Abstract
Despite its commonality in routine clinical practice, the approach to a diagnosis of atherosclerotic coronary artery disease remains complex and, in part, contentious. The traditional dogma linking ischaemia to hard clinical outcomes has been questioned and reframed over the years; rather than being a predictor of hard clinical outcomes, the degree of ischaemia may simply be a marker of atherosclerotic disease burden. A renewed interest in the imaging of plaque burden has spawned the contemporary role of CT imaging for not only diagnosis and prognosis, but also for dictating downstream management. As the technology develops and evidence expands, decisions on investigative modalities remain centred around patient factors, local availability, test performance and cost. This review summarizes the available methods for diagnosis in the symptomatic patient and provides an overview of the current evidence behind functional and anatomical approaches.
Keywords: coronary CT angiography, coronary angiography, coronary artery disease, myocardial ischaemia, stress testing
Introduction
Guidance on the diagnostic approach to coronary artery disease (CAD) has diverged as the increasing complexity of atherosclerotic clinicopathologic correlations has been revealed. Foundational concepts linking stenosis, the ischaemic cascade and prognosis have been re-evaluated in light of the underwhelming results from the percutaneous revascularization of stenotic vessels. Instead, observations from noninvasive anatomical imaging have redefined risk, shifting the focus away from discrete lesions towards total atherosclerotic burden, and with it elevating the role of computed tomography (CT) in contemporary diagnostic pathways. While guidelines attempt to keep pace with the advancing technology and expanding literature, clinicians are left to apply clinical acumen to decide on a vast (and increasing) array of investigative options. This review reiterates our understanding and limitations of the current approach to the diagnosis of CAD, provides a precis of the imaging techniques available and summarizes the current literature for both functional and anatomical approaches.
Scope of the problem
Stable chest pain is an extremely common reason for seeking care, with 1 in 10 persons over the age of 80 requiring evaluation in primary care or hospital for this symptom alone.1 Paradoxically, despite its commonality, a great deal of uncertainty persists about how to approach a diagnosis: who should be investigated, what test should be ordered and when should it be performed? The uncertainty is partially attributable to iterative imaging technology and varying levels of available evidence; however, there are also significant individual and population interests that add complexity. At an individual patient (and clinician) level, there is a desire to investigate thoroughly (and potentially defensively), to provide diagnostic clarity for both parties and to mitigate risk from future events. In comparison, with almost four million stress tests ordered annually in the USA,2 there is a population health need to evaluate patients in an efficient, evidence-based and outcome-driven manner. Achieving the balance between these two, at times competing, perspectives is but one challenge facing a diagnostic strategy for CAD.
Two other humbling statistics underline the difficulty of achieving an accurate diagnostic pathway for chest pain and suspected CAD. First, up to one-third of individuals ultimately referred for ‘gold standard’ elective coronary angiography consistently have little to no angiographic evidence of CAD, suggesting that the specificity of our current gate-keeping for invasive testing needs refinement.3 Second, of those provided a diagnosis of ‘noncardiac chest pain’ through any investigative means, up to one-third will still sustain an acute coronary syndrome or succumb to a cardiovascular death,4 highlighting the fallibility of our approach to date. Despite chest pain and CAD diagnosis being foundational components of clinical medicine and cardiology, these observations are a reminder of the contemporary challenges faced by clinicians. Finding the balance between diagnostic clarity and good clinical acumen, as well as managing patient- and system-level expectations, remain ongoing challenges in the diagnosis of CAD.
Pathophysiology of CAD: an evolution
The term coronary artery disease is used to describe the presence of atherosclerotic plaque within the epicardial coronary arteries. A fundamentally similar process occurs in other medium to large arteries such as the aorta, iliofemorals and carotids, and is now acknowledged to have an incontrovertible inflammatory basis.5 The earliest steps in atherogenesis appear to arise from the complex and dynamic interaction between an individual’s genetic susceptibility and exposure to environmental (or risk) factors. Endothelial dysfunction, one of the earliest detectable changes and a precursor to atherosclerosis, is a key initiator in permitting the passage of lipid material into the subintimal space, where a potent inflammatory response is mediated through macrophages, among other cells. Despite its reparative intent, what follows is a chronic, progressive accumulation of cell debris and cholesterol crystals which perpetuate inflammation and ultimately result in the development and progression of plaque. Environmental factors that have classically been associated with the development of atherosclerosis include advancing age, hyperlipidaemia, diabetes, hypertension and cigarette smoking. More recently, chronic kidney disease and inflammatory conditions, such as Crohn’s disease and rheumatoid arthritis, have been increasingly recognized as potent risk factors for atherogenesis.
Two fundamentally different clinicopathological entities occur in the context of coronary atherosclerosis: (stable) angina and acute coronary syndrome. Traditionally, the pathophysiologic basis for stable angina arises from the impediment to flow generated through the chronic, progressive growth of plaque into a vessel’s lumen. In the setting of increased myocardial oxygen demand such as occurs during exercise, the stenotic narrowing precludes a commensurate augmentation in flow, resulting in the subtended tissue becoming ischaemic; with rest, the myocardial oxygen demand reduces and the ischaemia abates. This syndrome of reproducible, exercise-induced pain (and sometimes shortness of breath, termed an angina ‘equivalent’) is called ‘stable angina’ and more broadly ‘stable ischaemic heart disease’. This process occurs in contrast to the development of rapidly progressive or rest symptoms, which suggest the development of local thrombosis and a consequent sudden, aggressive reduction in lumen calibre. Our current paradigm suggests a thrombus forms when the luminal side of an atherosclerotic plaque ruptures or erodes and exposes thrombogenic material in plaque to blood. Whether the thrombosis and secondary vasospasm completely occlude the vessel, and for how long, ultimately determines whether this event results in ‘unstable angina’ (i.e. incomplete occlusion with ischaemia but no cell death), or ‘myocardial infarction’ (i.e. sustained occlusion with cell death). The steps that lead a plaque to become ‘unstable’ through the erosion, fissure or rupture of its overlying cap remain incompletely understood and are covered in detail elsewhere.6
The term coronary artery disease has become synonymous with the atherosclerotic processes detailed above; however, there are a number of other diseases that affect the coronary arteries, including microvascular dysfunction, spontaneous coronary dissection and spasm. Although detailed descriptions of these clinical entities are critical to any discussion around the evaluation of chest pain,7 this review has remained focused on the diagnosis of patients with suspected atherosclerotic CAD.
Models of diagnosis
Despite the divergence in recommendations across medical societies’ guidelines,8–10 the broad aims of the diagnostic process are generally consistent: first, confirmation of the causality for symptoms; and second, risk stratification and prognostication, with a particular emphasis on the need for, or timing of, revascularization. With this in mind, there are two complementary but distinct approaches to achieving these aims, ischaemia and functional testing, compared with plaque and anatomical evaluation.
Stenosis: ischaemia focus
Our foundational understanding of CAD arose from seminal work by Gould in the 1970s.11 Elegant studies in dogs demonstrated a 50% obstruction of vessel diameter to be the threshold for impeding coronary flow reserve – that is, the inability to further augment blood flow in response to an increase in demand. Later shown in humans using nuclear imaging techniques,12 this construct linking ischaemia to a 50% narrowing in an epicardial coronary artery became the perhaps oversimplified, dichotomous definition of significant ‘coronary artery disease’. What followed was a series of occlusion studies that linked the temporal sequence of subclinical to clinical changes associated with ischaemia: the so-called ‘ischaemic cascade’.13 Typically (but not always) the cascade was described in a stepwise manner commencing with hypoperfusion, metabolic abnormalities, diastolic then systolic dysfunction, electrocardiographic abnormalities and eventually symptoms (angina or an equivalent).14
It is upon these principles that the premise for stress tests of inducible ischaemia were derived. Framing them within this ischaemic cascade provides general insight into their relative performance; stress perfusion imaging tests have high sensitivity, given their detection of early stage ischaemia, whereas the presence of wall motion abnormalities is likely to detect more sustained ischaemia and thus generally confer greater specificity. Despite the original aim being to evaluate the downstream effects of an epicardial stenosis, the additional insight afforded by ischaemia testing is the ‘whole of vessel’ function assessment. While this is somewhat beyond the scope of this review, there is increasing recognition of the disconnect between epicardial disease, symptoms and ischaemia – that is to say, patients with demonstrable ischaemia can have minimal or no symptoms, and patients without epicardial disease can have anginal symptoms. Although the former continues to provide management uncertainty for clinical cardiology, functional imaging may have a significant role in not only providing a diagnosis for the latter, but also to direct management decisions.
