Abstract
Practical relevance:
Urinary tract infection (UTI) is an important cause of feline lower urinary tract disease (FLUTD), particularly in female cats older than 10 years of age. In addition to cats with typical clinical signs of FLUTD or upper UTI, many cats have subclinical bacteriuria, but the clinical relevance of this is currently uncertain. UTIs are one of the most important indications for antimicrobial use in veterinary medicine and contribute to the development of antimicrobial resistance. Adherence to treatment guidelines and confinement to a few first-line antimicrobial agents is imperative to avoid further deterioration of the antimicrobial resistance situation. The decision to treat with antimicrobials should be based on the presence of clinical signs, and/or concurrent diseases, and the results of urine culture and susceptibility testing.
Clinical challenges:
Distinguishing between cats with bacterial cystitis, and those with idiopathic cystitis and concurrent clinical or subclinical bacteriuria, is challenging, as clinical signs and urinalysis results may be identical. Optimal treatment of subclinical bacteriuria requires clarification as there is currently no evidence that demonstrates a beneficial effect of routine treatment. Management of recurrent UTIs remains a challenge as evidence for most alternatives used for prevention in cats is mainly anecdotal, and no preventive treatment modality is currently recommended.
Evidence base:
This review draws on an extensive literature base in veterinary and human medicine, including the recently updated guidelines of the International Society for Companion Animal Infectious Diseases for the diagnosis and management of bacterial urinary tract infections in dogs and cats. Where published evidence is lacking, the authors describe their own approach; notably, for the bacteriuric cat with chronic kidney disease.
Keywords: Cystitis, feline lower urinary tract disease, FLUTD, pyelonephritis, antimicrobial resistance
Introduction
Urinary tract infection (UTI) refers to the adherence, multiplication and persistence of an infectious agent within the urogenital system that causes an associated inflammatory response and clinical signs. 1 In the vast majority of UTIs, bacteria are the infecting organisms; fewer than 1% of UTIs are due to parasitic, fungal or viral infections.
In addition to cats with typical clinical signs of feline lower urinary tract disease (FLUTD) or upper UTI, many cats have asymptomatic or subclinical bacteriuria. In human medicine, asymptomatic bacteriuria is defined as the isolation of a specified number of bacteria in a urine specimen from a patient without symptoms referable to UTI. 2 In veterinary medicine, use of the term subclinical UTI has been suggested for these cases, because clinical signs might be too subtle to be detected. 3 In humans and most of the studies on dogs and cats, the terms asymptomatic bacteriuria (in humans) and subclinical bacteriuria (in dogs and cats) are also used if there is evidence of inflammation (pyuria, haematuria) in the urine sediment.
Classification
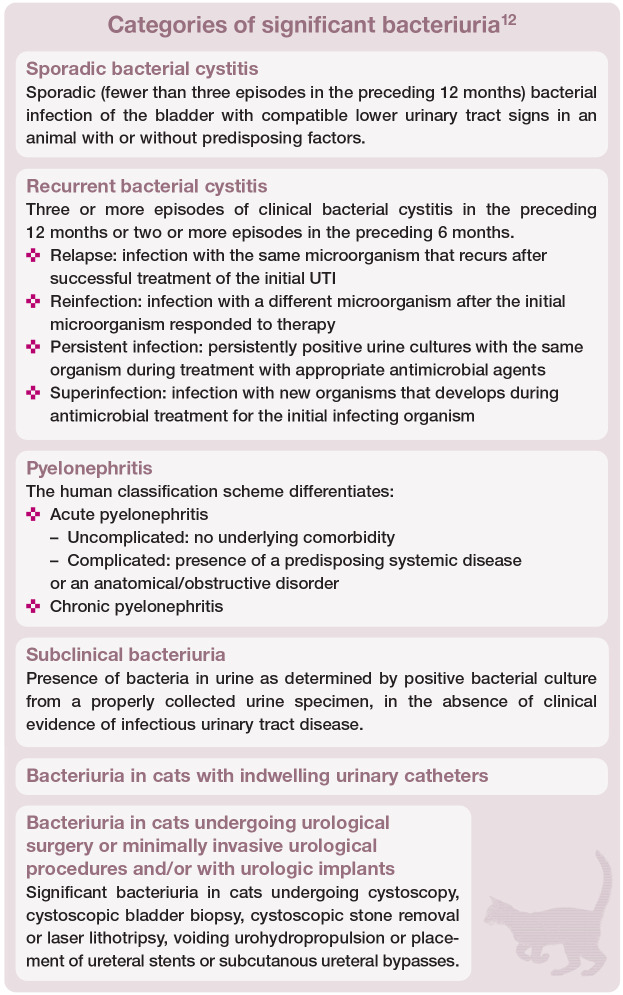
UTIs have traditionally been classified as uncomplicated or complicated. 8 An uncomplicated UTI was defined as a sporadic bacterial infection of the bladder in an otherwise healthy individual with normal urinary tract anatomy and function. UTIs in animals with any anatomic and/or functional abnormalities of the urinary tract, animals with comorbidities that predispose to persistent infection, recurrent infection or treatment failure, or animals experiencing more than three episodes within a 12-month period were categorised as complicated.
The recently updated guidelines of the International Society for Companion Animal Infectious Diseases (ISCAID) for the diagnosis and management of bacterial urinary tract infections in dogs and cats have adopted the following categories: sporadic bacterial cystitis, recurrent bacterial cystitis, pyelonephritis (see box below), bacterial prostatitis, subclinical bacteriuria and cats with indwelling urinary catheters. A new separate category is UTIs in dogs and cats receiving urological surgery, minimally invasive urological procedures and urologic implants. 12 The box on the right defines these categories (with the exception of bacterial prostatitis, which is of little relevance in cats).
Emphysematous, encrusting and polypoid cystitis are rare forms of complicated lower UTIs. Emphysematous cystitis is characterised by gas accumulation within the bladder wall and lumen secondary to infection with glucose-fermenting bacteria, mainly E coli. 13 Animals with diabetes mellitus are predisposed because of their high urinary glucose concentration.14,15 The hallmark of encrusting cystitis is adherent bladder mucosal plaques. Urea-splitting bacteria, such as Corynebacterium urealyticum, may lead to mineral precipitation, resulting in bladder wall encrustation.16–18 Treatment of encrusting cystitis can be challenging and long-term antimicrobial therapy, urine acidification and surgical debridement of plaques is often required. 16 Polypoid cystitis refers to masslike proliferations or diffuse thickening of the bladder mucosa induced by chronic inflammation, and is most commonly associated with Proteus species infections. 19 Although adequate antimicrobial treatment can lead to complete resolution, surgical intervention may be required.19,20
Prevalence
Urinary tract infections
Bacterial UTIs occur much less frequently in cats than in dogs, with only 1–2% of cats suffering from UTIs in their lifetime. 21 In cats with clinical signs of FLUTD, such as pollakiuria, macroscopic haematuria, stranguria, periuria or urethral obstruction, the proportion of cases with a UTI ranges from 2–19%.22–26 A low prevalence of UTI, <3%, has been identified in referral populations of cats of younger ages in the USA. 24 European studies incorporating higher proportions of primary cases and cats of all ages revealed higher rates of UTIs. While UTI is less common in younger cats, it is an important cause of FLUTD in cats older than 10 years, affecting 40–45% of the latter population.22,27
Subclinical bacteriuria
The reported prevalence of subclinical bacteriuria depends on the study population; for example, it was low (0.9%) in a cohort of 108 healthy cats from Norway with a mean age of 4 years. 28 In other studies, 6.2% 29 and 29% 30 of cats were affected. The median age of culture-positive cats in these three studies was 14 years. A prospective observational study that included 67 non-azotaemic cats that were at least 7 years old and tested on five occasions revealed a prevalence of 10–13%. 31 The most recent study, which included 179 cats over 6 years of age, revealed a prevalence of 6.1% for subclinical bacteriuria, with no significant difference between healthy cats and cats with different diseases. 32 In all of these studies, female cats were significantly more likely to be affected.28–30
Risk factors
A large case-controlled epidemiological study involving 22,908 cats with clinical signs of FLUTD revealed that UTIs were significantly more common in spayed female cats, Abyssinian cats and cats older than 10 years. 33 Other studies investigating cats with clinical signs of FLUTD or with subclinical bacteriuria have also identified female sex and age as risk factors.29,31,34 An increased risk for Abyssinian cats or Persian cats has only been identified in one study each.33,35 Possibly the Persian and Abyssinian cats’ longer hair and decreased grooming in cases of systemic illness could result in contamination of the fur in the anogenital area, allowing faecal bacteria to colonise the lower urinary tract.
