Abstract
Nonalcoholic steatohepatitis (NASH) is the second leading cause of liver transplantation in the US with a high risk of liver-related morbidities and mortality. Given the global burden of NASH, development of appropriate therapeutic strategies is an important clinical need. Where applicable, lifestyle modification remains the primary recommendation for the treatment of NASH, even though such changes are difficult to sustain and even insufficient to cure NASH. Bariatric surgery resolves NASH in such patients where lifestyle modifications have failed, and is recommended for morbidly obese patients with NASH. Thus, pharmacotherapies are of high value for NASH treatment. Though no drug has been approved by the US Food and Drug Administration for treatment of NASH, substantial progress in pharmacological development has been made in the last few years. Agents such as vitamin E and pioglitazone are recommended in patients with NASH, and yet concerns about their side effects remain. Many agents targeting various vital molecules and pathways, including those impacting metabolic perturbations, inflammatory cascades, and oxidative stress, are in clinical trials for the treatment of NASH. Some agents have shown promising results in phase II or III clinical trials, but more studies are required to assess their long-term effects. Herein, we review the potential strategies and challenges in therapeutic approaches to treating NASH.
Key words: Nonalcoholic steatohepatitis (NASH), Pharmacological therapy, Molecular targets, Lifestyle modification, Bariatric surgery
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD), which is a clinicopathological spectra of liver diseases including simple steatosis, nonalcoholic steatohepatitis (NASH), and cirrhosis, has become a major cause of chronic liver disease in adults1. NASH is characterized by steatosis, lobular inflammation, and hepatocellular ballooning and can potentially progress to cirrhosis2,3. It is the second leading cause of liver transplantation in the US with a high risk of liver-related morbidity and mortality4,5. At present, the global prevalence rate of NAFLD is approximately 25%, while the prevalence of NASH in general people is estimated to be 3%–5% worldwide6,7. NASH is highly related to obesity, dyslipidemia, and type 2 diabetes7. Due to the growing prevalence of obesity, the incidence of NASH may increase further. The high incidence and the poor clinical outcomes of NASH thus are being viewed as a serious global burden.
No US Food and Drug Administration (FDA)-approved medication currently exists for NASH8. Some available strategies, including lifestyle modification, bariatric surgery, and pharmacological therapies, have been recommended for the treatment of NASH in eligible and appropriate patients. The currently recommended treatments have their limitations, so new medical treatments are needed. To date, several novel therapies targeting different stages of NASH pathogenesis, including metabolic perturbations, inflammatory cascades, and oxidative stress, have entered clinical trials (Fig. 1). In addition, it is exciting that animal studies along with subsequent clinical trials have shown some promising results. This review discusses up-to-date therapies that have been developed to treat NASH and are mainly in phase II or III trials and their pharmacologic targets, in hopes of bringing new insights into the mechanisms and treatment of NASH.
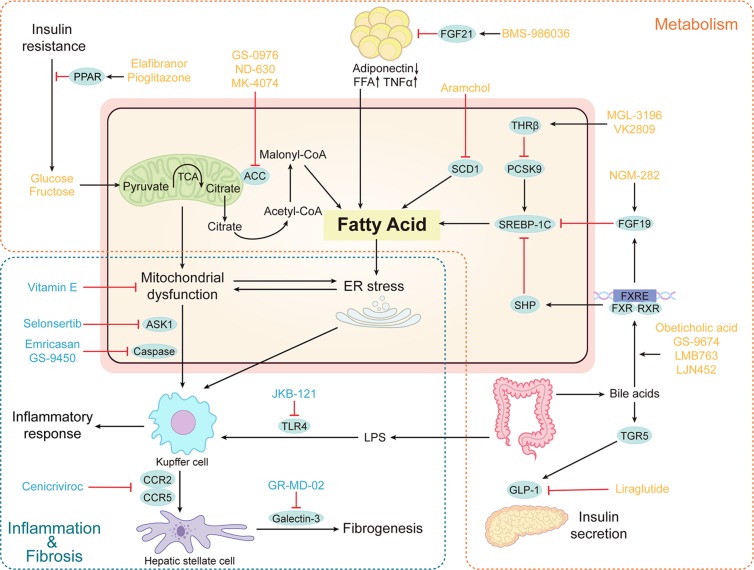
Figure 1.
Core mechanisms of therapeutic targets for nonalcoholic steatohepatitis (NASH). (1) Agents that target metabolic homeostasis in the liver. This group includes ACC inhibitors, FXR agonists, FGF 19/21 analog, PPAR agonist, THRβ agonists, SCD1 inhibitor, GLP-1 receptor agonist. (2) Agents that target inflammatory cascade and oxidative stress. This group includes vitamin E, CCR2/5 antagonist, TLR4 antagonist, ASK1 inhibition, galectin-3 inhibitor, and caspase inhibitor. Some therapeutic pathways (orange box) broadly regulate metabolic pathways. Other therapeutic pathways (blue box) regulate inflammatory cascade and oxidative stress. The blue circles indicate therapeutic targets. PPAR, peroxisome proliferator-activated receptor; TCA, tricarboxylic acid; ACC, acetyl-CoA carboxylase; FFA, free fatty acid; SCD1, stearyl coenzyme a desaturase 1; TNFα, tumor necrosis factor α; FGF21, fibroblast growth factor 21; SREBP, sterol regulatory element-binding proteins; PCSK9, proprotein convertase subtilisin/kexin 9; THRβ, thyroid hormone receptor β; FGF19, fibroblast growth factor 19; SHP, small heterodimer partner; FXR, farnesoid X receptor; RXR, retinoid-x receptor; FXRE, farnesoid X receptor element; TGR5, Takeda G protein-coupled receptor 5; GLP-1, glucagon-like peptide 1; ASK1, apoptosis signal-regulating kinase 1; CCR, C-C motif chemokine receptor; TLR4, toll-like receptor 4; LPS, lipopolysaccharide.
CURRENTLY RECOMMENDED THERAPIES
Lifestyle Modification
Lifestyle modification has represented the cornerstone of therapies for patients with NASH and includes weight loss, diet, and exercise. A comprehensive lifestyle intervention that includes reduced calorie intake and increased exercise can improve the histological features of NASH9.
Weight Loss
Modest weight loss reduced inflammation and altered homeostasis in humans, shedding light on its potential value for treating NASH10. The amount of weight loss is a determining factor in improving histological features of NASH patients. Specifically, weight reduction of 3%–5% is helpful for improving steatosis, while reductions of 5%–7% are necessary for attenuating inflammation11,12. Significant weight loss of 7% or more may improve the histologic features of NASH, involving hepatic steatosis, ballooning, and lobular inflammation13–15, while weight reductions of ≥10% are required to improve fibrosis and portal inflammation, as exhibited in a prospective trial11. Unfortunately, more than half of the subjects fail to achieve the goal of losing 7% of body weight at 12 months in the same study, underscoring the challenges of lifestyle modifications as a universal and practical intervention for NASH.
