Abstract
Simple Summary
In Mexico, the poultry industry uses antibiotics to improve meat production through increased feed conversion, growth rate promotion, and disease prevention. Nevertheless, due to the negative effects of antibiotic overuse and abuse, alternative strategies are required. Probiotics, Prebiotics, and Synbiotics are used as feed additives to maintain health and performance status in poultry production and have become a common method in preventing various gut diseases, but the mechanisms of how these mixtures promote animal health are still unclear. This work studies whether a Synbiotic, besides modulating the gut microbiota, can modify the intestinal mucosa ultrastructure, and if this modification can promote health conditions without affecting zootechnical parameters in broilers infected with Salmonella Typhimurium and Clostridium perfringens. Our results show that broilers treated with the Synbiotic, whether infected with pathogens or not, had healthier intestinal mucosa. The Synbiotic mix promotes structural changes in the intestinal mucosa, which in turn promotes the capacity to resist intestinal infections caused by S. Typhimurium and C. perfringens in broilers.
Abstract
Synbiotics can prevent gastrointestinal infections in broilers. This work studies the effect of a Synbiotic on broilers. One-day-old male broilers were divided into groups: Control; Synbiotic; Synbiotic + S. Typhimurium; Synbiotic + C. perfringens; Synbiotic + S. Typhimurium + C. perfringens; S. Typhimurium; C. perfringens; and S. Typhimurium + C. perfringens. Histopathological analysis revealed that the Synbiotic promoted longer villi, less deep crypts, and better villi-crypt ratio. Broilers treated with the Synbiotic, infected with pathogens or not, had healthier mucosa. In groups infected with pathogens, the frequency and intensity of histopathologic lesions were lessened often in groups treated with the Synbiotic. The Synbiotic group had higher lactic acid bacteria counts than the Control group on day 39, and the isolation frequency of S. Typhimurium was lower (p < 0.05) in the Synbiotic-treated groups. On day 18, mucosa, villi, villi-crypt ratio, crypt, and feed intake were influenced by Enterobacteriaceae. However, on day 39 (end of the trial), those parameters were influenced by lactic acid bacteria. The Synbiotic influenced morphological modifications in the duodenal mucosa, which in turn gave the broilers the ability to resist infections caused by S. Typhimurium and C. perfringens, by inhibiting their growth and decreasing the intensity and frequency of histopathological injuries.
Keywords: concurrent colonization, probiotic, prebiotic, broiler, Salmonella Typhimurium, Clostridium perfringens, intestinal mucosa
1. Introduction
Salmonella enterica subsp. enterica serovar Typhimurium (S. Typhimurium) and Clostridium perfringens (C. perfringens) are bacteria frequently associated with poultry. S. Typhimurium is a gastrointestinal pathogen and a frequent cause of food poisoning worldwide, being one of the most commonly isolated serotypes in chicken-related foodborne outbreaks [1]. It can cause morbidity and mortality in humans and poultry [2], but it can also live in poultry as a transient member of the intestinal microbial population without causing disease. Sometimes its colonisation does not affect poultry body-weight gain or performance, and this asymptomatic infection can increase the likelihood of zoonotic transmission to humans through the food chain [3]. Likewise, C. perfringens is a member of the normal microbiota in healthy birds but can cause myonecrotic and gastrointestinal diseases in humans and livestock, as well as in birds, under certain conditions [4]. For example, the presence of C. perfringens in the intestinal tract of chickens raised for meat production (broilers), even at high numbers, is not sufficient to produce necrotic enteritis. However, predisposing factors like intestinal epithelium damage, infectious bursal disease virus, high dietary levels of poorly digestible proteins, indigestible polysaccharides, feeding regime alterations, microbiota disturbances, overcrowding, and a variety of management and climatic conditions are all favorable conditions in which to develop the disease [5,6,7]. Clinical necrotic enteritis is characterized by a sudden increase in flock mortality, often without premonitory signs. Its symptoms include diarrhea, depression, reluctance to move, ruffled feathers, somnolence, decreased appetite or anorexia, huddling, and, in some cases, dribbling from the beak, dehydration, detrimental growth rate, and feeding efficiency. Notably necrotic intestinal lesions occur in the jejunum and ileum, but also in the duodenum and ceca [8,9]. Outbreaks of necrotic enteritis are common in chickens at 2–6 weeks of age, following the wane of maternal antibodies prior to the maturity of the broiler’s own immune system [8].
Subclinical necrotic enteritis can persist in broiler flocks without clinical manifestation [9], causing chronic damage to the intestinal mucosa by developing mucosal ulcerations and peripheral hyperemia [8], which leads to a decrease in digestion, absorption, and weight gain, as well as an increased feed conversion ratio and a subsequent increase in economic costs [4].
The undesired consequences of both S. Typhimurium and C. perfringens are prevented and treated by the addition of antimicrobials to the feed. However, due to the emergence of microbes resistant to antibiotics used to treat human and animal infections, the European Union decided to phase out, and finally ban, the marketing and use of antibiotics as growth promoters in feed in 2006; and the United States of America adopted these policies in 2008 [10,11]. Since the ban on growth promoting antibiotics, a rise in the incidence of subclinical necrotic enteritis and salmonellosis has become a major problem in the poultry industry, along with the subsequent decrease in animal performance and the increase of feed conversion [3,12]. Therefore, poultry farmers are looking for alternatives to control and prevent diseases in broilers, through the addition of Probiotics, Prebiotics, and Synbiotics into feed and drinking water.
The Food and Agriculture Organization (FAO) and the World Health Organization (WHO) defined Probiotics as ‘live microorganisms that, when administered in adequate amounts, confer a health benefit on the host’ [13]. A variety of microbial species are used as Probiotics in broiler nutrition, including Lactobacillus, Streptococcus, Bacillus, Bifidobacterium, Enterococcus, Aspergillus, Candida, and Saccharomyces [14]. Prebiotics are generally defined as ‘nondigestible food ingredients that have a beneficial effect on the host by selectively stimulating the growth and/or activity of one or a limited number of bacterial species already established in the colon, and thus improving host health’ [13]. The most popular prebiotics are mannan oligosaccharides (derived from cell walls of Saccharomyces cerevisiae), β-glucans (derived from yeast or fungal cell walls), and fructans, inulin, levan, and the branched groups (extracted from different plants, hydrolyzed from polysaccharides, or produced by microorganism) [15]. Synbiotics are a combination of Probiotics and Prebiotics; they exhibit a synergistic relationship that positively affects the host by facilitating the implantation and survival of probiotic microorganisms in the gastrointestinal tract [16]. The use of Synbiotics in the poultry industry is based on their ability to balance the gut environment and its microbiota [17] by providing substrates for bacterial fermentation, generating antibacterial substances, competing for nutrients, modulating immune responses, and competing with pathogens for adhesion receptors on the intestinal epithelium [18]. Furthermore, Probiotics are able to modulate the intestinal permeability, mucosal immunity, and mucus layer, reducing gut permeability to molecules or bacteria and mucus degradation [14]. Based on the above, the aim of this work was to investigate the effect of a Synbiotic formulated with agave inulin as a prebiotic and Lactobacillus rhamnosus and Pediococcus acidilactici as Probiotics on duodenal morphology, content of lactic acid bacteria, and enterobacteria, as well as the growth performance in broilers of the COBBAvian48 line, infected with S. Typhimurium and C. perfringens.
2. Materials and Methods
2.1. Treatment Preparation
Fifty milliliters of a Synbiotic mix (provided by Kurago Biotek, Jalisco, Mexico) were administered via drinking water. Each dose (1 mL) contained 7 log CFU/g of Lactobacillus rhamnosus HN001 and Pediococcus acidilactici MA18/5M and 4.5% (0.045 g) of Agave tequilana fructans (Patent WO2017105186 A1).
Two pathogens were used: S. Typhimurium and C. perfringens. S. Typhimurium was isolated at our laboratory (from meat-food samples) and analyzed at the Mexican National Laboratory for Diagnosis and Epidemiological Reference for serotype identification according to the White–Kauffman scheme [19]. S. Typhimurium was subcultured, prior to broiler administration, in lactose broth with yeast extract and incubated for 24 h at 37 °C. C. perfringens ATCC 13124 was subcultured in thioglycolate broth and incubated for 24 h at 37 °C under anaerobic environment.
Pathogens were separated by centrifugation (thrice at 4000 g for 20 min) and washed in physiological saline solution (solution of NaCl 0.8% w/v). The pellets were suspended in physiological saline solution, and the number of bacteria in the suspension was calculated using a nephelometer (DensiCHEK, Model: OA009372, bioMérieux Inc, Missouri, MO, USA).
2.2. Bioassay for In Vivo Evaluation of the Synbiotic Mix
This research followed the guidelines of the Institutional Animal Care and Use Committee (IACUC) and was approved by the Bioethics Committee (CUCBA) of the University of Guadalajara (Permit Number: CINV.078/15). All surgery was performed under sodium pentobarbital anesthesia (PISA Agropecuaria, Mexico), and all efforts were made to minimize broilers’ suffering.
2.2.1. Housing
Two hundred and fifty-eight 1-d-old male broilers (Gallus gallus domesticus), line COBBAvian48 (free of growth-promoting antibiotics), were obtained from a local commercial hatchery (AVI–INC, Jalisco, Mexico) and housed in an experimental poultry shed. The surfaces of the experimental poultry shed were washed and sanitized with 200 ppm chlorine solution before the broilers arrived. Broilers were raised in stainless-steel pens (24 pens) to prevent contact between groups of birds (maximum stocking density of 30 kg/m2), distributed in an experimental poultry shed. They were maintained in continuous light conditions during the first two weeks, and 18 h light/6 h dark cycles for the rest of the experiment [20,21]. Room temperature was maintained at 33 ± 1 °C for the first 5 days, and then gradually reduced by 1 °C each day, until reaching 24 ± 1 °C, which was maintained for the rest of the experiment.
