Abstract
We aimed to assess cerebral autoregulation (CA) and neurovascular coupling (NVC) in stroke patients of differing severity comparing responses to healthy controls and explore the association between CA and NVC with functional outcome. Patients admitted with middle cerebral artery (MCA) stroke and healthy controls were recruited. Stroke severity was defined by the National Institutes of Health Stroke Scale (NIHSS) scores: ≤4 mild, 5–15 moderate and ≥16 severe. Transcranial Doppler ultrasound and Finometer recorded MCA cerebral blood flow velocity (CBFv) and blood pressure, respectively, over 5 min baseline and 1 min passive movement of the elbow to calculate the autoregulation index (ARI) and CBFv amplitude responses to movement. All participants were followed up for three months. A total of 87 participants enrolled in the study, including 15 mild, 27 moderate and 13 severe stroke patients, and 32 control subjects. ARI was lower in the affected hemisphere (AH) of moderate and severe stroke groups. Decreased NVC was seen bilaterally in all stroke groups. CA and NVC correlated with stroke severity and functional outcome. CBFv regulation is significantly impaired in acute stroke, and further compromised with increasing stroke severity. Preserved CA and NVC in the acute period were associated with improved three-month functional outcome.
Keywords: Stroke, severity, cerebral autoregulation, neurovascular coupling, outcome
Introduction
Adequate brain perfusion after ischemic stroke (IS) depends not only on patent cerebral arteries and collaterals, but also on compensatory mechanisms in the cerebral microcirculation.1 Neurovascular coupling (NVC) is an adaptive mechanism in which cerebral blood flow (CBF) is adjusted by the local microcirculation in accordance with underlying neuronal activity.1 An increase in cerebral activity triggers a vasodilatory response of blood vessels leading to a consequent increase in CBF. Cerebral autoregulation (CA) is another vascular control mechanism in the brain, representing the ability of cerebral vessels to regulate their own blood supply (by maintaining adequate and stable CBF), despite changes in cerebral perfusion pressure.2
The importance of these mechanisms in the pathogenesis and treatment of acute focal cerebral ischemia remains unclear.3 Since clinical trials are most effective when limited to patients who are most likely to benefit, a better understanding of cerebral hemodynamic regulation and its relationship to IS is critical for the proper design of such studies. While numerous studies in the setting of IS have been published (for review Jordan and Powers4 and Salinet et al.5), there is still a lack of consistent information regarding the relation between both regulatory mechanisms and clinical findings in acute and chronic IS.6,7
In light of this, we previously performed a pilot study based on four sequential assessments of CA and NVC in acute IS over a three-month period.8 This provided a comprehensive understanding of the temporal progression of both regulatory mechanisms after IS, revealing acute NVC impairment and CA deterioration after two weeks with normalization by three months. Further clinical associations with respect to stroke severity or outcome were limited by sample size. Therefore, we proposed to evaluate, in a larger cohort, the two main CBF regulatory mechanisms, CA and NVC, in three stroke severity groups compared to non-stroke control subjects. Moreover, we examined the relationship between these regulatory mechanisms, stroke severity assessed by the National Institute of Health Stroke Scale (NIHSS) and three-month functional recovery assessed by the modified Rankin scale (mRs).
Material and methods
Research participants
The local ethics committee of the University of Sao Paulo approved the study (approval number 982.280), and conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all patients and relatives. From January 2015 to March 2017, 127 consecutive patients with acute IS were initially identified in the emergency room at Hospital das Clinicas in São Paulo, Brazil. Patients meeting all of the following criteria were eligible: first-ever acute IS within 48 h of onset; identified signs of focal neurological dysfunction in the middle cerebral artery (MCA) with/without MCA stenosis. Patients were excluded due to: (1) presence of intracranial or subarachnoid hemorrhage on CT or MRI, (2) other significant intracranial or extracranial major vascular stenosis or occlusion (assessed by transcranial Doppler ultrasound (TCD) and transcranial color-coded duplex sonography (TCCD), respectively, (3) absence of sufficient temporal bone window for MCA insonation, (4) history of myocardial infarction within six months or other neurological disorders. Patients with impaired consciousness or inability to cooperate sufficiently to complete the hemodynamic assessment, or insufficient quality of hemodynamic recordings were also excluded.
Age- and sex-matched healthy control subjects were recruited into the study from the Department staff, relatives of recruited patients and researchers. Controls subjects were excluded if there was history of stroke or transient ischemic attack, diabetes mellitus, hypercholesterolemia, carotid artery stenosis and smoking in the last five years.
