Abstract
Rationale
In patients with acute cerebral ischemia, the rate of stroke, myocardial infarction, or death during 90 days was reported to be non-significantly lower with ticagrelor compared with aspirin, with no increase in major hemorrhage. Dual antiplatelet therapy may be more effective in this setting.
Aim
To investigate whether ticagrelor combined with aspirin are superior to aspirin alone in preventing stroke or death in patients with non-severe, non-cardioembolic ischemic stroke or high-risk transient ischemic attack.
Design
The Acute Stroke or Transient Ischemic Attack Treated with Ticagrelor and Aspirin for Prevention of Stroke and Death (THALES) trial is a randomized, placebo-controlled, double-blind, event-driven study. Patients will be randomized within 24 h of onset of acute ischemic symptoms. THALES is expected to randomize 13,000 at ∼450 sites worldwide, to collect 764 primary outcome events. Study treatments are ticagrelor 180 mg loading dose on day 1, then 90 mg twice daily on days 2–30, or matching placebo. All patients will also receive open-label aspirin 300–325 mg on day 1, then 75–100 mg once daily on days 2–30.
Study outcomes
The primary efficacy outcome is time to the composite endpoint of stroke or death through 30-day follow-up. The primary safety outcome is time to first severe bleeding event.
Discussion
The THALES trial will provide important information about the benefits and risks of dual antiplatelet therapy with ticagrelor and aspirin in patients with acute cerebral ischemia in a global setting (funding: AstraZeneca).
Clinical Trial Registration URL
http://www.clinicaltrials.gov. Unique identifier: NCT03354429.
Keywords: Stroke, TIA, cerebral ischemia, antiplatelet, ticagrelor, aspirin
Introduction and rationale
Patients with acute cerebral ischemia are at high risk of recurrent ischemic events, particularly ischemic stroke1–6 and current international guidelines recommend antiplatelet therapy for secondary prevention in patients with acute stroke or transient ischemic attack (TIA) of non-cardioembolic origin. Aspirin is the only antiplatelet agent that has received a class 1A recommendation.7–9
Ticagrelor is a reversibly binding, direct-acting, oral P2Y12 receptor antagonist that prevents adenosine diphosphate-mediated P2Y12 dependent platelet activation and aggregation.10 Ticagrelor has a faster onset and achieves greater and more consistent platelet inhibition than clopidogrel,11 which requires metabolism to its active form through a pathway that is genetically determined.12 Poor responsiveness to clopidogrel is common, with a frequency that may be as high as 20–50% in some populations.13
The Acute Stroke or Transient Ischemic Attack Treated with Aspirin or Ticagrelor and Patient Outcomes (SOCRATES) trial (NCT01994720) investigated whether ticagrelor was superior to aspirin, when initiated within 24 h after symptom onset in patients with acute cerebral ischemia.14 The rate of stroke, myocardial infarction, or death during 90 days were numerically lower with ticagrelor as compared with aspirin (hazard ratio (HR) 0.89; 95% confidence interval (CI) 0.78 to 1.01; p = 0.07), with no increase in major hemorrhage.4,15
These promising results prompted several secondary analyses of SOCRATES to guide the design of the present study. One planned secondary analysis indicated benefit in reducing recurrent stroke events; HR 0.86 (95% CI 0.75 to 0.99; nominal p = 0.03). Stroke constituted almost 90% of primary events in SOCRATES, and the highest risk for new stroke events was seen during the first 30 days of treatment.4 Regarding population, patients with an ABCD2 score of 4–5 had a lower event rate compared with an ABCD2 score of 6–7 or minor ischemic strokes, consistent with international registry data.6 Also, patients with acute stroke/TIA with ipsilateral large vessel stenosis seemed to benefit more from ticagrelor treatment compared with aspirin.16 In another subgroup analysis, the treatment effect of ticagrelor was more pronounced in patients who received aspirin within 7 days before randomization.17 Since the antiplatelet effect of aspirin persisted into the first week of the trial among this group, short-term dual antiplatelet therapy (DAPT) could account for the greater benefit of ticagrelor in patients taking aspirin prior to randomization. This observation is in line with other studies suggesting that DAPT with clopidogrel and aspirin may be more effective in reducing the high risk of stroke after an acute ischemic stroke or TIA compared with aspirin alone, including studies of microembolization from atherosclerotic cerebral arteries in patients with acute cerebral ischemic events18,19 and in trials of patients with minor stroke or TIA.3,5
The pharmacological properties of ticagrelor support the hypothesis that combining ticagrelor with aspirin would be an even more effective DAPT combination, without non-responders and without a clinically unacceptable risk of severe bleeding events. The aim of the THALES trial is, therefore, to test whether DAPT with ticagrelor and aspirin results in clinical benefit to patients with acute cerebral ischemia.