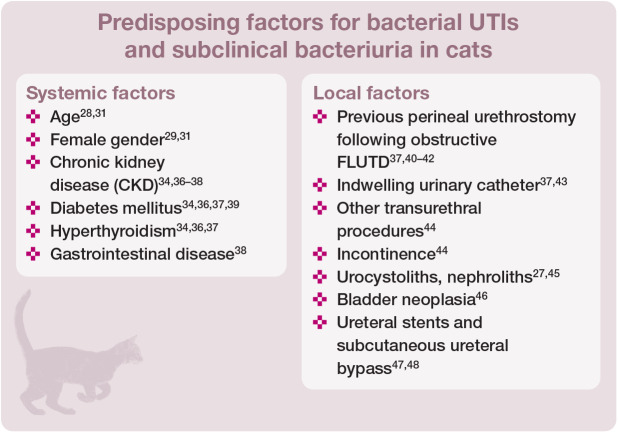
A predisposing comorbidity can be identified in 75–87% of cats with a UTI or subclinical bacteriuria (see box below).30,34,35,44
The most common systemic comorbidities in affected cats are CKD and endocrine diseases (diabetes mellitus and hyper-thyroidism). In two studies, approximately one-third of all culture-positive urine specimens were derived from cats with CKD.37,44 The reported prevalence of positive urine cultures in cats with CKD, diabetes mellitus and hyperthyroidism is 22–29%, 12–13% and 12%, respectively.36,38,39 Only 8–28% of cats with CKD displayed clinical signs of FLUTD; 20% of cats had clinical signs and haematological findings compatible with pyelonephritis (abdominal pain, pyrexia, renomegaly, neutrophilia with left shift).36,38 In cats with diabetes mellitus and hyperthyroidism, clinical signs of FLUTD were seen in 14–44% and 33% of cases with a positive urine culture, respectively.34,36,39
Impaired local defence mechanisms also predispose to the presence of positive urine cultures. One retrospective study investigating clinical features and risk factors predictive of positive urine cultures (defined as any growth of bacteria in cystocentesis samples or ⩾103 CFU/ml in catheter-derived urine samples) in cats, included 155 culture-positive cats and 186 control cats. Clinical signs of FLUTD were documented in 65% of cats with a positive urine culture, and 35% had sub-clinical bacteriuria. Factors associated with an increased risk of positive urine cultures in this study were urinary incontinence, transurethral procedures and urogenital surgery, gastrointestinal disease, decreased urine specific gravity (USG) and decreased body weight. 44 However, a low USG does not appear to be a consistent risk factor for positive urine cultures. 33
Hugonnard and colleagues investigated the incidence of catheter-associated UTIs in 18 cats with obstructive FLUTD. 43 In these cats, a transurethral catheter was placed using a standardised protocol and aseptic technique. The catheter was connected to a closed urine collection system. After 24 h and 48 h, urine culture was positive in 3/18 (17%) and 6/18 (33%) cats, respectively. 43 Another study reported that 8/37 (22%) cats had positive urine cultures after 48 h with an indwelling urinary catheter. 49
Perineal urethrostomy (PU) is a well-recognised risk factor for UTI, with 22-53% of affected cats having undergone the procedure.40–42 Bass et al reported that 23% of cats with a PU suffered from a bacterial UTI, and 15% of cats had up to 10 recurrent episodes of UTI. 40 In another study, 42 nine cats with recurrent or persistent urethral obstruction and 10 healthy castrated male cats underwent PU. Two of the nine cats with recurrent or persistent urethral obstruction developed recurrent UTIs after surgery, but none of the healthy castrated males did. Therefore, PU does not appear to predispose to UTI, but cats with an underlying uropathy undergoing surgery have an increased risk of infection. 42
In addition to obstructing the urinary outflow and injuring the renal parenchyma, nephroliths can predispose cats to pyelonephritis. 45 The reported prevalence of positive urine cultures in cats with ureteral obstructions before surgery is 2–33%.50–52 Bacterial UTIs are also among the most common complications after subcutaneous ureteral bypass and ureteral stent placement, with infection rates reaching 31%.47,48 Most culture-positive cats with a subcutaneous ureteral bypass have lower urinary tract signs and/or clinical signs of pyelonephritis. 52
To date, little evidence exists to suggest that feline immunodeficiency virus or feline leukaemia virus infection and immuno-suppressive drug treatment are predisposing conditions for significant bacteriuria. In one study, long-term treatment of 32 cats with glucocorticoids and ciclosporin was not associated with subclinical bacteriuria or UTI. 53
Pathogenesis
Development of UTI and subclinical bacteriuria is multifactorial and depends on the interplay between bacterial virulence and alterations in host defences.
Natural host defences
Owing to various host defence mechanisms, the healthy feline urinary tract is a remarkably hostile environment that is not conducive to bacterial migration and colonisation.54,55 A UTI develops when host defence mechanisms are transiently or permanently breached, allowing virulent microbes to adhere, multiply and persist within the urinary tract. 56 Major host defences against bacterial colonisation include frequent and complete voiding of an adequate urine volume, presence of a normal resident microflora, a physiological urinary tract anatomy, antimicrobial characteristics of the urine and systemic immuno-competence.54,56–58
Bacterial virulence factors
Bacterial virulence and fitness factors enable pathogens to colonise and to invade the urinary tract. Virulence factors determine not only the severity of a UTI, but also the infection site. Virulence factors are clustered on pathogenicity-associated islands, which can be easily spread among bacterial populations by horizontal gene transfer. 58
The virulence of uropathogenic E coli (UPEC) is enhanced by adhesins (eg, type 1 and P fimbriae), iron acquisition systems and toxins (eg, haemolysin). 59 P fimbriae are bacterial adhesion molecules found in most (80%) pathogens causing pyelonephritis, in 22% of cystitis strains, and in only 15% of asymptomatic strains in people.60,61 Bacterial invasion into epithelial cells often triggers the cell to undergo apoptosis and exfoliation. Some E coli strains in humans and mice are able to invade deeper tissues, persisting intracellularly, and may also form intracellular biofilm.61–63 Thus, those pathogens cannot be isolated from urine and can escape antimicrobial treatment.
Pathogens
Most commonly, significant bacteriuria is caused by pathogens from the host’s own enteric or distal urogenital flora. The bacteria ascend from the distal urethra into the normally sterile proximal urethra, urinary bladder and upper urinary tract. E coli clones causing UTIs can frequently be isolated from the same animal’s faeces as well. 62 Haematogenous spread is rare but can cause UTIs, in particular renal abscesses.
Most UTIs (>85%) are caused by a single bacterial pathogen, while two different species have previously been isolated in 13% of cats.6,23,30,38 Infections with multiple bacterial species are more common in cats with indwelling urinary catheters (27%) or other local comorbidities (20%). 37
In several studies, E coli was the most commonly isolated pathogen in feline urine, and was involved in 39–59% of positive cultures (Table 1).
Table 1.
Commonly isolated pathogens from feline urine
| Bailiff et al 63 | Martinez-Ruzafa et al 44 | Dorsch et al 35 | Lund et al 7 | Marques et al 6 | Teichmann-Knorrn et al 34 | ||
|---|---|---|---|---|---|---|---|
| Study period | 1995–2002 | 1989–2003 | 2000–2009 | 2003–2009 | 2008–2013 | 2009–2014 | |
| Number of cats | NA | 155 | 280 | 71 | NA | 150 | |
| Number of urine samples | NA | NA | 330 | 72 | 5963 | 169 | |
| Number of bacterial isolates | 101 | 198 | 375 | 82 | 6282 | 192 | |
| Gram-negative bacteria | Escherichia coli (%) | 59 | 50.3 | 42.3 | 38.8 | 59.3 | 50.5 |
| Proteus species (%) | 3.9 | 1.3 | 3.2 | 2.8 | 2.0 | 2.6 | |
| Klebsiella species (%) | 2.9 | 3.9 | 0.3 | 1.4 | 1.5 | NA | |
| Pasteurella species (%) | 2.0 | 1.9 | 1.3 | 5.5 | NA | 1.0 | |
| Pseudomonas species (%) | 1.0 | 5.2 | 1.6 | 1.4 | 1.5 | NA | |
| Enterobacter species (%) | 1.0 | 2.6 | NA | 2.8 | 1.8 | NA | |
| Gram-positive bacteria | Enterococcus species (%) | 13.9 | 21.3 | 6.6 | 9.7 | 12.1 | 15.1 |
| Staphylococcus species (%) | 7.8 | 17.4 | 16 | 15.3 | 16.8 | 22.9 | |
| Streptococcus species (%) | 5.9 | 12.9 | 19.2 | 4.2 | 2.0 | 3.6 | |
| Lactobacillus species (%) | NA | 3.2 | NA | NA | NA | NA | |
| Corynebacterium species (%) | NA | 1.9 | 2.1 | 1.4 | NA | NA | |
NA = not assessed
Other frequently documented microorganisms are Streptococcus species (2–19%), Enterococcus faecalis (5–27%) and Staphylococcus felis (17–20%).6–8,34,35,44,63 E faecalis was more likely to be present in cats with sub-clinical bacteriuria (23%) than in cats with UTIs (11%).29,34 Enterococcus species were previously considered commensal organisms of the gut flora with little clinical significance. Their emergence as leading causes of noso-comial infections worldwide has coincided with increased antimicrobial resistance.64,65 E faecalis has intrinsic resistance to beta-lactams, cephalosporins, trimethoprim/ sulfonamide, aminoglycosides, lincosamides and fluoroquinolones. 66 Acquired antibiotic resistance can develop through sporadic mutations within intrinsic genes or through horizontal gene transfer. Great caution is required when interpreting in vitro susceptibility testing results, because cephalosporins, clindamycin and trimethoprim/sulfonamide can appear active against E faecalis despite the intrinsic resistance of the pathogen and inactivity of these antibiotics in vivo. 67 Laboratories should not provide the results of drugs to which Enterococcus species are inherently resistant. Additionally, Enterococcus species form biofilms and can thus evade antimicrobials. 65
Clinical signs
Clinical signs should not be relied on alone for diagnosis. Rather, the presence of clinical abnormalities indicates that further work-up is needed.