All experiments were performed using a randomized complete block design, and the blocking variables were the experimental unit (pen of broiler) and the sampling time. Broilers were individually weighed and randomly divided into eight treatment groups with three replicates of each one. Each replicate was assigned to a pen physically separated from the other two of the same replicates and randomly placed in different sections of the shed. The number of birds per treatment was determined according to [22], considering the number of samples (six samples for all the treatments and three pre-samples for Control and Synbiotic treatments), number of replicas per sample (three for each treatment), and the percentage of mortality per treatment [22]. The treatments were: (1) Control group (n = 43); (2) Synbiotic (n = 35); (3) Synbiotic mix + S. Typhimurium (n = 25); (4) Synbiotic mix + C. perfringens (n = 25); (5) Synbiotic mix + S. Typhimurium + C. perfringens (n = 25); (6) S. Typhimurium (n = 30); (7) C. perfringens (n=30); and (8) S. Typhimurium + C. perfringens (n = 45).
2.2.2. Feeding and Vaccination
All broilers were fed ad libitum with two antibiotic-free basal diets. The starter diet was administered until the broilers were 21 days old, and the grower-finisher diet until the end of the study (6 weeks). Both diets consisted of a sorghum and soybean base, following the nutritional requirements for COBB chicken Avian48s [21] (Table 1). The traditional scheme of vaccination against avian pox, Gumboro, and Newcastle diseases was administered, the broilers weren’t vaccinated against Salmonella or Clostridium.
Table 1.
Ingredients and bromatological composition of the basal diets administered to broilers.
| Start | Grower-Finisher | |
|---|---|---|
| Ingredients(g/Kg) | ||
| Sorghum | 640 | 690 |
| Soybean | 255 | 210 |
| Vitamin and mineral Premix | 80 | 70 |
| Sunflower oil | 25 | 30 |
| Bromatological composition (%) | ||
| Dry material | 94.91 | 90.5 |
| Humidity | 5.09 | 9.5 |
| Ashes | 7.10 | 6.3 |
| Crude protein | 21.91 | 18.6 |
| Ether extract | 6.02 | 6.13 |
| Crude fiber | 2.90 | 3.1 |
| Nitrogen free elements | 56.98 | 56.37 |
| Calcium | 0.98 | 0.74 |
| Phosphorus | 0.48 | 0.40 |
| Metabolizable Energy (Mcal/kg) | 3.08 | 3.23 |
2.2.3. Oral Administration of the Synbiotic Mix and Pathogens
The Synbiotic mix was administered in drinking water the same day broilers arrived, following the manufacturer’s instructions (open and administer birds orally). The water containers with the mix were available for 2 h, based on the average water consumption of a one-day-old broiler of, which is 1.12 mL per hour [23]. For treatment groups 3 to 8, pathogens were administered on day 17 [24], where broilers were orally challenged with 5 log CFU of S. Typhimurium and/or 3 log CFU of C. perfringens per bird through their drinking water [25]. To calculate pathogen intake, we considered that, at 17 days old, each bird consumes 25 mL of water per hour, and, therefore, water containers with the pathogen were available for 2 h [23]. There were no other drinking troughs available during the administration of the treatments (Synbiotic and pathogenic microorganisms); technicians also walked around the house to encourage drinking [26].
Birds were kept under constant observation for any sign or symptom of Salmonella infection or subclinical necrotic enteritis (fever, huddling, diarrhea, dejection, ruffled feathers, closed eyes, loss of appetite, and thirst).
2.2.4. Slaughter and Collection of Samples
Six sampling times were scheduled with three replicas from each treatment at 18, 22, 25, 32, 36, and 39 days of life. In addition, three pre-samples were taken on broilers from Control and Synbiotic groups (at 4, 8, and 15 days of life) to know the state of its duodenal morphology, the intestinal content of Lactic Acid Bacteria and Enterobacteriaceae, and the absence or presence of S. Typhimurium and C. perfringens prior to the inoculation of pathogenic microorganisms. These pre-samples were not considered for statistical analysis.
Broilers were euthanized by intraperitoneal injection of 3 mL/2.5 kg of 6.3% sodium pentobarbital (PISA Agropecuaria, Mexico). Subsequently, broilers were eviscerated, and their gastrointestinal tracts were removed under aseptic conditions. Duodenum and caeca contents were transferred into sterile containers for microbiological analysis, and duodenum tissue samples of approximately 2 cm in length were taken for histological analysis.
2.3. Duodenal Morphology Evaluation
Duodenum tissue samples were preserved in neutral 10% formalin and processed by the conventional paraffin inclusion method. Five-microns-thick samples were stained with hematoxylin and eosin and observed with a 4× panoramic objective (optical microscope model E200 LED, Nikon, Tokyo), and the images were analyzed using the software Motic Images Plus 2.0 (Motic, Hong Kong). Mucosal thickness, villi height, and crypt depth of nine randomly selected villi per bird group were also measured. Mucosal thickness was measured from the lamina propria to the apex of the villi, while the villi height was measured from the apex of the villi to the villi crypt junction, and the crypt depth was defined as the invagination depth between adjacent villi. Once the measurements were obtained, the villi-crypt ratio (VCR) was calculated by dividing the length of the villi by the depth of the crypt [27,28]. A qualitative analysis was also carried out using the objectives 4×, 10×, and 40×.
2.4. Microbiological Isolation and Enumeration
Lactic Acid Bacteria was calculated by the spread plate technique from duodenal content on Difco Lactobacilli MRS Agar plates (Cat. No. 288210, BD, Franklin Lakes, NJ, USA) and incubated at 35 °C for 36 to 48 h in a microaerobic environment. At the end of the incubation period, the colony forming units (CFU) were enumerated. Three to five colonies showing typical morphology and biochemical tests (Gram stain, catalase, and oxidase activity) were analyzed on the API 50CHL identification system (Cat. No. 50410, bioMérieux, France) to determine Lactic Acid Bacteria species [29]. Enterobacteriaceae were also enumerated by the surface extension technique from ceca content, plated on Petrifilm Enterobacteriaceae Count Plates (Cat. No. 70-2006-7092-8, 3M, MN) and incubated at 35 °C for 24 h. The isolation of S. Typhimurium and C. perfringens was also evaluated qualitatively. S. Typhimurium isolation was from ceca contents, using buffered peptone water (Cat. No. DF1810-17-9, BD, NJ) as a pre-enrichment solution, Tetrathionate broth base (Cat. No. 210430, BD) and Rappaport-Vassiliadis R10 (Cat. No. 218581, BD) as selective enrichment, and XLT4 Agar base (Cat. No. 223420, BD) and HE Agar (Cat. No. 254009.08, BD) for isolation. From each agar plate (XLT4 and HE Agar), three to five colonies were tested on triple-sugar-iron agar (Cat. 221038, BD), lysine-iron agar (Cat. 284920, BD), citrate agar (Cat. L007504, BD), mobility-indole-ornithine agar (Cat. 221518, BD), and urea broth (Cat. 51463-500G, Merck, Germany). Isolates showing typical Salmonella biochemical reactions were streaked on Tryptic Soy Agar (Cat. No. 236940, BD) and tested for slide agglutination using polyvalent serum (A-Vi Difco, Franklin Lakes, NJ) [30]. C. perfringens was also isolated from ceca contents, added to 20 mL of NIH Thioglycollate broth (Cat. No. 225710, BD), heated at 70 °C for 20 min in a water bath, and incubated in an anaerobic jar with a GasPack (Cat. No. 260626, BD) at 35 °C for 24 h. After incubation, an aliquot was streaked onto Tryptone-Sulfite-Cycloserine agar (TSC) (Cat. 111972, Merk) and incubated at 35 °C for 36 to 48 h in an anaerobic environment. Typical colonies were subjected to biochemical tests, Gram staining, oxidase (Cat. 261181, BD) and catalase activity (Cat. 131510, Jaloma, Mexico), Triple Sugar Iron test (Cat. 221038, BD), and milk fermentation, to confirm the presence of C. perfringens [31].
2.5. Growth Performance
The body weight (BW) of each bird was measured weekly, and feed intake (FI) was assessed daily by averaging the intake of each pen among the broilers present in the pen. Feed conversion ratio (FCR) was calculated as the ratio of feed consumed to weight gained, as a measure of how efficiently a bird uses energy and nutrients from the feed for growth. Flocks with a lower FCR were considered better performers [32].
2.6. Statistical Analysis
Data analysis (except frequency of pathogens) was examined by ANOVA or MANOVA and Tukey test. Frequency of Lactobacillus species and pathogens was analyzed using the non-parametric chi-squared test. Principal component analysis (PCA) and hierarchical cluster analysis (HCA) were carried out to obtain correlations between all variables (mucosal thickness, villi height, crypt depth, villi-crypt ratio, body weight, feed intake, Lactic Acid Bacteria, and Enterobacteriaceae) and estimate the relationships between broiler groups (Control, Synbiotic, Synbiotic + S. Typhimurium, S. Typhimurium, Synbiotic + C. perfringens, C. perfringens, Synbiotic + S. Typhimurium + C. perfringens, S. Typhimurium + C. perfringens). All data were analyzed with Statistica 10 (TIBCO Statistica, Palo Alto, CA). Measurements were performed in triplicate. Results obtained were presented as means ± standard deviations (SD), and a p value less than 0.05 (p < 0.05) was considered significant where relevant.
