Abstract
Cholestasis results in blockage of bile flow whether the point of obstruction occurs extrahepatically or intrahepatically. Bile acids are a primary constituent of bile, and thus one of the primary outcomes is acute retention of bile acids in hepatocytes. Bile acids are normally secreted into the biliary tracts and then released into the small bowel before recirculating back to the liver. Retention of bile acids has long been hypothesized to be a primary cause of the associated liver injury that occurs during acute or chronic cholestasis. Despite this, a surge of papers in the last decade have reported a primary role for inflammation in the pathophysiology of cholestatic liver injury. Furthermore, it has increasingly been recognized that both the constituency of individual bile acids that make up the greater pool, as well as their conjugation status, is intimately involved in their toxicity, and this varies between species. Finally, the role of bile acids in drug-induced cholestatic liver injury remains an area of increasing interest. The purpose of this review is to critically evaluate current proposed mechanisms of cholestatic liver injury, with a focus on the evolving role of bile acids in cell death and inflammation.
Key words: Bile acid, Metabolism, Cholestasis, Liver, Neutrophil, Inflammation, Cytokine, Review, Cirrhosis, Drug-induced liver injury (DILI), Cholangiocytes
INTRODUCTION
Cholestatic liver injury is a common clinical occurrence wherein intrahepatic impairment of bile formation and excretion or extrahepatic blockade of the biliary tracts results in retention of bile acids or bile salts. Clinical obstructive cholestasis can be caused by events such as gallstones, impingement of pancreatic cancer onto the bile ducts, sclerosing cholangitis, or biliary stricture—all of which feature varying degrees of obstruction1–3. Acute cholestatic liver injury in patients, such as with gallstones, can commonly be resolved surgically. However, chronic diseases like biliary atresia, primary biliary cholangitis (PBC), progressive familial intrahepatic cholestasis (PFIC), and primary sclerosing cholangitis (PSC) are difficult to manage without liver transplantation. These diseases present with similar pathology including inflammation, major blockage of bile flow resulting in alterations in bile acid disposition, increases in serum transaminase levels indicative of liver injury, and substantial fibrosis eventually progressing to cirrhosis and liver failure. While the root cause is fundamentally different, these diseases converge upon obstruction of biliary flow as the primary instigator of liver damage. Unfortunately, therapeutic development in this area has lagged behind other areas for multiple reasons, and ursodeoxycholic acid (UDCA) remains one of few nonsurgical treatment options. Surgical treatments, including liver transplantation, are available, but the shortage of livers worldwide makes this option impossible for many patients. The lack of solid models for any of these diseases further makes development of therapeutics difficult4. Finally, many patients with these diseases present with significant obstruction, or even cirrhosis. At this point, it then becomes necessary to reverse the process rather than just limit damage to the liver, which complicates therapeutic development. New agents are in clinical trials, but limited success has been observed outside of recent success with bezafibrate in PBC5. Limiting hepatic injury, reducing fibrosis, and increasing choleresis to remove excessive bile acid levels remain likely mechanisms through which the disease states could be beneficially modulated; however, an increased understanding of how and why cholestasis causes liver injury is required to inform development of new therapeutics.
Normal enterohepatic circulation of bile acids results in the unobstructed flow from hepatocytes, the cellular point of synthesis, through the biliary tracts, into the intestines, and then back to the liver through the hepatic portal vein. Bile acid uptake and export through tissues is mediated by a series of uptake and export proteins selectively expressed on specific tissues to mediate this process (Table 1)6,7. Expression of these proteins is largely controlled by transcription factors such as the farnesoid X receptor (FXR), as well as circulating hormones like fibroblast growth factor 156–9. Ultimately, the purpose of this flow is to aid in digestion, assist in excretion of endogenous and exogenous compounds, and regulate metabolism for nutrient uptake and usage. This is a highly efficient process, with minimal bile acid loss daily6,7,9.
Table 1.
Expression Patterns of Multiple Transporters in Multiple Tissues
| Tissue | Uptake Transporters | Export Transporters |
|---|---|---|
| Liver (cell type) | ||
| Hepatocytes | From serum: OSTα/ß, NTCP, OATP | To bile: MRP2, MDR2, BSEP |
| To serum: OSTα/ß, MRPs | ||
| Cholangiocytes | From bile: ASBT | To serum: OSTα/ß |
| Colon | From feces: ASBT | To serum: OSTα/ß |
| Kidneys | From serum: ASBT | To serum/urine: MRP2, OSTα/ß |
NTCP, sodium taurocholate transporting polypeptide; MRP, multidrug resistance-associated protein; MDR, multidrug resistance protein; OST, organic solute transporters; ASBT, apical sodium-dependent bile acid transporters, organic anion-transporting polypeptide.
Obstruction of any point in the biliary tracts results in substantial pathology. Cholestasis occurs most commonly either intrahepatically in the small cholangioles of the liver, at the apical membrane of hepatocytes, or extrahepatically in the common bile duct. Similarly, a number of laboratory models have been developed that attempt to mimic this process through either direct physical obstruction or biochemical disruption of the biliary tracts10,11. Consistently, all of these models result in: 1) disruption of the bile acid pool in regard to its constituent members, the quantities in different compartments, and the molecular mechanisms that control bile acid uptake and export and 2) substantial liver inflammation, especially the recruitment and activation of cytotoxic neutrophils. Mechanisms of cholestatic liver injury described by our group and others have largely focused on these events as precipitating factors2,6,8,12–20. In spite of this, considerable debate still exists in the field as to what induces the characteristic hepatocyte cell death caused by cholestasis14,21. The purpose of this review is to critically evaluate mechanisms of cholestatic liver injury, with a focus on the interplay between bile acids, cell death, and inflammation. We will attempt to point out major differences between animal models and what is understood about the human condition, while focusing on gaps in knowledge that can be addressed to push the field forward.
BILE ACIDS IN CHOLESTATIC LIVER INJURY
Synthesis, Regulation, and Metabolism of Bile Acids
Bile acids are involved in a number of different diverse liver processes including metabolism, regeneration, and hormone signaling6,8. Bile acids are synthesized in the liver through well-described pathways involving oxidative metabolism by cytochrome P450s (Fig. 1)6,7. Cholesterol goes through a multienzymatic process ultimately controlled by CYP7A1 as the rate-limiting enzyme to produce one of two primary bile acids, cholic acid (CA) or chenodeoxycholic acid (CDCA)22,23. This process is acutely regulated by the transcription factor FXR, which is present in multiple tissues exposed to bile acids24–26. Activation of FXR by bile acids results in downregulation of CYP7A1 and other bile acid-metabolizing enzymes, downregulation of bile acid uptake proteins such as sodium taurocholate cotransporting polypeptide (NTCP), and simultaneous upregulation of proteins associated with bile acid export such as organic solute transporters (OSTs)26–34. These adaptive changes take place in multiple tissues and are designed to promote the excretion of bile acids to reduce systemic levels (Table 1). Primarily in hepatocytes, bile acids are exported by the bile salt export pump (BSEP) and multidrug resistance (mdr) family proteins6,7. BSEP is a major driving force for bile flow and, thus not surprisingly, BSEP inhibition by therapeutic compounds is a noted cause of cholestatic liver and a major source of toxicity in potential therapeutics7,14,35. Similarly, genetic loss of MDR3 in humans results in progressive familial intrahepatic cholestasis, a genetic condition with prominent cholestatic liver injury, which is recapitulated in the mouse10. In summary, bile acid synthesis is tightly controlled by FXR and other bile acid synthetic genes, both physiologically and during cholestasis, to control bile acid synthesis and enhance excretion, with the goal of maintaining normal metabolism and nutrient uptake.
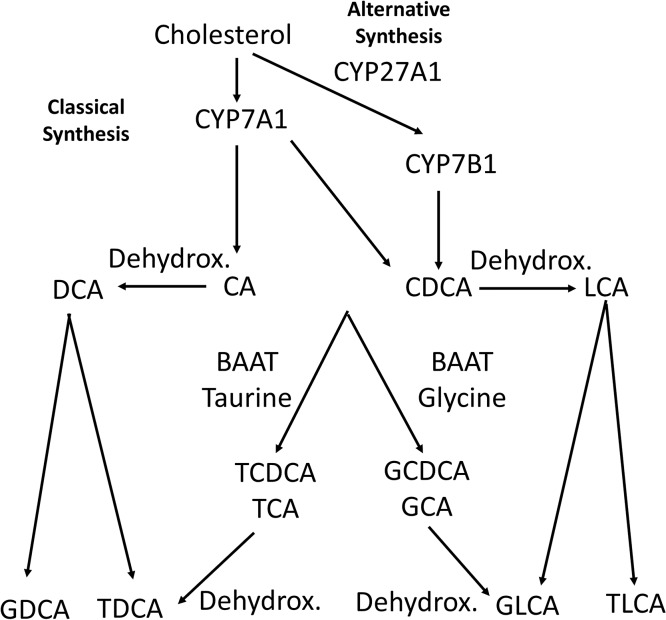
Figure 1.
Bile acid synthesis. Bile acids are synthesized from cholesterol by cytochrome p450s (CYP) to either cholic acid (CA) or chenodeoxycholic acid (CDCA). Dehydroxylation by gut bacteria results in lithocholic acid (LCA) or deoxycholic acid (DCA). Conjugation of CA or CDCA to taurine/glycine results in taurochenodeoxycholic/taurocholic acid (TCDCA/TCA) or glycochenodeoxycholic/glycocholic acid (GCA/GCDCA). Dehydroxylated bile acids can also be conjugated to taurine/glycine. Dehydrox., dehydroxylated.
