Abstract
Objective:
To clarify the reasons and beneficial effects and duration of arteriovenous fistula patency after radiological interventions in arteriovenous fistula. The patients investigated were referred due to arteriovenous fistula access flow problems.
Material and methods:
In 174 patients, 522 radiological investigations and endovascular treatments such as percutaneous transluminal angioplasty were analyzed, retrospectively. All investigations were performed due to clinical suspicion of impaired arteriovenous fistula function.
Results:
Arterial stenosis was significantly more frequent among patients with diabetic nephropathy (p < 0.001) and interstitial nephritis (p < 0.001). According to the venous stenosis, the diagnosis did not affect the frequency (p = 0.22) or the degree (p = 0.39) of stenosis. The degree of stenosis prior to percutaneous transluminal angioplasty correlated significantly with the degree of remaining stenosis after intervention (p < 0.001). Of the 174 patients, 123 (71%) performed a total of 318 investigations including percutaneous transluminal angioplasty. Repeated percutaneous transluminal angioplasty was performed significantly more often in patients with diabetic nephropathy. The median times to the first percutaneous transluminal angioplasty and to the subsequent percutaneous transluminal angioplasties were 9.5 and 5 months, respectively. Arteriovenous fistula in patients with diabetic nephropathy performed similar to most other diagnoses, although performing more percutaneous transluminal angioplasty/patient than most other diagnoses.
Conclusion:
Many patients could maintain long-term patency of arteriovenous fistula, including those with diabetic nephropathy, with repeated interventions; this motivates a closer follow-up for these patients. Clinically significant stenosis should be dilated as meticulously and as soon as possible. Occlusions of the arteriovenous fistula in most instances can be successfully thrombolyzed or dilated upon early diagnosis.
Keywords: Arteriovenous fistula, angiography, hemodialysis, percutaneous transluminal angioplasty, fistulography, phlebography
Introduction
Hemodialysis (HD) remains the most important treatment in end-stage renal failure when transplantation is not possible. Optimal function of vascular access and an adequate dialysis dose are two important factors for a successful HD. The golden standard of vascular access is a well-functioning native arteriovenous fistula (AVF).1,2 An AVF is recommended before arteriovenous graft (AVG) and central dialysis catheter (CDC). CDC constitutes the worst prognosis especially for the elderly.3 The differences in the uses of AVF throughout the world are presumed to contribute to survival differences between geographical areas.4–6 Therefore, it is important to perform and maintain a patent AVF.4 Maintaining a well-functioning AVF is crucial. In total, 20% of fistula creations have a primary non-function. AVFs cease their function over time, and it was shown in a meta-analysis that after 1 year only approximately 64% remain in function.7 The main reason for AVF failure is the development of stenotic formations that lead to radiological investigations and procedures such as percutaneous balloon dilation, endovascular stenting, thrombolysis, or reconstructive surgery. Early problems with AVF function are bleeding and hematoma that result in decreased flow, which thereby increases the risks for thrombosis and infection. Later problems are thrombosis, aneurysm, and stenosis with subsequent circulatory effects.8,9 Data vary in regard to the time to first intervention after placement of the AVF by surgery and in regard to the time to repeated intervention.10,11 The patency time is shorter for those above 65 years of age.10 Repeated percutaneous interventions on failing AVFs for HD are common, but the outcomes are largely unknown.12
The aim of this study was to clarify the reasons and beneficial effects and duration of AVF patency after radiological interventions in AVF. The patients investigated were referred due to AVF flow problems.
Materials and methods
The study included 174 patients that were on chronic HD and were referred to the radiological unit due to AVF flow problems. All patients had an AVF as the vascular access. The patients represented approximately 5.5% of the HD population in Sweden. The patients were consecutively included in the study based on the time of referral to the radiological unit. From 1 January 2006 to 31 December 2014, patients in the county and university hospital were identified consecutively from radiological registers based on radiological investigations such as angiography, phlebography, or balloon dilatation of a native AVF. Patients with grafts were excluded. All investigations were performed on clinical suspicion of AVF dysfunction such as recirculation, bleeding after HD, worsening Kt/V, access flow that during 3 months was 25% lower than before, or high venous pressure. The main author performed a retrospective radiological evaluation of all investigations. In total, 522 investigations were included and reviewed. All stenoses were evaluated. A significant stenosis that necessitated intervention was defined as an obstruction in the lumen by more than 50% in accordance with the KDOQI (Kidney Disease Outcomes Quality Initiative) guidelines.1 The length and placement of the stenosis were recorded in relation to the anastomosis and location at the arterial or venous side. The time from AVF surgical construction to the first and subsequent radiological investigation and/or procedures was recorded. Demographic data such as gender, age, diagnosis for end-stage renal disease, diabetic nephropathy (DN), primary fistula placement, and smoking habits were also recorded.
Routine estimation of AVF flow included clinical analyses such as increased venous pressure and transonic measurement of recirculation and flow. At intervals, Doppler investigations were performed to estimate whether there was a development of stenosis or not. Upon clinical suspicion, a Doppler investigation could be done on short notice to decide if admission for radiological investigation should be performed. On some occasions, acute problems developed.
Radiological investigations
Phlebography was performed through two needles that were placed in the AVF by a renal nurse at the location where the HD was normally done. A stenosis that was located toward the heart was dilated from the same location. A stenosis that was positioned near the anastomosis required a repeated puncture of the vein toward the anastomosis.
