Abstract
MiR-146a-5p plays different roles in different types of cancers. We showed that miR-146a-5p and long noncoding RNA HOTAIR were both upregulated in triple-negative breast cancer. Follow-up study showed that high levels of miR-146a-5p and HOTAIR in tumor tissues were closely correlated with poor survival. MiR-146a-5p and HOTAIR were positively correlated in tumor tissues. MiR-146a-5p positively regulated HOTAIR triple-negative breast cancer cells, while HOTAIR showed no regulatory effects on miR-146a-5p expression. MiR-146a-5p and HOTAIR positively regulated the migration and invasion of triple-negative breast cancer cells. In addition, HOTAIR silencing attenuated the effects of miR-146a-5p. Therefore, overexpression of miR-146a-5p may promote triple-negative breast cancer cell invasion and migration by upregulating HOTAIR.
Keywords: triple-negative breast cancer, miR-146a-5p, lncRNA HOTAIR, prognosis, cancer cell migration
Introduction
Breast cancer is a commonly diagnosed cancer among females and is responsible for about 20% deaths in women.1 Triple-negative breast cancer (TNBC) as a main subtype of breast cancer is characterized by the lack of hormone receptors and human epidermal growth factor receptor-2.2 Compared with other subtypes, TNBC is more aggressive and difficult to treat, and tumor spread and recur are more common.3,4 At present, overall survival of patients with TNBC is still poor, especially for those at advanced stages.5
The development and progression of TNBC requires the involvement of multiple internal and external factors.6 Besides protein-coding messenger RNA, the human genome also encodes a large set of noncoding RNAs (ncRNAs), which are critical players in human diseases.7 MicroRNAs (miRNAs) and long noncoding RNAs (lncRNAs) play essential roles in human cancers,8,9 including TNBC. As an oncogenic lncRNA, HOTAIR promotes the development of most, if not all, types of cancers.10 The function of miR-146a-5p in TNBC has also been characterized.11 However, the interaction between HOTAIR and miR-146a-5p is unknown. We showed that miR-146a-5p promoted cancer cell invasion and migration possibly by upregulating HOTAIR.
Materials and Methods
Patients and Cell Lines
Our study included 84 patients (29-69 years; mean: 49.1 ± 5.3 years) with TNBC who were admitted by Integrated Hospital of Traditional Chinese Medicine, Southern Medicine University, from April 2011 to July 2013. Inclusion criteria were as follows: (1) confirmed by pathological examinations; (2) patients who were diagnosed for the first time; and (3) patients participated and completed a 5-year follow-up study. Exclusion criteria include (1) patients complicated with other diseases; (2) patients died of other causes; and (3) patients who were lost during follow-up. Tumor and adjacent healthy tissue specimens were collected from each patient. According to American Joint Committee on Cancer stage, 10, 28, 22, and 24 patients were at stages I, II, III, and IV, respectively. All patients signed informed consent and this study passed the review of Ethic Committee of Integrated Hospital of Traditional Chinese Medicine, Southern Medicine University. BT-549 and HCC70 (ATCC) TNBC cell lines were used.
Real-Time Quantitative Polymerase Chain Reaction
MicroRNAs in cells and tissues were extracted using mirVana miRNA isolation kit (Thermo Fisher Scientific, San Jose, CA, USA), TaqMan assays (Applied Biosystems, Foster City, CA, USA) were performed to analyze the expression of miR-146-5p with U6 as endogenous control.
Total RNA in cells and tissues were extracted by RNeasy mini kit (Qiagen, Chatsworth, CA, USA), following reverse transcription (High-Capacity cDNA Reverse Transcription Kit, Applied Biosystems); qScript One-Step RT-qPCR kit (Quantabio, Beverly, MA, USA) was used to prepare quantitative polymerase chain reaction (qPCR) mixtures with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as endogenous control to analyze the expression of HOTAIR. According to 2−ΔΔCT, miR-146a-5p was normalized to U6, and HOTAIR was normalized to GAPDH.