Anatomic: plaque focus
CAD is defined by the presence of atherosclerotic plaque in the epicardial vessel, and thus has an intrinsic anatomic basis. It is for this reason that invasive coronary angiography (ICA) has remained the gold standard upon which other diagnostic tests are measured. As mentioned earlier, it has been the historical finding of a 50% (visual estimate) or 70% (by quantitative analysis) stenosis that confirms a diagnosis of CAD; however, this is inferred to be significant CAD. It has become somewhat circular that the performance of noninvasive diagnostic tests has been traditionally compared to the anatomic gold standard, which itself has been benchmarked against the observation of functional consequence. Moreover, the most recent data (covered later) suggest that rather than an arbitrary stenosis threshold, it is the number and severity of stenoses which are more important than consequent ischaemia per se. Adding to the complexity is that these percentage stenosis estimates in clinical practice (compared to their initial derivation in animal studies) only variably correspond to direct invasive measures of haemodynamic significance.15
While classical invasive angiography provides a ‘lumenogram’ (and thus an indirect, complementary view of atherosclerosis), the use of intravascular imaging has generated insight into not only plaque burden16 but more recently plaque composition.17,18 This detail has provided a unique and transformative endpoint for clinical research,19 yet its invasive nature prohibits a broader diagnostic application and instead has spawned growth in noninvasive alternatives. Correspondingly, in the last 15 years the anatomical approach to CAD diagnosis has been completely reshaped by iterative developments in CT. Coronary artery calcium (CAC) scoring has been elevated to a critical part of the American College of Cardiology (ACC) prevention guidelines through its coarse assessment of plaque burden and incremental capacity for risk stratification.20 Beyond CAC, improvements in spatial and temporal resolution, mitigation of both calcium blooming and movement artefacts, as well as a reduction in radiation dose, have promoted coronary CT angiography (CCTA) to an equal footing with stress testing for the evaluation of stable chest pain. Indeed, the most recent update to NICE (National Institute for Clinical Excellence) guidance recommends the use of CCTA upfront in patients with suspected angina.21
Diagnosis in the symptomatic patient
The symptomatic patient can be dichotomized into stable and unstable presentations. A patient presenting with crescendo pattern (increasing severity and/or with reducing exercise threshold) or rest symptoms should be referred to an emergency department for urgent evaluation of potential acute coronary syndrome. In contrast, the patient presenting with intermittent chest pain symptoms that are consistent with stable angina can undergo prompt outpatient evaluation. After performing a detailed history and physical examination, a baseline electrocardiogram (ECG) should be performed. In the absence of changes that indicate ischaemia or a prior infarct, an assessment of pre-test probability is the generally accepted next step.
Pre-test probability
Both the US and European guidelines recommend the concept of Bayesian probability when evaluating patients for symptomatic CAD: an initial assessment, or ‘pre-test’ probability, is estimated and then with the results of the test, a post-test probability of CAD is determined. Multiple methods have been described to assess pre-test probability;22–24 however, both the US and European guidelines utilize age, sex and chest pain ‘typicality’ (angina, atypical angina, non-anginal) to assign low-, intermediate- and high-risk diagnostic strata. Typical angina is characterized by all three of the following characteristics: retrosternal discomfort precipitated by physical exertion or stress; relief with rest or nitroglycerine in less than 10 min; and accompanied by radiation of the discomfort to the shoulder, jaw or the inner aspect of the arm. Atypical angina meets two of three criteria and non-anginal pain meets only one (or less).
Both US and ESC (European Society of Cardiology) guidelines note that noninvasive testing is of greatest benefit in those deemed at ‘intermediate’ likelihood of CAD, which, although somewhat arbitrary, has been defined in the US guidelines as those with 10–90% likelihood, and in the ESC guideline as those with 15–85% likelihood. These percentage estimates of likelihood were derived by Diamond and Forrester from seminal work that detailed age, sex and symptom correlates with the presence of angiographic CAD.23 Notably, the NICE guidance in 2016 moved away from a formal assessment of pre-test probability (see Table 1) and instead has been simplified to consider CCTA as the first-line approach in those with either typical or atypical symptoms (defined as above) or in the presence of non-anginal symptoms with an ECG that shows ST–T changes or Q waves. Although most studies have found an assessment of pre-test probability overestimates the burden of obstructive disease,25 this may be less pronounced with the NICE symptom focus than with the Bayesian approach.26 In those with low pre-test probability, alternative explanations for symptoms should be considered (e.g. gastroesophageal reflux, musculoskeletal); however, further investigation could be considered for risk stratification. Patients deemed of ‘high’ pre-test probability can be treated empirically for obstructive CAD and may proceed on to upfront angiography to facilitate revascularization in the event of refractory or progressive symptoms.
Table 1.
Society guidelines for diagnostic work up of coronary artery disease.
| ACC/AHA 20129 |
ESC 201310 |
NICE 20168 |
|||
|---|---|---|---|---|---|
| PTP | Test | PTP | Test | PTP | Test |
| <10% | Exercise ECG Exercise nuclear Stress echocardiography |
15–50% | CCTA | N/A | CCTA . . .then functional imaging if equivocal. . . .or ICA if functional imaging inconclusive |
| 10–90% | Exercise ECG Stress nuclear/echo/CMR CCTA |
15–65% | Exercise ECG Stress nuclear/echo/CMR |
||
| >90% | Stress nuclear/echo/CMR CCTA |
66–85% | Stress nuclear/echo/CMR | ||
Comparison of ACC/AHA 2012, ESC 2013 and NICE 2016 guidelines for selection of diagnostic modalities during work up of coronary artery disease.
ACC, American College of Cardiology; CCTA, coronary CT angiography; CMR, cardiac magnetic resonance; ECG, electrocardiogram; ESC, European Society of Cardiology; ICA, invasive coronary angiography; NICE, National Institute for Clinical Excellence; PTP, pre-test probability.
Diagnostic modalities
After making the decision to order a diagnostic test, there are a number of factors that are to be considered when choosing a modality. These include patient-level characteristics, availability, cost and local expertise. In order to provide background to broader commentary about each of the different approaches, a precis of the most widely used modalities is presented here. Figure 1 provides some representative images of nuclear perfusion, cardiac magnetic resonance (CMR) and CCTA, and Table 2 provides a summary of the strengths and limitations of each as well as their respective diagnostic performance. Performance measures are presented as estimates of sensitivity and specificity; however, these vary substantially depending on: the choice of reference ‘gold standard’ (i.e. diameter stenosis 50% or 70%, visual estimate or quantitated calculation, or the use of invasive physiology) and varying quality and design of studies leading to verification and post-test referral bias. While the former impacts both sensitivity and specificity, the latter overestimates sensitivity and underestimates specificity.
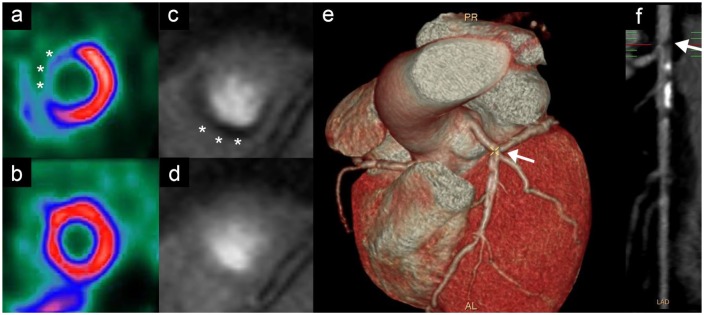
Figure 1.
Noninvasive imaging modalities for work up of coronary artery disease.
Nuclear imaging stress test demonstrating anteroseptal perfusion defect (asterisks) at stress (a), compared to rest (b). Cardiac magnetic resonance adenosine stress demonstrating inferior perfusion defect (asterisks) at stress (c), compared to rest (d). Cardiac computed tomography angiography demonstrating stenosis (arrow) on 3D reconstruction (e) and CT angiogram (f).
Table 2.
Current diagnostic methods for patients with suspected CAD.