Diet
A dietary fat with saturated fatty acids is associated with the development of NASH, but n − 3 polyunsaturated fatty acids have protective effects on NASH by mitigating hepatic oxidative stress and inflammation16,17. Recently, the Mediterranean diet, identified as a low-fat and restricted-calorie diet with ample n − 3 polyunsaturated fatty acids, has been demonstrated to reduce hepatic fat and improve hepatic insulin sensitivity, and adherence to the Mediterranean diet lower the risk for NASH. Moreover, this diet is recommended by the EASL-EASD-EASO Clinical Practice Guidelines18–20. Fructose intake should be limited in patients with NASH because its consumption was associated with NASH21. In addition, some dietary supplements contribute to the treatment of NASH, such as coffee and probiotics22–24.
Exercise
Exercise is beneficial for improving liver fat and histology of NASH25,26. Different forms of exercise have similar beneficial effects on hepatic fat, and vigorous exercise has the largest effects on NASH and fibrosis9. However, these effects disappear if patients do not exercise persistently. Vigorous exercise of no less than 250 min/week mitigates the pathophysiology of NASH better than exercise of less than 250 min/week, but excessive exercise is harmful for patients with cardiovascular complications12. Moderate intensity aerobic exercise over three to five sessions for 150–200 min/week is recommended by the EASL-EASD-EASO guidelines18. More studies on exercise are needed to verify its long-term effects on NASH and the related morbidity and mortality.
Although lifestyle modifications are considered generally safe, rapid weight loss achieved by any modality may increase the risk of cholelithiasis because of the increase in cholesterol flux through the biliary system, and many patients may experience problems regarding steadfast adherence27.
Bariatric Surgery
Bariatric surgery is an effective way to obtain long-term weight loss. Bariatric surgery prevented NASH progression in diet-induced obese rats of an animal research28, and the current evidence has demonstrated that liver steatosis, inflammation, and fibrosis were improved in NASH patients after bariatric surgery29,30. What is more, a recent study indicated that bariatric surgery has a long-term effect of mitigating NASH, and the effect could last for 10 years after various bariatric surgeries31. Bariatric surgery is applicable in morbidly obese patients with NASH and post-liver transplantation recurrent NASH32. However, insufficient clinical data exist to recommend bariatric surgery as a routine treatment for NASH considering the perioperative risks and cost effectiveness29. Larger randomized controlled trials (RCT) should be designed to confirm the efficacy and safety of bariatric surgery on NASH.
Pharmacological Therapy
Pharmacotherapies should be considered for NASH and fibrosis patients due to the limitations of lifestyle modification and bariatric surgery. Vitamin E and pioglitazone have been suggested as pharmacotherapies for histologically confirmed NASH patients in guidelines from the US, Europe, China, and Japan.
Vitamin E
Currently, vitamin E is the accepted first-line pharmacological treatment for NASH in nondiabetic patients. Vitamin E is an essential antioxidant that acts by inhibiting lipid peroxidation and improving lipid metabolism. In mouse models of NASH, vitamin E attenuated lipid peroxidation and inflammatory responses33. The potential effect of vitamin E on NASH has been evaluated in four clinical trials of which the PIVENS trial (Pioglitazone versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis) is the largest RCT34–37. In the PIVENS trial, 247 subjects with histologically confirmed NASH were randomly assigned to receive pioglitazone 30 mg/day (80 patients), vitamin E 800 IU/day (84 patients), or placebo (83 patients) for 96 weeks37. Vitamin E was superior to the placebo in improving alanine transaminase (ALT) levels, liver steatosis, and inflammation but did not significantly mitigate fibrosis. The long-term safety of vitamin E has been controversial, so its benefits need to be weighed against the increased risks of prostate cancer, hemorrhagic stroke, and even all-cause mortality38.
Pioglitazone
Peroxisome proliferator-activated receptors (PPARs), including PPARα, δ, and γ, are nuclear receptors involved in lipid metabolism. Pioglitazone, a PPARγ agonist, regulates plasma adiponectin and improves insulin sensitivity in the liver39. It could improve histologic alterations including hepatic injury and fibrosis in patients with NASH40. The function of alleviating histological components was also verified in the PIVENS trial, but this efficacy did not reach the predicted threshold37. It did resolve steatohepatitis in a subset of patients. A similar long-term RCT involving 101 prediabetic and diabetic patients with biopsy-proven NASH showed that pioglitazone treatment improved all histologic features and ameliorated NASH without a worsening of fibrosis41. Pioglitazone should be used carefully for NASH in diabetic patients with potential long-term risk concerns.
Some drugs such as statins, metformin, and lipase inhibitors seem to be safe but are not recommended because no histological data are available to support their effects on NASH. More novel pharmacological approaches are necessary for treating NASH because the long-term safety and impact on currently recommended pharmacotherapies should be further evaluated.
PROMISING MOLECULAR TARGETS FOR PHARMACOLOGICAL THERAPY
Some pharmacological therapies with different targets, including those impacting metabolic perturbations, inflammatory cascades, and oxidative stress, and are in phase II or III trials are promising for the treatment of NASH. Recently completed and ongoing RCTs for NASH are summarized in Table 1.
Table 1.
Recently Completed and Ongoing Randomized Controlled Trials for NASH.
| Agents | Mechanisms | Primary Endpoint(s) | Phase Completed | Patients (n) | Duration | Status | NCT |
|---|---|---|---|---|---|---|---|
| GS-0976 | ACC inhibitor | Overall safety | II | 125 | Up to12 weeks plus 30 days | Completed | NCT02856555 |
| Obeticholic acid | FXR agonist | Improvement in liver histology in non-cirrhotic NASH | III | 2500 | 18 months | Recruiting | NCT02548351 |
| Obeticholic acid | FXR agonist | Improvement in fibrosis | III | 540 | 12 months | Recruiting | NCT03439254 |
| GS-9674 | FXR agonist | Overall safety | II | 125 | Up to 24 weeks plus 30 days | Completed | NCT02854605 |
| LMB763 | FXR agonist | Adverse event profile and safety; change in transaminase levels | II | 100 | 12 weeks | Recruiting | NCT02913105 |
| LJN452 | FXR agonist | Adverse event profile; change in transaminase levels and fat in the liver | II | 250 | 12 weeks | Recruiting | NCT02855164 |
| NGM-282 | FGF19 analog | Change in hepatic fat | II | 75 | 24 weeks | Recruiting | NCT02443116 |
| BMS-986036 | FGF 21 analog | Improvement in fibrosis | II | 160 | 24 weeks | Recruiting | NCT03486899 |
| BMS-986036 | FGF 21 analog | Improvement in fibrosis | II | 100 | 48 weeks | Recruiting | NCT03486912 |
| Elafibranor | PPARα/δ agonist | Resolution of NASH without worsening fibrosis | III | 2000 | 72 weeks | Recruiting | NCT02704403 |
| MGL-3196 | THRβ agonist | Change in hepatic fat | II | 117 | 12 weeks | Active, not recruiting | NCT02912260 |
| Aramchol | SCD-1 inhibitor | Change in the hepatic fat | II | 240 | 52 weeks | Completed | NCT02279524 |
| Liraglutide | GLP-1 receptor agonist | Improvement in NASH | III | 36 | 12 months | Recruiting | NCT02654665 |
| Cenicriviroc | CCR2/5 antagonist | Improvement in liver histology | III | 2000 | 12 months | Recruiting | NCT03028740 |
| JKB-121 | TLR4 antagonist | Change in hepatic fat and improvement in ALT | II | 60 | 24 weeks | Completed | NCT02442687 |
| GS-4997 + Simtuzumab | ASK1 inhibitor, LOXL2 inhibitor | Adverse event profile | II | 70 | Up to 28 weeks | Completed | NCT02466516 |
| Selonsertib | ASK1 inhibitor | Improvement in fibrosis without worsening NASH | III | 800 | 48 weeks | Active, not recruiting | NCT03053050 |
| GR-MD-02 | Galectin-3 inhibitor | Improvement in fibrosis | II | 30 | 16 weeks | Completed | NCT02421094 |
| Emricasan | Caspase inhibitor | Improvement in fibrosis without worsening steatohepatitis | II | 330 | 72 weeks | Active, not recruiting | NCT02686762 |
| GS-9450 | Caspase inhibitor | Safety and tolerability | II | 110 | 8 weeks | Completed | NCT00740610 |
ACC, acetyl-CoA carboxylase; FXR, farnesoid X receptor; FGF19, fibroblast growth factor 19; FGF21, fibroblast growth factor 21; PPARα/δ, peroxisome proliferator-activated receptor α/δ; THRβ, thyroid hormone receptor β; LDL-C, low-density lipoprotein cholesterol; SCD1, stearyl coenzyme a desaturase 1; GLP-1, glucagon-like peptide 1; CCR2/5, C-C motif chemokine receptor 2/5; TLR4, toll-like receptor 4; ALT, alanine transaminase; ASK1, apoptosis signal-regulating kinase 1; LOXL 2, lysyl oxidase-like protein 2.