Clinical assessment
IS was defined as the occurrence of stroke confirmed by imaging (CT or MRI) and clinical diagnosis by a neurologist, blinded to cerebral hemodynamic analysis. The infarct volume was measured at 24 h following the ABC/2 formula that uses a pure ellipsoid model to estimate stroke and perfusion volumes.9 NIHSS score was assessed at admission: ≤4 defining mild stroke, 5–15 moderate and ≥16 severe.10
Clinical outcome
All participants were followed up at three months by a trained neurologist. Data on deaths, vascular events, blood pressure (BP) and mRS score were recorded. Poor functional outcome was defined as mRS > 2.
Peripheral and cerebral hemodynamic measurements
The researcher undertaking cerebral hemodynamic assessments was blinded to admission clinical examination, including NIHSS score. Recordings were performed as described previously.8 In brief, CBF velocity (CBFv) in the MCA was continuously assessed by TCD ultrasound (DWL Doppler Box) using a dual 2-MHz transducer fitted to a head frame. BP was continuously recorded by a Finapres device (Ohmeda 2300; Finapres, Louisville, Colo., USA). End-tidal CO2 (EtCO2) was monitored using a capnograph (MX-200, Transmai) with nasal sampling and heart rate was obtained from an electrocardiogram.
After stable values had been established, the servomechanism of the Finapres device was turned off. A data segment of 5 min baseline was recorded followed by a 4-min brain activation paradigm. The paradigm began with a 90-s baseline phase, followed by a passive motor paradigm (repetitive flexion and extension of the participant’s elbow undertaken by the examiner) over 60 s, with a 90-s recovery phase. The movements were driven by a metronome to ensure a standard frequency of 1 Hz. All participants performed the paradigm twice. The passive motor paradigm was executed only with the affected side in the stroke group and with either right or left chosen randomly in controls (activated hemisphere). Prior to recordings, the paradigm was trialled twice to provide training and avoid the need for any verbal instructions during the recordings.
Data analysis
Data were simultaneously recorded onto the DWL Doppler Box at a sampling rate of 100 samples/s. CBFv channels were filtered at 20 Hz (zero-phase eighth-order Butterworth), R-R interval was obtained from the electrocardiogram, and mean BP, CBFv and end-tidal carbon dioxide (EtCO2) were calculated for each cardiac cycle. The instantaneous relationship between BP and CBFv was used to estimate critical closing pressure (CrCP) and resistance area product (RAP) for each cardiac cycle using the first harmonic method.11 Beat-to-beat data were spline interpolated and resampled at five samples s−1 to produce signals with a uniform time-base.
Cerebral autoregulation
Autoregulation index (ARI) was adopted to quantify the status of CA.12 ARI consists of 10 levels from damaged (ARI = 0) to intact (ARI = 9) CA. A second-order differential equation simulates 10 sets of CBFv responses to an ideal step change in BP. By comparing the recorded CBFv with the 10 simulated velocities, the ARI is assigned to each subject for both hemispheres by using the best least squares fit.
NVC
Bilateral CBFv response to the passive paradigm was used to assess NVC, as previously described.8,13 The beginning of stimulation was used as the point of synchronism to obtain population averages of the first and second performance of each paradigm. From visual inspection, the paradigm that achieved the highest amplitude of contralateral CBFv response was chosen to represent the participant’s response at each visit.13 Mean CBFv, heart rate, BP and EtCO2 values were extracted from the 30 s preceding each paradigm for baseline comparisons.
Statistical analysis
To investigate the influence of the stroke severity on CA and NVC, patients were divided into groups according to the tertiles of admission NIHSS (mild, moderate and severe), and all of the systemic and cerebral hemodynamic parameters were subsequently compared among groups; the control group being used as reference.
Normality of distribution for continuous variables was evaluated using the Kolmogorov–Smirnov goodness-of-fit test. Variables with a positive skew were normalized using the base 10 logarithm of the variable in order to fit the variables in multivariable logistic models. For the control group, no significant differences between right and left cerebral hemispheres were found in CBFv, and ARI, and therefore the mean value for both hemispheres was used in further analyses.
Baseline demographics were compared among the four groups (mild, moderate, and severe stroke, and controls) using a one-way analysis of variance (ANOVA). Chi-square and Kruskal–Wallis tests were used for analysis of non-parametric data. Baseline CBFv and ARI (as a measure of CA) were compared using two-factor mixed-design ANOVA with subject group as the between factor, and side of recording (right, left, affected and unaffected cerebral hemispheres) as the within factor. To compare the effect of the passive paradigm on bilateral CBFv (as a measure of NVC), BP and EtCO2, the area-under-the-curve (AUC) was calculated for the duration of the paradigm. Again, two-factor mixed-design ANOVA was used to compare the AUC values. In case of a significant interaction effect, a one-factor ANOVA with the Bonferroni post hoc procedure was performed to identify the source of the difference.
