Abstract
Repetitive mild traumatic brain injury during adolescence can induce neurological dysfunction through undefined mechanisms. Interleukin-1 (IL-1) contributes to experimental adult diffuse and contusion TBI models, and IL-1 antagonists have entered clinical trials for severe TBI in adults; however, no such data exist for adolescent TBI. We developed an adolescent mouse repetitive closed head injury (rCHI) model to test the role of IL-1 family members in post-injury neurological outcome. Compared to one CHI, three daily injuries (3HD) produced acute and chronic learning deficits and emergence of hyperactivity, without detectable gliosis, neurodegeneration, brain atrophy, and white matter loss at one year. Mature IL-1β and IL-18 were induced in brain endothelium in 3HD but not 1HD, three hit weekly, or sham animals. IL-1β processing was induced cell-autonomously in three-dimensional human endothelial cell cultures subjected to in vitro concussive trauma. Mice deficient in IL-1 receptor-1 or caspase-1 had improved post-injury Morris water maze performance. Repetitive mild CHI in adolescent mice may induce behavioral deficits in the absence of significant histopathology. The endothelium is a potential source of IL-1β and IL-18 in rCHI, and IL-1 family members may be therapeutic targets to reduce or prevent neurological dysfunction after repetitive mild TBI in adolescents.
Keywords: Animal model, brain trauma, cerebral blood flow, endothelium, inflammation
Introduction
Mild traumatic brain injury (mTBI), defined as neurological impairment in the absence of structural brain damage on routine imaging studies, is a significant public health problem, affecting an estimated two million people per year in the U.S.1 The possible long-term consequences of sustaining mTBI during brain development in childhood and adolescence are particularly concerning.2,3 Although symptoms from a single mTBI resolve rapidly and completely in most cases, up to 30% of children and adolescents may have prolonged symptoms, causing significant social and academic impairment.4,5 Pediatric mTBI has become a national focus given its high incidence, a propensity for prolonged recovery and the potential for worse neurological outcomes vs. adults.5–9 Recent studies have shown chronic white matter alterations and an increased risk of cognitive and psychological impairments in adults who began participating in contact sports at an early age.9–12 Despite growing awareness of the potential dangers of mTBI in adolescence, relatively few animal studies use adolescent models to interrogate mTBI mechanisms.13–16
One mechanism that has been reported to contribute to neurological dysfunction after TBI is brain inflammation.17 Adult mouse single and repetitive mTBI models induce acute and chronic gliosis in injured brain,18,19 and neurological deficits in adult mTBI models can be manipulated by genetic and pharmacological inhibition of Akt/mTOR and tumor necrosis factor alpha/Fas receptor.18,20 Interleukin-1 (IL-1) family members, including IL-1 beta (IL-1β) and IL-18 are prototypic mediators of acute and chronic inflammation that are produced canonically by activation of a caspase-1 inflammasome, a multi-protein cytosolic complex that transduces inflammatory signals from toll-like receptors and other stimuli, or by caspase-8 in a ripoptosome complex.21 IL-1β and IL-18 signal in brain endothelial cells and glia via IL-1 receptor-1 (IL-1R1), whereas neurons have a truncated version of the IL-1R (IL-1R3) that mediates neuroprotection via Akt activation.22 IL-1β is increased in cerebrospinal fluid of humans with severe TBI23 and IL-1β antagonists reduce tissue damage and neurological dysfunction in rodent contusion and midline fluid percussion models.24–27 Though IL-1R1 function has not yet been reported in the context of mTBI, studies suggesting that IL-1β mediates synaptic dysfunction and learning deficits in models of systemic and central nervous system inflammation, including Alzheimer’s disease,28 support the hypothesis that IL-1R1 may be a therapeutic target to reduce cognitive dysfunction in adolescents with mTBI.
Here, we developed a model of repetitive closed head injury (rCHI) in adolescent mice that features neurological deficits in the absence of overt or microscopic tissue damage, and tested the hypothesis that IL-1β/IL-1R1 contribute to cognitive deficits observed during adulthood. We found that IL-1β and IL-18 are processed to their mature forms in brain endothelial cells in the subacute period after rCHI and in three-dimensional cultures of human brain endothelium subjected to concussive injury in vitro, and that genetic inhibition of IL-1R1 or caspase-1 improves post-injury cognitive outcome after 3HD in mice. The data are the first to describe an endothelial source of IL-1β in a repetitive mTBI model, and suggest that IL-1 signaling may be a therapeutic target to improve outcome after mTBI in adolescents.
Methods
Mice
Adolescent (day of life (DOL) 38), male wild-type (WT) C57/BL6 mice (Jackson Laboratories, Bar Harbor, ME), along with age matched inbred male IL-1 receptor knockout (IL-1R KO) mice (stock no. 003245, Jackson Laboratory) and male Caspase-1 knockout (Cas1 KO) mice (stock no. 016621, Jackson Laboratory) were used for experiments. Females were not used in this study because they weigh significantly less than males at DOL 38–40 and therefore would have differences in injury severity that cannot be easily estimated and controlled by scaling. Studies examining female mice will therefore be reported separately. Mice were housed for 12-h day/night cycles in laminar flow racks in a temperature-controlled room. Food and water were given ad libitum. All procedures were performed at Massachusetts General Hospital Institutional Animal Care and Use Committee in accordance with the NIH Guide for Care and Use of Laboratory Animals. Studies were performed according to ARRIVE guidelines and all data were obtained by investigators blinded to study groups. Mice were genotyped according to protocols published by Jackson Laboratories.
Closed head injury model
The CHI model was used as previously described with minor modifications.18 Mice were anesthetized with 2.5% Isoflurane (Anaquest, Memphis, TN) for 90 s in a 70% N2O-30% O2 Fluotec 3 vaporizer (Colonial Medical, Amherst, NH). Mice were placed on a Kimwipe (Kimberly-Clark, Irving, TX) and grasped by the tail, and the head was positioned under a hollow tube (diameter 10 mm). A metal bolt (53 g) was dropped 46 inches onto the dorsal aspect of the skull directly above the right (days 1, 3) or left (day 2) ear between the coronal and lambdoid sutures. Alternating injuries were performed to avoid apnea that sometimes results from injuring the vertex of the head. The head readily penetrated the Kimwipe following impact in the anterior–posterior plane. Sham injured mice were subjected to anesthesia without weight drop. Injured mice were recovered in room air in their cages. One-hit (1HD) mice were injured once, three-hit daily (3HD) mice were injured once daily for three consecutive days, whereas three-hit weekly (3HW) mice were injured on DOL 31, 38, and 45, alternating the side of the head injured on each day. Loss of consciousness time (LOC) was defined as latency to righting reflex.