Targeting Metabolic Perturbations
Acetyl-CoA Carboxylase (ACC) Inhibitors
ACC regulates the nonreversible conversion of acetyl-CoA to malonyl-CoA and includes the isoforms ACC1 and ACC242. Malonyl-CoA regulates fatty acid synthesis, which is a hallmark of various metabolic diseases including NASH. At present, several ACC inhibitors have shown beneficial effects in preclinical studies and clinical trials of NASH. Notably, GS-0976, an inhibitor of ACC1 and ACC2, decreased liver steatosis, markers of liver injury, and de novo lipogenesis (DNL) in a placebo-controlled trial of NASH patients43. ND-630, an isozyme-nonselective ACC inhibitor, can reduce fatty acid synthesis and promote fatty acid oxidation in cultured cells and animals. In animal models, ND-630 decreased liver steatosis, improved insulin sensitivity, as well as modulated dyslipidemia44. However, no clinical trial has tested the effect and safety of ND-630 in NASH patients. MK-4074, a liver-targeting inhibitor of ACC1 and ACC2, reduced hepatic triglyceride (TG) levels in preclinical studies and significantly reduced liver fat in human beings45. Unfortunately, the use of ACC inhibitors can increase the expression of glycerol-3-phosphate acyltransferase 1, which plays an important part in mediating triglyceride synthesis, ultimately leading to significant hypertriglyceridemia45.
Farnesoid X Receptor (FXR) Agonists
FXR was initially identified as a bile acid sensor that controls bile acid and lipid homeostasis through the small heterodimer partner (SHP)46,47. Obeticholic acid (OCA), an agonist of FXR, improves insulin sensitivity, inflammation, and fibrosis and reduces hepatic steatosis in obese rats48. In a recent clinical trial (FLINT), OCA induced a definite improvement in NASH activity, serum aminotransferase, and fibrosis score compared with placebo49. Furthermore, in a phase II study, OCA administered to NASH patients for 6 weeks increased insulin sensitivity and attenuated hepatic inflammation and fibrosis50. Two phase III trials involving 3,000 patients from more than 20 countries are upcoming to verify its influence (ClinicalTrials.gov Identifier: NCT02548351, NCT03439254). As exhibited in the interim results of phase III trials, OCA improved hepatic fibrosis with no worsening of NASH compared with placebo. However, OCA has several side effects, like pruritus, gastrointestinal events, and so on49. A nonsteroidal FXR agonist, GS-9674, has been exploited to address some of the side effects of OCA. GS-9674 mitigated liver steatosis and hepatic biochemistry in subjects with NASH in a phase II RCT (ClinicalTrials.gov Identifier: NCT02854605). Other FXR agonists, such as LMB763 and LJN452, with limited side effects, have also been tested in phase II trials (ClinicalTrials.gov Identifier: NCT02913105, NCT02855164).
Fibroblast Growth Factor 19/21 (FGF 19/21) Analog
A peptide hormone FGF19 is released from the intestine after bile acid binding to FXR and regulates bile acid synthesis and lipid metabolism51,52. In a preclinical study, treatment with FGF19 significantly decreased diet-induced hepatic steatosis53. NGM-282 is a recombinant FGF19 with the beneficial metabolic effects of reducing steatosis and lipotoxicity. Furthermore, a phase II study of 166 biopsy-confirmed NASH patients indicated that NGM-282 significantly reduced liver fat content with an acceptable safety profile as well as adverse events, including diarrhea, abdominal pain, and nausea54. Another phase II multiple-center study is ongoing to ascertain the safety and effectiveness of NGM282 in histologically confirmed NASH patients (ClinicalTrials.gov Identifier: NCT02443116).
FGF21, a 181-amino acid-secreted protein produced in the liver, enhances glycogen synthesis and ameliorates insulin sensitivity, which was beneficial in treating NASH in rodent models55–57. Due to the short half-life period of wild-type FGF21, the recombinant mutant FGF21, and the conjugation of FGF21 to polyethylene glycol (PEG), has been developed and was tested for its role in NASH. This variant of FGF21 (LY2405319) alleviated inflammation and reversed hepatofibrosis in an animal study of NASH58. Notably, a PEGylated FGF21 (BMS-986036) was demonstrated to significantly reduce hepatic fat in NASH patients and had good tolerance in a phase II clinical study59. Two phase II trials of BMS-986036 in NASH patients with stage 3 disease are currently ongoing (ClinicalTrials.gov Identifier: NCT03486899, NCT03486912).
PPARα/δ Agonist
PPARα activation regulates fatty acid β-oxidation and lipid transport, while PPARδ activation results in fatty acid oxidation60. Elafibranor (GFT505) is a dual PPARα/δ agonist that can improve peripheral insulin sensitivity. Treatment of an animal model of NASH demonstrated a decrease in hepatic fat and improvement in hepatic steatosis, inflammation, and fibrosis61. The antifibrotic effect of GFT-505 was independent of metabolic corrections. Elafibranor also reduced liver enzyme concentrations and had a reassuring safety profile62. Recent results from the phase IIb RCT demonstrated that Elafibranor resolved NASH and lowered ALT levels without worsening of fibrosis. It also exhibits beneficial effects on metabolic markers, including improving insulin sensitivity, lowering plasma triglycerides, and increasing high-density lipoprotein cholesterol63. Furthermore, it was well tolerated and thus may be a promising drug candidate for NASH therapy63. A pivotal phase III RESOLVE-IT trial of Elafibranor is currently recruiting patients (ClinicalTrials.gov Identifier: NCT02704403).