Cell Transfection
HOTAIR expression vector, as well as HOTAIR small interfering RNA (siRNA) and negative control (NC) siRNA, were from Sangon (Shanghai, China). Hsa-miR-146a-5p inhibitor and inhibitor NC, as well as hsa-miR-146a-5p miRNA mimic and scrambled miRNA NC, were purchased from Sigma-Aldrich (St. Louis, Missouri). Cells were harvested at confluence of 70% to 80%, followed by transfection of 10 nM vector (empty vector as NC group), 35 nM siRNA (NC siRNA as NC group), 35 nM miRNA (NC miRNA as NC group), or 35 nM inhibitor (NC inhibitor as NC group) using lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) into 1 × 106 cells. Following experiments were performed using cells harvested at 24 hours posttransfection. Control (C) cells were untransfected cells.
Transwell Assays
Cells were collected at 24 hours after transfection to perform Transwell migration and invasion assays in cases of overexpression rates of HOTAIR and miR-146a-5p were higher than 200% and HOTAIR and miR-146a-5p knockdown rate reach 50%. Cell suspensions (3 × 104 cells/mL) were prepared using serum-free cell culture medium. The upper chamber was filled with cell suspension and lower chamber with a mixture of 80% cell culture medium and 20% fetal bovine serum. Cells were cultivated for 3 hours and then the upper chamber membranes were stained with 0.5% crystal violet (Sigma-Aldrich) for 15 minutes at room temperature. Before cell invasion assay, membranes were precoated with Matrigel (Millipore, Billerica, MA, USA).
Statistical Analysis
Mean values (3 biological replicates) were presented. GraphPad Prism 6 software was used to perform all statistical analyses. Explorations of differences between 2 types of tissues were performed by paired t test. Explorations of differences among multiple groups were performed by 1-way analysis of variance in combination with Tukey test. Pearson correlation coefficient was used for correlation analyses. Patients were divided into high (n = 40) and low (n = 44) HOTAIR groups, as well as high (n = 38) and low (n = 46) miRNA-155 level groups according to Youden index. Survival curves were plotted using Kaplan-Meier test and compared by log-rank t test. Value of P < .05 was statistically significant.
Results
Expression of MiR-146a-5p and HOTAIR Were Upregulated in Patients With TNBC
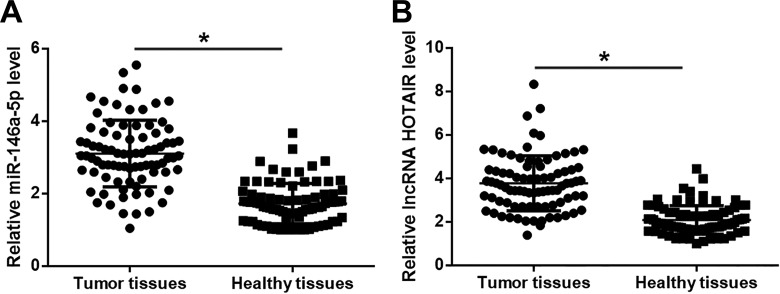
Before treatment, expression levels of miR-146a-5p and HOTAIR in 2 types of tissues of patients with TNBC were measured by real-time qPCR. Compared to healthy tissues, expression of miR-146a-5p (Figure 1A) and HOTAIR (Figure 1B) was significantly upregulated in tumor tissues (P < .05).
Figure 1.
Expression of miR-146a-5p and HOTAIR was upregulated in tumor tissues of TNBC. Compared with adjacent healthy tissues, RT-qPCR results showed that expression of miR-146a-5p (A) and HOTAIR (B) was significantly upregulated in tumor tissues. *P < .05. RT-qPCR indicates real-time quantitative polymerase chain reaction; TNBC, triple-negative breast cancer.
High Levels of HOTAIR and MiR-146a-5p in Tumor Tissues Were Closely Correlated With Poor Survival
Survival curves were plotted and compared using aforementioned methods. Analysis of survival curves showed that the overall survival rate of patients with high HOTAIR (Figure 2A) or miRNA-155 (Figure 2B) levels was significantly lower than patients with low HOTAIR or miRNA-155 level (P = .0007 or .0071).
Figure 2.