3. Results
The effect of a Synbiotic on the duodenal morphology, gut microbiota, and growth performance in broilers infected with S. Typhimurium and C. perfringens was investigated. At the end of the bioassay, no broiler died from S. Typhimurium and/or C. perfringens infection. Nevertheless, signs related to the colonization of these pathogens were observed. The broilers of the groups S. Typhimurium and S. Typhimurium + C. perfringens presented fever, huddling, diarrhea, dejection, loss of appetite, and thirst. These symptoms did not show up in the Synbiotic + S. Typhimurium and Synbiotic + S. Typhimurium + C. perfringens groups after day 22. Broilers of C. perfringens group presented huddling, diarrhea, and dejection until day 25. The only sign of Synbiotic + C. perfringens group was huddling, and it was present until day 22.
3.1. Duodenal Morphology Differs between Broilers Fed Synbiotic Mix
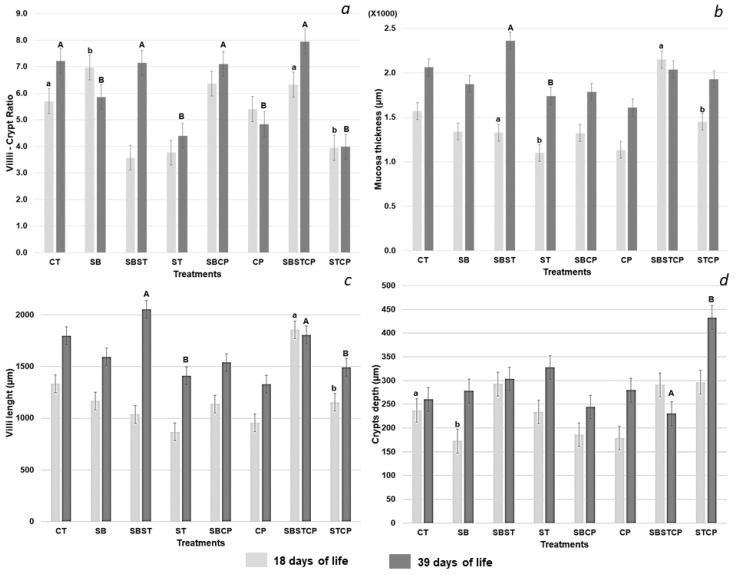
Duodenal morphology of the broilers, based on the VCR, villi length, crypt depth, and mucosa thickness, was evaluated on day 18 (24 h after inoculation with C. perfringens and S. Typhimurium) and on day 39. The average value of the VCR in the pre-samplings was 3.9 ± 0.9 for birds in the Control group and 5 ± 1.2 for those in the Synbiotic group. The VCR of the Synbiotic group was significantly higher (p < 0.05) compared to the Control group on day 18 (average of 7 ± 0.47 vs. 5.7 ± 0.47); however, at the end of the experiment (day 39), the VCR of the Control group was higher than the Synbiotic group’s (average of 7.2 ± 0.47 vs. 5.9 ± 0.47). On day 39, in groups inoculated with the pathogens, the VCR was higher (p < 0.05) in groups treated with the Synbiotic mix when compared to the S. Typhimurium group (average of 7.1 ± 0.47 vs. 4.4 ± 0.47), the C. perfringens group (average of 7.1 ± 0.47 vs. 4.8 ± 0.47), and the S. Typhimurium + C. perfringens group (average of 7.9 ± 0.5 vs. 4 ± 0.47) (Figure 1a) (File S2).
Figure 1.
Effect of dietary supplementation with a Synbiotic mix on duodenum morphology of broilers challenged with Salmonella Typhimurium and Clostridium perfringens. (a) Villi–crypt ratio; (b) mucosa thickness (µm); (c) villi length (µm); and (d) crypts depth (µm). CT: non-challenged Control group; SB: Synbiotic; SBST: Synbiotic + S. Typhimurium; ST: S. Typhimurium; SBCP: Synbiotic + C. perfringens; CP: C. perfringens; SBSTCP: Synbiotic + S. Typhimurium + C. perfringens; STCP: S. Typhimurium + C. perfringens. Means in the same row with different superscripts (a–b or A–B) differ (MANOVA p < 0.05).
Mucosal thickness of the broilers in the Synbiotic + S. Typhimurium group was significantly thicker (p < 0.05) when compared to the S. Typhimurium group on day 18 (average of 1328 ± 95.21 μm vs. 1100 ± 95.21 μm) and on day 39 (average of 2359 ± 95.21 μm vs. 1738 ± 95.21 μm) and to the S. Typhimurium + C. perfringens group at day 18 (average of 2148 ± 95.21 μm vs. 1450 ± 95.21 μm) (Figure 1b). The average value of mucosa thickness in pre-samples was 735 ± 191.3 μm in the Control group and 623 ± 61.3 μm in the Synbiotic group (Figure 1b) (File S2).
Villi length average in pre-samples was 594 ± 91.7 μm in the Control group and 528 ± 50.8 μm in the Synbiotic group. Villi length following Synbiotic treatment was significantly longer (p < 0.05) in groups inoculated with the pathogens compared to respective Controls on day 39 of treatment in both the S. Typhimurium (average of 2055 ± 85.79 μm vs. 1410 ± 85.79 μm), and S. Typhimurium + C. perfringens (average of 1807 ± 85.79 μm vs. 1494 ± 85.79 μm) groups (Figure 1c) (File S2).
The average of crypt depth in pre-samples was 140 ± 20.2 μm for broilers in the Control group and 95 ± 17.8 μm in the Synbiotic group. Crypt depth following Synbiotic treatment was smaller compared to respective Control, in the S. Typhimurium + C. perfringens group on day 39 (230 ± 24.98 μm vs. 433 ± 24.98 μm) (Figure 1d) (File S2).
There were no significant differences observed in any of the morphological features mentioned above between the C. perfringens and Synbiotic + C. perfringens groups (Figure 1a–d) (File S2).
No histopathological lesions were found in the non-pathogen Control or Synbiotic groups (Figure 2A,C). Broilers treated with S. Typhimurium showed multifocal epithelium hyperplasia with mucosa degeneration (Figure 2B), lymphocyte infiltration, and congested villi from day 22 until the end of the experiment, but those broilers receiving concurrent Synbiotic treatment showed no sign of lesions on day 32. Similarly, broilers treated with C. perfringens showed lymphocyte infiltration (Figure 2D), hemorrhagic villi (Figure 2E), and discreet multifocal necrosis of the mucosa from day 25 until the end of the experiment, whereas broilers receiving concurrent Synbiotic treatment showed no sign of lesions from day 32 and no sign of mucosal necrosis. Broilers receiving S. Typhimurium + C. perfringens exhibited all the above described lesions, as well as congested villi (Figure 2F), a feature not observed with S. Typhimurium or C. perfringens treatment alone, from day 22 until the end of the experiment. Broilers receiving concurrent Synbiotic treatment only exhibited these lesions on day 22 and 25, with no hemorrhagic villi observed.
Figure 2.
Histopathological lesions in duodenum of broilers challenged with Salmonella Typhimurium and Clostridium perfringens. (A) Epithelium without apparent lesions (4×), (B) calciform cells hyperplasia (10×), (C) villi without apparent lesions (10×), (D) lymphocyte infiltration (40×), (E) hemorrhagic villi (10×), and (F) congested villi (10×).
3.2. Microbiological Isolation and Enumeration from Intestinal Contents of Broilers Challenged with S. Typhimurium and C. Perfringens
Lactic Acid Bacteria were isolated from the duodenum, and the average load in the pre-samples (4, 8, and 15 days of life) was 5.3 ± 1.1 log CFU/g for the Control group and 5 ± 1.4 log CFU/g for the Synbiotic group. No significant differences (p > 0.05) in Lactic Acid Bacteria counts were observed among treatments until day 36, when broilers infected with S. Typhimurium had a significantly lower Lactic Acid Bacteria count (p < 0.05) (4.6 ± 1 log CFU/g) than in other groups (Synbiotic <6.5 ± 0.7 log CFU/g>, Synbiotic + C. perfringens <6.3 ± 0.1 log CFU/g> and Synbiotic + S. Typhimurium + C. perfringens <6.5 ± 0.1 log CFU/g>), and on day 39, significantly higher counts (p < 0.05) were observed in the Synbiotic group (6.5 ± 0.4 log CFU/g) compared to the Control group (4.1 ± 1.6 log CFU/g), the Synbiotic + S. Typhimurium group (3.9 ± 0.3 log CFU/g), and the Synbiotic + C. perfringens group (3.9 ± 0.3 log CFU/g) (Table 2) (File S1).
Table 2.
Effect of dietary supplementation with a Synbiotic mix on duodenal Lactic Acid Bacteria counts of broilers challenged with Salmonella Typhimurium and Clostridium perfringens.
| Mean Lactic Acid Bacteria (Log10 CFU/g) ± Standard Deviation | ||||||
|---|---|---|---|---|---|---|
| Treatment 1/Days | 18 d | 22 d | 25 d | 32 d | 36 d | 39 d |
| CT | 5.8 ± 0.7 | 6.3 ± 0.4 | 5.8 ± 1.7 | 5.3 ± 0.9 | 5.6 ± 0.4 ab | 4.1 ± 1.6 a |
| SB | 5.0 ± 1.9 | 6.2 ± 1.0 | 5.6 ± 1.3 | 5.2 ± 1.3 | 6.5 ± 0.7 a | 6.5 ± 0.4 b |
| SBST | 6.7 ± 0.3 | 5.6 ± 0.4 | 5.1 ± 1.2 | 4.4 ± 1.0 | 5.5 ± 0.2 ab | 3.9 ± 0.3 a |
| ST | 5.0 ± 1 | 5.3 ± 1 | 4.0 ± 0.6 | 4.5 ± 1.2 | 4.6 ± 1.0 b | 5.4 ± 0.8 ab |
| SBCP | 4.7 ± 1.6 | 4.9 ± 1.6 | 3.7 ± 0.8 | 5.9 ± 0.2 | 6.3 ± 0.1 a | 3.9 ± 0.3 a |
| CP | 5.9 | 5.3 | 4.2 ± 1.9 | 4.5 ± 1.2 | 5.2 ± 0.3 ab | 5.4 ± 0.3 ab |
| SBSTCP | 4.4 ± 0.7 | 6.0 ± 0.7 | 4.9 ± 0.4 | 5.0 ± 0.8 | 6.5 ± 0.1 a | 5.2 ± 0.6 ab |
| STCP | 4.8 ± 1.6 | 5.7 ± 1.6 | 4.8 ± 0.8 | 4.2 ± 0.2 | 5.2 ± 0.1 ab | 4.9 ± 0.3 a |
1 CT: Control group; SB: Synbiotic mix; SBST: Synbiotic mix + S. Typhimurium; ST: S. Typhimurium; SBCP: Synbiotic mix + C. perfringens; CP: C. perfringens; SBSTCP: Synbiotic mix + S. Typhimurium + C. perfringens; STCP: S. Typhimurium + C. perfringens. Mean values of three replicates with different letter (a,b) in the same column are significantly different (p < 0.05).