Angiography was performed with the following two methods:
Percutaneous transluminal angioplasty (PTA) was done from the femoral artery with the tip of the catheter in the axillary artery where the first dose of the contrast medium was injected (approximately 7 mL). This dose could be repeated. Thus, the angiography visualized the arterial side including the proximal part of the artery, the anastomosis, the distal venous side of the AVF, and the proximal and central veins. In the majority of the AVF cases, a pre-invasive ultrasound investigation was performed and guided intervention in the area of suspected stenosis.
The second technique was a micro-catheter technique. With the help of an ultrasound needle guide, a puncture was performed with a 0.8-mm needle to facilitate placement of a 4-Fr micro-introducer into the brachial or radial artery. Approximately 2–3 mL of contrast medium was used to localize and define the stenosis. This dose could be repeated. Performing a balloon dilatation from this location could result in substantial bleedings and a pseudoaneurysm that could require surgical reconstruction. Therefore, it was necessary to make a new puncture on the venous side to enable a dilatation of one or several stenoses. In the case of thrombosis, this location could be used for thrombolysis.13
Interventions were performed with various types (different blends) of balloons that included cutting balloons or stents (none drug eluting). Occasionally, the stenosis was dilated in addition to thrombolysis. A subsequent smoothening of the surface was easier to achieve by adding an intervention with a non-cutting conventional balloon. A stent was used in some central or rarely recurrent stenoses. A dilatation within stents was also performed.
The retrospective radiological evaluation included the identification of the anastomosis, and of all present stenoses. The degree of each stenosis was estimated as the percentage of the stenosis in relation to lumen size. The location of the stenosis in either the arterial or the venous part of the vessel was registered, as was the distance from the anastomosis and the length of the stenosis (measured in millimeters). After PTA, an estimation was made of the remaining degree of stenosis, the remaining length of the stenosis, and whether a stent was placed or complications occurred.
Primary unassisted patency was the time of AVF creation until the first radiological intervention such as angioplasty, thrombolysis, or thrombectomy to maintain or restore blood flow.
Recurrence was calculated for either radiological investigation, regardless of intervention or not, and separately for PTA intervention.
Statistics
The non-parametric Mann–Whitney U independent test was used for group comparison. Fisher’s test was used for comparison of ratios. The Wilcoxon paired test was used for intra-individual comparisons. Analysis of variance (ANOVA) calculations have been performed (Table 2). Mean values ± 1 standard deviation are presented, and the number of patients or investigations is shown in parenthesis. All statistical analyses were done with the SPSS package. A two-tailed p-value less than 0.05 was considered statistically significant.
Table 2.
Mean time interval in months (±SD) and number of episodes (in parenthesis) between the initial surgical placement of AVF and the first and follow-up radiological investigations (invest.) in relation to various background diagnoses.
| Surg. to 1st invest. (n = 174) | Follow-up invest. (n = 348) | Surg. to 1st PTA | Follow-up PTA (n = 320) | PTA, numbers (n = 484) | |
|---|---|---|---|---|---|
| Glomerulonephritisa | 24 ± 32 (28) | 9 ± 16 (48) | 46 ± 58 (26)b–f | 13 ± 19 (33)b | 2.0 ± 1.2 (59)b |
| Diabetic nephropathyb | 14 ± 25 (47) | 7 ± 9 (110) | 16 ± 23 (48)a | 7 ± 9 (136)a, c | 5.3 ± 5.3 (184)a, c–f |
| Interstitial nephritisc | 18 ± 18 (24) | 8 ± 11 (55) | 22 ± 20 (21)a | 12 ± 12 (40)b | 2.7 ± 1.7 (61)b |
| Hereditary diseased | 23 ± 29 (18) | 5 ± 7 (33) | 23 ± 28 (16)a | 8 ± 6 (16) | 1.7 ± 0.8 (32)b |
| Hypertension, nephrosclerosise | 16 ± 17 (38) | 7 ± 8 (82) | 19 ± 23 (37)a | 10 ± 8 (80) | 2.9 ± 2.0 (117)b |
| Other diagnosesf | 17 ± 23 (19) | 4 ± 7 (20) | 15 ± 17 (16)a | 10 ± 11 (15) | 1.6 ± 0.8 (31)b |
SD: standard deviation; AVF: arteriovenous fistula; PTA: percutaneous transluminal angioplasty; ANOVA: analysis of variance.
Data are also displayed for the mean time interval in months between the initial surgical placement of AVF and the first and follow-up episodes of PTA intervention. These data include all interventions at the same instance, for example, if three different stenoses were dilated at the same time, each dilatation was calculated as one separate event. Mean numbers of PTA/sessions (±SD) and total PTAs (in parenthesis) are also given. Data were generated with ANOVA.
Significant differences (p < 0.05) between groups.
Results
The study included 105 men (60%) and 69 women (40%). The median (range) age was 68 years (25–88 years), with a mean of 65 ± 14 years in both genders. Table 1 shows demographic data.
Table 1.
Demographic data with numbers given and percentage in parenthesis.
| Total (n = 174) | |
|---|---|
| Age (years), mean (±SD) | 65 (±14) |
| Men/women | 105/69 |
| Tobacco users | 48/167 (29%) |
| Diagnoses | |
| Glomerulonephritis | 28 (16%) |
| Diabetic nephropathy | 47 (27%) |
| Interstitial nephritis | 24 (14%) |
| Hereditary diseases | 18 (10%) |
| Nephrosclerosis/hypertensive disease | 38 (22%) |
| Others | 19 (11%) |
| Diabetic nephropathy | 55 (32%) |
| AV access location | |
| Radiocephalic forearm, left/right | 127/19 (84%) |
| Radiocephalic, unknown location | 8 (5%) |
| Brachiocephalic, left/right | 4/2 (3%) |
| Other | 14 (8%) |
| Investigation episodes | 522 |
| Fistulography | 251 (48%) |
| Angiography | 251 (48%) |
| Fistulo- and angiography | 60 (11%) |
| PTA | 318 (61%) |
SD: standard deviation; AV: arteriovenous; PTA: percutaneous transluminal angioplasty.
