Abstract
Background:
Trichomoniasis is a common sexually transmitted disease that is caused by infection with protozoan parasite called Trichomonas vaginalis. Metronidazole is the drug of choice for the treatment of this infection. In this study, design, formulation, and physicochemical evaluation of vaginal cream containing Eucalyptus camaldulensis, Viola odorata, and Mentha piperita extracts for the prevention and treatment of trichomoniasis has been investigated.
Methods:
Ethyl acetate extract of E. camaldulensis leaves, water fraction of V. odorata root, and hydroalcoholic extract of M. piperita leaves was prepared and used for anti-trichomonas experiments. Then, based on results, different formulations of vaginal cream containing mixed extracts were prepared and physicochemical evaluation was conducted. In the next step, anti-trichomonas effect of selective formulation was tested in vitro.
Results:
The mixed concentrates containing 2.5 mg/ml E. camaldulensis, 0.06 mg/ml V. odorata, and 1 mg/ml M. piperita showed 100% growth inhibition (GI) during 24 h. Furthermore, the mixture containing 1.25 mg/ml E. camaldulensis, 0.03 mg/ml V. odorata, and 0.5 mg/ml M. piperita showed 92% GI in the first 24 h. The selective formulation passed all of physicochemical test and also showed 100% GI for in vitro anti-trichomonas experiments in the first 24 h.
Conclusions:
The mixed concentrates containing 2.5 mg/ml E. camaldulensis, 0.06 mg/ml of V. odorata, and 1 mg/ml of M. piperita are the mixture which showed the highest percentage of GI (100%) after 24 h. The selective formulation of vaginal cream containing this mixture of extracts was detected 100% GI in the first 24 h.
Keywords: Eucalyptus camaldulensis, Mentha piperita, trichomoniasis, vaginal cream, Viola odorata
Introduction
Trichomoniasis is a sexually transmitted infection caused by Trichomonas vaginalis. In woman, trichomoniasis results in vaginitis. If untreated, it may lead to erosion of the epithelial lining.[1] Trichomoniasis, the most prevalent nonviral sexual infection, with roughly 170 million new infected people worldwide per year, has been related to various discomforts such as preterm delivery, high infant mortality, or low birth weight and makes patients more susceptible to HIV infection.[2,3,4,5] The drug of choice for the treatment of trichomoniasis is metronidazole, but some resistant strain to this medication has been detected.[6,7] It also has some side effects including metallic taste, nausea, and transient neutropenia.[8] Thus, efforts need to be taken for finding new alternative drugs, to control trichomoniasis. For a long time, there is widespread acceptance of using medical herbs in both developing and nondeveloping societies because of their significant benefits such as fewer side effects, better patient tolerance, lesser cost, and treating the main cause of disease.[9]
Eucalyptus, a large genus of Myrtaceae, represented by 900 species and subspecies, can be found the world over. Its leaves were used by Australians for its benefits such as wound healing and for treating fungal infection,[10,11] and it seems that the essential oils of Eucalyptus, containing different compounds such as terpenoid and phenolic ones, play an important role in its biological activities.[12] Moreover, it has been reported that the extracts of Eucalyptus camaldulensis have anti-trichomonas activity.[13,14,15]
Viola odorata is a species of the genus Viola native to Europe and Asia. It belongs to family Violaceae.[16] The phenolic glycosides, gaultherin, violutoside, saponins, flavonoids, odoratine, and violin were reported to occur in the plant.[17,18] The antibacterial and antifungal activities were reported for the V. odorata.[19,20] Moreover, it has been reported that the extracts of V. odorata have anti-trichomonas activity.[21]
Mentha piperita, a medicinally important plant belongs to the family Lamiaceae, is widely grown in temperate areas of the world, particularly in Europe, North America, and North Africa but nowadays cultivated throughout all regions of the world.[22,23] The alpha- and beta-pinene, menthol, menthone, monoterpenes, caffeic acid, flavonoids, and tannin were reported to occur in the plant.[24] The antiseptic, antispasmodic, antiemetic, analgesic, and antimicrobial activities were reported for the M. piperita.[25,26] Moreover, it has been reported that extracts of M. piperita have anti-trichomonas activity.[27]
Vaginal administration of drugs is mainly used for the treatment of local infection such as trichomoniasis. In addition, vaginal formulations have a great potential for the systemic absorption of drugs because of the large surface area and rich blood supply.[28]
The vanishing creams are oil in water emulsion-based formulations containing aqueous phase and oil phase.[29]
In this study, an effort to find a new formulation of vaginal cream containing E. camaldulensis, V. odorata, and M. piperita extracts was designed for the prevention and treatment of trichomoniasis and its effectiveness was evaluated in vitro.