A total of 110 strains of Lactic Acid Bacteria were identified from broiler groups both with and without Synbiotic treatment. Eight Lactobacillus species (L. delbrueckii, L. fermentum, L. acidophilus, L. brevis, L. crispatus, L. plantarum, L. lactis, and L. salivarius) were found in all treatment groups. In treatments with the Synbiotic mix, L. rhamnosus and L. curvatus were present. In broilers without the Synbiotic mix, only L. mesenteroides, L. pentosus, and L. buchneri were found.
Enterobacteriaceae were isolated from the ceca content of the broilers. Enterobacteriaceae counts across the Synbiotic group were lower than that of the Control group, but this did not reach statistical significance until day 32, where the Control (8.1 ± 0.3 log CFU/g) and S. Typhimurium (8.1 ± 0.1 log CFU/g) groups exhibited higher (p < 0.05) counts compared to the other groups (6–7.9 ± 0.4 log CFU/g). The C. perfringens group presented the lower counts (6 ± 0.3 log CFU/g), with similar trends observed until the end of the experiment (File S1).
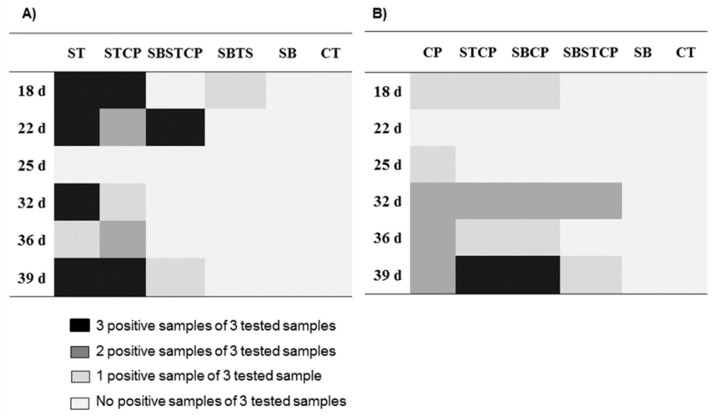
The isolation of the inoculated S. Typhimurium and C. perfringens was investigated in the cecal contents in groups inoculated with the pathogens, treated or non-treated with the Synbiotic mix. There were no S. Typhimurium or C. perfringens isolates in the samples taken before the administration of the pathogens. The isolation of the inoculated S. Typhimurium was significantly lower (p < 0.05) in the Synbiotic treatment groups (Synbiotic + S. Typhimurium and Synbiotic + S. Typhimurium + C. perfringens) compared to the S. Typhimurium, C. perfringens, and S. Typhimurium + C. perfringens groups. There was no difference in the isolation of the inoculated C. perfringens between groups treated or untreated with the Synbiotic mix (p > 0.05) (Figure 3) (File S1).
Figure 3.
Effect of dietary supplementation with a Synbiotic mix on the isolation of the inoculated Salmonella Typhimurium and Clostridium perfringens from ceca contents of broilers. (A) Isolation of the inoculated Salmonella Typhimurium and (B) isolation of the inoculated Clostridium perfringens. CT: non-challenged Control group; SB: Synbiotic; SBST: Synbiotic + S. Typhimurium; ST: S. Typhimurium; SBCP: Synbiotic + C. perfringens; CP: C. perfringens; SBSTCP: Synbiotic + S. Typhimurium + C. perfringens; and STCP: S. Typhimurium + C. perfringens.
3.3. Effect of the Synbiotic on Growth Performance
Body weight (BW), feed intake (FI), and feed conversion ratio (FCR) were also evaluated every week during the study (6 weeks). There was no significant difference in body weight with or without Synbiotic supplementation in birds (p > 0.05) (Table 3). Likewise, feed intake and FCR did not differ between treatment groups (File S3).
Table 3.
Effect of dietary supplementation with a Synbiotic mix on the body weight of birds challenged with Salmonella Typhimurium and Clostridium perfringens.
| Treatment 1 Days |
Mean Body Weight (g) ± Standard Deviation | ||||||
|---|---|---|---|---|---|---|---|
| 1 d | 7 d | 14 d | 21 d | 28 d | 35 d | 42 d | |
| CT | 43 ± 2.9 | 147 ± 11.8 | 424 ± 35.8 | 738 ± 74.2 a | 1148 ± 125.2 | 1734 ± 199.2 | 2416 ± 330 |
| SB | 44 ± 2.9 | 162 ± 13.2 | 432 ± 31.4 | 799 ± 54.5 | 1175 ± 70 | 1848 ± 93.7 | 2550 ± 26.4 |
| SBST | 44 ± 4.3 | 162 ± 2 | 448 ± 50.3 | 779 ± 96.6 | 1185 ± 121.8 | 1740 ± 197 | 2588 ± 106.8 |
| ST | 44 ± 3.1 | 152 ± 13.9 | 437 ± 63.2 | 747 ± 152.6 | 1071 ± 236.4 | 1836 ± 185.7 | 2491 ± 168.2 |
| SBCP | 44 ± 3.3 | 164 ± 18.5 | 460 ± 52.8 | 847 ± 115.8 b | 1306 ± 83.3 | 1969 ± 175.6 | 2693 ± 135 |
| CP | 44 ± 3.1 | 161 ± 12.4 | 465 ± 40.4 | 800 ± 70.3 | 1271 ± 114.2 | 1936 ± 218.7 | 2696 ± 360 |
| SBSTCP | 44 ± 2.7 | 153 ± 12.2 | 440 ± 39.6 | 812 ± 71.5 | 1206 ± 142.7 | 1860 ± 204.3 | 2295 ± 210.7 |
| STCP | 46 ± 4.6 | 155 ± 16.9 | 438 ± 51.2 | 794 ± 96.4 | 1211 ± 166.5 | 1780 ± 147 | 2453 ± 182.6 |
1 CT: Control group; SB: Synbiotic mix; SBST: Synbiotic mix + S. Typhimurium; ST: S. Typhimurium; SBCP: Synbiotic mix + C. perfringens; CP: C. perfringens; SBSTCP: Synbiotic mix + S. Typhimurium + C. perfringens; and STCP: S. Typhimurium + C. perfringens. Mean values of three replicates with different letter (a,b) in the same column are significantly different (p < 0.05).
3.4. Principal Component Analysis
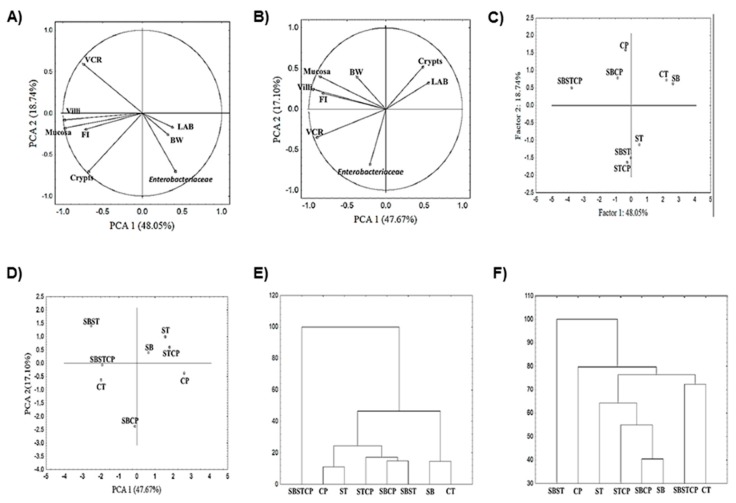
Principal component analysis (PCA) was applied to determine any pattern recognition between variables and treatments in addition to a comparison between the behavior of variables in broilers after 24 h of pathogenic administration (day 18) and at the end of trial (day 39). Principal components (PC) 1 and 2 had a high percentage of the total variance at 48% and 19%, respectively, on day 18, and 48% and 17%, respectively, on day 39. Parameters are presented in Figure 4A,B and the graph location of working groups are shown in Figure 4C,D. Thus, in a scatter plot of the parameter score values projected in the PC1 and PC2 planes (day 18), mucosa (−0.97), villi (−0.98), VCR (−0.74), crypt (−0.67) and feed intake (−0.71) were influenced by the presence of Enterobacteriaceae (0.41), proceeding from negative to positive values of PC1 (Figure 4A). On day 39, mucosa (−0.85), villi (−0.94), VCR (−0.89) and feed intake (−0.71) were influenced by the presence of Lactic Acid Bacteria (0.55), proceeding from negative to positive values of PC1 (Figure 4B). As indicated in Figure 4C,D, PCA can distinguish between broiler groups with or without Synbiotic treatment at both time-points. PC1 and PC2 showed a separation between broiler groups. According to the coordinate’s factor, on day 18, Control (2.20) and Synbiotic mix (2.60) have a closer relationship. Synbiotic mix + S. Typhimurium + C. perfringens (−3.75) showed a higher difference from both Control and Synbiotic mix compared to Synbiotic mix + S. Typhimurium (−0.04), Synbiotic mix + C. perfringens (−0.86), S. Typhimurium (0.49), C. perfringens (−0.37) and S. Typhimurium + C. perfringens (−0.26). On day 39, Control (−1.99) and Synbiotic mix + S. Typhimurium + C. perfringens (−1.91) exhibited a close relationship and were different with respect to S. Typhimurium (1.54), S. Typhimurium + C. perfringens (1.77) and C. perfringens (2.59), proceeding from negative to positive values of PC1 in both dates.
