Abstract
Contrast-induced acute kidney injury (CI-AKI) is an important consideration in patients undergoing cardiac catheterization. There has been a continuous strive to decrease morbidity and improve procedural safety. This review will address the pathophysiology, predictors, and clinical management of CI-AKI with a concise overview of the pathophysiology and a suggested association with left atrial appendage closure. Minimizing contrast administration and intravenous fluid hydration are the cornerstones of an effective preventive strategy. A few adjunctive pharmacotherapies hold promise, but there are no consensus recommendations on prophylactic therapies.
Keywords: Contrast-induced acute kidney injury, cardiac catheterization, pathophysiology, predictors, clinical management
Introduction
Since the advent of transcatheter diagnostic and interventional procedures, there has been a continuous strive for reduced morbidity and improved procedural safety. This continues to be a high priority within the growing field of structural heart interventions. Unfortunately, most procedures require intravascular administration of contrast media, which is associated with contrast-induced acute kidney injury (CI-AKI) and a commensurate increase in morbidity. We will review the pathophysiology, predictors, and clinical management of CI-AKI, in addition to a brief overview of the pathophysiology and a proposed association with left atrial appendage closure (LAAC) and renal dysfunction.
Pathophysiology of CI-AKI
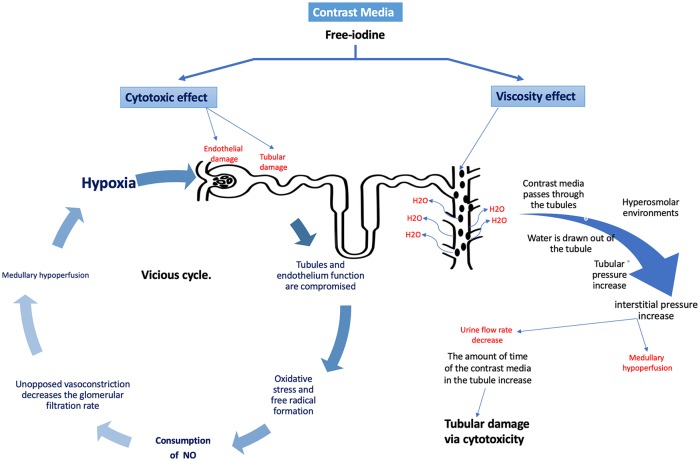
An association between radiocontrast agents and acute kidney injury has been well established. All formulations of contrast media have shown to be cytotoxic in vitro. Endothelial cells experience significant cell damage and/or apoptosis when exposed to contrast media1 (Figure 1). It is believed that free-iodine is released from the contrast during the procedure and provides a direct cytotoxic effect on the surrounding endothelial cells.2,3 In addition to damaging the surrounding endothelium, the contrast media have a cytotoxic effect on the tubules of the nephron.4 Cell damage on both fronts leads to oxidative stress and formation of free radicals. The generation of free radicals and reactive oxygen species (ROS) consumes NO and consequently prevents the protective effect of NO as a vasodilator.1 Tsarouhas et al5 emphasized that oxidative stress plays a role in CI-AKI; contrast mobilizes the antioxidant defense mechanism. In their study, a transradial approach reduces this risk. The sustained vasoconstriction, which could last for hours, decreases the glomerular filtration rate (GFR), causes medullary hypoperfusion, and increases blood viscosity through the nephron.2 Medullary hypoperfusion impedes oxygen delivery, resulting in ischemic injury to the tubules, which sustains the vicious cycle. In addition to its cytotoxic effects, the water-soluble contrast media can cause renal damage due to the difference in osmolarity relative to the surrounding tissue.2 Contrast media solutions with higher osmolarity are associated with higher cytotoxicity. As the contrast media pass through the tubules, it is exposed to increasingly hyperosmolar environments, especially in the medulla. Subsequently, water is drawn out of the tubules into the medulla, leading to an increase in the viscosity of the tubular fluid.2 This is especially problematic, as there is an increase in tubular pressure, while there is a decrease in the urine flow rate. With the increase in tubular pressure, interstitial pressure increases, further exacerbating the medullary hypoperfusion caused by the previously discussed mechanism. Decreased urine flow rate increases contrast media retention, thus increasing its cytotoxic impact.2 Mamoulakis et al6 evaluated the biomarkers that may reflect kidney damage in the blood and tissue samples of the animal model following administration of contrast. Iopromide is a molecule in the contrast media found to cause significant elevation serum creatinine (sCr) 68.2% within 48 hours, and serum symmetric-asymmetric dimethylarginine (SDMA-ADMA) levels. These markers were associated with an increase in renal-cell degeneration and apoptosis.
Figure 1.
The proposed mechanism of contrast-media-mediated nephrotoxicity.
Contrast media mediates renal toxicity via the interplay of (1) direct cytotoxicity to the renal endothelial and tubular cells, leading to a cycle of oxidative stress, hypoxia, and further tubular damage, and (2) viscous properties of contrast-triggering vasoconstriction, reduced urinary flow rate, and medullary hypoperfusion.
In summary, it is the interplay of cytotoxicity and viscosity that forms the pathophysiological basis of CI-AKI (Table 1). As will be discussed later, preprocedural hydration with normal saline (0.9% NaCl)7 and intravenous (IV) hydration reduces the adverse effects of contrast by flushing of the renal tubules.2
Table 1.