Spearman’s rho correlation analysis was performed to evaluate the relationship between CBF regulatory mechanisms (CA and NVC parameters) and clinical variables, such as stroke volume, stroke severity (admission NIHSS), functional outcome (three-month mRS), and history of AF, hypertension or diabetes. Logistic regression analysis was performed to determine variables independently associated with good functional recovery (mRS ≤ 2). Variables with values of p < 0.05 on univariate testing were included in this model. The measurement data are expressed as mean ± SD, and the count data are expressed as the rate (percentage). P-values < 0.05 were considered statistically significant.
Results
Participants’ characteristics
Thirty-five controls and 61 stroke participants gave consent to participate in the study. Six stroke and three control participants were excluded due to poor insonation of the temporal windows. Therefore, a total of 55 acute stroke subjects and 32 controls underwent data collection. In three mild stroke patients, no clear infarction could be demonstrated on early CT, but all patients had a clinical syndrome consistent with MCA territory IS. Demographic and clinical characteristics were similar among stroke subgroups (Table 1). MCA occlusion was detected at admission (after thrombolysis if applicable) in 12 (21%) of our cohort by TCD, allowing only the contralateral MCA to be monitored. Significantly higher mRS scores were seen in the severe stroke group compared to mild and moderate groups (0 (0–2), 2 (1–4) and 4 (3–6), respectively). Moreover, the mild group showed significantly lower mRS scores compared to moderate stroke group (p = 0.001).
Table 1.
Clinical characteristics according to admission stroke severity.
| Stroke |
Controls | |||
|---|---|---|---|---|
| Mild | Moderate | Severe | ||
| N = 15 | N = 27 | N = 13 | ||
| Age, years | 62.9 ± 13.0 | 62.1 ± 8.9 | 63.5 ± 15.6 | 63.6 ± 10.4 |
| Sex (male), n (%) | 7 (47) | 14 (52) | 6 (46) | 10 (31) |
| Smokers | ||||
| Current, n (%) | 5 (33) | 6 (22) | 9 (62) | 0 (0) |
| Former, n (%) | 10 (67) | 14 (52) | 4 (31) | 0 (0) |
| BMI, kg.m−2 | 27.3 ± 0.9 | 27.9 ± 1.2 | 28.0 ± 0.6 | 27.0 ± 1.0 |
| Co-morbidities, n (%) | ||||
| Diabetes mellitus | 3 (20) | 4 (15) | 3 (23) | 0 (0) |
| Hypertension | 5 (33) | 9 (33) | 8 (61)ɛ | 9 (32) |
| Hypercholesterolemia | 1 (7) | 4 (15) | 0 (0) | 0 (0) |
| Atrial fibrillation | 0 (0) | 3 (11) | 4 (31)* | 0 (0) |
| Transient ischemic attack | 0 (0) | 1 (4) | 0 (0) | |
| None | 7 (47) | 13 (48) | 4 (31) | |
| r-tPA, n (%) | 0 (0) | 10 (37) | 10 (77)* | – |
| Infarct volume, mL median (IQR) | 10 (0–43) | 93 (31–199)° | 158 (108–316)* | – |
| TOAST, n (%) | ||||
| Cardioembolism | 10 (67) | 18 (67) | 9 (62) | – |
| Undetermined | 5 (33) | 9 (33) | 5 (38) | – |
| Right stroke hemisphere, n (%) | 4 (27) | 14 (52) | 7 (54) | – |
| Time from stroke onset, h | 25.3 ± 10.4 | 22.0 ± 9.5 | 30.5 ± 13.5 | – |
| NIHSS, median (IQR) | 2 (0–4) | 9 (5–15)° | 18 (16–27)* | – |
BMI: body-mass index; r-tPA: recombinant tissue plasminogen activator; TOAST: trial of ORG 10172 in acute stroke treatment; NHISS: National Institutes of Health Stroke Scale.
p < 0.05 for post hoc Bonferroni, p value for differences between severe and the others subgroups.
p < 0.05 for post hoc Bonferroni, p value for differences between severe and moderate, and between severe and mild groups.
p < 0.05 for post hoc Bonferroni, p value for differences between mild and moderate groups.
Systemic and cerebral hemodynamics
Hemodynamic data are presented in Table 2. No differences between groups in systolic, mean or diastolic BP, or heart rate were found. Resting systolic, mean and diastolic CBFv was higher in controls compared to both hemispheres of the mild group (p < 0.02 for both AH and UH) and affected cerebral hemisphere of the severe group (p < 0.02). Whereas systolic CBFv of the unaffected hemisphere was higher than affected hemisphere CBFv in the moderate and severe groups (p < 0.02), higher mean CBFv of the unaffected hemisphere compared to the affected hemisphere was found only in the severe group (p = 0.009).