Methods
Design
The THALES trial (NCT03354429) is a randomized, placebo-controlled, double-blind, parallel-group, international, multicenter, phase III study to test the hypothesis that ticagrelor and aspirin is superior to placebo and aspirin in preventing stroke and death in patients with acute cerebral ischemia.
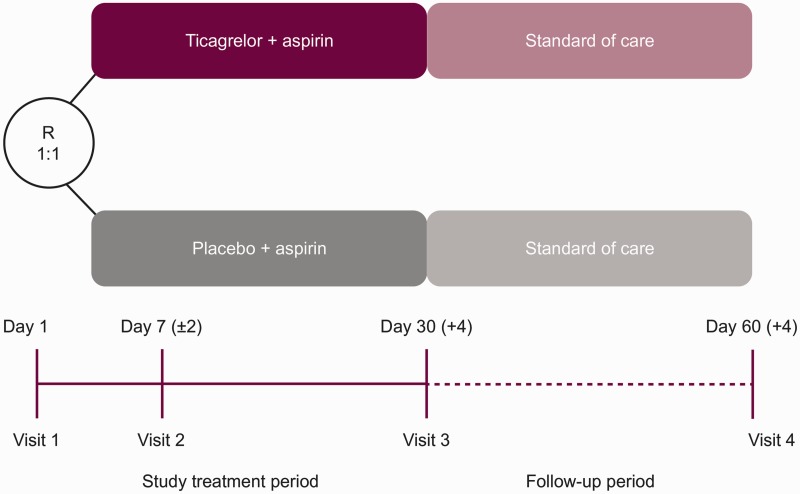
Patients will be randomized within 24 h of symptom onset to 30 days of treatment with ticagrelor or placebo on top of standard-of-care therapy with aspirin and followed up for 30 days for efficacy and 60 days for safety (Figure 1). Key design features of the THALES trial compared with the SOCRATES trial are presented in Table 1. In SOCRATES, the estimated treatment effect was similar whether adjudicated events or investigator-reported events were used in the analysis;20 therefore, investigator-reported outcomes will be used in THALES.
Figure 1.
THALES study design. R: randomization. Ticagrelor 180 mg loading dose (day 1) then 90 mg twice daily (days 2–30). Aspirin 300–325 mg loading dose (day 1) then 75–100 mg daily (days 2–30).
Table 1.
Comparison of THALES and SOCRATES study designs
| Comparison | THALES n ∼13,000 | SOCRATES n = 13,199 |
|---|---|---|
| Dose regimen | Ticagrelor vs. placebo on top of aspirin (DAPT) | Ticagrelor vs. aspirin (single antiplatelet therapy) |
| Study duration | 30 days + 30 days follow-up | 90 days + 30 days follow-up |
| Population | TIA with ABCD2 score ≥ 6 and/or ipsilateral stenosis and acute ischemic stroke NIHSS ≤5 | TIA with ABCD2 score ≥ 4 and/or ipsilateral stenosis and acute ischemic stroke NIHSS ≤5 |
| Endpoints | ||
| Primary efficacy Secondary efficacy Safety | Stroke + death Ischemic stroke, disability (mRS) Severe bleeding and AEs leading to discontinuation of study medication | Stroke + myocardial infarction + death Ischemic stroke, net clinical outcome Major bleeding and AEs leading to discontinuation of study medication |
DAPT: dual antiplatelet therapy; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; TIA: transient ischemic attack.