Thyroid Hormone Receptor β (THRβ) Agonists
THRβ is the crucial nuclear receptor for the action of thyroid hormones, especially triiodothyronine (T3), on hepatocytes. It has been confirmed to reduce hepatic lipotoxicity and improve hepatic function by lowering fatty acid accumulation and degeneration64. THRβ agonists have also been shown to induce liver regeneration via stimulation of Wnt/β-catenin pathway and inducing hepatocyte proliferation, while reducing hepatocellular cancer burden, which could all be beneficial in NASH65–67. A THRβ agonist prevented hepatic steatosis but impaired insulin sensitivity in high-fat diet-fed rats68. In a multicenter study in adults with biopsy-confirmed NASH, a highly selective liver-targeted THRβ agonist MGL-3196 for 12 weeks significantly decreased hepatic fat relative to placebo69. Moreover, VK2809, a liver-targeted THRβ agonist, was shown to reduce liver triglycerides and microvesicular steatosis in rats and mice70. Phase II trials of MGL-3196 and VK2809 are scheduled to begin enrolling patients soon (ClinicalTrials.gov Identifier: NCT02912260, NCT02927184).
Stearoyl Coenzyme A Desaturase 1 (SCD1) Inhibitor
SCD1 is the central enzyme that catalyzes the synthesis of fatty acids and decreases β-oxidation71. Aramchol, a novel SCD1 inhibitor, reduces hepatic fat content and mitigates steatohepatitis and fibrosis in mice72. In a phase II RCT of biopsy-proven NASH and NAFLD subjects, Aramchol given once a day for 3 months reduced hepatic fat with no adverse events73. However, this trial did not verify the effect of Aramchol on hepatic inflammation and fibrosis associated with NASH. In a 52-week phase IIb RCT of biopsy-proven NASH patients, two doses of Aramchol (400 and 600 mg) significantly reduced hepatic fat and improved histologic features with excellent safety and tolerability (ClinicalTrials.gov Identifier: NCT02279524). A phase III trial with a large sample size should be designed to determine the therapeutic effects of Aramchol.
Glucagon-Like Peptide 1 (GLP-1) Receptor Agonist
GLP-1, first described as a gut-derived hormone generated through the proteolytic processing of proglucagon74,75, has an influence on liver metabolism in NASH76,77. Preclinical studies observed reductions in liver enzymes and oxidative stress accompanied by improvements in liver histology in murine models of NASH after GLP-1 receptor agonist intervention78,79. The GLP-1 receptor agonist liraglutide was reported to ameliorate hepatic inflammation and ballooning in an animal study80. In humans, a randomized, placebo-controlled phase II trial showed liraglutide to cause histological resolution of NASH without deterioration of fibrosis81. It was found to be safe and well tolerated, but extensive, longer duration studies are warranted81,82. A phase III study to compare the therapeutic potency of liraglutide and bariatric surgery in NASH is currently enrolling cases (ClinicalTrials.gov Identifier: NCT02654665).
Targeting Inflammation and Oxidative Stress
C-C Motif Chemokine Receptor 2/5 (CCR2/5) Antagonist
The CCR2/5 chemokine axis recruits immune cells to the liver to initiate an immune response83. The CCR2/5 antagonist Cenicriviroc (CVC) displays anti-inflammatory and antifibrotic activities in preclinical models and may be useful in the treatment of NASH84. Presently, its efficacy and safety are being tested in a phase IIb RCT (CENTAUR trial) for treating NASH and hepatic fibrosis. CVC has shown a significant antifibrotic benefit with no worsening of steatohepatitis after 1 year. Compared with placebo, biomarkers of inflammation were significantly reduced after CVC intervention85. A phase III study is currently recruiting patients and evaluating the role of CVC for the treatment of NASH (ClinicalTrials.gov Identifier: NCT03028740).
Toll-Like Receptor 4 (TLR4) Antagonist
TLR4 is a crucial mediator of innate immunity and is involved in inflammation and insulin resistance86,87. It has been a potential target for amelioration of hepatic injury, insulin resistance, and progression to NASH in preclinical studies88,89. Notably, the TLR4 antagonist JKB-121 reduced LPS-induced inflammatory liver injury and inhibited hepatic stellate cell activation, supporting JKB-121 as a potential treatment for NASH. However, a phase II RCT of JKB-121 in 65 patients with NASH was completed with unsatisfactory results. Compared to placebo, JKB-121 did not further improve liver fat or ALT and showed mild drug-related adverse events (ClinicalTrials.gov Identifier: NCT02442687).
Apoptosis Signal-Regulating Kinase 1 (ASK-1) Inhibitor
ASK1 is a member of the MAP3K family and regulates the p38/JNK pathways90. ASK1 inhibition improves liver steatosis, inflammation, insulin resistance, and fibrosis in rodent and primate models of NASH91–93, suggesting CASP8, FADD-like apoptosis regulator (CFLAR), and TNF-α-induced protein 3 (TNFAIP3) as potential targets for the treatment of NASH94,95. Selonsertib (GS-4997), which targets ASK1, was assessed in a multicenter phase II trial in which selonsertib was shown to alleviate hepatic fibrosis in NASH patients and those with stage 2 or 3 fibrosis96. Therapy using a combination of selonsertib and simtuzumab improved fibrosis with high-dose selonsertib (ClinicalTrials.gov Identifier: NCT02466516). In addition, patient-reported outcomes displayed that selonsertib or selonsertib in combination with simtuzumab lowered hepatic collagen in NASH patients and stages 2–3 liver fibrosis97. Selonsertib is now being tested in a phase III trial for the treatment of NASH and F3 fibrosis (ClinicalTrials.gov Identifier: NCT03053050). Unfortunately, the latest report by Gilead shows that selonsertib failed to achieve the main goal of improving liver fibrosis in the phase III trial.
TGF-β-Activated Kinase 1 (TAK1) Inhibitor
TAK1 facilitates the activation of downstream JNK and NF-κB cascades, promoting the development of NASH87. Inhibition of TAK1 activity by deubiquitination or dephosphorylation protected against hepatic steatosis, insulin resistance, and inflammation and is thereby considered to be a therapeutic target for NASH98–101. In animal studies of NASH, specific proteins targeting TAK1, such as cylindromatosis (CYLD), ubiquitin-specific protease 4 (USP4), and dual-specificity phosphatase 14 (DUSP14), have been shown to hinder the progress of NASH101–103. However, no clinical trials have been designed to verify the effects of TAK1 inhibition on NASH.
Galectin-3 Inhibitor
Galectin-3, a β-galactoside-binding animal lectin, is expressed predominantly in immune cells, which regulates the progression of hepatic fibrosis104. Galactoarabinorhamnogalacturnate (GR-MD-02), a complex carbohydrate-based inhibitor that binds to galectin-3, ameliorated histopathological features of NASH and fibrosis in a preclinical study of NASH105. Furthermore, GR-MD-02 was safe and well tolerated, as demonstrated by the phase I human study of subjects with histologically confirmed NASH and stage 3 fibrosis106. Recently, one phase II randomized trial has already completed testing the role of GR-MD-02 in treating NASH patients. However, the results showed no apparent improvement in the levels of noninvasive biomarkers of hepatic inflammation or fibrosis after 16 weeks of therapy with GR-MD-02 (ClinicalTrials.gov Identifier: NCT02421094).