Lower UTI
Clinical signs of lower UTI include pollakiuria, gross haematuria, periuria, dysuria and stranguria. These are non-specific and can be seen in any disease of the lower urinary tract, of which idiopathic cystitis is the most common in cats.
Upper UTI
Pyelonephritis can have an acute or chronic clinical presentation and is commonly suspected in hospitalised patients. The diagnosis remains challenging and information in the literature is limited to a small number of case reports.68,69
Acute pyelonephritis may be associated with distinct clinical signs such as fever and painful kidneys, as well as anorexia, lethargy, polyuria and polydipsia, vomiting and diarrhoea. 70 According to one study of 17 histopathologically confirmed cases of pyelonephritis, the most common clinical signs were non-specific, such as anorexia, lethargy and vomiting; 71 renal pain and pyrexia were only observed in 3/17 and 2/17 cats, respectively. Azotaemia (11/11 cats with available serum chemistry), hyperphos-phataemia (8/11 cats) and non-regenerative anaemia (7/10 cats with available complete blood count) were the most common clinico-pathological abnormalities. 71 In addition, an inflammatory leukogram can be present. 72
In dogs, chronic pyelonephritis is considered to produce only mild or absent clinical signs.9,70 In cats, there is a lack of knowledge regarding this disease entity.
Diagnosis
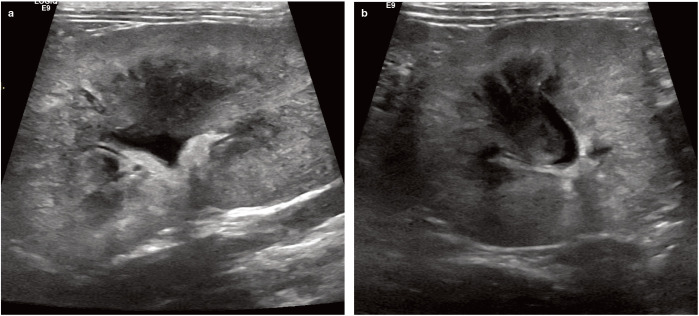
UTIs are diagnosed based on clinical signs, urinalysis findings and quantitative bacterial cultures. Distinguishing between cats with bacterial cystitis, and those with idiopathic cystitis and concurrent clinical or subclinical bacteriuria, is challenging, however, as clinical signs and urinalysis results may be identical.35,73 Particularly in cats, where other causes for clinical signs of lower urinary tract disease are common, positive urine cultures are indispensable for a reliable diagnosis. To select an effective antimicrobial, in vitro susceptibility testing should be performed on all isolates. Diagnostic imaging can help assess for complicating conditions (eg, upper urinary tract involvement) (Figure 1).
Figure 1.
Longitudinal (a) and transverse (b) ultrasound images of the left kidney of a cat with bilateral renomegaly and acute (grade V) kidney injury. The kidney is enlarged, there is poor corticomedullary differentiation, the cortex is patchy hyperechoic and the medulla has lost its typical hypoechogenic to anechoic appearance. The renal pelvis is dilated to 4 mm. Post-mortem examination of this cat revealed severe pyelonephritis
Urinalysis
When possible, urine specimens should be obtained by cystocentesis and before initiating antimicrobial treatment (Figure 2). Urine can be sampled by catheterisation, but the risk of contamination is higher and catheter placement requires sedation or anaesthesia. Free-catch samples (mid-stream voiding) are frequently contaminated and should not be used for bacterial cultures. 8 Note that manual expression is no longer recommended.
Figure 2.
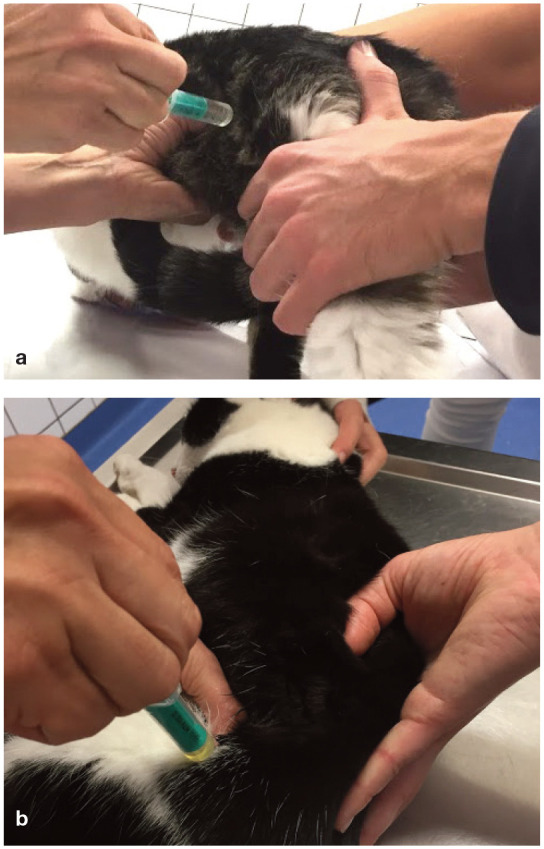
Cystocentesis performed with palpation of the urinary bladder in the standing cat (a) and the cat in lateral recumbency (b) through the lateral abdominal wall
Storage of urine specimens can influence the test results owing to altered pH, lysis of casts, leukocytes and epithelial cells, precipitation of substances and in vitro crystal formation. Furthermore, inadequate storage can lead to bacterial contamination and proliferation as well as bacterial death. 74
USG is variable in cats with UTIs. Gram-negative-infected specimens are reported to have a lower USG than Gram-positive-infected specimens and culture-negative specimens. 30 USG is higher in specimens infected with S felis and lower in specimens infected with E coli than in those infected with other uropathogens. 75 Dipstick analysis often reveals haematuria and proteinuria. Importantly, the leukocyte field is often falsely positive in cats, and the nitrite field is unreliable. 76
Urine sediment
Examining the urine sediment is an important diagnostic. There is, however, a large overlap between sediment findings in cats with UTIs and cats with other diseases of the lower urinary tract (Table 2). Haematuria (>5 red blood cells/high-power field [HPF]) and pyuria (>3–5 white blood cells/HPF) are seen in 28–77% and 35–100% of cats with subclinical bacteriuria or UTIs, respectively.29,31,73
Table 2.