The study is event-driven with approximately 13,000 patients expected to be randomized from approximately 450 sites in 28 countries worldwide to identify 764 outcome events. The first patient was recruited on 22 January 2018.
Patient population
Eligible are patients ≥ 40 years of age who have experienced a non-cardioembolic acute ischemic stroke with a National Institutes of Health Stroke Scale score ≤5 or high-risk TIA (defined as an ABCD2 score ≥ 6 or ipsilateral atherosclerotic stenosis ≥50% in an extra/intracranial artery) who can be randomized within 24 h of symptom onset or for wake-up strokes since last time known to be free of new ischemic symptoms. The complete inclusion and exclusion criteria are shown in Tables S1 and S2, respectively.
Randomization
Randomization codes are computer-generated by the AstraZeneca Global Randomization system and loaded into the Interactive Web Response System database. Patients are randomized as soon as possible after symptom onset, but within 24 h.
Treatments
Randomized patients receive either ticagrelor 180 mg loading dose on day 1, then 90 mg twice daily during the study treatment period or matching placebo, in addition to receiving standard-of-care open-label aspirin 300–325 mg on day 1, then 75–100 mg once daily during the study treatment period (Figure 1). The loading dose of ticagrelor/placebo should be given immediately after randomization. After the 30 days of study treatment, patients are treated with standard-of-care therapy at the discretion of the investigator and followed up for an additional 30 days with continued collection of endpoints and safety events.
Primary outcomes
The primary outcome is the time from randomization to first subsequent investigator-reported stroke or all-cause death at 30 days.
Secondary outcomes
Two secondary efficacy outcomes will be evaluated hierarchically after assessing the primary outcome. First, time from randomization to first subsequent ischemic stroke will be assessed, and then modified Rankin Scale (mRS) score > 1 at the end of treatment (visit 3).21,22
The main safety outcomes are: time from randomization to first bleeding event, categorized as severe based on criteria from the Global Utilization of Streptokinase and tissue-type plasminogen activator for Occluded Coronary Arteries (GUSTO) trial; time from randomization to first intracranial hemorrhage or fatal bleeding event; time from randomization to first bleeding event categorized as GUSTO moderate or severe; time from randomization to premature permanent discontinuation of study treatment due to bleeding.23 Asymptomatic hemorrhagic transformations of brain infarctions and microhemorrhages <10 mm evident only on gradient-echo magnetic resonance imaging are excluded from fulfilling intracranial hemorrhage criteria. This revision is an adaptation of the standard GUSTO definition to better distinguish clinically relevant events in the acute stroke population, and has been the common convention in recent large stroke trials.15 Serious adverse events (SAEs) and AEs leading to premature and permanent discontinuation of study medication will also be assessed.
Pre-defined exploratory outcomes are time from randomization to first subsequent stroke or death in patients with ipsilateral atherosclerotic stenosis; mRS score > 2 at visit 3 in patients with subsequent stroke; and generic health status (using the EQ-5D-5L questionnaire).
Data monitoring committee
An independent data monitoring committee (DMC) will review on an ongoing basis accumulating study data to safeguard the interests of the patients. The DMC will assess the benefit/risk profile of the intervention during the study, ensure the validity and integrity of the study, review the overall conduct of the study, and provide recommendations to the Executive Committee (EC) regarding the continued conduct of the study. The DMC will have access to the individual treatment codes and will be able to merge these with the collected study data while the study is ongoing.
One interim analysis for efficacy and futility will be performed by the DMC following the accrual of approximately 60% of planned primary events.
A DMC Charter details roles, responsibilities, and procedures to ensure maintenance of the blinding and integrity of the study.
Sample size estimates
The study is event-driven and the final number of randomized patients will be based on the blinded data review of overall primary endpoints. At least 764 primary endpoint events are needed to provide 85% power, assuming an HR of 0.805 (based on an HR of 0.8 for stroke and cardiovascular death, and an HR of 1.0 for non-cardiovascular death) in favor of ticagrelor at the significance level of 4.988%, adjusted for the planned interim analysis. Based on data from the SOCRATES study, a primary endpoint rate of 6.7% in the placebo group is assumed at 30 days following randomization. Hence, randomizing approximately 13,000 patients to ticagrelor or placebo, in a 1:1 ratio, is expected to yield the required 764 events.
