Abstract
Background:
Time matters in multiple sclerosis (MS). Irreversible neural damage and cell loss occur from disease onset. The MS community has endorsed a management strategy of prompt diagnosis, timely intervention and regular proactive monitoring of treatment effectiveness and disease activity to improve outcomes in people with MS.
Objectives:
We sought to develop internationally applicable quality standards for timely, brain health–focused MS care.
Methods:
A panel of MS specialist neurologists participated in an iterative, online, modified Delphi process to define ‘core’, ‘achievable’ and ‘aspirational’ time frames reflecting minimum, good and high care standards, respectively. A multidisciplinary Reviewing Group (MS nurses, people with MS, allied healthcare professionals) provided insights ensuring recommendations reflected perspectives from multiple stakeholders.
Results:
Twenty-one MS neurologists from 19 countries reached consensus on most core (25/27), achievable (25/27) and aspirational (22/27) time frames at the end of five rounds. Agreed standards cover six aspects of the care pathway: symptom onset, referral and diagnosis, treatment decisions, lifestyle, disease monitoring and managing new symptoms.
Conclusion:
These quality standards for core, achievable and aspirational care provide MS teams with a three-level framework for service evaluation, benchmarking and improvement. They have the potential to produce a profound change in the care of people with MS.
Keywords: Multiple sclerosis, quality improvement, consensus, standards, Delphi technique, benchmarking
Introduction
Time matters in multiple sclerosis (MS). Irreversible neural damage and cell loss occur from disease onset when the frequency of inflammatory attacks on the central nervous system is often greatest.1 Finite neurological reserves and plasticity compensate and maintain normal functioning in early disease. When reserves are exhausted, symptoms typically worsen in a progressive fashion and become irreversible.2 Manifestations include physical and cognitive decline, fatigue, reduced quality of life, compromised productivity and impaired functioning.
A policy report, Brain health: time matters in multiple sclerosis, the product of an international initiative by MS experts, delineates a strategy for preserving neurological reserve in people with MS.3 The planks of the strategy are as follows: increase the urgency of MS care; minimise delays in diagnosis and treatment initiation; monitor disease activity closely and proactively; set clear goals for treatment and ongoing management; underpin decision-making with robust up-to-date evidence; implement informed, shared decision-making; address lifestyle choices; collect and consult real world data.3 The MS community has widely endorsed this evidence-based strategy to reduce delays at all stages of the care pathway.3
Time to a diagnosis of MS is often protracted, delaying access to specialist healthcare advice and treatment initiation. Randomised controlled trials in patients with relapsing MS, for example, demonstrate that early treatment with a disease-modifying therapy (DMT) produces better outcomes than delayed treatment; specifically, lower relapse rates,4,5 reduced disability progression6–9 and improved survival.10,11 Conversely, unhealthy lifestyle choices and comorbidities can worsen MS outcomes. Smoking is associated with higher relapse rates, increased disability progression and greater cognitive impairment.12–15 Obesity is associated with increased lesion volume.16 Comorbidities can accelerate disability progression, increase mortality and reduce quality of life.17 Therefore, strategies seeking to preserve ‘brain health’ – a lay term for neurological reserve – should improve MS outcomes.
Quality standards and improvement programmes can improve patient outcomes and experiences. Introduction of the National Surgical Quality Improvement Programme was associated with decreases in mortality of 27% and 30-day morbidity of 45%.18 The Get With the Guidelines – Stroke programme was associated with a 1.18-fold yearly increase in the odds of receiving guideline-recommended care.19 In MS, several national quality standards describe aspects of care, but none comprehensively addresses brain health. Shared benchmarks are needed to formalise care standards and reduce global service provision disparities. We sought to develop internationally applicable quality standards for timely MS care.
Methods
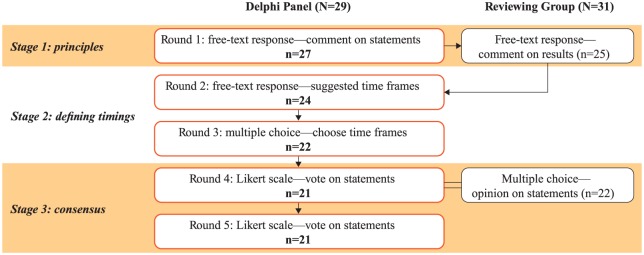
We conducted a modified Delphi consensus process to define timings for key brain health-related MS care milestones (Figure 1).20 The process involved three groups: the Delphi Chairs provided direction and identified potential participants; a Delphi Panel of MS neurologists proposed and agreed timings; a multidisciplinary Reviewing Group of MS nurses, allied healthcare professionals (aHCPs) and people with MS provided a broader perspective on the delivery and experience of MS care. Each of the four Chairs represented a different perspective: neurology (G.G.), patient-reported outcomes (J.H.), nursing/policy (A.B.) and the person with MS (G.P.). Analysts supported the Chairs by developing surveys, and collating and analysing responses.
Figure 1.
Study flow for the MS Brain Health Delphi process.
MS: multiple sclerosis.