Literature review—pathophysiology of contrast-induced acute kidney injury.
| S. No. | Author, journal | Year | Design | Conclusion |
|---|---|---|---|---|
| 1 | Sendeski Clin Exp Pharmacol Physiol |
2011 | Review | • Oxidative stress causes direct cell-membrane damage • Increased osmolarity of CM exacerbates cell damage |
| 2 | Seeliger Eur Heart J |
2012 | Review | • CM becomes concentrated in the tubules • Concentrated CM leads to reduced urine flow rate • Reduced urine flow rate increases timed exposure to CM • Hydration flushes tubules and prevents CI-AKI |
| 3 | Solomon and Dauerman Circulation |
2010 | Review | • Risk factors include diabetes mellitus, CHF, acute hypotension, STEMI, and volume depletion • Association between CI-AKI with poor short- and long-term outcomes |
| 4 | Au et al Ann Pharmacotherapy |
2014 | Review | • Insufficient clinical studies evaluating strategies for the prevention of CI-AKI • Best data support preprocedural hydration with normal saline solution |
Abbreviations: CHF, congestive heart failure; CI-AKI, contrast-induced acute kidney injury; CM, contrast media; STEMI, ST-elevation myocardial infarction.
Age-Related Changes in Renal Function
One of the most important risk factors for CI-AKI is age.8 Changes in renal physiology over time lead to a reduction in renal function (Table 2), which may explain why age is a strong risk factor for CI-AKI.
Table 2.
Literature review—age-related changes in renal function.
| S. No. | Author, journal | Year | Design | Conclusion |
|---|---|---|---|---|
| 1 | Bolignano et al Aging Res Rev |
2014 | Review | • Renal aging is multifactorial • Low-advanced glycosylation end-product diets with high content in antioxidants currently represent the most plausible approach to maintain kidney health |
| 2 | Muslem et al Am J Cardiol |
2017 | Retrospective | • Age >60 years is an independent predictor for an impaired renal function and mortality |
| 3 | Denegri et al Catheter Cardiovasc Interv |
2019 | Prospective | • Postprocedural risk stratification using the simple ACEF-7 score significantly better predicted long-term outcome than commonly used risk scores |
| 4 | Weinstein and Anderson Adv Chronic Kidney Dis |
2010 | Review | • GFR stays at about 140 mL/min/1.73 m2 until the fourth decade; then GFR declines by about 8 ml/min/1.73 m2 each decade |
| 5 | Fliser et al Kidney Int |
1997 | Prospective | • GFR is preserved at the expense of an increased filtration fraction in a vasoconstricted kidney • Age-related abnormalities of renal function are more marked in patients with comorbid conditions |
| 6 | Delp et al J Physiol |
2008 | Prospective | • The impairment of endothelium-dependent vasodilatation induced by old age is due to an altered nitric oxide signaling mechanism • The age-related deficit in flow-mediated vasodilatation appears to be the result, in part, of limited BH4 bioavailability |
Abbreviations: ACEF, the age, creatinine, and ejection fraction; BH4, tetrahydrobiopterin; GFR, glomerular filtration rate.
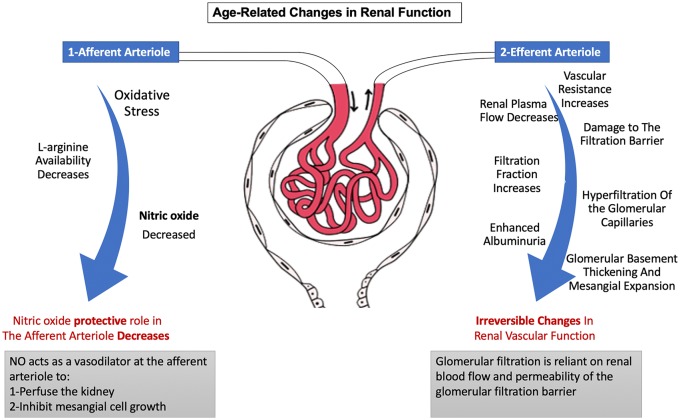
Glomerular filtration rate is determined by renal blood flow and permeability of the glomerular filtration barrier. Increasing age is accompanied by a decrease in renal plasma flow due to an increase in vascular resistance of the efferent arterioles9 (Figure 2). Consequently, the decrease in renal plasma flow causes an overall increase in the filtration fraction. Increased filtration fraction leads to hyperfiltration of the glomerular capillaries, which can damage the filtration barrier and lead to enhanced albuminuria.10,11 Over time, these processes lead to irreversible thickening of the glomerular basement membrane and expansion of the mesangium.12 A decline in nitric oxide—a vascular vasodilator that inhibits mesangial cell growth13—is also seen with advanced age due to oxidative stress and decreased L-arginine availability.14
Figure 2.
Changes in renal pathophysiology with age.
A gradual decline in nitric oxide leads to vasoconstriction of the afferent and efferent arterioles, increased mesangial cell growth and fibrosis, and an irreversible decline in renal function.
The magnitude of the decline in GFR varies across studies. In a systemic review, the yearly decline in estimated gomerular filtration rate (eGFR) ranged from 0.4 to 2.6 mL/min/1.73 m2.9 However, most studies used a GFR estimate to examine the natural history of renal function. In a study by Fliser et al15 using the gold standard method of inulin clearance, the GFR of a healthy group of volunteers with an average age of 68 ± 7 years was 85% lower than a similarly healthy group of volunteers with an average age of 26 ± 3.
The reduction in GFR with age and the pathophysiological basis for this reduction places it at an increased risk of AKI from other insults.