Statistical analyses
All efficacy and safety analyses will be based on the intention-to-treat principle. In time-to-event analyses, the treatment groups will be compared using a Cox proportional hazards model with a factor for treatment group, using the Efron method for ties. P-values and 95% CIs for the HRs will be based on the Wald statistic.
The primary and secondary efficacy outcomes will be included in a confirmatory testing procedure. Only if the analysis of the primary outcome is significant at the 4.988% level (adjusted for the interim analysis) will the secondary outcomes be tested in a confirmatory sense in the specified hierarchical order.
Study organization and funding
The EC, in collaboration with the sponsor, is responsible for the overall design, study protocol and amendments, interpretation, supervision, and reporting of the study results at international congresses and publishing in peer-reviewed journals. AstraZeneca is responsible for the operational study conduct. The EC will make recommendations to AstraZeneca regarding early stopping or modifications of the study based on the recommendations received from the DMC. The EC is comprised of six designated international academic experts including the Steering Committee chair and three non-voting AstraZeneca representatives, and operates under a separate charter.
The International Steering Committee is comprised of national lead investigators from each country where the study is conducted (Table S3) and will be supervised by the EC. Members of the Steering Committee will be responsible for providing clinical guidance on study implementation and conduct in their respective countries.
This study is sponsored by AstraZeneca.
Discussion
Acute cerebral ischemia most often presents as a non-severe stroke or TIA,24 and risk of recurrent ischemic events is very high, particularly in the first few days.1–6 Therefore, urgent assessment of these cases is required to initiate treatment to reduce the risk of subsequent severe, disabling stroke.
In this setting, aspirin is the only antiplatelet agent with a class 1A recommendation in international guidelines7–9 and the only antiplatelet therapy that has been shown to reduce disabling strokes.25 However, the status of aspirin as the standard-of-care for all non-cardioembolic strokes has been challenged by two trials investigating DAPT containing clopidogrel.3,5
The Platelet-Oriented Inhibition in New TIA and minor ischemic stroke (POINT) trial26 recently reported a benefit for clopidogrel and aspirin over aspirin alone.5 The POINT trial was stopped early by the Data and Safety Monitoring Board after an interim analysis with 84% of pre-planned events. POINT reported both a benefit in reduction of ischemic events and an increased risk of major bleeding events. However, the robustness of the POINT trial results suffers from a high rate of study-drug discontinuation and a large number of patients lost to follow-up without known vital status.
The Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events (CHANCE) trial reported similar efficacy for clopidogrel-aspirin compared with aspirin in reducing stroke risk but did not demonstrate an increase in major bleeding events.3 Concerns about differences in standards of care, stroke subtypes, duration of antiplatelet therapy, and genetics of clopidogrel metabolism limited generalization of CHANCE findings beyond China, where it was performed.
Thus, despite encouraging results in the POINT and CHANCE trials, clinically important questions remain in this setting. Treatment with clopidogrel in combination with aspirin in acute cerebral ischemia has not been evaluated by health authorities. Furthermore, clopidogrel as single antiplatelet therapy has not been approved for secondary prevention of stroke within 7 days after an acute stroke/TIA.
Internationally, health authorities have approved aspirin for the indication of reducing the risk of death and recurrent stroke in patients experiencing an ischemic stroke or TIA, noting that aspirin treatment can be started in the acute setting.27,28 Regulatory agencies in the US, Europe, and China were consulted during the planning of the THALES trial and all stated that placebo, rather than clopidogrel, was the appropriate comparator in patients receiving standard-of-care aspirin in acute minor stroke/TIA.