Table 2.
Physiological variables – Comparison between groups in systemic and cerebral hemodynamics.
| Stroke |
Control | |||
|---|---|---|---|---|
| Mild | Moderate | Severe | ||
| N = 15 | N = 27 | N = 13 | N = 32 | |
| Systemic hemodynamics | ||||
| Systolic BP, mm Hg | 147.1 ± 22.4 | 145.0 ± 20.3 | 134.7 ± 17.5 | 138.3 ± 20.2 |
| Mean BP, mmHg | 88.6 ± 8.4 | 91.4 ± 12.3 | 86.1 ± 8.7 | 93.1 ± 12.2 |
| Diastolic BP, mmHg | 63.9 ± 7.3 | 66.6 ± 10.2 | 62.4 ± 5.4 | 68.7 ± 9.7 |
| Heart rate, bpm | 69.3 ± 10.6 | 68.3 ± 6.2 | 67.6 ± 3.1 | 64.9 ± 3.3 |
| EtCO2, mmHg | 39.7 ± 1.6 | 38.0 ± 1.2 | 39.1 ± 2.1 | 38.9 ± 1.0 |
| Cerebral hemodynamics | ||||
| Affected hemisphere sys CBFv, cm.s−1 | 63.1 ± 11.9* | 72.3 ± 6.3 | 61.8 ± 24.3* | – |
| Unaffected hemisphere sys CBFv, cm.s−1 | 68.7 ± 12.1* | 87.4 ± 6.4ɛ | 82.2 ± 22.4ɛ | – |
| Mean sys CBFv, cm.s−1 | – | – | – | 83.1 ± 4.6 |
| Affected hemisphere mean CBFv, cm.s−1 | 39.7 ± 8.7* | 47.9 ± 10.8 | 38.7 ± 11.4* | – |
| Unaffected hemisphere mean CBFv, cm.s−1 | 42.4 ± 7.3* | 56.9 ± 14.7 | 56.9 ± 19.2ɛ | – |
| Mean CBFv, cm.s−1 | – | – | – | 58.0 ± 9.2 |
| Affected hemisphere dis CBFv, cm.s−1 | 26.1 ± 7.8* | 32.3 ± 4.6 | 29.5 ± 9.9* | – |
| Unaffected hemisphere dis CBFv, cm.s−1 | 25.8 ± 8.0* | 38.3 ± 12.2 | 33.2 ± 10.2 | – |
| Mean dis CBFv, cm.s−1 | – | – | – | 39.6 ± 2.4 |
| Affected hemisphere ARI | 5.4 ± 2.2 | 4.0 ± 2.7* | 3.2 ± 2.2* | – |
| Unaffected hemisphere ARI | 5.8 ± 2.0 | 5.3 ± 1.9 | 6.0 ± 1.8ɛ | – |
| Mean ARI | – | – | – | 6.3 ± 2.4 |
| Affected hemisphere NVC, %ΔCBFv | 8.3 ± 5.0*Ω | 5.8 ± 4.8* | 4.1 ± 4.3* | – |
| Unaffected hemisphere NVC, %ΔCBFv | 8.5 ± 5.1*δ | 5.6 ± 4.2* | 3.8 ± 3.9* | – |
| Activated hemisphere NVC, %ΔCBFv | – | – | – | 13.0 ± 6.0 |
| Non-activated hemisphere NVC, %ΔCBFv | – | – | – | 11.9 ± 5.8 |
BP: blood pressure; CBFv: cerebral blood flow velocity; ARI: autoregulation index.
p < 0.02 for post hoc Bonferroni for differences between stroke and control.
p = 0.009 for post hoc Bonferroni for differences between affected and unaffected hemisphere.
p = 0.005 and 0.003 for post hoc Bonferroni for differences between mild and moderate groups, and between mild and severe groups, respectively.
p = 0.005 and 0.001 for post hoc Bonferroni for differences between mild and moderate groups, and between mild and severe groups, respectively.
