Abstract
In routine upper and lower gastrointestinal endoscopy, overlooking neoplastic lesions is inevitable even for well-trained endoscopists. Various methods have been reported to improve the detection of gastrointestinal neoplasms including chromoendoscopy, special endoscopes, and processor and image enhanced technologies. Equipment-based image enhanced endoscopy (e-IEE) using narrow band imaging (NBI) and blue laser imaging (BLI) is useful to characterize known lesions with magnification at a close-up view. However, they are not useful for the early detection of superficial, pale neoplasms, or both because of the weak image at a distant view in a wide lumen such as the stomach or colon. Linked color imaging (LCI) is a novel pre- and post-processing technology developed by Fujifilm Corporation that has sufficient brightness to illuminate a wide lumen. LCI delineates early gastric cancers as orange–red and intestinal metaplasia as purple. LCI improves the adenoma detection rate in the colon and decreases the polyp miss rate. LCI contributes to the detection of superficial lesions throughout the gastrointestinal tract by enhancing the color contrast between the neoplasm and the surrounding mucosa. LCI can distinguish them by their specific color allocation based mainly on the distribution of capillaries. The authors believe that moving forward, LCI should be used in routine upper and lower gastrointestinal endoscopy.
Keywords: colon polyp, diagnosis, endoscopy, image enhanced endoscopy, linked color imaging
Introduction
Early endoscopic detection and resection of superficial gastrointestinal (GI) neoplasms is a mainstay of treatment and contributes significantly to patient’s quality of life. Routine esophagogastroduodenoscopy (EGD) and colonoscopy are the most useful strategies to detect early superficial neoplasms. Evolution of resection techniques including conventional snare polypectomy, endoscopic mucosal resection, and endoscopic submucosal dissection have extended the indications for endoscopic resection, largely obviating the need for surgical intervention in the 21st century. Therefore, the importance of early detection of superficial neoplasms continues to increase.
To improve endoscopic detection, dye-based image enhanced endoscopy (d-IEE) including chromoendoscopy using indigo carmine is useful because it enhances mucosal irregularities.1 However, it requires spraying indigo carmine through the working channel and it is not practical to spray the entire GI tract. Over the last two decades, the development and dissemination of equipment-based image enhanced endoscopy (e-IEE) modalities including narrow band imaging (NBI), blue laser imaging (BLI), and flexible spectral image color enhancement (FICE) have improved the rate of endoscopic diagnosis significantly. NBI or BLI with magnification are useful for detailed characterization of known lesions. These modalities are also useful for the detection of lesions in narrow areas of the GI tract including the esophagus. However, they are not suitable for detecting lesions in the stomach and colon because they do not have enough light intensity to illuminate a wide lumen. BLI-bright is an advanced version of standard BLI that provides brighter images and narrow band observation even in wide lumen organs including the stomach and colon. BLI-bright enhances mucosal vessels and structures more precisely than white light imaging (WLI) at a distant view. A Japanese prospective randomized study reported significantly higher real-time detection rates of early gastric cancer using BLI compared with WLI.2 Further comparative studies of BLI-bright versus other e-IEE are required to assess its role in detecting GI neoplasms. Unlike NBI, FICE has enough light intensity to illuminate a wide lumen. Therefore, it can be used for the detection of early neoplasms in some cases, but it does not show the details of mucosal structure and vessels sufficiently. FICE cannot distinguish neoplasms from intestinal metaplasia in the stomach at a distant view. There is insufficient evidence to support the use of e-IEE for the early detection of superficial neoplasms during routine endoscopic examinations. The evolution of e-IEE to improve the detection of superficial GI neoplasms at a distant view is of crucial importance to decrease the mortality from GI tract malignancies.
Characteristics of LCI
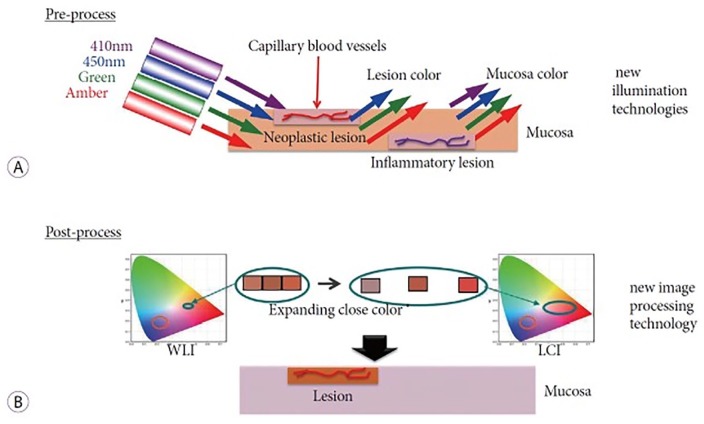
Novel laser imaging systems and multi-light technologies have been developed by Fujifilm Corporation (Tokyo, Japan). The laser endoscopic system (LASEREO) with BLI was available in Japan in 2011 and the system with LCI in 2014. The light-emitting diode endoscopic system (ELUXEO) was available in Europe in 2016. Unlike FICE, which only has post-processing, LCI uses both pre-processing narrow band radiation and post-processing color technology that separates imported colors into red, green, and blue. The separated colors are reallocated and adjusted to increase the observed color difference.3 The combination of pre- and post-processing technologies enhances color contrast between neoplastic lesions and the surrounding normal mucosa.4,5 Violet light at 410 nm wavelength can clearly elicit surface microstructures and microvasculature. In addition, LCI provides clear visualization of the vascular pattern and mucosal pits without magnification.