Renal diagnoses were registered and divided into six groups (Table 1). The distribution of the diagnoses was similar between genders. The group termed “Other” included patients with other than common renal disease such as damage of the kidneys due to sepsis, myocardial infarction, pregnancy complications, prostate cancer, or multiple myeloma. The proportion of these patients that needed radiological investigations and treatments was larger than the proportion of such patients in the general HD population.
The underlying diagnosis did not affect the number of investigations (p = 0.524).
Smokers (including snuff) were more prevalent among patients with the diagnosis of nephrosclerosis (50%, p < 0.001) and those with interstitial nephritis (IN; 43%, p = 0.028) as compared to other diagnoses where the prevalence of smoking varied from 12% to 22%.
The prevalence of HD patients with DN with AVF problems was more common in this study as compared with DN patients on HD (p = 0.008) within the national registry SNR (Swedish Renal Registry).14 A higher prevalence was also noted in this study for those with nephrosclerosis (p < 0.001), while for those with glomerulonephritis (GN) it was less (p = 0.004) in comparison to the SNR.
The location of the vascular access is presented in Table 1. There was 89% forearm AVF, radiocephalic anastomoses, mainly placed on the left side (i.e. 86% were placed on the non-dominant arm). The remaining type of AVF was not specified by side or upper arm AVF. Two AVFs were placed in the “fossa cubitii.”
In total, radiological examinations were performed 522 times (Table 1). The number of examinations was at a median of 2 (range 1–20) for each patient. Only one examination was performed in 65 patients. The maximum number of detected stenoses at the same time was 5 on the venous side (in one patient) and 2 on the arterial side.
The extent of venous stenosis (in percentage) could not be coupled to a specific diagnosis.
All stenoses
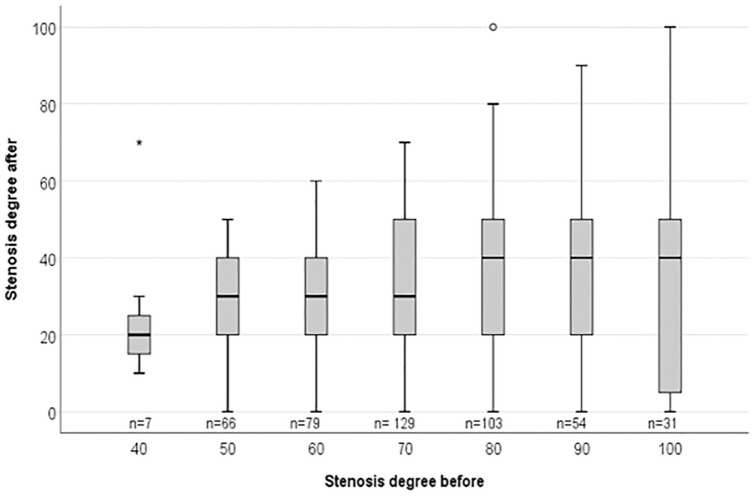
The degree of venous and arterial stenoses prior to PTA correlated with the degree of remaining stenoses after intervention (r = 0.20, p < 0.001). Figure 1 presents the distribution by box plot. This means that the more extensive stenoses were less completely resolved by PTA.
Figure 1.
Results of 469 PTAs displayed by box plot and the median value of the remaining stenoses. The x-axis shows the degree of stenosis (%) before intervention and the y-axis the degree of stenosis after PTA. The number of PTAs are given below the boxes (n=). The Spearman correlation of the median residual stenosis and the primary stenosis (r = 0.93, p = 0.003) indicates a greater residual stenosis after PTA in those with a more extensive primary stenosis.
The number of months from surgical placement of AVF to the first radiological investigation (all stenoses) did not differ between the diagnosis groups. The months to the next radiological investigation were shortest for the “Other” diagnoses, but no significant difference was found (Table 2).
The patency of the AVF from surgery to the first PTA (all stenoses) was the longest for patients with GN than for the other groups. The time interval for the whole material was at a median of 9.5 months (mean = 25 ± 36 months). Time is displayed in Figure 2.
Figure 2.
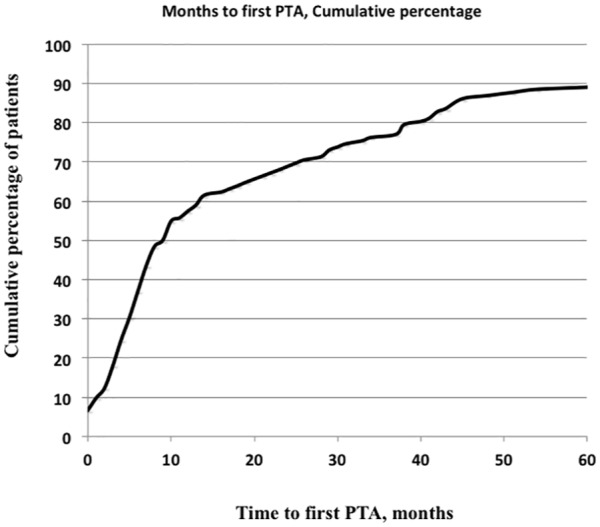
Months from surgery to first PTA distributed in cumulative percentage.