| Modality | Mechanism interpretation | Strengths | Limitations | Performance |
Considerations | Recommendations | |
|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | ||||||
| Functional testing | |||||||
| Stress ECG | Continuous 12-lead ECG acquired during exercise (treadmill or stationary bike) Abnormal: >1 mm ST depression in two contiguous leads, arrhythmia, below-average exercise capacity High risk: Duke treadmill score <–11 or abnormal haemodynamic response |
Noninvasive Low cost, quick Functional capacity assessed Widely available – often can be done at point of care or in the office/clinic |
Suboptimal sensitivity Does not localize ischemia Low detection rate of single-vessel disease Operator and patient dependence (wide range of sensitivity/specificity performance estimates) |
54 (51–66%) | 58 (51–69%) | LBBB, ST depression > 1mm, pre-excitation, paced rhythm Digoxin use Not appropriate for patients who cannot exercise (claudication, deconditioning, arthritis, pulmonary disease) Particularly poor performance in premenopausal women |
No longer solely recommended for evaluation of de novo CAD diagnosis Can be used to risk stratify (i.e. for discharge) while awaiting a higher fidelity test. |
| Stress Echo | Echocardiography with exercise or pharmacologically induced stress Ventricular size, wall motion and function (± valves) can be assessed Abnormal: new wall motional abnormalities or abnormal LVEF with stress |
Noninvasive No radiation Simultaneous structural information, localization of ischaemia Improved sensitivity and specificity compared to ECG alone |
Reduced performance in those with poor imaging windows Operator dependent Largely qualitative analysis Low(er) sensitivity for single-vessel disease |
76 (72–79)% | 80 (71–88)% | Poor imaging windows – morbid obesity, severe COPD, chest wall deformity. Now somewhat offset with contrast. Baseline regional wall motion abnormalities can complicate assessment |
Provides a coarse assessment of cardiac structure/function on baseline imaging |
| SPECT | Radionuclide (technetium, sestamibi, thallium, tetrofosmin) perfusion imaging using either vasodilator or chronotropic stress. Based on coronary ‘steal’ phenomenon. Coarse LV function assessment Abnormal: perfusion defect or coarse wall motion abnormality |
Perfusion evaluation (relative) Quantitative assessment possible Similar performance for exercise and pharmacological stimuli |
Radiation exposure (12–37 mSv) ‘Balanced ischaemia’ may lead to false negatives |
81 (74–86)% | 78 (70–85)% | Soft tissue attenuation: poorer images from obesity, breast artefacts, and liver artefacts Radiation implications for obese (higher dose) and women (breast tissue) Potentially less sensitive in ESRF |
Ideal in patients with poor echo windows or unable to exercise |
| Stress PET | PET under pharmacological stress Abnormal: perfusion defect |
Absolute quantitation of perfusion defect possible Higher image quality than SPECT |
Less available Pharmacological only High cost Radiation exposure (10–14 mSv) |
85 (71–99%) | 86% (65–97) | Limited availability/expensive | Women (less radiation, less breast attenuation) Obesity |
| Stress CMR | Magnetic resonance imaging of myocardium under pharmacological stress (perfusion using adenosine or regional wall motion using dobutamine) Abnormal: perfusion abnormalities with vasodilator; WMA with dobutamine |
High resolution Subendocardial perfusion and viability can be assessed No radiation Structural evaluation Can also evaluate viability Qualitative and semi-quantitative analysis possible |
Less available High cost Claustrophobia can be limiting Unable to give gadolinium with GFR < 30ml/min/m2 |
84 (76–90)% | 85 (77–90)% | Absolute: metallic foreign bodies. CMR-safe devices now mainstream but old ICDs are unsafe Significantly obese patients may not fit in scanner |
|
| Anatomical testing | |||||||
| CACS | Score calculated based on volume and density of calcification (Agatston score) Abnormal: >100 and >400 are traditional cut points although linear association with risk. Zero provides an excellent prognosis |
Quick, easy Widely available No contrast |
Very modest radiation dose Does not provide information on stenosis |
58 (46–69)% | 62 (54–69)% | Historically used for risk stratification rather than ‘diagnosis’ per se | Has been studied with functional testing to provide a combined assessment May be considered in low pre-test probability patients |
| CCTA | Structural luminal narrowing quantified. Abnormal: luminal irregularities or narrowing; >50% considered positive |
Noninvasive Detects obstructive CAD (versus CAC) Quick structural assessment Can assess CT-FFR |
Does not confirm ischaemia Motion artefacts high Calcification causes bloom artefacts, limiting lumen assessment Time-intensive interpretation and image construction Radiation exposure (1–5 mSv) |
96 (94–97)% | 79 (72–84)% | CKD and contrast Radiation implications for obese (higher dose) and women (breast tissue) Image quality less robust with AF or high heart rates Distal vessels sometimes not well seen |
Rule-out test in patients with low likelihood – very high negative predictive value. Could be considered in ‘triple rule out’ for CAD, PE and aortic dissection |
| ICA | High-resolution assessment of coronary lumen Gold standard |
Able to proceed with revascularization at the same sitting Can be paired with invasive functional assessment to have combined evaluation (FFR, iFR) |
Does not confirm ischaemia or degree of luminal narrowing Radiation exposure Invasive Resource intensive Associated with risk: 1 in 1000 of MI, stroke or death |
100% | 100% | CKD and contrast | Refractory or progressive symptoms High chance of needing revascularization |
AF, atrial fibrillation; CACS, coronary artery calcium score; CAD, coronary artery disease; CCTA, coronary CT angiography; CKD, chronic kidney disease; CMR, cardiac magnetic resonance; COPD, chronic obstructive pulmonary disease; CT-FFR, CT fractional flow reserve; ECG, electrocardiography; ESRF, end-stage renal failure; FFR, fractional flow reserve; iFR, instantaneous flow reserve; ICA, invasive coronary angiography; ICD, implantable cardiac defibrillator; LBBB, left bundle branch block; LV, left ventricle; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PE, pulmonary embolism; PET, positron emission tomography; SPECT, single photon emission computed tomography.27–30
Anatomical investigations
Invasive coronary angiography
ICA involves the introduction of radiopaque dye into the coronary ostia while obtaining fluoroscopic cine images. This provides a silhouette assessment of the lumen in 2D; however, by obtaining orthogonal views, a 3D pattern of obstructive disease can be estimated. Critically, there is minimal capacity to derive plaque characteristics (aside from overt calcification) and there is no ability to reliably quantitate plaque burden or positive remodelling beyond stenosis severity. In the clinical setting, stenosis severity has been assessed visually, with a threshold of 50% considered to be significant, and 70% when performed with offline quantitative techniques. Accurate assessment of stenosis severity is hampered by vessel tortuosity, overlapping of vessels and other radiodense anatomy, as well as consistency of vessel opacification.31 Conventionally performed via the femoral artery, over the last 10 years the radial artery has become the access site ‘gold standard’.32 Adverse reactions to dye were previously common, but with newer, low-ionic contrast material this has been significantly reduced.33 Kidney injury is also a known complication, not only from contrast dye which is nephrotoxic, but potentially also from systemic emboli dislodged during instrumentation. The overall risk of major complications has been reported to be as high as 2% and includes arterial dissection, perforation and systemic embolism (including death ~0.08%).34 Additionally, ICA is a comparatively expensive and resource-intensive investigation. For these collective reasons, most Society guidance reserves an early invasive anatomical assessment for those with refractory symptoms or high-risk features on noninvasive testing.
Coronary CT angiography
CCTA is recommended by NICE as the first-line investigation for patients with a stable chest pain syndrome. As displayed in Table 2, CCTA has a very high sensitivity (98.2%) with a moderate-to-high specificity (72–84%) for the diagnosis of significant CAD.35–38 CCTA is accessible, noninvasive and relatively quick. Although there have been significant technological advances, an irregular or elevated heart rate still limits interpretation in some patients and often requires preparatory medication. An additional consideration is the requirement for contrast dye, which can be limiting in those with hypersensitivity to iodinated compounds or significant renal dysfunction. Radiation doses were originally similar to those required for nuclear studies; however, these have dropped considerably to be between 1 and 3 mSv for most contemporary prospectively gated acquisitions.39 This is in comparison to 0.1 mSv for a plane radiography, and 5–15 mSv for diagnostic ICA.39
Coronary artery calcium scoring
CAC scoring is a noncontrast CT-based evaluation of coronary calcium deposition for quantification of atherosclerosis, most commonly calculated and interpreted through the Agatston score.40 CAC scoring is quick, easy to obtain, requires minimal preparation and is highly reproducible. Although some studies have used CAC as a way to increase the yield of stress testing in symptomatic patients,41 its role has become more established in stratifying risk in asymptomatic individuals in view of its high sensitivity. CAC scores can be a particularly useful adjunct when embarking on a shared decision-making process around preventive therapy as demonstrating the presence of established atherosclerosis can be a powerful arbiter for the commencement of medications like statins.42 Similarly, a CAC score of zero is associated with a reassuring prognosis and may alleviate costs, side effects and disutility of a lifelong therapy in patients at very low risk of events.43 Given its poor to modest specificity for clinically significant disease, CAC scoring is generally not recommended as a guide to revascularization in symptomatic patients as it does not produce a reliable (and measurable) estimate of luminal stenosis.44
Functional investigations
Exercise ECG
Exercise ECG has been the cornerstone of CAD diagnostic testing for several decades. The premise is straightforward: to induce sustained ventricular work through exercise such that the presence of a haemodynamically significant stenosis will overcome an ischaemic threshold and generate ECG changes and symptoms. Although traditionally performed on a treadmill, exercise can also be achieved through a stationary bike which reduces movement artefacts on the 12-lead ECG. Diagnostic ECG changes are generally >1 mm ST depression in two or more contiguous leads, although the results can be reported using the Duke treadmill score, which is a composite incorporating exercise time, symptom development, and ECG changes.45,46 A major advantage is its accessibility: there is no preparation, it requires no specialized imaging, contrast or radiation exposure, it is comparatively inexpensive and there is unmeasured, qualitative value in observing exercise capacity. However, a number of important disadvantages also exist. First, it is reliant on adequate exercise capacity and the attainment of adequate physical strain during the test (sustained maximal heart rate >85%), which is not always feasible (or predictable) and results in equivocal or nondiagnostic tests (and thus the requirement for further testing). Additionally, a number of baseline ECG abnormalities may mask ischaemic changes, including bundle branch blocks, left ventricular hypertrophy, ST depression at baseline, and pre-excitation, as well as changes that occur with digoxin use. Because of this, and the aforementioned reasons, both the sensitivity and specificity are relatively low.