Biomechanics of impact
We used a 0.8 g triaxial accelerometer (3133A1, Dytran Instruments Inc.) with sensitivity of 10 mV/g taped to the ventral jaw to assess impact biomechanics. Data were acquired at a rate of 20,000 Hz (USB-4432, National Instruments) during CHI. Linear acceleration was quantified as the magnitude of the three-dimensional acceleration vector. The corresponding rotational acceleration was obtained by dividing linear acceleration by the radius of rotation, measured for each mouse as the distance between the center of the skull and a point located between the cervical axis and the scapula. Impact time was defined as the duration between the point at which acceleration increased above 10 g (ignoring <10 g noise) to the first local minimum below 200 g following peak acceleration. Linear acceleration was integrated over this impact time to calculate the velocity change, which was again divided by radius of rotation to obtain rotational velocity change. A characteristic mass scaling ratio, , was used to scale measured values to equivalent human values.29
Behavioral testing
Prior to each test, mice were acclimatized to the room for at least 30 min. Mice were first tested in a Morris Water Maze (MWM) task five days after the last injury and again in a reverse MWM paradigm at six weeks. During weeks 6–7 after injury, mice were subjected to testing in the Y-maze, elevated plus maze, overnight open field, and forced swim test. Forced swim tests were conducted last to minimize effects of test-related anxiety. Behavioral tests were repeated at nine months after injury.
MWM
The MWM was performed as previously described with minor modifications.19 Each mouse was subjected to five hidden platform trials (one to two trials per day). Probe trials were performed 24 h after the last hidden platform trial by allowing the mice to swim in the tank for 60 s and recording the time spent in the target quadrant. For reverse MWM, the platform location was moved to the northwest quadrant and mice were subjected to three hidden platform trials. One trial per day was conducted for three days. Probe trials were not performed.
Elevated plus maze
The 24 inch high apparatus consisted of two 52 inch by 3 inch platforms with a 3 inch × 3 inch square area at their intersection. The closed arms of the platform had 4 inch walls, whereas the open arms had none. Each mouse was placed in the central square and video-recorded for 5 min. The apparatus was cleaned with 70% ethanol between trials. Video recordings were analyzed by Any Maze software for mean speed and percent time in closed and open arms.
Y-maze
The Y-shaped apparatus consisted of three 13″ arms with 6″ walls, and a 3″ × 3″ × 3″ triangular intersection. Each arm was identified with a symbol (square, circle, star). Mice were allowed access to the three arms and their movements recorded by Photobooth using a laptop computer. The number of times the mouse entered all three arms without re-entering the previous arm (i.e. triplets of ABC, ACB, BCA, etc. vs. ABA, CBC, etc.) and the total number of arm entries was recorded. The apparatus was cleaned with 70% ethanol between trials.
Open field test
Mice were placed in separate cages secured with metal wiring tops and movements were video-recorded in minimal lighting over night for 8 h beginning at 7–8 p.m. Any Maze software was used to assess mean speed and distance traveled.
Porsolt forced swim test
The apparatus consisted of a 4 L glass beaker filled with 2 L of water (25℃). Mice were placed in the water for 5 min and swimming movements were recorded by the Photo Booth program. Latency to freezing/immobility and total freezing time were experimental endpoints.
Assessment of cerebral blood flow index
Depilatory cream was used to remove hair on the scalp one day before the start of the experiment to improve the signal-to-noise ratio in DCS data. Mice were anesthetized with isoflurane, placed supine and allowed to stabilize for 60 s. Diffuse correlation spectroscopy (DCS) measurements were made as previously described.30 Data were acquired over each hemisphere in sequence for 30 s.
Edema
Brains were removed at 24 h after CHI (n = 5 per time point) and weighed (wet weight), then dried at 85℃ for 72 h, and dry weights were obtained. The percentage of brain water content was expressed as (wet-dry weight)/wet weight × 100%.
Assessment of blood–brain barrier permeability
Evans blue (5 ml/kg of a 2% solution) was injected intravenously immediately after the last CHI. Mice (n = 6 per group) were killed 24 h after injection by transcardial perfusion with phosphate-buffered saline (PBS). Their brains were removed and immersed in 1 ml of N,N-dimethylformamide for 72 h at room temperature. Evans blue concentration in N,N-dimethylformamide was analyzed by spectrophotometry (585 nm) from known standard concentrations, and results expressed as µg Evans blue/g brain.
Isolation of brain leukocytes by fluorescence activated cell sorting
After PBS perfusion, brains were removed and subjected to enzymatic digestion using the Neural Tissue Dissociation Kit (P) (Miltenyi Biotec, Auburn, CA). Myelin debris were then removed with myelin removal beads II (Miltenyi) and cells were labeled with fluorescence-labeled CD11b (M1/70) and CD45 (30-F11) antibodies (Biolegend, San Diego, CA). Microglia (CD11b+CD45+med), macrophage + neutrophils (CD11b+CD45+high) and lymphocytes (CD11b+lowCD45+high) were quantitated by FACS.
Histology
Mice were deeply anesthetized and transcardially perfused with PBS, followed by PBS containing 4% paraformaldehyde (PFA). Brains were post-fixed in 4% PFA at 4℃ for 24 h, cryoprotected in 30% sucrose for two days, and snap frozen in isopentane/dry ice and stored at −80℃. Brains were sectioned in the coronal plane at 200–300 µm intervals from anterior to posterior. Cryostat sections (12 um) were mounted on poly-L-lysine-coated slides. Some sections were stained with hematoxylin and eosin and photographed. For Fluoro Jade B (FJB) staining, sections were fixed in 100% ethanol for 10 min, and then labeled with 0.001% FJB (EMD Millipore, Billerica, MA) as per the manufacturer’s instruction. For immunohistochemistry, sections were washed in PBS, blocked in 10% normal goat serum in PBS for 1 h, and incubated overnight at 4℃ with primary antibody (rabbit anti-Iba1 antibody 1:200; Wako Pure Chemical Industries Ltd, Osaka, Japan; rabbit anti-GFAP antibody; Sigma, St. Louis, MO; mouse anti-mouse neuronal nuclei (NeuN), Millipore) followed by the appropriate Cy3 conjugated secondary antibody (1:300; Jackson ImmunoResearch, West Grove, PA). In the case of NeuN, we used an FITC-conjugated NeuN reagent, and thus no secondary antibody was needed. Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI), and coverslips were placed. Slides were analyzed using a Nikon Eclipse Ti-S fluorescence microscope (Micro Video Instruments; Avon, MA) using the appropriate excitation/emission spectra.
Assessment of cell counts
For each animal, coronal sections were stained and analyzed by an observer blinded to experimental conditions using ImageJ software (NIH). Brain regions assessed were all ×200 microscopic fields (1100 × 1100 um) in cortex (n = 4 fields/section, 4 sections per mouse = 16 fields/mouse; hippocampus (24 fields (in 5 sections) per mouse, corpus callosum (28 fields (in 4 sections) per mouse, thalamus and striatum (4 fields/section, 5 sections/mouse). Cell count data for each mouse (n = 5 per time point) were the average of the total brain fields counted.