Thirty-nine MS neurologists were invited by email to participate in the Delphi Panel. They were chosen to represent seven regions of high MS prevalence (North America, Northern Europe, Western Europe, Southern Europe, Eastern Europe and Russia, Australia and New Zealand, the Middle East and North Africa).21 Twenty-nine of the 39 (74%) agreed to participate and confirmed that they were based in MS clinics and spending at least half of their clinical time seeing people with MS; six did not respond, three declined because they did not meet the criteria and one did not have time to participate. Thirty-one of 39 (79%) invited individuals from the same regions agreed to participate in the Reviewing Group. Participants were contacted by email. Responses were collected between March and October 2017 via online surveys (SurveyMonkey Inc., San Mateo, CA, USA).
In brief, the Delphi process had three stages, each of which could have multiple rounds until considered complete. First, the scope was defined and agreed. Delphi Chairs and analysts derived, from Brain health: time matters in multiple sclerosis,3 principles of timely care. These were presented to the Delphi Panel to agree the content areas. Second, each Delphi participant independently proposed initial standards for the timing of key events in the MS care pathway and an iterative process was used to refine the timings. Third, consensus was reached via voting. Delphi panellists were required to participate in each round and, as standard for a Delphi process, remained anonymous throughout to analysts, Chairs and other participants.
Round 1: establishing principles of timely care
The Delphi Chairs and analysts derived 21 time-related principles from the evidence-based recommendations of Brain health: time matters in multiple sclerosis (Supplementary Table 1).3 The principles were grouped into five domains: onset of symptoms; referral and diagnosis; lifestyle and comorbidities; initiating DMT; monitoring. These were presented to the Delphi Panel who were asked if each was ‘an appropriate and accurate description of a good standard when considering brain health in people with MS’. Panellists had the opportunity to explain their opinions and/or propose additional principles. Responses were summarised and presented to the Reviewing Group for evaluation. The Chairs agreed that several principles did not require defined timings, for example, ‘Regular inclusion of patient data in MS database’. These were noted for inclusion later.
Rounds 2 and 3: setting time frames
In Round 2, Delphi Chairs derived variables reflecting timings of MS care pathway events from the principles (25 variables from 21 principles; Supplementary Table 2). For example, the variable ‘Frequency of magnetic resonance imaging (MRI) scans’ was derived from the principle ‘Regular MRI scans’. Each Delphi panellist was asked to assign (1) core, (2) achievable and (3) aspirational time frames for each variable using free text (Table 1). Analysts summarised the suggested timings as box plots showing the maximum, minimum, median and interquartile range. The Chairs developed multiple-choice options based on the grouped data.
Table 1.
Definitions used for consensus standards.
| Standard level | Definition |
|---|---|
| Core | This should currently be achieved by most MS teams worldwide, regardless of the local healthcare system, and will provide a minimum standard |
| Achievable | This is a realistic target for most MS teams and reflects a good standard of care |
| Aspirational | This might be achieved by only a few MS teams, where the local healthcare system allows, but should set the standard for high-quality care |
MS: multiple sclerosis.
In Round 3, Delphi panellists were presented with the box plots and multiple-choice options and asked to select core, achievable and aspirational time frames. Subsequently, the Chairs requested two new variables be included. These concerned timings of events following new or worsened symptoms and were intended to supplement timings for routine monitoring. Multiple-choice options for the two new variables were derived from clinical guidelines.22
Time frames for the final consensus rounds were determined based on the 75th percentile values achieved for each core, achievable and aspirational standard in the Round 3 voting. Four additional statements were derived from principles brought forwards from Round 1 that did not require defined timings, for example, ‘The MS team should regularly enter patient data into an MS database’.
Rounds 4 and 5: achieving consensus
In Round 4, the Delphi Panel was asked to indicate their level of agreement with the core, achievable and aspirational timings associated with each statement on a five-point scale (strongly disagree, disagree, neither agree nor disagree, agree, strongly agree). Data were analysed to quantify consensus. The threshold for Delphi Panel consensus was agreement (agree, strongly agree) by ⩾75% of panellists. For valid consensus, ⩾66% of panellists who completed Round 1 had to respond to all surveys. These percentages were predefined by the Chairs, based on a literature search.
In Round 5, statements for which consensus on time frame was not reached in Round 4 were shown alongside the associated voting results. The panellists were asked to vote again and to give an explanation if they still did not agree.
Reviewing Group opinion on consensus timings
The Reviewing Group was given the statements from Round 4 defining timings of MS care pathway events. Participants were asked to grade the ambition of each statement using a three-point scale (not ambitious enough, about right, too ambitious). Results will be presented elsewhere.
Post-Delphi feedback
After the Delphi process was completed, the full results were circulated to the unblinded panel. Two teleconferences were held in December 2017 to gain further insights into the thinking underlying panellists’ choices. Panellists unable to attend teleconferences were invited to provide feedback via email.
Results
Delphi Panel consensus
Twenty-one MS neurologists from 19 countries completed the modified Delphi process (78% of the 27 panellists from Round 1, Figure 1). Consensus was reached on the majority of core (25/27), achievable (25/27) and aspirational (22/27) time frames for events spanning the MS care pathway (Figure 2), thus defining a timeline for MS care (Figure 3).