The months elapsed from the previous PTA (all stenoses) to the next PTA were fewer for DN than those for GN (p = 0.007) and IN (p = 0.016) (Table 2). The time from the previous until the next PTA was at a median of 5 months (mean = 8.7 ± 10.2 months) and is displayed in Figure 3.
Figure 3.
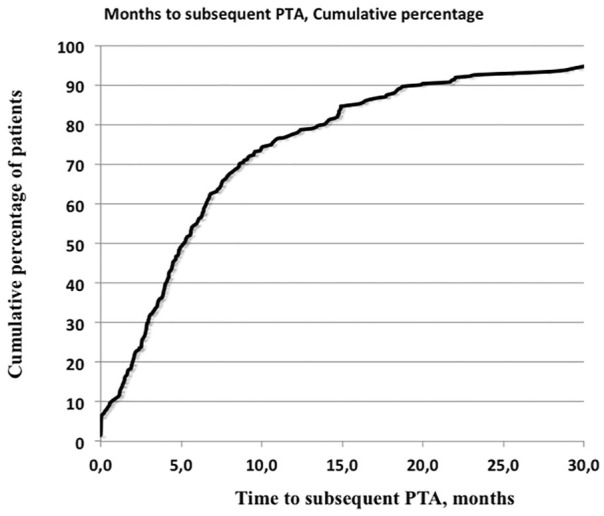
Months between the previous PTA and the next PTA given in cumulative percentage.
Of the 174 patients, 123 (71%) performed a total of 484 PTA investigations (each could include more than one PTA). These patients performed PTA either one (28.7%), two (17.2%), three (12.6%), four (2.3%), or five times or more (9.8%).
Recurrent PTA episodes were divided into three parts. The lowest tertial (shortest time to recurrence, n = 90, mean = 1.5 ± 1.1 months) was compared with the longest time to recurrence (n = 90, mean = 19.2 ± 11.9 months). Those with the shortest time between PTAs included a larger proportion of patients with DN and “Other” diagnoses, were younger (p = 0.031), and had a lower number of venous stenoses (p = 0.045). Venous stenosis number 2 was longer in those with the shortest time to the next PTA (p = 0.041). There were no differences in gender, presence of diabetes mellitus (DM) in general, tobacco usage, or in the percentage degree of stenosis of the artery or vein.
Arterial stenoses
Arterial stenoses were significantly more frequent among patients with DN (p < 0.001) and IN (p < 0.001) than with the other diagnoses. The degree of stenosis did not differ between the diagnoses. The underlying diagnosis did not influence the PTA result.
Of the arterial stenoses, 48 of 84 were considered significant. Intervention with dilatation was performed in 25 of these 48 cases (52%). The majority of the patients that underwent PTA of the feeding artery were men (n = 22, 88%).
Venous stenoses
The mean number of stenosis of the lumen of venous stenoses are given in Table 3. A total of 689 venous stenoses were present. Of these, 67% had one stenosis, 25% had two, and 8% had 3–5 stenoses. Most stenoses per patient and episode were present in patients with nephrosclerosis and DN, and the least were in those with hereditary disease and IN. Significant differences between some of the diagnoses existed (Table 3).
Table 3.
Mean number (±SD) of all venous stenoses (independent of whether dilated or not) detected at the same investigation.
| Venous stenoses (n = 689) | |
|---|---|
| Glomerulonephritisa | 1.5 ± 0.9 (94) |
| Diabetic nephropathyb | 1.7 ± 0.9 (228)c,d |
| Interstitial nephritisc | 1.3 ± 0.6 (93)b,e |
| Hereditary diseased | 1.1 ± 0.7 (57)b,e |
| Hypertension, nephrosclerosise | 1.8 ± 1.1 (165)c,d |
| Other diagnosesf | 1.4 ± 1.2 (52) |
SD: standard deviation.
The total numbers of venous stenoses are given in parentheses, even if no dilatation was performed.
Significant differences (p < 0.05) between groups.
The location of the venous stenosis from the anastomosis was possible to be calculated in 422 instances. The distance from the arteriovenous anastomosis was at a median of 5 (n = 236, 56%), 28 (n = 132, 31%), 70 (n = 46, 11%), and 160 (n = 8, 2%) mm for venous stenosis numbers 1, 2, 3, and 4, respectively (Figure 4). Of these, 36% were 5 mm or closer to the anastomosis and 98% were within 200 mm from the anastomosis. The lengths of the stenoses were at a median of 15, 17.5, 20, and 18 mm for venous stenosis numbers 1, 2, 3, and 4, respectively (Figure 5).
Figure 4.
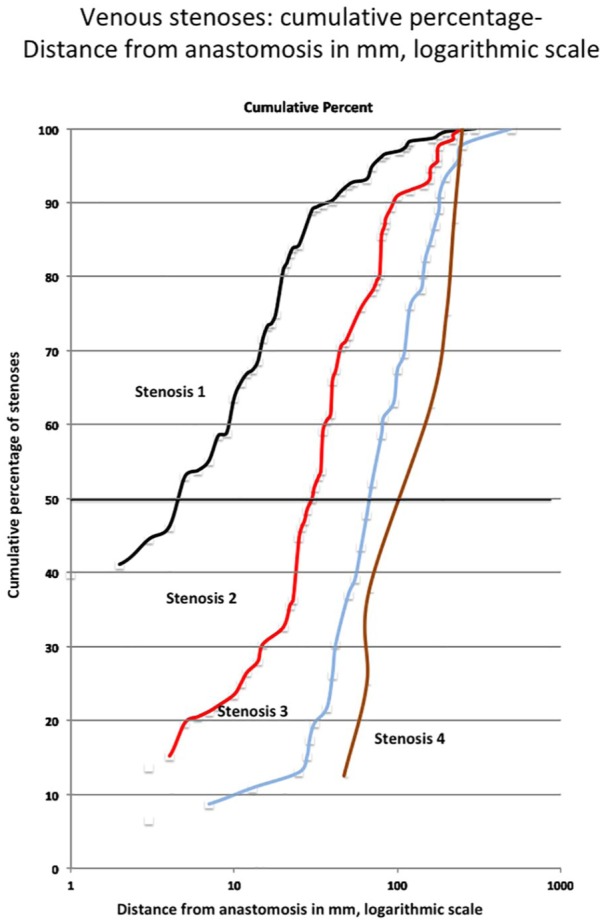
Distance (in mm) of venous stenosis from AVF anastomosis.
