney (U/mg protein) | 75.12±3.57 | 33.67±1.91a | 60.43±2.21a,b |
MDA=Malondialdehyde, GSH=Glutathione, NO=Nitric oxide, SOD=Superoxide dismutase, CAT=Catalase, GPX=Glutathione peroxidase, TA=Tioctic acid, IR=Ischemia–reperfusion. aIndicates significance of data comparing the sham group with other groups (P<0.001), bindicates significance of data comparing IR group with other groups (P<0.001)
Table 2.
Effect of thioctic acid on inflammatory markers in serum and renal homogenates of all groups
| Parameter | Sham | IR | IR + TA |
|---|---|---|---|
| IL-6 | |||
| Serum (ng/L) | 110.54±6.90 | 533.67±33.75a | 221.32±12.21a,b |
| Kidney (pg/mg) | 125.78±8.50 | 2055.67±177.75a | 1100.32±88.21a,b |
| TNF-α | |||
| Serum (ng/L) | 100.43±5.36 | 244.56±13.46a | 125.76±8.43a,b |
| Kidney (pg/mg) | 9.87±0.65 | 67.51±3.43a | 39.32±1.89a,b |
| MPO | |||
| Serum (U/mg protein) | 1.35±0.06 | 4.74±0.26a | 1.45±0.07b |
| Kidney (U/mg protein) | 0.55±0.11 | 3.01±0.62a | 0.61±0.13b |
IL-6=Interleukin-6, TNF-α=Tumor necrosis factor-α, MPO=Myeloperoxidase, TA=Tioctic acid, IR=Ischemia–reperfusion. aIndicates significance of data comparing the sham group with other groups (P<0.001), bindicates significance of data comparing IR group with other groups (P<0.001)
The correlation between MDA and other measurable parameters is presented in Figure 2. TNF-α, IL-6, MPO, and NO had a high positive correlation with MDA but vice versa about other cited parameters.
Figure 2.
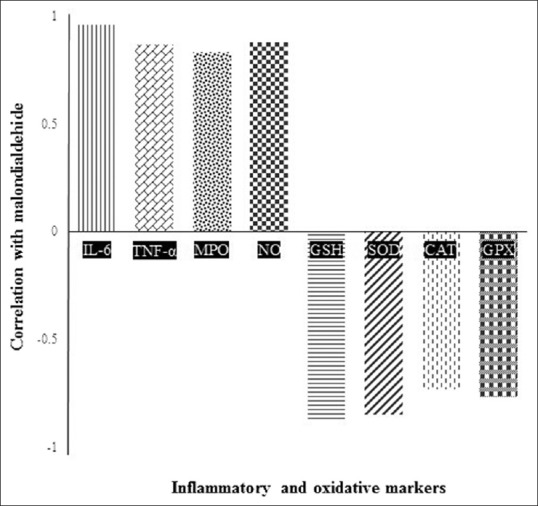
Correlation between malondialdehyde with inflammatory and oxidative stress markers
Discussion
We investigated the effect of two weeks’ pretreatment of i.p. injection of TA, 50 mg/kg, on kidney function tests, oxidative stress, and inflammatory markers in serum and kidney homogenates of the IR rat model. TA protected against kidney dysfunction due to an elevation of antioxidant property and reducing inflammatory responses in IR rat. Higher levels of urea and Cr in the serum of IR rats than the sham group following induction of IR propose substantial deterioration of kidney function [Figure 1a and 1b]. TA showed an improving effect on kidney function in IR rats due to lower levels of renal function parameters in the treated group than untreated (P < 0.001). The diminishing effect of TA on serum Cr and urea of IRI in simultaneous kidney–pancreas transplantation has been shown.[9]
Presumably, oxidative stress is a main initiator and motivator of IRI.[13] The raising levels of MDA and NO and diminishing of activities of antioxidant enzymes (SOD, CAT, and GPX) and level of GSH in serum and kidney homogenates indicated severe oxidative stress induction due to IR [Table 1]. Over and above, similar cited changes were observed in streptozotocin-induced diabetic rats exposed to renal IRI that confirms our results.[7,12] Based on our findings [Table 1], the treatment significantly compensated cited changes in the IR rat model (P < 0.001). The vitamin alleviated IRI by boosting the first lines of defense against free radicals with elevation of GSH level and SOD activity. The treatment may scavenge MDA and NO within IR tissue. NO convoy tissue versus IRI in low level, consequently its vasodilatory, antioxidant, and anti-inflammatory qualities. NO has an important role in the pathophysiology of renal IRI at a higher level.[14] Besides, inhibition of inducible nitric oxide synthase (iNOS) expression, iNOS activity, and scavenging of NO can attenuate or prevent NO-mediated renal injury.[15] A similar effect of the treatment on the levels of MDA and MPO as well as activities of GSH, SOD, and GPX, respectively, in renal sciatic homogenate on rats’ renal and sciatic nerve IR damage has been shown.[16,17] The activities of indigenous antioxidant enzymes, composed of SOD, CAT, and GPX, along with the level of GSH as ROS scavenger are the main contributors of reducing the tissue injury caused by IR.[18] Therefore, TA is a strong inhibitor of oxidative stress in IRI.