Methods
Materials
Leaves of E. camaldulensis, roots of V. odorata, and leaves of M. piperita collected from around Isfahan city, located in the center of Iran, in September 2015, and underwent a validation process by pharmacognosy specialists in the department of pharmacognosy. Methanol, HCl, NaOH, chloroform, diethyl ether, ethyl acetate, dimethyl sulfoxide (DMSO), stearic acid, beeswax, white soft paraffin, triethanolamine, propylene glycol, KOH, glycerol, and sorbitol were obtained from Merck Company (Germany).
Preparation of Eucalyptus camaldulensis phenolic extract
For the preparation of phenolic fraction of E. camaldulensis, the crude extract was prepared by maceration of 500 g of dried powder of E. camaldulensis leaves in 50% methanol and 1% HCl 6N for 3 days continuously at 25°C. The extract was concentrated using rotary evaporator. Then, the crude extract was partitioned into purified water and diethyl ether phase. The pH of aqueous phase was adjusted to 8–9 with 10% NaOH, which converted phenolic compound to its sodium salts. Then, chloroform was added, and using separating funnel, aqueous phase containing phenolic compounds was collected. Then, pH of residual aqueous phase was decreased to 3–4 using HCl 6N and extracted with ethyl acetate to provide phenolic fraction. Then, it was evaporated to dryness using vacuum rotary evaporator.[30]
Preparation of Viola odorata phenolic extract
For preparation of phenolic fraction of V. odorata, root parts of plant material were separated and shade dried. The cured extract was prepared by maceration of 150 g of dried powder of V. odorata root in 70 ml of 50% methanol and 1% HCl 6N for 3 days at 25°C.[31] The extract was processed as mention for E. camaldulensis extract to yield phenolic fraction.
Preparation of Mentha piperita hydroalcoholic extract
Menta aerial parts were separated and shade dried. The cured extract was prepared by maceration of 50 g of dried powder of M. piperita leaves in 500 ml water and 500 ml methanol for 3 days at 25°C. The extract was concentrated using rotary evaporator.[27]
Determination of 1,8-cineole by gas chromatography
Using gas chromatography (GC), 1,8-cineole as one of the major components of the formulation was standardized through internal standard calibration method. A standard calibration curve in the range of 2.5, 5, 10, 25, 50, and 75 μg/ml for quantitative analysis was prepared using different concentrations of 1,8-cineole (Sigma-Aldrich, USA). Dodecane (Sigma-Aldrich, USA) in constant amount (100 μg/mL) as the internal standard material was added to standards and samples. The rations of standards to internal standard responses (peak height) were then used to obtain calibration curve. Concentrations of 1,8-cineole in the samples were determined from the regression equations using the minimum square method (R2 value). For determination of 1,8-cineole in samples, 1 g sample formulation was dispersed in 2 mL ethyl acetate, filtered, and was evaporated to dryness under a stream of nitrogen. The dry residue was resolubilized in 1 mL ethyl acetate. Dodecane (100 μg/mL) as the internal standard material was added, and using Hamilton syringe (Reno, NV, USA), 2 μL of the sample was injected to GC with a spilt less time of 0.6 min. GC determinations were run on a GC-2550 (Teif Gostar Faraz, Iran) instrument using a TRB1 capillary column (30 m × 0.25 mm; film thickness: 1 μm). The carrier gas was hydrogen with a rate of 40 cm/min. The oven temperature was programmed to 80°C (hold 2.5 min) to 250°C @ 5°C/min. Injector and detector temperatures were 250 and 270°C, respectively.
Preparation of test microorganism
Test microorganism was obtained from parasitology laboratory of Isfahan University of Medical Sciences, which was isolated from vaginal discharged of female patients attending obstetrics and gynecology clinic in Shahr-e Kord, Iran.