Stress echocardiography
Stress echocardiography employs a mixed chronotropic and inotropic stress to induce ischaemia, and can be achieved by exercise (treadmill or stationary bike) or using dobutamine. Ventricular wall motion is observed at baseline and then at peak ‘stress’. While normally perfused segments will become increasingly contractile, ischaemic segments will become hypokinetic, and if sustained, dyskinetic. ECG monitoring is also performed and thus with the addition of wall motion imaging, has a higher sensitivity and specificity than ECG alone. Other benefits include the ability to assess ventricular and valvular function, as well as to semi-quantitatively assess the region and burden of ischaemia, should it be detected.47 Image quality can be a limiting feature owing to patient factors (e.g. obesity, chest deformity) and operator dependency, although some of this can be offset with the use of (relatively expensive) non-nephrotoxic intravenous contrast material.48
Nuclear perfusion imaging
Nuclear imaging techniques such as SPECT (single positron emission computed tomography) or stress PET (positron emission tomography) aim to induce hyperaemic perfusion defects using either indirect (exercise or dobutamine) or direct (adenosine or dipyridamole) microvascular dilatation. Exercise and dobutamine increase myocardial oxygen ‘demand’ and cause a secondary microvascular dilatation to increase ‘supply’. In contrast, adenosine (and dipyridamole through its ability to prevent the breakdown of endogenous adenosine) directly binds to receptors in the microvasculature, causing dilatation and hyperaemia. In the context of a haemodynamically significant coronary stenosis, there is obstruction to an increase in flow and thus patent epicardial vessels are able to ‘steal’ blood flow horizontally. By administering a radioactive tracer at stress and baseline, areas of reduced tracer at stress represent perfusion defects, and thus identify sites of potential epicardial stenosis. SPECT is more commonly available (and less expensive) than PET; however, the latter uses less radiation overall and may be favoured in women. The limitations of SPECT are that it is prone to artefacts, particularly from motion and soft tissue, and requires not insignificant radiation exposure. Furthermore, SPECT relies on relative perfusion of defects and thus may under-recognize ‘balanced’, or regions of simultaneous myocardial ischaemia in patients with multivessel disease.49 Adenosine (and therefore dipyridamole) is contraindicated in patients with high-grade atrioventricular nodal conduction disease, as well as bronchospastic airways disease. The features and metrics of SPECT and PET are compared in Table 2.49,50
Cardiac magnetic resonance imaging
CMR imaging has become a useful multifaceted modality for the diagnosis of CAD.50,51 Reversible perfusion defects can be detected using adenosine and gadolinium contrast in the same way adenosine and radioactive tracers are applied in nuclear imaging. This approach also allows the detection of late gadolinium enhancement, which is a useful marker of myocardial scarring and adds little to the overall duration of the study. The requirement for gadolinium can be limiting as it is contraindicated in patients with reduced kidney function (usually <30 ml/min/m2) as it has been associated with nephrogenic systemic fibrosis, a rare but untreatable scleroderma-like condition.52 In patients with either a gadolinium or adenosine contraindication, regional wall motion abnormalities can be evaluated with dobutamine which is anaologous to stress echocardiography. CMR tends to be expensive and less available, particularly in centres without a dedicated cardiac scanner. Moreover, the central magnet bore can cause limiting claustrophobia in up to 15% and may not comfortably accommodate morbidly obese patients.53
Choosing a diagnostic test
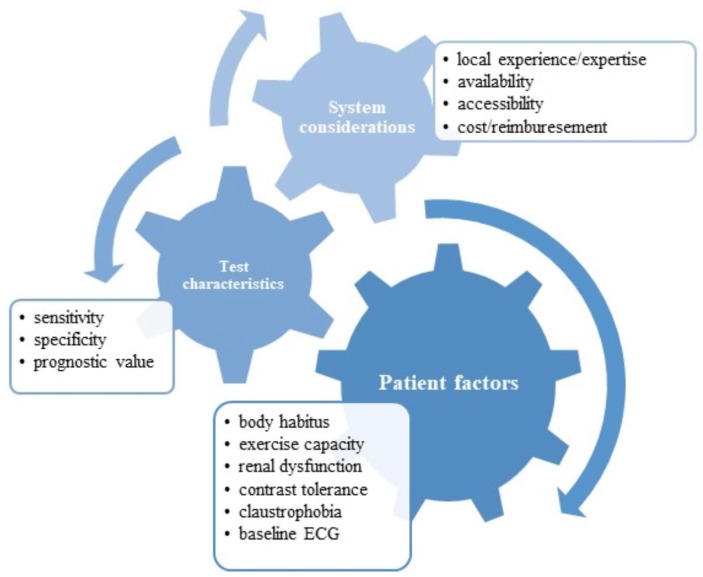
There is no convincing evidence that one particular test is significantly better than any other in the unselected population for the diagnosis of CAD. Instead, this decision needs to be made on a number of system-, patient- and test-related factors (Figure 2). Rather than recapitulate Society guidelines, the following are some key considerations when choosing a diagnostic test.
Figure 2.
Considerations when choosing an investigative modality for suspected coronary artery disease.
Can the patient exercise? If pursuing a functional test, using exercise as the method for stress is logistically simpler, less expensive and may be associated with a higher specificity than pharmacological stimulus.54 Of note, the percentage of patients undergoing pharmacological stress imaging relative to exercise stress imaging is increasing, at least in part due to the investigated population becoming increasingly frail and overweight.55 That being said, over 50% of patients referred for pharmacological stress tests in one study were able to complete a Bruce or Naughton protocol, suggesting many providers underestimate exercise capacity.56 This is of particular importance as adenosine and dobutamine, while generally well tolerated, are not without risk – complete heart block and bronchospasm in the former, arrhythmia in the latter.57 Exercise stress echocardiography and exercise nuclear scans are the preferred options over exercise ECG, given their higher sensitivity and specificity overall. The ability to exercise is not dichotomous, however, and for most stress tests to be diagnostic there is a need to obtain 85% maximal heart rate. When exercise capacity is suboptimal, there is a greater likelihood of the test being not only nondiagnostic and thereby requiring an additional test, but also insensitive and carrying the risk of a false-negative result. Furthermore, recent consumption of beta/calcium channel blockers or caffeine may hinder ‘urgent’ testing for exercise/dobutamine or vasodilator testing, respectively.
What are the local expertise and availability? Although exercise ECG offers the least sensitivity and specificity, it is widely available and in regional or resource-limited settings this may be a pragmatic first (but not definitive) step in the diagnostic approach to CAD. Some centres will have the full spectrum of imaging modalities while others may have only one (nuclear or stress echocardiography, for example). In these settings, ordering a test that can be performed in a timely manner and reliably reported by a team with experience is preferable. Costs for each of these tests vary widely and, particularly in health systems where patients are out-of-pocket, ought to be considered upfront and discussed directly with the patient.
Specific considerations for women. Most consensus statements suggest the avoidance of chest radiation in women and thus stress echocardiography is often preferred. However, there are some data emerging from recent clinical trials that CCTA may offer greater prognostic information than functional testing in women,58 although this awaits further confirmation. The use of ECG stress testing in women is strongly discouraged as most studies report reduced diagnostic performance overall,59 potentially mediated by a lower pre-test probability and reduced exercise capacity given a generally older age at presentation.60 Although outside the scope of this review, there is growing recognition of the broader range of coronary pathology that women present with beyond atherosclerotic CAD. To this end, it is perhaps unsurprising that classical diagnostic strategies aimed at discerning obstructive atherosclerosis are underwhelming when women are more likely than men to suffer from microvascular disease, spasm and spontaneous coronary dissection.61
Technical patient-level considerations. Stress echocardiography suffers from operator dependence and variable image quality. Obese patients or those with chest abnormalities (e.g. hyperexpansion from chronic obstructive pulmonary disease) may not have easily interpretable images using standard echocardiography. While this can be offset with contrast enhancement, this may not be available in all centres. In this context, nuclear imaging or CCTA may be preferable, although in obese patients this comes at a cost of greater proportional radiation exposure. While some studies have raised concerns about reduced CT image quality from soft tissue attenuation, more recent techniques seem to have overcome this limitation.62 As obesity reaches epidemic proportions, the diagnostic evaluation of overweight and obese patients is likely to become an increasingly common and challenging dilemma.
Evidence for a functional approach
Decades of large cohort studies in those with suspected and established CAD have demonstrated the extent and severity of ischaemic burden to be associated with a stepwise increase in the rates of myocardial infarction and cardiovascular death. This has been shown in exercise treadmill testing,63 stress echocardiography64 and nuclear perfusion imaging.65 In complement, a meta-analysis of over 40,000 patients with approximately 10 years of follow up demonstrated the absence of ischaemia (or normalcy) to confer an excellent prognosis, with cardiovascular event rates that approached those of the general population.66 Similarly large analyses have been performed with PET67 and more recently CMR,68,69 yielding similar results.
