Abstract
Thyroid nodules are common in the adult population where a majority are benign and only 4.0% to 6.5% are malignant. Fine needle aspiration (FNA) is a key method used in the early stages to evaluate and triage patients with thyroid nodules. While a definitive cytological diagnosis is provided in more than 70–75% of all thyroid FNA cases, the group of indeterminate lesions offers a challenge in terms of interpretation and clinical management. Molecular testing platforms have been developed, are recognized as an option by the 2015 American Thyroid Association Guidelines, and are frequently used in conjunction with FNA as an integral part of the cytologic evaluation. In this review, the utility of molecular testing options for nodules assigned to the group of indeterminate thyroid FNAs is described.
Keywords: fine needle aspiration, cytology, thyroid cancers, molecular testing, personalized medicine
1. Introduction
Fine-needle aspiration (FNA) is an important diagnostic tool for the initial evaluation of thyroid nodules because of its overall simplicity, safety, accuracy, and cost-effectiveness [1,2,3,4]. Approximately 70% of thyroid nodules are benign with only 5–10% reported as “malignant” [1,2,3,4,5,6]. The remaining 15–30% of thyroid FNAs are classified using the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) in the so-called “grey zone of indeterminate proliferations” for which a cytomorphological discrimination is not possible [7,8,9,10,11,12,13,14,15,16,17,18]. Thyroid nodules classified as indeterminate are the leading challenge for both cytopathologists and treating clinicians since the indeterminate diagnostic categories can lead to unnecessary surgical interventions and higher health care costs in almost 20–25% of indeterminate lesions.
The application of molecular analysis for thyroid FNA specimens was accepted in 2015 by the American Thyroid Association as well as other clinical endocrinology groups as a follow-up option for thyroid FNAs classified into one of the indeterminate categories [7,18]. The objective of molecular testing would be to provide more definitive guidance for the treating clinician to assist with decision making with regard to the management approach. Over the past decade, numerous studies have demonstrated the high sensitivity and overall diagnostic accuracy of molecular testing when applied to cytologic specimens [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. In fact, the application of ancillary molecular analysis is not unique to thyroid cytopathology, but it has become an integral part of the management of several different tumors such as lung and others. As molecular approaches continue to be improved, the molecular analysis of various tumors can be translated into clinical practice as an adjuvant tool for diagnosis, clinical management, specialized therapy, and prognosis [40].
While aspects of molecular testing were not sufficiently refined and vetted to be included in the first edition of TBSRTC, the role of molecular testing to assist in guiding management decisions including with the recently described non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), was included in the recent second edition of TBSRTC [41,42,43]. This review provides an overview of the key role that molecular testing is increasingly playing in the evaluation of thyroid FNA specimens, in particular those classified within the indeterminate categories of TBSRTC.
2. Molecular Testing and the Indeterminate Categories of the Bethesda System
In the USA, thyroid cancer (TC) accounts for 6% of cancers in women and less than 3% of cancers in men. Papillary TC (PTC) and Follicular TC (FTC) arise from follicular cells, and they constitute approximately 90% of all TC [1,2,3,4]. Data from the literature show that although TC generally has a very good prognosis, 10–15% of TCs are associated with recurrences and metastases to regional lymph nodes or distant sites including about 5% of patients with TCs that are not responsive to radioactive-iodine (RAI) and who may eventually die from their disease. For this reason, early diagnosis of TC and proper triaging of patients with thyroid nodules is needed. FNA plays a key role in differentiating benign from malignant thyroid nodules. However, morphology alone is not able to solve all of the diagnostic questions and the application of ancillary techniques, in particular molecular testing, is being increasingly used to improve the overall performance of FNA [42,43,44,45,46,47].
In 2014, the Thyroid Cancer Genome Atlas published findings that clarified our knowledge of the molecular pathology for PTC. The study examined a large cohort of PTCs and concluded that they can be separated into 2 distinct broad categories, those PTCs with a BRAFV600E-like profile and those that are RAS-like. In addition, most common mutations are shown to activate the mitogenic-activated protein kinase (MAPK) pathway in PTC [40]. In a meta-analysis by Trimboli et al., the authors found that a very low rate of lesions with indeterminate cytology are BRAF mutated. Thus, the role of this biomarker to detect or exclude cancers in patients with FNA reports of indeterminate lesions is limited [4]
As alluded to previously, in 2015, the American Thyroid Association (ATA) published revised management guidelines for patients with thyroid nodules and differentiated TCs (DTC) and recommended the use of molecular diagnostic as an option in FNA cases with an indeterminate cytology [7,19]. Specifically, the ATA Guidelines suggest that the performance of molecular panels (including markers such as BRAF, RAS, RET/PTC and PAX8-PPARγ) can significantly improve the accuracy of preoperative FNA in patients with indeterminate thyroid FNA samples. Furthermore, molecular testing could be used to stratify the risk of malignancy (ROM) of a particular indeterminate thyroid FNA, and when applied to those FNAs with a high negative predictive value (NPV), patients might avoid unnecessary surgical intervention, either lobectomy or total thyroidectomy. The 2015 ATA Guidelines did not endorse any specific molecular test, but, the recent 2nd edition of TBSRTC has suggested the potential role of different molecular tests in selected diagnostic categories and diagnostic scenarios. However, Ferris et al., in their ATA statement on surgical application of molecular profiling for thyroid nodules, did offer a suggestion for differential use of molecular testing in the diagnostic subcategories of TBSRTC indeterminate lesions. [19]. Familiarity with molecular testing options available for thyroid cytopathology as well as their indications, significance, and limitations is an important aspect of thyroid cytopathology, and may have implications for NIFTP [47,48,49,50,51,52,53,54,55,56,57,58,59,60].
Numerous studies have demonstrated that the detection of specific somatic mutations, gene rearrangements, and/or microRNA (miRNA) expression profiles can have high specificity and high positive predictive values for malignant thyroid disease [61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79]. The use of molecular testing panels rather than single gene mutation assays has provided the most successful results [61,62,63,64,65,66,67]. For example, Nikiforov et al., demonstrated the advantage of using a broad next-generation sequencing (NGS) panel that provides a highly accurate, informative and more comprehensive analysis of somatic mutations and chromosomal rearrangements in the diagnosis of nodules with AUS/FLUS and FN/SFN cytology, which ultimately facilitates the optimal management of these patients [68,69,70,71,72,73,74,75,76,77,78,79,80,81,82].
Despite the fact that currently there is no single, unequivocal molecular approach for the cytological evaluation of thyroid nodules, several molecular thyroid tests are commercially available in the USA including: a: ThyroSeq.v3 (University of Pittsburgh Medical Center [UPMC]/Cytopath Biopsy Lab [CBLPath], Pittsburgh, PA, USA); b: Afirma Gene Sequencing Classifier and Xpression Atlas (GSC & XA, Veracyte, South San Francisco, CA, USA); and c: ThyGenX and ThyraMIR (both from Interpace Diagnostics, Parsippany, NJ) [82,83]. These tests offer high NPV’s as well as reasonable positive predictive values (PPV) for the evaluation of indeterminate thyroid FNA cases [84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102]. The acknowledgement of the role of molecular testing formalizes its use in defining risk stratification of patients with thyroid nodules.
2.1. AUS/FLUS
The AUS/FLUS diagnostic category of TBSRTC was endorsed in the 2017 2nd edition of TBSRTC with the addition of only minor changes. The category continues to pose diagnostic challenges for cytopathologists and management challenges for treating clinicians. The AUS/FLUS category is often the subject of investigation of ancillary studies to improve the triage of patients receiving this diagnosis.