In all experiments, a pool of four isolated was used. The parasite T. vaginalis was cultured in vitro at 37°C in TYIS33. Log phase culture of T. vaginalis was diluted with TYIS33 medium for obtaining 104 cell/ml.
Growth inhibition assay
To explore anti-trichomonas effects of E. camaldulensis, V. odorata, and M. piperita, the extracts were diluted with DMSO or medium culture (depending on their solvents) and transferred to Eppendorf tubes for providing final concentration of 1.2, 2.5, 4.1, 12.5, and 25 mg/ml for E. camaldulensis, 0.15, 0.3, 0.5, 1.5, and 3 mg/ml for V. odorata, and 0.5, 1, 1.6, 5, and 10 mg/ml for M. piperita. The DMSO and medium culture were used as negative control accordingly, and metronidazole at concentration of 50 μg/ml was used as positive control. All tubes were incubated at 37°C. After 24, 48, and 72 h, the samples were taken from each tube and viable parasites were counted by hemocytometer. Complete active and flagella active parasites were considered as viable ones. Results of parasite counting have been reported as percentage of growth inhibition (GI)% using the following equation in which “a” stands for the mean number of viable parasites in negative control tube and “b” stands for the mean number of viable parasites in test tube.[30,32]
GI% = a−b/a × 100
For comparing GI% in case and control tubes, statistical values and tests such as mean, standard deviation, and analysis for variance test were used.
Based on the results, the extracts were diluted with DMSO or medium culture and transferred to Eppendorf tubes for providing different concentrations of certain fractions of three plants together including 4.1 mg/ml E. camaldulensis, 0.1 mg/ml V. odorata, and 1.6 mg/ml M. piperita; 2.5 mg/ml E. camaldulensis, 0.06 mg/ml V. odorata, and 1 mg/ml M. piperita; and 1.25 mg/ml E. camaldulensis, 0.03 mg/ml V. odorata, and 0.5 mg/ml M. piperita. The DMSO and medium culture were used as negative control accordingly, and metronidazole (50 μg/ml) was used as positive control. Results of parasite counting have been reported as percentage of GI.
Formulation of vaginal creams
In this study, vanishing cream was prepared by the addition of aqueous phase to the oily phase with continuous stirring. To prepare the base, an oily phase that consisted of stearic acid, beeswax, and white soft paraffin was heated up to 70°C ± 1°C. At the same time, aqueous phase consisting of triethanolamine, propylene glycol, NaOH, KOH, glycerol, sorbitol solution, and purified water was heated to 75°C ± 1°C. Then, the aqueous phase added in the oil phase with continuous stirring until congealing, and finally, the mixture of extracts added in cream bases at 40°C ± 1°C with continuous stirring until congealing well and cooling.
Physicochemical evaluation of vaginal creams
Physical appearance of formulations
Cream formulations were visually inspected for color, homogeneity, consistency, and presence of particles.[33] Homogeneity was examined by microscope.[34] To investigate the consistency of the formulations, a small quantity of cream was pressed between the thumb and the index fingers, and consistency of the creams was noticed.
pH determination
The pH of formulations was determined after diluting and dispersing 1 g of creams in 10 ml of purified water (10% w/v). All measurements were made in triplicate and mean was calculated. The measurement of pH was performed at 48 h, 1 week, 2 weeks, 1 month, 3 months, and 6 months after preparation to detect any pH fluctuation with time.[35]
Centrifugal test
To investigate the stability of the formulations against the centrifugal force, 48 h after preparation, formulations were transferred into tube and centrifugal at 2000 rpm for 60 min, and stability of formulations was evaluated at the time of 5, 15, 30, and 60 min.[34]
Thermal test
To investigate the stability of formulations in different seasons and climate conditions, 48 h after preparation, three samples were placed at 4°C, 25°C, and 45°C. Cream formulations were evaluated at the times of 24 h, 1 week, 2 weeks, 1 month, 3 months, and 6 months.[34]
Freeze and thaw test
To investigate the stability of the formulations in extreme cold, 48 h after preparation, 15 g cream was placed at −8°C for 48 h and then 48 h at 25°C for six periods. Then, stability of formulations was evaluated.[34]
Cooling and heating test
To investigate the stability of formulations against extreme temperature changes, 48 h after preparation, 15 g of formulations was placed at 45°C for 48 h and then 48 h at 4°C for six periods. Then, stability of formulations was evaluated.[34]
In vitro release study
Dissolution testing is an essential requirement for the development and establishment of the release of drug.[36] In this study, 1 g of creams was put on a 2.5 cm × 2.5 cm cellulose acetate membrane. A piece of stainless steel wire netting was used as supporter to tie the membrane to one end of glass pipe. The glass was filled with 25 ml phosphate buffer pH 4.5, agitated by a magnetic stirrer and temperature maintained at 37°C ± 0.5°C. Aliquots of 1 ml were withdrawn periodically at 30, 60, 120, 240, and 360 min. Each time of aliquots withdrawn, equal volume was replaced with fresh phosphate buffer previously heated to 37°C ± 0.5°C. The release percent of 1,8-cineole at selected time was determined after injection of sample to GC. All measurements were made in triplicate.