While they are commonly used experimentally as primary bile acids, the majority of circulating primary bile acids do not exist as CA or CDCA; rather, they are conjugated to either glycine or taurine by the enzyme bile acid-CoA:amino acid N-acyltransferase (BAAT) to promote hydrophilicity, presumably to help with solubility and reduce toxicity, or they are metabolized into other bile acids36–40. Taurocholic acid (TCA), taurochenodeoxycholic acid (TCDCA), glycocholic acid (GCA), and glycochenodeoxycholic acid (GCDCA) are the most common circulating bile acids in humans in bile and serum, especially during cholestasis whether this is due to general obstruction or chronic disease such as with primary sclerosing cholangitis (Fig. 2)2,39,40. While rats also conjugate bile acids to glycine, multiple groups have now noted that glycine conjugation in the mouse is nearly absent2,39–41. While the mechanism is not well understood, murine BAAT strongly prefers taurine as a substrate, and thus bile acid conjugation is likely due to the preference of BAAT, but may be in part linked to either dietary taurine/glycine intake, glycine metabolism from serine, or taurine synthetic metabolism36–38. Notably, the percentage of conjugated to unconjugated bile acids increases dramatically during liver injury2,39,40,42.
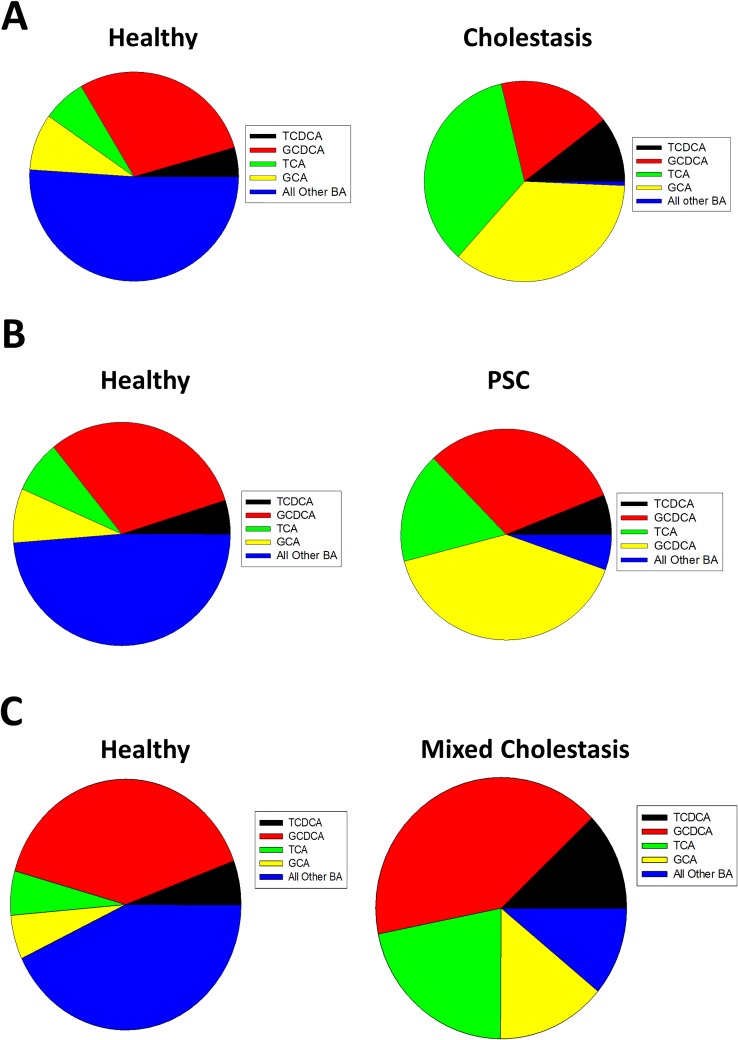
Figure 2.
Serum bile acid levels in human patients with or without cholestasis. Serum bile acids were measured in healthy or cholestatic patients (A), or healthy or patients with primary sclerosing cholangitis (B), or healthy or cholestatic patients with mixed etiology of cholestasis (C). Data adapted from References 2, 39–40. BA, bile acid.
Bile acids are also metabolized by gut bacteria, as CA is dehydroxylated to deoxycholic acid (DCA), while CDCA is dehydroxylated to lithocholic acid (LCA)6,8. These secondary bile acids can also be conjugated to glycine or taurine. While other metabolic processes including sulfation and other hydroxylation reactions have been established, levels of these bile acids are relatively minor in most species, and the predominant reactions are conjugation by BAAT and dehydroxylation by gut bacteria43–46.
Bile Acid Disposition in Cholestatic Liver Injury
Cholestasis dramatically alters bile acid concentrations in different compartments. Acute cholestasis in laboratory models results in dramatic increases in serum bile acid levels, that taper off, but remain elevated for a significant period47. The presence of high bile acid levels in serum alone is insufficient to induce liver injury though48–50. In case studies, patients with constitutive loss of NTCP had extremely high serum bile acid levels and hyperbilirubinemia, but with minimal signs of overt liver toxicity48,49. Similarly, Myrcludex B, an NTCP-inhibiting polypeptide in use in patients for treating hepatitis B infection, is well tolerated in both rodents and human patients despite drastic increases in serum bile acid levels associated with loss of hepatic uptake51,52. Moreover, Myrcludex B is protective against the primary laboratory cholestasis models including bile duct ligation (BDL), wherein the bile duct is surgically ligated to induce extrahepatic cholestasis, and 3.5-diethoxycarbonyl-1.4-dihydrocollidine (DDC) administration, wherein DDC induces intrahepatic loss of bile flow53. Surprisingly this did not extend to the MDR2−/− mouse53. While the mechanism has not been established, serum bile acids may also increase during noncholestatic liver injury42. This may be due to necrosis of hepatocytes with impaired uptake and increased release of intracellular stores, and is likely not a contributing mechanism to injury in all cases2,42.
Bile acids can be excreted into the urinary tracts and then removed from the body as an alternative means for excretion; however, long-term exposure results in kidney damage and indicates that the kidneys are not designed to continue this process chronically54,55. Cholemic nephropathy is largely understudied clinically, but a recent study indicates that this process likely occurs in patients as well, and may represent an area of underappreciated clinical concern56. Overall, it appears that shunting bile acids into the plasma is a protective measure used by the liver to prevent cholestatic liver injury by reducing overall bile acid levels via urinary excretion and preventing accumulation in hepatocytes.
In contrast to serum levels, biliary levels of bile acids can change depending upon the point of cholestasis. After lithocholic acid feeding, biliary levels can rise acutely, but can alter over time due to the influx of water and other solutes that alter total volume15,57. This is partially accommodated through expansion and filling of the gallbladder, which is also a noted aspect of BDL and gall bladder physiology. In contrast, loss of BSEP or mdr2 results in loss of normal bile acid export and reduced biliary bile acid levels acutely and chronically10,58,59. This is consistent with the proposed function of BSEP, which shunts bile acids into the biliary ducts and promotes biliary flow58,59. Notably, both extrahepatic and intrahepatic cholestasis dramatically reduce the formation of secondary bile acids as they do not reach the intestine and thus are not metabolized by gut bacteria2,47.
Cholestasis also results in alterations in intestinal bile acid levels (generally reducing them due to blockage in flow), which may be a therapeutic target8. Intestinal bile acids are typically reabsorbed by the apical sodium-dependent bile acid transporter (ABST)60,61. Recent efforts to reduce bile acid levels during cholestastic diseases using inhibition of ASBT have demonstrated that systemic reduction of bile acid levels can be highly beneficial to liver disease, including cholestasis62,63. This is recapitulated by the use of cholestyramine, an agent that binds bile acids and forces their excretion through defecation64,65. More work is needed in this area to determine if bile acid pools can safely be restricted via this method, although this is a promising area for therapeutic development.
Finally, cholestasis results in dramatic increases in bile acid levels within hepatocytes47. This is potently counterregulated by FXR, and likely other mechanisms, such that the initial rise in bile acid levels tapers off over time; however, the spike in intrahepatic bile acid levels has widely been attributed to be the primary cause of hepatocyte cell death18,46,66–68. Furthermore, the presence of infarctions in the biliary tracts results in localized increases in bile acids, potentially exposing hepatocyte to mM levels of bile acids acutely that generate small foci of cell death. In spite of these data, species differences in susceptibility, metabolism, and physiology have made it difficult to translate in vitro findings to human disease, and bile acid-induced toxicity continues to be widely studied.
Bile Acid-Induced Hepatocyte Cell Death
Different bile acids induce cell death at different concentrations, and this is due to both relative hydrophobicity and the current conjugation status of the individual bile acid2,18,69. Because it induces well-defined apoptosis in rat hepatocytes, or human hepatocellular carcinoma cells that overexpress NTCP, one of the most commonly used bile acid to induce in vitro cell death is GCDCA; however, neither mouse nor human hepatocytes undergo appreciable levels of apoptosis when exposed to these concentrations of GCDCA, and both require dramatically higher concentrations to undergo any degree of cell death at all2,70,71. Importantly, mouse and human hepatocytes undergo necrosis, and not apoptosis, when exposed to other bile acids2,57. Similarly, human patients have limited levels of caspase-cleaved cytokeratin-18 release associated with apoptosis, but dramatic increases in full-length cytokeratin-18 release associated with necrosis2. Blockade of apoptosis was cited as the primary mechanism of protection in a number of studies with interventions against cholestatic liver injury; however, a majority of the commonly used laboratory models do not demonstrate gold standard markers such as caspase cleavage, caspase activity, or histological evidence for apoptosis11,72–78. Even in the BDL model in the rat, wherein higher concentrations of intrahepatic GCDCA would be expected, only limited apoptosis is found, and this is countered by activation of nuclear factor κ light chain enhancer of activated B cells (NF-κB) that prevents widespread apoptosis79,80. As such, while bile acid-induced apoptosis was a leading hypothesis in the field for many years, the likelihood that it extends broadly to human disease is very unlikely. Although this does not preclude bile acid-induced cell death, the mechanisms that control this in both human and mouse hepatocytes are poorly explained because they do not follow the established mechanisms present in rat hepatocytes.