Figure 4.
Principal component analysis (PCA) plots of broilers treated with a Synbiotic mix. (A) Location of different variables at 18 days; (B) location of different variables at 39 days; (C) location of different treatments at 18 days; (D) location of different treatments at 39 days; (E) dendrogram of hierarchical cluster analysis at 18 days; (F) dendrogram of hierarchical cluster analysis at 39 days. BW: body weight, VCR: villi-crypt ratio, FI: feed intake, LAB: Lactic Acid Bacteria. CT: non-challenged Control group; SB: Synbiotic mix; SBST: Synbiotic mix + S. Typhimurium; ST: S. Typhimurium; SBCP: Synbiotic mix + C. Perfringens; CP: C. Perfringens; SBSTCP: Synbiotic mix + S. Typhimurium + C. Perfringens; and STCP: S. Typhimurium + C. Perfringens.
Additionally, hierarchical cluster analysis (HCA) was carried out and is displayed as a dendrogram in Figure 4E,F. This analysis indicated that the broilers within the same group are more like each other than samples in different groups. On day 18, Synbiotic mix + S. Typhimurium + C. Perfringens (first cluster) is clearly discernible from the other groups. A second cluster consists of C. Perfringens, S. Typhimurium, S. Typhimurium + C. Perfringens, Synbiotic mix + C. Perfringens, and Synbiotic mix + S. Typhimurium. The third cluster includes Synbiotic mix and Control. On day 39, differences in cluster grouping were observed. The first cluster is for Synbiotic mix + S. Typhimurium; the second cluster includes C. Perfringens, S. Typhimurium, S. Typhimurium + C. Perfringens, Synbiotic mix + C. Perfringens and Synbiotic mix; and the third cluster consists of Synbiotic mix + S. Typhimurium + C. Perfringens and Control.
4. Discussion
The presence of C. perfringens and S. Typhimurium in broilers can cause significant economic losses. These bacteria have traditionally been controlled using antibiotics, but due to the negative effects of antibiotic overuse and abuse, alternative strategies are required. Probiotics, Prebiotics, and Synbiotics are used as feed additives to maintain health and performance status in poultry production and have become a common method in preventing various gut diseases [33]. L. rhamnosus is one of the most widely used Probiotic strains, has the potential to be a good Probiotic choice thanks to its strong adhesive capacity, and is also able to enhance immunity [34,35,36]. It was shown to protect animals against gastrointestinal pathogens like E. coli O157:H7 [37] and S. Typhimurium [38]. P. acidilactici exert antagonism against other microorganisms like Clostridium spp. and Salmonella, primarily through the production of lactic acid, in addition to the production of antimicrobial peptides known as pediocins [39,40,41]. Fructans enhances the population of beneficial bacteria, such as bifidobacteria and lactobacilli, and suppresses levels of pathogenic bacteria, such as C. perfringens and E. coli, in the intestine of broilers [15]. Agave tequilana Fructans promotes the growth of probiotic bacteria such as Lactobacillus salivarius and Enterococcus faecium, and its Prebiotic effect surpasses chicory inulin [42].
In the current study, the intestinal tract mucosa of broilers infected with S. Typhimurium and C. perfringens and treated with the Synbiotic formulation was healthier, with higher VCR than their controls (S. Typhimurium, C. perfringens, and S. Typhimurium + C. perfringens groups). Colonization with the probiotic Lactobacillus rhamnosus promoted these ultrastructural changes by increasing epithelial cell turnover regulation [43]. Alternatively, the combination of the prebiotic (A. tequilana fructans) with Lactic Acid Bacteria in the intestine, could facilitate the implantation of other native Lactic Acid Bacteria species with a protective effect [44]. Some authors have reported that Probiotics such as L. johnsonii BSNE [45] and Synbiotics like E. faecium DSM 3530 and chicory prebiotic [46], or B. Subtilis and xylooligosaccharide [47] can enhance intestinal development.
It is known that shorter villi and deeper crypts are associated with the presence of bacterial toxins as the mucosa attempts to restore the epithelial cells affected by constant damage [48]. Concurrently, we found these shorter villi and deeper crypts in the groups infected with pathogens compared to the Control group, but not in pathogen groups receiving Synbiotic treatment.
The histological examination of the duodenum revealed lesions relating to subclinical necrotic enteritis and Salmonella colonization (epithelium hyperplasia, mucosa degeneration, lymphocyte infiltration, congested, and hemorrhage villi) in groups inoculated with the pathogens, but the frequency and intensity of lesions were lower in the Synbiotic-treated groups when compare to respective Controls. Our results indicate that the ultrastructural changes promoted by the Synbiotic mix could increase the resistance capacity of broilers to intestinal infections caused by S. Typhimurium and C. perfringens, as reported by Stanley et al. (2012) [49].
Host responses to infectious agents are often regulated through phosphorylation [50], and Prebiotics may impact host signaling affecting mucosal inflammation, independently of the presence of microbes [51]. Prebiotics were also shown to directly mediate changes in barrier function [52]. Synbiotics prevent infections by stimulating the microbiota of the host [53].
Lactic Acid Bacteria can alter gut dynamics and physiologic processes related to intestinal functions [18]. They improve bird nutrition by helping with the digestion process and synthesizing nutrients, which also stimulates the intestinal epithelium and reduces intestinal diseases by preventing the colonization of pathogenic microorganisms.
In our study, birds treated with the Synbiotic mix tended to show higher Lactic Acid Bacteria counts than those not treated (challenged or not with pathogens). However, these differences were not significant until 39 days of age, when birds of the Synbiotic group showed a higher Lactic Acid Bacteria count (6.5 log10 CFU/g) than the Control group (4.1 log10 CFU/g), which is comparable to a previous study, where counts of 6.68 log10 CFU/g were recorded in the ileum content of broilers treated with a commercial Synbiotic mix [54].
The fact that no differences were found prior to day 39 can be related to the development and natural establishment of the intestinal microbiota of broilers. It was reported that the microbial community of the small intestine is abundant in fecal streptococci and coliforms for the first 40 days of life and then lactobacilli become established and dominant [18,55]. Furthermore, the timing of probiotic administration may influence the beneficial effect. Nakphaichit et al. [56] administered Lactobacillus reuteri during the first week post-hatch, and, as in this study, they found no significant effects on day 18. However, on day 42, delayed effects were shown through the increase in diversity and abundance of Lactobacillus. Finding higher Lactic Acid Bacteria counts before 40 days of life in the Synbiotic group suggested that Synbiotic treatment introduced the conditions for enrichment of Lactobacillus species and modification of intestinal microbiota of broilers.
A broiler’s intestinal microbiota is composed of three major genera, Lactobacillus, Clostridium, and Bacteroides. A balanced intestinal microbiota and high microbial diversity (especially in lactobacilli) enhance resistant to infection [32,56,57]. Lactobacillus are sensitive to stress and tend to decrease when a bird is under stress, disturbing the microbiota balance and facilitating the development of gastrointestinal infections. Synbiotics selectively stimulate the growth and/or activity of those Lactobacillus [58,59].
In our study, L. rhamnosus and L. curvatus were only found in broilers treated with the Synbiotic mix, while L. pentosus, L. buchneri, and L. mesenteroides were found exclusively in broilers that did not receive the Synbiotics. L. rhamnosus improves growth performance, meat quality, ammonia emission, and intestinal microbiota in chickens [60]. Lactobacillus curvatus display antimicrobial activity against Klebsiella, E. coli, and Staphylococcus aureus [61]. Lactobacillus plantarum, which is associated with weight loss, and L. acidophilus and L. fermentum, which are associated with weight gain, were isolated from both broiler groups [62].
Probiotics and Prebiotics can influence the intestinal microbiota [15,63]; therefore, to evaluate the effect of the Synbiotic mix on the Gram-negative gut microbiota of broilers, Enterobacteriaceae counts were determined. However, these remained relatively constant throughout the six-week trial period, which is similar to a previous study where differences were observed only after 35 days [59].
The control of Salmonella is one of the major tasks in poultry production to ensure food safety, and the modulation of intestinal microbiota with Prebiotics, Probiotics, and Synbiotics in broilers has reduced farms contaminated with Salmonella [64]. In this study, the isolation of the inoculated S. Typhimurium was significantly lower in the group receiving S. Typhimurium and the Synbiotic mix, compare to S. Typhimurium alone. Previous research has shown a reduction of 58% of Salmonella in birds fed with B. subtilis [3], as well as a decreased in Salmonella numbers in the large and small intestine and in feces [65].
C. perfringens represents a hazardous risk to a broiler’s health when it is present in its small intestine. Its growth in the gastrointestinal tract depends on favorable conditions and subsequently extends pathogenicity [66]. During this study, C. perfringens was recovered from the ceca contents of inoculated broilers 24 hours post-inoculation and throughout the trial (39 days), but it was not recovered in non-inoculated groups. Not all broilers from which the pathogen was recovered showed signs of subclinical necrotic enteritis. However, it was reported that there is no direct relationship between the number of C. perfringens positive cells present in the gastrointestinal tract and the development of the disease [9,67].