High levels of miR-146a-5p and HOTAIR in tumor tissues were closely correlated with poor survival. Survival curve analysis showed that high levels of HOTAIR (A) and miR-146a-5p (B) in tumor tissues were closely correlated with poor survival.
Expression Levels of MiR-146a-5p and HOTAIR Were Positively Correlated
Pearson correlation coefficient was performed to investigate the correlations between HOTAIR and miR-146a-5p. As shown in Figure 3A, expression levels of HOTAIR and miR-146a-5p were significantly and positively correlated in tumor tissues (r = 0.86, P < .0001, Figure 3A). However, expression levels of HOTAIR and miR-146a-5p were not significantly correlated in adjacent healthy tissues (r = 0.23, P = .06, Figure 3B).
Figure 3.
MiR-146a-5p and HOTAIR were positively correlated in TNBC. Pearson correlation coefficient analysis showed that HOTAIR and miR-146a-5p were significantly and positively correlated in tumor tissues (A), but not in adjacent healthy tissues (B). TNBC indicates triple-negative breast cancer.
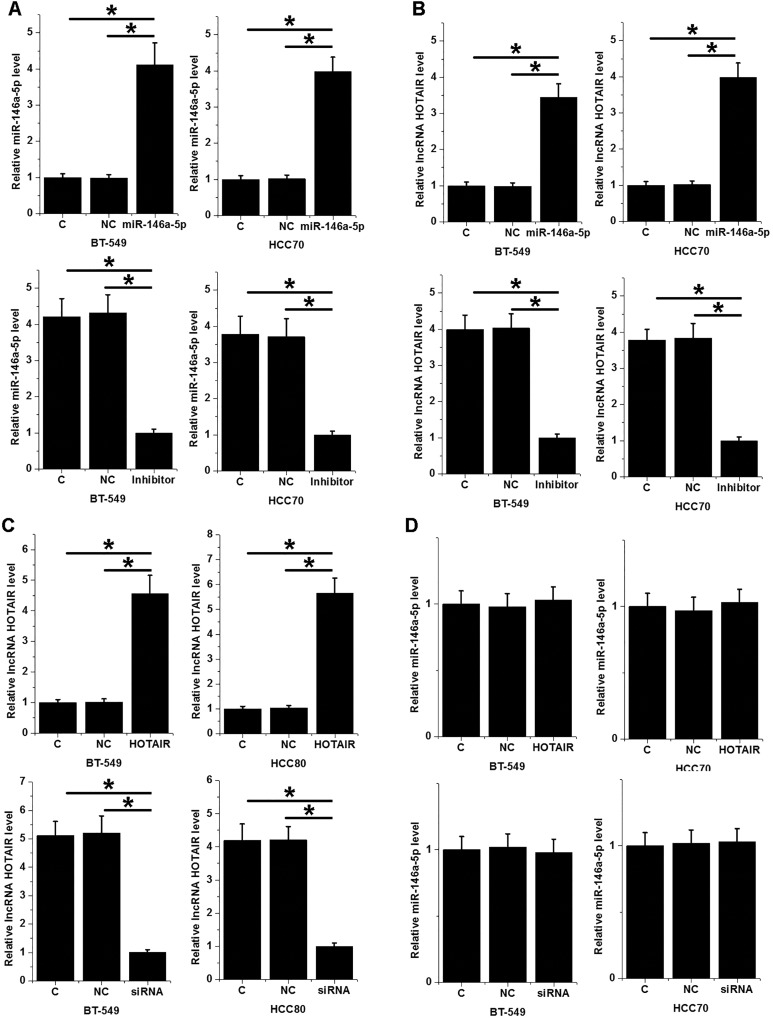
MiR-146a-5p Is a Potential Upstream Activator of HOTAIR in TNBC Cells
Overexpression and knockdown experiments were performed to further investigate the interactions between miR-146a-5p and HOTAIR in TNBC. Comparing to C and NC 2 controls, miR-146a-5p expression was significantly altered after transfection of mimic and inhibitor (Figure 4A, P < .05). Moreover, miR-146a-5p overexpression mediated the upregulated expression of HOTAIR, while miR-146a-5p inhibition mediated the downregulated expression of HOTAIR (Figure 4B, P < .05). Similarly, comparing to C and NC 2 controls, HOTAIR expression was also significantly altered after transfection of expression vector and siRNA (Figure 4C, P < .05). However, HOTAIR overexpression and knockdown failed to affect miR-146a-5p (Figure 4D, P < .05).