Urinalysis findings in cats with positive urine cultures
| Reference | n | Population of cats | Culture positive | Urinary tract infection | Subclinical bacteriuria | Haematuria | Pyuria | Bacteriuria |
|---|---|---|---|---|---|---|---|---|
| Gerber et al 23 | 77 | Signs of FLUTD | 5 | 5 | 0 | 5/5 | 3/5 | 3/5 |
| Bailiff et al 39 | 141 | Diabetes mellitus | 18 | 8/18 | 10/18 | 4/16 | 12/16 | 14/16 |
| Swenson et al 77 | 472 | No selection | 29 | NA | NA | NA | 10/29 | 24/29 |
| Lund et al 73 | 111 | Signs of FLUTD | 14 | 14 | 0 | 13/14 | 14/14 | NA |
| Dorsch et al 22 | 302 | Signs of FLUTD | 57 | 57 | 0 | 51/57 | 44/57 | 44/57 |
| Puchot et al 29 | 500 | No signs of FLUTD | 31 | 0 | 31 | 10/30 | 20/30 | 18/30 |
FLUTD = feline lower urinary tract disease; NA = not assessed
Haematuria is also present in more than 70% of cats with feline idiopathic cystitis, and in most cats with urolithiasis and bladder neoplasia.23,35,78 Pyuria is likewise a nonspecific finding and has been reported in up to 77% of cats with feline idiopathic cystitis and more than 50% of cats with urocystoliths. 73
In feline urine samples, bacteriuria identified on unstained or stained wet urine sediments is poorly correlated with positive culture results, whereas examination of air-dried Wright-stained urine sediments is much more reliable.73,77,79 In a study by Swenson et al, 29/472 urine specimens were culture-positive and 443 were culture-negative. 77 Considering the culture-positive samples as true positives and the culture-negative samples as true negatives, the wet unstained sediment had a sensitivity of 75.9% and a specificity of only 56.7%. Air-dried Wright-staining of the urine sediment improved sensitivity and specificity to 82.8% and 98.7%, respectively.
Quantitative bacterial cultures
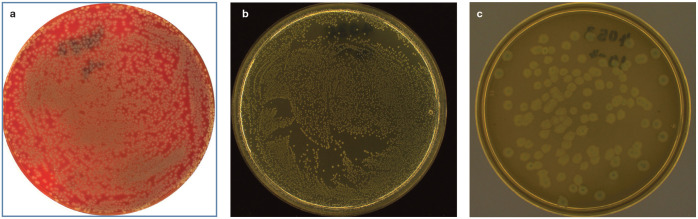
Storage at room temperature leads to rapid increases in bacterial numbers. 74 Therefore, urine samples for culture/susceptibility testing should be refrigerated as soon as possible and processed in a microbiology laboratory within 24 h. 80 Growth of ⩾103 CFU/ml in urine specimens obtained by cystocentesis or catheterisation is considered significant in cats (Figure 3; Table 3).81–83
Figure 3.
Positive aerobic urine cultures: (a) Escherichia coli on nutrient agar with 5% sheep blood; (b) Enterococcus faecalis on Mueller Hinton agar; and (c) Proteus mirabilis on Gassner agar. Images courtesy of Dr Georg Wolf, Institute for Infectious Diseases and Zoonoses, LMU Munich
Table 3.
| Sampling method | Likely contamination (CFU/ml) | Significant growth (CFU/ml) |
|---|---|---|
| Catheterisation | <103 | ⩾103 |
| Cystocentesis | <103 | ⩾103 |
CFU = colony-forming units
Most uropathogens can be cultivated over an 18–24 h incubation period. However, some slow-growing pathogens, such as Corynebacterium species, may require a longer time to appear in cultures. 16 This should be considered if bacteriuria was diagnosed on sediment analysis but bacteria cannot be cultivated. In these cases, incubation periods should be extended to at least 5 days.
Pyelonephritis
Culture of urine obtained by pyelocentesis is ideal for identifying the bacteria involved in pyelonephritis.70,72,81 Otherwise, cystocentesis (repeated cultures may be needed) or blood cultures may be used depending on the assumed infection route. Blood cultures should be considered in immunosuppressed cats, and in the presence of fever and azo-taemia in cats with negative urine cultures and no abnormalities in the urine sediment.84,85
Treatment
To prevent treatment failure and the development of antibiotic resistance, antimicrobials should ideally be selected based on in vitro susceptibility testing (see box), and antimicrobials with a narrow spectrum should be used. 8 If true bacterial UTIs are identified, the treatment plan depends on previous UTI history, affected structures, concurrent diseases and, to a lesser degree than in dogs, neutering status.
Empirical treatment is rarely indicated. As bacterial prevalence and antimicrobial resistance have strong regional differences, empirical treatment should be based on location-specific bacterial prevalence rates and antimicrobial resistance patterns.6,34
In the recently updated ISCAID guidelines, recommendations for the management of bacterial UTIs in dogs and cats were broadened to cover a more defined range of entities, including first-episode UTIs, recurrent UTIs, UTIs in compromised patients, pyelonephritis, subclinical bacteriuria, catheter-associated UTIs, medical management of infection-induced uroliths, as well as the manangement of dogs and cats with urinary catheters and those that receive urological surgery, minimally invasive urological procedures and urologic implants. 12
Antimicrobial treatment of sporadic/uncomplicated cystitis
Owing to the high frequency of comorbidities and an increased incidence of positive urine cultures among older cats, the actual occurrence of uncomplicated or sporadic cystitis in cats has been questioned. The 2019 ISCAID guidelines emphasise that there is no evidence indicating that sporadic cystitis should be more complicated to manage in cats vs dogs, as the presence of comorbidities does not necessarily imply a more complicated infection. 12
Given the low incidence of UTIs in cats with signs of FLUTD (especially among younger cats), empirical antimicrobial treatment is rarely indicated. To relieve patient discomfort, analgesics can be administered while awaiting culture results. If treatment must be initiated while culture and antimicrobial susceptibility test results are pending, a first-line antimicrobial, such as amoxicillin or trimethoprim/ sulfonamide, preferably with a known low local resistance rate, should be administered. 12
There is a lack of evidence to support the common recommendation for a treatment duration of 7–14 days and growing evidence indicates that shorter treatment periods (3–5 days) may be sufficient in veterinary medicine.89,90 The 2019 ISCAID guidelines recommend a 3–5 day treatment period in cases of sporadic cystitis. 12
If clinical signs disappear within the treatment period, and the antimicrobial was appropriate based on susceptibility testing, additional monitoring and diagnostic testing are generally not required. If clinical improvement is lacking and the chosen antimicrobial is insufficient based on susceptibility testing, the original antimicrobial should be discontinued and treatment adjusted as per the susceptibility test results. 12 The guidelines stress the importance of further investigation in cases of treatment failure; empirical changes of antimicrobials should not be performed. 12
Antimicrobial treatment of recurrent/complicated cystitis
In cases of complicated UTIs, identifying and addressing the primary reason for bacterial colonisation is critical to avoid treatment failure or recurrent infection. While the previous (2011) version of the ISCAID guidelines recommended long-duration treatment for all cases of recurrent cystitis, 8 the 2019 guidelines acknowledge the broad range of conditions encompassed in this category and state that long-term therapy is no longer automatically warranted. 12 Depending on the severity of clinical signs, analgesics could be considered for these patients as well, while awaiting culture results. 12
If empirical treatment of recurrent cystitis is necessary, the same drugs are recommended as for sporadic/uncomplicated UTIs. A treatment period of 3–5 days should be considered in cases of reinfection, while 7–14 days may be reasonable when treating persistent or potentially relapsing infections. 12 When longer treatment periods are applied, the benefit of urine cultures during treatment is unclear as the clinical outcome is of greater importance than the culture results per se. 12 Urine cultures 5–7 days post-treatment in animals where clinical cure is documented may, if positive, help to differentiate between relapse, reinfection and persistent infection, but do not necessarily indicate the need to continue treatment (see ‘Antimicrobial treatment of subclinical bacteriuria’ on page 10). 12
Antimicrobial treatment of pyelonephritis
If pyelonephritis is suspected (Figures 1 and 4), and empirical treatment is needed, antimicrobials with a good efficacy against Gram-negative bacteria (eg, fluoroquinolones) are recommended. The treatment must be adjusted according to susceptibility test results. Evidence-based recommendations on treatment duration are lacking, and while treatment durations of 4–6 weeks previously were considered reasonable, shorter periods of treatment have been reported to be effective in humans, 91 and the 2019 ISCAID guidelines recommend 10–14 days in veterinary patients. 12 Repeated cultures during the treatment period are no longer automatically warranted, but should be considered in cases of incomplete clinical response, while cultures 1–2 weeks post-treatment are recommended for all cases. 12 As for recurrent/complicated cystitis, consideration must be given to the clinical relevance of possible positive cultures post-treatment. Differentiation between sub-clinical bacteriuria and persistent infections may be challenging and reasons for potential persistence must be thoroughly investigated. 12
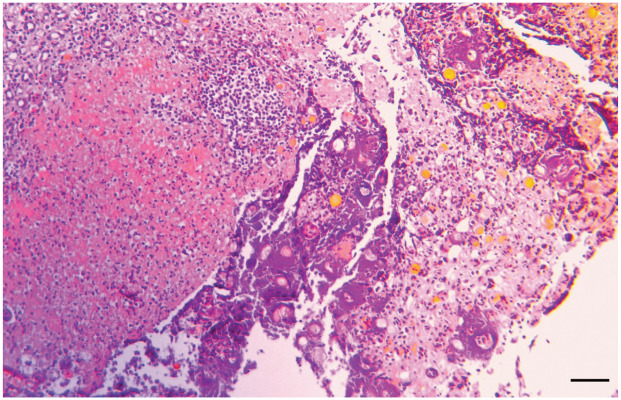
Figure 4.
