Epithelial–mesenchymal interactions are critical for organ development, function, and maintenance (1). In the developing lung, these interactions orchestrate branching morphogenesis during the pseudoglandular stage (2). The epithelium-derived soluble mediators SHH (sonic hedgehog), BMP4 (bone morphogenetic protein 4), and SPRY2 (sprouty homolog 2) are negative regulators of mesenchymal FGF10 (fibroblast growth factor 10), a key regulator of branching (3). Many of these developmental pathways are reactivated in fibrotic lung diseases, and most studies have focused on mesenchyme-derived factors that contribute to epithelial disrepair (4, 5). However, much less is understood about the epithelial factors that may maintain quiescence of the mesenchyme.
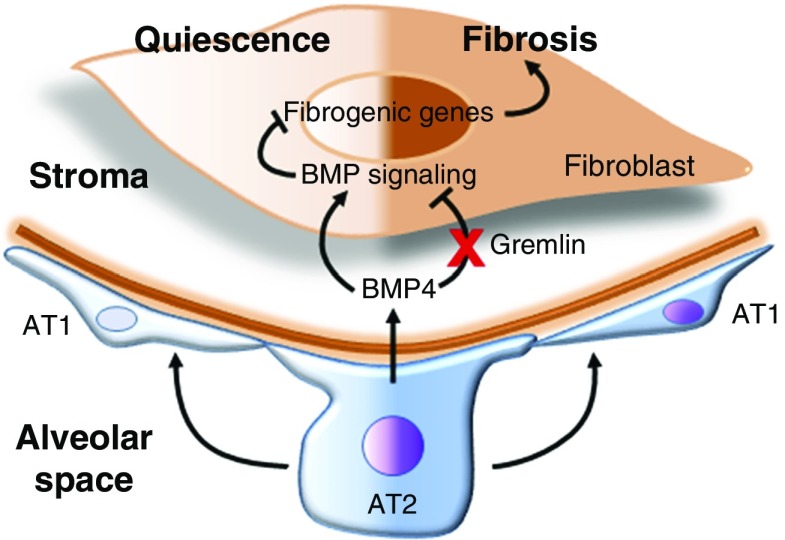
In a study presented in this issue of the Journal, Tan and colleagues (pp. 607–619) interrogated lung epithelial–mesenchymal cross-talk in a three-dimensional (3D) culture system (6). In this study, lung epithelial and mesenchymal cells were cultured on top of Matrigel, and a role for epithelial-derived BMP4 in suppressing profibrotic gene expression in fibroblasts was identified. A comparative whole-genome expression analysis of collagen-producing fibroblasts, grown alone or in combination with epithelial progenitors atop Matrigel, supported a critical role for BMP4-mediated signaling in suppressing fibrogenic activity. Fibrogenic stimuli, including transforming growth factor-β1 (TGF-β1) and the chemotherapeutic drug bleomycin, resulted in a suppression of epithelial BMP4 that led to derepression of fibroblast activation, as measured by enhanced proliferation and myogenic differentiation. These findings support a role for epithelium-derived BMP4 as a potential soluble mediator that maintains quiescence of the adjacent mesenchyme under homeostatic conditions in the lung. Epithelium-derived BMP4 signaling to the fibroblasts and its regulation in fibrosis are shown in Figure 1.
Figure 1.
Epithelium-derived BMP4 (bone morphogenetic protein 4) suppression of fibrogenic activation in lung fibroblasts. Under homeostatic conditions, fibroblasts remain quiescent under the influence of secreted factors from the epithelium. Epithelium-derived BMP4 is known to suppress mesenchymal activation during lung development to regulate epithelial branching. Findings from Tan and colleagues demonstrate that epithelium-secreted BMP4 maintains mesenchymal quiescence in the adult murine lung (6). BMP4 levels decline after epithelial injury, resulting in mesenchymal activation and fibrosis. AT1 = alveolar type 1 cells; AT2 = alveolar type 2 cells.
There are several notable findings in this study. First, the suppressive effect of the epithelium on mesenchymal activation was not observed in two-dimensional culture, but was clearly evident in the 3D organoid model. Organoids have shown great promise for decoding the conceptual foundations of cell polarity and emergence into complex functional tissue configurations (7). Lung organoids have been used to identify various regional stem and progenitor cells, intercellular communications, and regenerative responses (8). The majority of organoid approaches rely on coculturing epithelial and mesenchymal progenitors that are seeded in suitable matrices but lack other organ components, such as immune cells and blood vessels. In the study by Tan and colleagues, cells were not embedded within the matrix, but rather were studied on top of Matrigel. The resulting structural differences compared with the more traditional alveolosphere and bronchosphere models require further characterization (8).