There are potential benefits of ticagrelor therapy that should be acknowledged. Ticagrelor has a faster onset of action and achieves greater, more consistent, and predictable platelet inhibition than clopidogrel.11–13 Nearly all poor responders to clopidogrel will have platelet reactivity below the cut-off points associated with ischemic risk when treated with ticagrelor.12
The rapid and consistent onset of action of ticagrelor is expected to be important in all patients with acute cerebral ischemia, whereas the rapid offset may be more important for patients who are likely to undergo surgical intervention, such as patients with carotid stenosis. A subgroup analysis from the SOCRATES trial suggests that patients with ischemic events attributable to ipsilateral atherosclerotic stenosis may have a greater treatment effect with ticagrelor compared with those without ipsilateral stenosis.16 Patients with carotid stenosis were largely excluded from POINT,29 and were uncommon in the Chinese population enrolled in CHANCE.3 THALES has the opportunity to improve outcomes for these high-risk patients since patients with carotid stenosis are eligible for THALES, and the fast onset, fast offset, and reversible binding of ticagrelor11,12 may be an advantage compared with the irreversible inhibitor, clopidogrel. Moreover, preliminary analysis of the ticagrelor with aspirin on Platelet Reactivity In acute Non-disabling Cerebrovascular Events (PRINCE) trial showed a benefit of ticagrelor with aspirin compared with clopidogrel plus aspirin on platelet reactivity, which was the primary endpoint.30 Moreover, 21 strokes in 335 patients (6.3%) were reported in the ticagrelor-aspirin arm vs. 30 strokes in 339 patients (8.8%) in the clopidogrel-aspirin arm.
The ticagrelor dose in THALES is the same as that used in the target population of patients with ischemic stroke or TIA in the SOCRATES study, in which ticagrelor was well-tolerated and had a similar safety profile as aspirin with respect to major bleeding events.4,15 In patients with acute coronary syndrome, the same ticagrelor dose was used and treatment with ticagrelor compared with clopidogrel resulted not only in a higher degree of platelet inhibition31 but also in a reduced rate of myocardial infarction, stroke, or death from vascular causes, without an increase in the rate of overall major bleeding in the PLATO study.32 Importantly, the efficacy and bleeding results with ticagrelor in high-risk patients with a history of stroke or TIA were consistent with the overall PLATO trial population, with a favorable clinical net benefit and associated impact on mortality.33
Summary and conclusions
Overall, the available data support the potential for DAPT with ticagrelor and aspirin to improve outcomes in patients with acute ischemic stroke or high-risk TIA as compared with aspirin alone, the current standard of care. Despite advances in the field, there are still clinically important questions remaining about the benefits and risks of DAPT. The THALES trial will address these questions with a design and study conduct that will meet the rigorous standards of regulatory authorities.
Supplemental Material
Supplemental Material for The Acute Stroke or Transient Ischemic Attack Treated with Ticagrelor and Aspirin for Prevention of Stroke and Death (THALES) trial: Rationale and design by S Claiborne Johnston, Pierre Amarenco, Hans Denison, Scott R Evans, Anders Himmelmann, Stefan James, Mikael Knutsson, Per Ladenvall, Carlos A Molina, Yongjun Wang and for the THALES Investigators in International Journal of Stroke
Acknowledgments
Editorial support (formatting tables and figures, co-ordinating reviews, and preparing the manuscript for submission) was provided by Jackie Phillipson (Zoetic Science, an Ashfield company, part of UDG Healthcare plc, Macclesfield, UK).
Authors' contributions
All authors contributed to the design of the THALES study and will contribute to its oversight. Clay Johnston wrote the first draft of the manuscript, which was edited by all other authors.