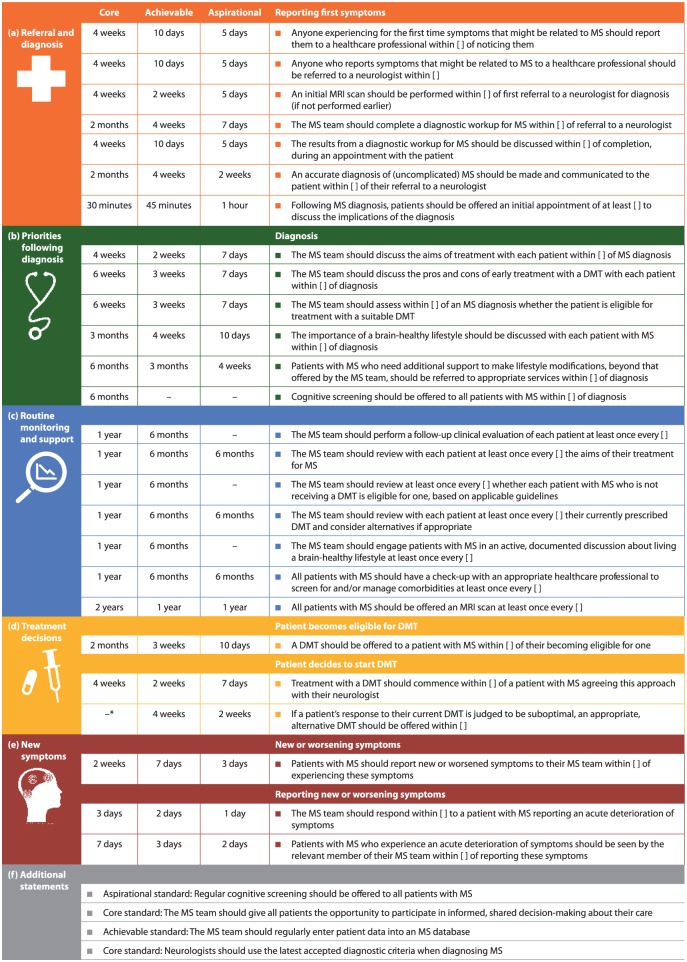
Figure 2.
Consensus standards for timely, brain health-focused MS care agreed by at least 75% of the Delphi Consensus Panel related to (a) referral and diagnosis, (b) priorities following diagnosis, (c) routine monitoring and support, (d) treatment decisions, (e) new symptoms and (f) additional statements that were not time limited.
DMT: disease-modifying therapy; MRI: magnetic resonance imaging; MS: multiple sclerosis.
*Time frame of 3 months was agreed by the Delphi Panel after completion of the Delphi process.
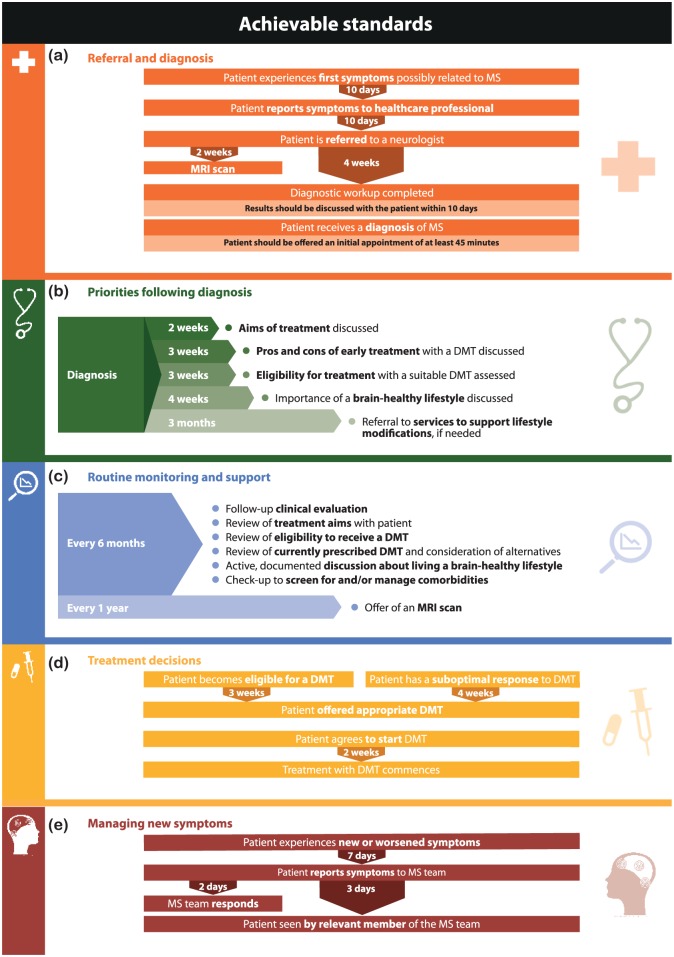
Figure 3.
Achievable consensus standards for the timing of key events in a brain health-focused MS care pathway related to (a) referral and diagnosis, (b) priorities following diagnosis, (c) routine monitoring and support, (d) treatment decisions and (e) new symptoms.
DMT: disease-modifying therapy; MRI: magnetic resonance imaging; MS: multiple sclerosis.