Severe necrosuppurative pyelonephritis. Haematoxylin and eosin stain of a histological section of the renal papilla of the same cat as in Figure 1, showing severe inflammation with multiple neutrophils and bacterial colonies; bar = 50 μm. Courtesy of Dr Monir Majzoub-Altweck, Institute of Veterinary Pathology, LMU Munich
Antimicrobial treatment of catheter-associated UTIs
Prophylactic antimicrobial treatment is never warranted and no evidence supports routine urine cultures after catheter removal. The 2019 ISCAID guidelines recommend bacterial cultures only in cats with ongoing clinical signs of FLUTD after the catheter has been removed. 12
Cats with indwelling urinary catheters and clinical signs of UTI may be difficult to identify as many will have been catheterised for signs of FLUTD initially. Routine cytological evaluation of urine is not recommended as haematuria, pyuria and bacteriuria can occur in the absence of cystitis. Urine cultures should be performed in all cases with suspected bacterial cystitis, in the presence of fever and bacteraemia, and if a sudden change in urine character is seen. 12 Because treatment is more likely to be effective if the catheter is removed prior to treatment, the catheter should be removed if possible and urine for culture should be collected by cystocentesis. If the catheter must remain in place, it should be replaced and urine for culture should be obtained through the new catheter. 12
Antimicrobial treatment of subclinical bacteriuria
Currently, the clinical relevance of subclinical bacteriuria is uncertain, and its optimal treatment requires clarification. 12 In healthy women, and in women and men with comorbidities, asymptomatic bacteriuria is common. 92 Several randomised clinical trials and meta-analyses with high numbers of patients have shown that antimicrobial treatment does not benefit asymptomatic individuals but is instead associated with negative effects, such as adverse drug reactions and increased antimicrobial resistance.93–95 Therefore, guidelines for antimicrobial use in human medicine contain strong recommendations against screening for and treating asymptomatic bacteriuria. In one study of older cats, subclinical bacteriuria was not adversely associated with survival despite withholding antimicrobial treatment. 31
The current recommendation for cats and dogs is to consider treatment of subclinical bacteriuria only in animals with suspected pyelonephritis, patients undergoing surgical procedures of the urinary tract, patients undergoing endoscopic procedures of the urinary tract with associated bleeding, in patients where the bladder is thought to be the focus of extraurinary tract infection, and in diabetic animals if subclinical bacteriuria is thought to be the reason for insulin antagonism or ketosis. 12 Treatment should also be considered in cats with C urealyticum bacteri-uria 96 because this has been associated with encrusting cystitis in cats (see earlier) 17 as well as an increased risk of obstructive uropathy in human transplant patients. 97 The presence of multidrug-resistant bacteria should not affect the decision of whether or not to treat subclinical bacteriuria. 12
Preventive therapy
Currently, no preventive treatment modality is recommended by ISCAID. 12 Evidence for most of the alternative approaches used to prevent recurrent UTIs in cats, as discussed below, is mainly anecdotal.
Preventive therapy in the form of pulse or chronic low-dose antimicrobial administration is, at best, controversial.12,98,99
Proanthocyanidin from cranberry inhibits UPEC adherence to human uroepithelium and can reduce the prevalence of recurrent UTIs.100–102 Cranberry extracts have demonstrated in vitro efficacy in preventing bacterial attachment to the uroepithelium of dogs and cats in a dose-dependent way, but evidence of a clinical effect is inconsistent.103–106 D-mannose may disrupt bacterial adhesion to the urothelium, but evidence of a clinical effect in cats and dogs is lacking as well. 107
Based on positive results for preventing recurrent UTIs by orally and vaginally administering probiotics in women,108,109 probiotics have also been recommended for cats and dogs. One prospective controlled clinical study in dogs, however, demonstrated no benefit from orally administered Lactobacillus, Bifidobacterium and Bacillus species. 110 In cats, no clinical studies have, to date, provided data on the efficacy of this therapy.
There is no evidence for a clinical effect of direct instillation of various components such as antimicrobials, antiseptics or dimethyl sulfoxide into the feline urinary bladder.111–114 Urinary antiseptics in the form of methenamine salts require acidic urine to convert methenamine to bacteriostatic concentrations of formalde-hyde.113,114 No evidence-based recommendations for this treatment exist in veterinary medicine.
Intravesical application of non-pathogenic E coli strains reduced symptoms of UTI in humans with urine retention by approximately 50%.115,116 In similar studies in healthy dogs, consistent establishment of bladder colonisation was less successful.117,118 However, a recent pilot study applying non-pathogenic E coli intravesically in dogs with recurrent UTI showed promising results. 119 To the authors’ knowledge, use of non-pathogenic bacteria has not been examined in cats.
Possible future treatment strategies include the introduction of bacteriophages able to lyse UPEC. This has been studied in both feline and canine UPEC isolates. 120
Key Points
UTI and subclinical bacteriuria are most common in older, female cats.
Most culture-positive cats have underlying predisposing diseases, principally CKD and endocrine diseases.
Urine sediment analysis alone is inadequate for diagnosing UTI because urine sediment findings largely overlap with findings in cats with other diseases of the lower urinary tract.
Antimicrobial treatment while culture and susceptibility test results are pending is only indicated in selected cases, such as cats with suspected acute pyelonephritis.
Adherence to treatment guidelines and confinement to a few first-line antimicrobial agents is imperative to avoid the development of antimicrobial-resistant bacteria.
Antimicrobials with a narrow spectrum should be used as first-line drugs.
Screening selected populations for bacteriuria, such as cats with diabetes mellitus or hyperthyroidism, without clinical signs of UTI is questionable, as no evidence demonstrating a beneficial effect of routine treatment in culture-positive cats exists.
Acknowledgments
We thank Julia Harrer for her editorial help with this article. The case notes were provided courtesy of Dr Nina Ottesen, Section for Anaesthesia and Radiology, Department of Companion Animal Clinical Sciences, Norwegian University of Life Sciences.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Contributor Information
Roswitha Dorsch, Clinic of Small Animal Medicine, LMU Munich, Veterinärstrasse 13, 80539 Munich, Germany.
Svenja Teichmann-Knorrn, Clinic of Small Animal Medicine, LMU Munich, Veterinärstrasse 13, 80539 Munich, Germany.
Heidi Sjetne Lund, Small Animal Section, Department of Companion Animal Clinical Sciences, Faculty of Veterinary Medicine, Norwegian University of Life Sciences, PO Box 369 Sentrum, 0102 Oslo, Norway.