Figure 5.
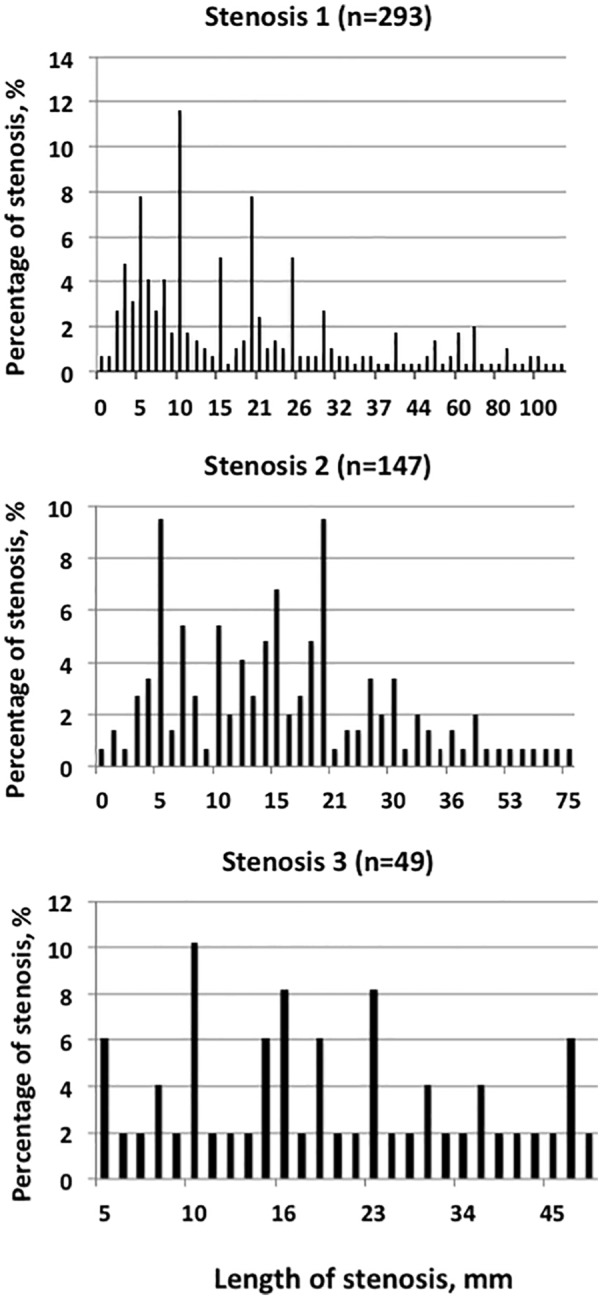
Distribution (percentage) of the various lengths of venous stenosis numbers 1–3 (in mm).
There was no difference in the distribution of renal diagnoses in relation to the percentage of those who had the first stenosis within 10 mm from the anastomosis versus those with a more distant first stenosis. The exit angle (as a possible reason for turbulent flow) of the venous part of the AVF from the artery showed a similar (n.s.) ratio of the first versus all stenoses (angle 0–30°: 20/35 = 57%; angle 31–60°: 43/58 = 74%; angle 61–90°: 43/71 = 61%; angle 91–120°: 16/20 = 80%; angle 121–150°: 1/2 = 50%).
PTA was performed in 459 of the venous stenoses. Four stenoses (1%) were impaired after PTA due to bleeding, thrombosis, or dissection. The underlying diagnosis did not affect the risk for these complications. Smoking did not influence the time span between interventions.
According to the venous stenosis, the diagnosis did not impact the frequency (p = 0.22), or the degree of venous stenosis (p = 0.39). The long-term survival of AVF is shown in Figure 6. There was a significantly poorer outcome only for those suffering from the “Other” diagnoses versus GN (p = 0.017), DN (p = 0.003), and IN (p = 0.036). AVF in patients with DN performed similar to most other diagnoses, although they had performed more PTAs/patient than the other diagnoses (p = 0.036).
Figure 6.
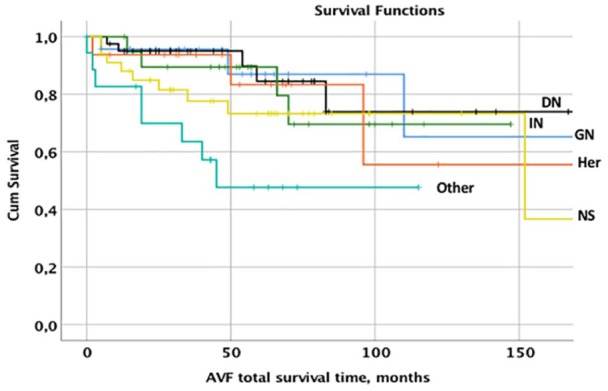
Distribution of cumulative survival of AVF related to the various diagnoses glomerulonephritis (GN), diabetic nephropathy (DN), interstitial nephritis (IN), hereditary diseases (Her), nephrosclerosis (NS), and other diagnoses (Other).