Cerebral autoregulation
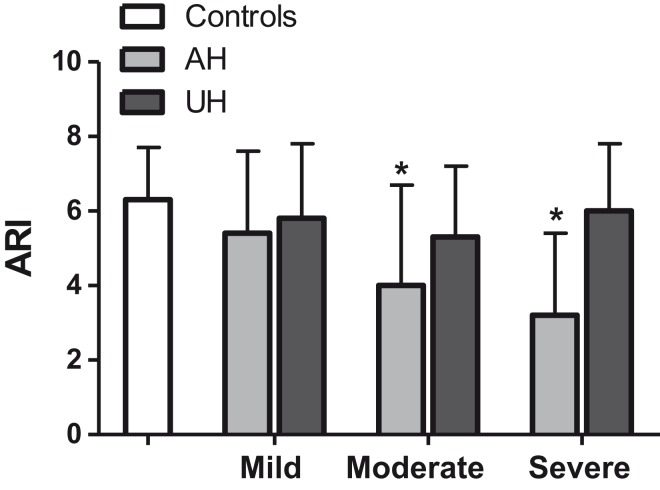
Population average values of ARI across the different groups are compared in Figure 1 and Table 2 showing that ARI was significantly lower in the affected cerebral hemisphere following moderate and severe stroke compared to controls (Table 2, p < 0.02, respectively). No differences in ARI were found between the unaffected hemispheres and controls. There were significant autoregulatory differences between the affected and unaffected hemispheres only following severe stroke (post hoc p = 0.009).
Figure 1.
Comparison of autoregulatory index (ARI) among controls, mild, moderate and severe stroke sub-groups. Bars/whisker represents one SD. * p < 0.05 compared to controls.
NVC
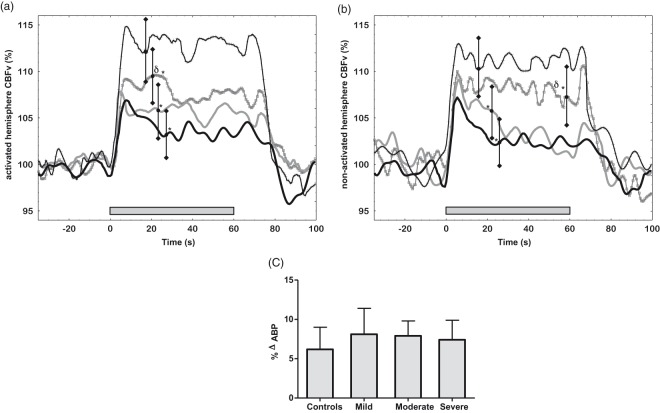
All participants performed the passive paradigm. It led to a bilateral increase in CBFv in both control and stroke participants (Figure 2(a) and (b)). AUC revealed lower bilateral CBFv responses in all three stroke sub-groups compared to controls (Table 2). Moreover, higher NVC values were found in the mild IS group compared to moderate and severe strokes (Table 2).
Figure 2.
Cerebral and systemic hemodynamic responses to the passive paradigm (grey bar). (a) CBFv responses in the activated hemisphere of control (continuous black line + crosses), mild stroke (continuous grey line + squares), moderate stroke (continuous grey line) and severe stroke (continuous black line); (b) CBFv percentage responses in the non-activated cerebral hemispheres; (c) Variation in BP during the paradigm performance. Bars represent one SD. δ p < 0.006 between mild and the other two stroke groups *p < 0.05 between stroke and control.
Figure 2(c) shows the effect of the passive paradigm on BP. By comparing baseline and AUC values, the paradigm led to a significant increase in BP in all three sub-groups (p < 0.001). Though higher BP amplitude was seen in the AUC of mild stroke sub-group, no difference was found between sub-groups (ANOVA F = 2.1 p = 0.1).
Correlation between clinical and hemodynamic variables
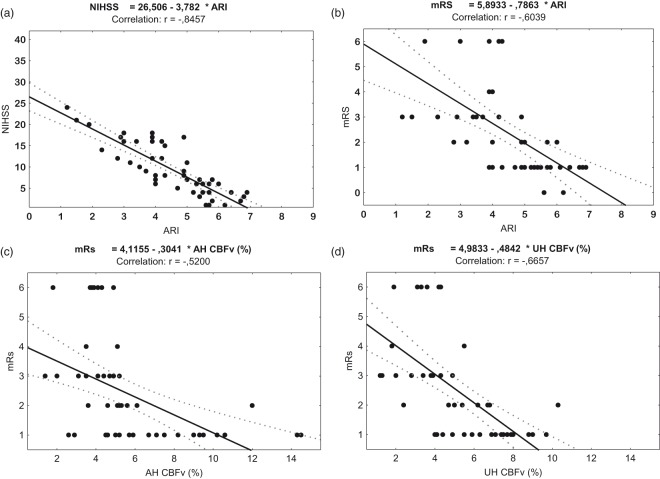
Reduced ARI in the affected hemisphere was associated with increasing stroke severity (Figure 3(a); r = −0.845, p = 0.03) and poor three-month functional outcome (Figure 3(b); r = −0.604, p = 0.001). A lower CBFv response to neural activation in both the unaffected (Figure 3(c); r = −0.665, p = 0.02) and affected hemispheres (Figure 3(d); r = −0.520, p = 0.04) was also associated with poor three-month outcome. However, no significant correlations were identified between CA and NVC responses, and CT/MRI stroke volume and both ARI and CBFv responses. Logistic regression analysis showed that absence of AF, lower stroke severity and higher affected hemisphere ARI were the independent predictive factors of good functional recovery, after adjusting for significant variables in the univariate analysis (Table 3).