References
- 1. Wood MW. Lower urinary tract infections. In: Ettinger SJ, Feldman EC, Côté E. (eds). Textbook of veterinary internal medicine. 8th ed. St Louis, MO: Elsevier, 2017, pp 1992–1996. [Google Scholar]
- 2. Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005; 40: 643–654. [DOI] [PubMed] [Google Scholar]
- 3. Sykes JE, Westropp JL. Bacterial infections of the genito – urinary tract. In: Sykes JE. (ed). Canine and feline infectious diseases. St Louis, MO: Elsevier Saunders, 2014, pp 871–885. [Google Scholar]
- 4. Hardefeldt LY, Selinger J, Stevenson MA, et al. Population wide assessment of antimicrobial use in dogs and cats using a novel data source – a cohort study using pet insurance data. Vet Microbiol 2018; 225: 34–39. [DOI] [PubMed] [Google Scholar]
- 5. Schmitt K, Lehner C, Schuller S, et al. Antimicrobial use for selected diseases in cats in Switzerland. BMC Vet Res 2019; 15: 94. DOI: 10.1186/s12917-019-1821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marques C, Gama LT, Belas A, et al. European multicenter study on antimicrobial resistance in bacteria isolated from companion animal urinary tract infections. BMC Vet Res 2016; 12: 213. DOI: 10.1186/s12917-016-0840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lund HS, Skogtun G, Sorum H, et al. Anti microbial susceptibility in bacterial isolates from Norwegian cats with lower urinary tract disease. J Feline Med Surg 2015; 17: 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weese JS, Blondeau JM, Boothe D, et al. Antimicrobial use guidelines for treatment of urinary tract disease in dogs and cats: antimicrobial guidelines working group of the International Society for Companion Animal Infectious Diseases. Vet Med Int 2011; 2011. DOI: 10.4061/2011/263768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bouillon J, Snead E, Caswell J, et al. Pyelonephritis in dogs: retrospective study of 47 histologically diagnosed cases (2005-2015). J Vet Intern Med 2018; 32: 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smee N, Loyd K, Grauer G. UTIs in small animal patients: part 1: etiology and pathogenesis. J Am Anim Hosp Assoc 2013; 49: 1–7. [DOI] [PubMed] [Google Scholar]
- 11. Faucher MR, Theron ML, Reynolds BS. Renal abscesses in cats: six cases. J Feline Med Surg 2017; 19: 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weese JS, Blondeau J, Boothe D, et al. International Society for Companion Animal Infectious Diseases (ISCAID) guidelines for the diagnosis and management of bacterial urinary tract infections in dogs and cats. Vet J 2019; 247: 8–25. [DOI] [PubMed] [Google Scholar]
- 13. Quint HJ, Drach GW, Rappaport WD, et al. Emphysematous cystitis: a review of the spectrum of disease. J Urol 1992; 147: 134–137. [DOI] [PubMed] [Google Scholar]
- 14. Root CR, Scott RC. Emphysematous cystitis and other radio-graphic manifestations of diabetes mellitus in dogs and cats. J Am Vet Med Assoc 1971; 158: 721–728. [PubMed] [Google Scholar]
- 15. Davies NL, Williams JH. Emphysematous cystitis in a non-diabetic cat. J S Afr Vet Assoc 1993; 64: 162–164. [PubMed] [Google Scholar]
- 16. Bailiff NL, Westropp JL, Jang SS, et al. Corynebacterium urealyticum urinary tract infection in dogs and cats: 7 cases (1996-2003). J Am Vet Med Assoc 2005; 226: 1676–1680. [DOI] [PubMed] [Google Scholar]
- 17. Briscoe KA, Barrs VR, Lindsay S, et al. Encrusting cystitis in a cat secondary to Corynebacterium urealyticum infection. J Feline Med Surg 2010; 12: 972–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Raab O, Béraud R, Tefft KM, et al. Successful treatment of Corynebacterium urealyticum encrusting cystitis with systemic and intravesical antimicrobial therapy. Can Vet J 2015; 56: 471–475. [PMC free article] [PubMed] [Google Scholar]
- 19. Martinez I, Mattoon JS, Eaton KA, et al. Polypoid cystitis in 17 dogs (1978-2001). J Vet Intern Med 2003; 17: 499–509. [DOI] [PubMed] [Google Scholar]
- 20. Wolfe TM, Hostutler RA, Chew DJ, et al. Surgical management of diffuse polypoid cystitis using submucosal resection in a dog. J Am Anim Hosp Assoc 2010; 46: 281–284. [DOI] [PubMed] [Google Scholar]
- 21. Von Vopelius-Feldt C. Bakterielle Harnwegsinfektionen bei der Katze – eine Retrospektive tiber 10 Jahre. Doctoral thesis, Ludwig Maximilian University Munich, Germany, 2012. [Google Scholar]
- 22. Dorsch R, Remer C, Sauter-Louis C, et al. Feline lower urinary tract disease in a German cat population. A retrospective analysis of demographic data, causes and clinical signs. Tierarztl Prax Ausg K Kleintiere Heimtiere 2014; 42: 231–239. [PubMed] [Google Scholar]
- 23. Gerber B, Boretti FS, Kley S, et al. Evaluation of clinical signs and causes of lower urinary tract disease in European cats. J Small Anim Pract 2005; 46: 571–577. [DOI] [PubMed] [Google Scholar]
- 24. Kruger JM, Osborne CA, Goyal SM, et al. Clinical evaluation of cats with lower urinary tract disease. J Am Vet Med Assoc 1991; 199: 211–216. [PubMed] [Google Scholar]
- 25. Saevik BK, Trangerud C, Ottesen N, et al. Causes of lower urinary tract disease in Norwegian cats. J Feline Med Surg 2011; 13: 410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buffington CA, Chew DJ, Kendall MS, et al. Clinical evaluation of cats with nonobstructive urinary tract diseases. J Am Vet Med Assoc 1997; 210: 46–50. [PubMed] [Google Scholar]
- 27. Bartges JW, Barsanti JA. Bacterial urinary tract infections in cats. In: Bonagura JD. (ed). Current veterinary therapy XIII. Philadelphia: Saunders, 2000, pp 880–883. [Google Scholar]
- 28. Eggertsdottir AV, Saevik BK, Halvorsen I, et al. Occurrence of occult bacteriuria in healthy cats. J Feline Med Surg 2011; 13: 800–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Puchot ML, Cook AK, Pohlit C. Subclinical bacteriuria in cats: prevalence, findings on contemporaneous urinalyses and clinical risk factors. J Feline Med Surg 2017; 19: 1238–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Litster A, Moss S, Platell J, et al. Occult bacterial lower urinary tract infections in cats – urinalysis and culture findings. Vet Microbiol 2009; 136: 130–134. [DOI] [PubMed] [Google Scholar]
- 31. White JD, Cave NJ, Grinberg A, et al. Subclinical bacteriuria in older cats and its association with survival. J Vet Intern Med 2016; 30: 1824–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moberg FS, Langhorn R, Bertelsen PV, et al. Subclinical bacteriuria in a mixed population of 179 middle-aged and elderly cats: a prospective cross-sectional study. J Feline Med Surg. Epub ahead of print 20 September 2019. DOI: 10.1177/1098612X19874141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lekcharoensuk C, Osborne CA, Lulich JP. Epidemiologic study of risk factors for lower urinary tract diseases in cats. J Am Vet Med Assoc 2001; 218: 1429–1435. [DOI] [PubMed] [Google Scholar]
- 34. Teichmann-Knorrn S, Reese S, Wolf G, et al. Prevalence of feline urinary tract pathogens and antimicrobial resistance over five years. Vet Rec 2018; 183: 21. [DOI] [PubMed] [Google Scholar]
- 35. Dorsch R, Von Vopelius-Feldt C, Wolf G, et al. Feline urinary tract pathogens: prevalence of bacterial species and antimicrobial resistance over a 10-year period. Vet Rec 2015; 176: 201. [DOI] [PubMed] [Google Scholar]
- 36. Mayer-Roenne B, Goldstein RE, Erb HN. Urinary tract infections in cats with hyperthyroidism, diabetes mellitus and chronic kidney disease. J Feline Med Surg 2007; 9: 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dorsch R, Von Vopelius-Feldt C, Wolf G, et al. Urinary tract infections in cats. Prevalence of comorbidities and bacterial species, and determination of antimicrobial susceptibility to commonly used antimicrobial agents. Tierarztl Prax Ausg K Kleintiere Heimtiere 2016; 44: 227–236. [DOI] [PubMed] [Google Scholar]
- 38. White JD, Stevenson M, Malik R, et al. Urinary tract infections in cats with chronic kidney disease. J Feline Med Surg 2013; 15: 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bailiff NL, Nelson RW, Feldman EC, et al. Frequency and risk factors for urinary tract infection in cats with diabetes mellitus. J Vet Intern Med 2006; 20: 850–855. [DOI] [PubMed] [Google Scholar]
- 40. Bass M, Howard J, Gerber B, et al. Retrospective study of indications for and outcome of perineal urethrostomy in cats. J Small Anim Pract 2005; 46: 227–231. [DOI] [PubMed] [Google Scholar]