Unlike existing modalities that use NBI and BLI, where images are too dark in distant views to allow detection of superficial lesions, LCI provides sufficient light intensity with bright imaging even at a distant view using novel processing technology. On first impression, LCI produces images that are similar to WLI, while the color tones provided by NBI and BLI are quite different from those provided by WLI. The strong light intensity and enhanced red color provided by LCI clearly demonstrates the vascular patterns of the GI tract compared with WLI. For example, discontinuation of the vascular pattern visualized by LCI facilitates the recognition of pale serrated polyps.
LCI contributes to the improved diagnosis of various GI tract lesions that is difficult using WLI alone. LCI enables the differentiation of the colors of neoplastic lesions from inflammatory lesions by using differences in microvascular distribution. We can explain one of the possible phenomena as follows, neoplastic lesions have superficial dilated and concentrated capillaries on the mucosal surface, and inflammatory lesions have dilated capillaries in the deeper layer of the mucosa (Figure 1). In neoplastic lesions 410 nm violet light is likely to be absorbed by hemoglobin in the capillaries in the shallow layer, and then the violet light is not visible. Therefore, neoplastic lesions appear orange–red when using LCI. In inflammatory lesions, 410 nm violet light cannot reach the capillaries in the deeper layer and is reflected from the surface of the mucosa without absorption. Therefore, inflammatory lesions appear violet using LCI. Therefore, LCI can differentiate neoplastic lesions from inflammatory lesions even at a distant view. These color patterns could be influenced by other factors including the various types of glands that are present in the shallow layer. The color contrast enhancement and absorption of 410 nm violet light when using the LCI mode facilitates the early detection of superficial lesions in the GI tract. Unlike the cumbersome application of conventional chromoendoscopy that requires spraying dye through the working channel, the LCI mode is easily applied by the simple push of a button on the endoscope.
Figure 1.
(a) Pre-processing by linked color imaging (LCI) technology. In neoplastic lesions, the capillaries are located in the shallow layer. 410 nm violet light is absorbed by capillaries, therefore, the violet light is not visible and the neoplastic lesion appears red. In inflammatory lesions, capillaries are in the deep layer. 410 nm violet light is not absorbed, and the lesion appears violet. (b) Post-processing by LCI technology. The colors obtained are separated and reallocated for color enhancement. This makes red and white lesions become more red and more white, respectively.3
WLI: white light imaging.
LCI has three grades of color enhancement ranging from C1 to C3. Color enhancement stronger than C1 (e.g. C2–C3) when using LCI is helpful to detect neoplastic lesions. We often use the C3 mode. For structural enhancement, a strong level of B-mode (B7 or B8) is preferred to the A-mode.
Detection of early gastric cancer
Early gastric cancer behind chronic mucosal inflammation is frequently overlooked by routine observation using WLI alone. Unlike the esophagus, the stomach has a wide lumen that NBI and BLI cannot sufficiently illuminate. Therefore, these e-IEE modalities are not useful for routine EGD. LCI has sufficient light intensity to illuminate a wide lumen that facilitates the recognition of superficial neoplastic lesions in the stomach. Before the development of LCI, NBI and BLI were infrequently used for screening endoscopy of early gastric cancers because their weak light intensity produces dark images.