Figure 4.
MiR-146a-5p is a potential upstream activator of HOTAIR in TNBC cells. Comparing to C and NC 2 controls, miR-146a-5p expression was significantly altered after transfection of mimic and inhibitor (A). Moreover, miR-146a-5p overexpression mediated the upregulated expression of HOTAIR, while miR-146a-5p inhibition mediated the downregulated expression of HOTAIR (B). Similarly, comparing to C and NC 2 controls, HOTAIR expression was also significantly altered after transfection of expression vector and siRNA (C). However, HOTAIR overexpression and knockdown failed to affect miR-146a-5p (D). *P < .05. C indicates control; NC, negative control; siRNA, small interfering RNA; TNBC, triple-negative breast cancer.
MiR-146a-5p Promoted TNBC Cell Migration and Invasion Through HOTAIR
Comparing to C and NC group, overexpression of miR-146a-5p and HOTAIR led to accelerated, while HOTAIR siRNA silencing and miR-146a-5p inhibitor led to inhibited migration (Figure 5A) and invasion (Figure 5B) of cells of TNBC cell lines BT-549 and HCC70 (P < .05). In addition, HOTAIR silencing attenuated the inhibitory effects of miR-146a-5p on cancer cell migration and invasion (P < .05).
Figure 5.
MiR-146a-5p promoted TNBC cell migration and invasion through HOTAIR. Transwell migration and invasion assays showed that, compared with control (C) and negative control (NC) groups, overexpression of miR-146a-5p and HOTAIR led to accelerated, while HOTAIR siRNA silencing and miR-146a-5p inhibitor led to inhibited migration (A) and invasion (B) of cells of TNBC cell lines BT-549 and HCC70 (P < .05). In addition, HOTAIR silencing attenuated the inhibitory effects of miR-146a-5p on cancer cell migration and invasion. (P < .05. TNBC indicates triple-negative breast cancer.
Discussion
We found that miR-146a-5p can upregulate HOTAIR, which is a well-characterized oncogenic lncRNA, to promote the development of TNBC. MiR-146a-5p plays different roles in different types of cancers.12,13 During the progression of esophageal squamous cell carcinoma, miR-146a-5p promotes epithelial–mesenchymal transition, so as to mediate the metastasis of tumors.12 In contrast, miR-146a-5p inhibits non-small cell lung cancer by inhibiting cancer cell proliferation and cell-cycle progression.13 A recent study claimed that miR-146a-5p was downregulated in TNBC, and upregulation of miR-146a-5p inhibited cancer metastasis and growth.11 However, in another study, Gao et al reported that miR-146a 5p was upregulated in breast cancer, and the downregulation of miR-146a-5p led to inhibited cancer cell proliferation.14 Our findings are consistent with Gao et al’ study,14 which showed the overexpressed expression pattern of miR-146a-5p in TNBC. However, our study also showed that miR-146a-5p promoted invasion and migration, but not proliferation (data not shown) of TNBC cells. This is possibly due to the different cell lines used in our study, which also support TNBC as a heterogeneous disease.15
HOTAIR is an oncogenic lncRNA that promotes development and progression many cancers, including TNBC.10 In effect, inhibition of HOTAIR expression now is considered as a promising therapeutic target for the treatment of TNBC.16 Our study further confirmed the oncogenic role of HOTAIR in TNBC. The crosstalk between miRNAs and lncRNAs is frequently observed during the development and progression of human cancers, especially during cancer metastasis.17 Our study first reported that miR-146a-5p is likely an upstream activator of HOTAIR in TNBC, and this crosstalk between miR-146a-5p and HOTAIR participated in the regulation of cancer cell invasion and migration. Our finding provided new insights to the pathogenesis of TNBC. However, our data also support the hypothesis that miR-146a-5p may upregulate HOTAIR in TNBC through indirect pathways due to the lack of significant correlation between miR-146a-5p and HOTAIR in adjacent healthy tissues. In conclusion, overexpression of miR-146a-5p may promote cancer cell migration and invasion by upregulating HOTAIR.