Despite differences in interpretation, a number of studies support the ideas that excessive bile acid levels can kill hepatocytes. While rats undergo apoptosis at GCDCA concentrations above 50 μM, human cells are largely resistant until concentrations above 500 μM to 1 mM in vitro2,70. Whether this difference in susceptibility between rat and human hepatocytes is due to relatively higher exposure to glycine-conjugated bile acid under normal conditions or other mechanisms is not currently known. As the normal bile acid pool in the mouse consists primarily of taurine-conjugated bile acids, the relative toxicity of the pool on its own is minimal2,39,40,81. In contrast, toxification of the bile acid pool by feeding hydrophobic bile acids results in considerable toxicity15,57,69. Both cholic acid feeding and lithocholic acid feeding result in cholestasis and cell death when given at high concentrations in the feed15,57,69. Notably in the lithocholic acid model, a role for inflammation was ruled out, and administration of bile acid concentrations equivalent to what hepatocytes are predicted to be exposed to was toxic in vitro supporting the idea that hydrophobic bile acids, when present in sufficient concentrations in vivo, can be directly toxic57. Importantly in this model, direct cholestasis, that is a reduction in bile flow due to obstruction, was observed confirming the cholestatic phenotype15. Notably though, LCA levels either conjugated or unconjugated are usually very low in cholestasis, and the likely reason is that gut bacteria metabolism is necessary for their generation and cholestasis blocks flow to the gut, and thus this model is highly artificial relative to human disease39,40.
Just as toxification of the bile acid pool increases cell death, detoxification of the bile acid pool reduces cell death. NorUrsodeoxycholic acid (nUDCA), a UDCA derivative, has been used to reduce liver injury caused by bile acids in a number of different models as it is a relatively mild bile acid in toxicity that also promotes biliary flow, which has been proposed to help with excretion of bile acids and consequently prevent mitochondrial damage induced by bile acid retention82. A recent experiment noted that mdr2−/− animals had higher levels of cholic acid, a more hydrophobic bile acid; however, when this mouse was fed hydrophilic bile acids, or when this mouse was crossed to the BSEP−/− mouse that produces largely hydrophilic bile acids, liver injury was reduced83. Experiments directly feeding a dose response of different bile acids, even up to 3% UDCA, did not produce a significant increase in serum ALT values, despite the fact that it increased liver bile acid concentrations69. In this same study though, 1% cholic acid feeding produced significant increases in serum ALT, although this occurred without a change in overall liver bile acid levels28. Serum bile acid levels were dramatically increased though, and as such, the point measured may have occurred after FXR counterregulation and shunting of bile acid to serum40. These experiments largely combine to indicate alterations in bile acid pool size, and constituency dramatically affects the capacity of the liver to detoxify and prevent bile acid-induced injury.
Intrahepatic cholestasis has varying results with bile acid toxicity. Since its initial understanding, BSEP inhibition has routinely been proposed as a major cause of drug withdrawal due to drug-induced cholestatic liver injury84. The proposed mechanism was increased intrahepatic bile acid accumulation; however, BSEP knockout alone does not result in liver injury in mice or in cell lines59,85,86. In fact, many drugs that are BSEP inhibitors in vitro demonstrate alteration in the bile acid pool and bile acid concentrations in media at concentrations significantly below their toxicity level87. Furthermore, when troglitazone, a known BSEP inhibitor, was given to BSEP KO HepaRG cells, the toxicity was enhanced88. Troglitazone can reduce conjugation of bile acid to taurine and further polarizes the bile acid pool toward glycine-conjugated bile acid acids89. This may be a mechanism through which troglitazone induces cholestasis and subsequent liver injury89. As such, while excessive intrahepatic bile acid stores are likely a potential cause of cholestatic liver injury, simply blocking export is insufficient to induce toxicity in vitro, and thus other mechanisms may also be at play.
One of the characteristic histological findings with many types of cholestatic liver injury, including in laboratory models, is the presence of foci of liver necrosis (Fig. 3). These foci are thought to be due to infarction of the surrounding biliary tracts and leakage of bile into the hepatic parenchyma and are commonly referred to as bile infarcts or Charcot–Gombault necrosis90. Biliary infarcts are noted in the BDL model, the mdr2−/− model, the LCA administration model, and in human patients2,16. It has been hypothesized that mechanical stress weakens the small cholangioles in the liver, which increases susceptibility to infarction of the biliary tree resulting in leakage of bile91. A recent study has confirmed this using intravital two-photon imaging90. Biliary infarcts are initiated at the apical membrane of hepatocytes and expand from there along with hepatocyte cell death68. These infarcts are especially notable in the LCA model wherein electron microscopy pictures have detailed the formation of LCA precipitates that aggregate and irritate the cholangioles leading to rupture15. As these infarcts are commonly associated with major obstruction, it was surprisingly noted that UDCA levels actually worsened injury levels in these animals16. As UDCA is thought to be far less toxic and potentially even helpful to biliary injury, these data indicate that biliary rupture is likely highly damaging to hepatocytes, even when the bile is relatively detoxified71. In contrast, the use of nUDCA benefited mice with partial obstruction, although not complete obstruction92. Supporting these data, a recent study noted that knockout of sortilin, a trafficking protein that can affect bile acid metabolism, reduced bile acid pool size, which led to less infarction of the biliary tracts and a reduction in injury consistent with the idea that reduction in the bile acid pool size leads to reduced injury, likely due to reduced intrahepatic biliary pressure and reduced bile acid leakage into the parenchyma93.
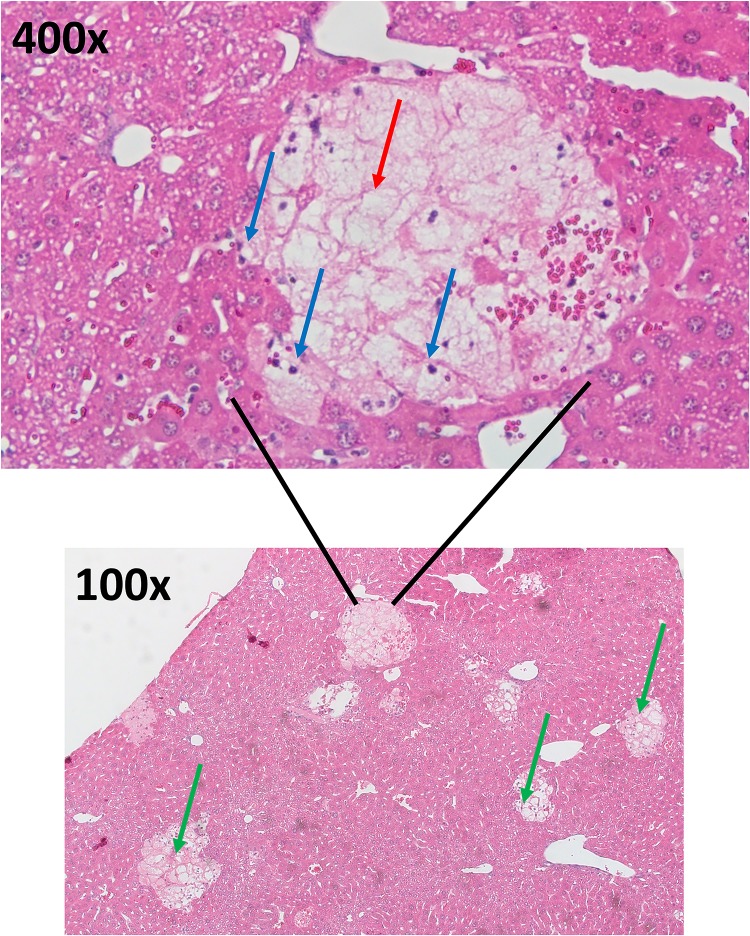
Figure 3.
Bile duct ligation histology. H&E stain of a mouse liver 24 h post-bile duct ligation. Blue arrows represent inflammatory cells. Red arrows represent areas of feathery necrosis inside the infarct. Green arrows on the 100× images represent obvious areas of infarction and cell death.
Bile acid-induced toxicity is clearly dependent on a number of factors relating to individual bile acid levels, bile acid disposition, and relative degree of obstruction. The majority of the data points toward the same idea: reducing pool size, promoting conjugation to taurine, pushing bile acids into serum or alternate excretion routes, and preventing complete obstruction all minimize liver injury.
CHOLANGIOCYTES: CRITICAL MEDIATORS OF THE EPITHELIAL BARRIER TO BILE ACID TOXICITY
Cholangiocytes are also critical mediators of cholestatic liver injury, and their relationship with cholestatic liver injury cannot be overlooked. The liver is interlaced internally with small biliary vessels termed bile canaliculi that are lined with cholangiocytes. Canaliculi dump bile acids generated by hepatocytes into larger cholangioles and then into the greater biliary tracts. Export of bile acids generated by hepatocytes is mediated by transporters such as BSEP and mdr2 as aforementioned. Bile acids such as TCA stimulate proliferation of the cholangioles in order to handle increased bile load and prevent infarction94. One of the most important effects of cholangiocytes is maintenance of bile acid-independent bile flow via the hormone secretin and the protein anion exchanger 2 in addition to glutathione95,96. These proteins regulate secretion of bicarbonate and chloride anion, which then regulate water flux and drive biliary flow95. Cholangiocytes also express a number of bile acid receptors including the apical sodium bile acid transporter (ASBT), sphingosine 1 phosphate receptor 2 (S1PR2), and TGR597. These receptors can mediate cholestatic liver injury. Loss of S1PR2 reduces BDL-induced cholestasis and fibrosis, but had minimal effect on hepatic injury as measured by ALT/AST97. Activation of TGR5 stimulates proliferation of cholangiocytes and protects against death receptor-induced cell death, which may be protective through maintenance of normal epithelial barrier against biliary infarction via stabilization of junctional adhesion molecule-A, and thus TGR5 agonism may represent a therapeutic target in cholestatic liver injury98,99. ASBT shunts bile acids from bile back into the liver through as a cholehepatic shunt, which may yield alternate excretory mechanisms100. Secretin upregulates ASBT and prolongs bile acid transit time by enhancing the shunt of bile acids back into hepatocytes providing a potential feedback loop101. Overall, cholangiocytes express a number of bile acid transporters that react to alterations in biliary flow by altering bile acid uptake and bile acid-independent flow. Moreover, cholangiocytes provide the critical epithelial barrier against biliary rupture necessary for safely removing excess bile acid levels.