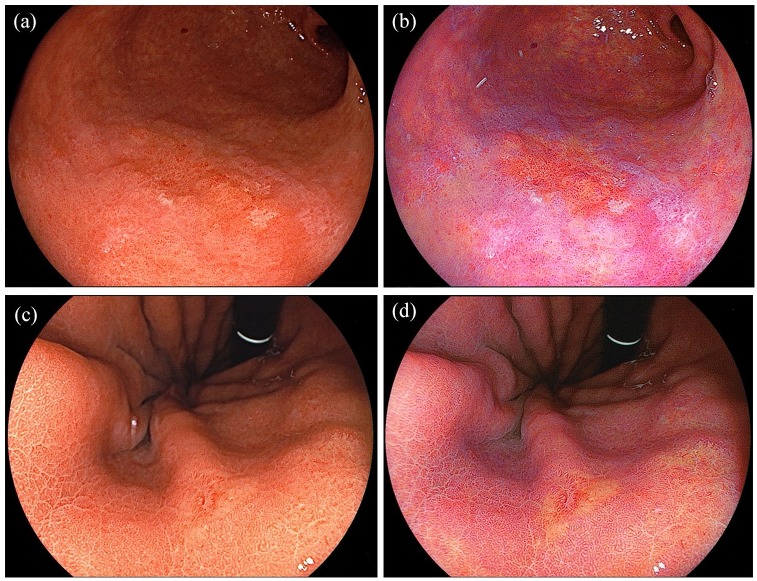
It is difficult to distinguish neoplastic lesions from inflammatory mucosa caused by chronic Helicobacter pylori infection even for well-trained endoscopists. In general, most gastric cancers are surrounded by intestinal metaplasia. The presence of widely spreading intestinal metaplasia makes the diagnosis of gastric cancer difficult because both gastric neoplasia and metaplasia have a similar appearance using WLI. Although the recent evolution of magnifying endoscopy has helped to alleviate this problem, gastroenterologists cannot always observe all multiple gastric lesions using magnification. As shown in Figure 2, early gastric cancer surrounded by intestinal metaplasia has an orange–red appearance surrounded by purple mucosa in LCI. As described previously, the microvasculature in the shallow layer of the mucosa in early gastric cancer can absorb violet light, and gastric cancer appears orange–red using LCI unlike inflammatory mucosa (Figure 2). In general, gastric cancer has an orange–red appearance using LCI.4 We first reported the superiority of LCI over WLI that shows a clear demarcation line between a flat gastric cancer (orange) and the surrounding intestinal metaplasia (purple) that was difficult to detect using WLI.6 A Japanese study reported that the color difference between gastric cancer and the surrounding mucosa using LCI was significantly greater than when using WLI. The color difference was more accentuated when a gastric cancer was surrounded by intestinal metaplasia.5,7 With regard to the comparison with other e-IEE modalities, improved visibility of early gastric cancer using LCI compared with BLI-bright regardless of the endoscopist’s experience was reported.8
Figure 2.
Detection of early gastric cancers. (a) A well-differentiated adenocarcinoma of the stomach imaged using white light imaging (WLI). (b) A clear line of demarcation is observed using linked color imaging (LCI). (c) An undifferentiated adenocarcinoma of the stomach using WLI. (d) LCI enhances the color contrast between the cancer and the surrounding normal mucosa.
This is applicable to well-differentiated adenocarcinomas rather than undifferentiated adenocarcinomas (Figure 2a and b). For undifferentiated adenocarcinoma, the capillary vessel concentration is not evident because of their depth beneath normal mucosa (Figure 2c and d). Although the color enhancement provided by LCI may be useful to detect undifferentiated adenocarcinoma by enhancing the pale white appearance of the lesion compared with the surrounding normal mucosa, further studies are necessary to clarify this concept.
Endoscopists, in general, detect early gastric cancer based on morphologic appearance, and LCI adds a new detection method, a so-called ‘color differentiation,’ to distinguish neoplastic lesions from inflammation. High color contrast and differences in violet light absorption between malignant lesions and the surrounding mucosa greatly facilitate the detection of early gastric cancers in atrophic gastritis with intestinal metaplasia.
Detection of early gastric cancer after successful H. pylori eradication
LCI is useful to estimate the current state of H. pylori infection by enhancing erythema in the fundic gland mucosa.9 The Japanese national health insurance system started reimbursement for H. pylori eradication therapy for patients with H. pylori-associated gastritis in 2013. While the prevention of gastric cancer by H. pylori eradication therapy was confirmed in a recent systematic review,10 gastric cancer occurs even after successful eradication. The incidence of gastric cancer following eradication was reported to be 1.1% at a median of 4.5 years, and all patients with new gastric cancers had severe atrophic gastritis.11 In comparison with gastric cancer before eradication, early gastric cancer after eradication has a gastritis-like appearance and is frequently covered with nonneoplastic epithelium making both malignant characteristics and the line of demarcation unclear.12 Therefore, endoscopists should carefully observe gastritis-like lesions using magnifying endoscopy. In practice, it is difficult to observe all gastritis-like lesions using magnifying endoscopy with e-IEE at screening EGD, which implies the need for novel e-IEE modalities to overcome this difficulty.