Second, this study highlights the critical role of a healthy epithelium in restraining overexuberant mesenchymal activation. With its identification as a critical suppressive factor, BMP4 is added to the list of other mediators, such as PGE2 (prostaglandin E2) (9) and SHH (10), that have been previously described. In this regard, it is important to define the precise role of these potential antifibrotic mediators in affected tissues of human subjects with particular fibrotic diseases. Although PGE2 has been shown to be decreased in the lungs of subjects with idiopathic pulmonary fibrosis (11), SHH signaling appears to be (paradoxically) elevated (5, 12). It is possible that signaling by certain mediators can be pleiotropic, and even antagonistic, in the setting of chronic injury and aging (4).
The authors should be commended for using a novel 3D cell culture model to study cell–cell interactions. These models could be further refined to take into account the unique cellular microenvironments and niches in the lung, such as the air–liquid interface, changes in gas tensions (e.g., oxygen and carbon dioxide), nutrient availability, and metabolite concentrations. Organoids are typically grown ex vivo from pluripotent stem cells or isolated progenitors seeded in gels containing component(s) of the extracellular matrix (8). Within the matrix, cells proliferate, self-organize, and differentiate to form isolated functional units mirroring basic organ structure and function (13). To date, organoids have been generated from both murine and human stem and progenitor cells for a number of organs, including the lung (8, 14). Organoids are commonly used in developmental studies, disease modeling, and drug testing, and hold promise for tissue replacement in the future (15).
A deficiency in epithelium-derived factors that suppress mesenchymal activation, such as BMP4 in the study by Tan and colleagues, PGE2 (9), and SHH (10), suggests that repletion of these factors may be beneficial in supporting tissue/organ regeneration. However, many of these factors may be pleiotropic in their actions in diseased tissues (4), and it is important to verify their deficient signaling activity in vivo. In support of the role of deficient BMP4 signaling in progressive fibrosis is the finding that gremlin, a negative regulator of BMP4, is highly expressed in idiopathic pulmonary fibrosis tissues (16). Application of innovative approaches to study cell–cell interactions in the lung will uncover bidirectional signaling between cellular compartments that would not be evident with traditional cell culture models.
Supplementary Material
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogan BL, Yingling JM. Epithelial/mesenchymal interactions and branching morphogenesis of the lung. Curr Opin Genet Dev. 1998;8:481–486. doi: 10.1016/s0959-437x(98)80121-4. [DOI] [PubMed] [Google Scholar]
- 3.Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chanda D, Otoupalova E, Smith SR, Volckaert T, De Langhe SP, Thannickal VJ. Developmental pathways in the pathogenesis of lung fibrosis. Mol Aspects Med. 2019;65:56–69. doi: 10.1016/j.mam.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chanda D, Kurundkar A, Rangarajan S, Locy M, Bernard K, Sharma NS, et al. Developmental reprogramming in mesenchymal stromal cells of human subjects with idiopathic pulmonary fibrosis. Sci Rep. 2016;6:37445. doi: 10.1038/srep37445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan Q, Ma XY, Liu W, Meridew JA, Jones DL, Haak AJ, et al. Nascent lung organoids reveal epithelium- and bone morphogenetic protein–mediated suppression of fibroblast activation Am J Respir Cell Mol Biol 201961607–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolodsick JE, Peters-Golden M, Larios J, Toews GB, Thannickal VJ, Moore BB. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via E. prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. Am J Respir Cell Mol Biol. 2003;29:537–544. doi: 10.1165/rcmb.2002-0243OC. [DOI] [PubMed] [Google Scholar]
- 10.Peng T, Frank DB, Kadzik RS, Morley MP, Rathi KS, Wang T, et al. Hedgehog actively maintains adult lung quiescence and regulates repair and regeneration. Nature. 2015;526:578–582. doi: 10.1038/nature14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilborn J, Crofford LJ, Burdick MD, Kunkel SL, Strieter RM, Peters-Golden M. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J Clin Invest. 1995;95:1861–1868. doi: 10.1172/JCI117866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolaños AL, Milla CM, Lira JC, Ramírez R, Checa M, Barrera L, et al. Role of Sonic Hedgehog in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;303:L978–L990. doi: 10.1152/ajplung.00184.2012. [DOI] [PubMed] [Google Scholar]
- 13.Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345:1247125. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- 14.Dutta D, Heo I, Clevers H. Disease modeling in stem cell-derived 3d organoid systems. Trends Mol Med. 2017;23:393–410. doi: 10.1016/j.molmed.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Surolia R, Li FJ, Wang Z, Li H, Liu G, Zhou Y, et al. 3D pulmospheres serve as a personalized and predictive multicellular model for assessment of antifibrotic drugs. JCI Insight. 2017;2:e91377. doi: 10.1172/jci.insight.91377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koli K, Myllärniemi M, Vuorinen K, Salmenkivi K, Ryynänen MJ, Kinnula VL, et al. Bone morphogenetic protein-4 inhibitor gremlin is overexpressed in idiopathic pulmonary fibrosis. Am J Pathol. 2006;169:61–71. doi: 10.2353/ajpath.2006.051263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.