Abstract
Hypoxia has been associated with multiple sclerosis (MS) and is an important area of research. Hypoxia can exacerbate inflammation via the prolylhydroxylase pathway. Inflammation can also trigger hypoxia by damaging mitochondria and endothelial cells to impair blood flow regulation. We hypothesize that there is a “hypoxia–inflammation cycle” in MS which plays an important role in MS disease progression. Therapies that break this cycle may be an interesting area of exploration for treatment of MS.
Keywords: Biomarkers, hypoxia, inflammation
Introduction
Hypoxia has been associated with multiple sclerosis (MS) for years.1 Type III lesions in MS brain are histologically similar to hypoxic/ischemic lesions, and lesions tend to form in areas that are more susceptible to hypoxia.2 There is evidence for reduction in global metabolic rate in MS,3 which could suggest that hypoxia/ischemia is not restricted to lesions, but rather a diffuse event. Inflammation is also distributed throughout the brain. Is there a link between hypoxia and inflammation?
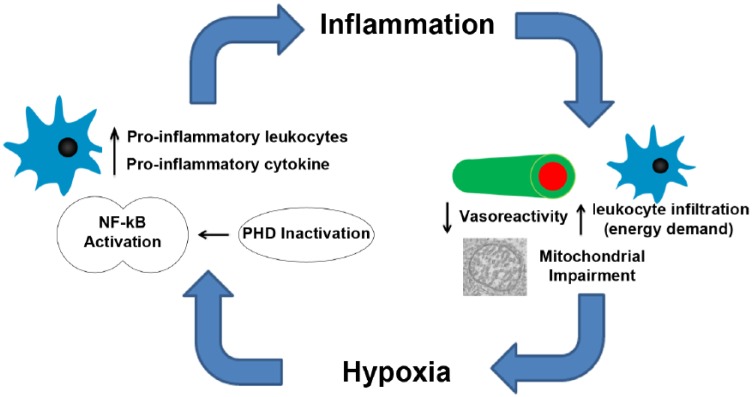
Hypoxia may be critical in the context of MS. Signaling pathways for hypoxia and inflammation are intricately connected by the enzyme prolylhydroxylase (PHD).4 PHDs are responsible for breaking down hypoxia inducible factor 1 alpha (HIF-1α), the master regulator in the hypoxia response. Concurrently, PHD-related pathways impact nuclear factor kappa B (NF-κB), which is involved in driving the production of pro-inflammatory cytokines. Hypoxia inhibits PHD, allowing both HIF-1α and NF-κB to become active.4 This not only triggers the up-regulation of genes involved in the hypoxia response, but also exacerbates the inflammatory response. Inflammation can damage the vascular endothelium, reduce vasoreactivity, and promote the influx of leukocytes. An increased metabolic demand from inflammatory cells may exceed the supply of oxygen, thus exacerbating hypoxia. Here, we review the evidence suggesting that diffuse hypoxia is present in MS and propose that there is an “hypoxia–inflammation cycle” (Figure 1) that contributes to MS disease progression.
Figure 1.
Hypoxia and inflammation form a positive feedback loop. The presence of low oxygen will lead to PHD inactivation, which will eventually result in the activation of NF-κB, the master regulator of inflammation. Increased levels of pro-inflammatory leukocytes and cytokines can damage vascular endothelial cells, decrease vasoreactivity, and impair mitochondrial function, which could in turn exacerbate hypoxia.
Presence of hypoxia in MS
Accumulating evidence indicates that hypoxia is not restricted to the focal type III lesions, but is in fact a diffuse event. Using a rat experimental autoimmune encephalomyelitis (EAE) model of MS, it has been shown that partial pressure of oxygen (pO2) in the spinal cord significantly decreased after induction of EAE.5 We used implanted pO2 probes to quantify oxygen in the cerebellum and cortex of EAE mice and detected hypoxia compared to Complete Freund’s Adjuvant (CFA) controls.6 These results show that the EAE model of MS, an inflammatory model, shows significant levels of hypoxia.