INFLAMMATION IN CHOLESTATIC LIVER INJURY: CAUSE OR CONSEQUENCE
Neutrophils and Cholestatic Liver Injury
Although cholestatic liver injury was hypothesized to be due to bile acid toxicity, other hypotheses also exist that explain why biliary rupture would be damaging to hepatocytes. Primarily, a number of studies have begun to elucidate intricate signaling networks mediated in part by the presence of high levels of bile acids that initiate a potent neutrophil-mediated inflammatory response. While a consensus has formed that inflammation occurs after biliary rupture or hepatic exposure to high levels of bile acids, the mechanisms that dictate inflammatory progression and its precise role in the injury process remain areas of intense study.
The most commonly cited inflammatory process after either BDL, bile acid feeding, or in the mdr2−/− model is the recruitment of neutrophils to areas of injury10,102–104. Initial results indicated a potential CXCL-mediated neutrophil recruitment pathway after BDL, which has largely been confirmed in the mouse105,106. Knockout of CCL2 resulted in sustained inhibition of BDL-induced liver injury and injury after cholic acid feeding107,108. Neutrophil recruitment happens as early as 6 h after BDL, consistent with the initial points of tissue damage, and continues throughout the disease11. Studies have shown that prevention of neutrophil adhesion through either knockout of CD18 or knockout of intercellular adhesion molecule-1 (ICAM-1) was protective against BDL-induced liver injury, as was knockout of P-selectin glycoprotein ligand-1 necessary for neutrophil adherence103,104,109. Neutrophils are hypothesized to kill hepatocytes through release of potent ROS forms such as hypochlorous acid, which induces cell death through increasing oxidative stress102,110,111. Furthermore, the lpr mutant mouse, which has a deficient immune response and autoimmune dysfunction due to mutation in Fas receptor, is also protected, independent of fas-induced apoptosis76. Similar results were obtained in the plasminogen activator inhibitor (PAI1−/−) knockout mouse, or Egr-1−/− mouse, both of which have knockouts for proteins involved in initiating inflammation112,113. Knockout of osteopontin yielded an early decrease in liver injury after BDL that was not sustained, indicating it may have an acute role in the injury process114. The biliary release of osteopontin by cholangiocytes and cleavage by matrix metalloproteases generates a potent chemotactic factor, which is responsible for the initial recruitment of neutrophils and the early inflammatory injury114. In addition, biliary levels of bile acids induce chemokine formation in hepatocytes81. Notably, interleukin (IL)-17−/− animals had reduced injury, but this did not affect levels of bilirubin, serum bile acids, or alkaline phosphatase, indicating that the reduction in injury was purely associated with a change in inflammation, and not with the relative level of cholestasis in the animal115. As such, several lines of research have converged on the idea that inflammation can mediate a portion of the injury, especially after the initial biliary rupture1,14,16,90. Even still, this may be dependent on the model, and no data to the authors’ knowledge has fundamentally demonstrated a role for inflammation in hepatocyte death in human patients with any specific form of cholestatic liver injury. Moreover, in human patients, significant quantities of glycine-conjugated bile acids are present that could justifiably induce cell death during cholestasis. Figure 4 depicts mechanisms of neutrophil-induced liver injury in the mouse BDL model.
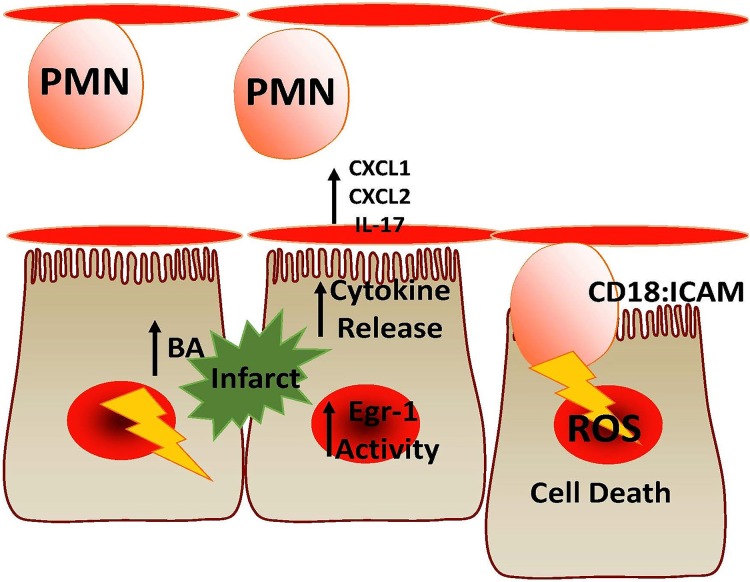
Figure 4.
Proposed model of neutrophil-induced injury. Infarction of the biliary tract results in hepatocyte damage and release of bile acids (BA) and damage-associated molecular patterns (DAMPs). This releases cytokines like CXC-ligands 1 and 2 or IL-17 and increased early growth factor response-1 activity. Neutrophils (PMN) recognize these signals and adhere firmly to hepatocytes via CD18/intercellular adhesion molecule-1 (ICAM-1) and induce cell death through ROS production.
Chronic administration of α-naphthyl isothiocyanate (ANIT) shares many aspects of BDL-induced liver injury. Inflammation is also prominent in the model, and blockade of inflammation reduces liver injury. This is a neutrophil- and Egr-1-dependent injury process similar to BDL116–118. These data support the idea that inflammation, especially neutrophils, can promote cholestatic liver injury.
Kupffer Cells and Inflammatory Mediators in Cholestatic Liver Injury
A number of other inflammatory cells might be involved in inflammation during cholestasis. Kupffer cells are resident tissue macrophages in the liver that also have implications in BDL-induced liver injury. Kupffer cell inactivation with gadolinium chloride protects against BDL-induced liver injury and reduces neutrophil recruitment leading to reduced liver injury73,119. In contrast, Kupffer cell depletion with clodronate liposomes enhances injury120. Notably, IL-6 depletion also worsens injury and is thought to be mediated by Kupffer cells in the model, indicating that IL-6 might have an anti-inflammatory role in the model, which is recapitulated by the fact that recombinant IL-6 administration is also protective120. Both dendritic cells and T cells have also been observed to cause differences in BDL-exposed animals; however, the role of the adaptive immune system generally is less well understood121–123.
Inflammation is also a likely consequence of most types of liver injury. Necrosis of hepatocyte results in release of sterile mediators referred to as damage-associated molecular patterns (DAMPs) that can initiate inflammation including mitochondrial DNA, nuclear DNA fragments, ATP, and more124,125. Receptors for many of these products are present on Kupffer cells and even hepatocytes and can initiate an inflammatory response124. Notably, bile acid levels are also increased dramatically in noncholestatic forms of liver injury such as with acetaminophen overdose42. Bile acids also have signaling pathways mediated by receptors such as G-protein-coupled bile acid receptor (TGR5) on Kupffer cells that mediate inflammation, indicating that bile acids themselves may be an underappreciated DAMP65. Moreover, bile acids also directly induce inflammation in hepatocyte in murine hepatocytes in an Egr-1-dependent manner81,126. Regardless, a causative role for neutrophil-mediated liver injury is implied through experiments with knockout of mediators of neutrophil adherence and recruitment that implicate inflammation directly103,104,113,127. Inflammation is directly tied to biliary pressure and degree of cholestasis, and thus, completely separating bile acid accumulation and inflammation is nearly impossible in determining a concrete mechanism.
Cholangiocytes and the Senescence-Associated Secretory Phenotype
Cholangiocytes are known to undergo senescence-like changes during cholestasis, resulting in the senescence-associated secretory phenotype that promotes inflammation. Biliary cells are especially prone to cellular senescence, and their presence is noted in chronic cholestatic diseases such as PBC128. Cellular senescence in the liver initiates a paracrine signaling pathway that exacerbates DDC-induced liver injury through enhanced secretion of proinflammatory and profibrotic mediators128–130. Senescence is known to promote inflammation, and thus biliary senescence may be a major mediator of inflammation, especially in the later stages of advanced cholestasis.
BILE ACID-INDUCED PROINFLAMMATORY SIGNALING: A NEW HYPOTHESIS?