The alteration of the microbiota, especially the decrease in the number of Lactic Acid Bacteria, could help in the development of the disease [68]. Qing et al. proved that Lactobacillus johnsonii BS15 can prevent subclinical necrotic enteritis caused by C. perfringens through adjusting the intestinal microbiota composition [45]. Wang et al. concluded that feed supplementation with L. johnsonii BS15 may prevent subclinical necrotic enteritis by the enhancement of small intestinal immunity [69].
It was demonstrated that the use of additives in the diet of broilers affects the intestinal microbial balance and subsequently improves the growth performance and reduces the mortality rate [70]. In the present study, no differences in the body weight of the broilers were found (p > 0.05). Weight gain discrepancies may be due to the use of diverse strains of Probiotics, different prebiotic fibers, environmental conditions, and the genetic lines of the chickens. Furthermore, Synbiotic additives can be more effective under stress conditions, extreme temperatures, crowding, and poor management conditions [71], which were avoided during this study.
Multivariate tools such as Principal Components Analysis and Hierarchical Cluster are used in poultry research. These uses include analyzing performance and measuring carcass traits [72,73]; measuring morphostructural traits [74]; observing how the laying cycle can be divided and what the relationships of the breeding values of egg production are between the partial periods and the total period [75]; and comparing rep-PCR genomic fingerprinting methods for differentiation of fecal Escherichia coli from humans, poultry, and wild birds [76]. However, to our knowledge, this is the first time that it has been used to evaluate the effect of a Synbiotic mix on broilers’ health and growth.
Our analysis revealed different patterns of variables and treatments on day 18 and 39. On day 18, mucosa thickness, villi, crypt depth, villi-crypt ratio, and feed intake were influenced by the presence of Enterobacteria, whereas at the end of the trial, the same parameters were influenced by the Lactic Acid Bacteria. According to Cisek and Binek [77], the intestinal microbiota affects, either negatively or positively, intestinal maturation and development.
The hierarchical cluster analysis grouped the broilers from the different treatments into three clusters on day 18 and three on day 39. On day 18, the broilers of the cluster Synbiotic mix + S. Typhimurium + C. Perfringens were characterized by showing the thickest intestinal mucosa, followed by the cluster formed by Synbiotic mix and Control, and finally the cluster formed by C. Perfringens, S. Typhimurium, S. Typhimurium + C. Perfringens, Synbiotic mix + C. Perfringens, Synbiotic mix + S. Typhimurium groups, whose mucosa was the thinnest compared to that of the two previous clusters. On day 39, the grouping of the clusters was different, being the one formed by Synbiotic mix + S. Typhimurium the one with the thickest mucosa, followed by the cluster Synbiotic mix + S. Typhimurium + C. Perfringens and Control, and the cluster with the thinnest mucosa was formed by C. Perfringens, S. Typhimurium, S. Typhimurium + C. Perfringens, and Synbiotic mix + C. Perfringens and Synbiotic mix. Probiotics are regarded as modifying agents of the intestinal-wall thickness due to the resulting elimination of harmful bacteria [59].
5. Conclusions
The Synbiotic composed of Lactobacillus rhamnosus HN001, Pediococcus acidilactici MA18/5M, and Agave tequilana fructans influenced morphological modifications (longer villi and less-deep crypts) in the duodenal mucosa, which in turn gave the broilers the ability to resist infections caused by S. Typhimurium and C. perfringens, by inhibiting the growth of S. Typhimurium and decreasing the intensity and frequency of histopathological injuries associated with subclinical necrosis caused by C. perfringens. However, the productive parameters (body weight, feed intake, and feed conversion ratio) were not modified with the administration of the Synbiotic.
On the other hand, the broilers treated with the Synbiotic showed a tendency toward having higher counts of Lactic Acid Bacteria throughout the bioassay, and different strains of groups with and without Synbiotic treatment were identified.
Acknowledgments
We are grateful for the support given by the histology laboratory of CUCBA and its staff: Victor Barragan Cano and Xóchitl Rocío Ávila Dávila.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2615/9/10/777/s1. File S1: Raw data from microbiological isolation and enumeration. File S2: Raw data from Duodenal morphology evaluation. File S3: Raw data from growth performance evaluation.
Author Contributions
Conceptualization, F.A. and A.V.-L.; data curation, Z.V.-d.l.M.; formal analysis, Z.V.-d.l.M., O.V.-P., J.C.-R., C.G.-A., and C.A.; funding acquisition, A.V.-L.; investigation, Z.V.-d.l.M., K.N., O.V.-P., H.A., and A.V.-L.; methodology, K.N., C.A., and A.V.-L.; project administration, A.V.-L.; resources, J.C.-R., C.G.-A., and A.V.-L.; supervision, K.N., O.V.-P., F.A., and A.V.-L.; writing—original draft, Z.V.-d.l.M.; writing—review and editing, K.N., F.A., and A.V.-L.
Funding
This work is part of the project ‘Synbiotic avian vector for the improvement of feed efficiency and prevention of infections’. Project number 220311. Funded under the call for proposals PEI/CONACyT 2015 and by the company Kurago Biotek. Zuami Villagrán-de la Mora holds a doctorate scholarship from the Teacher Professional Development Program (PRODEP).
Conflicts of Interest
The authors declare no conflict of interest, and the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- 1.Finstad S., O’Bryan C.A., Marcy J.A., Crandall P.G., Ricke S.C. Salmonella and broiler processing in the United States: Relationship to foodborne salmonellosis. Food Res. Int. 2012;45:789–794. doi: 10.1016/j.foodres.2011.03.057. [DOI] [Google Scholar]
- 2.Li J., Hao H., Cheng G., Liu C., Ahmed S., Shabbir M.A.B., Hussain H.I., Dai M., Yuan Z. Microbial shifts in the intestinal microbiota of Salmonella infected chickens in response to enrofloxacin. Front. Microbiol. 2017;8:1–14. doi: 10.3389/fmicb.2017.01711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knap I., Kehlet A.B., Bennedsen M., Mathis G.F., Hofacre C.L., Lumpkins B.S., Jensen M.M., Raun M., Lay A. Bacillus subtilis ( DSM17299 ) significantly reduces Salmonella in broilers. Poult. Sci. 2011;90:1690–1694. doi: 10.3382/ps.2010-01056. [DOI] [PubMed] [Google Scholar]
- 4.Van Immerseel F., Rood J.I., Moore R.J., Titball R.W. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. 2009;17:32–36. doi: 10.1016/j.tim.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Timbermont L., Haesebrouck F., Ducatelle R., Van Immerseel F. Necrotic enteritis in broilers: An updated review on the pathogenesis. Avian Pathol. 2011;40:341–347. doi: 10.1080/03079457.2011.590967. [DOI] [PubMed] [Google Scholar]
- 6.Moore R.J. Necrotic enteritis predisposing factors in broiler chickens. Avian Pathol. 2016;45:275–281. doi: 10.1080/03079457.2016.1150587. [DOI] [PubMed] [Google Scholar]
- 7.Antonissen G., Eeckhaut V., Van Driessche K., Onrust L., Haesebrouck F., Ducatelle R., Moore R.J., Van Immerseel F. Microbial shifts associated with necrotic enteritis. Avian Pathol. 2016;45:308–312. doi: 10.1080/03079457.2016.1152625. [DOI] [PubMed] [Google Scholar]
- 8.Cooper K.K., Songer J.G., Uzal F.A. Diagnosing clostridial enteric disease in poultry. J. Vet. Diagnostic Investig. 2013;25:314–327. doi: 10.1177/1040638713483468. [DOI] [PubMed] [Google Scholar]
- 9.Olkowski A.A., Wojnarowicz C., Chirino-Trejo M., Laarveld B., Sawicki G. Sub-clinical necrotic enteritis in broiler chickens: Novel etiological consideration based on ultra-structural and molecular changes in the intestinal tissue. Res. Vet. Sci. 2008;85:543–553. doi: 10.1016/j.rvsc.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Huyghebaert G., Ducatelle R., Van Immerseel F. Van An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011;187:182–188. doi: 10.1016/j.tvjl.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Gaucher M.-L., Quessy S., Letellier A., Arsenault J., Boulianne M. Impact of a drug-free program on broiler chicken growth performances, gut health, Clostridium perfringens and Campylobacter jejuni occurrences at the farm level. Poult. Sci. 2015;94:1791–1801. doi: 10.3382/ps/pev142. [DOI] [PubMed] [Google Scholar]
- 12.Engster H.M., Marvil D., Stewart-Brown B. The effect of withdrawing growth promoting antibiotics from broiler chickens: A long-term commercial industry study. J. Appl. Poult. Res. 2002;11:431–436. doi: 10.1093/japr/11.4.431. [DOI] [Google Scholar]
- 13.Food and Agriculture Organization, (FAO) World Health Organization, (WHO) Probiotics in Food. Health and Nutritional Properties and Guidelines for Evaluation. Volume 85. 2006. [Google Scholar]
- 14.Awad W.A., Ghareeb K., Böhm J. Effect of addition of a probiotic micro-organism to broiler diet on intestinal mucosal architecture and electrophysiological parameters. J. Anim. Physiol. Anim. Nutr. (Berl). 2010;94:486–494. doi: 10.1111/j.1439-0396.2009.00933.x. [DOI] [PubMed] [Google Scholar]
- 15.Teng P., Kim W.K. Review: Roles of Prebiotics in Intestinal Ecosystem of Broilers. Front. Vet. Sci. 2018;5:1–18. doi: 10.3389/fvets.2018.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naghi A.S., Ghasemi H.A., Taherpour K. Evaluation of Aloe vera and synbiotic as antibiotic growth promoter substitutions on performance, gut morphology, immune responses and blood constitutes of broiler chickens. Anim. Sci. J. 2017;88:306–313. doi: 10.1111/asj.12629. [DOI] [PubMed] [Google Scholar]
- 17.Dhama K., Verma V., Sawant P., Tiwari R., Vaid R., Chauhan R. Applications of Probiotics in Poultry: Enhancing Immunity and Beneficial Effects on Production Performances and Health - A Review. J. Immunol. Immunopathol. 2011;13:1–19. [Google Scholar]
- 18.Adil S., Magray S.N. Impact and manipulation of gut microflora in poultry: A review. J. Anim. Vet. Adv. 2012;6:873–877. doi: 10.3923/javaa.2012.873.877. [DOI] [Google Scholar]
- 19.Kauffmann F. In: Serological Diagnosis of Salmonella-Species Kauffmann-White-Schema. Williams & Wilkins, editor. CABI; Denmark, MI, USA: 1972. [Google Scholar]
- 20.ADSA. ASAS. PSA . Guide for the care and use of agricultural animals in research and teaching. ADSA; ASAS; PSA; Champaing, IL, USA: 2010. [Google Scholar]
- 21.Cobb-vantress COBB Broiler Management Guide. [(accessed on 25 June 2018)]; Available online: http://www.cobb-vantress.com/docs/default-source/management-guides/broiler-management-guide.pdf.