Altogether, there were 57 occlusions. One occlusion was not dilated because of infection. More than 80% of all interventions of the occlusions of the AVFs were successful. The distribution of interventions of occlusions and thrombolysis is displayed in Table 4.
Table 4.
Distribution (in percentage) of interventions of all total occlusions (n = 57).
| Interventions | Percentage |
|---|---|
| Thrombolysis | 9 |
| Surgical correction | 37 |
| Stent placement | 5 |
| PTA | 25 |
| PTA + stent | 4 |
| PTA failed | 7 |
| Thrombolysis + dilatation | 14 |
PTA: percutaneous transluminal angioplasty.
Data show more than 100%, since in some more than one intervention was made at the same time.
Discussion
This study included only referrals that were based on AVF access flow problems. This study showed that patients with AVF suffer mainly from venous stenosis, whereas arterial stenosis was more common in men and those with DN and nephrosclerosis. In this study, the risk to develop venous stenosis could not be coupled to a specific diagnosis or to gender, while others found a more general relation between cardiovascular arterial diseases and diabetes nephropathy.15 The present data support the fact that arterial and venous stenoses are due to different pathophysiological mechanisms. This is further strengthened by the data showing that the number of months from surgery to the first radiological investigation or PTA was similar for all diagnoses. Notably, a large proportion of patients suffered from a short patency of the AVF both after primary placement and after subsequent PTA. Also, if a PTA was very successful, reoccurrence of significant stenosis could be found within a few months; this indicates strong individual differences in the development of stenosis. Therefore, follow-up investigations and PTA were performed at a mean of within a 5-month interval in those with the “Other” diagnoses compared to less than 12 months in the residual patients. Notably, the smokers were not overrepresented in the number of interventions. The lack of such findings may be due to the absence of information on who was a current and who was a previous smoker, as well as the lack of information of daily doses of tobacco consumption. The locations and sizes of the stenoses in our material were consistent with the results of a previous study by Maya et al.16 who showed that venous anastomosis stenoses were the most frequent and arterial the least frequent. The present data indicate that the stenoses close to the anastomosis are more due to the effect of shear stress in the lumen of the anastomosis and less due to the angle of exit itself. Stenoses at the second and third sites indicate lesions and stenoses caused by repeated needle punctures along the fistula area, which suggests other modifiable factors. However, the different puncture techniques were not registered in our material although most were performed with a buttonhole technique (using the same entrance as before).
The extent of stenosis and location of venous stenosis could be partly related to AVF access sites. In some patients, only one dilatation was necessary, whereas in other patients repeated PTAs were needed. Although the first stenosis in DN was found as frequent as for other diagnoses, recurrent venous stenosis after PTA was more common among patients with DN. The latter is in line with other studies.15,17–20 This motivates a closer follow-up, especially for this group of patients.
The present data revealed that recurrent venous stenosis appeared after approximately 6 months. This interval was similar even after several interventions. In some cases, the medical condition of the patient limited the number of interventions, and CDC placement was performed.
Our data support the fact that PTA should be performed early in the course and with the intention to normalize the lumen size to achieve the best resolution of stenosis. A residual stenosis will cause increased fistula flow in the stenosis area and will thereby favor endothelial stress and recurrent stenosis. By aiming at zero stenosis after the intervention, the risks for shear stress and recurrent stenosis of vessels are reduced. This is in line with other studies.8,20–22 We also promote that all interventions should be performed as soon as possible, perhaps already before the stenosis reaches 50% of the diameter.
In patients with total occlusions of the AVF, we showed a surprisingly high success rate of 80% after radiological interventions. The benefit of such actions was also shown in a previous study that had 70% success using AVF thrombectomy.23 This supports attempts of interventions if occlusions are detected early.
In this study, the mean time from access creation to endovascular intervention was similar to data from a previous study where a mean patency of 23.5 months was reported.24 However, the median time for our patients was much shorter than reported previously since many needed interventions early in the course, and only a few had excellent patency. This study does not clarify if prior CDC use was a negative reason for longevity, as shown in a previous study.25 Other risk factors are older age, diastolic hypotension, diabetes,15,18,20,26 long lesion length, and a younger age of fistulae.27 The laboratory markers noted were serum albumin,26 increased level of the neutrophil–lymphocyte ratio,28 serum C-reactive protein (CRP),29 and coagulation–fibrinolysis imbalance after intervention,30 but there were few other blood markers.31
Another study showed that higher doses of erythrocyte-stimulating agents (ESA) were used in patients who suffered from AVF stenosis problems.32 Since ESA may stimulate endothelial growth and fibrosis, future studies have to clarify whether this contributes to stenosis or is a confounding factor.
We did not use drug-eluting balloon angioplasty that could prolong patency,28,33 whereas repeated follow-up of ultrasound and blood flow of AVF was done according to others.34,35
Although stent grafts for AVF stenosis have a positive impact,36,37 this was only used to a limited extent.