Declaration of conflicting interests
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: S Claiborne Johnston reports receiving research grants from the National Institutes of Neurological Disorders and Stroke (NINDS) and the National Institutes of Health (NIH), including for the POINT trial; Sanofi provided free drug and placebo to the NIH for patients in the POINT trial. His institution received research support (significant) for the SOCRATES and THALES trials. Pierre Amarenco reports receiving significant research grant support from AstraZeneca, Sanofi, and Bristol-Myers Squibb (for TIAregistry.org), the French Government and Pfizer (for the TST trial), and Boston Scientific (for the WATCH-AF registry). He has received modest consultant/advisory board fees from Amgen and Bristol-Myers Squibb. He has also received modest honoraria from Amgen (speaker activities), Pfizer (SPIRE Program Executive Committee), AstraZeneca (SOCRATES Trial Executive Committee), and Kowa (PROMINENT Executive Committee), and significant honoraria from Bayer (XANTUS Executive Committee), AstraZeneca (THALES Executive Committee), and Fibrogen (ALPINE program trials DSMB member). Hans Denison, Anders Himmelmann, Mikael Knutsson, and Per Ladenvall are employees of AstraZeneca (all significant). Scott R Evans is a statistical consultant to AstraZeneca (significant) for the SOCRATES and THALES trials. Stefan James has received institutional research grants and lecture fees from AstraZeneca. Carlos A Molina reports serving on the Steering Committee (significant) of the Combined lysis of thrombus with ultrasound and systemic tissue plasminogen activator for emergent revascularization in acute ischemic stroke trial (Cerevast); SOCRATES (AstraZeneca), Implant Augmenting Cerebral Blood Flow Trial 24 hours from stroke onset trial (Brainsgate), Endovascular Revascularization With Solitaire Device Versus Best Medical Therapy in Anterior Circulation Stroke Within 8 Hours trial (Fundació Ictus Malaltia Vascular). He has received honoraria for participation in clinical trials, and contribution to advisory boards or oral presentations from: AstraZeneca (modest), Boehringer Ingelheim, Daiichi Sankyo, Bristol-Myers Squibb, Covidien, Cerevast, and Brainsgate. He has no ownership interest and does not own stocks of any pharmaceutical or medical device company. Yongjun Wang reports research grant support from AstraZeneca.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The THALES study is funded by AstraZeneca.
References
- 1.Johnston SC, Gress DR, Browner WS, et al. Short-term prognosis after emergency department diagnosis of TIA. JAMA 2000; 284: 2901–2906. [DOI] [PubMed] [Google Scholar]
- 2.Coull AJ, Lovett JK, Rothwell PM. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ 2004; 328: 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med 2013; 369: 11–19. [DOI] [PubMed] [Google Scholar]
- 4.Johnston SC, Amarenco P, Albers GW, et al. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med 2016; 375: 35–43. [DOI] [PubMed] [Google Scholar]
- 5.Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med 2018; 379: 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amarenco P, Lavallée PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med 2016; 374: 1533–1542. [DOI] [PubMed] [Google Scholar]
- 7.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018; 49: e46–e110. [DOI] [PubMed] [Google Scholar]
- 8.European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis 2008; 25: 457–507. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Liu M, Pu C. 2014 Chinese guidelines for secondary prevention of ischemic stroke and transient ischemic attack. Int J Stroke 2017; 12: 302–320. [DOI] [PubMed] [Google Scholar]
- 10.Husted S, van Giezen JJ. Ticagrelor: the first reversibly binding oral P2Y12 receptor antagonist. Cardiovasc Ther 2009; 27: 259–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurbel PA, Bliden KP, Butler K, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation 2009; 120: 2577–2585. [DOI] [PubMed] [Google Scholar]
- 12.Gurbel PA, Bliden KP, Butler K, et al. Response to ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies: the RESPOND study. Circulation 2010; 121: 1188–1199. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Zhao X, Lin J, et al. Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. JAMA 2016; 316: 70–78. [DOI] [PubMed] [Google Scholar]
- 14.Johnston SC, Amarenco P, Albers GW, et al. Acute stroke or transient ischemic attack treated with aspirin or ticagrelor and patient outcomes (SOCRATES) trial: rationale and design. Int J Stroke 2015; 10: 1304–1308. [DOI] [PubMed] [Google Scholar]