Although these data support the prognostic value of a functional assessment, it remains of ongoing interest whether it can be used to guide subsequent management. A detailed discussion on the approach to stable ischaemia is outside the scope of this review, but an understanding of the downstream implications of a diagnostic strategy is critical to a discussion of its overall performance. To this end, while risk stratification is of significant clinical interest, it is of greater value if the finding is both modifiable and on the causal pathway of outcomes, i.e. reducing or mitigating a functional abnormality will theoretically lead to improved outcomes. One of the earliest studies to evaluate this in a cohort without known CAD analysed >10,000 patients from a nuclear imaging registry.70 Participants were followed for 2 years and on subgroup analysis of those who received percutaneous coronary intervention (PCI) there was a graded reduction in cardiovascular death in those with >10% ischaemic burden. Although detailed propensity scoring was performed, it is nearly impossible to account for all of the factors that may have impacted on the likelihood of receiving revascularization. Consistent with unmeasured confounding is that only 39% of participants in the study that had >10% ischaemic burden on nuclear imaging actually received PCI, casting doubts on the generalizability of this finding. Recently, an upfront CMR-guided revascularization strategy at a 6% inducible ischaemia threshold was associated with lower rates of angiography (and presumably unnecessary revascularization), but did not show a difference in hard clinical endpoints.71
Additional insight into the ischaemia–prognosis relationship comes from two key clinical trials – COURAGE and BARI-2D. The COURAGE (Clinical Outcomes Using Revascularization and Aggressive Drug Evaluation) trial randomized participants with objective ischaemia confirmed on angiography to receive either medical therapy or PCI for a median follow up of 4.6 years.72 In a subgroup analysis of the main trial, participants who had nuclear perfusion testing at baseline (1381 of 2287) were dichotomized by ischaemic burden – ‘no or mild’ versus ‘moderate to severe’. Although PCI was associated with a significant reduction in ischaemic burden in those who had repeat studies,73 there was no reduction in the rates of myocardial infarction (MI) or death in either group.74 The BARI-2D (Bypass Angioplasty Revascularization 2 Diabetes) study randomized patients with type 2 diabetes and evidence of ischaemia on stress testing (confirmed on invasive angiography) to similarly receive medical therapy or revascularization.75 As with the COURAGE results, there was significantly less residual ischaemia observed in the revascularization arm, but no difference in the hard endpoints of MI or cardiovascular mortality.74 Meta-analysis of these and three other studies of patients with demonstrable ischaemia in the context of stable, symptomatic coronary disease showed that revascularization provided no significant reduction in clinical endpoints.76
In summary, the available data suggest the relationship between ischaemia and prognosis is not altered by PCI and questions the validity of an ischaemia-driven revascularization approach. By extension, the basis of clinical events in these patients appears unlikely to arise from functionally significant lesions which generate ischaemia in subtended myocardium. Instead, it is more likely ischaemia is a marker for greater atherosclerotic burden and thus a greater volume of plaque potentially at risk of rupture and subsequent hard clinical events. This is supported by studies that show a strong relationship between atherosclerotic burden assessed with CAC score, and functional ischaemia on nuclear imaging.77,78 Indeed, a direct comparison in a further subanalysis of the COURAGE trial showed that plaque burden independently predicted outcome, whereas ischaemia burden did not.79 Given that plaque events frequently occur on lesions that are not functionally significant,80,81 it is entirely consistent that a discrete intervention on a process removed from the causal pathway fails to reduce events. Although most recent studies have been directionally consistent with this theory, both COURAGE and BARI-2D may have potentially suffered from selection bias in that all patients were randomized after angiography. This could have resulted in the highest risk patients being excluded from the studies and thus negating the potential benefit of revascularization. This has been the basis for the ISCHEMIA trial, which will randomize patients with a functional test demonstrating moderate-to-high burden of ischaemia with blinded CT angiography to either PCI or medical therapy.82 While the results will provide insight into the utility of PCI in this cohort, particularly in light of recent data suggesting a placebo effect of PCI,83 it will also help clarify the prognostic role of functional testing in the management of stable ischaemia.
Evidence for an anatomical approach
Historically the anatomical approach to diagnosis has focused around the finding of a ‘significant’ stenosis and thereby a categorical diagnosis of CAD. Although this approach has traditionally provided a useful construct with which to guide revascularization, there is growing recognition that the detection of a ‘significant’ stenosis is probably just a coarse marker for total atherosclerotic burden. Elegant demonstration of this comes from over 11,000 participants undergoing ICA who were followed for 7 years. Those with ‘nonobstructive disease’ (i.e. stenosis <50%) were at significantly higher risk than those without disease, and experienced cardiovascular events at the same rates as those with single-vessel disease.84
Although ICA provides a coarse, semi-quantitative assessment of atherosclerotic burden, the advent of CCTA has provided even deeper insight into this relationship. Registry data from over 20,000 symptomatic individuals without established cardiovascular disease undergoing CCTA demonstrated a linear risk continuum between cardiovascular events and plaque burden.85 Critically in this study, and other CAC data,86 there was no evidence of a threshold effect at either end of the spectrum, nor was there an inflexion point in those with lesions demonstrating >70% luminal obstruction. These observations provide insight into the potential superiority of an anatomical assessment. For example, individuals diagnosed with nonobstructive atheroma, a group with up to sixfold risk of cardiovascular death than those without atheroma,87 would remain undifferentiated by a functional approach. It is potentially these individuals who may derive the most benefit from the administration of preventive therapies such as statins or aspirin.
Leveraging this potential benefit, two key studies have evaluated the role of an anatomical assessment on clinical outcomes. The PROMISE (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) study randomized 10,003 symptomatic outpatients to either CCTA or a functional test and were followed for 25 months. Similar clinical outcomes were observed in both arms, suggesting that an upfront CCTA approach was safe and may have improved the yield of ICA.88 If the cohort had a higher event rate (overall rate was ~3%) with a greater burden of disease (12% had typical angina), there may have been an opportunity to demonstrate superior clinical outcomes from the institution of preventive therapy.89 Support for this comes from a post hoc analysis of the SCOT-HEART trial which randomized 4,146 patients to standard care (of which 85% received ECG stress and 9% received an imaging stress test) or standard care plus CCTA. At 4.8 years there were similar rates of revascularization; however, the CCTA arm had a lower rate of death or nonfatal MI [2.3% (n = 48) versus 3.9% (n = 81); HR, 0.59 (95% CI 0.41–0.84), p = 0.004].90 An earlier post hoc analysis of the same signal suggested this benefit may have been, at least in part, associated with the introduction of preventive therapies, such as a statin and aspirin, as this was mandated in the CCTA arm.91
In summary, the current evidence suggests that while rates of ICA may be slightly higher for a CCTA-based approach, there may be net benefit derived through the detection of individuals with atheroma that would not have otherwise have been identified on functional imaging and subsequently receive preventive management. Functional testing continues to have a role in delineating.
For those with moderate to severe stenoses on CCTA, functional testing continues to have a general role in prognosis and to identify patients likely to benefit from ICA and subsequently symptom-driven revascularization.
Evolving approaches
Given the complementary information provided by both anatomical and functional testing, there is growing interest in approaches that provide both plaque and ischaemic data. In the setting of ICA, techniques using pressure or flow wires have been described to evaluate the haemodynamic significance of an ‘intermediate’ lesion, thereby influencing a decision to perform PCI. These techniques have generally required the use of adenosine (in the same way perfusion imaging is performed, above); however, more recent applications have used the intrinsic characteristics of blood flow alone,92 or under the influence of saline or contrast injections.93 Studies utilizing some form of intravascular functional assessment compared to ICA alone have generally shown greater specificity of CAD ‘significance’, and thus a tendency to reduce likely unnecessary PCI,,94,95 and the potential to meaningfully change management and revascularization strategy when performed upfront in ICA (CABG versus PCI versus optimal medical therapy).96,97 In line with the earlier discussion around functional significance, it remains unclear whether an invasive functional assessment significantly impacts hard outcomes beyond urgent revascularization.98,99
In the noninvasive hybrid imaging sphere, CCTA can be augmented by quantitating perfusion [CT myocardial perfusion imaging (CT-MPI), analogous to CMR by detecting passage of iodinated contrast into the myocardium] and/or deriving an index of flow reserve (CT fractional flow reserve, or CT-FFR, analogous to invasive FFR) using computational fluid dynamics. Both approaches provide incremental prognostic information with comparable cost-effectiveness compared to ICA.100,101 As computational requirements improve and costs fall, broader availability may see hybrid CT becoming the premier gatekeeper for ICA.102,103 However, whether a hybrid CT as the dominant noninvasive test upfront improves hard clinical endpoints will be informed by ongoing clinical trials [FORECAST (Fractional Flow Reserve Derived from Computed Tomography Angiography in the Assessment and Management of Stable Chest Pain; ClinicalTrials.gov identifier: NCT03187639) and PRECISE (Prospective Randomized Trial of the Optimal Evaluation of Cardiac Symptoms and Revascu-larization; ClinicalTrials.gov identifier: NCT03702244)] studies.
Beyond conventional anatomical and haemodynamic assessments of plaque burden and lesion severity, respectively, molecular imaging techniques are now providing deeper insight into both plaque behaviour and composition. If the premise linking plaque burden to outcomes is driven by the propensity for plaque ‘events’, molecular imaging theoretically could evaluate this metric directly, with higher fidelity and potentially over time. Two nuclear tracers, 18F-sodium fluoride (18F-NaF) and 18F-flurodeoxyglucose (18F-FDG) have been shown to concentrate in areas of microcalcification, inflammation and macrophage burden, as well as to identify sites of recent clinical plaque rupture.104,105 Whether or not this information can predict sites of disease progression or those plaques at risk of causing myocardial infarction is being tested in the multicenter PREFFIR trial (Prediction of Recurrent Events with 18F-Fluoride) due for completion in the middle of 2020 [ClinicalTrials.gov identifier: NCT02278211].