Definition, Clinical Implication, Controversies, and Risk Assessment
Contrast-induced acute kidney injury is defined as an increase in sCr by 0.3 mg/dL or an increase in creatinine to ⩾1.5 times baseline within 3 to 5 days following contrast exposure.16 Using this definition of CI-AKI, a study of 33 249 patients with acute myocardial infarction undergoing cardiac catheterization demonstrated a dramatic decline in the incidence of CI-AKI from 24.6% in 2000 to 16.5% in 2008.17 The National Cardiovascular Data Registry (NCDR) Cath-PCI registry data have provided an excellence data source for examining contemporary trends, predictors, and outcomes of CI-AKI in patients undergoing percutaneous coronary intervention (PCI). Data collected between 2009 and 2011 from 985 737 patients in the Cath-PCI registry revealed an overall CI-AKI incidence of 7.1%, with 0.3% requiring dialysis18 Other studies of the NCDR Cath-PCI registry since have demonstrated similar rates of CI-AKI between 7% and 8%,19,20 likely suggesting a leveling off in this decline in the incidence of CI-AKI.
Although the incidence of the need for dialysis remains low, the occurrence of CI-AKI is a significant sentinel event. It is associated with prolonged hospitalization and increased short- and long-term mortality.21 Contrast-induced acute kidney injury risk of in-hospital mortality (9.7%; adjusted odds ratio [OR]: 7.8; 95% CI: 7.4-8.1, P < .001).18 Contrast-induced acute kidney injury is also associated with an increase in postdischarge adverse events. A study linking the Cath-PCI registry to the Center for Medicare & Medicaid Services (CMS) billing data, found a higher postdischarge rate of death, myocardial infarction, and bleeding at 1 year, with the highest rates occurring within 30 days.19 Patients with CI-AKI requiring dialysis have higher rates (34%) of in-hospital mortality (OR: 21.7; 95% CI: 19.6-24.1; P < .001)18 and postdischarge adverse events19 compared with subjects without CI-AKI.
Furthermore, CI-AKI is associated with accelerated progression of chronic kidney disease (CKD). In a retrospective cohort study of 11 249 patients undergoing coronary angiography, patients with CI-AKI were more likely to have sustained reduction in renal function at 3 months. In adjusted analysis, the odds of a decline in renal function were higher in those with moderate to severe CI-AKI (OR: 17.31, 95% confidence interval [CI]: 12.03-24.90) compared with milder AKI (OR: 4.74, 95% CI: 3.92-5.74).22
It should be noted that although the entity of CI-AKI is relatively well accepted in the cardiology and nephrology community, there is skepticism regarding the true incidence of the disease. Rates of CI-AKI in patients undergoing contrast-enhanced computed tomography exam are lower (5%-6%). Wilhelm-Leen et al23 found that patients to whom radiocontrast was and was not administered developed AKI at rates of 5.5% and 5.6%, respectively. Moreover, adverse outcomes in these patients is negligible (~0%) compared with the cardiac studies above.24 A meta-analysis of studies comparing contrasted and noncontrasted radiology exams demonstrated no difference in the rates of CI-AKI.25 There are multiple potential explanations for these discrepant findings. Patients undergoing cardiac catheterization likely have more comorbidities or present in the setting of acute coronary syndrome with tenuous hemodynamic status, all of which contribute to a higher risk of CI-AKI. Moreover, it has long been known that injection of contrast in the arterial bed or near the renal arteries is associated with a higher risk of CI-AKI compared with IV administration.24 Atherosclerotic emboli could also partly explain these findings. Coronary angiography had traditionally been done via an access through the femoral arteries, which requires wire and catheter manipulation across the renal arteries. This could lead to embolization of plaque to the distal renal vascular bed and contribute to the onset of CI-AKI. This proposed mechanism is supported by a meta-analysis of studies comparing radial versus femoral artery access for coronary angiography that demonstrated a lower risk of CI-AKI in the radial access group.26
As discussed below, therapeutic options for CI-AKI are limited. Therefore, it is imperative to identify risk factors and establish preventive strategies to minimize CI-AKI in all patient populations. The most critical risk factor for CI-AKI is preexisting renal impairment.27,28 In patients undergoing cardiac catheterization, moderate to severe CKD (eGFR < 30), ST-elevation myocardial infarction, and cardiogenic shock are associated with more than quadrupling of risk.18 Other predictors of CI-AKI include diabetes, heart failure, contrast volume, and anemia.29 Mehran et al30 and Tsai et al18 developed two separate validated risk-prediction tools that incorporate the above risks in addition to other variables with modest discrimination. These tools are useful in clinical decision-making with patients and families, in addition to guide the clinician in determining preventive therapies.
Prevention and Management of CI-AKI
Minimizing contrast
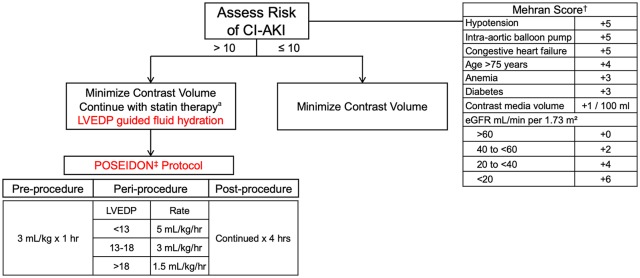
As there is no treatment for CI-AKI, prevention is the optimal management strategy. Multiple studies have demonstrated a direct correlation between the volume of contrast administered and risk of CI-AKI.30 Therefore, it is imperative that the volume of contrast administered is the lowest amount reasonable for a successful procedure (Figure 3). A contrast volume to creatinine clearance ratio of >2.44 is shown to be associated with CI-AKI.31 Automated injection systems are available to minimize “excessive” contrast injection by the operator; however, these systems have not been shown to reduce the rate of CI-AKI.32
Figure 3.
Proposed algorithm for CI-AKI prevention in cardiac Cath patients.