LCI can display sharp color contrasts. It has been reported that an early gastric cancer after eradication looks like orange–red mucosa surrounded by purple mucosa at a distant view.4 LCI does not require a dedicated endoscope. LCI may enable the operator to distinguish a neoplastic lesion from the surrounding mucosa even in mucosa following H. pylori eradication at a distant view without magnification.5 In such patients, it may be important to evaluate the whole areas of cancer using characteristic color (orange–red) and color contrast with the surrounding mucosa without magnification. Further studies are required to clarify the utility of LCI in patients with early gastric cancer who have undergone H. pylori eradication. Transnasal ultrathin endoscopy has been widely used in the 21st century for routine EGD in Japan, but it does not have a magnifying capability. However, LCI can be used in screening transnasal ultrathin endoscopy. When LCI is used for screening for gastric cancer, endoscopists should use it from the beginning of the observation and examine mucosa suspicious of being neoplastic.
Usefulness of LCI for the diagnosis of H. pylori gastritis and premalignant lesions
Gastric mucosal inflammation, severe atrophy, and intestinal metaplasia are closely associated with gastric cancer, and detection of these changes are important, especially after successful H. pylori eradication. Recently, the value of LCI for detecting these changes has been reported. LCI facilitates the recognition of mucosal changes after H. pylori eradication. A Japanese study reported the usefulness of LCI for detecting predictors of gastric cancer. First, map-like redness, a positive predictor of gastric cancer after successful H. pylori eradication, is more frequently found using LCI than when using WLI. Second, a regular arrangement of collecting venules, a negative predictor of gastric cancer, is more frequently identified using LCI than when using WLI.13 The usefulness of LCI to delineate the borders of gastric atrophic and nonatrophic mucosa by evaluating their color differences was reported, and the color difference at the atrophic border is significantly greater when using LCI than when using WLI.14 Regarding the detectability of intestinal metaplasia, that is considered as a risk factor for intestinal-type gastric cancer, a few prospective studies reported that gastric intestinal metaplasia was shown as purple (lavender color) using LCI and as a white flat/elevated lesion using WLI. The diagnostic accuracies of biopsy was increased when using LCI compared with using WLI.5,15 Therefore, LCI is useful to detect premalignant lesion of the stomach.
Artificial intelligence technology in combination with LCI
Artificial intelligence (AI) technology in combination with e-IEE in routine EGD is expected to assist in detecting the presence of H. pylori infection and in the early detection of gastric neoplasia. AI deep learning is a standard method for creating a neural network and can generate a new classification automatically after training with known lesions. A Japanese study demonstrated the excellent ability of AI in combination with LCI or BLI-bright to diagnose H. pylori infection status.16 Another Japanese study reported the ability of AI in combination with LCI to establish the diagnosis of existing H. pylori infections better than inexperienced endoscopists. However, there was some difficulty in diagnosing post-eradication status using AI.17 Further improvements and evolution are necessary to effectively utilize AI for the diagnosis of H. pylori infection status and the detection of gastric lesions.
Early detection of colorectal neoplasms
The adenoma to adenocarcinoma sequence is the main pathway for the formation of colorectal cancer. The removal of colorectal polyps decreases colorectal cancer-related mortality.18 Routine total colonoscopy is the best method for early detection and resection. For colorectal polyps <10 mm, cold snare polypectomy (snare polypectomy without electrocauterization) reduces procedure time, medical costs, and the rate of adverse events more than conventional snare polypectomy with electrocauterization.19 The extended cold snare polypectomy technique (resection with >1 mm surrounding mucosa) improves the rate of R0 resections without increasing the rate of delayed bleeding.20 Cold snare polypectomy for sub-centimeter colorectal polyps is safely performed even in patients taking oral anticoagulants.21 Therefore, polypectomy for polyps <10 mm has become easier for colonoscopists to perform. However, the miss rate of small polyps remains a serious concern for colonoscopists.
The adenoma detection rate (ADR) is a reliable quality indicator for screening colonoscopy and is a good predictor of developing interval cancer.22 An increase in ADR of 1% is associated with a 3% decrease in the rate of interval cancer.23 In addition, the ADR is associated with the experience of the endoscopist.24 Improvement of the ADR may result in improved long-term survival by facilitating high-quality colonoscopy. Colonoscopists recognize that colorectal polyps can be frequently missed, especially flat, small, faded polyps, or both. A systematic review of six studies reported that adenoma miss rates by size were 2.1% (⩾10 mm), 13% (5–10 mm) and 26% (1–5 mm), respectively.25 The miss rate for colorectal polyps <10 mm is remarkably higher than that for polyps ⩾10 mm.