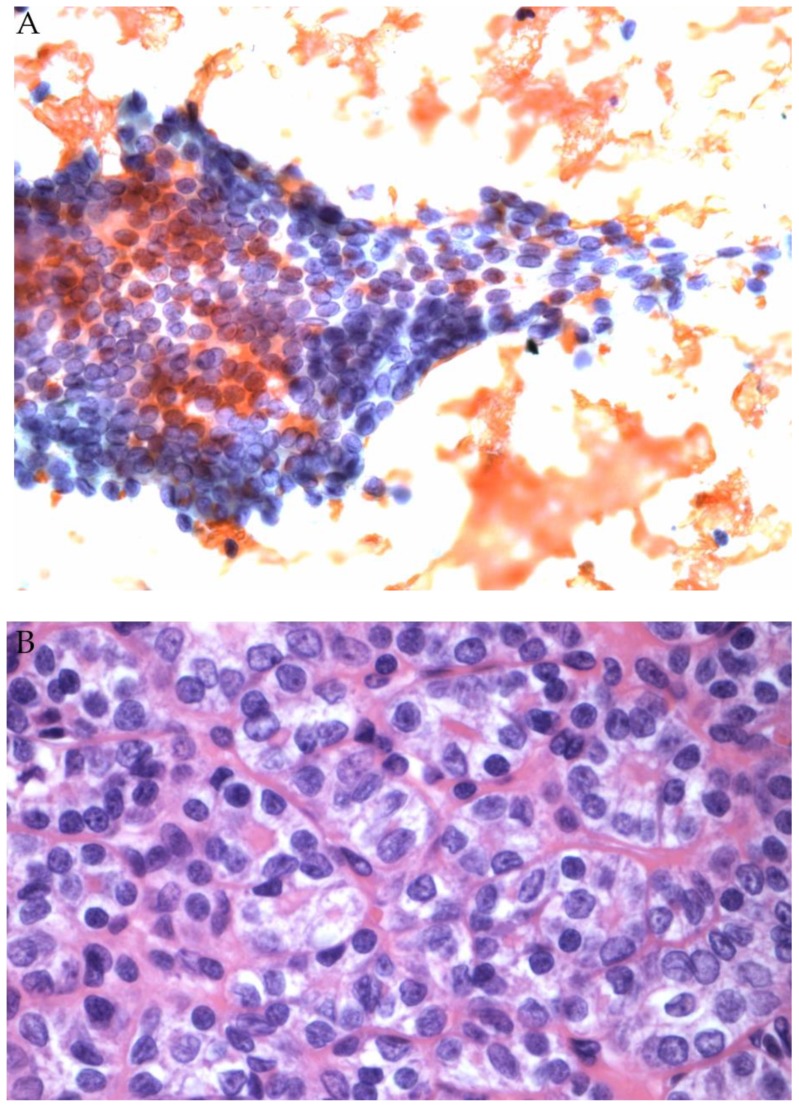
In agreement with the 2015 ATA guidelines, an initial AUS/FLUS interpretation is typically followed by either repeat FNA or molecular testing [7]. Over 50% of nodules initially diagnosed as AUS/FLUS would be reclassified as Benign by repeat FNA; however, approximately 10–30% of cases would be classified as AUS/FLUS by repeat FNA. In those cases and other scenarios with an AUS/FLUS classification, the application of mutational testing could provide additional diagnostic information that could impact clinical management decisions including clinical follow-up versus surgical resection (Figure 1).
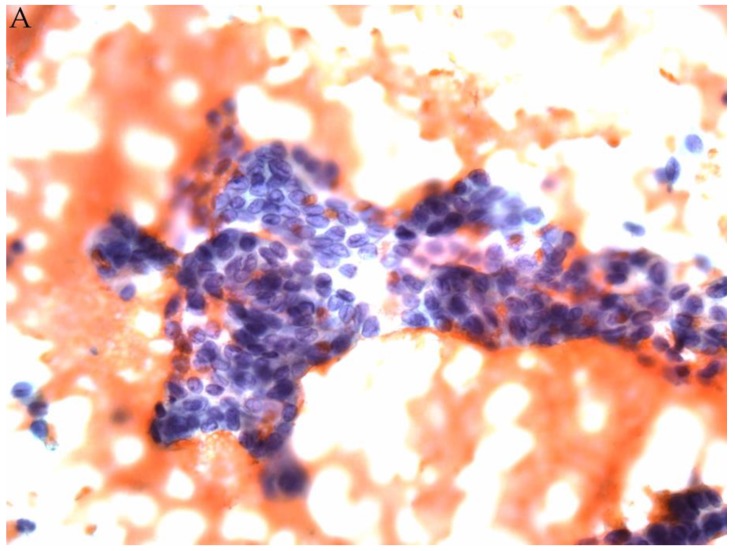
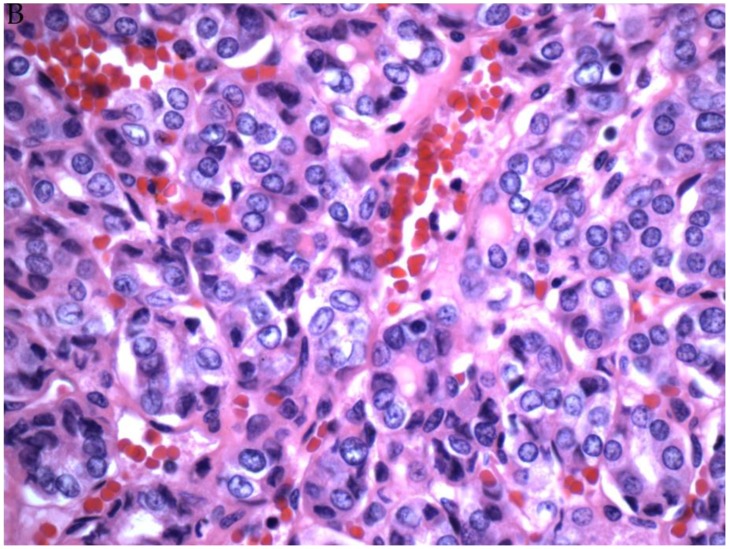
Figure 1.
(A) FNA of a thyroid nodule classified as AUS/FLUS (Papanicolaou stain). Afirma testing was Suspicious. (B) Corresponding histology showed an encapsulated follicular variant of papillary thyroid carcinoma (H&E stain).
The second edition of suggests that decisions regarding surgery (typically lobectomy) vs. continued observation should be based on a combination of cytologic, molecular, clinical, and radiologic findings that cannot exclude the evaluation of clinical risk factors and patient preferences [41]. It is also worth noting that the ROM associated with a thyroid nodule classified as AUS/FLUS varies significantly with the subtype of AUS/FLUS, ranging from a mean ROM of 47% for AUS/FLUS with cytologic atypia to only 5% for AUS/FLUS with Hürthle cell features [6,7,8,9,10,11].
Several studies have confirmed that the AUS/FLUS category represents a low risk of malignancy and, when malignant, the follow-up histological diagnosis is most often FVPTC, so that an expanded mutation panel might have higher sensitivity than BRAFV600E alone but diminished specificity due to the increased prevalence of RAS mutations.
In 2007, Nikiforov et al., developed a 7-gene molecular test (ThyroSeq v0), composed of a panel of mutations (BRAF, N-/H-/K-RAS) as well as the translocations RET/PTC and PAX8/PPAR [32]. The authors included a series of 1056 indeterminate thyroid lesions, classified according to TBSRTC, and they found an increased ROM for mutated AUS/FLUS, FN, and SM cases (88%, 87%, and 95% respectively), compared to 6%, 14%, and 28% in mutation-negative thyroid nodules [32]. Among the 1056 indeterminate lesions, the cohort included 653 cytological cases of AUS/FLUS with 247 that had histological follow-up. The detection of any mutations increased the risk of cancer from 14% to 87%, whereas the absence of any mutations was linked with a cancer risk of 6%.
In 2013, Nikiforov et al., began applying next generation sequencing (NGS) technology as a new approach for testing a broad spectrum of point mutations [34]. In 2014, the first mutational panel, ThyroSeq v1 including 15-genes was followed by a new and superior NGS-based assay, ThyroSeq v2, that was applied initially to 143 cases of FN/SFN with the evaluation of an expanded 56-gene panel to include several point mutations and gene fusions with high NPV [35]. In 2015, Nikiforov et al., evaluated the impact of ThyroSeq v2 on the AUS/FLUS category showing that the test performance was dependent on the pre-test probability of malignancy for this category [36]. Their analysis demonstrated a sensitivity of 90.9%, specificity of 92.1%, PPV of 76.9%, and NPV of 97.2% with an overall accuracy of 91.8%. The disease prevalence was in agreement with the Bethesda diagnostic categories.
In 2017, the same group released their most recent version, the ThyroSeq v.3 test, which includes more than 12000 mutation hotspots and more than 120 gene fusion types. In a recent prospective study, including 10 medical centers with 286 cytologically indeterminate lesions, Steward et al., found that in the Bethesda III and IV categories combined, ThyroSeq v.3 demonstrated a 94% sensitivity and 82% specificity [84]. The conclusion is that application of ThyroSeq v.3 could potentially obviate surgery in up to 61% of those patients with an indeterminate thyroid cytology [84].
The Afirma Gene Expression Classifier (GEC) is a commonly used molecular test for indeterminate thyroid proliferations based on the concept of predicting benign thyroid lesions [37,85,86,87,88,89,90,91]. In 2012, the Afirma GEC was validated in a key study published by Alexander et al., including 265 indeterminate thyroid lesions out of 4812 FNA cases from a multicenter trial [37]. The original Afirma test was based on the expression of 167 genes including 142 genes in the main classifier (benign or suspicious) and 25 smaller gene expression panels to filter out rare neoplasms [37]. Alexander and colleagues in 2012 demonstrated a 95% NPV for AUS/FLUS lesions and a 94% NPV for FN with an associated malignancy rate of 24% and 25%, respectively [37]. Of note, the authors concluded that the Afirma GEC identified thyroid nodules as benign with a very low ROM in the AUS/FLUS and SFN/FN categories. In contrast, the test’s NPV was much lower for the suspicious for malignancy (SFM) category indicating that it would not be as useful for nodules classified as SFM.