Inflammatory events raise in IR owing to expanding neutrophil infiltration and oxidative stress.[7] Inflammation is a principal player in renal IRI, so a constraint of inflammatory reactions is a strategy for convoy renal tissue. Cytokines such as IL6 and TNF-α play a major role in renal dysfunction of IRI.[19] MPO is the most abundant proinflammatory enzyme stored in the azurophilic granules of neutrophilic granulocytes.[20] Furthermore, MPO has a role in the pathogenesis of renal IRI, and it is generally used as a marker of local and systemic inflammatory.[21] The levels of inflammatory markers, TNF-α and IL-6, in addition to the activity of MPO in serum and renal tissues of the IR group were noticeably higher than the sham group [Table 2]. The treatment showed potent anti-inflammatory effect (P < 0.001). In our research, between MPO activity in serum and kidney homogenates of the treated IR rats and sham rats, there was no difference. Previously, the reducing effect of TA preconditioning on inflammatory markers such as TNF-α and IL-6 as well as early kidney dysfunction and clinical posttransplant pancreatitis was represented.[9] It is suggested that any treatment that could lead to augment of activity of antioxidant enzymes and GSH level, but reduces the activity of MPO and NO level may prevent or mitigate the consequences of IR. Therefore, TA is a perfect treatment for prevention of IRI. IR induces oxidative stress and inflammation by reduction of expression of nuclear-factor-E2-related factor 2 (Nrf2) and thioredoxin reductase-2 (TrxR2) as well as an elevation of expression of nuclear-factor-kappa B (NF-kB).[2,22,23] Furthermore, the elevation of the Nrf2/heme oxygenase-1 (HO-1) pathway played an antiapoptotic role.[24] Thus, the antioxidant, anti-inflammatory, and antiapoptosis effects of the treatment probably are based on the promotion of expression of Nrf2, HO-1, and TrxR2 along with the reduction of expression of NF-kB.
In this research, the correlation between MDA as a general marker of oxidative stress with other cited parameters is reported in Figure 2. Renal IRI increases with an increase in MPO, IL-6, and NO as well as a decrease in SOD, CAT, GPX, and GSH (P < 0.005). Therefore, TA as a multifunctional compound with antioxidant and anti-inflammatory activities ameliorated IRI.
Conclusions
TA improved kidney dysfunction in the renal IRI rat model following decrement levels of oxidative stress and inflammatory markers along with boosting endogenous antioxidant system.
Financial support and sponsorship
Lorestan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors wish to thank Deputy of Research and Razi Herbal Research Center of Lorestan Medical University, Lorestan, Iran.
References
- 1.Feng W, Tang R, Ye X, Xue C, Liao Y. Identification of genes and pathways associated with kidney ischemia-reperfusion injury by bioinformatics analyses. Kidney Blood Press Res. 2016;41:48–54. doi: 10.1159/000368546. [DOI] [PubMed] [Google Scholar]
- 2.Hu B, Wu Y, Liu J, Shen X, Tong F, Xu G, et al. GSK-3beta inhibitor induces expression of nrf2/TrxR2 signaling pathway to protect against renal ischemia/Reperfusion injury in diabetic rats. Kidney Blood Press Res. 2016;41:937–46. doi: 10.1159/000452598. [DOI] [PubMed] [Google Scholar]
- 3.Li X, Hu X, Wang J, Xu W, Yi C, Ma R, et al. Short-term hesperidin pretreatment attenuates rat myocardial ischemia/Reperfusion injury by inhibiting high mobility group box 1 protein expression via the PI3K/Akt pathway. Cell Physiol Biochem. 2016;39:1850–62. doi: 10.1159/000447884. [DOI] [PubMed] [Google Scholar]
- 4.Zhao L, Xu L, Tao X, Han X, Yin L, Qi Y, et al. Protective effect of the total flavonoids from rosa laevigata michx fruit on renal ischemia-reperfusion injury through suppression of oxidative stress and inflammation. Molecules. 2016;21 doi: 10.3390/molecules21070952. pii: E952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu B, Wu Y, Tong F, Liu J, Shen X, Shen R, et al. Apocynin alleviates renal ischemia/Reperfusion injury through regulating the level of zinc and metallothionen. Biol Trace Elem Res. 2017;178:71–8. doi: 10.1007/s12011-016-0904-z. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Xie Z, Lin M, Huang R, Liang Z, Huang W, et al. Renalase protects the cardiomyocytes of Sprague-Dawley rats against ischemia and reperfusion injury by reducing myocardial cell necrosis and apoptosis. Kidney Blood Press Res. 2015;40:215–22. doi: 10.1159/000368497. [DOI] [PubMed] [Google Scholar]