Colonoscopists have struggled to improve the ADR using various special devices, colonoscopy techniques, or both. The EndoCuff (Arc Medical Design Ltd., Leeds, United Kingdom) is a disposable attachment for the tip of the endoscope that has finger-like thorns and enables the operator to visualize small polyps behind the haustra. EndoCuff-assisted colonoscopy improved the ADR in both the left and right sides of the colon in a randomized crossover study. Of note, it is more effective for the detection of adenomas <5 mm.26 A full-spectrum endoscopy with a 330° angle of view using three side-by-side displays increased the polyp detection rate and decreased the polyp miss rate compared with standard forward-viewing endoscopy.27 The Third Eye Retroscope (Avantis Medical Systems, CA, USA) improved adenoma detection compared with a standard forward-viewing colonoscope.28 However, it requires expensive dedicated endoscopes and systems.
Autofluorescence imaging (AFI) was developed by Olympus Corporation (Tokyo, Japan) and is expected to improve the polyp detection rate. A recent systematic review reported that AFI improved the adenoma and polyp miss rate in routine colonoscopy compared with WLI, although it did not increase the overall ADR.29 A randomized controlled trial (RCT) from Japan reported the superiority of AFI for the detection of flat colonic lesions over WLI.30 It requires a dedicated endoscope.
In the 21st century, e-IEE including NBI, BLI, and FICE are expected to improve the detection of colorectal polyps. A recent systematic review of NBI that included 2936 patients demonstrated no significant difference in the overall ADR or the number of adenomas per patient comparing NBI and WLI.31 FICE is a post-processing technology developed by Fujifilm Corporation (Tokyo, Japan), and does not require a special endoscope. An RCT did not show a difference in overall ADR with a similar withdrawal time comparing patients evaluated with FICE and WLI.32 Existing e-IEE modalities do not have enough power to illuminate a wide colonic lumen. They are not useful for early detection at a distant view but are useful for characterizing known lesions with magnification at a close-up view. Therefore, a new generation of e-IEE technology and processing that improves colorectal polyp detection is required.
Utility of LCI for detection of colorectal polyps
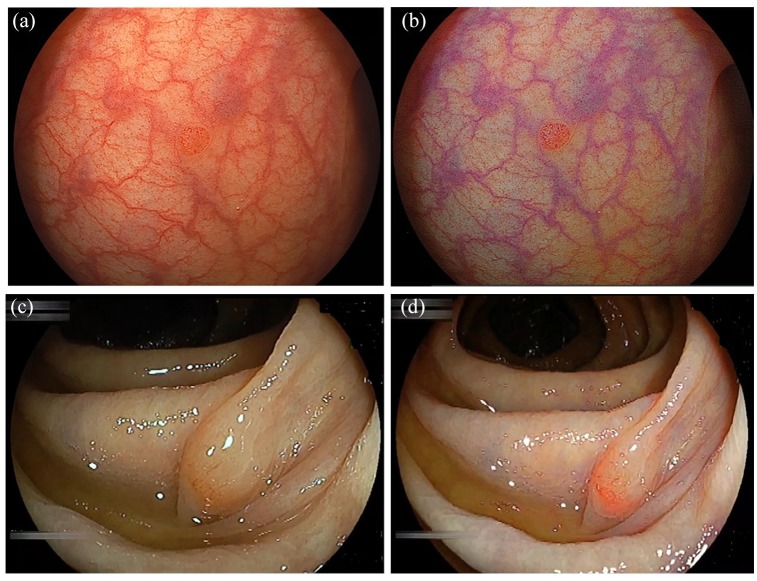
The brightness and color enhancement provided by LCI improves the detection of superficial colorectal neoplasms (Figure 3). The usefulness of LCI for polyp detection has been reported. A multicenter RCT reported that LCI significantly improved the overall polyp detection rate compared with WLI with equal withdrawal time.33 In addition, LCI demonstrated superiority in detecting sessile serrated adenoma/polyps (SSA/P). Fujimoto and colleagues reported a higher detection rate of SSA/P using LCI compared with WLI using still colonoscopic images.34 Therefore, LCI is useful for colorectal polyp detection. When LCI is used for detection, it should be used from the start of the colonoscopic observation.
Figure 3.
Detection of colorectal neoplastic lesions. (a) A small polyp in the sigmoid colon seen using white light imaging (WLI). (b) Linked color imaging (LCI) provides clear visualization of the small polyp by enhancing the color contrast. (c) A sessile serrated adenoma/polyp (SSA/P) of the ascending colon seen using WLI. (d) LCI demonstrates a clear line of demarcation by enhancing the color contrast.