Preparation of brain microvessels
Mice were transcardially perfused with PBS and the brain was removed, digested in collagenase A (Roche Diagnostic GmbH, Germany), and mechanically dissociated with a plastic pipette. After centrifugation at 1000 × g (7 min), the cell pellet was resuspended and incubated with CD31 antibody (BD pharmingen)-coated Dynabeads (Invitrogen) and a magnetic separator was used to recover the bead-bound microvessels.31 Unbound cells were used as CD31-fraction after myelin removal by centrifugation with percoll (Sigma Aldrich, St. Louis, MI).
Western blot
Isolated microvessels were homogenized in RIPA buffer (EMD Millipore) with phosphatase and protease inhibitors (Thermo Fisher Scientific) and subjected to Western blotting as previously described.18 Membranes were incubated overnight at 4℃ with the following primary antibodies: Anti-β-actin antibody (1:10000, Cell signaling Technology), anti-IL-1β antibody (1:1000, Abcam), anti-IL-18 antibody (1:1000, Abcam), anti-NeuN (1:3000, Millipore), anti-Iba1(1:3000, Wako), or anti-GFAP (1:3000, Invitrogen). After incubation with peroxide-conjugated secondary antibodies (1:5000, Cell signaling Technology), visualization was enhanced by ECL (EMD Millipore) detection. The results were normalized to β-actin. Optical density was assessed using ImageJ software.
Human primary brain microvascular endothelial cell culture on silk scaffolds
Porous silk scaffolds were prepared as previously reported,32,33 hpBMEC was purchased from Cell Systems (Kirkland, WA) and cultured according to the manufacturer’s protocol. The silk scaffolds were coated with 20 µg/mL fibronectin (Sigma Aldrich) for 1 h at 37℃ before cell seeding. The coating solution was removed by vacuum aspiration and scaffolds allowed to dry in the hood for 15 min. The scaffolds were individually transferred to 24-well, ultra-low attachment plates (Corning, Corning, NY) and 2.5 × 105 cells were seeded for each scaffold in 50 µl of media, allowing the silk sponges to fully absorb the cell suspension. The plates were then stored in the incubator for 30 min, to allow cell adhesion, prior to adding enough media to each well (complete classic medium with serum and cultureboost, cell systems) to fully cover the scaffolds (1 mL). Cell-seeded sponges were cultured for seven days to allow formation of a confluent endothelial layer covering the whole surface of the scaffolds, replacing the media every three days. Cell viability and confluence were confirmed by Calcein-AM staining (Thermo Fisher Scientific, Waltham, MA).
3D in vitro closed head injury model
Three-dimensional cultures of endothelial cells were placed in media in a 1″ × 1″ × 1″ plastic cube through an opening that was sealed with wax paper. The plastic cube was placed on a Kimwipe and subjected to weight drop using the same apparatus used for in vivo CHI (53 g, 46″) three times or to sham injury (no weight drop). Cultures were then returned to media and incubated at 37℃ for 24 h.
Statistical analysis
Results are mean ± standard deviation and were analyzed using Graphpad PRISM 7 (La Jolla, CA). When only two groups were compared, an unpaired t-test was used. Multiple comparisons were evaluated by one-way ANOVA followed by Tukey’s test. Overnight open field test and MWM were analyzed using two-factor repeated measures ANOVA (group × time). p < 0.05 was considered significant.
Results
A total of 256 mice were used in the studies. Supplemental Table 1 shows the schedule of injuries and behavioral tests. None of the mice examined exhibited skull fractures, contusion, or intraparenchymal brain hemorrhage. One mouse in the 3HD cohort died in between the two series of MWM testing. Weights of representative sham and CHI mice on DOL 38 did not differ (Figure 1(a)). Accelerometer data describing the biomechanics of CHI are given in Table 1 along with scaled human equivalent values. Peak linear acceleration was 610 ± 190 g across an average impact time of 1.5 ± 0.4 ms, which corresponded to a peak rotational acceleration 5.2 × 105 ± 1.6 × 105 rad/s2. Time to righting reflex after the first CHI was significantly increased vs. sham (Figure 1(b)).
Figure 1.
Closed head injury (CHI) produces early behavioral deficits. (a) Weights of the mice after sham and CHI in representative cohorts (n = 15–18/group, p = 0.8 sham vs. injured groups) (b) Time to righting reflex after the first hit in mice after sham and CHI in representative cohorts (n = 10–15/group, *p < 0.001). (c) Morris water maze (MWM) performance did not differ between 1HD and 1HD sham groups (n = 9/group, p = ns for group for hidden and visible platform trials). (d) Hidden (but not visible) platform performance was worse in 2HD vs. 2HD sham groups (n = 12–13/group, p < 0.05 for group) initially but no effect of group was seen in reversal trials six weeks after 2HD. (e) MWM deficits were observed in hidden platform trials after 3HD (n = 10–11/group, p < 0.05) as well as in reversal trials (n = 10–11/group, p < 0.001 for group). (f) Wire grip score did not differ between sham and 3HD on days 1 and 4 after the third injury. (g) Percent alternation was not different in sham versus 3HD groups in Y maze (n = 10–11/group, p = ns). (h) Percent time spent in the open arm and (I) speed on the elevated plus maze were not different in sham versus 3HD groups (n = 10–11/group, p = ns). (j) Distance in the open field test was not different in sham versus 3HD mice (n = 10–11/group, p = ns). (k) No difference in MWM performance in 3HW mice vs. 3HW shams (n = 11/group, p = ns).
Table 1.
Biomechanical parameters.
| Adolescent animal model | Equivalent human scaled value | ||
|---|---|---|---|
| Parameter | Mean ± SD | Scaling Factora | Mean ± SD |
| Peak linear acceleration (g) | 610 + 190 | 1/λ | 39 ± 12 |
| Peak rotational acceleration (rad/s2) | 5.2e5 + 1.6e5 | 1/λ2 | 2000 ± 620 |
| Impact duration (ms) | 1.5 + 0.4 | λ | 24 + 6.5 |
| Velocity change (m/s) | 3.6 + 0.59 | 1 | 3.6 + 0.59 |
| Rotational velocity change (rad/s) | 310 + 50 | 1/λ | 21 + 3.3 |
| Kinetic energy transfer (J)b | 2.6e-2 + 7.0e-4 | λ3 | 100 + 2.8 |
Scaling factor λ = λM = 15.
Mouse head mass is a measured value including accelerometer mass (∼0.004 kg total). Human head mass assumed to be 5 kg.