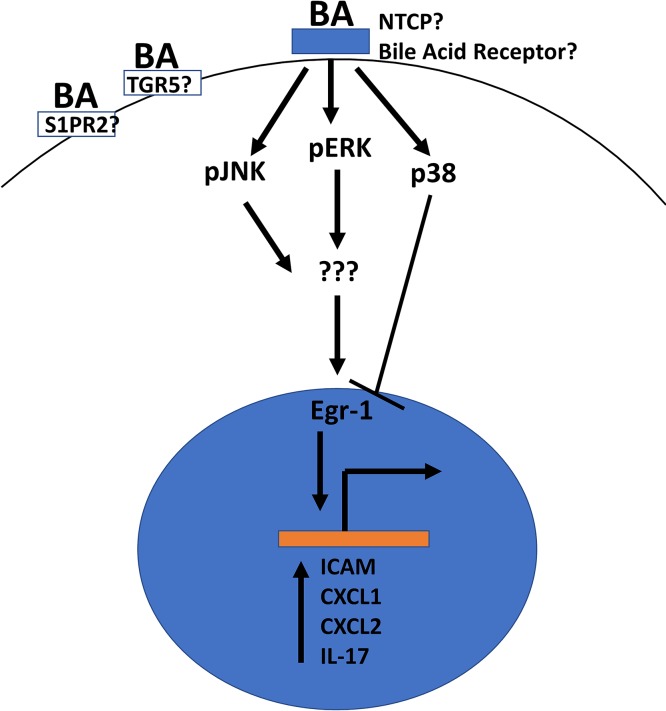
In contrast to experimental evidence in favor of a neutrophil-mediated injury response, defining the signaling pathway is polluted and made more difficult by activation of a number of proinflammatory signaling cascades through generalized inflammation and cell death. As such, detailed investigations into the inflammatory process have proven difficult. One area where a consensus is building is in proinflammatory signaling induced by bile acids in isolated hepatocytes. Initial studies indicated that a number of different conjugated and unconjugated bile acids can induce proinflammatory signaling changes in isolated murine hepatocytes81. This was most prevalent with TCA, which dramatically enhances expression and secretion of cytokines such as CXCL1 and CXCL2 as well as expression of ICAM-181. This pathway is dependent on Egr-1126. These same cytokines have been directly implicated in BDL-induced injury, and this process was noted to occur independently of FXR, meaning an entirely separate bile acid signaling pathway is present in hepatocytes that mediates this interaction independently, which does not occur with isolated nonparenchymal cells81,107. TCA-mediated increases in CCL2 may also mediate other forms of injury such as carbon tetrachloride, wherein the process is dependent on c-jun N-terminal kinase signaling131. Subsequent studies showed increases in IL-17A and IL-23A expression after TCA exposure in hepatocytes, indicating the hepatocyte-mediated proinflammatory pathway likely has a direct linkage to the subsequent inflammation found in the BDL model115. The receptor or mechanism responsible for the initiation of this signaling pathway is not currently well understood, although multiple receptors have been established as bile acid receptors including S1PR2, TGR5, and likely more65,132. Figure 5 depicts a simplified version of this pathway.
Figure 5.
Proposed model of bile acid inflammatory signaling. Bile acids activate an unknown receptor, potentially sphingosine 1 phosphate receptor 2 (S1PR2) or G-protein-coupled bile acid receptor (TGR5), to initiate mitogen-activated protein kinase and c-Jun N-terminal kinase pathways. These increase early growth factor response-1 (Egr1) activity and induce proinflammatory gene induction.
Bile acid-induced proinflammatory signaling changes as observed in mouse hepatocytes were not repeatable in primary human hepatocytes or in HepaRG cells, a hepatocyte-like cell line that expresses some bile acid transporters, exposed to TCA2,133. However, later studies indicated that GCDCA did induce expression of human cytokines at concentrations of 50 μM107. A diverse array of diseases present with increased inflammation and increased serum bile acid levels, and subsequent enhancement of inflammation may be involved broadly in liver inflammation in addition to the role of bile acids in metabolism.
FUTURE PERSPECTIVES
Cholestasis definitively results in considerable hepatocyte cell death. Recent studies have indicated separate roles for bile acids and inflammatory cells, but both likely contribute to the disease. Moreover, bile acids themselves are likely a highly proinflammatory DAMP-like molecule, and their removal may benefit other disease states. Depending on the model, the degree of inflammation may be sharply tied to the degree of cholestasis, and thus, the degree to which inflammation contributes is likely dependent on the location of obstruction and pathological sequelae. As such, therapeutics that enhance excretion of bile acids in these patients are likely to be of benefit for both the reduction in inflammation and the reduction in intrahepatic bile acid levels. Reducing levels of toxic bile acids may also benefit patients. Critical questions remain in the field though, primarily: 1) How do we safely alter conjugation status of bile acid pools to promote conjugation to taurine and reduce direct bile acid toxicity? 2) What is the role of inflammation in human diseases with prominent cholestasis and can reduction in inflammation acutely or chronically benefit patients and/or stave off liver transplantation? 3) Can alterations in bile acid-induced inflammation reduce injury in other disease states with increased bile acid levels? 4) What is the most effective way to reduce systemic bile acid levels without inducing toxic effects? Novel studies answering these questions could potentially reshape patient treatment in this disease space in the near future.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (grant R01 DK102142) and the National Institute of General Medical Sciences (P20 GM103549 and P30 GM118247) from the National Institutes of Health to H.J. The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Woolbright BL, Jaeschke H. Therapeutic targets for cholestatic liver injury. Expert Opin Ther Targets 2016;20(4):463–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Woolbright BL, Dorko K, Antoine DJ, Clarke JI, Gholami P, Li F, Kumer SC, Schmitt TM, Forster J, Fan F, et al. Bile acid-induced necrosis in primary human hepatocytes and in patients with obstructive cholestasis. Toxicol Appl Pharmacol. 2015;283(3):168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Virani S, Akers A, Stephenson K, Smith S, Kennedy L, Alpini G, Francis H. Comprehensive review of molecular mechanisms during cholestatic liver injury and cholangiocarcinoma. J Liver 2018;7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pollheimer MJ, Trauner M, Fickert P. Will we ever model PSC?—“It’s hard to be a PSC model!”. Clin Res Hepatol Gastroenterol. 2011;35(12):792–804. [DOI] [PubMed] [Google Scholar]
- 5. Corpechot C, Chazouilleres O, Rousseau A, Le Gruyer A, Habersetzer F, Mathurin P, Goria O, Potier P, Minello A, Silvain C, et al. A placebo-controlled trial of bezafibrate in primary biliary cholangitis. N Engl J Med. 2018;378(23):2171–81. [DOI] [PubMed] [Google Scholar]
- 6. Chiang JY. Bile acid metabolism and signaling. Compr Physiol. 2013;3(3):1191–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li T, Chiang JY. Nuclear receptors in bile acid metabolism. Drug Metab Rev. 2013;45(1):145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chiang JYL, Ferrell JM. Bile acid metabolism in liver pathobiology. Gene Expr. 2018;18(2):71–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kliewer SA, Mangelsdorf DJ. Bile acids as hormones: The FXR-FGF15/19 pathway. Dig Dis. 2015;33(3):327–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Nieuwerk CM, Groen AK, Ottenhoff R, van Wijland M, van den Bergh Weerman MA, Tytgat GN, Offerhaus JJ, Oude Elferink RP. The role of bile salt composition in liver pathology of mdr2 (−/−) mice: Differences between males and females. J Hepatol. 1997;26(1):138–45. [DOI] [PubMed] [Google Scholar]
- 11. Woolbright BL, Antoine DJ, Jenkins RE, Bajt ML, Park BK, Jaeschke H. Plasma biomarkers of liver injury and inflammation demonstrate a lack of apoptosis during obstructive cholestasis in mice. Toxicol Appl Pharmacol. 2013;273(3):524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jones H, Alpini G, Francis H. Bile acid signaling and biliary functions. Acta Pharm Sin B 2015;5(2):123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Woolbright BL, Jaeschke H. Critical factors in the assessment of cholestatic liver injury in vitro. Methods Mol Biol. 2015;1250:363–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Woolbright BL, Jaeschke H. Novel insight into mechanisms of cholestatic liver injury. World J Gastroenterol. 2012;18(36):4985–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fickert P, Fuchsbichler A, Marschall HU, Wagner M, Zollner G, Krause R, Zatloukal K, Jaeschke H, Denk H, Trauner M. Lithocholic acid feeding induces segmental bile duct obstruction and destructive cholangitis in mice. Am J Pathol. 2006;168(2):410–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fickert P, Zollner G, Fuchsbichler A, Stumptner C, Weiglein AH, Lammert F, Marschall HU, Tsybrovskyy O, Zatloukal K, Denk H, et al. Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology 2002;123(4):1238–51. [DOI] [PubMed] [Google Scholar]
- 17. Zollner G, Fickert P, Silbert D, Fuchsbichler A, Stumptner C, Zatloukal K, Denk H, Trauner M. Induction of short heterodimer partner 1 precedes downregulation of Ntcp in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2002;282(1):G184–91. [DOI] [PubMed] [Google Scholar]
- 18. Spivey JR, Bronk SF, Gores GJ. Glycochenodeoxycholate-induced lethal hepatocellular injury in rat hepatocytes. Role of ATP depletion and cytosolic free calcium. J Clin Invest. 1993;92(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65(2):166–76. [DOI] [PubMed] [Google Scholar]
- 20. Oude Elferink RP, Paulusma CC. Function and pathophysiological importance of ABCB4 (MDR3 P-glycoprotein). Pflugers Arch. 2007;453(5):601–10. [DOI] [PubMed] [Google Scholar]
- 21. Guicciardi ME, Gores GJ. Cholestatic hepatocellular injury: What do we know and how should we proceed. J Hepatol. 2005;42(3):297–300. [DOI] [PubMed] [Google Scholar]