Minimizing contrast administration and intravenous fluid hydration for effective preventive strategies. CI-AKI indicates contrast-induced acute kidney injury; GFR, glomerular filtration rate; LVEDP, left ventricular end-diastolic pressure.
aNo guideline recommendations on statin initiation for CI-AKI prevention.
High-osmolar agents had previously been shown to be associated with high risk of CI-AKI, and therefore, their use has been virtually eliminated for coronary angiography.33 Iso-osmolar agents were shown to further reduce the rate of CI-AKI compared with low-osmolar agents in high-risk patients with underlying renal impairment undergoing coronary angiography.34 However, these findings were not replicated in subsequent studies and meta-analyses comparing the two agents,35,36 and consensus documents do not recommend the use of either agent.37
IV fluid hydration
As mentioned previously, normal saline (0.9% NaCl) flushes the renal tubules and facilitates the transition of the water-soluble contrast through nephrons, thereby reducing its cytotoxic impact. Hydration is the only accepted practice for prevention and management of CI-AKI. In a study of 408 patients presenting with ST-elevation myocardial infarction (STEMI), patients randomized to routine hydration using IV normal saline (1 mL/kg/min) from the onset of the procedure till 24 hours postprocedure had a significant reduction in CI-AKI than the no hydration group (11% vs 21%, P = .016).38 Although normal saline appears to be superior to half-normal hydration,39 studies have failed to show superiority of sodium bicarbonate compared with normal saline hydration.40,41
Although adequate hydration is an important preventive strategy, volume overload should be avoided, particularly in patients presenting with left ventricular dysfunction (i.e. preexisting heart failure or acute coronary syndrome). One hydration strategy features the use of an invasive hemodynamic measure as a guide for fluid administration. The POSEIDON (Prevention of Contrast Renal Injury with Different Hydration Strategies) trial randomized 396 patients with preexisting renal impairment (eGFR < 60) and an additional risk factor (diabetes, heart failure, hypertension, and age >75) to left ventricular end-diastolic pressure (LVEDP)-guided hydration strategy or standard fluid administration. Standard group received a bolus infusion of 3 mL/kg for 1 hour prior to the procedure, followed by 1.5 mL/kg/hr for 4 hours. The LVEDP-guided group received the same preprocedural hydration, but the periprocedural and postprocedural hydration were guided by LVEDP. There was a significant reduction in the rate of CI-AKI with the LVEDP-guided group relative to the standard group (relative risk [RR] = 0.41; 95% CI: 0.22-0.79; P = .005, which paralleled a reduction in the 6-month major adverse events rate—a composite of all-cause mortality, myocardial infarction, and hemodialysis (RR: 0.32; 95% CI: 0.13-0.79; P = .008; Figure 3).42
Pharmacotherapies
Multiple adjunctive pharmacotherapies for CI-AKI prevention have been tested with mixed outcomes (Table 3). N-acetylcysteine (NAC) was hypothesized to reduce the risk of CI-AKI via ROS scavenging and is recommended by Kidney Disease Improving Global Outcomes (KDIGO) for contrast prophylaxis in high-risk patients. However, two of the largest studies to date, PRESERVE and ACT trials, have failed to demonstrate a reduction in adverse renal outcomes with oral NAC.41 Routine use of NAC for CI-AKI prevention is class III in the ACC/AHA guidelines (Table 4).43
Table 3.
Literature review—risk assessment, prevention, and management of CI-AKI.
| S. No. | Author, journal | Year | Design | Conclusion |
|---|---|---|---|---|
| 1 | McDonald et al Radiology |
2013 | Metaanalysis | • Controlled contrast medium-induced nephropathy studies show similar incidences of acute kidney injury and death between the control and contrast medium groups |
| 2 | Owen et al Can Assoc Radiol J |
2014 | Review | • The most important risk factor is preexisting renal impairment • Serum creatinine is not an accurate indicator of renal function • eGFR should be used to assign risk levels and implement proper prevention strategies |
| 3 | Andreucci et al ScientificWorldJournal |
2014 | Review | • The incidence of CIN is less than it was in the past • Clinicians must identify risk factors and follow precautions • Most important risk factors are kidney function before the procedure and adequate hydration |
| 4 | Mehran et al J Am Coll Cardiol |
2004 | Retrospective | • The risk of CIN after PCI can be simply assessed using readily available information • This risk score can be used for both clinical and investigational purposes |
| 5 | Mehran et al JACC Cardiovasc Interv |
2018 | Prospective | • AVERT significantly reduced the amount of contrast media used • No significant differences in CI-AKI were observed with AVERT in this trial |
| 6 | Deek et al Aust Crit Care |
2014 | Review | • Administer IV fluid of 1 mL/kg/hr of 0.9% saline for 12 hours before the procedure and 6 hours after the procedure • Use of NAC and sodium bicarbonate were controversial • Beta-blocker before the diagnostic test was found to be effective • Theophylline administration prevents CIN in moderate- to high-risk patients |
| 7 | Jurado-Roman et al Am J Cardiol |
2015 | Prospective | • Intravenous saline hydration during procedure reduced the risk of CIN to 48% • Patients who experienced CIN had increased mortality and required dialysis • Preventive hydration should be mandatory in all patients unless contraindicated |
| 8 | Mueller et al Arch Intern Med |
2002 | Prospective | • Isotonic hydration is superior to half-isotonic hydration in the prevention of contrast media-associated nephropathy |
| 9 | Liu et al Circ Cardiovasc Interv |
2015 | Prospective | • The V/CrCl ratio adjusted for HV/W may be a more reliable predictor of CIN and even long-term outcomes after cardiac catheterization |
Abbreviations: CI-AKI, contrast-induced acute kidney injury; CIN, contrast-induced injury; eGFR, estimated gomerular filtration rate; NAC, N-acetylcysteine; PCI, percutaneous coronary intervention; V/CrCl, the ratios of contrast volume-to-creatinine clearance; HV/W, hydration volume to body weight.