Determination of viscosity
A Brookfield viscometer DVIII was used with spindle number 74 to determine the viscosity of formulations. The tests were carried out at 25°C. The spindle was rotated at 100 rpm. All measurements were made in triplicate.[37]
Growth inhibition assay
For evaluation of anti-trichomonas effect of selected formulation, 2 g of cream was applied on the bottom of the Petri dish and was covered with filter paper. Then, TYIS33 medium and 104 cell/1 g of cream were added. The free extracts’ cream was used negative control and metronidazole (50 μg/ml) was used as positive control. All Petri dishes were incubated at 37°C. After 24, 48, and 72 h, the samples were taken from each Petri dish and viable parasites were counted by hemocytometer. Results of parasite counting have been reported as GI%.
Results
Ethyl acetate extract of E. camaldulensis leaves in a concentration of 12.5 mg/ml showed 100% GI during 24 h. Detail results about the effect of different concentrations of E. camaldulensis have been showed in Figure 1.
Figure 1.
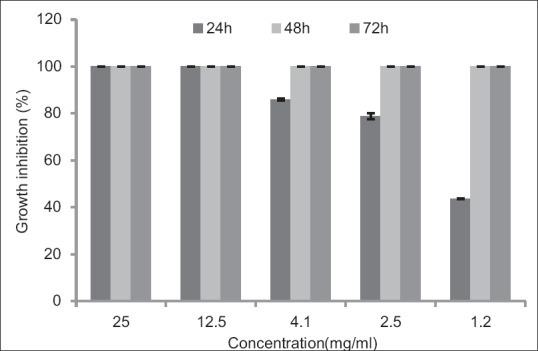
Effects of different concentrations of Eucalyptus camaldulensis on Trichomonas vaginalis in culture medium following 24, 48, and 72 h. Results are presented as mean ± standard deviation (P<0.05)
Water extract of V. odorata root in a concentration of 0.3 mg/ml showed 100% GI after 24 h. Results of different concentrations of V. odorata have been presented in Figure 2.
Figure 2.
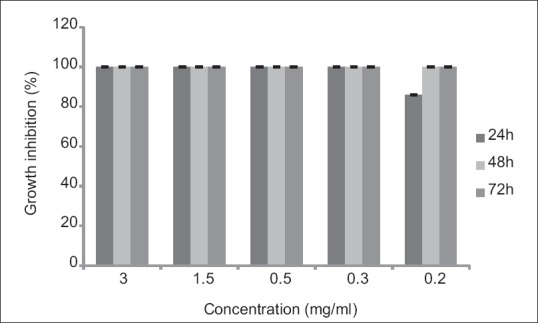
Effects of different concentrations of Viola odorata on Trichomonas vaginalis in culture medium following 24, 48, and 72 h. Results are presented as mean ± standard deviation
Hydroalcoholic extract of M. piperita in a concentration of 5 mg/ml showed 100% GI during 24 h. Results of different concentrations of M. piperita have been presented in Figure 3.
Figure 3.