- 41. Corgozinho KB, De Souza HJM, Pereira AN, et al. Catheter-induced urethral trauma in cats with urethral obstruction. J Feline Med Surg 2007; 9: 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Griffin DW, Gregory CR. Prevalence of bacterial urinary tract infection after perineal urethrostomy in cats. J Am Vet Med Assoc 1992; 200: 681–684. [PubMed] [Google Scholar]
- 43. Hugonnard M, Chalvet-Monfray K, Dernis J, et al. Occurrence of bacteriuria in 18 catheterised cats with obstructive lower urinary tract disease: a pilot study. J Feline Med Surg 2013; 15: 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Martinez-Ruzafa I, Kruger JM, Miller R, et al. Clinical features and risk factors for development of urinary tract infections in cats. J Feline Med Surg 2012; 14: 729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Adams LG. Nephroliths and ureteroliths: a new stone age. N Z Vet J 2013; 61: 212–216. [DOI] [PubMed] [Google Scholar]
- 46. Wilson HM, Chun R, Larson VS, et al. Clinical signs, treatments, and outcome in cats with transitional cell carcinoma of the urinary bladder: 20 cases (1990-2004). J Am Vet Med Assoc 2007; 231: 101–106. [DOI] [PubMed] [Google Scholar]
- 47. Wormser C, Clarke DL, Aronson LR. Outcomes of ureteral surgery and ureteral stenting in cats: 117 cases (2006-2014). J Am Vet Med Assoc 2016; 248: 518–525. [DOI] [PubMed] [Google Scholar]
- 48. Wolff ED, Dorsch R, Knebel J, et al. Initial outcomes and complications of the subcutaneous ureteral bypass procedure at two university hospitals (2012-2015) [abstract]. J Vet Intern Med 2016; 30: 1489. [Google Scholar]
- 49. Dorsch R, Zellner F, Schulz B, et al. Evaluation of meloxicam for the treatment of obstructive feline idiopathic cystitis. J Feline Med Surg 2016; 18: 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Berent AC, Weisse CW, Todd K, et al. Technical and clinical outcomes of ureteral stenting in cats with benign ureteral obstruction: 69 cases (2006-2010). J Am Vet Med Assoc 2014; 244: 559–576. [DOI] [PubMed] [Google Scholar]
- 51. Culp WT, Palm CA, Hsueh C, et al. Outcome in cats with benign ureteral obstructions treated by means of ureteral stenting versus ureterotomy. J Am Vet Med Assoc 2016; 249: 1292–1300. [DOI] [PubMed] [Google Scholar]
- 52. Kopecny L, Palm CA, Drobatz KJ, et al. Risk factors for positive urine cultures in cats with subcutaneous ureteral bypass and ureteral stents (2010-2016). J Vet Intern Med 2019; 33: 178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lockwood SL, Schick AE, Lewis TP, et al. Investigation of subclinical bacteriuria in cats with dermatological disease receiving long-term glucocorticoids and/or ciclosporin. Vet Dermatol 2018; 29: 25-e12. [DOI] [PubMed] [Google Scholar]
- 54. Litster A, Thompson M, Moss S, et al. Feline bacterial urinary tract infections: an update on an evolving clinical problem. Vet J 2011; 187: 18–22. [DOI] [PubMed] [Google Scholar]
- 55. Bartges JW. Bacterial urinary tract infections – simple and complicated. Vet Med 2005; 100: 224–232. [Google Scholar]
- 56. Schaeffer AJ. What do we know about the urinary tract infection-prone individual? J Infect Dis 2001; 183: 66–69. [DOI] [PubMed] [Google Scholar]
- 57. Osborne CA, Lees GE. Bacterial infections of the canine and feline urinary tract. In: Osborne CA, Finco DR. (eds). Canine and feline nephrology and urology. Baltimore, MD: Williams and Wilkins, 1995, pp 759–797. [Google Scholar]
- 58. Oelschlaeger TA, Dobrindt U, Hacker J. Virulence factors of uropathogens. Curr Opin Urol 2002; 12: 33–38. [DOI] [PubMed] [Google Scholar]
- 59. Guyer DM, Gunther NW, 4th, Mobley HL. Secreted proteins and other features specific to uropathogenic Escherichia coli. J Infect Dis 2001; 183: 32–35. [DOI] [PubMed] [Google Scholar]
- 60. Lane MC, Mobley HLT. Role of P-fimbrial-mediated adherence in pyelonephritis and persistence of uropathogenic Escherichia coli (UPEC) in the mammalian kidney. Kidney Int 2007; 72: 19–25. [DOI] [PubMed] [Google Scholar]
- 61. Vejborg RM, Hancock V, Schembri MA, et al. Comparative genomics of Escherichia coli strains causing urinary tract infections. Appl Environ Microbiol 2011; 77: 3268–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Johnson JR, Clabots C, Kuskowski MA. Multiple-host sharing, long-term persistence, and virulence of Escherichia coli clones from human and animal household members. J Clin Microbiol 2008; 46: 4078–4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bailiff NL, Westropp JL, Nelson RW, et al. Evaluation of urine specific gravity and urine sediment as risk factors for urinary tract infections in cats. Vet Clin Pathol 2008; 37: 317–322. [DOI] [PubMed] [Google Scholar]
- 64. Bonten MJ, Willems R, Weinstein RA. Vancomycin-resistant enterococci: why are they here, and where do they come from? Lancet Infect Dis 2001; 1: 314–325. [DOI] [PubMed] [Google Scholar]
- 65. Reyes K, Bardossy AC, Zervos M. Vancomycin-resistant enterococci: epidemiology, infection prevention, and control. Infect Dis Clin North Am 2016; 30: 953–965. [DOI] [PubMed] [Google Scholar]
- 66. Hollenbeck BL, Rice LB. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence 2012; 3: 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Papich MG. Antimicrobial susceptibility testing for feline urinary tract isolates. J Feline Med Surg 2016; 18: 183–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gould EN, Cohen TA, Trivedi SR, et al. Emphysematous pyelonephritis in a domestic shorthair cat. J Feline Med Surg 2016; 18: 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Watson AD, Culvenor JA, Middleton DJ, et al. Distal renal tubular acidosis in a cat with pyelonephritis. Vet Rec 1986; 119: 65–68. [DOI] [PubMed] [Google Scholar]
- 70. Olin SJ, Bartges JW. Urinary tract infections: treatment/ comparative therapeutics. Vet Clin North Am Small Anim Pract 2015; 45: 721–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cheney A, Palerme J-S, Van Vertloo LR, et al. A multi-institutional retrospective study of 17 cases of histopathologically confirmed feline pyelonephritis. ACVIM Forum; 2018 June 14-16; Seattle, WA, USA. [Google Scholar]
- 72. Smee N, Loyd K, Grauer GF. UTIs in small animal patients: part 2: diagnosis, treatment, and complications. J Am Anim Hosp Assoc 2013; 49: 83–94. [DOI] [PubMed] [Google Scholar]
- 73. Lund HS, Krontveit RI, Halvorsen I, et al. Evaluation of urinaly-ses from untreated adult cats with lower urinary tract disease and healthy control cats: predictive abilities and clinical relevance. J Feline Med Surg 2013; 15: 1086–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Padilla J, Osborne C, Ward G. Effects of storage time and temperature on quantitative culture of canine urine. J Am Vet Med Assoc 1981; 178: 1077–1081. [PubMed] [Google Scholar]
- 75. Litster A, Moss SM, Honnery M, et al. Prevalence of bacterial species in cats with clinical signs of lower urinary tract disease: recognition of Staphylococcus felis as a possible feline urinary tract pathogen. Vet Microbiol 2007; 121: 182–188. [DOI] [PubMed] [Google Scholar]
- 76. Klausner JS, Osborne CA, Stevens JB. Clinical evaluation of commercial reagent strips for detection of significant bacteri-uria in dogs and cats. Am J Vet Res 1976; 37: 719–722. [PubMed] [Google Scholar]
- 77. Swenson CL, Boisvert AM, Gibbons-Burgener SN, et al. Evaluation of modified Wright-staining of dried urinary sediment as a method for accurate detection of bacteriuria in cats. Vet Clin Pathol 2011; 40: 256–264. [DOI] [PubMed] [Google Scholar]
- 78. Defauw PA, Van de Maele I, Duchateau L, et al. Risk factors and clinical presentation of cats with feline idiopathic cystitis. J Feline Med Surg 2011; 13: 967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. O’Neil E, Horney B, Burton S, et al. Comparison of wet-mount, Wright-Giemsa and Gram-stained urine sediment for predicting bacteriuria in dogs and cats. Can Vet J 2013; 54: 1061–1066. [PMC free article] [PubMed] [Google Scholar]
- 80. Saunders A, Bartges JW, Bemis DA, et al. Evaluation of blood agar plates and incandescent lighting for aerobic bacterial urine cultures. J Vet Intern Med 2002; 16: 379. DOI: 10.1111/j.1939-1676.2002.tb02376.x. [DOI] [Google Scholar]
- 81. Bartges JW. Diagnosis of urinary tract infections. Vet Clin North Am Small Anim Pract 2004; 34: 923–933. [DOI] [PubMed] [Google Scholar]
- 82. Lees GE, Simpson RB, Green RA. Results of analyses and bacterial cultures of urine specimens obtained from clinically normal cats by three methods. J Am Vet Med Assoc 1984; 184: 449–454. [PubMed] [Google Scholar]