Caspase Inhibitor
Caspases are a class of enzymes that play a critical role in executing apoptotic pathways or programmed cell death. Emricasan (IDN-6556), a currently available irreversible pancaspase inhibitor, has shown an influence on improving NAS and fibrosis in preclinical trials107. In a phase II study, 38 subjects with proven NAFLD/NASH were randomly assigned to receive Emricasan 25 mg twice a day or placebo for 28 days with a primary result of reduction in ALT and biomarkers108. Recently, a phase IIb study of Emricasan will begin enrolling cases soon (ClinicalTrials.gov Identifier: NCT02686762). Another caspase inhibitor, GS-9450, can act selectively on caspases 1, 8, and 9. As exhibited in a phase II, double-blind trial, GS-9450 significantly reduced ALT levels in patients with NASH109. The safety and efficacy of GS-9450 for NASH need to be assessed in larger-scale clinical trials.
CHALLENGES IMPEDING TRANSLATION FROM BENCH TO BEDSIDE IN DRUG DISCOVERY FOR NASH
Drug development for NASH requires clear mechanisms, appropriate animal models, progressive clinical trials, convenient efficacy evaluation, and follow-up methods. Many challenges impeding the translation from bench to bedside remain in this drug discovery process. The pathogenesis of NASH has not been entirely elucidated and has been debated for a long time, which is one of the bottlenecks in drug development. The “multiple-parallel hit” hypothesis was recently proposed, providing a more adequate explanation of how fatty acids and their metabolites promote NASH through multiple sequential or parallel cytotoxic pathways110. The pathogenesis of human NASH involves varied molecular pathways and complex progression with a dynamic bidirectional nature, which is unlikely to be the same in all patients, raising concern about individual differences.
The perfect animal model that mimics the pathophysiology of NASH and displays the most clinical characteristics of human disease as closely as possible does not exist, further increasing the difficulty in identifying and validating potential drug targets for human NASH. Many species, including mice, rabbits, pigs, and monkeys, are used to develop models with a liver phenotype resembling human NASH, and each has its advantages and disadvantages111. Recently, monkeys have been recommended as models of NASH due to similarities in liver anatomy, physiology, metabolism, and genetics to humans112,113. Monkey breeding has specific disadvantages including sophisticated genetic methods, housing, cost, and logistics. Moreover, the ideal pharmacodynamics of drugs in animal models do not necessarily replicate in human NASH.
Because of the slow progression to clinically significant outcomes in NASH, drug development has been delayed, and the potential time to market for drugs has been extended. The use of optimal surrogate endpoints for clinical trials in NASH is imperative for evaluating pharmacologic agents114. For purposes of accelerated approval, the surrogate endpoints used by the FDA can be achieved in a reasonably short timeframe. Biomarkers of NASH are helpful for the diagnosis, monitoring, and prognosis of disease progression and evaluation of the effects of new regimens, which is urgent in the field of NASH. In addition, existing methods of pharmacodynamic evaluation and follow-up are still insufficient to assess NASH progression and regression for drug development.
PROSPECTIVE AND FUTURE
Currently, no FDA-approved drug is available to treat NASH. Lifestyle modification rarely leads to the resolution of NASH, while bariatric surgery can resolve NASH but is just recommended for morbidly obese patients with NASH. Many efforts have been made to develop pharmacotherapies based on the core mechanism of NASH, and some agents have entered phase III favorably. Encouragingly, promising results have been obtained for some medications, including FXR agonists, PPAR-α/δ agonists, GLP-1 agonists, CCR2/5 chemokine receptor antagonists, ASK-1 inhibitors, galectin-3 inhibitors, and caspase inhibitors. However, these drugs are still investigative and experimental. None of these experimental medicines has a perfect function of improving all histological features of NASH and reversing NASH with no drug-related adverse events in the existing clinical trials. Long-term effects and safety on NASH and liver-related mortality and morbidity should be tested in more clinical trials, particularly given concerns about the potential adverse events of some drugs. In the coming years, it is expected that some of these agents alone or in combination will provide the optimal outcomes and new treatment options for NASH patients. Furthermore, improved animal models and scientific research are needed to identify the complete pathogenesis and find new targets for therapy, while specific markers, optimized clinical trial design, suitable surrogate endpoints, and follow-up investigation are required for further drug development.
ACKNOWLEDGMENTS
This work was supported by grants from the National Science Fund for Distinguished Young Scholars (No. 81425005; H.L.), the Key Project of the National Natural Science Foundation (Nos. 81330005 and 81630011; H.L.), the Major Research Plan of the National Natural Science Foundation of China (Nos. 1729303 and91639304; H.L.), the Creative Groups Project of Hubei Province (No. 2016CFA010; H.L.), the National Key R&D Program of China (No. 2016YFF0101504; Z.-G.S.), and the National Natural Science Foundation of China (No. 81770053; Z.-G.S.).