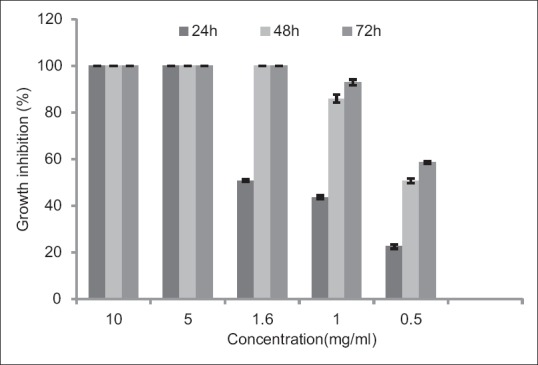
Effects of different concentrations of Mentha piperita on Trichomonas vaginalis in culture medium following 24, 48, and 72 h. Results are presented as mean ± standard deviation (P<0.05)
Mixed extracts with different concentrations including 4.1 mg E. camaldulensis, 0.1 mg V. odorata, 1.6 mg M. piperita (a); 2.5 mg E. camaldulensis, 0.06 mg V. odorata, 1 mg M. piperita (b); and 1.25 mg E. camaldulensis, 0.03 mg V. odorata, 0.5 mg M. piperita (c) showed 100%, 100%, and 92% GI, respectively, during 24 h. Results of different concentrations of mixed extracts have been presented in Figure 4.
Figure 4.
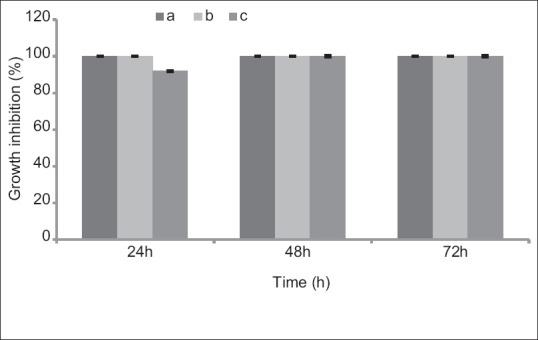
Effects of different concentrations of mixed extracts on Trichomonas vaginalis in culture medium following 24, 48, and 72 h. Results are presented as mean ± standard deviation. (a: 4.1 mg Eucalyptus camaldulensis, 0.1 mg Viola odorata, and 1.6 mg Mentha piperita. b: 2.5 mg Eucalyptus camaldulensis, 0.06 mg Viola odorata, and 1 mg Mentha piperita. c: 1.25 mg Eucalyptus camaldulensis, 0.03 mg Viola odorata, and 0.5 mg Mentha piperita)
Four of the six formulations passed all of physicochemical tests and one of them is the best formulation. The best formulation designated as selected formulation forin vitro GI test.
GC method developed in this study was applied for quantification of 1,8-cineole as the bioactive marker in formulations. 1,8-cineole and dodecane peaks in GC chromatogram appeared at a retention time of 11.17 and 14.52 min, respectively. The calibration curve was determined by linear regression in the range of 2.5–75 μg/ml. The regression equation was y = 0.0121× −0.019, where × was the concentration of 1,8-cineole in sample (μg/ml) with the correlation cofactor (R2) of 0.983.
Based on standard curve, each gram of cream was contained 40.08 μg of 1,8-cineole, and after 8 h, 97.6% ± 0.2% of 1,8-cineole was released. Results of this test have been presented in Figure 5.
Figure 5.
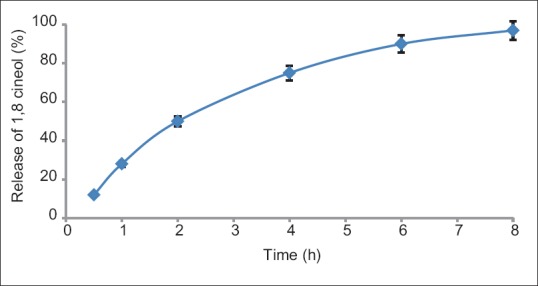
In vitro release of selected formulation containing stearic acid, triethanolamine, propylene glycol, purified water and 2.5 mg Eucalyptus camaldulensis, 0.06 mg Viola odorata, and 1 mg Mentha piperita/1 g of cream. Results are presented as mean ± standard deviation
The selective formulation and positive control showed 100% GI after 24 h.