Table 4.
Literature review—efficacy of percutaneous coronary intervention and associated kidney injury and mortality.
| S. No. | Author, journal | Year | Design | Conclusion |
|---|---|---|---|---|
| 1 | Valsson et al Intensive Care Med |
1996 | Prospective | • The improvement in renal blood flow and glomerular filtration rate may be of potential therapeutic value to prevent or treat exaggerated renal vasoconstriction in patients with acute renal impairment following cardiac surgery |
| 2 | Liu et al Biomed Res Int |
2016 | Prospective | • Combined with hydration, exogenous BNP administration before CM effectively decreases CIN incidence in CKD patients |
| 3 | Xing et al Heart Vessels |
2016 | Prospective | • The incidence of renal injury was not different between rhBNP and nitroglycerin in STEMI-HF patients with mild renal insufficiency • Infusion of rhBNP was associated with a decline in the rate of CIN |
| 4 | Morikawa et al J Am Coll Cardiol |
2009 | Prospective | • ANP administration is effective in the prevention of CIN in patients with chronic renal failure, and the effect was maintained for 1 month |
| 5 | Sezai et al J Am Coll Cardiol |
2011 | Prospective | • The improvement in renal blood flow and glomerular filtration rate may be of potential therapeutic value to prevent or treat exaggerated renal vasoconstriction in patients with acute renal impairment following cardiac surgery |
Abbreviations: ANP, atrial natriuretic peptide; BNP, B-type natriuretic peptide; CIN, contrast-induced injury; CKD, chronic kidney disease; CM, contrast media; rhBNP, recombinant human brain natriuretic peptide; STEMI-HF, ST-segment elevation myocardial infarction—heart failure.
Similarly, the anti-inflammatory effects of statin, in addition to other potential hypothesized reno-protective pathways, are thought to position it well as an adjunctive therapy in prevention of renal injury. Two randomized trials of rosuvastatin administered to patients undergoing coronary or peripheral angiography demonstrated significant reduction in the rates of CI-AKI.44,45 Few meta-analyses of randomized trials confirmed these findings, and further demonstrated a significant reduction with high-dose versus low-dose statins.46,47 It is important to note that most patients who are referred for coronary angiography, however, are likely on statin therapy or will be prescribed a statin regardless of the renal benefit. There are no guideline recommendations on the use of statins for CI-AKI prevention.
Studies of renin-angiotensin-aldosterone inhibitor (RASi) on CI-AKI have mixed findings, with harm, benefit, and neutral signals all demonstrated. The CAPTAIN trial of 208 patients with CKD undergoing cardiac catheterization randomized to hold (>24 hours) versus continue RASi failed to demonstrate a significant reduction in CI-AKI in the hold group (hazard ratio = 0.59; 95% CI: 0.30-1.19; P = .16), possibly due to the small sample size and beta error.48 Current ACC/AHA guidelines do not make any recommendations on the preprocedural management of RASi, and KDIGO guidelines posit that there is insufficient evidence to recommend discontinuation of these agents.
Finally, initial concerns regarding withholding metformin stemmed from isolated case reports of death from CI-AKI and lactic acidosis in patients on metformin undergoing IV contrast studies. However, all reports of lactic acidosis have occurred in patients with severe underlying cardiac or renal disease.49 There are no ACC/AHA or KDIGO guidelines regarding withholding metformin, and the 2018 American college of radiology guidelines recommends holding metformin only in patients with CI-AKI or GFR <30.50
Proposed Association of LAAC and With Kidney Injury
The impact of new percutaneous catheter-based procedures on acute kidney injury is not well explored. In particular, the impact of LAAC on renal function is not well explored. Left atrial appendage closure is a transcatheter procedure for stroke risk reduction in patients with atrial fibrillation who are not candidates for long-term anticoagulation. Left atrial appendage closure is thought to reduce risk of stroke by excluding the appendage—where thrombus formation can occur in atrial fibrillation—from the remaining cardiac chambers; however, it also uncouples the appendage from hemodynamic changes in the left atrium. Two important regulatory peptides, atrial natriuretic peptide (ANP) and to a lesser extent brain natriuretic peptide (BNP), are stored and released from the left atrial appendage in response to stretch mechanoreceptors. Levels of both these peptides have been shown to decrease acutely after LAAC.51 Atrial natriuretic peptide and BNP are key regulatory peptides that cause vasodilation of the afferent arterioles, decrease renal vascular resistance, increase renal blood flow, and consequently increase natriuresis and diuresis.52,53 These down-stream actions of the peptides may have an important reno-protective quality.