Since its initial introduction, many published studies have demonstrated the utility of the Afirma GEC for AUS/FLUS lesions. The data confirm that approximately one-half of AUS/FLUS cases have a “Benign” GEC result, and that it is more frequently ascribed to those AUS/FLUS cases with isolated architectural atypia than those AUS/FLUS cases with nuclear atypia or nuclear plus architectural atypia. Specifically, the rate of Benign GEC results was higher for AUS/FLUS thyroid nodules with architectural atypia (65%) than in AUS/FLUS nodules with nuclear atypia (59%) or AUS/FLUS with both nuclear and architectural atypia (38%). In patients who had GEC Suspicious nodules, the ROM is higher in cases with both architectural and nuclear atypia (57%) than in cases with architectural or nuclear atypia alone (19% and 45% respectively) [87]. Based on evidence that a benign result is associated with a ROM decreasing from 24% to 5%, observation over surgery is considered an appropriate choice for patients with a Benign GEC test result [48].
Recently, a next-generation Afirma Genomic Sequencing Classifier (GSC) was introduced as a new advancement. The Afirma GSC combines not only gene expression, but also the presence of DNA variants, fusions, copy number variants, and other information that may be predictive of thyroid cancer. Specifically, the Afirma GSC does not use expression pattern (RNA microarray) but it uses RNA-seq analysis [92]. The purpose of GSC is to maintain the high original test sensitivity while also significantly increasing its specificity. This results in approximately 70% of patients with indeterminate cytology avoiding unnecessary surgery [92,93]. Endo et al., from a single academic tertiary center, found an improved specificity and PPV while maintaining high sensitivity and NPV for GSC compared with GEC. A statistically significant increase in benign call rates was observed in GSC compared with GEC, likely indicating fewer false positive results. After implementation of the Afirma GSC, surgical interventions have been reduced by 68% [94].
2.2. Follicular Neoplasm (FN/SFN)
The FN/SFN diagnostic category is another indeterminate category of TBSRTC where the application of molecular testing has been useful. In a study analyzing the use of Thyroseq v0, Nikiforov et al., included 247 FN/SFN cases with 214 having histological follow-up [32]. Among the 38 resected nodules that were associated with a defined molecular alteration, 33 (87%) were found to be histologically malignant, and all BRAF and PAX8/PPARγ-positive nodules were malignant [32]. The ThyroSeq mutational analysis from these FNA studies was associated with 57% sensitivity, 97% specificity, 86% diagnostic accuracy, 87% PPV and 86% NPV [32].
In 2014, the analysis of 143 FN/SFN with ThyroSeq v2 in a series of 143 retrospectively and prospectively collected FN/SFN nodules showed a performance of 90% sensitivity, 93% specificity, 83% PPV and 96% NPV [35]. The authors confirmed in this series that point mutations in BRAFV600E, TERT, TP53, PIK3CA and any gene fusion were associated with cancer in 100% of thyroid FNA cases [35]. The results of this study indicate that, due to its high PPV and NPV, ThyroSeq v2 and the more recent ThyroSeq v3 are essentially able to perform as both “rule-out” and “rule-in” tests for the FN/SFN category (Figure 2) [35,65,66,67,68,69,70,71,72,73,74,75,76,95,96,97,98,99]. While the application of molecular testing identifying point mutations and rearrangements was proving to be useful for identifying those FN/SFNs with a microfollicular architecture, there were signs that its ability to distinguish Hurthle cell carcinomas from Hurthle cell adenomas was more challenging. However, these issues were addressed by Thyroseq v3, which includes assessment of gene copy number variations, a feature useful in the evaluation of Hurthle cell neoplasms. The Afirma GSC test also addressed the problem of overclassifying Hurthle cell lesions as Suspicious. Several studies reported a lower specificity or higher false positive rate in GEC tests among indeterminate nodules with Hurthle cell predominance. Brauner et al., included a cohort of 122 Hurthle cell-predominant nodules identified as GEC Suspicious with a corresponding benign histopathology [95]. Nonetheless, the development of the Afirma GSC was able to improve the test performance for Hürthle-cell lesions with increased specificity of 59% compared with just 12% with the original Afirma GEC [92,93].
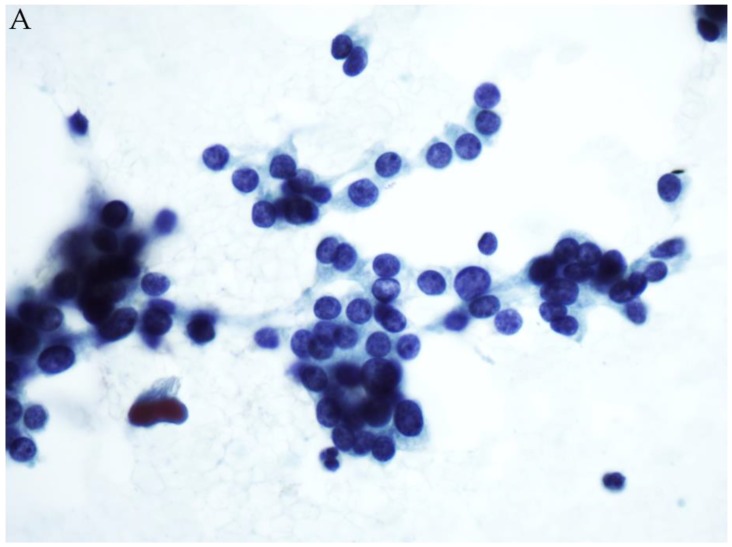
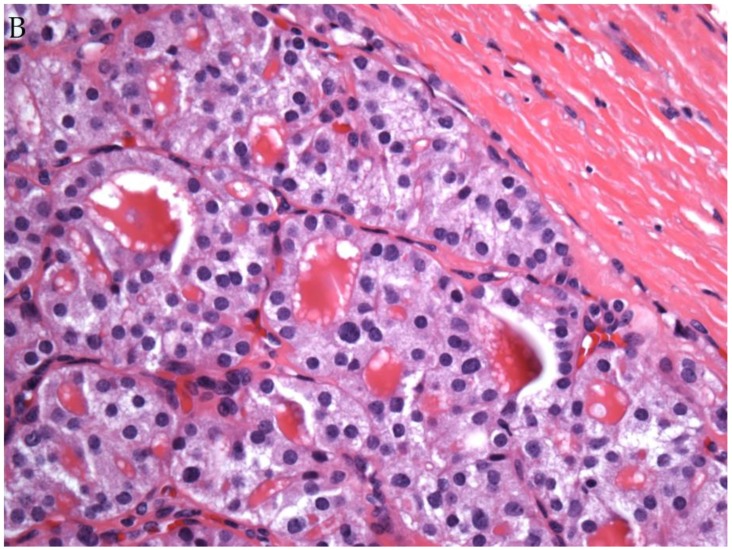
Figure 2.
(A) FNA of a thyroid nodule classified as FN/SFN (Papanicolaou stain). ThyroSeq v3 testing showed an H-RAS mutation. (B) Corresponding histology showed a follicular adenoma (H&E stain).
Other authors found that RosettaGX Reveal, which was a microRNA-based diagnostic assay, was also used to further evaluate cytologically indeterminate thyroid nodules and that it might prevent over 75% of unnecessary surgeries for initial indeterminate diagnoses. Lithwick-Yanai et al., using the microRNA-based assay for the AUS/FLUS and FN/SFN categories, showed that the sensitivity and specificity were both 74%, with a NPV of 92% and PPV of 43% [94]. RosettaGXReveal had the advantage of using FNA material obtained by scraping archival cytology slides. Although having several promising advantages, the test is unfortunately no longer commercially available.
Another molecular testing option which has received some attention is ThyGenX (Interpace Diagnostics, Parsippany, NJ), a thyroid 8-gene panel representing a “newer version” of the original gene panel test to detect genetic alterations. This is another NGS-based technology, commercially known as miRInform (Asuragen, Austin, Tex, USA). Based on its specific methodology, the detection of BRAFV600E or RET/PTC is associated with 100% ROM, but the ROM is lower and wider for RAS mutations (range, 12–87.5%) and PAX8/PPARg rearrangements (range, 50–100%). The ROM for wild type AUS/FLUS is only slightly higher than that of an FNA classified as benign while the ROM for a wild type FN/SFN is identical to the non-tested cases.
Based on these results, Interpace Diagnostics propose ThyraMIR (from Interpace Diagnostics, Parsippany, NJ, USA) as an additional reflex test, for those cases with a wild type/negative ThyGenX result [96,97]. ThyraMIR is a thyroid microRNA (miRNA) classifier that is able to divide results into “positive” or “negative” categories. Different studies have demonstrated the successful application of miRNA detection on different cytological material, including indeterminate thyroid FNA lesions. Aberrant expression of specific miRNAs (e.g., miR-146, 221, 222) are considered a clue to thyroid well differentiated carcinomas [65,66,67,68,69,70,71,72,73,74,75,76].
High sensitivity and specificity are obtained using the combination testing of ThyGenX and ThyraMIR as demonstrated in two different studies that combined these tests for indeterminate thyroid nodules [96,97]. They demonstrated high sensitivity (94% for AUS/FLUS and 82% for SFN/FN) and specificity (80% for AUS/FLUS and 91% for SFN/FN), with a PPV of 74% and NPV of 94% [96,97]. The application of multi-panel testing not only provides important information about specific mutations being present, but also the prognostic relevance of some of these mutations that would suggest management option such as a total thyroidectomy versus lobectomy [96,97].