The Delphi Panel agreed that uncomplicated MS ought to be diagnosed in all clinics within 3 months of symptoms first being reported to a healthcare professional, and that diagnosis within 1 month would be expected of the best clinics (Figure 2(a)). These time frames include referral and completion of diagnostic workup (Figure 2(a)). There was consensus that all clinics should as a minimum standard assess DMT eligibility within 6 weeks of diagnosis (Figure 2(b)) and then offer an appropriate DMT within 2 months (Figure 2(d)). Consensus was that routine check-ups to assess MS status, review treatment plans and discuss lifestyle issues ought to take place at least annually, with 6-monthly check-ups being an achievable target for most MS teams (Figure 2(c)). The Delphi Panel also agreed that offering a routine annual MRI scan was achievable for most clinics (Figure 2(c)). In addition, there was consensus that all patients should be seen by a member of the MS team within 7 days of reporting new or worsened symptoms, or within 2 days in leading centres (Figure 2(e)). Supplementary Table 3 shows the nine statements for which consensus was not reached on time frames.
The Panel also reached agreement on four statements that were not time limited (Figure 2(f)). Applying the latest MS diagnostic criteria and offering all people with MS the opportunity to participate in informed, shared decision-making were agreed to be minimum standards of good care. There was consensus that routine entry of patient data into an MS database was achievable for most MS teams. Offering regular cognitive screening to all patients with MS was agreed to be an aspirational target.
Post-Delphi agreement
All 21 Delphi panellists fed back on the results (12 via teleconference; 9 via email). Reasons for non-agreement with suggested time frames included impact on healthcare costs and potential inconvenience to patients (Supplementary Table 3). Panellists agreed a time frame for one further core statement: when the response to a DMT is suboptimal, an appropriate alternative DMT should be offered within 3 months. In the Delphi process, the panel had been offered a time frame of 4 months; 6/21 considered this time frame too long.
Discussion
A modified Delphi process has established a comprehensive set of globally applicable quality standards for timely MS care. This is the first time a multinational group of neurologists has reached consensus on the timing of events across the MS care pathway. Given that participants practise in a wide range of countries with differing healthcare systems, it is reassuring that consensus was reached. The standards defined here provide benchmarks for MS services, while the three levels – core, achievable and aspirational – offer all clinics a standard to aim for. Taken together, the standards outline a practical and reasonable timeline for brain health-focused MS care.
The Delphi Panel agreed that MS teams should provide prompt diagnosis and treatment of MS, followed by regular monitoring, regardless of the local healthcare system. Across the standards, there is a focus on MS teams engaging patients in informed, shared decision-making and offering services promptly. This is particularly important when discussing DMTs, where each person has unique circumstances, needs and attitudes to risk.23 Healthcare professionals with expertise in MS are best placed to explain the pros and cons of various treatment options, given the increasing number of DMTs available.24 The panel agreed alternative DMTs should be offered and provided promptly when a treatment response is suboptimal. Naturally, some people with MS may require a longer time for decision-making and the standards allow for this. Informing, encouraging and empowering people to lead a brain-healthy lifestyle was regarded as a priority following diagnosis and regularly thereafter. Face-to-face discussions were considered more effective and less intrusive than written reminders. MS nurses are well placed to lead these discussions, so this does not necessitate longer consultations with a neurologist, which may be challenging in many centres.
Routine, 6-monthly appointments were agreed to reflect high-quality care, providing that MS teams respond quickly to patients reporting new or worsened symptoms. Annual MRI was considered a realistic target. More frequent ‘routine’ MRI was not recommended, due to concerns regarding lack of proven added benefit, expense and inconvenience to people with MS. Naturally, this guidance does not apply to ad hoc scanning for new or worsening symptom evaluation, which should be conducted as needed. Contrast-enhanced scanning was not specified because of recent concerns around gadolinium accumulation.25
The panel agreed that regular cognitive screening was an aspirational, rather than core, standard, with participants noting that emotions immediately post-diagnosis can affect results. In general, cognitive screening is not routine, which may be a consequence of healthcare costs and limited availability of neuropsychologists. There are several rapid, inexpensive, effective cognitive screening tests,26–28 but none are used as standard. Agreement among the MS community on a test to measure and monitor longitudinal cognitive changes would encourage acceptance of cognitive screening as a standard of MS care.
We recognise the limitations of this work. The standards are not exhaustive – we have not been explicit about what constitutes shared decision-making, MRI sequences and the extent of diagnostic testing, for example, because our focus was on timing. The strengths of the Delphi method include the anonymity of respondents and the equal weight given to all opinions, but because it can be difficult to reconcile widely varying opinions, it is recommended that panellists have similar backgrounds and experience.20 Hence, only practising MS neurologists were invited to participate in the panel. Improving MS care involves multiple stakeholders; so, the Delphi process was modified to include a Reviewing Group of MS nurses, aHCPs and people with MS. Subgroup analysis could provide additional insights into geographical or stakeholder group differences in expectations.
Many healthcare systems are now focusing on quality and improving patient care, and so we developed these standards to facilitate formal care quality improvement. Other standards for MS care exist, including local clinical guidelines, treatment algorithms and recommendations on MRI use.22,24,29–33 Our quality standards differ in that they focus on timings rather than specific treatment strategies; as such, they should be considered in conjunction with existing local guidance. We have defined global standards that are not specific to any one country. To ensure these standards were relevant in a range of healthcare systems, we agreed upon three levels. MS clinics therefore have the flexibility to work towards standards that are realistic within the constraints of their systems. The National Institute for Health and Care Excellence quality standards, for example, recommend annual clinical evaluations;34 this is consistent with the core standard in the present study, but we have gone further and defined an achievable standard of 6-monthly evaluations and hope that MS clinics will strive for this higher standard.