Decreasing the polyp miss rate using LCI
LCI decreases the miss rate of polyps in the right colon. To evaluate the miss rate, a prospective RCT with tandem colonoscopy is considered the gold standard. Patients were randomly allocated to WLI–LCI (WLI first) and LCI–WLI (LCI first) groups, and then additional polyps detected during the second observation were compared. An RCT from Italy reported that the adenoma miss rate of LCI–WLI was significantly lower than WLI–LCI in the right colon.35 An RCT from Japan reported that the additional polyp detection rate of SSA/P with WLI–LCI was significantly higher than with LCI–WLI.34 A retrospective study with a video eye-tracking system reported that LCI has a significantly lower rate of polyp miss rate and shorter detection time than WLI.36
Color differences between neoplasms and normal mucosa
LCI enables the operator to distinguish superficial colorectal lesions from surrounding normal mucosa due to enhanced color contrast, even in faded lesions. We first reported a clearer line of delineation in laterally spreading tumors with LCI compared with WLI.37 Suzuki and colleagues reported higher visibility scores using LCI compared with WLI in laterally spreading nongranular tumors.38 Yoshida and colleagues reported improved polyp visibility scores and color differences for 2–20 mm colorectal polyps using LCI compared with WLI using still colonoscopic images.39 A recent study from Japan documented a significantly higher color difference using LCI compared with WLI when imaging SSA/P and surrounding colonic mucosa.34 LCI demonstrated significant superiority in both polyp visibility scores and color differences compared with WLI. Flat type colorectal polyps are more likely to be overlooked than nonflat type polyps.33 We believe that LCI may provide noticeable effects for the detection of flat, or faded polyps, or both.
We suggest three reasons for improved detection of colorectal polyps using LCI compared with WLI or other e-IEE modalities. First, LCI provides a clear line of demarcation between the neoplastic lesion and surrounding mucosa due to color enhancement with a bright distant view. Second, discontinuation of the vascular pattern is easily recognized by LCI because the red vascular pattern is strongly enhanced by LCI, especially for the detection of SSA/P. Third, with existing e-IEE technologies including NBI and BLI, residual colonic fluid appears red which prevents detection of small or flat lesions at the bottom of the fluid. With LCI, any residual fluid remains yellow, which is similar to WLI. Therefore, colonoscopists can detect small polyps through the yellow fluid using LCI.
Usefulness of LCI in patients with ulcerative colitis
Mucosal healing and early detection of malignancies is important to control and improve the long-term outcomes of patients with ulcerative colitis. The degree of mucosal inflammation is a good predictor of long-term remission and a surgery-free period.40 A recent study from Japan reported that colonic mucosal erythema visualized by LCI correlates with the histological inflammation of ulcerative colitis, and the LCI classification of mucosal damage can predict long-term nonrelapse rates.41 The color enhancement of LCI may be useful to evaluate mucosal inflammation and detection of neoplastic lesions surrounded by inflammatory GI mucosa. Further studies are necessary to confirm the usefulness of LCI for the early detection of neoplastic lesions in patients with inflammatory bowel disease.
Conclusion
LCI provides significant color differentiation between neoplastic and inflammatory lesions in addition to the pre-existing morphological diagnosis. The histological difference in the shallow layer of the mucosa, including the depth and density of capillaries and changes in the glands, may be a key factor in this color differentiation. Enhanced color contrast contributes significantly to the ability to distinguish neoplastic lesions from nonneoplastic mucosa. However, we need to be cautious because LCI can only improve the detection of lesions in visualized GI mucosa. LCI cannot improve the detection of lesions located in blind spots including behind folds or areas with acute bends. Because LCI cannot resolve the miss rate of all GI lesions, basic endoscopic training and careful observation taking adequate time are still quite important. Despite a lack of rigorous evidence, the usefulness of LCI for the detection of GI neoplasms at a distant view is obvious. The authors recommend the use of LCI from the start of routine EGD and colonoscopy.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: H.Y. has a consultant relationship with Fujifilm Corporation and has received honoraria, grants, and royalties from the company. H.O. and Y.H. have received honoraria from Fujifilm Corporation. The other authors have no financial conflicts of interest.
ORCID iD: Hironori Yamamoto  https://orcid.org/0000-0002-3601-1153
https://orcid.org/0000-0002-3601-1153
Contributor Information
Satoshi Shinozaki, Department of Medicine, Division of Gastroenterology, Jichi Medical University, Shimotsuke, Japan; Shinozaki Medical Clinic, Utsunomiya, Japan.
Hiroyuki Osawa, Department of Medicine, Division of Gastroenterology, Jichi Medical University, Shimotsuke, Japan.
Yoshikazu Hayashi, Department of Medicine, Division of Gastroenterology, Jichi Medical University, Shimotsuke, Japan.
Alan Kawarai Lefor, Department of Surgery, Jichi Medical University, Shimotsuke, Japan.
Hironori Yamamoto, Department of Medicine, Division of Gastroenterology, Jichi Medical University, 3311-1 Yakushiji, Shimotsuke, Tochigi 329-0498, Japan.