- 83. Sorensen TM, Jensen AB, Damborg P, et al. Evaluation of different sampling methods and criteria for diagnosing canine urinary tract infection by quantitative bacterial culture. Vet J 2016; 216: 168–173. [DOI] [PubMed] [Google Scholar]
- 84. Kim Y, Seo MR, Kim SJ, et al. Usefulness of blood cultures and radiologic imaging studies in the management of patients with community-acquired acute pyelonephritis. Infect Chemother 2017; 49: 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Westropp JL, Sykes JE. Diagnostics and management of pyelonephritis. ACVIM Forum; 2017 June 8-10; National Harbor, MD. [Google Scholar]
- 86. Love BC, Jones RL. Laboratory diagnosis of bacterial infections. In: Greene CE. (ed). Infectious diseases of the dog and cat. 4th ed. St Louis, MO: Elsevier Saunders, 2013, pp 277–282. [Google Scholar]
- 87. Reller LB, Weinstein M, Jorgensen JH, et al. Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin Infect Dis 2009; 49: 1749–1755. [DOI] [PubMed] [Google Scholar]
- 88. Brooks MB, Morley PS, Dargatz DA, et al. Survey of antimicrobial susceptibility testing practices of veterinary diagnostic laboratories in the United States. J Am Vet Med Assoc 2003; 222: 168–173. [DOI] [PubMed] [Google Scholar]
- 89. Clare S, Hartmann FA, Jooss M, et al. Short- and long-term cure rates of short-duration trimethoprim-sulfamethoxazole treatment in female dogs with uncomplicated bacterial cystitis. J Vet Intern Med 2014; 28: 818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Westropp JL, Sykes JE, Irom S, et al. Evaluation of the efficacy and safety of high dose short duration enrofloxacin treatment regimen for uncomplicated urinary tract infections in dogs. J Vet Intern Med 2012; 26: 506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Morello W, La Scola C, Alberici I, et al. Acute pyelonephritis in children. Pediatr Nephrol 2016; 31: 1253–1265. [DOI] [PubMed] [Google Scholar]
- 92. Nicolle LE. Asymptomatic bacteriuria: when to screen and when to treat. Infect Dis Clin North Am 2003; 17: 367–394. [DOI] [PubMed] [Google Scholar]
- 93. Nicolle LE. Asymptomatic bacteriuria: review and discussion of the IDSA guidelines. Int J Antimicrob Agents 2006; 28 Suppl 1: 42–48. [DOI] [PubMed] [Google Scholar]
- 94. Köves B, Cai T, Veeratterapillay R, et al. Benefits and harms of treatment of asymptomatic bacteriuria: a systematic review and meta-analysis by the European Association of Urology Urological Infection Guidelines Panel. Euro Urol 2017; 72: 865–868. [DOI] [PubMed] [Google Scholar]
- 95. Bengtsson C, Bengtsson U, Björkelund C, et al. Bacteriuria in a population sample of women: 24-year follow-up study. Results from the prospective population-based study of women in Gothenburg, Sweden. Scand J Urol Nephrol 1998; 32: 284–289. [DOI] [PubMed] [Google Scholar]
- 96. Weese JS, Blondeau JM, Boothe D, et al. ISCAID consensus statement: antimicrobial guidelines for the treatment of urinary tract infections in dogs and cats. ACVIM Forum; 2016 June 8-11; Denver, CO, USA. [Google Scholar]
- 97. López-Medrano F, García-Bravo M, Morales JM, et al. Urinary tract infection due to Corynebacterium urealyticum in kidney transplant recipients: an underdiagnosed etiology for obstructive uropathy and graft dysfunction – results of a prospective cohort study. Clin Infect Dis 2008; 46: 825–830. [DOI] [PubMed] [Google Scholar]
- 98. Gibson JS, Morton JM, Cobbold RN, et al. Risk factors for multidrug-resistant Escherichia coli rectal colonization of dogs on admission to a veterinary hospital. Epidem Infect 2011; 139: 197–205. [DOI] [PubMed] [Google Scholar]
- 99. Hernandez J, Bota D, Farbos M, et al. Risk factors for urinary tract infection with multiple drug-resistant Escherichia coli in cats. J Feline Med Surg 2014; 16: 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Gupta K, Chou MY, Howell A, et al. Cranberry products inhibit adherence of p-fimbriated Escherichia coli to primary cultured bladder and vaginal epithelial cells. J Urol 2007; 177: 2357–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Howell AB, Botto H, Combescure C, et al. Dosage effect on uropathogenic Escherichia coli anti-adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: a multicentric randomized double blind study. BMC Infect Dis 2010; 10: 94. DOI: 10.1186/1471-2334-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev 2012; 10. DOI: 10.1002/14651858.CD001321.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Smee N, Grauer GF, Schermerhorn T. Investigations into the effect of cranberry extract on bacterial adhesion to canine uro-epithelial cells [abstract]. J Vet Intern Med 2011; 25: 716. [Google Scholar]
- 104. Olby NJ, Vaden SL, Williams K, et al. Effect of cranberry extract on the frequency of bacteriuria in dogs with acute thora-columbar disk herniation: a randomized controlled clinical trial. J Vet Intern Med 2017; 31: 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chou H-I, Chen K-S, Wang H-C, et al. Effects of cranberry extract on prevention of urinary tract infection in dogs and on adhesion of Escherichia coli to Madin-Darby canine kidney cells. Am J Vet Res 2016; 77: 421–427. [DOI] [PubMed] [Google Scholar]
- 106. Mayot G, Secher C, Di Martino P. Inhibition of adhesion of uropathogenic Escherichia coli to canine and feline uroepithe-lial cells by an extract from cranberry. J Microbiol Biotechnol Food Sci 2018; 7: 404–406. [Google Scholar]
- 107. Wellens A, Garofalo C, Nguyen H, et al. Intervening with urinary tract infections using anti-adhesives based on the crystal structure of the FimH-oligomannose-3 complex. PloS One 2008; 3. DOI: 10.1371/journal.pone.0002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus pro-biotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis 2011; 52: 1212–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mastromarino P, Macchia S, Meggiorini L, et al. Effectiveness of Lactobacillus-containing vaginal tablets in the treatment of symptomatic bacterial vaginosis. Clin Microbiol Infect 2009; 15: 67–74. [DOI] [PubMed] [Google Scholar]
- 110. Hutchins RG, Bailey CS, Jacob ME, et al. The effect of an oral probiotic containing Lactobacillus, Bifidobacterium, and Bacillus species on the vaginal microbiota of spayed female dogs. J Vet Intern Med 2013; 27: 1368–1371. [DOI] [PubMed] [Google Scholar]
- 111. Ball AJ, Carr TW, Gillespie WA, et al. Bladder irrigation with chlorhexidine for the prevention of urinary infection after transurethral operations: a prospective controlled study. J Urol 1987; 138: 491–494. [DOI] [PubMed] [Google Scholar]
- 112. Farca AM, Piromalli G, Maffei F, et al. Potentiating effect of EDTA-Tris on the activity of antibiotics against resistant bacteria associated with otitis, dermatitis and cystitis. J Small Anim Pract 1997; 38: 243–245. [DOI] [PubMed] [Google Scholar]
- 113. Greenwood D, Slack RCB. The antibacterial activity of hexamine (methenamine), hexamine hippurate and hexamine mandelate. Infection 1981; 9: 223–227. [DOI] [PubMed] [Google Scholar]
- 114. Mayrer AR, Andriole VT. Urinary tract antiseptics. Med Clin North Am 1982; 66: 199–208. [DOI] [PubMed] [Google Scholar]
- 115. Hull R, Rudy D, Donovan W, et al. Urinary tract infection prophylaxis using Escherichia coli 83972 in spinal cord injured patients. J Urol 2000; 163: 872–877. [PubMed] [Google Scholar]
- 116. Sundén F, Håkansson L, Ljunggren E, et al. Escherichia coli 83972 bacteriuria protects against recurrent lower urinary tract infections in patients with incomplete bladder emptying. J Urol 2010;184: 179–185. [DOI] [PubMed] [Google Scholar]
- 117. Thompson MF, Schembri MA, Mills PC, et al. A modified three-dose protocol for colonization of the canine urinary tract with the asymptomatic bacteriuria Escherichia coli strain 83972. Vet Microbiol 2012; 158: 446–450. [DOI] [PubMed] [Google Scholar]
- 118. Thompson MF, Totsika M, Schembri MA, et al. Experimental colonization of the canine urinary tract with the asymptomatic bacteriuria Escherichia coli strain 83972. Vet Microbiol 2011; 147: 205–208. [DOI] [PubMed] [Google Scholar]
- 119. Segev G, Sykes JE, Klumpp DJ, et al. Evaluation of the live biotherapeutic product, asymptomatic bacteriuria Escherichia coli 2-12, in healthy dogs and dogs with clinical recurrent UTI. J Vet Intern Med 2018; 32: 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Freitag T, Squires RA, Schmid J. Naturally occurring bacterio-phages lyse a large proportion of canine and feline uropathogenic Escherichia coli isolates in vitro. Res Vet Sci 2008; 85: 1–7. [DOI] [PubMed] [Google Scholar]