In a study of 106 patients with mild underlying CKD undergoing elective coronary angiography or PCI, subjects were randomized to a recombinant form of BNP versus placebo. There was a significant reduction in the primary outcome of CI-AKI (6.6% vs 16.5%, P = .025). Patients with CI-AKI in the BNP arm had less deterioration and faster recovery of eGFR. In multivariable analysis, BNP administration was protective and contrast volume >100 mL was associated with CI-AKI.54 A study of STEMI patients with heart failure and mild CKD also demonstrated a similar reduction in the rate of contrast-induced injury (CIN) in patients randomized to recombinant BNP infusion (12.28 vs 28.81%, P < .05).55
In a randomized controlled trial, Morikawa et al56 randomized 254 patients undergoing coronary angiography to infusion of ANP or lactated ringers. There was a significant reduction in the rate of CIN in the ANP group compared with the control group (3.2% vs 11.7%; P = .015), with a persistently lower creatinine in the ANP group at 1-month follow-up. A randomized study of patients after coronary artery bypass graft demonstrated a significant reduction in creatinine increase rate, in addition to a significant reduction in the need for dialysis and cardiac events in the ANP arm compared to placebo.57
The rate of CI-AKI in a small study of 355 patients undergoing LAAC was 9%, which is a slightly higher rate compared with patients undergoing cardiac catheterization (~7%).58 This is despite the IV hydration in almost all patients undergoing LAAC, with a goal of mean left atrial pressure >12 mmHg. In this population, CI-AKI was associated with higher in-hospital bleeding events, out-of-hospital bleeding events, systemic embolic events, and mortality. The higher rate of CI-AKI can be attributed to a patient population with more risk factors. In our single-center experience of 106 patients undergoing LAAC, we noted a significant decline in eGFR at 6 month follow-up (71.9% vs 62.8%; P < .001). Collectively, these studies add traction to a possible reno-protective role for ANP and BNP by reducing the risk of CI-AKI in patients undergoing cardiac procedures. Hence, in addition to the intra-cardiac iodinated contrast administration, LAAC may increase the risk of CI-AKI by reducing circulating levels of ANP and BNP. Additional larger studies are needed to assess the short- and long-term impacts of LAAC on renal function.
Conclusions
Contrast-induced acute kidney injury continues to be a major comorbidity in patients undergoing cardiac catheterization. It is associated with major adverse clinical outcomes and death. Minimizing contrast administration and IV fluid hydration are the cornerstones of an effective preventive strategy. Although a few adjunctive pharmacotherapies hold promise, there are no consensus recommendations on prophylactic therapies. There is a theoretical concern for an increased risk of renal injury with LAAC that is mediated by a reduction in natriuretic peptides. Further studies are needed to investigate this proposed link.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author’s note: Priya Bansal, Haider Al Taii, Houman Khalili, Brijeshwar Maini are also affiliated to Department of Cardiovascular Diseases, Florida Atlantic University, Boca Raton, FL 3348.
Author Contributions: All authors contributed extensively to the work presented in this literature review.
ORCID iD: Ramez Morcos  https://orcid.org/0000-0002-7234-6364
https://orcid.org/0000-0002-7234-6364
References
- 1. Sendeski MM. Pathophysiology of renal tissue damage by iodinated contrast media. Clin Exp Pharmacol Physiol. 2011;38:292-299. [DOI] [PubMed] [Google Scholar]
- 2. Seeliger E, Sendeski M, Rihal CS, Persson PB. Contrast-induced kidney injury: mechanisms, risk factors, and prevention. Eur Heart J. 2012;33:2007-2015. [DOI] [PubMed] [Google Scholar]
- 3. Mamoulakis C, Tsarouhas K, Fragkiadoulaki I, et al. Contrast-induced nephropathy: basic concepts, pathophysiological implications and prevention strategies. Pharmacol Ther. 2017;180:99-112. [DOI] [PubMed] [Google Scholar]
- 4. Solomon R, Dauerman HL. Contrast-induced acute kidney injury. Circulation. 2010;122:2451-2455. [DOI] [PubMed] [Google Scholar]
- 5. Tsarouhas K, Tsitsimpikou C, Papantoni X, et al. Oxidative stress and kidney injury in trans-radial catheterization. Biomed Rep. 2018;8:417-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mamoulakis C, Fragkiadoulaki I, Karkala P, et al. Contrast-induced nephropathy in an animal model: evaluation of novel biomarkers in blood and tissue samples. Toxicol Rep. 2019;6:395-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Au TH, Bruckner A, Mohiuddin SM, Hilleman DE. The prevention of contrast-induced nephropathy. Ann Pharmacother. 2014;48:1332-1342. [DOI] [PubMed] [Google Scholar]
- 8. O’Sullivan ED, Hughes J, Ferenbach DA. Renal aging: causes and consequences. J Am Soc Nephrol. 2017;28:407-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bolignano D, Mattace-Raso F, Sijbrands EJ, Zoccali C. The aging kidney revisited: a systematic review. Ageing Res Rev. 2014;14:65-80. [DOI] [PubMed] [Google Scholar]
- 10. Muslem R, Caliskan K, Akin S, et al. Effect of age and renal function on survival after left ventricular assist device implantation. Am J Cardiol. 2017;120:2221-2225. [DOI] [PubMed] [Google Scholar]
- 11. Denegri A, Mehran R, Holy E, et al. Post procedural risk assessment in patients undergoing trans aortic valve implantation according to the age, creatinine, and ejection fraction-7 score: advantages of age, creatinine, and ejection fraction-7 in stratification of post-procedural outcome. Catheter Cardiovasc Interv. 2019;93: 141-148. [DOI] [PubMed] [Google Scholar]