This study favors the fact that a specific diagnosis such as DN is connected with arterial but not with the initial development of venous stenosis. However, recurrent venous stenoses are more common in these patients. As we show, the location of venous stenosis is mainly in three areas, and frequently there are numerous stenoses in the same patient. Our study shows the time that can be expected to elapse between the primary operation and the first PTA necessary, as well as the episodes and time elapsed between PTA recurrences. The angles of the venous anastomosis toward the feeding artery appear not to be of importance for the development of the stenoses. Most stenoses were within 5 mm of the anastomosis, and the subsequent stenoses seemed to be located where the arterial and venous needles were located. The latter indicates a traumatic reason for stenosis more so than turbulence. We feel that the dilatation of the stenosis should be performed before the lumen becomes too narrow since the effect of dilatation is less at this stage. Our data indicate that there may be a benefit to dilate stenoses below the 50% limit as recommended by guidelines. The effect of dilatation could be maintained even when repeated dilatations were performed and the time delay between these recurrent stenoses, albeit short, did not change. The data also give hope for radiological interventions when a total occlusion is present that includes thrombectomy, thrombolysis, and dilatations.
In conclusion, AVF stenoses develop and reoccur differently depending on the renal diagnosis as well as stage of intervention. Radiological interventions are effective and can be repeatedly performed with success. Repeated follow-up of risk patients and earlier interventions will help patients stay on native AVF. It is important to consider the investigation and endovascular treatment in one sequence. In case of occlusion, thrombolysis with or without PTA should be attempted before surgery. All interventions should be performed as soon as possible since late intervention results in a larger remaining stenosis. Interventionists should not hesitate to intervene in occluded fistulae since results are favorable.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors received financial support from Skaraborg Hospital and Skaraborg Research and development Council, Skövde Sweden and from Njurforeningarna i Vasterbotten, Norrbotten, Vasternottland and Jamtland-Harjedalen.
ORCID iD: Bernd Stegmayr  https://orcid.org/0000-0003-2694-7035
https://orcid.org/0000-0003-2694-7035
References
- 1. National Kidney Foundation. National Kidney Foundation K/DOQI clinical practice guidelines for vascular access 2006. New York: National Kidney Foundation, 2018. [Google Scholar]
- 2. Ozeki T, Shimizu H, Fujita Y, et al. The type of vascular access and the incidence of mortality in Japanese dialysis patients. Intern Med 2017; 56(5): 481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Raimann JG, Barth C, Usvyat LA, et al. Dialysis access as an area of improvement in elderly incident hemodialysis patients: results from a cohort study from the international monitoring dialysis outcomes initiative. Am J Nephrol 2017; 45(6): 486–496. [DOI] [PubMed] [Google Scholar]
- 4. Robinson BM, Akizawa T, Jager KJ, et al. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet 2016; 388(10041): 294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robinson BM, Bieber B, Pisoni RL, et al. Dialysis Outcomes and Practice Patterns Study (DOPPS): its strengths, limitations, and role in informing practices and policies. Clin J Am Soc Nephrol 2012; 7(11): 1897–1905. [DOI] [PubMed] [Google Scholar]
- 6. Polkinghorne KR, McDonald SP, Atkins RC, et al. Vascular access and all-cause mortality: a propensity score analysis. J Am Soc Nephrol 2004; 15(2): 477–486. [DOI] [PubMed] [Google Scholar]
- 7. Bylsma LC, Gage SM, Reichert H, et al. Arteriovenous fistulae for haemodialysis: a systematic review and meta-analysis of efficacy and safety outcomes. Eur J Vasc Endovasc Surg 2017; 54: 513–522. [DOI] [PubMed] [Google Scholar]
- 8. Schwab SJ, Raymond JR, Saeed M, et al. Prevention of hemodialysis fistula thrombosis. Kidney Int 1989; 36(4): 707–711. [DOI] [PubMed] [Google Scholar]
- 9. Kazemzadeh GH, Modaghegh MHS, Ravari H, et al. Primary patency rate of native AV fistula: long term follow up. Int J Clin Exp Med 2012; 5(2): 173–178. [PMC free article] [PubMed] [Google Scholar]
- 10. Misskey J, Faulds J, Sidhu R, et al. An age-based comparison of fistula location, patency, and maturation for elderly renal failure patients. J Vasc Surg 2018; 67(5): 1491–1500. [DOI] [PubMed] [Google Scholar]
- 11. Kim SM, Ko HK, Noh M, et al. Factors affecting patency following successful percutaneous intervention for dysfunctional hemodialysis vascular access. Ann Vasc Surg 2018; 47: 54–61. [DOI] [PubMed] [Google Scholar]
- 12. Malka KT, Flahive J, Csizinscky A, et al. Results of repeated percutaneous interventions on failing arteriovenous fistulas and grafts and factors affecting outcomes. J Vasc Surg 2016; 63(3): 772–777. [DOI] [PubMed] [Google Scholar]
- 13. Cansu A, Soyturk M, Ozturk MH, et al. Diagnostic value of color Doppler ultrasonography and MDCT angiography in complications of hemodialysis fistulas and grafts. Eur J Radiol 2013; 82(9): 1436–1443. [DOI] [PubMed] [Google Scholar]
- 14. Stendahl M. Svenskt Njurregister. Årsrapport 2016 (Swedish Renal Registry, Report 2016; in Swedish Only), 2016, https://www.medscinet.net/snr/