Repetitive CHI produces early behavioral deficits
No differences in MWM testing were observed in sham and 1HD mice (Figure 1(c)) including probe trials (sham, 28.8 ± 9.5%; CHI 21.1 ± 7.5%, p = 0.07, n = 9/group). Mice subjected to 2HD performed worse in hidden trials vs. sham within one week after injury, but did not differ in reversal trials performed at three weeks (Figure 1(d)), nor did they differ in probe trials (sham, 20.2 ± 12.3%; 2HD 15 ± 7.3%, p = 0.2, n = 12–13/group). However, 3HD mice had significant deficits in MWM and reversal trials compared to 3HD shams (Figure 1(e)), but probe trials did not differ between groups (sham, 31.4 ± 9.7%; 3HD 29.4 ± 18.3%, n = 11/group; p = 0.8). Wire grip scores on days 1 and 4 (Figure 1(f), n = 8/group) did not differ between 3HD and sham. No differences were observed between sham and 3HD in the Y maze, elevated plus maze, and overnight open field tests at six to seven weeks (Figure 1(g) to (j)). Sham and 3HD also did not differ in time to first freeze in the forced swim test (sham 73.9 ± 22.9 s and 3HD 61.3 ± 33.1 s, p = 0.3) or total time freezing (sham 145.9 ± 25.5 s; 3HD 132.4 ± 39.5 s; p = 0.4, n = 10–11/group).
Effect of one-week rest interval on cognitive deficits after repetitive CHIs
To determine whether a safe rest interval might exist in our adolescent rCHI model, mice were subjected to three injuries at one-week intervals beginning on DOL 31. Body weight in DOL 31 sham (15.4 + 1.5 g) and injured (16.4 + 1 g) groups was similar (p = 0.07, n = 11/group) but time to righting reflex was greater in DOL 31 CHI (210.2 + 69.4 s) vs. sham (29.4 + 6.2) (p < 0.0001, n = 11/group). In contrast to 3HD, MWM testing revealed no difference between sham and injured 3HW groups (Figure 1(k)) including probe trials (sham 30 ± 11.6%; 3HW 29.0 ± 9.2%, p = 0.8, n = 12/group).
Long-term effects of 3HD on neurological function and cortical cerebral blood flow
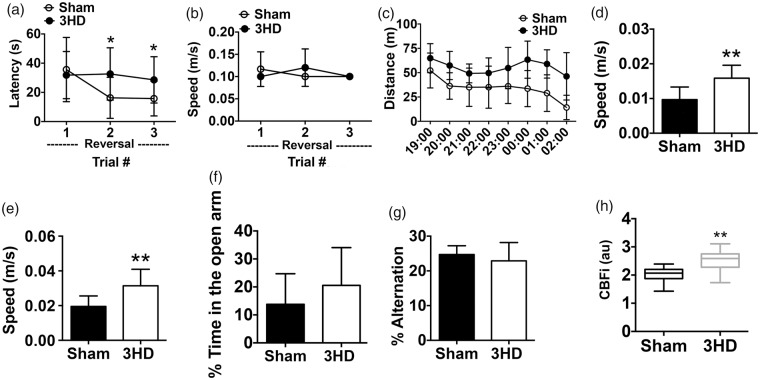
At nine months, sham and 3HD mice were again assessed in behavior tests. Reversal trials in the MWM continued to show deficits in 3HD mice in the absence of swim speed differences (Figure 2(a) and (b)). Moreover, open field and elevated plus maze testing at nine months revealed marked hyperactivity in 3HD mice, assessed by distance and speed in the open field and speed in the elevated plus maze (Figure 2(c) to (e)). However, no differences between groups were observed in time spent in the open arms of the elevated plus maze or in Y maze tests (Figure 2(f) and (g)). At four months after injury, CBFi assessed with DCS was significantly increased in 3HD mice compared to sham (Figure 2(h)).
Figure 2.
Long-term effects of 3HD on neurological function and cortical cerebral blood flow. (a) At nine months, 3HD mice demonstrated worse performance in reversal platform trials compared to sham-injured mice. (n = 10–12/group, *p < 0.05 for group × time interaction), with no difference in (b) swim speed. (c) 3HD mice had increased distance traveled in an overnight open field test (n = 10–12/group, p < 0.001 for group) as well as (d) higher speed (n = 10–12/group, **p < 0.01 for group). (e) In the elevated plus maze, 3HD mice exhibited higher speed vs. sham (n = 10–12/group, **p < 0.01), but not time in the open arms (f). (g) Y-maze performance did not differ between 3HD and sham groups (n = 10–12/group, p = ns). (h) Average cerebral blood flow index assessed by diffuse correlation spectroscopy was higher in 3HD vs. sham groups at three to six months after injuries (n = 9–11/group, **p < 0.01).
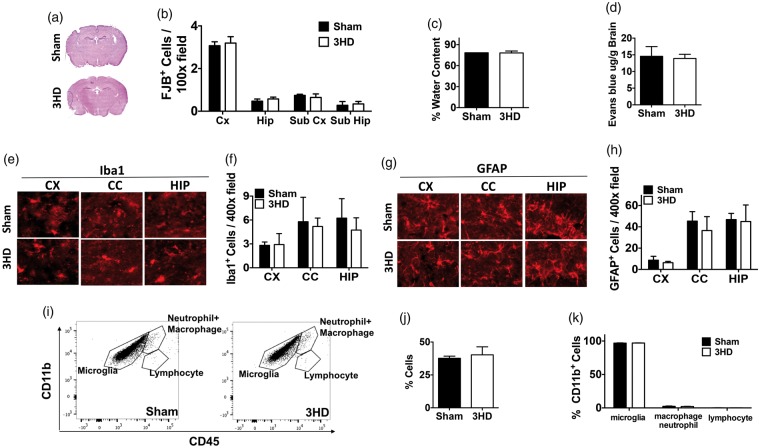
Acute and chronic histopathology of adolescent rCHI
Adolescent 3HD produced no obvious brain damage at 4 h after the last injury (Figure 3(a)). Despite robust choroid plexus staining with PI indicating delivery to brain, no PI+ cells were detected in injured cortex, hippocampus, or striatal brain regions in any mice at 4 h after 3HD (data not shown). Likewise, no degenerative cells were detected in cortex, hippocampus, striatum, or any other brain regions (not shown) by hematoxylin and eosin criteria (shrunken, agyrophilic nuclei, eosinophilic cytoplasm). Only an occasional fluoro-Jade B+ cell was observed in brain sections of sham and 3HD mice, but no differences were observed between groups in any of the above brain regions (Figure 3(b)). Brain edema and blood–brain barrier leakage to Evans blue also did not differ between 3HD and sham groups at 24 h (Figure 3(c) and (d)). Likewise, no differences were observed in microglia and astrocyte counts at 4 h after 3HD or sham injury (Figure 3(e) to (h)). Flow cytometry data showed no accumulation of leukocytes in 3HD brain at 24 h vs. sham (Figure 3(i) to (k)). These data suggested that our 3HD adolescent model is one of mild TBI.
Figure 3.