- 12. Weinstein JR, Anderson S. The aging kidney: physiological changes. Adv Chronic Kidney Dis. 2010;17:302-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baylis C. Sexual dimorphism in the aging kidney: differences in the nitric oxide system. Nat Rev Nephrol. 2009;5:384-396. [DOI] [PubMed] [Google Scholar]
- 14. Delp MD, Behnke BJ, Spier SA, Wu G, Muller-Delp JM. Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J Physiol. 2008;586:1161-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fliser D, Franek E, Joest M, Block S, Mutschler E, Ritz E. Renal function in the elderly: impact of hypertension and cardiac function. Kidney Int. 1997;51: 1196-1204. [DOI] [PubMed] [Google Scholar]
- 16. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179-c184. [DOI] [PubMed] [Google Scholar]
- 17. Amin AP, Salisbury AC, McCullough PA, et al. Trends in the incidence of acute kidney injury in patients hospitalized with acute myocardial infarction. Arch Intern Med. 2012;172:246-253. [DOI] [PubMed] [Google Scholar]
- 18. Tsai TT, Patel UD, Chang TI, et al. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the NCDR Cath-PCI registry. JACC Cardiovasc Interv. 2014;7:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Valle JA, McCoy LA, Maddox TM, et al. Longitudinal risk of adverse events in patients with acute kidney injury after percutaneous coronary intervention: insights from the National Cardiovascular Data Registry [published online ahead of print April 2017]. Circ Cardiovasc Interv. doi:10.1161/CIRCINTERVEN-TIONS.116.004439. [DOI] [PubMed] [Google Scholar]
- 20. Amin AP, Bach RG, Caruso ML, Kennedy KF, Spertus JA. Association of variation in contrast volume with acute kidney injury in patients undergoing percutaneous coronary intervention. JAMA Cardiol. 2017;2:1007-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Neyra JA, Shah S, Mooney R, Jacobsen G, Yee J, Novak JE. Contrast-induced acute kidney injury following coronary angiography: a cohort study of hospitalized patients with or without chronic kidney disease. Nephrol Dial Transplant. 2013;28:1463-1471. [DOI] [PubMed] [Google Scholar]
- 22. James MT, Ghali WA, Tonelli M, et al. Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney Int. 2010;78:803-809. [DOI] [PubMed] [Google Scholar]
- 23. Wilhelm-Leen E, Montez-Rath ME, Chertow G. Estimating the risk of radiocontrast-associated nephropathy. J Am Soc Nephrol. 2017;28:653-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Katzberg RW, Newhouse JH. Intravenous contrast medium-induced nephrotoxicity: is the medical risk really as great as we have come to believe. Radiology. 2010;256:21-28. [DOI] [PubMed] [Google Scholar]
- 25. McDonald JS, McDonald RJ, Comin J, et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267:119-128. [DOI] [PubMed] [Google Scholar]
- 26. Ando G, Capodanno D. Radial versus femoral access in invasively managed patients with acute coronary syndrome: a systematic review and meta-analysis. Ann Intern Med. 2015;163:932-940. [DOI] [PubMed] [Google Scholar]
- 27. Owen RJ, Hiremath S, Myers A, Fraser-Hill M, Barrett BJ. Canadian Association of Radiologists consensus guidelines for the prevention of contrast-induced nephropathy: update 2012. Can Assoc Radiol J. 2014;65:96-105. [DOI] [PubMed] [Google Scholar]
- 28. Andreucci M, Faga T, Pisani A, Sabbatini M, Russo D, Michael A. Prevention of contrast-induced nephropathy through a knowledge of its pathogenesis and risk factors. ScientificWorldJournal. 2014;2014:823169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sato A, Aonuma K, Watanabe M, et al. Association of contrast-induced nephropathy with risk of adverse clinical outcomes in patients with cardiac catheterization: from the CINC-J study. Int J Cardiol. 2017;227:424-429. [DOI] [PubMed] [Google Scholar]
- 30. Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393-1399. [DOI] [PubMed] [Google Scholar]
- 31. Liu Y, Chen JY, Tan N, et al. Safe limits of contrast vary with hydration volume for prevention of contrast-induced nephropathy after coronary angiography among patients with a relatively low risk of contrast-induced nephropathy [published online ahead of print June 2015]. Circ Cardiovasc Interv. doi:10.1161/CIRCINTERVENTIONS.114.001859. [DOI] [PubMed] [Google Scholar]
- 32. Mehran R, Faggioni M, Chandrasekhar J, et al. Effect of a contrast modulation system on contrast media use and the rate of acute kidney injury after coronary angiography. JACC Cardiovasc Interv. 2018;11:1601-1610. [DOI] [PubMed] [Google Scholar]
- 33. Deek H, Newton P, Sheerin N, Noureddine S, Davidson PM. Contrast media induced nephropathy: a literature review of the available evidence and recommendations for practice. Aust Crit Care. 2014;27:166-171. [DOI] [PubMed] [Google Scholar]
- 34. Jo SH, Youn TJ, Koo BK, et al. Renal toxicity evaluation and comparison between visipaque (iodixanol) and hexabrix (ioxaglate) in patients with renal insufficiency undergoing coronary angiography: the RECOVER study: a randomized controlled trial. J Am Coll Cardiol. 2006;48:924-930. [DOI] [PubMed] [Google Scholar]
- 35. Solomon RJ, Natarajan MK, Doucet S, et al. Cardiac Angiography in Renally Impaired Patients (CARE) study: a randomized double-blind trial of contrast-induced nephropathy in patients with chronic kidney disease. Circulation. 2007; 115:3189-3196. [DOI] [PubMed] [Google Scholar]
- 36. Reed M, Meier P, Tamhane UU, Welch KB, Moscucci M, Gurm HS. The relative renal safety of iodixanol compared with low-osmolar contrast media: a meta-analysis of randomized controlled trials. JACC Cardiovasc Interv. 2009;2:645-654. [DOI] [PubMed] [Google Scholar]