- 22. Song KH, Li T, Owsley E, Strom S, Chiang JY. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology 2009;49(1):297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li T, Jahan A, Chiang JY. Bile acids and cytokines inhibit the human cholesterol 7 alpha-hydroxylase gene via the JNK/c-jun pathway in human liver cells. Hepatology 2006;43(6):1202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kong B, Wang L, Chiang JY, Zhang Y, Klaassen CD, Guo GL. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology 2012;56(3):1034–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thomas AM, Hart SN, Kong B, Fang J, Zhong XB, Guo GL. Genome-wide tissue-specific farnesoid X receptor binding in mouse liver and intestine. Hepatology 2010;51(4):1410–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem. 2001;276(31):28857–65. [DOI] [PubMed] [Google Scholar]
- 27. Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell 2000;6(3):507–15. [DOI] [PubMed] [Google Scholar]
- 28. Schuetz EG, Strom S, Yasuda K, Lecureur V, Assem M, Brimer C, Lamba J, Kim RB, Ramachandran V, Komoroski BJ, et al. Disrupted bile acid homeostasis reveals an unexpected interaction among nuclear hormone receptors, transporters, and cytochrome P450. J Biol Chem. 2001;276(42):39411–8. [DOI] [PubMed] [Google Scholar]
- 29. Donner MG, Keppler D. Up-regulation of basolateral multidrug resistance protein 3 (Mrp3) in cholestatic rat liver. Hepatology 2001;34(2):351–9. [DOI] [PubMed] [Google Scholar]
- 30. Soroka CJ, Lee JM, Azzaroli F, Boyer JL. Cellular localization and up-regulation of multidrug resistance-associated protein 3 in hepatocytes and cholangiocytes during obstructive cholestasis in rat liver. Hepatology 2001;33(4):783–91. [DOI] [PubMed] [Google Scholar]
- 31. Slitt AL, Allen K, Morrone J, Aleksunes LM, Chen C, Maher JM, Manautou JE, Cherrington NJ, Klaassen CD. Regulation of transporter expression in mouse liver, kidney, and intestine during extrahepatic cholestasis. Biochim Biophys Acta 2007;1768(3):637–47. [DOI] [PubMed] [Google Scholar]
- 32. Boyer JL, Trauner M, Mennone A, Soroka CJ, Cai SY, Moustafa T, Zollner G, Lee JY, Ballatori N. Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTalpha-OSTbeta in cholestasis in humans and rodents. Am J Physiol Gastrointest Liver Physiol. 2006;290(6):G1124–30. [DOI] [PubMed] [Google Scholar]
- 33. Schlattjan JH, Winter C, Greven J. Regulation of renal tubular bile acid transport in the early phase of an obstructive cholestasis in the rat. Nephron Physiol. 2003;95(3):p49–56. [DOI] [PubMed] [Google Scholar]
- 34. Lee J, Azzaroli F, Wang L, Soroka CJ, Gigliozzi A, Setchell KD, Kramer W, Boyer JL. Adaptive regulation of bile salt transporters in kidney and liver in obstructive cholestasis in the rat. Gastroenterology 2001;121(6):1473–84. [DOI] [PubMed] [Google Scholar]
- 35. Chatterjee S, Annaert P. Drug-induced cholestasis: Mechanisms, models, and markers. Curr Drug Metab. 2018;19(10):808–18. [DOI] [PubMed] [Google Scholar]
- 36. Shonsey EM, Sfakianos M, Johnson M, He D, Falany CN, Falany J, Merkler DJ, Barnes S. Bile acid coenzyme A: Amino acid N-acyltransferase in the amino acid conjugation of bile acids. Methods Enzymol. 2005;400:374–94. [DOI] [PubMed] [Google Scholar]
- 37. Falany CN, Fortinberry H, Leiter EH, Barnes S. Cloning, expression, and chromosomal localization of mouse liver bile acid CoA: Amino acid N-acyltransferase. J Lipid Res. 1997;38(6):1139–48. [PubMed] [Google Scholar]
- 38. Falany CN, Johnson MR, Barnes S, Diasio RB. Glycine and taurine conjugation of bile acids by a single enzyme. Molecular cloning and expression of human liver bile acid CoA: Amino acid N-acyltransferase. J Biol Chem. 1994;269(30):19375–9. [PubMed] [Google Scholar]
- 39. Trottier J, Bialek A, Caron P, Straka RJ, Heathcote J, Milkiewicz P, Barbier O. Metabolomic profiling of 17 bile acids in serum from patients with primary biliary cirrhosis and primary sclerosing cholangitis: A pilot study. Dig Liver Dis. 2012;44(4):303–10. [DOI] [PubMed] [Google Scholar]
- 40. Trottier J, Bialek A, Caron P, Straka RJ, Milkiewicz P, Barbier O. Profiling circulating and urinary bile acids in patients with biliary obstruction before and after biliary stenting. PLoS One 2011;6(7):e22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang YK, Guo GL, Klaassen CD. Diurnal variations of mouse plasma and hepatic bile acid concentrations as well as expression of biosynthetic enzymes and transporters. PLoS One 2011;6(2):e16683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Woolbright BL, McGill MR, Staggs VS, Winefield RD, Gholami P, Olyaee M, Sharpe MR, Curry SC, Lee WM, Jaeschke H and others. Glycodeoxycholic acid levels as prognostic biomarker in acetaminophen-induced acute liver failure patients. Toxicol Sci. 2014;142(2):436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thakare R, Alamoudi JA, Gautam N, Rodrigues AD, Alnouti Y. Species differences in bile acids II. Bile acid metabolism. J Appl Toxicol. 2018;38(10):1336–52. [DOI] [PubMed] [Google Scholar]
- 44. Thakare R, Alamoudi JA, Gautam N, Rodrigues AD, Alnouti Y. Species differences in bile acids I. Plasma and urine bile acid composition. J Appl Toxicol. 2018;38(10):1323–35. [DOI] [PubMed] [Google Scholar]
- 45. Alnouti Y. Bile acid sulfation: A pathway of bile acid elimination and detoxification. Toxicol Sci. 2009;108(2):225–46. [DOI] [PubMed] [Google Scholar]
- 46. Alnouti Y, Csanaky IL, Klaassen CD. Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;873(2):209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang Y, Hong JY, Rockwell CE, Copple BL, Jaeschke H, Klaassen CD. Effect of bile duct ligation on bile acid composition in mouse serum and liver. Liver Int. 2012;32(1):58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vaz FM, Paulusma CC, Huidekoper H, de Ru M, Lim C, Koster J, Ho-Mok K, Bootsma AH, Groen AK, Schaap FG and others. Sodium taurocholate cotransporting polypeptide (SLC10A1) deficiency: Conjugated hypercholanemia without a clear clinical phenotype. Hepatology 2015;61(1):260–7. [DOI] [PubMed] [Google Scholar]
- 49. Qiu JW, Deng M, Cheng Y, Atif RM, Lin WX, Guo L, Li H, Song YZ. Sodium taurocholate cotransporting polypeptide (NTCP) deficiency: Identification of a novel SLC10A1 mutation in two unrelated infants presenting with neonatal indirect hyperbilirubinemia and remarkable hypercholanemia. Oncotarget 2017;8(63):106598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Slijepcevic D, Roscam Abbing RLP, Katafuchi T, Blank A, Donkers JM, van Hoppe S, de Waart DR, Tolenaars D, van der Meer JHM, Wildenberg M, et al. Hepatic uptake of conjugated bile acids is mediated by both sodium taurocholate cotransporting polypeptide and organic anion transporting polypeptides and modulated by intestinal sensing of plasma bile acid levels in mice. Hepatology 2017;66(5):1631–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Blank A, Eidam A, Haag M, Hohmann N, Burhenne J, Schwab M, van de Graaf S, Meyer MR, Maurer HH, Meier K, et al. The NTCP-inhibitor Myrcludex B: Effects on bile acid disposition and tenofovir pharmacokinetics. Clin Pharmacol Ther. 2018;103(2):341–8. [DOI] [PubMed] [Google Scholar]
- 52. Blank A, Markert C, Hohmann N, Carls A, Mikus G, Lehr T, Alexandrov A, Haag M, Schwab M, Urban S, et al. First-in-human application of the novel hepatitis B and hepatitis D virus entry inhibitor myrcludex B. J Hepatol. 2016;65(3):483–9. [DOI] [PubMed] [Google Scholar]
- 53. Slijepcevic D, Roscam Abbing RLP, Fuchs CD, Haazen LCM, Beuers U, Trauner M, Oude Elferink RPJ, van de Graaf SFJ. Na(+)-taurocholate cotransporting polypeptide inhibition has hepatoprotective effects in cholestasis in mice. Hepatology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Krones E, Eller K, Pollheimer MJ, Racedo S, Kirsch AH, Frauscher B, Wahlstrom A, Stahlman M, Trauner M, Grahammer F, et al. NorUrsodeoxycholic acid ameliorates cholemic nephropathy in bile duct ligated mice. J Hepatol. 2017;67(1):110–9. [DOI] [PubMed] [Google Scholar]
- 55. Fickert P, Krones E, Pollheimer MJ, Thueringer A, Moustafa T, Silbert D, Halilbasic E, Yang M, Jaeschke H, Stokman G, et al. Bile acids trigger cholemic nephropathy in common bile-duct-ligated mice. Hepatology 2013;58(6):2056–69. [DOI] [PubMed] [Google Scholar]
- 56. Brasen JH, Mederacke YS, Schmitz J, Diahovets K, Khalifa A, Hartleben B, Person F, Wiech T, Steenbergen E, Grosshennig A, et al. Cholemic nephropathy causes acute kidney injury and is accompanied by loss of aquaporin 2 in collecting ducts. Hepatology 2019;69(5):2107–19. [DOI] [PubMed] [Google Scholar]
- 57. Woolbright BL, Li F, Xie Y, Farhood A, Fickert P, Trauner M, Jaeschke H. Lithocholic acid feeding results in direct hepato-toxicity independent of neutrophil function in mice. Toxicol Lett. 2014;228(1):56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lam P, Wang R, Ling V. Bile acid transport in sister of P-glycoprotein (ABCB11) knockout mice. Biochemistry 2005;44(37):12598–605. [DOI] [PubMed] [Google Scholar]
- 59. Wang R, Lam P, Liu L, Forrest D, Yousef IM, Mignault D, Phillips MJ, Ling V. Severe cholestasis induced by cholic acid feeding in knockout mice of sister of P-glycoprotein. Hepatology 2003;38(6):1489–99. [DOI] [PubMed] [Google Scholar]