Abbreviations
- C
control
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- lncRNA
long noncoding RNA
- miRNA
microRNA
- NC
negative control
- ncRNA
noncoding RNA
- RT-qPCR
real-time quantitative polymerase chain reaction
- siRNA
small interfering RNA
- TNBC
triple-negative breast cancer
Footnotes
Authors’ Note: This study was approved by the Ethic Committee of Integrated Hospital of Traditional Chinese Medicine, Southern Medicine University (approval no. IHTCMSMU2011017695). All patients provided written informed consent prior to enrollment in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by the National Natural Science Foundation of China (grant no. 81502380), the Natural Science Foundation of Guangdong Province, China (grant no. 2016A030310379), the Medicine Scientific Research Foundation of Guangdong Province, China (A2015285, B2016054), and the Scientific Research Foundation of Southern Medicine University (PY2015N032).
ORCID iD: Lixian Zeng  https://orcid.org/0000-0001-8710-7641
https://orcid.org/0000-0001-8710-7641
References
- 1. DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67(6):439–448. [DOI] [PubMed] [Google Scholar]
- 2. Cetin I, Topcul M. Triple negative breast cancer. Asian Pac J Cancer Prev. 2014;15(6):2427–2431. [DOI] [PubMed] [Google Scholar]
- 3. Denkert C, Liedtke C, Tutt A, von Minckwitz G. Molecular alterations in triple-negative breast cancer—the road to new treatment strategies. Lancet. 2017;389(10087):2430–2442. [DOI] [PubMed] [Google Scholar]
- 4. Dent R, Trudeau M, Pritchard K I, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15):4429–4434. [DOI] [PubMed] [Google Scholar]
- 5. Nanda R, Chow L Q, Dees E C, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34(21):2460–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bosch A, Eroles P, Zaragoza R, Viña JR, Lluch A. Triple-negative breast cancer: molecular features, pathogenesis, treatment and current lines of research. Cancer Treat Rev. 2010;36(3):206–215. [DOI] [PubMed] [Google Scholar]
- 7. Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861–874. [DOI] [PubMed] [Google Scholar]
- 8. Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9(6):703–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calin G A, Croce C M. MicroRNA–cancer connection: the beginning of a new tale. Cancer Res. 2006;66(15):7390–7394. [DOI] [PubMed] [Google Scholar]
- 10. Bhan A, Mandal SS. HOTAIR: a master regulator of chromatin dynamics and cancer. Biochim Biophys Acta. 2015;1856(1):151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Si C, Yu Q, Yao Y. Effect of miR-146a-5p on proliferation and metastasis of triple-negative breast cancer via regulation of SOX5. Exp Ther Med. 2018;15(5):4515–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang C, Zhang W, Zhang L. et al. miR-146a-5p mediates epithelial–mesenchymal transition of oesophageal squamous cell carcinoma via targeting Notch2. Br J Cancer. 2018;118(6):e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li YL, Wang J, Zhang CY, et al. MiR-146a-5p inhibits cell proliferation and cell cycle progression in NSCLC cell lines by targeting CCND1 and CCND2. Oncotarget, 2016;7(37):59287–59298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gao W, Hua J, Jia Z, et al. Expression of miR 146a 5p in breast cancer and its role in proliferation of breast cancer cells. Oncol Lett. 2018;15(6):9884–9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bianchini G, Balko JM, Mayer IA, Sanders M, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13(11):674–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang YL, Overstreet AM, Chen MS, et al. Combined inhibition of EGFR and c-ABL suppresses the growth of triple-negative breast cancer growth through inhibition of HOTAIR. Oncotarget. 2015;6(13):11150–11161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cao M, Jiang Y, Tang Y, Liang XH. The crosstalk between lncRNA and microRNA in cancer metastasis: orchestrating the epithelial–mesenchymal plasticity. Oncotarget. 2017;8(7):12472–12483. [DOI] [PMC free article] [PubMed] [Google Scholar]