- 15.Easton JD, Aunes M, Albers GW, et al. Risk for major bleeding in patients receiving ticagrelor compared with aspirin after transient ischemic attack or acute ischemic stroke in the SOCRATES study (Acute Stroke or Transient Ischemic Attack Treated With Aspirin or Ticagrelor and Patient Outcomes). Circulation 2017; 136: 907–916. [DOI] [PubMed] [Google Scholar]
- 16.Amarenco P, Albers GW, Denison H, et al. Efficacy and safety of ticagrelor versus aspirin in acute stroke or transient ischaemic attack of atherosclerotic origin: a subgroup analysis of SOCRATES, a randomised, double-blind, controlled trial. Lancet Neurol 2017; 16: 301–310. [DOI] [PubMed] [Google Scholar]
- 17.Wong KSL, Amarenco P, Albers GW, et al. Efficacy and safety of ticagrelor in relation to aspirin use within the week before randomization in the SOCRATES trial. Stroke 2018; 49: 1678–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markus HS, Droste DW, Kaps M, et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using Doppler embolic signal detection: the Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic carotid Stenosis (CARESS) trial. Circulation 2005; 111: 2233–2240. [DOI] [PubMed] [Google Scholar]
- 19.Wong KS, Chen C, Fu J, et al. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol 2010; 9: 489–497. [DOI] [PubMed] [Google Scholar]
- 20.Easton JD, Denison H, Knutsson M, et al. Estimated treatment effect of ticagrelor versus aspirin by investigator-assessed events compared with judgment by an independent event adjudication committee in the SOCRATES trial. Eur Stroke J 2018; 3(Suppl 1): 36. [DOI] [PubMed] [Google Scholar]
- 21.van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19: 604–607. [DOI] [PubMed] [Google Scholar]
- 22.Bruno A, Akinwuntan AE, Lin C, et al. Simplified modified Rankin scale questionnaire: reproducibility over the telephone and validation with quality of life. Stroke 2011; 42: 2276–2279. [DOI] [PubMed] [Google Scholar]
- 23.GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993; 329: 673–682. [DOI] [PubMed] [Google Scholar]
- 24.Béjot Y, Mehta Z, Giroud M, et al. Impact of completeness of ascertainment of minor stroke on stroke incidence: implications for ideal study methods. Stroke 2013; 44: 1796–1802. [DOI] [PubMed] [Google Scholar]
- 25.Rothwell PM, Algra A, Chen Z, et al. Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: time-course analysis of randomised trials. Lancet 2016; 388: 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston SC, Easton JD, Farrant M, et al. Platelet-oriented inhibition in new TIA and minor ischemic stroke (POINT) trial: rationale and design. Int J Stroke 2013; 8: 479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CAST (Chinese Acute Stroke Trial) Collaborative Group. CAST: randomized placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. Lancet 1997; 349: 1641–1649. [PubMed] [Google Scholar]
- 28.International Stroke Trial Collaborative Group. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. Lancet 1997; 349: 1569–1581. [PubMed] [Google Scholar]
- 29.Grotta JC. Antiplatelet therapy after ischemic stroke or TIA. N Engl J Med 2018; 379: 291–292. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Meng X, Chen W, et al. Ticagrelor with aspirin on platelet reactivity in acute non-disabling cerebrovascular events (PRINCE) trial: final analysis. In: The ISC 2018. LBA8, Los Angeles, CA, USA; Jan 24 –26 2018.
- 31.Storey RF, Angiolillo DJ, Patil SB, et al. Inhibitory effects of ticagrelor compared with clopidogrel on platelet function in patients with acute coronary syndromes: the PLATO (PLATelet inhibition and patient Outcomes) PLATELET substudy. J Am Coll Cardiol 2010; 56: 1456–1462. [DOI] [PubMed] [Google Scholar]
- 32.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361: 1045–1057. [DOI] [PubMed] [Google Scholar]
- 33.James SK, Storey RF, Khurmi NS, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes and a history of stroke or transient ischemic attack. Circulation 2012; 125: 2914–2921. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for The Acute Stroke or Transient Ischemic Attack Treated with Ticagrelor and Aspirin for Prevention of Stroke and Death (THALES) trial: Rationale and design by S Claiborne Johnston, Pierre Amarenco, Hans Denison, Scott R Evans, Anders Himmelmann, Stefan James, Mikael Knutsson, Per Ladenvall, Carlos A Molina, Yongjun Wang and for the THALES Investigators in International Journal of Stroke