Acute histopathology of adolescent rCHI. (a) Representative image of H&E staining of sham and 3HD mice at 4 h after the last injury. No differences were observed between groups at 4 h after 3HD in (b) Fluoro Jade B + cells, (c) brain edema, (d) blood–brain barrier leakage of Evans blue, (e, f) Iba1+ cells, or (g, h) GFAP+ cells (n = 5–6/group except FJB which was n = 4 shams and 5 injured, p = ns for all comparisons). (i) Representative flow cytometry plots showing microglia (CD11bhigh, CD45med), macrophages + neutrophils (CD11bhigh, CD45high), and lymphocytes (CD11blow, CD45high) in sham and 3HD brain at 24 h after the last injury. Numbers represent percentages of cells in each gate relative to total plotted cells. (j) Percentage of total brain leukocytes (microglia, macrophages, neutrophils, and lymphocytes) in sham- and 3HD mouse brain at 24 h (n = 4/group, p = ns). (k) Of total numbers of CD11b+ cells isolated by FACS, percentages of microglia, macrophages + neutrophils, and lymphocytes did not differ between 3HD and sham 3HD groups at 24 h (n = 4/group, p = ns).
Figure 4 shows results for histopathology in the chronic period (one year) after 3HD. No differences in IBA-1+ microglia and GFAP+ astrocyte numbers or gross morphology were observed in cortex, hippocampus, corpus callosum, and striatum of sham and 3HD mice (Figure 4(a) to (d)). Likewise, NeuN+ cell counts in cortex regions did not differ between groups (Figure 4(e) and (f)). Corpus callosum white matter area stained with anti-neurofilament antibody did not differ between groups (Figure 4(g) and (h)). DAPI+ cell counts in cortex, hippocampus, and striatum also did not differ between groups (Figure 4(i) and (j)). Volumetric analyses of cortex, hippocampus, and lateral ventricles showed no differences between sham and 3HD groups at one year (Figure 4(k) to (m)). These data confirm our impression that, in terms of histopathology, the 3HD adolescent model is one of mild TBI.
Figure 4.
Chronic histopathology of adolescent rCHI. (a) Representative image and (b) quantification of Iba1+ cells in 3HD and sham groups. (c) Representative image and (d) quantification of GFAP+ cells in 3HD and sham groups. (e) Representative image and (f) quantification of NeuN+ cells in cortex. (g) Representative image and (h) quantification of CMI+ area in corpus callosum. (i) Representative image and (j) quantification of DAPI+ cells in brain regions analyzed. No differences were observed in any of the aforementioned parameters between sham- and 3HD- injured mice. Additionally, no differences were observed between groups in (k) cortex (Cx) volume, (l) hippocampus (Hip) volume, and (m) lateral ventricle volume. For all assessments, n = 5/group. CC: corpus callosum; Str: striatum.
Endothelial IL-1 and IL-18 processing and behavioral protection in IL-1R1 and caspase-1 KO mice after 3HD in adolescent mice
We next sought a mechanistic basis for the observed cognitive deficits in the 3HD model with a focus on IL-1β and IL-18. Lectin and DAPI staining on isolated brain microvessels (Figure 5(a)) and Western blot of CD31+ cell lysates (Figure 5(b)) showed that CD31+ cell isolation is specific for brain endothelial cells. We began by examining whole brain microvessel isolates obtained 11 days after 3HD or sham injury, a time when MWM deficits were apparent in 3HD mice. Western blot analyses showed increased processing of IL-1β and Il-18 with significant reduction in their full-length forms in CD31+ microvessels isolated from brains of 3HD but not 1HD or 3HW mice (Figure 5(c) to (f)). Notably, IL-1β and IL-18 processing was not readily detected in CD31-cells (representative blots shown in Figure 5(c) and (e)), and no difference between sham and 3HD in CD31+ IL-1β processing was seen at 48 h (Supplemental Figure 1). Similar to in vivo data, three-dimensional cultured human brain endothelial cells subjected to weight drop trauma also showed increased expression of cleaved IL-1β (Figure 5(g) and (h)). Performance in the MWM was similar between naïve IL-1R1 KO vs. WT and caspase-1 KO vs. WT mice, but following 3HD, adolescent IL-1R1 KO and caspase-1 KO mice performed significantly better than WT mice in MWM hidden platform trials (Figure 5(i) and (j)), with performance in injured caspase-1 KO indistinguishable from that of naïve caspase-1 KO mice.
Figure 5.
Endothelial inflammation after repetitive CHI, and improved cognitive outcome in adolescent IL-1R1 and caspase-1 KO mice. (a) Representative image of Lectin/DAPI staining of isolated CD31+ microvessels. (b) Western blot of isolated CD31+ microvessels or mouse cortex homogenate using NeuN, GFAP and Iba1 antibodies. (c) At 11 days after the last CHI, expression of pro-IL-1β was decreased in endothelial cells isolated from 3HD and 3HW but not 1HD mice, whereas cleaved IL-1β was increased in endothelium only after 3HD but not 3HW or 1 HD. (d) Densitometry data for panel A: 1HD (n = 5/group), 3HD (n = 5/group, *p < 0.05 for pro- IL 1β and **p < 0.001 for cleaved- IL 1β vs. sham), 3HW (n = 3/group, *p < 0.05 for pro- IL 1β). Note that cleaved IL-1β was not detected in the CD31- fraction. (e) Western blot and (f) densitometry of IL-18 in endothelial cells in sham vs. 1HD (p = ns), 3HD (*p < 0.05) and 3HW (p = ns). IL-18 was not readily detected in the CD31-fraction. (g) Western blot results in three-dimensional cultures of human brain endothelial cells subjected to concussive trauma ×3 in one day. Expression of cleaved IL-1β (but not pro- IL-1β) at 24 h was significantly increased in injured cultures. (h) Densitometry data for panel G (n = 4/group, *p < 0.05 for cleaved IL-1β vs. sham). (i) Naïve IL-1R1 KO mice had similar Morris water maze performance as naïve WT in hidden platform trials but slightly worse performance in visible platform trials (p = 0.02 for group, n = 10–11/group). However, after 3HD, IL-1R1 KO mice performed better in hidden trials compared to injured WT mice (p < 0.05 for group, n = 10/group) with no difference in visible platform trials (p = 0.05 vs. injured WT). (j) Naïve Cas1 KO and WT mice did not differ in MWM performance in the hidden or visible platform (p = ns, n = 11–12/group). After 3HD, Cas1 KO mice performed better in hidden trials compared to WT mice (p < 0.05 for group, n = 9–10/group).
Discussion
Herein, we report the longest longitudinal preclinical study of an adolescent repetitive TBI model to date. Using CHI in DOL 38 mice, we found that three daily (but not weekly) injuries induced persistent and emergent neurological deficits for up to one year in the absence of overt and microscopic histopathology. Acute cognitive deficits were associated with processing of IL-1β and IL-18 in the cerebrovascular endothelium of 3HD, but not 1HD or 3HW mice, and IL-1β was processed in a cell autonomous manner in cultured human endothelial cells subjected to three traumatic injuries. Mice with global deletion of IL-1R1 or caspase-1 had improved post-injury MWM performance early after 3HD, suggesting a functional role for IL-1R1 in acute post-injury cognitive deficits. These data are the first to identify an endothelial source for IL-1 and IL-18 in an rCHI model, and to link inflammatory mechanisms and neurological deficits in an adolescent rCHI model. These findings may have mechanistic and functional implications for adolescents who sustain repetitive mTBI.