REFERENCES
- 1. Bellentani S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017;37(Suppl 1):81–4. [DOI] [PubMed] [Google Scholar]
- 2. Wong RJ, Liu B, Bhuket T. Significant burden of non-alcoholic fatty liver disease with advanced fibrosis in the US: A cross-sectional analysis of 2011–2014 National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 2017;46(10):974–80. [DOI] [PubMed] [Google Scholar]
- 3. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64(1):73–84. [DOI] [PubMed] [Google Scholar]
- 4. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148(3):547–55. [DOI] [PubMed] [Google Scholar]
- 5. Zhang XJ, She ZG, Li H. Time to step-up the fight against NAFLD. Hepatology 2018;67(6):2068–71. [DOI] [PubMed] [Google Scholar]
- 6. Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. [DOI] [PubMed] [Google Scholar]
- 7. Younossi ZM. Non-alcoholic fatty liver disease—A global public health perspective. J Hepatol. 2019;70(3):531–44. [DOI] [PubMed] [Google Scholar]
- 8. Younossi Z, Tacke F, Arrese M, et al. Global perspectives on non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Hepatology 2019;69(6):2672–82. [DOI] [PubMed] [Google Scholar]
- 9. Romero-Gomez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67(4):829–46. [DOI] [PubMed] [Google Scholar]
- 10. Magkos F, Fraterrigo G, Yoshino J, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23(4):591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015;149(2):367–78.e5. [DOI] [PubMed] [Google Scholar]
- 12. Oh S, Shida T, Yamagishi K, et al. Moderate to vigorous physical activity volume is an important factor for managing nonalcoholic fatty liver disease: A retrospective study. Hepatology 2015;61(4):1205–15. [DOI] [PubMed] [Google Scholar]
- 13. Vilar Gomez E, Rodriguez De Miranda A, Gra Oramas B, et al. Clinical trial: A nutritional supplement Viusid, in combination with diet and exercise, in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2009;30(10):999–1009. [DOI] [PubMed] [Google Scholar]
- 14. Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on non-alcoholic steatohepatitis. Hepatology 2010;51(1):121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wong VW, Chan RS, Wong GL, et al. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: A randomized controlled trial. J Hepatol. 2013;59(3):536–42. [DOI] [PubMed] [Google Scholar]
- 16. Musso G, Gambino R, De Michieli F, et al. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology 2003;37(4):909–16. [DOI] [PubMed] [Google Scholar]
- 17. Jeyapal S, Kona SR, Mullapudi SV, Putcha UK, Gurumurthy P, Ibrahim A. Substitution of linoleic acid with alpha-linolenic acid or long chain n-3 polyunsaturated fatty acid prevents Western diet induced non-alcoholic steatohepatitis. Sci Rep. 2018;8(1):10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. European Association for the Study of the L, European Association for the Study of D, European Association for the Study of O. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–402. [DOI] [PubMed] [Google Scholar]
- 19. Zelber-Sagi S, Salomone F, Mlynarsky L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: Evidence and plausible mechanisms. Liver Int. 2017;37(7):936–49. [DOI] [PubMed] [Google Scholar]
- 20. Della Corte C, Mosca A, Vania A, Alterio A, Iasevoli S, Nobili V. Good adherence to the Mediterranean diet reduces the risk for NASH and diabetes in pediatric patients with obesity: The results of an Italian Study. Nutrition 2017;39–40:8–14. [DOI] [PubMed] [Google Scholar]
- 21. Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377(21):2063–72. [DOI] [PubMed] [Google Scholar]
- 22. Molloy JW, Calcagno CJ, Williams CD, Jones FJ, Torres DM, Harrison SA. Association of coffee and caffeine consumption with fatty liver disease, nonalcoholic steatohepatitis, and degree of hepatic fibrosis. Hepatology 2012;55(2):429–36. [DOI] [PubMed] [Google Scholar]
- 23. Abdel Monem SM. Probiotic therapy in patients with nonalcoholic steatohepatitis in Zagazig University Hospitals. Euroasian J Hepatogastroenterol. 2017;7(1):101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manzhalii E, Virchenko O, Falalyeyeva T, Beregova T, Stremmel W. Treatment efficacy of a probiotic preparation for non-alcoholic steatohepatitis: A pilot trial. J Dig Dis. 2017;18(12):698–703. [DOI] [PubMed] [Google Scholar]
- 25. Kwak MS, Kim D. Non-alcoholic fatty liver disease and lifestyle modifications, focusing on physical activity. Korean J Intern Med. 2018;33(1):64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van der Windt DJ, Sud V, Zhang H, Tsung A, Huang H. The effects of physical exercise on fatty liver disease. Gene Expr. 2018;18(2):89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Issa D, Wattacheril J, Sanyal AJ. Treatment options for nonalcoholic steatohepatitis—A safety evaluation. Expert Opin Drug Saf. 2017;16(8):903–13. [DOI] [PubMed] [Google Scholar]
- 28. Yu HH, Hsieh MC, Wu SY, Sy ED, Shan YS. Effects of duodenal–jejunal bypass surgery in ameliorating nonalcoholic steatohepatitis in diet-induced obese rats. Diabetes Metab Syndr Obes. 2019;12:149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lassailly G, Caiazzo R, Buob D, et al. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology 2015;149(2):379–88. [DOI] [PubMed] [Google Scholar]
- 30. Schneck AS, Anty R, Patouraux S, et al. Roux-En Y gastric bypass results in long-term remission of hepatocyte apoptosis and hepatic histological features of non-alcoholic steatohepatitis. Front Physiol. 2016;7:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tan CH, Al-Kalifah N, Ser KH, Lee YC, Chen JC, Lee WJ. Long-term effect of bariatric surgery on resolution of nonalcoholic steatohepatitis (NASH): An external validation and application of a clinical NASH score. Surg Obes Relat Dis. 2018;14(10):1600–6. [DOI] [PubMed] [Google Scholar]
- 32. Fan JG, Wei L, Zhuang H, National Workshop on Fatty L, Alcoholic Liver Disease CSoHCMA, Fatty Liver Disease Expert Committee CMDA. Guidelines of prevention and treatment of nonalcoholic fatty liver disease (2018, China). J Dig Dis. 2019;20(4):163–73. [DOI] [PubMed] [Google Scholar]
- 33. Chung MY, Yeung SF, Park HJ, Volek JS, Bruno RS. Dietary alpha- and gamma-tocopherol supplementation attenuates lipopolysaccharide-induced oxidative stress and inflammatory-related responses in an obese mouse model of nonalcoholic steatohepatitis. J Nutr Biochem. 2010;21(12):1200–6. [DOI] [PubMed] [Google Scholar]
- 34. Sanyal AJ, Mofrad PS, Contos MJ, et al. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2(12):1107–15. [DOI] [PubMed] [Google Scholar]
- 35. Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children: A pilot study. J Pediatr. 2000;136(6):734–8. [PubMed] [Google Scholar]
- 36. Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98(11):2485–90. [DOI] [PubMed] [Google Scholar]
- 37. Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Klein EA, Thompson IM Jr., Tangen CM, et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011;306(14):1549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: Pathophysiology and clinical implications. Gastroenterology 2012;142(4):711–25.e16. [DOI] [PubMed] [Google Scholar]
- 40. Aithal GP, Thomas JA, Kaye PV, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 2008;135(4):1176–84. [DOI] [PubMed] [Google Scholar]
- 41. Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: A randomized trial. Ann Intern Med. 2016;165(5):305–15. [DOI] [PubMed] [Google Scholar]