Figure 3.
Scatter graphs of Spearman’s correlation analysis. Correlation coefficient (r-value) between stroke affected hemisphere ARI and severity (a) and outcome (b), and between mRS and affected (c) and unaffected hemispheres (d).
Table 3.
Adjusted odds ratios of good functional recovery for clinical and hemodynamic variables.
| O. R. | 95% C.I. | p | |
|---|---|---|---|
| Age | 1.00 | 0.13–1.88 | 0.34 |
| Sex (female) | 1.06 | 0.99–1.45 | 0.10 |
| Previous Hypertension | 6.91 | 2.92–19.31 | 0.07 |
| Acute hypertension | 0.99 | 0.65–1.22 | 0.43 |
| AF | 0.15 | 0.99–5.30 | 0.001 |
| Stroke severity (<5) | 9.12 | 2.45–24.87 | 0.004 |
| AH ARI (>4) | 10.54 | 5.98–15.11 | 0.007 |
AF: history of atrial fibrillation; AH ARI: autoregulatory index of the stroke affected hemisphere.
Note: Categorical variables were included as 1 = yes or 0 = no. Acute hypertension was considered present when above of 140/90 mmHg. Stroke severity and AH ARI were dichotomized in mild (admission NIHSS < 5) versus moderate/severe stroke (admission NIHSS > 5), and intact CA (ARI > 4 and impaired CA (ARI ≤ 4).
Discussion
This study provides evidence that cerebral hemodynamics are compromised with increasing stroke severity assessed by admission NIHSS. Moderate and severe stroke was associated with greater CBFv asymmetries between affected and unaffected hemispheres, CA deterioration in the affected stroke hemisphere only, and bilateral NVC impairment. CA impairment in the affected hemisphere was associated with increasing stroke severity, and predicted poor three-month mRS outcome. Additionally, lower CBFv responses to brain activation correlated significantly with poorer functional outcome.
Average side-to-side differences in hemodynamic parameters in healthy adults were reported to be negligible,14 though substantial interhemispheric asymmetry after stroke is a sign of cerebral hemodynamic deterioration. Higher CBF (or CBFv) values in the unaffected hemisphere are commonly interpreted as a sign of activation of the collateral circulation.15,16 In line with our results, the effectiveness of the collateral circulation has been previously associated with reduced stroke severity and more favourable outcomes in severe stroke.17–19
Hitherto, there has been no consensus in the literature concerning CA status after stroke. Some authors have postulated an acute impairment,20–22 and others deterioration over time.8,23–25 Moreover, no agreement is found whether ischemia leads to only focal8,21,22 or more global CA impairment.24–27 This inconsistency may be attributed in part to the heterogeneity of the stroke populations studied previously. In particular, few previous studies have considered the influence of stroke subtype26,27 and severity.28 Our study is the first to evaluate CA by stroke severity and to demonstrate its deterioration in the affected cerebral hemisphere with increasing severity. These results may have implications for the management of acute stroke-associated physiological perturbations.
NVC data and their prognostic significance in acute stroke are even more limited in the literature, with considerable methodological variation.5 The present study reported acute impairment in NVC, consistent with our previous report,8,29 but additionally confirmed that this impairment was irrespective of stroke severity. Moreover, lower CBFv responses to the passive paradigm were correlated with poor three-month functional outcome. This has important implications for patient management in the acute stroke phase, particularly the role played by intensive therapy. Previous randomized trials have failed to show a positive impact of intensive training very early after stroke.30,31 Based on our results, we could speculate that these unfavourable outcomes may be consequence of inappropriate flow adaptation to neuronal demands (NVC impairment) increasing the risk of secondary damage to the ischemic area.