2.3. The Application of Molecular Testing to NIFTP
The new terminology of “noninvasive follicular thyroid neoplasm with papillary-like nuclear features” (NIFTP) was introduced to replace the encapsulated-noninvasive follicular variant of PTC (FVPTC). NIFTPs are biologically similar to follicular adenomas, lacking features of carcinoma such as lymph node metastasis and/or recurrence. NIFTP is a histological diagnosis defined by strict major and minor histological criteria. FNA cannot be used to specifically diagnose NIFTP because the diagnosis is made based on encapsulation of the tumor. However, NIFTP has had significant impact on thyroid cytology including on the ROM of the different diagnostic categories in TBSRTC. A wide range of studies have demonstrated that the majority of NIFTP are classified cytologically in the indeterminate categories with 31% in the AUS/FLUS, 26.6% in the FN/SFN and 24.3% in the SFM [56,57,58,59,62,63,64].
As stated in TBSRTC, a definitive diagnosis of NIFTP is not possible based upon the cytomorphologic features found in thyroid FNA samples. However, the presence of nuclear pseudoinclusions and papillary cytoarchitecture which are typical features of classical PTC can be used to exclude NIFTP. A predominant follicular pattern and less frequent nuclear elongation and grooves increase the likelihood that the lesion sampled by FNA will be NIFTP [56,98,99,100,101,102,103,104,105,106,107]. Given the prevalence of NIFTP among indeterminate thyroid FNA samples, caution should be exercised to avoid overtreatment. The evaluation of somatic mutations and/or chromosomal rearrangements have shown that NIFTP has a molecular profile that usually differs from classical PTC. In fact, while certain molecular features such as BRAFv600E mutation are diagnostic of PTC, specific molecular signatures are not available for NIFTP (Figure 3). Despite the absence of any specific genetic alterations linked with NIFTP, molecular testing has been used to distinguish potential cases of NIFTP from other neoplasms [108,109,110,111,112,113]. In general, molecular testing strategies including Afirma GSC and Thyroseq v.3 will likely identify potential FNA cases of NIFTP as atypical, and will triage patients for surgery [108,109,110,111,112,113].
Figure 3.
(A) FNA of a thyroid nodule classified as AUS/FLUS (Papanicolaou stain). Afirma testing was Suspicious. (B) Histology revealed NIFTP (H&E stain).
Like encapsulated FVPTC, NIFTP has a different molecular profile from classical PTC [113,114,115]. However, the distinction between invasive forms of FVPTC and NIFTP (i.e., encapsulated non-invasive FVPTC) can only be made based upon histomorphological evaluation. In a study by Kim TH et al., including 177 consecutive FVPTCs (74 non-invasive encapsulated, 51 invasive encapsulated, 52 infiltrative), they showed that any type of RAS mutation (NRAS, HRAS and KRAS mutations) was observed more often in encapsulated FVPTC (48.6% in non-invasive and 66.7% in invasive) and NIFTP than in infiltrative FVPTC (15.4%). Concerning BRAFV600E mutation, its identification in a thyroid FNA sample can be used to exclude NIFTP. This is also true for RET-PTC rearrangements. Molecular features of NIFTP include alterations in RAS, PAX8/PPAηo or BRAFK601E, in contrast to the frequent BRAFV600E and RET/PTC alterations observed in classical PTC. For this reason, molecular testing such as ThyroSeq v3, the Afirma Xpression Atlas, or ThyGenX could serve as a guide in selected thyroid cases for surgical management (total thyroidectomy vs. hemithyroidectomy).
3. Conclusions
The diagnosis of indeterminate lesions of the thyroid is a challenge in cytopathology practice. The evaluation of morphological features alone is unable to provide definitive classification in many cases. The application of ancillary molecular testing for indeterminate thyroid FNA specimens has provided better stratification and triage of patients. Several mutation analysis panels are not only helpful as diagnostic tests, but may also serve as prognostic markers. Much progress has been made in the continual development of molecular testing platforms including the Afirma GSC & Xpression Atlas, ThyroSeq v.3, and ThyGenX/ThyroMIR. Each test has different advantages and limitations in the evaluation of indeterminate thyroid FNA samples. As these molecular tests are improved further making them more accurate and less expensive, they will continue to become a more integral part of the thyroid nodule evaluation.
Funding
This research was funded in part by the Elizabeth and Michael Ruane Center for Endocrine Tumors Fund.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Cramer H. Fine-needle aspiration cytology of the thyroid: An appraisal. Cancer. 2000;90:325–329. doi: 10.1002/1097-0142(20001225)90:6<325::AID-CNCR1>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 2.Gharib H., Papini E., Paschke R. Thyroid nodules: A review of current guidelines, practices, and prospects. Eur. J. Endocrinol. 2008;159:493–505. doi: 10.1530/EJE-08-0135. [DOI] [PubMed] [Google Scholar]
- 3.Trimboli P., Treglia G., Condorelli E., Romanelli F., Crescenzi A., Bongiovanni M., Giovanella L. BRAF-mutated carcinomas among thyroid nodules with prior indeterminate FNA report: A systematic review and meta-analysis. Clin. Endocrinol. 2016;84:315–320. doi: 10.1111/cen.12806. [DOI] [PubMed] [Google Scholar]
- 4.Broome J.T., Solorzano C.C. The impact of atypia/follicular lesion of undetermined significance on the rate of malignancy in thyroid fine-needle aspiration: Evaluation of the Bethesda System for Reporting Thyroid Cytopathology. Surgery. 2011;150:1234–1241. doi: 10.1016/j.surg.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Ravetto C., Colombo L., Dottorini M.E. Usefulness of fine-needle aspiration in the diagnosis of thyroid carcinoma: A retrospective study in 37,895 patients. Cancer. 2000;90:357–363. doi: 10.1002/1097-0142(20001225)90:6<357::AID-CNCR6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Poller D.N., Ibrahim A.K., Cummings M.H., Mikell J.J., Boote D., Perry M. Fine-needle aspiration of the thyroid. Importance of an indeterminate diagnostic category. Cancer Cytopathol. 2000;90:239–244. doi: 10.1002/1097-0142(20000825)90:4<239::AID-CNCR7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 7.Haugen B.R., Alexander E., Bible K.C., Doherty G.M., Mandel S.J., Nikiforov Y.E., Pacini F., Randolph G.W., Sawka A.M., Schlumberger M., et al. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho A.S., Sarti E.E., Jain K.S., Wang H., Nixon I.J., Shaha A.R., Shah J.P., Kraus D.H., Ghossein R., Fish S.A., et al. Malignancy Rate in Thyroid Nodules Classified as Bethesda Category III (AUS/FLUS) Thyroid. 2014;24:832–839. doi: 10.1089/thy.2013.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song J.Y., Chu Y.C., Kim L., Park I.S., Han J.Y., Kim J.M. Reclassifying Formerly Indeterminate Thyroid FNAs Using the Bethesda System Reduces the Number of Inconclusive Cases. Acta Cytol. 2012;56:122–129. doi: 10.1159/000334200. [DOI] [PubMed] [Google Scholar]
- 10.Shi Y., Ding X., Klein M., Sugrue C., Matano S., Edelman M., Wasserman P. Thyroid fine-needle aspiration with atypia of undetermined significance: A necessary or optional category? Cancer. 2009;117:298–304. doi: 10.1002/cncy.20039. [DOI] [PubMed] [Google Scholar]
- 11.Damiani D., Suciu V., Vielh P. Cytopathology of Follicular Cell Nodules. Endocr. Pathol. 2015;26:286–290. doi: 10.1007/s12022-015-9386-3. [DOI] [PubMed] [Google Scholar]
- 12.Nagarkatti S.S., Faquin W.C., Lubitz C.C., Garcia D.M., Barbesino G., Ross D.S., Hodin R.A., Daniels G.H., Parangi S. Management of Thyroid Nodules with Atypical Cytology on Fine-needle Aspiration Biopsy. Ann. Surg. Oncol. 2013;20:60–65. doi: 10.1245/s10434-012-2601-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olson M.T., Clark D.P., Erozan Y.S., Ali S.Z. Spectrum of Risk of Malignancy in Subcategories of ‘Atypia of Undetermined Significance’. Acta Cytol. 2011;55:518–525. doi: 10.1159/000333232. [DOI] [PubMed] [Google Scholar]