Implementing the quality standards first requires individual MS centres to evaluate their service. A quality improvement tool – which helps MS clinics to compare their current practice with the standards – is in development and will be piloted in several international sites. Given the number of standards, a key action is to identify a representative subgroup of standards, allowing refinement of the tool to make it brief enough for easy use in routine clinical practice. The final tool will be available for interested MS teams to use locally. MS clinics could use this as an opportunity for patient engagement by asking patients to complete a survey about their experiences, complementing a formal review of patient records. We encourage centres to seek specific funding, where needed, to carry out an assessment. Following service evaluation, MS teams should analyse their integrated care pathways to understand the processes underpinning any delays identified and have targeted discussions with local development teams to identify solutions.
Using a quality improvement cycle, MS clinics will be able to demonstrate service improvement. We encourage MS teams to incorporate data collection into routine care to support re-evaluation, and to share examples of best practice. In some countries, local budget holders may want further evidence of the clinical benefit of timely care – as described by the quality standards. To generate evidence, MS clinics will need to link results from routine service evaluations with long-term clinical outcomes. MS clinics could collaborate with other centres to allow comparisons and, in the future, MS databases could enable large-scale analysis by adding relevant data fields.
Other stakeholders will contribute to improving care. MS neurologists and MS nurses should educate colleagues – including general neurologists and primary care practitioners – on the importance of timely, specialist MS care, and highlight best practice examples to local budget holders, managers and service providers. Professional organisations could promote the standards by incorporating the ‘time matters’ message into training programmes. Charities representing people with MS could use the quality standards in advocacy work with decision-makers, to demonstrate the care people with MS should be receiving. If accompanying data show that standards are not being met, this could be a powerful motivator for increased resources, particularly if the standards are met in comparable countries. The standards also present an opportunity for empowering individuals to ask for high-quality care. We encourage stakeholders to collaborate nationally and speak to decision-makers with a united voice.
We know these standards will be challenging for all of us to meet, particularly within current healthcare climates. However, we believe this is no justification for disregarding timely care. These standards provide an opportunity to identify strengths and weaknesses, focus problem-solving and highlight areas requiring investment.
Conclusion
Multinational MS neurologists have used a Delphi process to agree quality standards for timely MS care focused on preserving brain health. These new global benchmarks have the potential to help individual clinics and national healthcare systems maximise outcomes for people with MS. We anticipate vigorous debate of these standards in the wider MS care community.
Supplemental Material
Supplemental material, MSJ809326_supplementary_material for International consensus on quality standards for brain health-focused care in multiple sclerosis by Jeremy Hobart, Amy Bowen, George Pepper, Harriet Crofts, Lucy Eberhard, Thomas Berger, Alexey Boyko, Cavit Boz, Helmut Butzkueven, Elisabeth Gulowsen Celius, Jelena Drulovic, José Flores, Dana Horáková, Christine Lebrun-Frénay, Ruth Ann Marrie, James Overell, Fredrik Piehl, Peter Vestergaard Rasmussen, Maria José Sá, Carmen-Adella Sîrbu, Eli Skromne, Øivind Torkildsen, Vincent van Pesch, Timothy Vollmer, Magd Zakaria, Tjalf Ziemssen and Gavin Giovannoni in Multiple Sclerosis Journal
Acknowledgments
The authors are grateful to Ruth Bentley of Oxford Health Policy Forum CIC, UK for critical review of the study design and manuscript, the Reviewing Group participants for their insights and contribution, and Danielle Emery of Oxford PharmaGenesis for co-ordinating the blinded aspects of the Delphi process. J.H., A. Bowen., G.P. and G.G. – Chairs of the MS Brain Health Delphi process; H.C. and L.E. – Analysts; T.B., A. Boyko., C.B., H.B., E.G.C., J.D., J.F., D.H., C.L.-F., R.A.M., J.O., F.P., P.V.R., M.J.S., C.-A.S., E.S., Ø.T., V.v.P., T.V., M.Z. and T.Z. – Members of the Delphi Consensus Panel.