Cost-effectiveness
Cost-effectiveness analyses are challenging to generalize given they are inherently dependent on local and regional economic factors, availability of imaging techniques, local expertise and disease prevalence – all of which are dynamic. Although a number of analyses have been performed comparing one imaging technique to another, few have been done across all modalities and even fewer have captured the complexity of different temporal approaches to an investigative strategy. Furthermore, few studies have successfully captured all of the downstream subtleties – an expensive test may be justified by the avoidance of downstream testing but even more so if it facilitates improved clinical outcomes through upstream management decisions, i.e. the adoption of preventive therapies where indicated, or the avoidance of disutility from long-term medication use where not required. One of the more complete studies in this area developed a microsimulation model to compare CCTA with stress imaging and was analysed from the perspective of three different health systems: the Netherlands, the UK and the USA.106 A target population of 60-year-old patients with an intermediate pre-test probability of CAD was selected and 16 different diagnostic pathways studied involving the following permutations: no imaging, CCTA, stress imaging, CCTA followed by stress imaging and direct invasive angiography. The analysis utilized Markov state-transition modelling with a lifetime horizon for long-term prognosis (alive, post-MI and dead) as well as the impact on quality of life and outcomes from treatment of inducible ischaemia based on COURAGE data, as well as the implications of false-positive results. The summary suggested in the modelled cohort that CCTA was a cost-effective initial strategy prior to stress imaging across all of the studied regions. The comparisons between stress imaging tests suggested. There performance of the stress imaging approaches was similar however, owing to its lower cost, stress echocardiography was deemed the most cost-effective stress imaging modality. These results, and internal NICE modelling,107 resulted in the adoption of CCTA as the initial strategy for investigating intermediate-risk stable chest pain.8
Conclusion
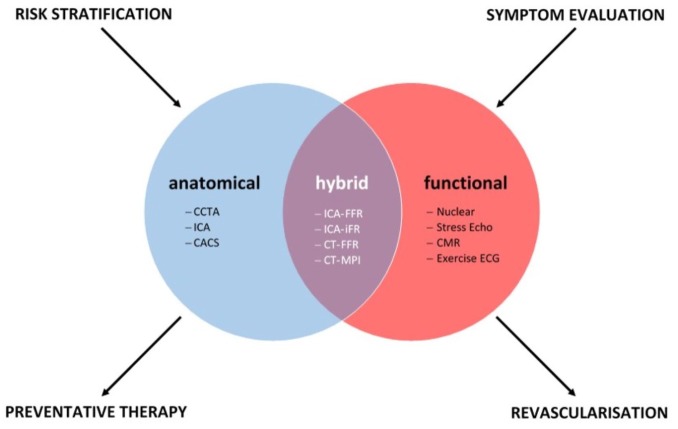
Despite being a foundational pillar in clinical medicine, the optimal approach to the diagnosis of CAD remains contentious. Historical goalposts set against the faithful determination of a functional or anatomical threshold for ischaemia have been challenged by clinical trial evidence, which has failed to demonstrate improved outcomes through its mitigation. These studies have revealed ischaemia to be an important marker for cardiovascular outcomes, but likely separate from the causal pathway of hard clinical events. Instead the growth of noninvasive CT anatomical assessment has provided new insights into the risk continuum between plaque burden and adverse outcomes. The lack of durability observed with a lesion-level, ischaemia-focused strategy to date lends further support to the theory that a systemic approach to preventive therapy is likely to result in improved cardiovascular outcomes. As it currently stands, functional and anatomical approaches provide complementary information; stress testing continues to provide guidance for potential revascularization in current guidelines, yet anatomical testing may additionally identify individuals likely to benefit from preventive therapy (Figure 3). Decisions surrounding which test, in whom, in what order, and when, continue to have patient-, modality- and system-level considerations. Outcome trials evaluating hard clinical endpoints comparing not only different testing modalities but also care pathways are likely to provide critical insight into some of these unanswered questions.
Figure 3.
Complementary roles of anatomical, functional and hybrid imaging modalities.
CACS, coronary artery calcium scoring; CCTA, coronary CT angiography; CT, computed tomography; FFR, fractional flow reserve; ICA, invasive coronary angiography; MPI, myocardial perfusion imaging.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by a project grant from the National Health and Medical Research Council of Australia (GNT1127159, PJP) and fellowship funding from the National Heart Foundation of Australia (Future Leader Fellowship FLF102056, PJP) and National Health and Medical Research Council of Australia (CDF1161506, PJP).
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: PJP has received research support from Abbott Vascular, consulting fees from Amgen and Esperion and speaker honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Merck Schering-Plough and Pfizer.
Ethical statement: This manuscript did not require an ethical board approval because it did not contain human or animal trials.
ORCID iD: Peter J. Psaltis  https://orcid.org/0000-0003-0222-5468
https://orcid.org/0000-0003-0222-5468
Contributor Information
Adam J. Nelson, Duke Clinical Research Institute, Durham, NC, USA Vascular Research Centre, Lifelong Health Theme, South Australian Health and Medical Research Institute, Adelaide, Australia; Adelaide Medical School, University of Adelaide, Adelaide, Australia.
Maddalena Ardissino, Duke Clinical Research Institute, Durham, NC, USA; School of Medicine, Imperial College, London, UK.
Peter J. Psaltis, South Australian Health and Medical Research Institute, North Terrace, Adelaide, SA 5005, Australia; Adelaide Medical School, University of Adelaide, Adelaide, Australia.
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics – 2015 update: a report from the American Heart Association. Circulation 2015; 131: e29–e322. [DOI] [PubMed] [Google Scholar]
- 2. Ladapo JA, Blecker S, Douglas PS. Physician decision making and trends in the use of cardiac stress testing in the United States: an analysis of repeated cross-sectional data. Ann Intern Med 2014; 161: 482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med 2010; 362:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sekhri N, Feder GS, Junghans C, et al. How effective are rapid access chest pain clinics? Prognosis of incident angina and non-cardiac chest pain in 8762 consecutive patients. Heart 2007; 93: 458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Libby P. Inflammation in atherosclerosis. Nature 2002; 420: 868–874. [DOI] [PubMed] [Google Scholar]
- 6. Libby P, Pasterkamp G, Crea F, et al. Reassessing the mechanisms of acute coronary syndromes. Circ Res 2019; 124: 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bairey Merz CN, Pepine CJ, Walsh MN, et al. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation 2017; 135: 1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Institute for Health and Care Excellence. Chest pain of recent onset: assessment and diagnosis (NICE Guideline 95), https://www.nice.org.uk/guidance/cg95/resources/chest-pain-of-recent-onset-assessment-and-diagnosis-pdf-975751036117 (2016, accessed 1 June 2019). [PubMed]
- 9. Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2012; 126: e354–e471. [DOI] [PubMed] [Google Scholar]
- 10. Task Force Members, Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the Management of Stable Coronary Artery Disease of the European Society of Cardiology. Eur Heart J 2013; 34: 2949–3003. [DOI] [PubMed] [Google Scholar]
- 11. Gould KL, Lipscomb K. Effects of coronary stenoses on coronary flow reserve and resistance. Am J Cardiol 1974; 34: 48–55. [DOI] [PubMed] [Google Scholar]
- 12. Uren NG, Melin JA, De Bruyne B, et al. Relation between myocardial blood flow and the severity of coronary-artery stenosis. N Engl J Med 1994; 330: 1782–1788. [DOI] [PubMed] [Google Scholar]
- 13. Nesto RW, Kowalchuk GJ. The ischemic cascade: temporal sequence of hemodynamic, electrocardiographic and symptomatic expressions of ischemia. Am J Cardiol 1987; 59: 23C–30C. [DOI] [PubMed] [Google Scholar]
- 14. Detry JM. The pathophysiology of myocardial ischaemia. Eur Heart J 1996; 17(Suppl. G): 48–52. [DOI] [PubMed] [Google Scholar]
- 15. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009; 360: 213–224. [DOI] [PubMed] [Google Scholar]
- 16. Kataoka Y, Puri R, Nicholls SJ. Inflammation, plaque progression and vulnerability: evidence from intravascular ultrasound imaging. Cardiovasc Diagn Ther 2015; 5: 280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Psaltis PJ, Nicholls SJ. Imaging: focusing light on the vulnerable plaque. Nat Rev Cardiol 2016; 13: 253–255. [DOI] [PubMed] [Google Scholar]
- 18. Puri R, Tuzcu EM, Nissen SE, et al. Exploring coronary atherosclerosis with intravascular imaging. Int J Cardiol 2013; 168: 670–679. [DOI] [PubMed] [Google Scholar]
- 19. Andrews J, Puri R, Kataoka Y, et al. Therapeutic modulation of the natural history of coronary atherosclerosis: lessons learned from serial imaging studies. Cardiovasc Diagn Ther 2016; 6: 282–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. Epub ahead of print 10 November 2018. DOI: 10.1016/j.jacc.2018.11.003. [DOI] [Google Scholar]
- 21. Cooper A, Timmis A, Skinner J, et al. Assessment of recent onset chest pain or discomfort of suspected cardiac origin: summary of NICE guidance. BMJ 2010; 340: c1118. [DOI] [PubMed] [Google Scholar]