- 22.Rojo A. Cálculo del tamaño muestral en procedimientos experimentales con animales. Valoración de las incidencias. Anim. Lab. 2014;62:31–33. [Google Scholar]
- 23.Garden M., Singleton R. Manejo del Pollo de Engorde para un Peso Liviano al Mercado (de 1.5 a 1.8 Kg/de 3.3 a 4.0 lb) [(accessed on June 25 2018)]; Available online: http://cn.aviagen.com/assets/Tech_Center/BB_Foreign_Language_Docs/Spanish_TechDocs/A-Acres-Boletin-de-Servicio-Abr-08-Manejo-Pollo-Engorde-Peso-Liviano-Mercado.pdf.
- 24.Cooper K.K., Songer J.G. Necrotic enteritis in chickens: A paradigm of enteric infection by Clostridium perfringens type A. Anaerobe. 2009;15:55–60. doi: 10.1016/j.anaerobe.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 25.La Ragione R.M., Narbad A., Gasson M.J., Woodward M.J. In vivo characterization of Lactobacillus johnsonii FI9785 for use as a defined competitive exclusion agent against bacterial pathogens in poultry. Lett. Appl. Microbiol. 2004;38:197–205. doi: 10.1111/j.1472-765X.2004.01474.x. [DOI] [PubMed] [Google Scholar]
- 26.Ganapathy K., Bufton A., Pearson A., Lemiere S., Jones R.C. Vaccination of commercial broiler chicks against avian metapneumovirus infection: a comparison of drinking-water, spray and oculo-oral delivery methods. Vaccine. 2010;28:3944–3948. doi: 10.1016/j.vaccine.2010.03.065. [DOI] [PubMed] [Google Scholar]
- 27.Pelicano E., Souza P., Souza H., Figueiredo D., Boiago M., Carvalho S., Bordon V. Intestinal mucosa development in broiler chickens fed natural growth promoters. Rev. Bras. Ciência Avícola. 2005;7:221–229. doi: 10.1590/S1516-635X2005000400005. [DOI] [Google Scholar]
- 28.Tian X., Shao Y., Wang Z., Guo Y. Effects of dietary yeast B-glucans supplementation on growth performance, gut morphology, intestinal Clostridium perfringens population and immune response of broiler chickens challenged with necrotic enteritis. Anim. Feed Sci. Technol. 2016;215:144–155. doi: 10.1016/j.anifeedsci.2016.03.009. [DOI] [Google Scholar]
- 29.Mookiah S., Sieo C.C., Ramasamy K., Abdullah N., Ho Y.W. Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations and caecal fermentation concentrations of broiler chickens. J. Sci. Food Agric. 2014;94:341–348. doi: 10.1002/jsfa.6365. [DOI] [PubMed] [Google Scholar]
- 30.Mikoleit M.L. WHO Global Foodborne Infections Network “A WHO Network Building Capacity to Detect, Control and Prevent Foodborne and Other Enteric Infections from Farm to Table” Laboratory Protocol: “Isolation of Salmonella and Shigella from Faecal Specimens.”. [(accessed on 25 June 2018)]; Available online: http://antimicrobialresistance.dk/CustomerData/Files/Folders/6-pdf-protocols/63_18-05-isolation-of-salm-220610.pdf.
- 31.Heikinheimo A., Lindström M., Granum P.E., Korkeala H. Humans as Reservoir for Enterotoxin Gene – carrying Clostridium perfringens Type A. Emergin Infect. Dis. 2006;12:1724–1729. doi: 10.3201/eid1211.060478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanley D., Denman S.E., Hughes R.J., Geier M.S., Crowley T.M., Chen H., Haring V.R., Moore R.J. Intestinal microbiota associated with differential feed conversion efficiency in chickens. Appl. Microbiol. Biotechnol. 2012;96:1361–1369. doi: 10.1007/s00253-011-3847-5. [DOI] [PubMed] [Google Scholar]
- 33.Eeckhaut V., Wang J., Van Parys A., Haesebrouck F., Joossens M., Falony G., Raes J., Ducatelle R., Van Immerseel F. The probiotic butyricicoccus pullicaecorum reduces feed conversion and protects from potentially harmful intestinal microorganisms and necrotic enteritis in broilers. Front. Microbiol. 2016;7:1–9. doi: 10.3389/fmicb.2016.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Segers M.E., Lebeer S. Towards a better understanding of Lactobacillus rhamnosus GG - host interactions. Microb. Cell Fact. 2014;13:S7. doi: 10.1186/1475-2859-13-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gill H.S., Rutherfurd K.J., Prasad J., Gopal P.K. Enhancement of natural and acquired immunity by Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019) Br. J. Nutr. 2000;83:167–176. doi: 10.1017/S0007114500000210. [DOI] [PubMed] [Google Scholar]
- 36.Peña J.A., Versalovic J. Lactobacillus rhamnosus GG decreases TNF-α production in lipopolysaccharide-activated murine macrophages by a contact-independent mechanism. Cell. Microbiol. 2003;5:277–285. doi: 10.1046/j.1462-5822.2003.t01-1-00275.x. [DOI] [PubMed] [Google Scholar]
- 37.Shu Q., Gill H.S. Immune protection mediated by the probiotic Lactobacillus rhamnosus HN001 (DR20TM) against Escherichia coli O157:H7 infection in mice. FEMS Immunol. Med. Microbiol. 2002;34:59–64. doi: 10.1111/j.1574-695X.2002.tb00603.x. [DOI] [PubMed] [Google Scholar]
- 38.De Keersmaecker S.C.J., Verhoeven T.L.A., Desair J., Marchal K., Vanderleyden J., Nagy I. Strong antimicrobial activity of Lactobacillus rhamnosus GG against Salmonella typhimurium is due to accumulation of lactic acid. FEMS Microbiol. Lett. 2006;259:89–96. doi: 10.1111/j.1574-6968.2006.00250.x. [DOI] [PubMed] [Google Scholar]
- 39.Papagianni M., Anastasiadou S. Pediocins: The bacteriocins of Pediococci. Sources, production, properties and applications. Microb. Cell Fact. 2009;8:3. doi: 10.1186/1475-2859-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakaridis I., Soultos N., Dovas C.I., Papavergou E., Ambrosiadis I., Koidis P. Lactic acid bacteria from chicken carcasses with inhibitory activity against Salmonella spp. and Listeria monocytogenes. Anaerobe. 2012;18:62–66. doi: 10.1016/j.anaerobe.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Lee S., Lillehoj H.S., Park D.W., Hong Y.H., Lin J.J. Effects of Pediococcus- and Saccharomyces-based probiotic (MitoMax®) on coccidiosis in broiler chickens. Comp. Immunol. Microbiol. Infect. Dis. 2007;30:261–268. doi: 10.1016/j.cimid.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Ayala Monter M.A., Sánchez D.H., Ruiz R.P., González Muñoz S.S., Bárcena Gama J.R., Mendo O.H., Salado N.T. Prebiotic effect of two sources of inulin on in vitro growth of Lactobacillus salivarius and Enterococcus faecium. Rev. Mex. Ciencias Pecu. 2018;9:346–361. doi: 10.22319/rmcp.v9i2.4488. [DOI] [Google Scholar]
- 43.Yitbarek A., Rodriguez-Lecompte J.C., Echeverry H.M., Munyaka P., Barjesteh N., Sharif S., Camelo-Jaimes G. Performance, histomorphology, and toll-like receptor, chemokine, and cytokine profile locally and systemically in broiler chickens fed diets supplemented with yeast-derived macromolecules. Poult. Sci. 2013;92:2299–2310. doi: 10.3382/ps.2013-03141. [DOI] [PubMed] [Google Scholar]
- 44.M’Sadeq S.A., Wu S., Swick R.A., Choct M. Towards the control of necrotic enteritis in broiler chickens with in-feed antibiotics phasing-out worldwide. Anim. Nutr. 2015;1:1–11. doi: 10.1016/j.aninu.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qing X., Zeng D., Wang H., Ni X., Liu L., Lai J., Khalique A., Pan K., Jing B. Preventing subclinical necrotic enteritis through Lactobacillus johnsonii BS15 by ameliorating lipid metabolism and intestinal microflora in broiler chickens. AMB Express. 2017;7:139. doi: 10.1186/s13568-017-0439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Awad W.A., Ghareeb K., Abdel-Raheem S., Bohm J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 2009;88:49–56. doi: 10.3382/ps.2008-00244. [DOI] [PubMed] [Google Scholar]
- 47.Min Y.N., Yang H.L., Xu Y.X., Gao Y.P. Effects of dietary supplementation of synbiotics on growth performance, intestinal morphology, sIgA content and antioxidant capacities of broilers. J. Anim. Physiol. Anim. Nutr. (Berl). 2016;100:1073–1080. doi: 10.1111/jpn.12479. [DOI] [PubMed] [Google Scholar]
- 48.Laudadio V., Passantino L., Perillo a., Lopresti G., Passantino A., Khan R.U., Tufarelli V. Productive performance and histological features of intestinal mucosa of broiler chickens fed different dietary protein levels. Poult. Sci. 2012;91:265–270. doi: 10.3382/ps.2011-01675. [DOI] [PubMed] [Google Scholar]