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.H. has received consulting fees, honoraria, support to attend meetings or research support from Acorda, Asubio, Bayer Schering, Biogen Idec, F. Hoffmann-La Roche, Genzyme, Merck Serono, Novartis, Oxford Health Policy Forum, Oxford PharmaGenesis and Teva. G.P. has received consulting fees from Biogen, Novartis, Oxford Health Policy Forum, Oxford PharmaGenesis and Teva. H.C. and L.E. are employees of Oxford PharmaGenesis Ltd., and work on this study was carried out as part of their role at the company. A.Boyko. has received honoraria as a member of working groups and advisory boards and participated in clinical trials for Biogen, Schering, Merck, Teva, Novartis, Sanofi Genzyme, Actelion, Biocad and Generium. H.B. has received personal fees from Biogen, Merck, Novartis, Oxford Health Policy Forum, Oxford PharmaGenesis and Teva, and research support from Biogen and Novartis. E.G.C. has received personal fees from Almirall, Biogen, Merck, Roche and Teva, and grants and personal fees from Novartis and Sanofi. J.D. has received fees for serving on scientific advisory boards for Bayer Schering Pharma, Merck Serono, Teva, Genzyme, a Sanofi Company, Roche; has received speaker honoraria from Merck Serono, Teva, Bayer Schering, Genzyme, a Sanofi Company, Medis and Roche; and has received research grant support from the Ministry of Education and Science, Republic of Serbia (project no. 175031). D.H. has received grants from Czech Ministry of Education project Progres Q27/LF1, and speaker honoraria, travel expenses and consultancy fees from Biogen, Novartis, Merck, Roche, Sanofi Genzyme and Teva. J.O. has received grants, personal fees and non-financial support from Biogen, Novartis, Roche and Genzyme, and personal fees and non-financial support from Allergan, Merck and Teva. F.P. has received research grants from Biogen, Novartis and Genzyme, and fees for serving as Chair of DMC in clinical trials with Parexel. P.V.R. has received grant support from Novartis; has received fees for acting as a consultant or advisory board member for Allergan, Biogen, Merck, Novartis, Roche, Sanofi-Aventis and Teva; and has received speaker fees from Teva. M.J.S. has received speaker and/or consulting fees from Bayer, Biogen, CSL Behring, Merck, Novartis, Roche, Sanofi Genzyme and Teva. C.-A.S. has received conference expenses from Bayer, Medison Pharma, Merck, Novartis and Teva. Ø.T. has received speaker honoraria and fees for serving on scientific advisory boards from Biogen, Merck and Sanofi-Aventis, and has received speaker honoraria from Novartis. V.v.P. has received travel grants from Biogen, Genzyme, Merck, Teva, Roche and Novartis Pharma. His institution receives honoraria for consultancy and lectures from Biogen, Genzyme, Merck, Roche, Teva and Novartis Pharma as well as research grants from Sanofi, Novartis Pharma and Roche. T.V. has received compensation for acting as a consultant, speaker or advisory board member for Academic CME, Alcimed, Anthem Blue Cross, Genentech/Roche, Biogen IDEC, Novartis, CellGene, Epigene, Rocky Mountain MS Center, GLG Consulting, Ohio Health, Oxford Health Policy Forum, Oxford PharmaGenesis, TG Therapeutics, Topaz Therapeutics, Dleara Lawyers and Teva Neuroscience, and has received research support from Teva Neuroscience, NIH/NINDS, Rocky Mountain MS Center, Actelion, Biogen, Novartis, Roche/Genentech and TG Therapeutics, Inc. T.Z. has received grants and personal fees from Biogen, Novartis, Sanofi and Teva, and personal fees from Almirall, Bayer, Merck, Oxford Health Policy Forum and Roche. G.G. has received consulting fees from AbbVie, Atara Biotherapeutics, Almirall, Biogen, Celgene, GlaxoSmithKline, MedDay Pharmaceuticals, Merck and Company (USA), Merck Group (Europe), Novartis, Oxford Health Policy Forum, Oxford PharmaGenesis, Roche, Sanofi Genzyme, Synthon, Takeda, Teva Pharmaceutical Industries Ltd. and UCB, and has received research support from Biogen, Sanofi Genzyme and Takeda. A.Bowen., C.B., T.B., J.F., C.L.-F., R.A.M., E.S. and M.Z. declare no competing interests.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was commissioned by Oxford Health Policy Forum, on behalf of the MS Brain Health initiative. Grants to support MS Brain Health were provided by Actelion Pharmaceuticals, Biogen, Celgene, F. Hoffmann-La Roche, Merck KGaA and Sanofi Genzyme, all of whom had no role in study design, data collection, data analysis, data interpretation, writing of the report or in the decision to submit the paper for publication. All authors had full access to the data in the study, and the corresponding author had the final responsibility for the decision to submit for publication.
Contributor Information
Jeremy Hobart, Plymouth University Peninsula Schools of Medicine and Dentistry, University of Plymouth, Plymouth, UK.
Amy Bowen, NHS RightCare, NHS England, London, UK.
George Pepper, Shift.ms, Leeds, UK.
Harriet Crofts, PharmaGenesis London, London, UK.
Lucy Eberhard, PharmaGenesis London, London, UK.
Thomas Berger, Clinical Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria.
Alexey Boyko, Department of Neurology, Neurosurgery and Medical Genetics, Pirogov Russian National Research Medical University, Moscow, Russia/Demyelinating Diseases Center, Yusupov Hospital, Moscow, Russia.
Cavit Boz, Department of Neurology, Karadeniz Technical University, Trabzon, Turkey.
Helmut Butzkueven, MS and Neuroimmunology Unit, Alfred Health and Eastern Health, Monash University, Melbourne, VIC, Australia.
Elisabeth Gulowsen Celius, Department of Neurology, Oslo University Hospital, Oslo, Norway.
Jelena Drulovic, Department for Immune-Mediated Disorders of the Central Nervous System, Clinic of Neurology, Faculty of Medicine, University of Belgrade, Belgrade, Serbia.