Discussion
In this investigation, design, formulation, and physicochemical evaluation of vaginal cream containing E. camaldulensis, V. odorata, and M. piperita extracts as an alternative to metronidazole for the treatment of trichomoniasis has been investigated. At concentrations of 12.5, 25 mg/ml of Eucalyptus camaldulensis, 0.3, 0.5, 1.5, 3 mg/ml of Viola odorota, 5, 10 mg/ml of Mentha piperita and mixed extracts (a) and (b) showed 100% of GI after 24 h. Mixed extract (c) showed 92% and 100% GI after 24 and 48 h, respectively. The least GI% was shown by concentration of 0.5 mg/ml of M. piperita. M. piperita in a concentration of 1 mg/ml showed 85.9% and 92.9% of GI after 48 and 72 h, respectively, and in a concentration of 0.5 mg/ml showed 22.5%, 50.7%, and 58.5% of GI after 24, 48, and 72 h, respectively.
Ethyl acetate with a polarity of 4.4 and water with a polarity of 10.2 extracted the polar compounds of E. camaldulensis, V. odorata, and M. piperita including phenolic compounds. Furthermore, other polar compounds such as allicin and ajoene, which exist in Allium hirtifolium, could exhibit anti-trichomonas activity in comparison to metronidazole.[38] Furthermore, it had been reported in another study that aqueous extract of Mosla chinensis maxim at a concentration of 62.5 mg/ml had activity against T. vaginalis.[39] Another study reported that ethyl acetate extract of Arbutus unedo leaves showed 100% GI at a concentration of 500 μg/ml against T. vaginalis.[40] Furthermore, aqueous extract of Nigella sativa in a concentration of 10 mg/ml showed 100% inhibited trichomonas growth after 24 h.[32] It has been reported in another study that berberine isolated from Berberis aristata hadin vitro activity compared with metronidazole on T. vaginalis.[41] Another study showed that methanol extract of Vernonia amygdalina had activity against T. vaginalis.[42]
Physical appearance, uniformity, and homogeneity of selective formulation containing stearic acid, triethanolamine, propylene glycol, purified water, and mixed extracts were good. The formulation remained stable after centrifugal test. This formulation showed no change in visual quality after stability tests such as temperature changes, heating and cooling, and melting and freezing tests. The selective formulation in anti-trichomonas assay showed 100% GI after 24 h in vitro anti-trichomonas test.
Conclusions
The prepared vaginal formulation containing stearic acid, triethanolamine, propylene glycol, purified water and 2.5 mg E. camaldulensis, 0.06 mg V. odorata, and 1 mg M. piperita/1 g of cream passed all pharmacopeial tests and showed 100% GI after 24 h in vitro anti-trichomonas test.
Financial support and sponsorship
This study was supported by Isfahan University of Medical Sciences as a thesis research project numbered 394487.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Top EV, Gentry-Nielsen M, Knoop FC. Omaha, USA: Elsevier Inc; 2007. Trichomoniasis. [Google Scholar]
- 2.Patel SR, Wiese W, Patel SC, Ohl C, Byrd JC, Estrada CA, et al. Systematic review of diagnostic tests for vaginal trichomoniasis. Infect Dis Obstet Gynecol. 2000;8:248–57. doi: 10.1155/S1064744900000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrin D, Delgaty K, Bhatt R, Garber G. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300–17. doi: 10.1128/cmr.11.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwebke JR, Burgess D. Trichomoniasis. Clin Microbiol Rev. 2004;17:794–803. doi: 10.1128/CMR.17.4.794-803.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Upcroft P, Upcroft JA. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin Microbiol Rev. 2001;14:150–64. doi: 10.1128/CMR.14.1.150-164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards DI. Nitroimidazole drugs-action and resistance mechanisms. I. Mechanism of action. J Antimicrob Chemother. 1993;31:9–20. doi: 10.1093/jac/31.1.9. [DOI] [PubMed] [Google Scholar]