- 7.Hu B, Tong F, Xu L, Shen Z, Yan L, Xu G, et al. Role of calcium sensing receptor in streptozotocin-induced diabetic rats exposed to renal ischemia reperfusion injury. Kidney Blood Press Res. 2018;43:276–86. doi: 10.1159/000487685. [DOI] [PubMed] [Google Scholar]
- 8.Deng H, Zuo X, Zhang J, Liu X, Liu L, Xu Q, et al. A-lipoic acid protects against cerebral ischemia/reperfusion-induced injury in rats. Mol Med Rep. 2015;11:3659–65. doi: 10.3892/mmr.2015.3170. [DOI] [PubMed] [Google Scholar]
- 9.Ambrosi N, Arrosagaray V, Guerrieri D, Uva PD, Petroni J, Herrera MB, et al. A-lipoic acid protects against ischemia-reperfusion injury in simultaneous kidney-pancreas transplantation. Transplantation. 2016;100:908–15. doi: 10.1097/TP.0000000000000981. [DOI] [PubMed] [Google Scholar]
- 10.Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr J. 2016;15:71. doi: 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosquesi PL, Melo TR, Vizioli EO, Santos JL, Chung MC. Anti-inflammatory drug design using a molecular hybridization approach. Pharmaceuticals (Basel) 2011;4:1450–74. doi: 10.3390/ph4111450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu B, Xu G, Zheng Y, Tong F, Qian P, Pan X, et al. Chelerythrine attenuates renal ischemia/Reperfusion-induced myocardial injury by activating CSE/H2S via PKC/NF-κB pathway in diabetic rats. Kidney Blood Press Res. 2017;42:379–88. doi: 10.1159/000477948. [DOI] [PubMed] [Google Scholar]
- 13.Scindia Y, Dey P, Thirunagari A, Liping H, Rosin DL, Floris M, et al. Hepcidin mitigates renal ischemia-reperfusion injury by modulating systemic iron homeostasis. J Am Soc Nephrol. 2015;26:2800–14. doi: 10.1681/ASN.2014101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aksu U, Demirci C, Ince C. The pathogenesis of acute kidney injury and the toxic triangle of oxygen, reactive oxygen species and nitric oxide. Contrib Nephrol. 2011;174:119–28. doi: 10.1159/000329249. [DOI] [PubMed] [Google Scholar]
- 15.Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, et al. A unified theory of sepsis-induced acute kidney injury: Inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41:3–11. doi: 10.1097/SHK.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sehirli O, Sener E, Cetinel S, Yüksel M, Gedik N, Sener G, et al. Alpha-lipoic acid protects against renal ischaemia-reperfusion injury in rats. Clin Exp Pharmacol Physiol. 2008;35:249–55. doi: 10.1111/j.1440-1681.2007.04810.x. [DOI] [PubMed] [Google Scholar]
- 17.Turamanlar O, Özen OA, Songur A, Yaǧmurca M, Akçer S, Mollaoǧlu H, et al. Protective effect of alpha lipoic acid on rat sciatic nerve ischemia reperfusion damage. Balkan Med J. 2015;32:196–202. doi: 10.5152/balkanmedj.2015.15619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang PR, Wang JS, Zhang C, Song XF, Tian N, Kong LY, et al. Huang-lian-jie-du-decotion induced protective autophagy against the injury of cerebral ischemia/reperfusion via MAPK-mTOR signaling pathway. J Ethnopharmacol. 2013;149:270–80. doi: 10.1016/j.jep.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 19.Voss A, Bode G, Kerkhoff C. Double-stranded RNA induces IL-8 and MCP-1 gene expression via TLR3 in haCaT-keratinocytes. Inflamm Allergy Drug Targets. 2012;11:397–405. doi: 10.2174/187152812803251042. [DOI] [PubMed] [Google Scholar]
- 20.Aratani Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch Biochem Biophys. 2018;640:47–52. doi: 10.1016/j.abb.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Andelid K, Bake B, Rak S, Lindén A, Rosengren A, Ekberg-Jansson A, et al. Myeloperoxidase as a marker of increasing systemic inflammation in smokers without severe airway symptoms. Respir Med. 2007;101:888–95. doi: 10.1016/j.rmed.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 22.Markó L, Vigolo E, Hinze C, Park JK, Roël G, Balogh A, et al. Tubular epithelial NF-κB activity regulates ischemic AKI. J Am Soc Nephrol. 2016;27:2658–69. doi: 10.1681/ASN.2015070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen X, Hu B, Xu G, Chen F, Ma R, Zhang N, et al. Activation of nrf2/HO-1 pathway by glycogen synthase kinase-3β inhibition attenuates renal ischemia/Reperfusion injury in diabetic rats. Kidney Blood Press Res. 2017;42:369–78. doi: 10.1159/000477947. [DOI] [PubMed] [Google Scholar]
- 24.Ma W, Hu J, Cheng Y, Wang J, Zhang X, Xu M, et al. Ginkgolide B protects against cisplatin-induced ototoxicity: Enhancement of akt-nrf2-HO-1 signaling and reduction of NADPH oxidase. Cancer Chemother Pharmacol. 2015;75:949–59. doi: 10.1007/s00280-015-2716-9. [DOI] [PubMed] [Google Scholar]