- 22. Chaitman BR, Bourassa MG, Davis K, et al. Angiographic prevalence of high-risk coronary artery disease in patient subsets (CASS). Circulation 1981; 64: 360–367. [DOI] [PubMed] [Google Scholar]
- 23. Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med 1979; 300: 1350–1358. [DOI] [PubMed] [Google Scholar]
- 24. Pryor DB, Harrell FE, Jr, Lee KL, et al. Estimating the likelihood of significant coronary artery disease. Am J Med 1983; 75: 771–780. [DOI] [PubMed] [Google Scholar]
- 25. Cheng VY, Berman DS, Rozanski A, et al. Performance of the traditional age, sex, and angina typicality-based approach for estimating pretest probability of angiographically significant coronary artery disease in patients undergoing coronary computed tomographic angiography: results from the multinational coronary CT angiography evaluation for clinical outcomes: an international multicenter registry (CONFIRM). Circulation 2011; 124: 2423–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adamson PD, Newby DE, Hill CL, et al. Comparison of international guidelines for assessment of suspected stable angina: insights from the PROMISE and SCOT-HEART. JACC Cardiovasc Imaging 2018; 11: 1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Jong MC, Genders TS, van Geuns RJ, et al. Diagnostic performance of stress myocardial perfusion imaging for coronary artery disease: a systematic review and meta-analysis. Eur Radiol 2012; 22: 1881–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hecht HS, Shaw L, Chandrashekhar YS, et al. Should NICE guidelines be universally accepted for the evaluation of stable coronary disease? A debate. Eur Heart J 2019; 40: 1440–1453. [DOI] [PubMed] [Google Scholar]
- 29. Knuuti J, Ballo H, Juarez-Orozco LE, et al. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: a meta-analysis focused on post-test disease probability. Eur Heart J 2018; 39: 3322–3330. [DOI] [PubMed] [Google Scholar]
- 30. Stolzmann P, Donati OF, Desbiolles L, et al. Coronary artery plaques and myocardial ischaemia. Eur Radiol 2011; 21: 1628–1634. [DOI] [PubMed] [Google Scholar]
- 31. Topol EJ, Nissen SE. Our preoccupation with coronary luminology: the dissociation between clinical and angiographic findings in ischemic heart disease. Circulation 1995; 92: 2333–2342. [DOI] [PubMed] [Google Scholar]
- 32. Mason PJ, Shah B, Tamis-Holland JE, et al. An update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome: a scientific statement from the American Heart Association. Circ Cardiovasc Interv 2018; 11: e000035. [DOI] [PubMed] [Google Scholar]
- 33. Kim MH, Lee SY, Lee SE, et al. Anaphylaxis to iodinated contrast media: clinical characteristics related with development of anaphylactic shock. PLoS One 2014; 9: e100154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tavakol M, Ashraf S, Brener SJ. Risks and complications of coronary angiography: a comprehensive review. Glob J Health Sci 2012; 4: 65–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 2008; 52: 1724–1732. [DOI] [PubMed] [Google Scholar]
- 36. Meijboom WB, Meijs MF, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 2008; 52: 2135–2144. [DOI] [PubMed] [Google Scholar]
- 37. Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008; 359: 2324–2336. [DOI] [PubMed] [Google Scholar]
- 38. Stein PD, Yaekoub AY, Matta F, et al. 64-slice CT for diagnosis of coronary artery disease: a systematic review. Am J Med 2008; 121: 715–725. [DOI] [PubMed] [Google Scholar]
- 39. Cademartiri F, Maffei E, Arcadi T, et al. CT coronary angiography at an ultra-low radiation dose (<0.1 mSv): feasible and viable in times of constraint on healthcare costs. Eur Radiol 2013; 23: 607–613. [DOI] [PubMed] [Google Scholar]
- 40. Pletcher MJ, Tice JA, Pignone M, et al. Using the coronary artery calcium score to predict coronary heart disease events: a systematic review and meta-analysis. Arch Intern Med 2004; 164: 1285–1292. [DOI] [PubMed] [Google Scholar]
- 41. Berman DS, Rozanski A. Value-based imaging: combining coronary artery calcium with myocardial perfusion imaging. J Nucl Cardiol 2016; 23: 939–941. [DOI] [PubMed] [Google Scholar]
- 42. Hecht HS, Cronin P, Blaha MJ, et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: a report of the society of cardiovascular computed tomography and society of thoracic radiology. J Cardiovasc Comput Tomogr 2017; 11: 74–84. [DOI] [PubMed] [Google Scholar]
- 43. Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the multi-ethnic study of atherosclerosis (MESA). Circulation 2016; 133: 849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Villines TC, Hulten EA, Shaw LJ, et al. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography: results from the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: an International Multicenter) registry. J Am Coll Cardiol 2011; 58: 2533–2540. [DOI] [PubMed] [Google Scholar]
- 45. Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to update the 1997 exercise testing guidelines). J Am Coll Cardiol 2002; 40: 1531–1540. [DOI] [PubMed] [Google Scholar]
- 46. Mark DB, Hlatky MA, Harrell FE, Jr, et al. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med 1987; 106: 793–800. [DOI] [PubMed] [Google Scholar]
- 47. Fleischmann KE, Hunink MG, Kuntz KM, et al. Exercise echocardiography or exercise SPECT imaging? A meta-analysis of diagnostic test performance. JAMA 1998; 280: 913–920. [DOI] [PubMed] [Google Scholar]
- 48. Hamilton-Craig C, Boga T, West C, et al. Contrast echocardiography in Australian clinical practice. Heart Lung Circ 2010; 19: 385–394. [DOI] [PubMed] [Google Scholar]
- 49. Mc Ardle BA, Dowsley TF, deKemp RA, et al. Does rubidium-82 PET have superior accuracy to SPECT perfusion imaging for the diagnosis of obstructive coronary disease? a systematic review and meta-analysis. J Am Coll Cardiol 2012; 60: 1828–1837. [DOI] [PubMed] [Google Scholar]
- 50. Greenwood JP, Maredia N, Younger JF, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet 2012; 379: 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nagel E, Lehmkuhl HB, Bocksch W, et al. Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: comparison with dobutamine stress echocardiography. Circulation 1999; 99: 763–770. [DOI] [PubMed] [Google Scholar]
- 52. Reiter T, Ritter O, Prince MR, et al. Minimizing risk of nephrogenic systemic fibrosis in cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2012; 14: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dewey M, Schink T, Dewey CF. Claustrophobia during magnetic resonance imaging: cohort study in over 55,000 patients. J Magn Reson Imaging 2007; 26: 1322–1327. [DOI] [PubMed] [Google Scholar]
- 54. Gonzalez JA, Beller GA. Choosing exercise or pharmacologic stress imaging, or exercise ECG testing alone: how to decide. J Nucl Cardiol 2017; 24: 555–557. [DOI] [PubMed] [Google Scholar]
- 55. Argulian E, Po JRF, Uretsky S, et al. Comparison of the current reasons for undergoing pharmacologic stress during echocardiographic and radionuclide stress testing. J Nucl Cardiol 2017; 24: 546–554. [DOI] [PubMed] [Google Scholar]
- 56. Ross MI, Wu E, Wilkins JT, et al. Safety and feasibility of adjunctive regadenoson injection at peak exercise during exercise myocardial perfusion imaging: the Both Exercise and Regadenoson Stress Test (BERST) trial. J Nucl Cardiol 2013; 20: 197–204. [DOI] [PubMed] [Google Scholar]
- 57. Dilsizian V, Gewirtz H, Paivanas N, et al. Serious and potentially life threatening complications of cardiac stress testing: physiological mechanisms and management strategies. J Nucl Cardiol 2015; 22: 1198–213; quiz 1195–1197. [DOI] [PubMed] [Google Scholar]
- 58. Pagidipati NJ, Hemal K, Coles A, et al. Sex differences in functional and CT angiography testing in patients with suspected coronary artery disease. J Am Coll Cardiol 2016; 67: 2607–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kwok Y, Kim C, Grady D, et al. Meta-analysis of exercise testing to detect coronary artery disease in women. Am J Cardiol 1999; 83: 660–666. [DOI] [PubMed] [Google Scholar]
- 60. Stangl V, Witzel V, Baumann G, et al. Current diagnostic concepts to detect coronary artery disease in women. Eur Heart J 2008; 29: 707–717. [DOI] [PubMed] [Google Scholar]
- 61. Franke KB, Wong DT, Baumann A, et al. Current state-of-play in spontaneous coronary artery dissection. Cardiovasc Diagn Ther 2019; 9: 281–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ben-Haim S, Almukhailed O, Neill J, et al. Clinical value of supine and upright myocardial perfusion imaging in obese patients using the D-SPECT camera. J Nucl Cardiol 2014; 21: 478–485. [DOI] [PubMed] [Google Scholar]
- 63. Shaw LJ, Peterson ED, Shaw LK, et al. Use of a prognostic treadmill score in identifying diagnostic coronary disease subgroups. Circulation 1998; 98: 1622–1630. [DOI] [PubMed] [Google Scholar]
- 64. Marwick TH, Case C, Sawada S, et al. Prediction of mortality using dobutamine echocardiography. J Am Coll Cardiol 2001; 37: 754–760. [DOI] [PubMed] [Google Scholar]
- 65. Berman DS, Hachamovitch R, Kiat H, et al. Incremental value of prognostic testing in patients with known or suspected ischemic heart disease: a basis for optimal utilization of exercise technetium-99m sestamibi myocardial perfusion single-photon emission computed tomography. J Am Coll Cardiol 1995; 26: 639–647. [DOI] [PubMed] [Google Scholar]