Clinical studies also support the theory that hypoxia exists in patients with MS. Several groups have used magnetic resonance imaging (MRI) and showed that patients with MS have reduced cerebral blood flow (CBF) compared to controls, which is likely caused by impaired vasodilation. Impaired vasodilation7 and decreased CBF are significantly correlated with gray matter (GM) loss and cognitive impairment,8 suggesting that insufficient blood flow (and thus hypoxic stress) may play an important role in neurodegeneration. These results are consistent with a hypoxia/ischemia-induced metabolic stress, which ultimately lead to impairment of neuronal function.
We measured brain oxygenation in MS patients using near-infrared spectroscopy (NIRS) and confirmed that approximately half of all MS patients have low microvascular hemoglobin saturation, consistent with GM hypoxia. NIRS is a non-invasive technique that utilizes the differential absorption properties of oxygenated and deoxygenated hemoglobin to calculate the tissue hemoglobin oxygen saturation (StO2). We found that relapse–remitting patients with higher levels of impairment, as well as secondary progressive patients, had significantly lower StO2 in their brains compared to controls.9 These evidence suggests that hypoxia is present in MS, and that it is relatively common phenomenon.
Inflammation drives hypoxia—evidence from other inflammatory diseases
Inflammation may be one of the key causes of hypoxia in many conditions, such as Crohn’s disease, sepsis,10 and the no-reflow phenomenon phenomenon seen in stroke.11 Inflammation can cause hypoxia through several mechanisms. Reactive oxygen species (ROS) that are released during inflammation can damage the vascular endothelium and reduce the endothelial smooth muscles’ ability to relax and cause vasodilation. This may be one of the mechanisms causing reduced CBF and hypoxia in MS. Using MRI to measure CBF, it has been shown that vasodilation in response to CO2 is significantly decreased in MS patients.
Inflammation can also cause physical obstruction of small blood vessels, a phenomenon that has been documented in ischemic stroke.11 In experimental animal models of stroke, blood oxygenation levels in mice treated with Interleukin 1 beta (IL-1β) continued to decline even after the reversal of the occlusion. Immuno-histochemistry showed a significant increase in leukocytes, platelets, and vascular adhesion molecules. Leukocytes and platelets aggravated in small blood vessels, which likely created physical ischemia in the microvasculature. It is possible that such events are occurring in patients with MS.
The idea that inflammation can cause physical obstruction of capillaries has been documented in the sepsis literature. It has been shown that administration of lipopolysaccharide (LPS) causes decreased CBF in the microvasculature as well as cerebral tissue pO2.10 The decrease in tissue oxygenation is concurrent with a decrease in the proportion of small perfused vessels, which indicates that circulating inflammatory cells may be occluding small blood vessels. The decrease in CBF, combined with the influx of immune cells into the brain parenchyma, may cause oxygen demand to far exceed the supply available, thus creating a hypoxic condition.
Hypoxia in MS: a detrimental phenomenon
The pathway initiating the hypoxic response is intricately connected with the inflammatory pathway through a class of oxygen sensing molecules called PHDs.4 There are three types of PHDs (PHD-1, 2, 3), and they all require oxygen as a co-factor to function.4 Under normal levels of oxygen, PHDs prevent the stabilization of HIF1-α and inhibit the hypoxia response. PHDs under normal oxygen conditions also inhibit the NF-κB (a master regulator of inflammation) by targeting the NF-κB activation molecule inhibitor of nuclear factor kappa-B kinase subunit beta (IKKβ) for degradation. When oxygen levels fall, PHDs are no longer active, resulting in activation of the HIF pathway as well as an accumulation of Ikkβ, leading to the activation of NF-κB.4 As NF-κB is responsible for polarization innate and adaptive immune cells into a pro-inflammatory state, one could argue that hypoxia is detrimental and will exacerbate inflammation in MS.
In the context of MS, there is a large body of literature showing that NF-κB activation is detrimental. Many of the new Food and Drug Administration (FDA) approved MS medications, such as fingolimod, dimethyl fumarate, and teriflunomide all modulate the NF-κB signaling pathway in some aspects.12 NF-κB is clearly important in the progression of MS, and measurement of tissue hypoxia may provide insights into the activity of this signaling pathway.