References
- 1. Kaltenbach T, Sano Y, Friedland S, et al. American gastroenterological association (AGA) institute technology assessment on image enhanced endoscopy. Gastroenterology 2008; 134: 327–340. [DOI] [PubMed] [Google Scholar]
- 2. Dohi O, Yagi N, Naito Y, et al. Blue laser imaging-bright improves the real-time detection rate of early gastric cancer: a randomized controlled study. Gastrointest Endosc 2019; 89: 47–57. [DOI] [PubMed] [Google Scholar]
- 3. Yamamoto H, Shinozaki S, Hayashi Y, et al. Advanced treatment and imaging in colonoscopy: the pocket-creation method for complete resection and linked color imaging for better detection of early neoplastic lesions by colonoscopy. Clin Endosc 2019; 52: 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Osawa H, Miura Y, Takezawa T, et al. Linked color imaging and blue laser imaging for upper gastrointestinal screening. Clin Endosc 2018; 51: 513–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fukuda H, Miura Y, Osawa H, et al. Linked color imaging can enhance recognition of early gastric cancer by high color contrast to surrounding gastric intestinal metaplasia. J Gastroenterol 2019; 54: 396–406. [DOI] [PubMed] [Google Scholar]
- 6. Fukuda H, Miura Y, Hayashi Y, et al. Linked color imaging technology facilitates early detection of flat gastric cancers. Clin J Gastroenterol 2015; 8: 385–389. [DOI] [PubMed] [Google Scholar]
- 7. Kanzaki H, Takenaka R, Kawahara Y, et al. Linked color imaging (LCI), a novel image-enhanced endoscopy technology, emphasizes the color of early gastric cancer. Endosc Int Open 2017; 5: E1005–E1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yoshifuku Y, Sanomura Y, Oka S, et al. Evaluation of the visibility of early gastric cancer using linked color imaging and blue laser imaging. BMC Gastroenterol 2017; 17: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dohi O, Yagi N, Onozawa Y, et al. Linked color imaging improves endoscopic diagnosis of active Helicobacter pylori infection. Endosc Int Open 2016; 4: E800–E805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sugano K. Effect of Helicobacter pylori eradication on the incidence of gastric cancer: a systematic review and meta-analysis. Gastric Cancer 2018; 22: 435–445. [DOI] [PubMed] [Google Scholar]
- 11. Kamada T, Hata J, Sugiu K, et al. Clinical features of gastric cancer discovered after successful eradication of Helicobacter pylori: results from a 9-year prospective follow-up study in Japan. Aliment Pharmacol Ther 2005; 21: 1121–1126. [DOI] [PubMed] [Google Scholar]
- 12. Saka A, Yagi K, Nimura S. Endoscopic and histological features of gastric cancers after successful Helicobacter pylori eradication therapy. Gastric Cancer 2016; 19: 524–530. [DOI] [PubMed] [Google Scholar]
- 13. Majima A, Dohi O, Takayama S, et al. Linked color imaging identifies important risk factors associated with gastric cancer after successful eradication of Helicobacter pylori. Gastrointest Endosc. Epub ahead of print 9 July 2019. DOI: 10.1016/j.gie.2019.06.043. [DOI] [PubMed] [Google Scholar]
- 14. Mizukami K, Ogawa R, Okamoto K, et al. Objective endoscopic analysis with linked color imaging regarding gastric mucosal atrophy: a pilot study. Gastroenterol Res Pract 2017; 2017: 5054237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ono S, Kato M, Tsuda M, et al. Lavender color in linked color imaging enables noninvasive detection of gastric intestinal metaplasia. Digestion 2018; 98: 222–230. [DOI] [PubMed] [Google Scholar]
- 16. Nakashima H, Kawahira H, Kawachi H, et al. Artificial intelligence diagnosis of Helicobacter pylori infection using blue laser imaging-bright and linked color imaging: a single-center prospective study. Ann Gastroenterol 2018; 31: 462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yasuda T, Hiroyasu T, Hiwa S, et al. Potential of automatic diagnosis system with linked color imaging for diagnosis of Helicobacter pylori infection. Dig Endosc. Epub ahead of print 9 August 2019. DOI: 10.1111/den.13509. [DOI] [PubMed] [Google Scholar]
- 18. Zauber AG, Winawer SJ, O’brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012; 366: 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shinozaki S, Kobayashi Y, Hayashi Y, et al. Efficacy and safety of cold versus hot snare polypectomy for resecting small colorectal polyps: systematic review and meta-analysis. Dig Endosc 2018; 30: 592–599. [DOI] [PubMed] [Google Scholar]