- 60. Oelkers P, Kirby LC, Heubi JE, Dawson PA. Primary bile acid malabsorption caused by mutations in the ileal sodium-dependent bile acid transporter gene (SLC10A2). J Clin Invest. 1997;99(8):1880–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Christie DM, Dawson PA, Thevananther S, Shneider BL. Comparative analysis of the ontogeny of a sodium-dependent bile acid transporter in rat kidney and ileum. Am J Physiol. 1996;271(2 Pt 1):G377–85. [DOI] [PubMed] [Google Scholar]
- 62. Rao A, Kosters A, Mells JE, Zhang W, Setchell KD, Amanso AM, Wynn GM, Xu T, Keller BT, Yin H and others. Inhibition of ileal bile acid uptake protects against nonalcoholic fatty liver disease in high-fat diet-fed mice. Sci Transl Med. 2016;8(357):357ra122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Miethke AG, Zhang W, Simmons J, Taylor AE, Shi T, Shanmukhappa SK, Karns R, White S, Jegga AG, Lages CS, et al. Pharmacological inhibition of apical sodium-dependent bile acid transporter changes bile composition and blocks progression of sclerosing cholangitis in multidrug resistance 2 knockout mice. Hepatology 2016;63(2):512–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bhushan B, Borude P, Edwards G, Walesky C, Cleveland J, Li F, Ma X, Apte U. Role of bile acids in liver injury and regeneration following acetaminophen overdose. Am J Pathol. 2013;183(5):1518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pean N, Doignon I, Garcin I, Besnard A, Julien B, Liu B, Branchereau S, Spraul A, Guettier C, Humbert L and others. The receptor TGR5 protects the liver from bile acid overload during liver regeneration in mice. Hepatology 2013;58(4):1451–60. [DOI] [PubMed] [Google Scholar]
- 66. Botla R, Spivey JR, Aguilar H, Bronk SF, Gores GJ. Ursodeoxycholate (UDCA) inhibits the mitochondrial membrane permeability transition induced by glycochenodeoxycholate: A mechanism of UDCA cytoprotection. J Pharmacol Exp Ther. 1995;272(2):930–8. [PubMed] [Google Scholar]
- 67. Sommerfeld A, Reinehr R, Haussinger D. Tauroursodeoxycholate protects rat hepatocytes from bile acid-induced apoptosis via beta1-integrin- and protein kinase A-dependent mechanisms. Cell Physiol Biochem. 2015;36(3):866–83. [DOI] [PubMed] [Google Scholar]
- 68. Reinehr R, Graf D, Haussinger D. Bile salt-induced hepatocyte apoptosis involves epidermal growth factor receptor-dependent CD95 tyrosine phosphorylation. Gastroenterology 2003;125(3):839–53. [DOI] [PubMed] [Google Scholar]
- 69. Song P, Zhang Y, Klaassen CD. Dose-response of five bile acids on serum and liver bile acid concentrations and hepatotoxicty in mice. Toxicol Sci. 2011;123(2):359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Galle PR, Theilmann L, Raedsch R, Otto G, Stiehl A. Ursodeoxycholate reduces hepatotoxicity of bile salts in primary human hepatocytes. Hepatology 1990;12(3 Pt 1):486–91. [DOI] [PubMed] [Google Scholar]
- 71. Tamaki N, Hatano E, Taura K, Tada M, Kodama Y, Nitta T, Iwaisako K, Seo S, Nakajima A, Ikai I, et al. CHOP deficiency attenuates cholestasis-induced liver fibrosis by reduction of hepatocyte injury. Am J Physiol Gastrointest Liver Physiol. 2008;294(2):G498–505. [DOI] [PubMed] [Google Scholar]
- 72. Canbay A, Feldstein AE, Higuchi H, Werneburg N, Grambihler A, Bronk SF, Gores GJ. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology 2003;38(5):1188–98. [DOI] [PubMed] [Google Scholar]
- 73. Canbay A, Guicciardi ME, Higuchi H, Feldstein A, Bronk SF, Rydzewski R, Taniai M, Gores GJ. Cathepsin B inactivation attenuates hepatic injury and fibrosis during cholestasis. J Clin Invest. 2003;112(2):152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Canbay A, Higuchi H, Bronk SF, Taniai M, Sebo TJ, Gores GJ. Fas enhances fibrogenesis in the bile duct ligated mouse: A link between apoptosis and fibrosis. Gastroenterology 2002;123(4):1323–30. [DOI] [PubMed] [Google Scholar]
- 75. Miyoshi H, Rust C, Roberts PJ, Burgart LJ, Gores GJ. Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterology 1999;117(3):669–77. [DOI] [PubMed] [Google Scholar]
- 76. Gujral JS, Liu J, Farhood A, Jaeschke H. Reduced oncotic necrosis in Fas receptor-deficient C57BL/6J-lpr mice after bile duct ligation. Hepatology 2004;40(4):998–1007. [DOI] [PubMed] [Google Scholar]
- 77. Fickert P, Trauner M, Fuchsbichler A, Zollner G, Wagner M, Marschall HU, Zatloukal K, Denk H. Oncosis represents the main type of cell death in mouse models of cholestasis. J Hepatol. 2005;42(3):378–85. [DOI] [PubMed] [Google Scholar]
- 78. Nalapareddy P, Schungel S, Hong JY, Manns MP, Jaeschke H, Vogel A. The BH3-only protein bid does not mediate death-receptor-induced liver injury in obstructive cholestasis. Am J Pathol. 2009;175(3):1077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schoemaker MH, Conde de la Rosa L, Buist-Homan M, Vrenken TE, Havinga R, Poelstra K, Haisma HJ, Jansen PL, Moshage H. Tauroursodeoxycholic acid protects rat hepatocytes from bile acid-induced apoptosis via activation of survival pathways. Hepatology 2004;39(6):1563–73. [DOI] [PubMed] [Google Scholar]
- 80. Schoemaker MH, Gommans WM, Conde de la Rosa L, Homan M, Klok P, Trautwein C, van Goor H, Poelstra K, Haisma HJ, Jansen PL, et al. Resistance of rat hepatocytes against bile acid-induced apoptosis in cholestatic liver injury is due to nuclear factor-kappa B activation. J Hepatol. 2003;39(2):153–61. [DOI] [PubMed] [Google Scholar]
- 81. Allen K, Jaeschke H, Copple BL. Bile acids induce inflammatory genes in hepatocytes: A novel mechanism of inflammation during obstructive cholestasis. Am J Pathol. 2011;178(1):175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fickert P, Wagner M, Marschall HU, Fuchsbichler A, Zollner G, Tsybrovskyy O, Zatloukal K, Liu J, Waalkes MP, Cover C, et al. 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology 2006;130(2):465–81. [DOI] [PubMed] [Google Scholar]
- 83. Wang R, Sheps JA, Liu L, Han J, Chen PSK, Lamontagne J, Wilson PD, Welch I, Borchers CH, Ling V. Hydrophilic bile acids prevent liver damage caused by lack of biliary phospholipid in Mdr2(−/−) mice. J Lipid Res. 2019;60(1):85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Stieger B, Fattinger K, Madon J, Kullak-Ublick GA, Meier PJ. Drug- and estrogen-induced cholestasis through inhibition of the hepatocellular bile salt export pump (Bsep) of rat liver. Gastroenterology 2000;118(2):422–30. [DOI] [PubMed] [Google Scholar]
- 85. Trauner M, Fuchs CD, Halilbasic E, Paumgartner G. New therapeutic concepts in bile acid transport and signaling for management of cholestasis. Hepatology 2017;65(4):1393–404. [DOI] [PubMed] [Google Scholar]
- 86. Fuchs CD, Paumgartner G, Wahlstrom A, Schwabl P, Reiberger T, Leditznig N, Stojakovic T, Rohr-Udilova N, Chiba P, Marschall HU, et al. Metabolic preconditioning protects BSEP/ABCB11(−/−) mice against cholestatic liver injury. J Hepatol. 2017;66(1):95–101. [DOI] [PubMed] [Google Scholar]
- 87. Burban A, Sharanek A, Humbert L, Eguether T, Guguen-Guillouzo C, Rainteau D, Guillouzo A. Predictive value of cellular accumulation of hydrophobic bile acids as a marker of cholestatic drug potential. Toxicol Sci. 2019;168(2):474–85. [DOI] [PubMed] [Google Scholar]
- 88. Qiu X, Zhang Y, Liu T, Shen H, Xiao Y, Bourner MJ, Pratt JR, Thompson DC, Marathe P, Humphreys WG, et al. Disruption of BSEP function in HepaRG cells alters bile acid disposition and is a susceptive factor to drug-induced cholestatic injury. Mol Pharm. 2016;13(4):1206–16. [DOI] [PubMed] [Google Scholar]
- 89. Ogimura E, Nakagawa T, Deguchi J, Sekine S, Ito K, Bando K. Troglitazone inhibits bile acid amidation: A possible risk factor for liver injury. Toxicol Sci. 2017;158(2):347–55. [DOI] [PubMed] [Google Scholar]
- 90. Ghallab A, Hofmann U, Sezgin S, Vartak N, Hassan R, Zaza A, Godoy P, Schneider KM, Guenther G, Ahmed YA, et al. Bile microinfarcts in cholestasis are initiated by rupture of the apical hepatocyte membrane and cause shunting of bile to sinusoidal blood. Hepatology 2019;69(2):666–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fickert P, Fuchsbichler A, Wagner M, Zollner G, Kaser A, Tilg H, Krause R, Lammert F, Langner C, Zatloukal K, et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology 2004;127(1):261–74. [DOI] [PubMed] [Google Scholar]
- 92. Fickert P, Pollheimer MJ, Silbert D, Moustafa T, Halilbasic E, Krones E, Durchschein F, Thuringer A, Zollner G, Denk H, et al. Differential effects of norUDCA and UDCA in obstructive cholestasis in mice. J Hepatol. 2013;58(6):1201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Li J, Woolbright BL, Zhao W, Wang Y, Matye D, Hagenbuch B, Jaeschke H, Li T. Sortilin 1 Loss-of-function protects against cholestatic liver injury by attenuating hepatic bile acid accumulation in bile duct ligated mice. Toxicol Sci. 2018;161(1):34–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Alpini G, Glaser SS, Ueno Y, Rodgers R, Phinizy JL, Francis H, Baiocchi L, Holcomb LA, Caligiuri A, LeSage GD. Bile acid feeding induces cholangiocyte proliferation and secretion: Evidence for bile acid-regulated ductal secretion. Gastroenterology 1999;116(1):179–86. [DOI] [PubMed] [Google Scholar]