The idea that hypoxia can exacerbate inflammation in MS is supported by the observation that lesions are more common in areas that may be susceptible to hypoxia.2,13,14 Lesions are commonly formed at the lateral ventricles. Arterioles in this region are long and narrow, which could reduce the capacity to modulate flow and increase the susceptibility of these regions to hypoxic stress.14 Lesions are also commonly formed in areas known as watersheds, or borders of arterial territories. These areas have significantly lower perfusion and have an increased susceptibility to hypoxia.13,14 Others have also postulated that inflammatory events occurring in hypoperfused areas are likely to result in more damage and are less likely to resolve.13
In summary, there is a growing body of evidence suggesting that hypoxic regions exist in the brain of some MS patients. It is difficult to determine whether hypoxia or inflammation is the primary event. However, it is likely that hypoxia exacerbates inflammation and vice versa, thereby creating a “hypoxia–inflammation cycle.” Thus, hypoxia could not only be a potential biomarker of pro-inflammatory activities, but also a therapeutic target.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: R.Y. is funded by an Alberta Innovates Health Solutions MD-PhD Studentship; programmatic funding was received from Alberta Innovates Health Solutions - CRIO Team Grant.
Contributor Information
Runze Yang, Department of Radiology, University of Calgary, Calgary, AB, Canada/Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada/Department of Clinical Neurosciences, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada.
Jeff F Dunn, Department of Radiology, University of Calgary, Calgary, AB, Canada/Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada/Department of Clinical Neurosciences, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada.
References
- 1. Gottlieb SF, Smith JE, Neubauer RA. The etiology of multiple sclerosis: A new and extended vascular-ischemic model. Med Hypotheses 1990; 33: 23–29. [DOI] [PubMed] [Google Scholar]
- 2. Brownell B, Hughes JT. The distribution of plaques in the cerebrum in multiple sclerosis. J Neurol Neurosurg Psychiatry 1962; 25: 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun X, Tanaka M, Kondo S, et al. Clinical significance of reduced cerebral metabolism in multiple sclerosis: A combined PET and MRI study. Ann Nucl Med 1998; 12: 89–94. [DOI] [PubMed] [Google Scholar]
- 4. Bartels K, Grenz A, Eltzschig HK. Hypoxia and inflammation are two sides of the same coin. Proc Natl Acad Sci U S A 2013; 110: 18351–18352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davies AL, Desai RA, Bloomfield PS, et al. Neurological deficits caused by tissue hypoxia in neuroinflammatory disease. Ann Neurol 2013; 74: 815–825. [DOI] [PubMed] [Google Scholar]
- 6. Johnson TW, Wu Y, Nathoo N, et al. Gray matter hypoxia in the brain of the experimental autoimmune encephalomyelitis model of multiple sclerosis. PLoS ONE 2016; 11: e0167196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marshall O, Chawla S, Lu H, et al. Cerebral blood flow modulation insufficiency in brain networks in multiple sclerosis: A hypercapnia MRI study. J Cereb Blood Flow Metab 2016; 36: 2087–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Inglese M, Adhya S, Johnson G, et al. Perfusion magnetic resonance imaging correlates of neuropsychological impairment in multiple sclerosis. J Cereb Blood Flow Metab 2008; 28: 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang R, Dunn JF. Reduced cortical microvascular oxygenation in multiple sclerosis: A blinded, case-controlled study using a novel quantitative near-infrared spectroscopy method. Sci Rep 2015; 5: 16477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taccone FS, Su F, De Deyne C, et al. Sepsis is associated with altered cerebral microcirculation and tissue hypoxia in experimental peritonitis. Crit Care Med 2014; 42: e114–e122. [DOI] [PubMed] [Google Scholar]
- 11. Burrows F, Haley MJ, Scott E, et al. Systemic inflammation affects reperfusion following transient cerebral ischaemia. Exp Neurol 2016; 277: 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leibowitz SM, Yan J. NF-κB pathways in the pathogenesis of multiple sclerosis and the therapeutic implications. Front Mol Neurosci 2016; 9: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haider L, Zrzavy T, Hametner S, et al. The topograpy of demyelination and neurodegeneration in the multiple sclerosis brain. Brain 2016; 139: 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martinez Sosa S, Smith KJ. Understanding a role for hypoxia in lesion formation and location in the deep and periventricular white matter in small vessel disease and multiple sclerosis. Clin Sci (Lond) 2017; 131: 2503–2524. [DOI] [PubMed] [Google Scholar]