This study has limitations, including the use of non-invasive measurements of CBF and BP32 and the relatively small sample size of the stroke sub-groups. Though we limited the eligible population to patients with ICA stenosis<40% and MCA infarcts, it was nonetheless a heterogenous population with differing pathological mechanisms, which limited our ability to investigate the effect of stroke subtype. Another limitation to consider is the missing data in the ipsilateral hemisphere in 21% of the stroke subjects. MCA occlusion is one of the most common and well-known forms of IS and the authors believe this might have not introduced bias into the results. In addition, 18% and 9% of the studied stroke participants had a history of diabetes and hypercholesterolemia, whereas these comorbidities were excluded from the control participants group. Future studies will benefit from a second control group composed of non-healthy subjects, in order to determine the separate contributions of stroke and diabetes and hypercholesterolemia. Other point is the accuracy of ARI estimates based on spontaneous fluctuations in BP and CBFv. Despite its methodological limitations (ex. redefined linear and stationary relationship between BP and CBFv), ARI reliability has been mitigated to some extend by the transfer function analysis robustness and standardized data collection. Finally, the sensorimotor paradigms led to significant CBFv responses in both MCAs rather than a lateralized response. The limited lateralization in NVC studies using TCD has been reported previously in several studies, not only using passive arm movement, but also cognitive or sensorimotor paradigms.5,8,13,29,33–35 We have previously addressed the possible reasons behind this characteristic of TCD-based studies, including an additional diffuse and nonspecific mental stimulus regarding attention, concentration and motivation during the lateralized and focal motor paradigm.
In conclusion, the present study identified an uncoupling between CBF and neuronal activation bilaterally in all stroke sub-groups, and a deterioration of autoregulatory mechanisms in the affected stroke hemisphere following moderate and severe stroke. Importantly, preserved CA may be associated with good functional outcome, although it was not possible to assess the causality of association with stroke severity. Further studies to improve the understanding of CBF regulation after stroke should focus on its natural history, the influence of pathological subtype, the prediction of long-term functional outcome, and the implications for early intensive rehabilitation and pharmacological strategies.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: São Paulo Research Foundation (FAPESP, Grant nos. 2013/25953-0, 2014/04955-8 and 2016/20787-3) supported this work.
Acknowledgements
The authors would like to thank Dr. João Loures Salinet Júnior for their help with the data collection and analysis. Prof. Robinson is an NIHR Senior Investigator.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
Salinet – Study concept and design, acquisition of data, analysis and interpretation of data, drafted the manuscript. Silva – acquisition of data, analysis and interpretation of data, drafted the manuscript. Caldas – acquisition of data, critical revision of the manuscript for important intellectual content. de Azevedo – acquisition of data, critical revision of the manuscript for important intellectual content. de-Lima-Oliveira – acquisition of data, critical revision of the manuscript for important intellectual content. Nogueira – critical revision of the manuscript for important intellectual content. Conforto – critical revision of the manuscript for important intellectual content. Teixeira – study supervision. Robinson – critical revision of the manuscript for important intellectual content, study supervision. Panerai – critical revision of the manuscript for important intellectual content, study supervision. Bor-Seng-Shu – critical revision of the manuscript for important intellectual content, study supervision.
References
- 1.Lin WH, Hao Q, Rosengarten B, et al. Impaired neurovascular coupling in ischaemic stroke patients with large or small vessel disease. Eur J Neurol 2011; 18: 5731–5736. [DOI] [PubMed] [Google Scholar]
- 2.Paulson OB, Strandgaard S, Edvinsson L, et al. Cerebral autoregulation. Cerebrovasc Brain Metab Rev 1990; 2: 161–192. [PubMed] [Google Scholar]
- 3.González RG, Schwamm LH. Imaging acute ischemic stroke. Handb Clin Neurol 2016; 135: 293–315. [DOI] [PubMed] [Google Scholar]
- 4.Jordan JD, Powers WJ. Cerebral autoregulation and acute ischemic stroke. Am J Hypertens 2012; 25: 946–950. [DOI] [PubMed] [Google Scholar]
- 5.Salinet ASM, Haunton VJ, Panerai RB, et al. A systematic review of cerebral hemodynamic responses to neural activation following stroke. J Neurology 2013; 260: 2715–2721. [DOI] [PubMed] [Google Scholar]
- 6.Tsivgoulis G, Apostolidou N, Giannopoulos S, et al. Hemodynamic causes of deterioration in acute ischemic stroke. Perspect Med 2012; 1: 177–184. [Google Scholar]