- 42. Foster DW. Malonyl-CoA: The regulator of fatty acid synthesis and oxidation. J Clin Invest. 2012;122(6):1958–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lawitz EJ, Coste A, Poordad F, et al. Acetyl-CoA carboxylase inhibitor GS-0976 for 12 weeks reduces hepatic de novo lipogenesis and steatosis in patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2018;16(12):1983–91.e83. [DOI] [PubMed] [Google Scholar]
- 44. Harriman G, Greenwood J, Bhat S, et al. Acetyl-CoA carboxylase inhibition by ND-630 reduces hepatic steatosis, improves insulin sensitivity, and modulates dyslipidemia in rats. Proc Natl Acad Sci USA 2016;113(13):E1796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim CW, Addy C, Kusunoki J, et al. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: A bedside to bench investigation. Cell Metab. 2017;26(3):576. [DOI] [PubMed] [Google Scholar]
- 46. Adorini L, Pruzanski M, Shapiro D. Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis. Drug Discov Today 2012;17(17–18):988–97. [DOI] [PubMed] [Google Scholar]
- 47. Chiang JYL, Ferrell JM. Bile acid metabolism in liver pathobiology. Gene Expr. 2018;18(2):71–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cipriani S, Mencarelli A, Palladino G, Fiorucci S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipid Res. 2010;51(4):771–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): A multicentre, randomised, placebo-controlled trial. Lancet 2015;385(9972):956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mudaliar S, Henry RR, Sanyal AJ, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 2013;145(3):574–82.e71. [DOI] [PubMed] [Google Scholar]
- 51. Nies VJ, Sancar G, Liu W, et al. Fibroblast growth factor signaling in metabolic regulation. Front Endocrinol. (Lausanne) 2015;6:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. You M, Zhou Z, Daniels M, Jogasuria A. Endocrine adiponectin–FGF15/19 axis in ethanol-induced inflammation and alcoholic liver injury. Gene Expr. 2018;18(2):103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Alvarez-Sola G, Uriarte I, Latasa MU, et al. Fibroblast growth factor 15/19 (FGF15/19) protects from diet-induced hepatic steatosis: Development of an FGF19-based chimeric molecule to promote fatty liver regeneration. Gut 2017;66(10):1818–28. [DOI] [PubMed] [Google Scholar]
- 54. Harrison SA, Rinella ME, Abdelmalek MF, et al. NGM282 for treatment of non-alcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2018;391(10126):1174–85. [DOI] [PubMed] [Google Scholar]
- 55. Cicione C, Degirolamo C, Moschetta A. Emerging role of fibroblast growth factors 15/19 and 21 as metabolic integrators in the liver. Hepatology 2012;56(6):2404–11. [DOI] [PubMed] [Google Scholar]
- 56. Zhang F, Yu L, Lin X, et al. Minireview: Roles of fibroblast growth factors 19 and 21 in metabolic regulation and chronic diseases. Mol Endocrinol. 2015;29(10):1400–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Markan KR, Naber MC, Ameka MK, et al. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 2014;63(12):4057–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lee JH, Kang YE, Chang JY, et al. An engineered FGF21 variant, LY2405319, can prevent non-alcoholic steatohepatitis by enhancing hepatic mitochondrial function. Am J Transl Res. 2016;8(11):4750–63. [PMC free article] [PubMed] [Google Scholar]
- 59. Sanyal A, Charles ED, Neuschwander-Tetri BA, et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: A randomised, double-blind, placebo-controlled, phase 2a trial. Lancet 2019;392(10165):2705–17. [DOI] [PubMed] [Google Scholar]
- 60. Barbara M, Scott A, Alkhouri N. New insights into genetic predisposition and novel therapeutic targets for nonalcoholic fatty liver disease. Hepatobiliary Surg Nutr. 2018;7(5):372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Staels B, Rubenstrunk A, Noel B, et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 2013;58(6):1941–52. [DOI] [PubMed] [Google Scholar]
- 62. Cariou B, Hanf R, Lambert-Porcheron S, et al. Dual peroxisome proliferator-activated receptor alpha/delta agonist GFT505 improves hepatic and peripheral insulin sensitivity in abdominally obese subjects. Diabetes Care 2013;36(10):2923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ratziu V, Harrison SA, Francque S, et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology 2016;150(5):1147–59.e45. [DOI] [PubMed] [Google Scholar]
- 64. Kelly MJ, Pietranico-Cole S, Larigan JD, et al. Discovery of 2-[3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy)phenyl]-3,5-dio xo-2,3,4,5-tetrahydro[1,2,4]triazine-6-carbonitrile (MGL-3196), a highly selective thyroid hormone receptor beta agonist in clinical trials for the treatment of dyslipidemia. J Med Chem. 2014;57(10):3912–23. [DOI] [PubMed] [Google Scholar]
- 65. Puliga E, Min Q, Tao J, et al. Thyroid hormone receptor-beta agonist GC-1 inhibits met-beta-catenin-driven hepatocellular cancer. Am J Pathol. 2017;187(11):2473–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Alvarado TF, Puliga E, Preziosi M, et al. Thyroid hormone receptor beta agonist induces beta-catenin-dependent hepatocyte proliferation in mice: Implications in hepatic regeneration. Gene Expr. 2016;17(1):19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fanti M, Singh S, Ledda-Columbano GM, Columbano A, Monga SP. Tri-iodothyronine induces hepatocyte proliferation by protein kinase A-dependent beta-catenin activation in rodents. Hepatology 2014;59(6):2309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vatner DF, Weismann D, Beddow SA, et al. Thyroid hormone receptor-beta agonists prevent hepatic steatosis in fat-fed rats but impair insulin sensitivity via discrete pathways. Am J Physiol Endocrinol Metab. 2013;305(1):E89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Harrison S, Moussa S, Bashir M, Alkhouri N. MGL-3196, a selective thyroid hormone receptor-beta agonist significantly decreases hepatic fat in NASH patients at 12 weeks the primary endpoint in a 36 week serial liver biopsy study. J Hepatol. 2018;68:S38. [Google Scholar]
- 70. Cable EE, Finn PD, Stebbins JW, et al. Reduction of hepatic steatosis in rats and mice after treatment with a liver-targeted thyroid hormone receptor agonist. Hepatology 2009;49(2):407–17. [DOI] [PubMed] [Google Scholar]
- 71. Issandou M, Bouillot A, Brusq JM, et al. Pharmacological inhibition of stearoyl-CoA desaturase 1 improves insulin sensitivity in insulin-resistant rat models. Eur J Pharmacol. 2009;618(1–3):28–36. [DOI] [PubMed] [Google Scholar]
- 72. Iruarrizaga-Lejarreta M, Varela-Rey M, Fernandez-Ramos D, et al. Role of Aramchol in steatohepatitis and fibrosis in mice. Hepatol Commun. 2017;1(9):911–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Safadi R, Konikoff FM, Mahamid M, et al. The fatty acid-bile acid conjugate Aramchol reduces liver fat content in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2014;12(12):2085–91.e2081. [DOI] [PubMed] [Google Scholar]
- 74. Salehi M, Vahl TP, D’Alessio DA. Regulation of islet hormone release and gastric emptying by endogenous glucagon-like peptide 1 after glucose ingestion. J Clin Endocrinol Metab. 2008;93(12):4909–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mojsov S, Weir GC, Habener JF. Insulinotropin: Glucagon-like peptide I (7–37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest. 1987;79(2):616–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bifari F, Manfrini R, Dei Cas M, et al. Multiple target tissue effects of GLP-1 analogues on non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). Pharmacol Res. 2018;137:219–29. [DOI] [PubMed] [Google Scholar]