- 14.Horne M.J., Chhieng D.C., Theoharis C., Schofield K., Kowalski D., Prasad M.L., Hammers L., Udelsman R., Adeniran A.J. Thyroid follicular lesion of undetermined significance: Evaluation of the risk of malignancy using the two-tier sub-classification. Diagn. Cytopathol. 2012;40:410–415. doi: 10.1002/dc.21790. [DOI] [PubMed] [Google Scholar]
- 15.Hyeon J., Ahn S., Shin J.H., Oh Y.L. The prediction of malignant risk in the category “atypia of undetermined significance/follicular lesion of undetermined significance” of the Bethesda System for Reporting Thyroid Cytopathology using subcategorization and BRAF mutation results. Cancer Cytopathol. 2014;122:368–376. doi: 10.1002/cncy.21396. [DOI] [PubMed] [Google Scholar]
- 16.Dincer N., Balci S., Yazgan A., Guney G., Ersoy R., Cakir B., Guler G. Follow-up of atypia and follicular lesions of undetermined significance in thyroid fine needle aspiration cytology. Cytopathology. 2013;24:385–390. doi: 10.1111/cyt.12021. [DOI] [PubMed] [Google Scholar]
- 17.Gocun P.U., Karakuş E., Bulutay P., Akturk M., Akin M., Poyraz A., Gocun P.U. What is the malignancy risk for atypia of undetermined significance? Three years’ experience at a university hospital in Turkey. Cancer Cytopathol. 2014;122:604–610. doi: 10.1002/cncy.21434. [DOI] [PubMed] [Google Scholar]
- 18.Correia-Rodrigues H.G., Nogueira De Pontes A.A., Adan L.F. Use of molecular markers in samples obtained from preoperative aspiration of thyroid. End. J. 2012;59:417–424. doi: 10.1507/endocrj.EJ11-0410. [DOI] [PubMed] [Google Scholar]
- 19.Ferris R.L., Baloch Z.W., Bernet V., Chen A., Fahey T.J., 3rd, Ganly I., Hodak S.P., Kebebew E., Patel K.N., Shaha A., et al. For the American Thyroid Association Surgical Affairs Committee. American Thyroid Association Statement on Surgical Application of Molecular Profiling for Thyroid Nodules: Current Impact on Perioperative Decision Making. Thyroid. 2015;25:760–768. doi: 10.1089/thy.2014.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartolazzi A., Orlandi F., Saggiorato E., Volante M., Arecco F., Rossetto R., Palestini N., Ghigo E., Papotti M., Bussolati G., et al. Italian Thyroid Cancer Study Group (ITCSG). Galectin-3 expression analysis in the surgical selection of follicular thyroid nodules with indeterminate fine-needle aspiration cytology: A prospective multicentre study. Lancet Oncol. 2008;9:543–549. doi: 10.1016/S1470-2045(08)70132-3. [DOI] [PubMed] [Google Scholar]
- 21.Longatto-Filho A., Goncalves A.E., Martinho O., Schmitt F.C., Reis R.M. Liquid based cytology in DNA-based molecular research. Anal. Quant. Cytol. Histol. 2009;31:395–400. [PubMed] [Google Scholar]
- 22.Nikiforova M.N., Nikiforov Y.E. Molecular Diagnostics and Predictors in Thyroid Cancer. Thyroid. 2009;19:1351–1361. doi: 10.1089/thy.2009.0240. [DOI] [PubMed] [Google Scholar]
- 23.Nikiforov Y.E., Steward D.L., Robinson-Smith T.M., Haugen B.R., Klopper J.P., Zhu Z., Fagin J.A., Falciglia M., Weber K., Nikiforova M.N. Molecular Testing for Mutations in Improving the Fine-Needle Aspiration Diagnosis of Thyroid Nodules. J. Clin. Endocrinol. Metab. 2009;94:2092–2098. doi: 10.1210/jc.2009-0247. [DOI] [PubMed] [Google Scholar]
- 24.Ohori N.P., Nikiforova M.N., Schoedel K.E., Lebeau S.O., Hodak S.P., Seethala R.R., Carty S.E., Ogilvie J.B., Yip L., Nikiforov Y.E. Contribution of molecular testing to thyroid fine-needle aspiration cytology of “follicular lesion of undetermined significance/atypia of undetermined significance”. Cancer Cytopathol. 2010;118:17–23. doi: 10.1002/cncy.20063. [DOI] [PubMed] [Google Scholar]
- 25.Krane J.F., Cibas E.S., Alexander E.K., Paschke R., Eszlinger M. Molecular analysis of residual ThinPrep material from thyroid FNAs increases diagnostic sensitivity. Cancer Cytopathol. 2015;123:356–361. doi: 10.1002/cncy.21546. [DOI] [PubMed] [Google Scholar]
- 26.Rossi E.D., Martini M., Capodimonti S., Lombardi C.P., Pontecorvi A., Vellone V.G., Zannoni G.F., Larocca L.M., Fadda G. BRAF (v600e) mutation analysis on LBC-processed aspiration biopsies predicts bilaterality and nodal involvement in papillary thyroid microcarcinoma. Cancer Cytopathol. 2013;121:291–297. doi: 10.1002/cncy.21258. [DOI] [PubMed] [Google Scholar]
- 27.Soares P., Trovisco V., Rocha A.S., Lima J., Castro P., Preto A., Máximo V., Botelho T., Seruca R., Sobrinho-Simões M. BRAF mutations and RET/PTC rearrangements are alternative events in the ethiopathogenesis of PTC. Oncogene. 2003;22:4578–4580. doi: 10.1038/sj.onc.1206706. [DOI] [PubMed] [Google Scholar]
- 28.Cheung C.C., Carydis B., Ezzat S., Bedard Y.C., Asa S.L. Analysis of ret/PTC Gene Rearrangements Refines the Fine Needle Aspiration Diagnosis of Thyroid Cancer. J. Clin. Endocrinol. Metab. 2001;86:2187–2190. doi: 10.1210/jcem.86.5.7504. [DOI] [PubMed] [Google Scholar]
- 29.Moses W., Weng J., Sansano I., Peng M., Khanafshar E., Ljung B.-M., Duh Q.-Y., Clark O.H., Kebebew E. Molecular Testing for Somatic Mutations Improves the Accuracy of Thyroid Fine-needle Aspiration Biopsy. World J. Surg. 2010;34:2589–2594. doi: 10.1007/s00268-010-0720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musholt T.J., Fottner C., Weber M., Eichhorn W., Pohlenz J., Musholt P.B., Springer E., Schad A. Detection of Papillary Thyroid Carcinoma by Analysis of BRAF and RET/PTC1 Mutations in Fine-needle Aspiration Biopsies of Thyroid Nodules. World J. Surg. 2010;34:2595–2603. doi: 10.1007/s00268-010-0729-4. [DOI] [PubMed] [Google Scholar]
- 31.Colanta A., Lin O., Tafe L., Ghossein R., Nafa K., Mitchell T., Ladanyi M., Arcila M. BRAF Mutation Analysis of Fine-Needle Aspiration Biopsies of Papillary Thyroid Carcinoma: Impact on Diagnosis and Prognosis. Acta Cytol. 2011;55:563–569. doi: 10.1159/000333272. [DOI] [PubMed] [Google Scholar]
- 32.Nikiforov Y.E., Ohori N.P., Hodak S.P., Carty S.E., Lebeau S.O., Ferris R.L., Yip L., Seethala R.R., Tublin M.E., Stang M.T., et al. Impact of Mutational Testing on the Diagnosis and Management of Patients with Cytologically Indeterminate Thyroid Nodules: A Prospective Analysis of 1056 FNA Samples. J. Clin. Endocrinol. Metab. 2011;96:3390–3397. doi: 10.1210/jc.2011-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radkay L., Chiosea S.I., Seethala R.R., Hodak S.P., LeBeau S.O., Yip L., McCoy K.L., Carty S.E., Schoedel K.E., Nikiforova M.N., et al. Thyroid nodules with KRAS mutations are different from nodules with NRAS and HRAS mutations with regard to cytopathologic and histopathologic outcome characteristics. Cancer Cytopathol. 2014;122:873–882. doi: 10.1002/cncy.21474. [DOI] [PubMed] [Google Scholar]
- 34.Nikiforova M.N., Wald A.I., Roy S., Durso M.B., Nikiforov Y.E. Targeted next-generation sequencing panel (Thyro Seq) for detection of mutations in thyroid cancer. J. Clin. Endocrinol. Metab. 2013;98:E1852–E1860. doi: 10.1210/jc.2013-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nikiforov Y.E., Carty S.E., Chiosea S.I., Coyne C., Duvvuri U., Ferris R.L., Gooding W.E., Hodak S.P., LeBeau S.O., Ohori N.P., et al. Highly accurate diagnosis of cancer in thyroid nodules with follicolar neoplasm/suspicious for a follicular neoplasm cytology by Thyroseq v2 next generation sequencing assay. Cancer. 2014;120:3627–3634. doi: 10.1002/cncr.29038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikiforov Y.E., Carty S.E., Chiosea S.I., Coyne C., Duvvuri U., Ferris R.L., Gooding W.E., Lebeau S.O., Ohori N.P., Seethala R.R., et al. Impact of the Multi-Gene ThyroSeq Next-Generation Sequencing Assay on Cancer Diagnosis in Thyroid Nodules with Atypia of Undetermined Significance/Follicular Lesion of Undetermined Significance Cytology. Thyroid. 2015;25:1217–1223. doi: 10.1089/thy.2015.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexander E.K., Kennedy G.C., Baloch Z.W., Chudova D., Friedman L., Rosai J., Wilde J.I., Cibas E.S., Diggans J., Kloos R.T., et al. Preoperative Diagnosis of Benign Thyroid Nodules with Indeterminate Cytology. N. Engl. J. Med. 2012;367:705–715. doi: 10.1056/NEJMoa1203208. [DOI] [PubMed] [Google Scholar]