Repetitive CHI recapitulated clinically relevant features of human mTBI, including impact and angular acceleration forces, long-term cognitive and motor learning deficits, and absence of structural brain injury or skull fracture.34 CHI produced mean rotational accelerations of 5.2e5 rad/s2. Recognizing that significant controversy exists scaling mouse TBI models to humans,29 when scaled to human equivalents, these values are approximately 1/3 the thresholds published for concussed adults (4600–8200 rad/s2).35 Additional data consistent with a mild degree of injury include lack of an MWM deficit after a single CHI, and lack of cell death, gliosis, and white matter and brain volume changes acutely and at one year after 3HD. Nonetheless, CHI induced an apparent LOC that is only observed in a subset of concussed athletes. Differentiating between an effect of CHI on consciousness vs. interaction with isoflurane that prolongs emergence would require comparison to an unanesthetized CHI control group. Alternatively, adolescent rodents may have a lower threshold for traumatic LOC compared to humans.
Whereas two CHI produced an initial MWM deficit that resolved (in reversal trials) at one month, three daily CHIs produced MWM hidden platform deficits measureable for up to one year, in the absence of differences in motor performance, swim speeds, and depression/anxiety phenotypes. These results are reminiscent of behavioral deficits observed in studies that lesioned the dorsomedial striatum.36 Delayed onset of hyperactivity in 3HD mice also suggests striatal dysfunction.37 With regard to MWM deficits, the timing of CHIs was important as MWM performance in rCHI mice was similar to sham when injuries were spaced one week apart. We recently reported a safe rest interval of one to two weeks in a 5HD adult rCHI model similar to the one used herein.19 In contrast to adults, 3HW in adolescent mice (DOL 31, 38, and 45) occurs at significantly different stages of brain development. Accordingly, resistance to CHI at DOL 31 or 45 (or both) might explain the safe rest interval of seven days. Further studies with 3HD beginning on DOL 31 vs. 45 are needed to resolve this question. Regardless, the 3HW paradigm models the real-life scenario in which TBI occurs at multiple stages of adolescent brain development.
In contrast to early and persistent MWM deficits, increased locomotor activity emerged by nine months after 3HD. Although we did not measure attention in the current study, the data are consistent with secondary attention deficit hyperactivity disorder reported in children and juvenile rodents with TBI.38,39 The emergence of an abnormal locomotor phenotype in the chronic period suggests evolving striatal injury, with potential implications for later development of neurodegenerative disease such as chronic traumatic encephalopathy (CTE).40,41 However, even at one year after injuries, 3HD did not induce “polypathology” of CTE, such as cortical neuron loss, gliosis, or white matter loss.42 In other studies, repetitive CHI in adolescent mice14 and rats43 induced gliosis and axon damage detectable by three months, findings that are likely attributable to different TBI models43 and numbers of injuries14 as well as time points examined. Similar to our results, repetitive low-level blast TBI in adult rats did not induce gliosis and overt histopathology in the chronic stages.44 Indeed, the current study shows that repetitive TBI in adolescent mice can lead to long-term, progressive neurological dysfunction in the absence of significant histopathology. These findings have important implications for adolescents who play contact sports or are otherwise at risk of repetitive head injury, inasmuch as mechanisms other than or in addition to those associated with CTE may be relevant to development of impaired neurological function later in life.
We did not assess female mice in the current study. Female DOL 38–40 mice weigh significantly less than males and would therefore be subjected to different biomechanical forces using our injury apparatus. In clinical studies, females display greater cognitive impairment following injuries.45 However, in experimental concussion models, injured female mice and rats often have better cognitive outcome than their male counterparts.46–48 Although female sex may confer resistance to cognitive effects of repetitive CHI,48 further experiments controlling for body weight and potential effects of endogenous female sex hormones during MWM acquisition are needed to conclusively answer this question. These studies are ongoing in our lab.
Using DCS, we found that 3HD mice had increased cortical cerebral blood flow (CBF) vs. sham in the chronic period (three to six months), suggesting cerebrovascular dysfunction. We previously reported transient decreases in cortical CBF with DCS at 4 h after CHI that returned to baseline at 24 h in an adult rCHI model.30 In that study, lower baseline (pre-injury) CBF correlated with worse post-injury MWM performance, suggesting a close relationship between cerebrovascular function and cognitive outcome.30 Recent clinical studies have also demonstrated increased CBF in concussed adolescents with persistent neurological symptoms,49,50 and others have demonstrated impaired cerebrovascular activation51 and autoregulation52 that may occur even in the absence of symptoms in children and adolescents with mTBI. Although the relationship between rmTBI and pathogenesis of cognitive dysfunction is poorly understood, findings from these aforementioned studies and our data suggest that the cerebrovasculature may contribute to physiological and neurological deficits after rCHI.
The finding that 3HD produced lasting neurological deficits and increased cortical microcirculatory blood flow without detectable neuronal loss suggests a vascular pathophysiology fundamentally different from the small vessel disease of “vascular dementias” associated with hypoperfusion and neuronal death.53 Although microcirculatory changes in the rCHI model require further characterization especially at later time points, to our knowledge, the current study is the first to examine endothelial mechanisms of cognitive dysfunction in a repetitive concussion model. Mature IL-1β and IL-18, known mediators of synaptic and cognitive dysfunction,28 were increased in brain endothelial cells at 11 days after 3HD rCHI, a time when MWM deficits were apparent. We did not observe differences in mature IL-1β at 48 h, nor did we perform MWM analyses at this early time point after injury to test for a possible relationship between the two. The lack of increased mature IL-1β and IL-18 in brains of 3HW, 1HD, and sham-injured mice also corresponded to a lack of early MWM deficits in these groups. These findings, and the finding that IL-1R1 and caspase-1 (an upstream activator of IL-1β and IL-18) KO mice had improved post-injury MWM performance early after 3HD suggest that IL-1/IL-18 might be drivers of cognitive dysfunction at least in the sub-acute period after 3HD in adolescent mice.
Because in vivo models cannot easily distinguish between cell-autonomous and non-autonomous responses to TBI, we used a three-dimensional human brain endothelial cell culture system to study cell autonomous responses to concussive forces similar to those experienced by mice in the in vivo studies. Importantly, this system reproduces the mechanical properties of brain cortical tissue.33 Though cell cultures were not directly impacted by the weight drop (because they were floating in media within a protective plastic cube), we found that three sequential weight drop hits induced endothelial cell IL-1β processing by 24 h. These data demonstrate cell autonomous IL-1 signaling by brain vascular endothelial cells in response to concussive trauma and suggest that human brain endothelial cells might respond similarly in vivo. These studies add translational value to human mTBI for which brain samples are almost never available for direct study in the acute period.