- 49.Stanley D., Keyburn A.L., Denman S.E., Moore R.J. Changes in the caecal microflora of chickens following Clostridium perfringens challenge to induce necrotic enteritis. Vet. Microbiol. 2012;159:155–162. doi: 10.1016/j.vetmic.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 50.Kogut M.H., Swaggerty C.L., Byrd J.A., Selvaraj R., Arsenault R.J. Chicken-Specific Kinome Array Reveals that Salmonella enterica Serovar Enteritidis Modulates Host Immune Signaling Pathways in the Cecum to Establish a Persistence Infection. Int. J. Mol. Sci. 2016;17:1207. doi: 10.3390/ijms17081207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu R.Y., Määttänen P., Napper S., Scruten E., Li B., Koike Y., Johnson-Henry K.C., Pierro A., Rossi L., Botts S.R., et al. Non-digestible oligosaccharides directly regulate host kinome to modulate host inflammatory responses without alterations in the gut microbiota. Microbiome. 2017;5:135. doi: 10.1186/s40168-017-0357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu R.Y., Abdullah M., Määttänen P., Pilar A.V.C., Scruten E., Johnson-Henry K.C., Napper S., O’Brien C., Jones N.L., Sherman P.M. Protein kinase Cσ signaling is required for dietary prebiotic-induced strengthening of intestinal epithelial barrier function. Sci. Rep. 2017;7:1–10. doi: 10.1038/srep40820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alloui M.N., Szczurek W., Świątkiewicz S. The usefulness of prebiotics and probiotics in modern poultry nutrition: a review. Ann. Anim. Sci. 2013;13:17–32. doi: 10.2478/v10220-012-0055-x. [DOI] [Google Scholar]
- 54.Salehimanesh A., Mohammadi M., Roostaei-Ali Mehr M. Effect of dietary probiotic, prebiotic and synbiotic supplementation on performance, immune responses, intestinal morphology and bacterial populations in broilers. J. Anim. Physiol. Anim. Nutr. (Berl). 2016;100:694–700. doi: 10.1111/jpn.12431. [DOI] [PubMed] [Google Scholar]
- 55.Stanley D., Hughes R.J., Moore R.J. Microbiota of the chicken gastrointestinal tract: Influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014;98:4301–4310. doi: 10.1007/s00253-014-5646-2. [DOI] [PubMed] [Google Scholar]
- 56.Nakphaichit M., Thanomwongwattana S., Phraephaisarn C., Sakamoto N., Keawsompong S., Nakayama J., Nitisinprasert S. The effect of including Lactobacillus reuteri KUB-AC5 during post-hatch feeding on the growth and ileum microbiota of broiler chickens. Poult. Sci. 2011;90:2753–2765. doi: 10.3382/ps.2011-01637. [DOI] [PubMed] [Google Scholar]
- 57.Patterson J.A., Burkholder K.M. Application of prebiotics and probiotics in poultry production. Poult. Sci. 2003;82:627–631. doi: 10.1093/ps/82.4.627. [DOI] [PubMed] [Google Scholar]
- 58.Mao X., Gu C., Hu H., Tang J., Chen D., Yu B., He J., Yu J., Luo J., Tian G. Dietary lactobacillus rhamnosus GG supplementation improves the mucosal barrier function in the intestine of weaned piglets challenged by porcine rotavirus. PLoS ONE. 2016;11:1–14. doi: 10.1371/journal.pone.0146312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olnood C.G., Beski S.S.M., Choct M., Iji P.A. Novel probiotics: Their effects on growth performance, gut development, microbial community and activity of broiler chickens. Anim. Nutr. 2015;1:184–191. doi: 10.1016/j.aninu.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen F., Gao S.S., Zhu L.Q., Qin S.Y., Qiu H.L. Effects of dietary Lactobacillus rhamnosus CF supplementation on growth, meat quality, and microenvironment in specific pathogen-free chickens. Poult. Sci. 2018;97:118–123. doi: 10.3382/ps/pex261. [DOI] [PubMed] [Google Scholar]
- 61.Ouled-Haddar H., Idoui T., Sifour M., Guezira M., Bouthabet M. Isolation, characterization and microencapsulation of probiotic Lactobacillus curvatus G7 from chicken crop. Online J. Sci. Technol. 2012;2:6. [Google Scholar]
- 62.Drissi F., Merhej V., Angelakis E., El Kaoutari A., Carrière F., Henrissat B., Raoult D. Comparative genomics analysis of Lactobacillus species associated with weight gain or weight protection. Nutr. Diabetes. 2014;4:e109. doi: 10.1038/nutd.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salim H.M., Kang H.K., Akter N., Kim D.W., Kim J.H., Kim M.J., Na J.C., Jong H.B., Choi H.C., Suh O.S., et al. Supplementation of direct-fed microbials as an alternative to antibiotic on growth performance, immune response, cecal microbial population, and ileal morphology of broiler chickens. Poult. Sci. 2013;92:2084–2090. doi: 10.3382/ps.2012-02947. [DOI] [PubMed] [Google Scholar]
- 64.Chambers J.R., Gong J. The intestinal microbiota and its modulation for Salmonella control in chickens. Food Res. Int. 2011;44:3149–3159. doi: 10.1016/j.foodres.2011.08.017. [DOI] [Google Scholar]
- 65.Park J.H., Kim I.H. The effects of the supplementation of Bacillus subtilis RX7 and B2A strains on the performance, blood profiles, intestinal Salmonella concentration, noxious gas emission, organ weight and breast meat quality of broiler challenged with Salmonella typhimuri. J. Anim. Physiol. Anim. Nutr. (Berl). 2015;99:326–334. doi: 10.1111/jpn.12248. [DOI] [PubMed] [Google Scholar]
- 66.Moran E.T. Intestinal events and nutritional dynamics predispose Clostridium perfringens virulence in broilers. Poult. Sci. 2014;93:3028–3036. doi: 10.3382/ps.2014-04313. [DOI] [PubMed] [Google Scholar]
- 67.Cooper K.K., Songer J.G. Virulence of Clostridium perfringens in an experimental model of poultry necrotic enteritis. Vet. Microbiol. 2010;142:323–328. doi: 10.1016/j.vetmic.2009.09.065. [DOI] [PubMed] [Google Scholar]
- 68.Shojadoost B., Vince A.R., Prescott J.F. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: A critical review. Vet. Res. 2012;43:1. doi: 10.1186/1297-9716-43-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang H., Ni X., Qing X., Liu L., Lai J., Khalique A., Li G., Pan K., Jing B., Zeng D. Probiotic Enhanced Intestinal Immunity in Broilers against Subclinical Necrotic Enteritis. Front. Immunol. 2017;8:1–14. doi: 10.3389/fimmu.2017.01592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Talebi A., Amani A., Pourmahmod M., Saghaei P., Rezaie R. Synbiotic enhances immune responses against infectious bronchitis, infectious bursal disease, Newcastle disease and avian influenza in broiler chickens. Vet. Res. Forum. 2015;6:191–197. [PMC free article] [PubMed] [Google Scholar]
- 71.Midilli M., Alp M., Kocabaǧli N., Muǧlali Ö.H., Turan N., Yilmaz H., Çakir S. Effects of dietary probiotic and prebiotic supplementation on growth performance and serum IgG concentration of broilers. S. Afr. J. Anim. Sci. 2008;38:21–27. doi: 10.4314/sajas.v38i1.4104. [DOI] [Google Scholar]
- 72.Pinto L.F.B., Packer I.U., De Melo C.M.R., Ledur M.C., Coutinho L.L. Principal components analysis applied to performance and carcass traits in the chicken. Anim. Res. 2006;55:419–425. doi: 10.1051/animres:2006022. [DOI] [Google Scholar]
- 73.Udeh I., Ogbu C.C. Principal Component Analysis of Body Measurements in Three Strains of Broiler Chicken. Sci. World J. 2012;6:11–14. [Google Scholar]
- 74.Ogah D.M., Alaga A.A., Momoh M.O. Principal component factor analysis of the morphostructural traits of Muscovy duck. Int. J. Poult. Sci. 2009;8:1100–1103. doi: 10.3923/ijps.2009.1100.1103. [DOI] [Google Scholar]
- 75.Venturini G.C., Savegnago R.P., Nunes B.N., Ledur M.C., Schmidt G.S., El Faro L., Munari D.P. Genetic parameters and principal component analysis for egg production from White Leghorn hens. Poult. Sci. 2013;92:2283–2289. doi: 10.3382/ps.2013-03123. [DOI] [PubMed] [Google Scholar]
- 76.Mohapatra B.R., Broersma K., Mazumder A. Comparison of five rep-PCR genomic fingerprinting methods for differentiation of fecal Escherichia coli from humans, poultry and wild birds. FEMS Microbiol. Lett. 2007;277:98–106. doi: 10.1111/j.1574-6968.2007.00948.x. [DOI] [PubMed] [Google Scholar]
- 77.Cisek A.A., Binek M. Chicken intestinal microbiota function with a special emphasis on the role of probiotic bacteria. Pol. J. Vet. Sci. 2014;17:385–394. doi: 10.2478/pjvs-2014-0057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.