- 38.Xing M. Braf mutationin papillary thyroid cancer: Pathogenic role, molecular bases, and clinical implications. Endocr. Rev. 2007;28:742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 39.Puxeddu E., Durante C., Avenia N., Filetti S., Russo D. Clinical implications of BRAF mutation in thyroid carcinoma. Trends Endocrinol. Metab. 2008;19:138–145. doi: 10.1016/j.tem.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Cancer Genome Atlas Research Network Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676–690. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ali S., Cibas E.S. The Bethesda System for Reporting Thyroid Cytopathology. 2nd ed. Springer; Berlin, Germany: 2018. [Google Scholar]
- 42.Hang J.F., Westra W.H., Cooper D.S., Ali S.Z. The impact of noninvasive follicular thyroid neoplasm with papillary-like nuclear features on the performance of the Afirma gene expression classifier. Cancer. 2017;125:683–691. doi: 10.1002/cncy.21879. [DOI] [PubMed] [Google Scholar]
- 43.Poller D.N., Glaysher S. Molecular pathology and thyroid FNA. Cytopathology. 2017;28:475–481. doi: 10.1111/cyt.12492. [DOI] [PubMed] [Google Scholar]
- 44.Dejmek A., Zendehrokh N., Tomaszewska M., Edsjö A. Preparation of DNA from cytological material. Cancer Cytopathol. 2013;121:344–353. doi: 10.1002/cncy.21276. [DOI] [PubMed] [Google Scholar]
- 45.Catherwood M.A., Schmitt F., Salto-Tellez M. Molecular diagnostics and the training of future tissue- and cell-based pathologists. Cytopathology. 2012;23:283–285. doi: 10.1111/cyt.12015. [DOI] [PubMed] [Google Scholar]
- 46.Malapelle U., De Rosa N., Bellevicine C., Rocco D., Vitiello F., Piantedosi F.V., Illiano A., Nappi O., Troncone G. EGFR mutations detection on liquid-based cytology: Is microscopy still necessary? J. Clin. Pathol. 2012;65:561–564. doi: 10.1136/jclinpath-2011-200659. [DOI] [PubMed] [Google Scholar]
- 47.Malapelle U., De Rosa N., Rocco D., Crispino C., Illiano A., Piantedosi F.V., Nappi O., Troncone G. EGFR and KRAS mutations detection on lung liquid based cytology: A pilot study. J. Clin. Pathol. 2012;65:87–91. doi: 10.1136/jclinpath-2011-200296. [DOI] [PubMed] [Google Scholar]
- 48.Killian J.K., Walker R.L., Suuriniemi M. Archival fine-needle aspiration cytopathology (FNAC) samples: Untapped resource for clinical molecular profiling. J. Mol. Diagn. 2010;12:739–745. doi: 10.2353/jmoldx.2010.090238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fadda G., Rossi E.D. Liquid-Based Cytology in Fine-Needle Aspiration Biopsies of the Thyroid Gland. Acta Cytol. 2011;55:389–400. doi: 10.1159/000329029. [DOI] [PubMed] [Google Scholar]
- 50.Rossi E.D., Schmitt F. Pre-analytic steps for molecular testing on thyroid fine-needle aspirations: The goal of good results. CytoJournal. 2013;28:10–24. doi: 10.4103/1742-6413.122300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang H., Lee H., Yoon S.O., Kim H., Kim A., Kim B.H. BRAF (V600E) mutation analysis of liquid-based preparation-processed fine needle aspiration sample improves the diagnostic rate of papillary thyroid carcinoma. Hum. Pathol. 2012;43:89–95. doi: 10.1016/j.humpath.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 52.Kwon H.J., Kim E.-K., Kwak J.Y. Cytomorphologic features in thyroid nodules read as “suspicious for malignancy” on cytology may predict thyroid cancers with the BRAF mutation. Pathol. Res. Pr. 2015;211:671–676. doi: 10.1016/j.prp.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 53.Krane J.F., Vanderlaan P.A., Faquin W.C., Renshaw A.A. The atypia of undetermined significance/follicular lesion of undetermined significance:malignant ratio. Cancer Cytopathol. 2012;120:111–116. doi: 10.1002/cncy.20192. [DOI] [PubMed] [Google Scholar]
- 54.Trimboli P., Fulciniti F., Zilioli V., Ceriani L., Giovanella L. Accuracy of international ultrasound risk stratification systems in thyroid lesions cytologically classified as indeterminate. Diagn. Cytopathol. 2017;45:113–117. doi: 10.1002/dc.23651. [DOI] [PubMed] [Google Scholar]
- 55.Nikiforov Y.E., Seethala R.R., Tallini G., Baloch Z.W., Basolo F., Thompson L.D.R., Barletta J.A., Wenig B.M., Al Ghuzlan A., Kakudo K., et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA Oncol. 2016;2:1023–1029. doi: 10.1001/jamaoncol.2016.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maletta F., Massa F., Torregrossa L., Duregon E., Casadei G.P., Basolo F., Tallini G., Volante M., Nikiforov Y.E., Papotti M. Cytological features of “invasive follicular thyroid neoplasm with papillary-like nuclear features” and their correlation with tumor histology. Hum. Pathol. 2016;13:134–142. doi: 10.1016/j.humpath.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 57.Faquin W.C., Wong L.Q., Afrogheh A.H., Ali S.Z., Bishop J.A., Bongiovanni M., Pusztaszeri M.P., VandenBussche C.J., Gourmaud J., Vaickus L.J., et al. Impact of reclassifying noninvasive follicular variant of papillary thyroid carcinoma on the risk of malignancy in the Bethesda system for reporting thyroid cytopathology. Cancer Cytopathol. 2016;124:181–187. doi: 10.1002/cncy.21631. [DOI] [PubMed] [Google Scholar]
- 58.Wong K.S., Angell T.E., Strickland K.C., Alexander E.K., Cibas E.S., Krane J.F., Barletta J.A. Noninvasive Follicular Variant of Papillary Thyroid Carcinoma and the Afirma Gene-Expression Classifier. Thyroid. 2016;26:911–915. doi: 10.1089/thy.2015.0644. [DOI] [PubMed] [Google Scholar]
- 59.Strickland K.C., Howitt B.E., Marqusee E., Alexander E.K., Cibas E.S., Krane J.F., Barletta J.A. The Impact of Noninvasive Follicular Variant of Papillary Thyroid Carcinoma on Rates of Malignancy for Fine-Needle Aspiration Diagnostic Categories. Thyroid. 2015;25:987–992. doi: 10.1089/thy.2014.0612. [DOI] [PubMed] [Google Scholar]
- 60.Zhou H., Baloch Z.W., Nayar R., Bizzarro T., Fadda G., Adhikari-Guragain D., Hatem J., Larocca L.M., Samolczyk J., Slade J., et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): Implications for the risk of malignancy (ROM) in the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) Cancer. 2017;126:20–26. doi: 10.1002/cncy.21926. [DOI] [PubMed] [Google Scholar]
- 61.Mazeh H., Mizrahi I., Halle D., Ilyayev N., Stojadinovic A., Trink B., Mitrani-Rosenbaum S., Roistacher M., Ariel I., Eid A., et al. Development of a microRNA-based molecular assay for the detection of papillary thyroid carcinoma in aspiration biopsy samples. Thyroid. 2011;21:111–118. doi: 10.1089/thy.2010.0356. [DOI] [PubMed] [Google Scholar]
- 62.Bongiovanni M., Trimboli P., Rossi E.D., Fadda G., Nobile A., Giovanella L. Diagnosis of endocrine disease: High-yield thyroid fine-needle aspiration cytology: An update focused on ancillary techniques improving its accuracy. Eur. J. Endocrinol. 2016;174:R53–R63. doi: 10.1530/EJE-15-0817. [DOI] [PubMed] [Google Scholar]
- 63.Bongiovanni M., Giovanella L., Romanelli F., Trimboli P. Cytological Diagnoses Associated with Noninvasive Follicular Thyroid Neoplasms with Papillary-Like Nuclear Features According to the Bethesda System for Reporting Thyroid Cytopathology: A Systematic Review and Meta-Analysis. Thyroid. 2019;29:222–228. doi: 10.1089/thy.2018.0394. [DOI] [PubMed] [Google Scholar]