Our work has a number of important limitations relevant to data interpretation. We did not measure systemic or brain temperature, or other physiological variables such as blood pressure and blood gases requiring arterial access that would be challenging given the relatively small size of the mice. Indirect and/or off target effects of brain development in the absence of IL-1R1 or caspase-1 could have influenced outcome in KO mice, as could off-target systemic effects unique to these mice. MWM performance was assessed in the acute but not the chronic period after injury, and further studies are required to determine whether IL-1R1 or caspase-1 mediates longer term functional outcome. Our study is also limited by use of global IL-1R1 KO and caspase-1 KO mice, which prohibits conclusions about which cell type(s) are critical for neurological outcome. Future studies will incorporate cell specific IL-1R1 deletion using Cre-Lox technology to attempt to identify cell types critical for IL-1R1 and caspase-1 signaling. These approaches are important because glial and other cell types may also signal through IL-1R1.
In conclusion, rCHI in adolescent mice induced long-term neurological deficits in the absence of overt and microscopic histopathology, and endothelial IL-1/IL-18 processing and IL-1R1 were linked to acute cognitive outcome. Further studies of endothelial mechanisms in repetitive adolescent concussion models are warranted that could yield novel therapeutic options to prevent or ameliorate long-term consequences of repetitive TBI in this vulnerable population.
Supplemental Material
Supplemental material for Repetitive head injury in adolescent mice: A role for vascular inflammation by Limin Wu, Joon Y Chung, Shivani Saith, Lorenzo Tozzi, Erin M Buckley, Bharat Sanders, Maria A Franceschini, Sevda Lule, Saef Izzy, Josephine Lok, William J Edmiston III, Lauren M McAllister, Sloane Mebane, Gina Jin, Jiaxi Lu, John S Sherwood, Sarah Willwerth, Suzanne Hickman, Joseph El Khoury, Eng H Lo, David Kaplan and Michael J Whalen in Journal of Cerebral Blood Flow & Metabolism
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was funded by NIH/NINDS 1RO1NS092847-01 (DK), 5RO1NS091573 (JL), R21HD086385 (MJW), NIA RF1AG051506 (JEK)
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
LW and MW designed, analyzed and interpreted the data and wrote the manuscript. LW, JYC, SS, LT, EB, BS, WJE, LMM, SM, GJ, JL, JSS, SW and MAF performed the experiments. SL, SI, JL, SH, JEK, EHL, DK and MW assisted in revising the manuscript and approved the final version.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Bryan MA, Rowhani-Rahbar A, Comstock RD, et al. Sports- and recreation-related concussions in US youth. Pediatrics 2016; 138: pii: e20154635. [DOI] [PubMed] [Google Scholar]
- 2.McKinlay A, Grace RC, Horwood LJ, et al. Prevalence of traumatic brain injury among children, adolescents and young adults: prospective evidence from a birth cohort. Brain Inj 2008; 22: 175–181. [DOI] [PubMed] [Google Scholar]
- 3.Ryu WH, Feinstein A, Colantonio A, et al. Early identification and incidence of mild TBI in Ontario. Can J Neurol Sci 2009; 36: 429–435. [DOI] [PubMed] [Google Scholar]
- 4.Barlow KM. Postconcussion syndrome: a review. J Child Neurol 2016; 31: 57–67. [DOI] [PubMed] [Google Scholar]
- 5.Barlow KM, Crawford S, Stevenson A, et al. Epidemiology of postconcussion syndrome in pediatric mild traumatic brain injury. Pediatrics 2010; 126: e374–e381. [DOI] [PubMed] [Google Scholar]
- 6.Fay TB, Yeates KO, Taylor HG, et al. Cognitive reserve as a moderator of postconcussive symptoms in children with complicated and uncomplicated mild traumatic brain injury. J Int Neuropsychol Soc 2010; 16: 94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baillargeon A, Lassonde M, Leclerc S, et al. Neuropsychological and neurophysiological assessment of sport concussion in children, adolescents and adults. Brain Inj 2012; 26: 211–220. [DOI] [PubMed] [Google Scholar]
- 8.McCrory P, Collie A, Anderson V, et al. Can we manage sport related concussion in children the same as in adults? Br J Sports Med 2004; 38: 516–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stamm JM, Koerte IK, Muehlmann M, et al. Age at first exposure to football is associated with altered corpus callosum white matter microstructure in former professional football players. J Neurotrauma 2015; 32: 1768–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schultz V, Stern RA, Tripodis Y, et al. Age at first exposure to repetitive head impacts is associated with smaller thalamic volumes in former professional american football players. J Neurotrauma 2018; 35: 278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alosco ML, Kasimis AB, Stamm JM, et al. Age of first exposure to American football and long-term neuropsychiatric and cognitive outcomes. Transl Psychiatry 2017; 7: e1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montenigro PH, Alosco ML, Martin BM, et al. Cumulative head impact exposure predicts later-life depression, apathy, executive dysfunction, and cognitive impairment in former high school and college football players. J Neurotrauma 2017; 34: 328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant DA, Serpa R, Moattari CR, et al. Repeat mild traumatic brain injury in adolescent rats increases subsequent beta-amyloid pathogenesis. J Neurotrauma 2017; 35: 94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mannix R, Berkner J, Mei Z, et al. Adolescent mice demonstrate a distinct pattern of injury after repetitive mild traumatic brain injury. J Neurotrauma 2017; 34: 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prins ML, Alexander D, Giza CC, et al. Repeated mild traumatic brain injury: mechanisms of cerebral vulnerability. J Neurotrauma 2013; 30: 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Semple BD, Sadjadi R, Carlson J, et al. Long-term anesthetic-dependent hypoactivity after repetitive mild traumatic brain injuries in adolescent mice. Dev Neurosci 2016; 38: 220–238. [DOI] [PubMed] [Google Scholar]
- 17.Jassam YN, Izzy S, Whalen M, et al. Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron 2017; 95: 1246–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khuman J, Meehan WP, Zhu X, et al. Tumor necrosis factor alpha and Fas receptor contribute to cognitive deficits independent of cell death after concussive traumatic brain injury in mice. J Cereb Blood Flow Metab 2011; 31: 778–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mannix R, Meehan WP, Mandeville J, et al. Clinical correlates in an experimental model of repetitive mild brain injury. Ann Neurol 2013; 74: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu X, Park J, Golinski J, et al. Role of Akt and mammalian target of rapamycin in functional outcome after concussive brain injury in mice. J Cereb Blood Flow Metab 2014; 34: 1531–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol 2012; 28: 137–161. [DOI] [PubMed] [Google Scholar]