- 37. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. 2014;130:e344-e426. [DOI] [PubMed] [Google Scholar]
- 38. Jurado-Román A, Hernández-Hernández F, García-Tejada J, et al. Role of hydration in contrast-induced nephropathy in patients who underwent primary percutaneous coronary intervention. Am J Cardiol. 2015;115:1174-1178. [DOI] [PubMed] [Google Scholar]
- 39. Mueller C, Buerkle G, Buettner HJ, et al. Prevention of contrast media-associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002;162:329-336. [DOI] [PubMed] [Google Scholar]
- 40. Solomon R, Gordon P, Manoukian SV, et al. Randomized trial of bicarbonate or saline study for the prevention of contrast-induced nephropathy in patients with CKD. Clin J Am Soc Nephrol. 2015;10:1519-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weisbord SD, Gallagher M, Jneid H, et al. Outcomes after angiography with sodium bicarbonate and acetylcysteine. N Engl J Med. 2018;378:603-614. [DOI] [PubMed] [Google Scholar]
- 42. Brar SS, Aharonian V, Mansukhani P, et al. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: the POSEIDON randomised controlled trial. Lancet. 2014;383:1814-1823. [DOI] [PubMed] [Google Scholar]
- 43. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American college of cardiology foundation/American heart association task force on practice guidelines and the society for cardiovascular angiography and interventions. Circulation. 2011;124:e574-e651. [DOI] [PubMed] [Google Scholar]
- 44. Han Y, Zhu G, Han L, et al. Short-term rosuvastatin therapy for prevention of contrast-induced acute kidney injury in patients with diabetes and chronic kidney disease. J Am Coll Cardiol. 2014;63:62-70. [DOI] [PubMed] [Google Scholar]
- 45. Leoncini M, Toso A, Maioli M, Tropeano F, Villani S, Bellandi F. Early high-dose rosuvastatin for contrast-induced nephropathy prevention in acute coronary syndrome: results from the PRATO-ACS Study (Protective Effect of Rosuvastatin and Antiplatelet Therapy On contrast-induced acute kidney injury and myocardial damage in patients with Acute Coronary Syndrome). J Am Coll Cardiol. 2014;63:71-79. [DOI] [PubMed] [Google Scholar]
- 46. Li H, Wang C, Liu C, Li R, Zou M, Cheng G. Efficacy of short-term statin treatment for the prevention of contrast-induced acute kidney injury in patients undergoing coronary angiography/percutaneous coronary intervention: a meta-analysis of 21 randomized controlled trials. Am J Cardiovasc Drugs. 2016;16: 201-219. [DOI] [PubMed] [Google Scholar]
- 47. Zhou X, Dai J, Xu X, et al. Comparative efficacy of statins for prevention of contrast-induced acute kidney injury in patients with chronic kidney disease: a network meta-analysis. Angiology. 2019;70:305-316. [DOI] [PubMed] [Google Scholar]
- 48. Bainey KR, Rahim S, Etherington K, et al. Effects of withdrawing vs continuing renin-angiotensin blockers on incidence of acute kidney injury in patients with renal insufficiency undergoing cardiac catheterization: results from the angiotensin converting enzyme inhibitor/angiotensin receptor blocker and contrast induced nephropathy in patients receiving cardiac catheterization (CAPTAIN) trial. Am Heart J. 2015;170:110-116. [DOI] [PubMed] [Google Scholar]
- 49. Wolffenbuttel BH, Nijst L, Sels JP, Menheere PP, Muller PG, Kruseman AC. Effects of a new oral hypoglycaemic agent, repaglinide, on metabolic control in sulphonylurea-treated patients with NIDDM. Eur J Clin Pharmacol. 1993;45:113-116. [DOI] [PubMed] [Google Scholar]
- 50. Kodzwa R. Updates to the ACR manual on contrast media. Radiol Technol. 2017;89:186-189. [PubMed] [Google Scholar]
- 51. Majunke N, Sandri M, Adams V, et al. Atrial and brain natriuretic peptide secretion after percutaneous closure of the left atrial appendage with the watchman device. J Invasive Cardiol. 2015;27:448-452. [PubMed] [Google Scholar]
- 52. Tabata T, Oki T, Yamada H, Abe M, Onose Y, Thomas JD. Relationship between left atrial appendage function and plasma concentration of atrial natriuretic peptide. Eur J Echocardiogr. 2000;1:130-137. [DOI] [PubMed] [Google Scholar]
- 53. Valsson F, Ricksten SE, Hedner T, Lundin S. Effects of atrial natriuretic peptide on acute renal impairment in patients with heart failure after cardiac surgery. Intensive Care Med. 1996;22:230-236. [DOI] [PubMed] [Google Scholar]
- 54. Liu J, Xie Y, He F, et al. Recombinant brain natriuretic peptide for the prevention of contrast-induced nephropathy in patients with chronic kidney disease undergoing nonemergent percutaneous coronary intervention or coronary angiography: a randomized controlled trial. Biomed Res Int. 2016;2016:5985327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xing K, Fu X, Wang Y, et al. Effect of rhBNP on renal function in STEMI-HF patients with mild renal insufficiency undergoing primary PCI. Heart Vessels. 2016;31:490-498. [DOI] [PubMed] [Google Scholar]
- 56. Morikawa S, Sone T, Tsuboi H, et al. Renal protective effects and the prevention of contrast-induced nephropathy by atrial natriuretic peptide. J Am Coll Cardiol. 2009;53:1040-1046. [DOI] [PubMed] [Google Scholar]
- 57. Sezai A, Hata M, Niino T, et al. Results of low-dose human atrial natriuretic peptide infusion in nondialysis patients with chronic kidney disease undergoing coronary artery bypass grafting: the NU-HIT (Nihon University working group study of low-dose HANP Infusion Therapy during cardiac surgery) trial for CKD. J Am Coll Cardiol. 2011;58:897-903. [DOI] [PubMed] [Google Scholar]
- 58. Nombela-Franco L, Rodes-Cabau J, Cruz-Gonzalez I, et al. Incidence, predictors, and prognostic value of acute kidney injury among patients undergoing left atrial appendage closure. JACC Cardiovasc Interv. 2018;11:1074-1083. [DOI] [PubMed] [Google Scholar]