- 64.Bongiovanni M., Faquin W., Giovanella L., Durante C., Kopp P., Trimboli P. Impact of non-invasive follicular thyroid neoplasms with papillary-like nuclear features (NIFTP) on risk of malignancy in patients undergoing lobectomy/thyroidectomy for suspicious for malignancy or malignant fine-needle aspiration cytology findings: A systematic review and meta-analysis. Eur. J. Endocrinol. 2019 doi: 10.1530/EJE-19-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saiselet M., Pita J.M., Augenlicht A., Dom G., Tarabichi M., Fimereli D., Dumont J.E., Detours V., Maenhaut C. miRNA expression and function in thyroid carcinomas: A comparative and critical analysis and a model for other cancers. Oncotarget. 2016;7:52475–52492. doi: 10.18632/oncotarget.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen R., Liyanarachchi S., Li W., Wakely P.E., Saji M., Huang J., Nagy R., Farrell T., Ringel M.D., De La Chapelle A., et al. MicroRNA Signature in Thyroid Fine Needle Aspiration Cytology Applied to “Atypia of Undetermined Significance” Cases. Thyroid. 2012;22:9–16. doi: 10.1089/thy.2011.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nikiforova M.N., Chiosea S.I., Nikiforov Y.E. MicroRNA expression profiles in thyroid microRNAs in thyroid fine needle aspiration biopsy samples. Thyroid. 2012;22:285–291. doi: 10.1089/thy.2011.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nikiforova M.N., Tseng G.C., Steward D., Diorio D., Nikiforov Y.E. MicroRNA Expression Profiling of Thyroid Tumors: Biological Significance and Diagnostic Utility. J. Clin. Endocrinol. Metab. 2008;93:1600–1608. doi: 10.1210/jc.2007-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mazeh H., Levy Y., Mizrahi I., Appelbaum L., Ilyayev N., Halle D., Freund H.R., Nissan A. Differentiating benign from malignant thyroid nodules using micro ribonucleic acid amplification in residual cells obtained by fine needle aspiration biopsy. J. Surg. Res. 2013;180:216–221. doi: 10.1016/j.jss.2012.04.051. [DOI] [PubMed] [Google Scholar]
- 70.Keutgen X.M., Filicori F., Fahey T.J., 3rd Molecular diagnosis for indeterminate thyroid nodules on fine needle aspiration: Advances and limitations. Expert Rev. Mol. Diagn. 2013;13:613–623. doi: 10.1586/14737159.2013.811893. [DOI] [PubMed] [Google Scholar]
- 71.Pallante P., Visone R., Ferracin M., Ferraro A., Berlingieri M.T., Troncone G., Chiappetta G., Liu C.G., Santoro M., Negrini M., et al. MircoRNA deregulation in human thyroid papillary carcinomas. Endocr. Relat. Cancer. 2006;13:497–508. doi: 10.1677/erc.1.01209. [DOI] [PubMed] [Google Scholar]
- 72.Agretti P., Ferrarini E., Rago T., Candelieri A., De Marco G., Dimida A., Niccolai F., Molinaro A., Di Coscio G., Pinchera A., et al. MicroRNA expression profile helps to distinguish benign nodules from papillary thyroid carcinomas starting from cells of fine-needle aspiration. Eur. J. Endocrinol. 2012;167:393–400. doi: 10.1530/EJE-12-0400. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y., Zhong Q., Chen X., Fang J., Huang Z. Diagnostic value of microRNAs in discriminating malignant thyroid nodules from benign ones on fine-needle aspiration samples. Tumor Boil. 2014;35:9343–9353. doi: 10.1007/s13277-014-2209-1. [DOI] [PubMed] [Google Scholar]
- 74.Dettmer M., Perren A., Moch H., Komminoth P., Nikiforov Y.E., Nikiforova M.N. Comprehensive MicroRNA Expression Profiling Identifies Novel Markers in Follicular Variant of Papillary Thyroid Carcinoma. Thyroid. 2013;23:1383–1389. doi: 10.1089/thy.2012.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dettmer M., Vogetseder A., Durso M.B., Moch H., Komminoth P., Perren A., Nikiforov Y.E., Nikiforova M.N. MicroRNA expression array identifies novel diagnostic markers for conventional and oncocytic follicular thyroid carcinomas. J. Clin. Endocrinol. Metab. 2013;98:E1–E7. doi: 10.1210/jc.2012-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rossi E.D., Martini M., Bizzarro T., Capodimonti S., Sarti D., Cenci T., Bilotta M., Fadda G., Larocca L.M. The evaluation of miRNAs on thyroid FNAC: The promising role of miR-375 in follicular neoplasms. Endocrine. 2016;54:723–732. doi: 10.1007/s12020-016-0866-0. [DOI] [PubMed] [Google Scholar]
- 77.Burch H.B., Burman K.D., Cooper D.S., Hennessey J.V., Vietor N.O. A 2015 Survey of Clinical Practice Patterns in the Management of Thyroid Nodules. J. Clin. Endocrinol. Metab. 2016;101:2853–2862. doi: 10.1210/jc.2016-1155. [DOI] [PubMed] [Google Scholar]
- 78.Benjamin H., Schnitzer-Perlman T., Shtabsky A., Vandenbussche C.J., Ali S.Z., Kolar Z., Pagni F., Bar D., Meiri E. Analytical validity of a microRNA-based assay for diagnosing indeterminate thyroid FNA smears from routinely prepared cytology slides. Cancer Cytopathol. 2016;124:711–721. doi: 10.1002/cncy.21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Le Mercier M., D’Haene N., De Neve N., Blanchard O., Degand C., Rorive S., Salmon I. Next-generation sequencing improves the diagnosis of thyroid FNA specimens with indeterminate cytology. Histopathology. 2015;66:215–224. doi: 10.1111/his.12461. [DOI] [PubMed] [Google Scholar]
- 80.Nikiforov Y.E., Baloch Z.W. Clinical validation of the ThyroSeq V3 genomic classifier in thyroid nodules with indeterminate FNA cytology. Cancer Cytopathol. 2019;127:225–230. doi: 10.1002/cncy.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nikiforova M., Mercurio S., Wald A., Barbi de Moura M., Callenberg K., Santana-Santos L., Gooding W.E., Yip L., Ferris R.L., Nikiforov Y.E. Analytical performance of the ThyroSeq v3 genomic classifier for cancer diagnosis in thyroid nodules. Cancer. 2018;124:1682–1690. doi: 10.1002/cncr.31245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nishino M. Molecular cytopathology for thyroid nodules: A review of methodology and test performance. Cancer. 2016;124:14–27. doi: 10.1002/cncy.21612. [DOI] [PubMed] [Google Scholar]
- 83.Rossi E.D., LaRocca L.M., Pantanowitz L. Ancillary molecular testing of indeterminate thyroid nodules. Cancer Cytopathol. 2018;126:654–671. doi: 10.1002/cncy.22012. [DOI] [PubMed] [Google Scholar]
- 84.Ohori N.P., Landau M.S., Carty S.P., Yip L., Lebeau S.O., Manroa P., Seethala R.R., Schoedel K.E., Nikiforova M.N., Nikiforov Y.E. Benign call rate and molecular test result distribution in ThyroSeq V3. Cancer Cytopathol. 2019;127:161–168. doi: 10.1002/cncy.22088. [DOI] [PubMed] [Google Scholar]
- 85.McIver B., Castro M.R., Morris J.C., Bernet V., Smallridge R., Henry M., Kosok L., Reddi H. An Independent Study of a Gene Expression Classifier (Afirma) in the Evaluation of Cytologically Indeterminate Thyroid Nodules. J. Clin. Endocrinol. Metab. 2014;99:4069–4077. doi: 10.1210/jc.2013-3584. [DOI] [PubMed] [Google Scholar]
- 86.Dedhia P.H., Rubio G.A., Cohen M.S., Miller B.S., Gauger P.G., Hughes D.T. Potential Effects of Molecular Testing of Indeterminate Thyroid Nodule Fine Needle Aspiration Biopsy on Thyroidectomy Volume. World J. Surg. 2014;38:634–638. doi: 10.1007/s00268-013-2430-x. [DOI] [PubMed] [Google Scholar]
- 87.Kloos R.T. Molecular Profiling of Thyroid Nodules: Current Role for the Afirma Gene Expression Classifier on Clinical Decision Making. Mol. Imaging Radionucl. Ther. 2017;26:36–49. doi: 10.4274/2017.26.suppl.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harrison G., Sosa J.A., Jiang X. Evaluation of the Afirma Gene Expression Classifier in Repeat Indeterminate Thyroid Nodules. Arch. Pathol. Lab. Med. 2017;141:985–989. doi: 10.5858/arpa.2016-0328-OA. [DOI] [PubMed] [Google Scholar]
- 89.Baca S.C., Wong K.S., Strickland K.C., Heller H.T., Kim M.I., Barletta J.A., Cibas E.S., Krane J.F., Marqusee E., Angell T.E. Qualifiers of atypia in the cytologic diagnosis of thyroid nodules are associated with different Afirma gene expression classifier results and clinical outcomes. Cancer Cytopathol. 2017;125:313–322. doi: 10.1002/cncy.21827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marti J.L., Avadhani V., Donatelli L.A., Niyogi S., Wang B., Wong R.J., Shaha A.R., Ghossein R.A., Lin O., Morris L.G.T., et al. Wide Inter-institutional Variation in Performance of a Molecular Classifier for Indeterminate Thyroid Nodules. Ann. Surg. Oncol. 2015;22:3996–4001. doi: 10.1245/s10434-015-4486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Balentine C.J., Vanness D.I., Schneider D.F. Cost-effectiveness of lobectomy versus genetic testing (Afirma®) for indeterminate thyroid nodules: Considering the costs of surveillance. Surgery. 2018;163:88–96. doi: 10.1016/j.surg.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hao Y., Duh Q.Y., Kloos R.T., Babiarz J., Harrell R.M., Traweek S.T., Kim S.Y., Fedorowicz G., Walsh P.S., Sadow P.M., et al. Identification of Hürthle cell cancers: Solving a clinical challenge with genomic sequencing and a trio of machine learning algorithms. BMC Syst. Boil. 2019;13:27. doi: 10.1186/s12918-019-0693-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Angell T.E., Heller H.T., Cibas E.S., Barletta J.A., Kim M.I., Krane J.F., Marqusee E. Independent Comparison of the Afirma Genomic Sequencing Classifier and Gene Expression Classifier for Cytologically Indeterminate Thyroid Nodules. Thyroid. 2019;29:650–656. doi: 10.1089/thy.2018.0726. [DOI] [PubMed] [Google Scholar]