- 95. Afroze S, Meng F, Jensen K, McDaniel K, Rahal K, Onori P, Gaudio E, Alpini G, Glaser SS. The physiological roles of secretin and its receptor. Ann Transl Med. 2013;1(3):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ballatori N, Truong AT. Relation between biliary glutathione excretion and bile acid-independent bile flow. Am J Physiol. 1989;256(1 Pt 1):G22–30. [DOI] [PubMed] [Google Scholar]
- 97. Wang Y, Aoki H, Yang J, Peng K, Liu R, Li X, Qiang X, Sun L, Gurley EC, Lai G, et al. The role of sphingosine 1-phosphate receptor 2 in bile-acid-induced cholangiocyte proliferation and cholestasis-induced liver injury in mice. Hepatology 2017;65(6):2005–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Reich M, Deutschmann K, Sommerfeld A, Klindt C, Kluge S, Kubitz R, Ullmer C, Knoefel WT, Herebian D, Mayatepek E, et al. TGR5 is essential for bile acid-dependent cholangiocyte proliferation in vivo and in vitro. Gut 2016;65(3):487–501. [DOI] [PubMed] [Google Scholar]
- 99. Merlen G, Kahale N, Ursic-Bedoya J, Bidault-Jourdainne V, Simerabet H, Doignon I, Tanfin Z, Garcin I, Pean N, Gautherot J, et al. TGR5-dependent hepatoprotection through the regulation of biliary epithelium barrier function. Gut 2019. (E-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 100. Xia X, Francis H, Glaser S, Alpini G, LeSage G. Bile acid interactions with cholangiocytes. World J Gastroenterol. 2006;12(22):3553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Alpini G, Glaser S, Baiocchi L, Francis H, Xia X, Lesage G. Secretin activation of the apical Na+-dependent bile acid transporter is associated with cholehepatic shunting in rats. Hepatology 2005;41(5):1037–45. [DOI] [PubMed] [Google Scholar]
- 102. Gujral JS, Hinson JA, Jaeschke H. Chlorotyrosine protein adducts are reliable biomarkers of neutrophil-induced cytotoxicity in vivo. Comp Hepatol. 2004;3(Suppl 1):S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gujral JS, Liu J, Farhood A, Hinson JA, Jaeschke H. Functional importance of ICAM-1 in the mechanism of neutrophil-induced liver injury in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2004;286(3):G499–507. [DOI] [PubMed] [Google Scholar]
- 104. Gujral JS, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology 2003;38(2):355–63. [DOI] [PubMed] [Google Scholar]
- 105. Saito JM, Maher JJ. Bile duct ligation in rats induces biliary expression of cytokine-induced neutrophil chemoattractant. Gastroenterology 2000;118(6):1157–68. [DOI] [PubMed] [Google Scholar]
- 106. Georgiev P, Navarini AA, Eloranta JJ, Lang KS, Kullak-Ublick GA, Nocito A, Dahm F, Jochum W, Graf R, Clavien PA. Cholestasis protects the liver from ischaemic injury and post-ischaemic inflammation in the mouse. Gut 2007;56(1):121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Cai SY, Ouyang X, Chen Y, Soroka CJ, Wang J, Mennone A, Wang Y, Mehal WZ, Jain D, Boyer JL. Bile acids initiate cholestatic liver injury by triggering a hepatocyte-specific inflammatory response. JCI Insight 2017;2(5):e90780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cai SY, Boyer JL. The role of inflammation in the mechanisms of bile acid-induced liver damage. Dig Dis. 2017;35(3):232–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Dold S, Laschke MW, Zhau Y, Schilling M, Menger MD, Jeppsson B, Thorlacius H. P-selectin glycoprotein ligand-1-mediated leukocyte recruitment regulates hepatocellular damage in acute obstructive cholestasis in mice. Inflamm Res. 2010;59(4):291–8. [DOI] [PubMed] [Google Scholar]
- 110. Gujral JS, Hinson JA, Farhood A, Jaeschke H. NADPH oxidase-derived oxidant stress is critical for neutrophil cytotoxicity during endotoxemia. Am J Physiol Gastrointest Liver Physiol. 2004;287(1):G243–52. [DOI] [PubMed] [Google Scholar]
- 111. Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290(6):G1083–8. [DOI] [PubMed] [Google Scholar]
- 112. Wang H, Vohra BP, Zhang Y, Heuckeroth RO. Transcriptional profiling after bile duct ligation identifies PAI-1 as a contributor to cholestatic injury in mice. Hepatology 2005;42(5):1099–108. [DOI] [PubMed] [Google Scholar]
- 113. Kim ND, Moon JO, Slitt AL, Copple BL. Early growth response factor-1 is critical for cholestatic liver injury. Toxicol Sci. 2006;90(2):586–95. [DOI] [PubMed] [Google Scholar]
- 114. Yang M, Ramachandran A, Yan HM, Woolbright BL, Copple BL, Fickert P, Trauner M, Jaeschke H. Osteopontin is an initial mediator of inflammation and liver injury during obstructive cholestasis after bile duct ligation in mice. Toxicol Lett. 2014;224(2):186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. O’Brien KM, Allen KM, Rockwell CE, Towery K, Luyendyk JP, Copple BL. IL-17A synergistically enhances bile acid-induced inflammation during obstructive cholestasis. Am J Pathol. 2013;183(5):1498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sullivan BP, Cui W, Copple BL, Luyendyk JP. Early growth response factor-1 limits biliary fibrosis in a model of xenobiotic-induced cholestasis in mice. Toxicol Sci. 2012;126(1):267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Luyendyk JP, Kassel KM, Allen K, Guo GL, Li G, Cantor GH, Copple BL. Fibrinogen deficiency increases liver injury and early growth response-1 (Egr-1) expression in a model of chronic xenobiotic-induced cholestasis. Am J Pathol. 2011;178(3):1117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Luyendyk JP, Flanagan KC, Williams CD, Jaeschke H, Slusser JG, Mackman N, Cantor GH. Tissue factor contributes to neutrophil CD11b expression in alpha-naphthylisothiocyanate-treated mice. Toxicol Appl Pharmacol. 2011;250(3):256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Canbay A, Taimr P, Torok N, Higuchi H, Friedman S, Gores GJ. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest. 2003;83(5):655–63. [DOI] [PubMed] [Google Scholar]
- 120. Gehring S, Dickson EM, San Martin ME, van Rooijen N, Papa EF, Harty MW, Tracy TF Jr., Gregory SH. Kupffer cells abrogate cholestatic liver injury in mice. Gastroenterology 2006;130(3):810–22. [DOI] [PubMed] [Google Scholar]
- 121. Lukacs-Kornek V, Schuppan D. Dendritic cells in liver injury and fibrosis: Shortcomings and promises. J Hepatol. 2013;59(5):1124–6. [DOI] [PubMed] [Google Scholar]
- 122. Katz SC, Ryan K, Ahmed N, Plitas G, Chaudhry UI, Kingham TP, Naheed S, Nguyen C, Somasundar P, Espat NJ, et al. Obstructive jaundice expands intrahepatic regulatory T cells, which impair liver T lymphocyte function but modulate liver cholestasis and fibrosis. J Immunol. 2011;187(3):1150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Bleier JI, Katz SC, Chaudhry UI, Pillarisetty VG, Kingham TP, 3rd, Shah AB, Raab JR, DeMatteo RP. Biliary obstruction selectively expands and activates liver myeloid dendritic cells. J Immunol. 2006;176(12):7189–95. [DOI] [PubMed] [Google Scholar]
- 124. Chen GY, Nunez G. Inflammasomes in intestinal inflammation and cancer. Gastroenterology 2011;141(6):1986–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Woolbright BL, Jaeschke H. The impact of sterile inflammation in acute liver injury. J Clin Transl Res. 2017;3(Suppl 1):170–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Allen K, Kim ND, Moon JO, Copple BL. Upregulation of early growth response factor-1 by bile acids requires mitogen-activated protein kinase signaling. Toxicol Appl Pharmacol. 2010;243(1):63–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wang H, Zhang Y, Heuckeroth RO. Tissue-type plasminogen activator deficiency exacerbates cholestatic liver injury in mice. Hepatology 2007;45(6):1527–37. [DOI] [PubMed] [Google Scholar]
- 128. Sasaki M, Ikeda H, Yamaguchi J, Miyakoshi M, Sato Y, Nakanuma Y. Bile ductular cells undergoing cellular senescence increase in chronic liver diseases along with fibrous progression. Am J Clin Pathol. 2010;133(2):212–23. [DOI] [PubMed] [Google Scholar]
- 129. Sasaki M, Miyakoshi M, Sato Y, Nakanuma Y. Modulation of the microenvironment by senescent biliary epithelial cells may be involved in the pathogenesis of primary biliary cirrhosis. J Hepatol. 2010;53(2):318–25. [DOI] [PubMed] [Google Scholar]
- 130. Ferreira-Gonzalez S, Lu WY, Raven A, Dwyer B, Man TY, O’Duibhir E, Lewis PJS, Campana L, Kendall TJ, Bird TG, et al. Paracrine cellular senescence exacerbates biliary injury and impairs regeneration. Nat Commun. 2018;9(1):1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Takahashi S, Tanaka N, Golla S, Fukami T, Krausz KW, Polunas MA, Weig BC, Masuo Y, Xie C, Jiang C, et al. Editor’s highlight: Farnesoid X receptor protects against low-dose carbon tetrachloride-induced liver injury through the taurocholate-JNK pathway. Toxicol Sci. 2017;158(2):334–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Liu R, Zhao R, Zhou X, Liang X, Campbell DJ, Zhang X, Zhang L, Shi R, Wang G, Pandak WM, et al. Conjugated bile acids promote cholangiocarcinoma cell invasive growth through activation of sphingosine 1-phosphate receptor 2. Hepatology 2014;60(3):908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Woolbright BL, McGill MR, Yan H, Jaeschke H. Bile acid-induced toxicity in HepaRG cells recapitulates the response in primary human hepatocytes. Basic Clin Pharmacol Toxicol. 2016;118(2):160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]