- 66. Shaw LJ, Iskandrian AE. Prognostic value of gated myocardial perfusion SPECT. J Nucl Cardiol 2004; 11: 171–185. [DOI] [PubMed] [Google Scholar]
- 67. Dorbala S, Di Carli MF. Cardiac PET perfusion: prognosis, risk stratification, and clinical management. Semin Nucl Med 2014; 44: 344–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bertaso AG, Richardson JD, Wong DT, et al. Prognostic value of adenosine stress perfusion cardiac MRI with late gadolinium enhancement in an intermediate cardiovascular risk population. Int J Cardiol 2013; 167: 2055–2060. [DOI] [PubMed] [Google Scholar]
- 69. Heitner JF, Kim RJ, Kim HW, et al. Prognostic value of vasodilator stress cardiac magnetic resonance imaging: a multicenter study with 48000 patient-years of follow-up. JAMA Cardiol 2019; 4: 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hachamovitch R, Hayes SW, Friedman JD, et al. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003; 107: 2900–2907. [DOI] [PubMed] [Google Scholar]
- 71. Nagel E, Greenwood JP, McCann GP, et al. Magnetic resonance perfusion or fractional flow reserve in coronary disease. N Engl J Med 2019; 380: 2418–2428. [DOI] [PubMed] [Google Scholar]
- 72. Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356: 1503–1516. [DOI] [PubMed] [Google Scholar]
- 73. Shaw LJ, Berman DS, Maron DJ, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the clinical outcomes utilizing revascularization and aggressive drug evaluation (COURAGE) trial nuclear substudy. Circulation 2008; 117: 1283–1291. [DOI] [PubMed] [Google Scholar]
- 74. Shaw LJ, Weintraub WS, Maron DJ, et al. Baseline stress myocardial perfusion imaging results and outcomes in patients with stable ischemic heart disease randomized to optimal medical therapy with or without percutaneous coronary intervention. Am Heart J 2012; 164: 243–250. [DOI] [PubMed] [Google Scholar]
- 75. Group BDS, Frye RL, August P, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360: 2503–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Stergiopoulos K, Boden WE, Hartigan P, et al. Percutaneous coronary intervention outcomes in patients with stable obstructive coronary artery disease and myocardial ischemia: a collaborative meta-analysis of contemporary randomized clinical trials. JAMA Intern Med 2014; 174: 232–240. [DOI] [PubMed] [Google Scholar]
- 77. Chang SM, Nabi F, Xu J, et al. The coronary artery calcium score and stress myocardial perfusion imaging provide independent and complementary prediction of cardiac risk. J Am Coll Cardiol 2009; 54: 1872–1882. [DOI] [PubMed] [Google Scholar]
- 78. Schenker MP, Dorbala S, Hong EC, et al. Interrelation of coronary calcification, myocardial ischemia, and outcomes in patients with intermediate likelihood of coronary artery disease: a combined positron emission tomography/computed tomography study. Circulation 2008; 117: 1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mancini GBJ, PM Hartigan, Shaw LJ, et al. Predicting outcome in the COURAGE trial (clinical outcomes utilizing revascularization and aggressive drug evaluation): coronary anatomy versus ischemia. JACC Cardiovasc Interv 2014; 7: 195–201. [DOI] [PubMed] [Google Scholar]
- 80. Falk E, Nakano M, Bentzon JF, et al. Update on acute coronary syndromes: the pathologists’ view. Eur Heart J 2013; 34: 719–728. [DOI] [PubMed] [Google Scholar]
- 81. Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011; 364: 226–235. [DOI] [PubMed] [Google Scholar]
- 82. Maron DJ, Harrington RA, Hochman JS. Planning and conducting the ISCHEMIA trial. Circulation 2018; 138: 1384–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Al-Lamee R, Thompson D, Dehbi HM, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet 2018; 391: 31–40. [DOI] [PubMed] [Google Scholar]
- 84. Jespersen L, Hvelplund A, Abildstrom SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012; 33: 734–744. [DOI] [PubMed] [Google Scholar]
- 85. Chow BJ, Small G, Yam Y, et al. Prognostic and therapeutic implications of statin and aspirin therapy in individuals with nonobstructive coronary artery disease: results from the CONFIRM (COronary CT Angiography EvaluatioN for clinical outcomes: an InteRnational Multicenter registry) registry. Arterioscler Thromb Vasc Biol 2015; 35: 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol 2007; 49: 1860–1870. [DOI] [PubMed] [Google Scholar]
- 87. Habib PJ, Green J, Butterfield RC, et al. Association of cardiac events with coronary artery disease detected by 64-slice or greater coronary CT angiography: a systematic review and meta-analysis. Int J Cardiol 2013; 169: 112–120. [DOI] [PubMed] [Google Scholar]
- 88. Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015; 372: 1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hoffmann U, Ferencik M, Udelson JE, et al. Prognostic value of noninvasive cardiovascular testing in patients with stable chest pain: insights from the PROMISE trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017; 135: 2320–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. SCOT-HEART Investigators, Newby DE, Adamson PD, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med 2018; 379: 924–933. [DOI] [PubMed] [Google Scholar]
- 91. Williams MC, Hunter A, Shah ASV, et al. Use of coronary computed tomographic angiography to guide management of patients with coronary disease. J Am Coll Cardiol 2016; 67: 1759–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sen S, Escaned J, Malik IS, et al. Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis: results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J Am Coll Cardiol 2012; 59: 1392–1402. [DOI] [PubMed] [Google Scholar]
- 93. Spagnoli V, Picard F, Tadros VX, et al. Simplifying the assessment of coronary artery stenosis by enhancing instantaneous wave free ratio. Cardiovasc Diagn Ther 2018; 8: 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bech GJ, De Bruyne B, Pijls NH, et al. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation 2001; 103: 2928–2934. [DOI] [PubMed] [Google Scholar]
- 95. Sen S, Ahmad Y, Dehbi HM, et al. Clinical events after deferral of LAD revascularization following physiological coronary assessment. J Am Coll Cardiol 2019; 73: 444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Curzen N, Rana O, Nicholas Z, et al. Does routine pressure wire assessment influence management strategy at coronary angiography for diagnosis of chest pain? The RIPCORD study. Circ Cardiovasc Interv 2014; 7: 248–255. [DOI] [PubMed] [Google Scholar]
- 97. Van Belle E, Rioufol G, Pouillot C, et al. Outcome impact of coronary revascularization strategy reclassification with fractional flow reserve at time of diagnostic angiography: insights from a large French multicenter fractional flow reserve registry. Circulation 2014; 129: 173–185. [DOI] [PubMed] [Google Scholar]
- 98. Ahn JM, Park DW, Shin ES, et al. Fractional flow reserve and cardiac events in coronary artery disease: data from a prospective IRIS-FFR registry (Interventional Cardiology Research Incooperation Society Fractional Flow Reserve). Circulation 2017; 135: 2241–2251. [DOI] [PubMed] [Google Scholar]
- 99. De Bruyne B, Fearon WF, Pijls NH, et al. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med 2014; 371: 1208–1217. [DOI] [PubMed] [Google Scholar]
- 100. Douglas PS, Pontone G, Hlatky MA, et al. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: the prospective longitudinal trial of FFR(CT): outcome and resource impacts study. Eur Heart J 2015; 36: 3359–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hlatky MA, Saxena A, Koo BK, et al. Projected costs and consequences of computed tomography-determined fractional flow reserve. Clin Cardiol 2013; 36: 743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Chang HJ, Lin FY, Gebow D, et al. Selective referral using CCTA versus direct referral for individuals referred to invasive coronary angiography for suspected CAD: a randomized, controlled, open-label trial. JACC Cardiovasc Imaging. Epub ahead of print 12 December 2018. DOI: 10.1016/j.jcmg.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 103. Patel MR, Norgaard BL, Fairbairn TA, et al. 1-year impact on medical practice and clinical outcomes of FFRCT: the ADVANCE registry. JACC Cardiovasc Imaging 2019; pii: S1936-878X(19)30227-X. [DOI] [PubMed] [Google Scholar]
- 104. Joshi NV, Vesey AT, Williams MC, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet 2014; 383: 705–713. [DOI] [PubMed] [Google Scholar]
- 105. Scherer DJ, Psaltis PJ. Future imaging of atherosclerosis: molecular imaging of coronary atherosclerosis with 18F positron emission tomography. Cardiovasc Diagn Ther 2016; 6: 354–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Genders TS, Petersen SE, Pugliese F, et al. The optimal imaging strategy for patients with stable chest pain: a cost-effectiveness analysis. Ann Intern Med 2015; 162: 474–484. [DOI] [PubMed] [Google Scholar]
- 107. Moss AJ, Williams MC, Newby DE, et al. The updated NICE guidelines: cardiac CT as the first-line test for coronary artery disease. Curr Cardiovasc Imaging Rep 2017; 10: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]