- 94.Endo M., Nabhan F., Porter K., Roll K., Shirley L.A., Azaryan I., Tonkovich D., Perlick J., Ryan L.E., Khawaja R. Afirma Gene Sequencing Classifier Compared with Gene Expression Classifier in Indeterminate Thyroid Nodules. Thyroid. 2019;29:1115–1124. doi: 10.1089/thy.2018.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brauner E., Holmes B.J., Krane J.F., Nishino M., Zurakowski D., Hennessey J.V., Faquin W.C., Parangi S. Performance of the Afirma Gene Expression Classifier in Hürthle Cell Thyroid Nodules Differs from Other Indeterminate Thyroid Nodules. Thyroid. 2015;25:789–796. doi: 10.1089/thy.2015.0049. [DOI] [PubMed] [Google Scholar]
- 96.Lithwick-Yanai G., Dromi N., Shtabsky A., Morgenstern S., Strenov Y., Feinmesser M., Kravtsov V., Leon M.E., Hajdúch M., Ali S.Z. Multicentre validation of a microRNA-based assay for diagnosing indeterminate thyroid nodules utilizing fine needle aspirate smears. J. Clin. Pathol. 2017;70:500–507. doi: 10.1136/jclinpath-2016-204089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Keutgen X.M., Filicori F., Crowley M.J., Wang Y., Scognamiglio T., Hoda R., Buitrago D., Cooper D., Zeiger M.A., Zarnegar R., et al. A Panel of Four miRNAs Accurately Differentiates Malignant from Benign Indeterminate Thyroid Lesions on Fine Needle Aspiration. Clin. Cancer Res. 2012;18:2032–2038. doi: 10.1158/1078-0432.CCR-11-2487. [DOI] [PubMed] [Google Scholar]
- 98.Labourier E., Shifrin A., Busseniers A.E., Lupo M.A., Manganelli M.L., Andruss B., Wylie D., Beaudenon-Huibregtse S. Molecular Testing for miRNA, mRNA, and DNA on Fine-Needle Aspiration Improves the Preoperative Diagnosis of Thyroid Nodules With Indeterminate Cytology. J. Clin. Endocrinol. Metab. 2015;100:2743–2750. doi: 10.1210/jc.2015-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wylie D., Beaudenon-Huibregtse S., Haynes B.C., Giordano T.J., Labourier E. Molecular classification of thyroid lesions by combined testing for miRNA gene expression and somatic gene alterations. J. Pathol. Clin. Res. 2016;2:93–103. doi: 10.1002/cjp2.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ohori N.P., Wolfe J., Hodak S.P., LeBeau S.O., Yip L., Carty S.E., Duvvuri U., Schoedel K.E., Nikiforova M.N., Nikiforov Y.E. “Colloid-rich” follicular neoplasm/suspicious for follicular neoplasm thyroid fine-needle aspiration specimens: Cytologic, histologic, and molecular basis for considering an alternate view. Cancer Cytopathol. 2013;121:718–728. doi: 10.1002/cncy.21333. [DOI] [PubMed] [Google Scholar]
- 101.Paskas S., Jankovic J., Živaljević V., Tatic S., Bozic V., Nikolić A., Radojkovic D., Savin S., Cvejiċ D. Malignant risk stratification of thyroid FNA specimens with indeterminate cytology based on molecular testing. Cancer Cytopathol. 2015;123:471–479. doi: 10.1002/cncy.21554. [DOI] [PubMed] [Google Scholar]
- 102.Rossi E.D., Bizzarro T., Martini M., Larocca L.M., Schmitt F., Vielh P. Cytopathology of Follicular Cell Nodules. Adv Anat Pathol. 2017;24:45–55. doi: 10.1097/PAP.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 103.Straccia P., Rossi E.D., Bizzarro T., Brunelli C., Cianfrini F., Damiani D., Fadda G. A meta-analytic review of the Bethesda System for Reporting Thyroid Cytopathology: Has the rate of malignancy in indeterminate lesions been underestimated? Cancer Cytopathol. 2015;123:713–722. doi: 10.1002/cncy.21605. [DOI] [PubMed] [Google Scholar]
- 104.Jara S.M., Bhatnagar R., Guan H., Gocke C.D., Ali S.Z., Tufano R.P. Utility of BRAF mutation detection in fine-needle aspiration biopsy samples read as “suspicious for papillary thyroid carcinoma”. Head Neck. 2015;37:1788–1793. doi: 10.1002/hed.23829. [DOI] [PubMed] [Google Scholar]
- 105.Lau R.P., Paulsen J.D., Brandler T.C., Liu C.Z., Simsir A., Zhou F. Impact of the Reclassification of “Noninvasive Encapsulated Follicular Variant of Papillary Thyroid Carcinoma” to “Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features” on the Bethesda System for Reporting Thyroid Cytopathology: A Large Academic Institution’s Experience. Am. J. Clin. Pathol. 2017 doi: 10.1093/ajcp/aqx136. [DOI] [PubMed] [Google Scholar]
- 106.Esebua M., Kannuswamy R., Layfield L.J., Baloch Z.W., Schmidt R.L. Impact of the Reclassification of the Non-Invasive Follicular Variant of Papillary Carcinoma as Benign on the Malignancy Risk of the Bethesda System for Reporting Thyroid Cytopathology: A Meta-Analysis Study. Acta Cytol. 2017;61:187–193. doi: 10.1159/000469654. [DOI] [PubMed] [Google Scholar]
- 107.Shahi M., Yousaf H., Amin K., Li F. Impact of New Nomenclature “Non-Invasive Follicular Neoplasm with Papillary Like Nuclear Features” on the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC). An Institutional Experience. Mod. Pathol. 2017;30:116A. [Google Scholar]
- 108.Bizzarro T., Martini M., Capodimonti S., Straccia P., Lombardi C.P., Pontecorvi A., LaRocca L.M., Rossi E.D. Young investigator challenge: The morphologic analysis of noninvasive follicular thyroid neoplasm with papillary-like nuclear features on liquid-based cytology: Some insights into their identification. Cancer Cytopathol. 2016;124:699–710. doi: 10.1002/cncy.21777. [DOI] [PubMed] [Google Scholar]
- 109.Yang G.C.H., Fried K.O., Scognamiglio T. Sonographic and cytologic differences of NIFTP from infiltrative or invasive encapsulated follicular variant of papillary thyroid carcinoma: A Review of 179 Cases. Diagn. Cytopathol. 2017;45:533–541. doi: 10.1002/dc.23709. [DOI] [PubMed] [Google Scholar]
- 110.Jiang X.S., Harrison G.P., Datto M.B. Young Investigator Challenge: Molecular testing in noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Cancer Cytopathol. 2016;124:893–900. doi: 10.1002/cncy.21802. [DOI] [PubMed] [Google Scholar]
- 111.Song S.J., LiVolsi V.A., Montone K., Baloch Z. Pre-operative features of non-invasive follicular thyroid neoplasms with papillary-like nuclear features: An analysis of their cytological, Gene Expression Classifier and sonographic findings. Cytopathology. 2017;28:488–494. doi: 10.1111/cyt.12501. [DOI] [PubMed] [Google Scholar]
- 112.Basolo F., Macerola E., Ugolini C., Poller D.N., Baloch Z. The molecular landscape of non invasive follicular thyroid neoplasm with papillary like-nuclear features (NIFTP): A literature review. Adv. Anat. Pathol. 2017;24:252–258. doi: 10.1097/PAP.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 113.Kim T.H., Lee M., Kwon A.Y., Choe J.H., Kim J.H., Kim J.S., Hahn S.Y., Shin J.H., Chung M.K., Son Y.I. Molecular genotyping of the non-invasive encapsulated follicular variant of papillary thyroid carcinoma. Histopathology. 2017 doi: 10.1111/his.13401. [DOI] [PubMed] [Google Scholar]
- 114.Steward D.L., Carty S.E., Sippel R.S., Yang S.P., Sosa J.A., Sipos J.A., Figge J.J., Mandel S., Haugen B.R., Burmen K.D. Performance of a Multigene Genomic Classifier in Thyroid Nodules With Indeterminate Cytology: A Prospective Blinded Multicenter Study. JAMA Oncol. 2018 doi: 10.1001/jamaoncol.2018.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sahli Z.T., Smith P.W., Umbricht C.B., Zeiger M.A. Preoperative Molecular Markers in Thyroid Nodules. Front. Endocrinol. (Lausanne) 2018;9:179. doi: 10.3389/fendo.2018.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]