- 77. Armstrong MJ, Hull D, Guo K, et al. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J Hepatol. 2016;64(2):399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ding X, Saxena NK, Lin S, Gupta NA, Anania FA. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology 2006;43(1):173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Trevaskis JL, Griffin PS, Wittmer C, et al. Glucagon-like peptide-1 receptor agonism improves metabolic, biochemical, and histopathological indices of nonalcoholic steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2012;302(8):G762–72. [DOI] [PubMed] [Google Scholar]
- 80. Ipsen DH, Rolin B, Rakipovski G, et al. Liraglutide decreases hepatic inflammation and injury in advanced lean non-alcoholic steatohepatitis. Basic Clin Pharmacol Toxicol. 2018;123(6):704–13. [DOI] [PubMed] [Google Scholar]
- 81. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016;387(10019):679–90. [DOI] [PubMed] [Google Scholar]
- 82. Armstrong MJ, Houlihan DD, Rowe IA, et al. Safety and efficacy of liraglutide in patients with type 2 diabetes and elevated liver enzymes: Individual patient data meta-analysis of the LEAD program. Aliment Pharmacol Ther. 2013;37(2):234–42. [DOI] [PubMed] [Google Scholar]
- 83. Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17(5):306–21. [DOI] [PubMed] [Google Scholar]
- 84. Lefebvre E, Moyle G, Reshef R, et al. Antifibrotic effects of the dual CCR2/CCR5 antagonist cenicriviroc in animal models of liver and kidney fibrosis. PLoS One 2016;11(6):e0158156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Friedman SL, Ratziu V, Harrison SA, et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology 2018;67(5):1754–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Xu M, Liu PP, Li H. Innate immune signaling and its role in metabolic and cardiovascular diseases. Physiol Rev. 2019;99(1):893–948. [DOI] [PubMed] [Google Scholar]
- 87. Cai J, Zhang XJ, Li H. Role of innate immune signaling in non-alcoholic fatty liver disease. Trends Endocrinol Metab. 2018;29(10):712–22. [DOI] [PubMed] [Google Scholar]
- 88. Zhao GN, Zhang P, Gong J, et al. Tmbim1 is a multivesicular body regulator that protects against non-alcoholic fatty liver disease in mice and monkeys by targeting the lysosomal degradation of Tlr4. Nat Med. 2017;23(6):742–52. [DOI] [PubMed] [Google Scholar]
- 89. Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 2007;56(8):1986–98. [DOI] [PubMed] [Google Scholar]
- 90. Cai J, Xu M, Zhang X, Li H. Innate immune signaling in nonalcoholic fatty liver disease and cardiovascular diseases. Annu Rev Pathol. 2019;14:153–84. [DOI] [PubMed] [Google Scholar]
- 91. Xiang M, Wang PX, Wang AB, et al. Targeting hepatic TRAF1-ASK1 signaling to improve inflammation, insulin resistance, and hepatic steatosis. J Hepatol. 2016;64(6):1365–77. [DOI] [PubMed] [Google Scholar]
- 92. Zhang P, Wang PX, Zhao LP, et al. The deubiquitinating enzyme TNFAIP3 mediates inactivation of hepatic ASK1 and ameliorates nonalcoholic steatohepatitis. Nat Med. 2018;24(1):84–94. [DOI] [PubMed] [Google Scholar]
- 93. Wang PX, Ji YX, Zhang XJ, et al. Targeting CASP8 and FADD-like apoptosis regulator ameliorates nonalcoholic steatohepatitis in mice and nonhuman primates. Nat Med. 2017;23(4):439–49. [DOI] [PubMed] [Google Scholar]
- 94. Kovalic AJ, Satapathy SK, Chalasani N. Targeting incretin hormones and the ASK-1 pathway as therapeutic options in the treatment of non-alcoholic steatohepatitis. Hepatol Int. 2018;12(2):97–106. [DOI] [PubMed] [Google Scholar]
- 95. Schuster S, Feldstein AE. NASH: Novel therapeutic strategies targeting ASK1 in NASH. Nat Rev Gastroenterol Hepatol. 2017;14(6):329–30. [DOI] [PubMed] [Google Scholar]
- 96. Loomba R, Lawitz E, Mantry PS, et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology 2018;67(2):549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Younossi ZM, Stepanova M, Lawitz E, et al. Improvement of hepatic fibrosis and patient-reported outcomes in non-alcoholic steatohepatitis treated with selonsertib. Liver Int. 2018;38(10):1849–59. [DOI] [PubMed] [Google Scholar]
- 98. Wang PX, Zhang XJ, Luo P, et al. Hepatocyte TRAF3 promotes liver steatosis and systemic insulin resistance through targeting TAK1-dependent signalling. Nat Commun. 2016;7:10592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. An S, Zhao LP, Shen LJ, et al. USP18 protects against hepatic steatosis and insulin resistance through its deubiquitinating activity. Hepatology 2017;66(6):1866–84. [DOI] [PubMed] [Google Scholar]
- 100. Yan FJ, Zhang XJ, Wang WX, et al. The E3 ligase tripartite motif 8 targets TAK1 to promote insulin resistance and steatohepatitis. Hepatology 2017;65(5):1492–511. [DOI] [PubMed] [Google Scholar]
- 101. Wang S, Yan ZZ, Yang X, et al. Hepatocyte DUSP14 maintains metabolic homeostasis and suppresses inflammation in the liver. Hepatology 2018;67(4):1320–38. [DOI] [PubMed] [Google Scholar]
- 102. Ji YX, Huang Z, Yang X, et al. The deubiquitinating enzyme cylindromatosis mitigates nonalcoholic steatohepatitis. Nat Med. 2018;24(2):213–23. [DOI] [PubMed] [Google Scholar]
- 103. Zhao Y, Gao L, Xu L, et al. Ubiquitin-specific protease 4 is an endogenous negative regulator of metabolic dysfunctions in nonalcoholic fatty liver disease. Hepatology 2018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 104. Henderson NC, Mackinnon AC, Farnworth SL, et al. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci USA 2006;103(13):5060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Traber PG, Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS One 2013;8(12):e83481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Harrison SA, Marri SR, Chalasani N, et al. Randomised clinical study: GR-MD-02, a galectin-3 inhibitor, vs. placebo in patients having non-alcoholic steatohepatitis with advanced fibrosis. Aliment Pharmacol Ther. 2016;44(11–12):1183–98. [DOI] [PubMed] [Google Scholar]
- 107. Barreyro FJ, Holod S, Finocchietto PV, et al. The pan-caspase inhibitor Emricasan (IDN-6556) decreases liver injury and fibrosis in a murine model of non-alcoholic steatohepatitis. Liver Int. 2015;35(3):953–66. [DOI] [PubMed] [Google Scholar]
- 108. Shiffman M, Freilich B, Vuppalanchi R, et al. Randomised clinical trial: Emricasan versus placebo significantly decreases ALT and caspase 3/7 activation in subjects with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2019;49(1):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ratziu V, Sheikh MY, Sanyal AJ, et al. A phase 2, randomized, double-blind, placebo-controlled study of GS-9450 in subjects with nonalcoholic steatohepatitis. Hepatology 2012;55(2):419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016;65(8):1038–48. [DOI] [PubMed] [Google Scholar]
- 111. Jahn D, Kircher S, Hermanns HM, Geier A. Animal models of NAFLD from a hepatologist’s point of view. Biochim Biophys Acta Mol Basis Dis. 2019;1865(5):943–53. [DOI] [PubMed] [Google Scholar]
- 112. Cai J, Zhang XJ, Li H. Progress and challenges in the prevention and control of nonalcoholic fatty liver disease. Med Res Rev. 2019;39(1):328–48. [DOI] [PubMed] [Google Scholar]
- 113. Nagarajan P, Venkatesan R, Kumar M, Usmani A, Majumdar SS. Macaca radiata (bonnet monkey): A spontaneous model of nonalcoholic fatty liver disease. Liver Int. 2008;28(6):856–64. [DOI] [PubMed] [Google Scholar]
- 114. Hannah WN Jr., Torres DM, Harrison SA. Nonalcoholic steatohepatitis and endpoints in clinical trials. Gastroenterol Hepatol, (N Y) 2016;12(12):756–63. [PMC free article] [PubMed] [Google Scholar]