José Flores, National Institute of Neurology and Neurosurgery, ABC Medical Center, Mexico City, Mexico.
Dana Horáková, Department of Neurology and Center of Clinical Neuroscience, First Faculty of Medicine, Charles University and General University Hospital, Prague, Czech Republic.
Christine Lebrun-Frénay, CRCSEP Neurologie Pasteur 2, Université Côte d’Azur, Nice, France.
Ruth Ann Marrie, Departments of Internal Medicine and Community Health Sciences, Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, MB, Canada.
James Overell, Glasgow MS Clinical Research Centre, Queen Elizabeth University Hospital, Glasgow, UK.
Fredrik Piehl, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden.
Peter Vestergaard Rasmussen, Department of Neurology, Aarhus University Hospital, Aarhus, Denmark.
Maria José Sá, MS Clinic, Department of Neurology, Centro Hospitalar São João, Faculty of Health Sciences, University Fernando Pessoa, Porto, Portugal.
Carmen-Adella Sîrbu, Clinic of Neurology, Central Military Emergency University Hospital, Bucharest, Romania.
Eli Skromne, Instituto Mexicano de Neurociencias, Hospital Angeles Lomas, Mexico City, Mexico.
Øivind Torkildsen, Department of Neurology, Haukeland University Hospital, Bergen, Norway.
Vincent van Pesch, Cliniques Universitaires Saint-Luc, Université Catholique de Louvain, Brussels, Belgium.
Timothy Vollmer, Department of Neurology, University of Colorado, Denver, CO, USA.
Magd Zakaria, Department of Neurology, Ain Shams University, Cairo, Egypt.
Tjalf Ziemssen, Department of Neurology, MS Center Dresden, Center of Clinical Neuroscience, University Hospital Carl Gustav Carus, Dresden University of Technology, Dresden, Germany.
Gavin Giovannoni, Queen Mary University of London, Blizard Institute, Barts and The London School of Medicine and Dentistry, London, UK.
References
- 1. Tremlett H, Zhao Y, Joseph J, et al. Relapses in multiple sclerosis are age- and time-dependent. J Neurol Neurosurg Psychiatry 2008; 79: 1368–1374. [DOI] [PubMed] [Google Scholar]
- 2. Sumowski JF, Rocca MA, Leavitt VM, et al. Brain reserve and cognitive reserve in multiple sclerosis: What you’ve got and how you use it. Neurology 2013; 80: 2186–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Giovannoni G, Butzkueven H, Dhib-Jalbut S, et al. Brain health: Time matters in multiple sclerosis. Mult Scler Relat Disord 2016; 9(Suppl. 1): S5–S48. [DOI] [PubMed] [Google Scholar]
- 4. Kappos L, O’Connor P, Radue EW, et al. Long-term effects of fingolimod in multiple sclerosis: The randomized FREEDOMS extension trial. Neurology 2015; 84: 1582–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kappos L, Traboulsee A, Constantinescu C, et al. Long-term subcutaneous interferon beta-1a therapy in patients with relapsing-remitting MS. Neurology 2006; 67: 944–953. [DOI] [PubMed] [Google Scholar]
- 6. Johnson KP, Ford CC, Lisak RP, et al. Neurologic consequence of delaying glatiramer acetate therapy for multiple sclerosis: 8-year data. Acta Neurol Scand 2005; 111: 42–47. [DOI] [PubMed] [Google Scholar]
- 7. Rovaris M, Comi G, Rocca MA, et al. Long-term follow-up of patients treated with glatiramer acetate: A multicentre, multinational extension of the European/Canadian double-blind, placebo-controlled, MRI-monitored trial. Mult Scler 2007; 13: 502–508. [DOI] [PubMed] [Google Scholar]
- 8. Trojano M, Pellegrini F, Paolicelli D, et al. Real-life impact of early interferon beta therapy in relapsing multiple sclerosis. Ann Neurol 2009; 66: 513–520. [DOI] [PubMed] [Google Scholar]
- 9. O’Connor P, Goodman A, Kappos L, et al. Long-term safety and effectiveness of natalizumab redosing and treatment in the STRATA MS Study. Neurology 2014; 83: 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goodin DS, Reder AT, Ebers GC, et al. Survival in MS: A randomized cohort study 21 years after the start of the pivotal IFNβ-1b trial. Neurology 2012; 78: 1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Merkel B, Butzkueven H, Traboulsee AL, et al. Timing of high-efficacy therapy in relapsing-remitting multiple sclerosis: A systematic review. Autoimmun Rev 2017; 16: 658–665. [DOI] [PubMed] [Google Scholar]
- 12. D’Hooghe MB, Nagels G, Bissay V, et al. Modifiable factors influencing relapses and disability in multiple sclerosis. Mult Scler 2010; 16: 773–785. [DOI] [PubMed] [Google Scholar]
- 13. Pittas F, Ponsonby AL, van der Mei IA, et al. Smoking is associated with progressive disease course and increased progression in clinical disability in a prospective cohort of people with multiple sclerosis. J Neurol 2009; 256: 577–585. [DOI] [PubMed] [Google Scholar]