- 7.Grossman JH, 3rd, Galask RP. Persistent vaginitis caused by metronidazole-resistant trichomonas. Obstet Gynecol. 1990;76:521–2. [PubMed] [Google Scholar]
- 8.Kurohara ML, Kwong FK, Lebherz TB, Klaustermeyer WB. Metronidazole hypersensitivity and oral desensitization. J Allergy Clin Immunol. 1991;88:279–80. doi: 10.1016/0091-6749(91)90341-k. [DOI] [PubMed] [Google Scholar]
- 9.Vermani K, Garg S. Herbal medicines for sexually transmitted diseases and AIDS. J Ethnopharmacol. 2002;80:49–66. doi: 10.1016/s0378-8741(02)00009-0. [DOI] [PubMed] [Google Scholar]
- 10.Brooker MI, Kleinig DA. South-Eastern Australia: Inkata Press Pty Ltd; 1983. Field Guide to Eucalypts. Vol. 1. [Google Scholar]
- 11.Chevallier A, Association RsD. London: Dorling Kindersley; 1996. The Encyclopedia of Medicinal Plants. [Google Scholar]
- 12.Conner D. New York: Marcel Dekker; 1993. Naturally occurring compounds. Food Science and Technology; p. 441. [Google Scholar]
- 13.Mahdi NK, Gany ZH, Sharief M. Alternative drugs against Trichomonas vaginalis. East Mediterr Health J. 2006;12:679–84. [PubMed] [Google Scholar]
- 14.Youse HA, Kazemian A, Sereshti M, Rahmanikhoh E, Ahmadinia E, Rafaian M, et al. Effect of Echinophora platyloba, Stachys lavandulifolia, and Eucalyptus camaldulensis plants on Trichomonas vaginalis growth in vitro . Adv Biomed Res. 2012;1:79. doi: 10.4103/2277-9175.102987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassani S, Asghari G, Yousefi H, Kazemian A, Rafieiean M, Darani HY, et al. Effects of different extracts of Eucalyptus camaldulensis on Trichomonas vaginalis parasite in culture medium. Adv Biomed Res. 2013;2:47. doi: 10.4103/2277-9175.114187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asakawa B, Asakawa S. Franklin: Cool Springs Press; 2001. California Gardener's Guide. [Google Scholar]
- 17.Ireland DC, Colgrave ML, Craik DJ. A novel suite of cyclotides from Viola odorata: Sequence variation and the implications for structure, function and stability. Biochem J. 2006;400:1–2. doi: 10.1042/BJ20060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karioti A, Furlan C, Vincieri FF, Bilia AR. Analysis of the constituents and quality control of Viola odorata aqueous preparations by HPLC-DAD and HPLC-ESI-MS. Anal Bioanal Chem. 2011;399:1715–23. doi: 10.1007/s00216-010-4473-2. [DOI] [PubMed] [Google Scholar]
- 19.Pränting M, Lööv C, Burman R, Göransson U, Andersson DI. The cyclotide cycloviolacin O2 from Viola odorata has potent bactericidal activity against Gram-negative bacteria. J Antimicrob Chemother. 2010;65:1964–71. doi: 10.1093/jac/dkq220. [DOI] [PubMed] [Google Scholar]
- 20.Amin GR, Surmaghi MS, Yasa N, Sharifabadi AD, Emami M, Shidfar M, et al. Screening of Iranian Plants for Antifungal Activity: Part 2. Daru Journal of faculty of pharmacy. 2002;10:78–89. [Google Scholar]
- 21.Salehi L, Asghari G, Yousofi H, Yousofi-Darani H. The effects of different extracts of Viola odorata on Trichomonas vaginalis in culture medium. J Isfahan Med Sch. 2014;31:2139–48. [Google Scholar]
- 22.Pharmacopoeia A. 1st ed. Lagos- Nigeria: 1985. Pharmacopee Africanine OAU/STR Scientific Publication. Prepared by Inter African Committee on Medicinal Plants and African Traditional Medicine; p. 1. [Google Scholar]
- 23.Singh R, Shushni MA, Belkheir A. Antibacterial and antioxidant activities of Mentha piperita L. Arabian J Chem. 2015;8:322–8. [Google Scholar]
- 24.Karuza L, Blažević N, Šoljić Z. Isolation and structure of flavonoids from peppermint (Mentha x piperita) leaves. Acta Pharm. 1996;46:315–20. [Google Scholar]