- 20. Abe Y, Nabeta H, Koyanagi R, et al. Extended cold snare polypectomy for small colorectal polyps increases the R0 resection rate. Endosc Int Open 2018; 6: E254–E258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takeuchi Y, Mabe K, Shimodate Y, et al. Continuous anticoagulation and cold snare polypectomy versus heparin bridging and hot snare polypectomy in patients on anticoagulants with subcentimeter polyps: a randomized controlled trial. Ann Intern Med. Epub ahead of print 16 July 2019. DOI: 10.7326/M19-0026. [DOI] [PubMed] [Google Scholar]
- 22. Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med 2010; 362: 1795–1803. [DOI] [PubMed] [Google Scholar]
- 23. Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014; 370: 1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bressler B, Paszat LF, Chen Z, et al. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology 2007; 132: 96–102. [DOI] [PubMed] [Google Scholar]
- 25. Van Rijn JC, Reitsma JB, Stoker J, et al. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol 2006; 101: 343–350. [DOI] [PubMed] [Google Scholar]
- 26. De Palma GD, Giglio MC, Bruzzese D, et al. Cap cuff-assisted colonoscopy versus standard colonoscopy for adenoma detection: a randomized back-to-back study. Gastrointest Endosc 2018; 87: 232–240. [DOI] [PubMed] [Google Scholar]
- 27. Gralnek IM, Siersema PD, Halpern Z, et al. Standard forward-viewing colonoscopy versus full-spectrum endoscopy: an international, multicentre, randomised, tandem colonoscopy trial. Lancet Oncol 2014; 15: 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Demarco DC, Odstrcil E, Lara LF, et al. Impact of experience with a retrograde-viewing device on adenoma detection rates and withdrawal times during colonoscopy: the third eye retroscope study group. Gastrointest Endosc 2010; 71: 542–550. [DOI] [PubMed] [Google Scholar]
- 29. Zhao ZY, Guan YG, Li BR, et al. Detection and miss rates of autofluorescence imaging of adenomatous and polypoid lesions during colonoscopy: a systematic review and meta-analysis. Endosc Int Open 2015; 3: E226–E235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takeuchi Y, Sawaya M, Oka S, et al. Efficacy of autofluorescence imaging for flat neoplasm detection: a multicenter randomized controlled trial (a-Flat Trial). Gastrointest Endosc 2019; 89: 460–469. [DOI] [PubMed] [Google Scholar]
- 31. Dinesen L, Chua TJ, Kaffes AJ. Meta-analysis of narrow-band imaging versus conventional colonoscopy for adenoma detection. Gastrointest Endosc 2012; 75: 604–611. [DOI] [PubMed] [Google Scholar]
- 32. Aminalai A, Rosch T, Aschenbeck J, et al. Live image processing does not increase adenoma detection rate during colonoscopy: a randomized comparison between fice and conventional imaging (Berlin Colonoscopy Project 5, Becop-5). Am J Gastroenterol 2010; 105: 2383–2388. [DOI] [PubMed] [Google Scholar]
- 33. Min M, Deng P, Zhang W, et al. Comparison of linked color imaging and white-light colonoscopy for detection of colorectal polyps: a multicenter, randomized, crossover trial. Gastrointest Endosc 2017; 86: 724–730. [DOI] [PubMed] [Google Scholar]
- 34. Fujimoto D, Muguruma N, Okamoto K, et al. Linked color imaging enhances endoscopic detection of sessile serrated adenoma/polyps. Endosc Int Open 2018; 6: E322–E334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paggi S, Mogavero G, Amato A, et al. Linked color imaging reduces the miss rate of neoplastic lesions in the right colon: a randomized tandem colonoscopy study. Endoscopy 2018; 50: 396–402. [DOI] [PubMed] [Google Scholar]
- 36. Kumahara K, Ikematsu H, Shinmura K, et al. Objective evaluation of the visibility of colorectal lesions using eye tracking. Dig Endosc 2019; 31: 552–557. [DOI] [PubMed] [Google Scholar]
- 37. Okada M, Sakamoto H, Takezawa T, et al. Laterally spreading tumor of the rectum delineated with linked color imaging technology. Clin Endosc 2016; 49: 207–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Suzuki T, Hara T, Kitagawa Y, et al. Linked-color imaging improves endoscopic visibility of colorectal nongranular flat lesions. Gastrointest Endosc 2017; 86: 692–697. [DOI] [PubMed] [Google Scholar]
- 39. Yoshida N, Naito Y, Yasuda R, et al. Linked color imaging improves the visibility of various featured colorectal polyps in an endoscopist’s visibility and color difference value. Int J Colorectal Dis 2017; 32: 1253–1260. [DOI] [PubMed] [Google Scholar]
- 40. Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut 2012; 61: 1619–1635. [DOI] [PubMed] [Google Scholar]
- 41. Uchiyama K, Takagi T, Kashiwagi S, et al. Assessment of endoscopic mucosal healing of ulcerative colitis using linked colour imaging, a novel endoscopic enhancement system. J Crohns Colitis 2017; 11: 963–969. [DOI] [PubMed] [Google Scholar]