- 22.Smith DE, Lipsky BP, Russell C, et al. A central nervous system-restricted isoform of the interleukin-1 receptor accessory protein modulates neuronal responses to interleukin-1. Immunity 2009; 30: 817–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helmy A, Carpenter KL, Menon DK, et al. The cytokine response to human traumatic brain injury: temporal profiles and evidence for cerebral parenchymal production. J Cereb Blood Flow Metab 2011; 31: 658–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clausen F, Hanell A, Bjork M, et al. Neutralization of interleukin-1beta modifies the inflammatory response and improves histological and cognitive outcome following traumatic brain injury in mice. Eur J Neurosci 2009; 30: 385–396. [DOI] [PubMed] [Google Scholar]
- 25.Clausen F, Hanell A, Israelsson C, et al. Neutralization of interleukin-1beta reduces cerebral edema and tissue loss and improves late cognitive outcome following traumatic brain injury in mice. Eur J Neurosci 2011; 34: 110–123. [DOI] [PubMed] [Google Scholar]
- 26.Semple BD, O'Brien TJ, Gimlin K, et al. Interleukin-1 receptor in seizure susceptibility after traumatic injury to the pediatric brain. J Neurosci 2017; 37: 7864–7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekmark-Lewen S, Flygt J, Fridgeirsdottir GA, et al. Diffuse traumatic axonal injury in mice induces complex behavioural alterations that are normalized by neutralization of interleukin-1beta. Eur J Neurosci 2016; 43: 1016–1033. [DOI] [PubMed] [Google Scholar]
- 28.Mendiola AS, Cardona AE. The IL-1beta phenomena in neuroinflammatory diseases. J Neural Transm 2018; 125: 781–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ommaya AK, Hirsch AE, Yarnell P, et al. Scaling of experimental data on cerebral concussion in subhuman primates to concussion threshold for man, Fort Belvoir, Virginia: Defense Technical Information Center, 1967, pp. 1–11. [Google Scholar]
- 30.Buckley EM, Miller BF, Golinski JM, et al. Decreased microvascular cerebral blood flow assessed by diffuse correlation spectroscopy after repetitive concussions in mice. J Cereb Blood Flow Metab 2015; 35: 1995–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim YC, Garcia-Cardena G, Allport JR, et al. Heterogeneity of endothelial cells from different organ sites in T-cell subset recruitment. Am J Pathol 2003; 162: 1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rockwood DN, Preda RC, Yucel T, et al. Materials fabrication from Bombyx mori silk fibroin. Nat Protoc 2011; 6: 1612–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang-Schomer MD, White JD, Tien LW, et al. Bioengineered functional brain-like cortical tissue. Proc Natl Acad Sci U S A 2014; 111: 13811–13816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCrory P, Meeuwisse W, Dvorak J, et al. Consensus statement on concussion in sport-the 5(th) international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med 2017; 51: 838–847. [DOI] [PubMed] [Google Scholar]
- 35.Viano DC, Casson IR, Pellman EJ. Concussion in professional football: biomechanics of the struck player – part 14. Neurosurgery 2007; 61: 313–327; discussion 27–28. [DOI] [PubMed] [Google Scholar]
- 36.Pooters T, Gantois I, Vermaercke B, et al. Inability to acquire spatial information and deploy spatial search strategies in mice with lesions in dorsomedial striatum. Behav Brain Res 2016; 298(Pt B): 134–141. [DOI] [PubMed] [Google Scholar]
- 37.Kostrzewa RM, Brus R, Kalbfleisch JH, et al. Proposed animal model of attention deficit hyperactivity disorder. Brain Res Bull 1994; 34: 161–167. [DOI] [PubMed] [Google Scholar]
- 38.Max JE, Schachar RJ, Levin HS, et al. Predictors of secondary attention-deficit/hyperactivity disorder in children and adolescents 6 to 24 months after traumatic brain injury. J Am Acad Child Adolesc Psychiatry 2005; 44: 1041–1049. [DOI] [PubMed] [Google Scholar]
- 39.Mychasiuk R, Hehar H, Esser MJ. A mild traumatic brain injury (mTBI) induces secondary attention-deficit hyperactivity disorder-like symptomology in young rats. Behav Brain Res 2015; 286: 285–292. [DOI] [PubMed] [Google Scholar]
- 40.McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013; 136(Pt 1): 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stern RA, Daneshvar DH, Baugh CM, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology 2013; 81: 1122–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol 2013; 9: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prins ML, Hales A, Reger M, et al. Repeat traumatic brain injury in the juvenile rat is associated with increased axonal injury and cognitive impairments. Dev Neurosci 2010; 32: 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gama Sosa MA, De Gasperi R, Perez Garcia GS, et al. Lack of chronic neuroinflammation in the absence of focal hemorrhage in a rat model of low-energy blast-induced TBI. Acta neuropathol Commun 2017; 5: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Resch JE, Rach A, Walton S, et al. Sport concussion and the female athlete. Clin Sports Med 2017; 36: 717–739. [DOI] [PubMed] [Google Scholar]
- 46.Velosky AG, Tucker LB, Fu AH, et al. Cognitive performance of male and female C57BL/6J mice after repetitive concussive brain injuries. Behav Brain Res 2017; 324: 115–124. [DOI] [PubMed] [Google Scholar]
- 47.O'Connor CA, Cernak I, Vink R. Interaction between anesthesia, gender, and functional outcome task following diffuse traumatic brain injury in rats. J Neurotrauma 2003; 20: 533–541. [DOI] [PubMed] [Google Scholar]
- 48.Wright DK, O'Brien TJ, Shultz SR, et al. Sex matters: repetitive mild traumatic brain injury in adolescent rats. Ann Clin Transl Neurol 2017; 4: 640–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stephens JA, Liu P, Lu H, et al. Cerebral blood flow after mild traumatic brain injury: associations between symptoms and post-injury perfusion. J Neurotrauma 2018; 35: 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barlow KM, Marcil LD, Dewey D, et al. Cerebral perfusion changes in post-concussion syndrome: a prospective controlled cohort study. J Neurotrauma 2017; 34: 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Talavage TM, Nauman EA, Breedlove EL, et al. Functionally-detected cognitive impairment in high school football players without clinically-diagnosed concussion. J Neurotrauma 2014; 31: 327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mutch WA, Ellis MJ, Ryner LN, et al. Brain magnetic resonance imaging CO2 stress testing in adolescent postconcussion syndrome. J Neurosurg 2016; 125: 648–660. [DOI] [PubMed] [Google Scholar]
- 53.Iadecola C. The pathobiology of vascular dementia. Neuron 2013; 80: 844–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for Repetitive head injury in adolescent mice: A role for vascular inflammation by Limin Wu, Joon Y Chung, Shivani Saith, Lorenzo Tozzi, Erin M Buckley, Bharat Sanders, Maria A Franceschini, Sevda Lule, Saef Izzy, Josephine Lok, William J Edmiston III, Lauren M McAllister, Sloane Mebane, Gina Jin, Jiaxi Lu, John S Sherwood, Sarah Willwerth, Suzanne Hickman, Joseph El Khoury, Eng H Lo, David Kaplan and Michael J Whalen in Journal of Cerebral Blood Flow & Metabolism