- 25.Hoffmann D. London: Element Books LTD; 1996. The Complete Illustrated Holistic Herbal: A Safe and Practical Guide to Making and Using Herbal Remedies. [Google Scholar]
- 26.Bove M. New Canan, CT: Keats Publishing, Inc; 1996. An Encyclopedia of Natural Healing for Children and Infants. [Google Scholar]
- 27.Yousefi M, Arefkhah N, Rahimian R, Davoudian A, Rafiean M, Yousefi Darani H. In vitro effect of Mentha piperita and Salvia officinalis extracts on Trichomonas vaginalis. J Isfahan Med Sch. 2013;31:811–4. [Google Scholar]
- 28.Vermani K, Garg S. The scope and potential of vaginal drug delivery. Pharm Sci Technolo Today. 2000;3:359–64. doi: 10.1016/s1461-5347(00)00296-0. [DOI] [PubMed] [Google Scholar]
- 29.Das K, Dang R, Machale M, Ugandar R, Lalitha B. Evaluation for safety assessment of formulated vanishing cream containing aqueous Stevia extract for topical application. Indian J Novel Drug Deliv. 2012;4:43–51. [Google Scholar]
- 30.Moon T, Wilkinson JM, Cavanagh HM. Antiparasitic activity of two lavandula essential oils against Giardia duodenalis, Trichomonas vaginalis and Hexamita inflata. Parasitol Res. 2006;99:722–8. doi: 10.1007/s00436-006-0234-8. [DOI] [PubMed] [Google Scholar]
- 31.Verma AR, Vijayakumar M, Mathela CS, Rao CV. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem Toxicol. 2009;47:2196–201. doi: 10.1016/j.fct.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Tonkal A. In vitro antitrichomonal effect of Nigella sativa aqueous extract and wheat germ agglutinin. J King Abdulaziz Univ Med Sci. 2009;16:17–34. [Google Scholar]
- 33.Jelvehgari M, Rashidi MR, Samadi H. Mucoadhesive and drug release properties of benzocaine gel. Iran J Pharm Sci. 2006;2:185–94. [Google Scholar]
- 34.Aslani A, Emami S, Ghannadi A, Ajdari M. Formulation and physicochemical evaluation of an herbal antihemorrohid ointment from Quercus, Black cumin and Fenugreek for the treatment of internal anal hemorrhoids. Pharm Sci Tabriz Univ Med Sci. 2009;14:247–57. [Google Scholar]
- 35.Saleem MA, Bala S, Liyakat, Aeajaz A. Effect of different carriers on in vitro permeation of meloxicam through rat skin. Indian J Pharm Sci. 2010;72:710–8. doi: 10.4103/0250-474X.84579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ariyana A, Sinurat D, Ervina I, Bangun H. Formulation and in vitro evaluation of alginate based Metronidazole periodontal gel. Asian J Pharm Clin Res. 2013;1:224–8. [Google Scholar]
- 37.Chen MX, Alexander KS, Baki G. Formulation and evaluation of antibacterial creams and gels containing metal ions for topical application. J Pharm (Cairo) 2016;2016:5754349. doi: 10.1155/2016/5754349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taran M, Rezaeian M, Izaddoost M. In vitro antitrichomonas activity of Allium hirtifloium (Persian Shallot) in comparison with Metronidazole. Iran J Public Health. 2006;35:92–4. [Google Scholar]
- 39.Zheng LL, Cui Y, Qin YH, Ren YX, Liu X, Tao L, et al. Effect of Mosla chinensis maxim on Trichomonas vaginalis in vitro . J Dalian Med Univ. 2009;31:282–5. [Google Scholar]
- 40.Ertabaklar H, Kivçak B, Mert T, Ozensoy Töz S. In vitro activity of Arbutus unedo leaf extracts against Trichomonas vaginalis trophozoites. Turkiye Parazitol Derg. 2009;33:263–5. [PubMed] [Google Scholar]
- 41.Soffar SA, Metwali DM, Abdel-Aziz SS, el-Wakil HS, Saad GA. Evaluation of the effect of a plant alkaloid (berberine derived from Berberis aristata) on Trichomonas vaginalis in vitro . J Egypt Soc Parasitol. 2001;31:893–904. [PubMed] [Google Scholar]
- 42.Hakizamung EP, Wery M. Screening of rwanndex medicinal plant for anti-trichomonal activity. J Ethnopharmacol. 1992;36:143–6. doi: 10.1016/0378-8741(92)90014-i. [DOI] [PubMed] [Google Scholar]