- 15. Da Cruz RN, Retzlaff G, Zanetti GR, et al. The influence of diabetes mellitus on patency of arteriovenous fistulas for hemodialysis. J Vasc Bras 2015; 14: 217–223. [Google Scholar]
- 16. Maya ID, Oser R, Saddekni S, et al. Vascular access stenosis: comparison of arteriovenous grafts and fistulas. Am J Kidney Dis 2004; 44(5): 859–865. [PubMed] [Google Scholar]
- 17. Hall RK, Myers ER, Rosas SE, et al. Choice of hemodialysis access in older adults: a cost-effectiveness analysis. Clin J Am Soc Nephrol 2017; 12(6): 947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park YJ, Gloviczki P, Kim YW, et al. The influence of cephalic vein diameter and diabetes on primary maturation and patency of autogenous radiocephalic arteriovenous fistulas. J Vasc Surg 2015; 62(4): 1003–1009. [DOI] [PubMed] [Google Scholar]
- 19. Lamprou A, de Bruin C, van Roon A, et al. Patient-related factors influencing patency of autogenous brachiocephalic haemodialysis fistulas. J Vasc Access 2017; 18(Suppl. 1): 104–109. [DOI] [PubMed] [Google Scholar]
- 20. Aktas A, Bozkurt A, Aktas B, et al. Percutaneous transluminal balloon angioplasty in stenosis of native hemodialysis arteriovenous fistulas: technical success and analysis of factors affecting postprocedural fistula patency. Diagn Interv Radiol 2015; 21(2): 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bountouris I, Kristmundsson T, Dias N, et al. Is repeat PTA of a failing hemodialysis fistula durable. Int J Vasc Med 2014; 2014: 369687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Veroux P, Giaquinta A, Tallarita T, et al. Primary balloon angioplasty of small (</=2 mm) cephalic veins improves primary patency of arteriovenous fistulae and decreases reintervention rates. J Vasc Surg 2013; 57: 131–136. [DOI] [PubMed] [Google Scholar]
- 23. Ghaffarian AA, Al-Dulaimi R, Kraiss LW, et al. Clinical effectiveness of open thrombectomy for thrombosed autogenous arteriovenous fistulas and grafts. J Vasc Surg 2018; 68(1): 189–196. [DOI] [PubMed] [Google Scholar]
- 24. Senthoor D, Thant KZ, Ng TK, et al. Clinical course of hemodialysis access after initial endovascular intervention for stenosis in Asian renal failure patients. Vasc Endovascular Surg 2017; 51(6): 363–367. [DOI] [PubMed] [Google Scholar]
- 25. Asano M, Thumma J, Oguchi K, et al. Vascular access care and treatment practices associated with outcomes of arteriovenous fistula: international comparisons from the Dialysis Outcomes and Practice Patterns Study. Nephron Clin Pract 2013; 124(1–2): 23–30. [DOI] [PubMed] [Google Scholar]
- 26. Zhou L, Liu H, Liu F, et al. Survival analysis and risk factors for arteriovenous fistula in 472 patients. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2015; 40(8): 902–906. [DOI] [PubMed] [Google Scholar]
- 27. Maeda K, Furukawa A, Yamasaki M, et al. Percutaneous transluminal angioplasty for brescia-cimino hemodialysis fistula dysfunction: technical success rate, patency rate and factors that influence the results. Eur J Radiol 2005; 54(3): 426–430. [DOI] [PubMed] [Google Scholar]
- 28. Cildag MB, Cildag S, Koseoglu OF. The relationship between neutrophil-lymphocyte ratio and primary patency of percutaneous transluminal angioplasty in hemodialysis arteriovenous fistula stenosis when using conventional and drug-eluting balloons. Cardiovasc Intervent Radiol 2016; 39(12): 1702–1707. [DOI] [PubMed] [Google Scholar]
- 29. Chou CY, Kuo HL, Yung YF, et al. C-reactive protein predicts vascular access thrombosis in hemodialysis patients. Blood Purif 2006; 24(4): 342–346. [DOI] [PubMed] [Google Scholar]
- 30. Hasuike Y, Kakita N, Aichi M, et al. Imbalance of coagulation and fibrinolysis can predict vascular access failure in patients on hemodialysis after vascular access intervention. J Vasc Surg 2019; 69: 174–180.e2. [DOI] [PubMed] [Google Scholar]
- 31. Morton SK, Rodriguez AJ, Morris DR, et al. A systematic review and meta-analysis of circulating biomarkers associated with failure of arteriovenous fistulae for haemodialysis. PLoS ONE 2016; 11(7): e0159963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Warme A, Hadimeri U, Hadimeri H, et al. High doses of erythropoietin stimulating agents may be a risk factor for AV-fistula stenosis. Clin Hemorheol Microcirc 2019; 71: 53–57. [DOI] [PubMed] [Google Scholar]
- 33. Irani FG, Teo TKB, Tay KH, et al. Hemodialysis arteriovenous fistula and graft stenoses: randomized trial comparing drug-eluting balloon angioplasty with conventional angioplasty. Radiology 2018; 289(1): 238–247. [DOI] [PubMed] [Google Scholar]
- 34. Ishii T, Suzuki Y, Nakayama T, et al. Duplex ultrasound for the prediction of vascular events associated with arteriovenous fistulas in hemodialysis patients. J Vasc Access 2016; 17(6): 499–505. [DOI] [PubMed] [Google Scholar]
- 35. Polkinghorne KR, Lau KK, Saunder A, et al. Does monthly native arteriovenous fistula blood-flow surveillance detect significant stenosis—a randomized controlled trial. Nephrol Dial Transplant 2006; 21(9): 2498–2506. [DOI] [PubMed] [Google Scholar]
- 36. Nassar GM, Beathard G, Rhee E, et al. Management of transposed arteriovenous fistula swing point stenosis at the basilic vein angle of transposition by stent grafts. J Vasc Access 2017; 18(6): 482–487. [DOI] [PubMed] [Google Scholar]
- 37. Sprouse LR, Lesar CJ, Meier GH, et al. Percutaneous treatment of symptomatic central venous stenosis [corrected]. J Vasc Surg 2004; 39(3): 578–582. [DOI] [PubMed] [Google Scholar]