- 14. Ozcan ME, Ince B, Bingol A, et al. Association between smoking and cognitive impairment in multiple sclerosis. Neuropsychiatr Dis Treat 2014; 10: 1715–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paz-Ballesteros WC, Monterrubio-Flores EA, de Jesus Flores-Rivera J, et al. Cigarette smoking, alcohol consumption and overweight in multiple sclerosis: Disability progression. Arch Med Res 2017; 48: 113–120. [DOI] [PubMed] [Google Scholar]
- 16. Kappus N, Weinstock-Guttman B, Hagemeier J, et al. Cardiovascular risk factors are associated with increased lesion burden and brain atrophy in multiple sclerosis. J Neurol Neurosurg Psychiatry 2016; 87: 181–187. [DOI] [PubMed] [Google Scholar]
- 17. Marrie RA. Comorbidity in multiple sclerosis: Implications for patient care. Nat Rev Neurol 2017; 13: 375–382. [DOI] [PubMed] [Google Scholar]
- 18. Khuri SF, Daley J, Henderson WG. The comparative assessment and improvement of quality of surgical care in the Department of Veterans Affairs. Arch Surg 2002; 137: 20–27. [DOI] [PubMed] [Google Scholar]
- 19. Schwamm LH, Fonarow GC, Reeves MJ, et al. Get With the Guidelines-Stroke is associated with sustained improvement in care for patients hospitalized with acute stroke or transient ischemic attack. Circulation 2009; 119: 107–115. [DOI] [PubMed] [Google Scholar]
- 20. Hsu C-C, Sandford BA. The Delphi technique: Making sense of consensus. Pract Assess Res Eval 2007; 12: 1–8. [Google Scholar]
- 21. Multiple Sclerosis International Federation. Atlas of MS 2013: Mapping multiple sclerosis around the world, https://www.msif.org/wp-content/uploads/2014/09/Atlas-of-MS.pdf (2013, accessed 14 December 2017).
- 22. National Institute for Health and Care Excellence (NICE). Multiple sclerosis in adults: Management. NICE Clinical Guideline, CG. 186, London, UK: NICE, October 2014. [Google Scholar]
- 23. Reen GK, Silber E, Langdon DW. Multiple sclerosis patients’ understanding and preferences for risks and benefits of disease-modifying drugs: A systematic review. J Neurol Sci 2017; 375: 107–122. [DOI] [PubMed] [Google Scholar]
- 24. Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler 2018; 24: 96–120. [DOI] [PubMed] [Google Scholar]
- 25. U.S. Food & Drug Administration. FDA Drug Safety Communication: FDA warns that gadolinium-based contrast agents (GBCAs) are retained in the body; requires new class warnings, https://www.fda.gov/Drugs/DrugSafety/ucm589213.htm (2017, accessed 8 January 2018).
- 26. Ruet A, Deloire MS, Charre-Morin J, et al. A new computerised cognitive test for the detection of information processing speed impairment in multiple sclerosis. Mult Scler 2013; 19: 1665–1672. [DOI] [PubMed] [Google Scholar]
- 27. Benedict RH, DeLuca J, Phillips G, et al. Validity of the symbol digit modalities test as a cognition performance outcome measure for multiple sclerosis. Mult Scler 2017; 23: 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Langdon DW, Amato MP, Boringa J, et al. Recommendations for a brief international cognitive assessment for multiple sclerosis (BICAMS). Mult Scler 2012; 18: 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. American Association of Neuroscience Nurses, Association of Rehabilitation Nurses and International Organization of Multiple Sclerosis Nurses. Nursing management of the patient with multiple sclerosis: AANN, ARN, and IOMSN clinical practice guideline series. AANN, Chicago, IL, USA, 2011. [Google Scholar]
- 30. Yamout B, Alroughani R, Al-Jumah M, et al. Consensus recommendations for the diagnosis and treatment of multiple sclerosis: The Middle East North Africa Committee for Treatment and Research In Multiple Sclerosis (MENACTRIMS). Curr Med Res Opin 2015; 31: 1349–1361. [DOI] [PubMed] [Google Scholar]
- 31. Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology 2018; 90: 777–788. [DOI] [PubMed] [Google Scholar]
- 32. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 33. Traboulsee A, Simon JH, Stone L, et al. Revised recommendations of the Consortium of MS Centers Task Force for a standardized MRI protocol and clinical guidelines for the diagnosis and follow-up of multiple sclerosis. AJNR Am J Neuroradiol 2016; 37: 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. National Institute for Health and Care Excellence (NICE). Multiple sclerosis. NICE Quality Standard, QS. 108, London, UK: NICE, January 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MSJ809326_supplementary_material for International consensus on quality standards for brain health-focused care in multiple sclerosis by Jeremy Hobart, Amy Bowen, George Pepper, Harriet Crofts, Lucy Eberhard, Thomas Berger, Alexey Boyko, Cavit Boz, Helmut Butzkueven, Elisabeth Gulowsen Celius, Jelena Drulovic, José Flores, Dana Horáková, Christine Lebrun-Frénay, Ruth Ann Marrie, James Overell, Fredrik Piehl, Peter Vestergaard Rasmussen, Maria José Sá, Carmen-Adella Sîrbu, Eli Skromne, Øivind Torkildsen, Vincent van Pesch, Timothy Vollmer, Magd Zakaria, Tjalf Ziemssen and Gavin Giovannoni in Multiple Sclerosis Journal