- 7.Iannotti F, Hoff J. Ischemic brain edema with and without reperfusion: an experimental study in gerbils. Stroke 1983; 14: 562–567. [DOI] [PubMed] [Google Scholar]
- 8.Salinet ASM, Panerai RB, Robinson TG. The longitudinal evolution of cerebral blood flow regulation after acute ischaemic stroke. Cerebrovasc Dis Extra 2014; 4: 186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sims R, Rezai GL, Schaefer PW, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology 2009; 72: 2104–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brott T, Adams HP Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989; 20: 864–870. [DOI] [PubMed] [Google Scholar]
- 11.Panerai RB. The critical closing pressure of the cerebral circulation. Med Eng Phys 2003; 25: 621–632. [DOI] [PubMed] [Google Scholar]
- 12.Tiecks FP, Lam AM, Aaslid R, et al. Comparison of static and dynamic cerebral autoregulation measurements. Stroke 1995; 26: 1014–1019. [DOI] [PubMed] [Google Scholar]
- 13.Salinet ASM, Robinson TG, Panerai RB. Reproducibility of cerebral and peripheral haemodynamic responses to active, passive and motor imagery paradigms in older healthy volunteers: a fTCD study. J Neurosci Methods 2012; 206: 143–150. [DOI] [PubMed] [Google Scholar]
- 14.Krejza J, Chen R, Romanowicz G, et al. Sickle cell disease and transcranial Doppler imaging: inter-hemispheric differences in blood flow Doppler parameters. Stroke 2011; 42: 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winship IR. Cerebral collaterals and collateral therapeutics for acute ischemic stroke. Microcirculation 2015; 22: 228–236. [DOI] [PubMed] [Google Scholar]
- 16.Liebeskind DS. Collateral circulation. Stroke 2003; 34: 2279–2284. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Prabhakar P, Sealock R, et al. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cereb Blood Flow Metab 2010; 30: 923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lima FO, Furie KL, Silva GS, et al. The pattern of leptomeningeal collaterals on CT angiography is a strong predictor of long-term functional outcome in stroke patients with large vessel intracranial occlusion. Stroke 2010; 41: 2316–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miteff F, Levi CR, Bateman GA, et al. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain 2009; 132: 2231–2238. [DOI] [PubMed] [Google Scholar]
- 20.Xiong L, Tian G, Lin W, et al. Is dynamic cerebral autoregulation bilaterally impaired after unilateral acute ischemic stroke? J Stroke Cerebrovasc Dis 2017; 26: 1081–1087. [DOI] [PubMed] [Google Scholar]
- 21.Petersen N, Ortega-Gutierrez S, Reccius A, et al. Dynamic cerebral autoregulation is transiently impaired for one week after large-vessel acute ischemic stroke. Cerebrovasc Dis 2015; 39: 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castro P, Azevedo E, Serrador J, et al. Hemorrhagic transformation and cerebral edema in acute ischemic stroke: link to cerebral autoregulation. J Neurol Sci 2017; 372: 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Proescholdt MA, Faltermeier R, Bele S, et al. Detection of impaired cerebral autoregulation using selected correlation analysis: a validation study. Comput Math Methods Med 2017; 2017: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawson SL, Panerai RB, Potter JF. Serial changes in static and dynamic cerebral autoregulation after acute ischaemic stroke. Cerebrovasc Dis 2003; 16: 69–75. [DOI] [PubMed] [Google Scholar]
- 25.Reinhard M, Roth M, Guschlbauer B, et al. Dynamic cerebral autoregulation in acute ischemic stroke assessed from spontaneous blood pressure fluctuations. Stroke 2005; 36: 1684–1689. [DOI] [PubMed] [Google Scholar]
- 26.Guo ZN, Liu J, Xing Y, et al. Dynamic cerebral autoregulation is heterogeneous in different subtypes of acute ischemic stroke. PLoS One 2014; 9: e93213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Immink RV, van Montfrans GA, Stam J, et al. Dynamic cerebral autoregulation in acute lacunar and middle cerebral artery territory ischemic stroke. Stroke 2005; 36: 2595–2600. [DOI] [PubMed] [Google Scholar]
- 28.Dohmen C, Bosche B, Graf R, et al. Identification and clinical impact of impaired cerebrovascular autoregulation in patients with malignant middle cerebral artery infarction. Stroke 2007; 38: 56–61. [DOI] [PubMed] [Google Scholar]
- 29.Salinet ASM, Robinson TG, Panerai RB. Cerebral blood flow response to neural activation after acute ischemic stroke: a failure of myogenic regulation? J Neurol 2013; 260: 2588–2595. [DOI] [PubMed] [Google Scholar]
- 30.Yelnik AP, Quintaine V, Andriantsifanetra Cet al. AMOBES Group AMOBES (Active Mobility Very Early After Stroke): a randomized controlled trial. Stroke 2017; 48: 400–405. [DOI] [PubMed] [Google Scholar]
- 31.Bernhardt J, Langhorne P, Lindley RI, et al. AVERT Trial Collaboration group. Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. Lancet 2015; 386: 46–55. [DOI] [PubMed] [Google Scholar]
- 32.Panerai RP. Assessment of cerebral perfusion autoregulation in humans – a review of measurements methods. Physiol Meas 1998; 19: 305–338. [DOI] [PubMed] [Google Scholar]
- 33.Maggio P, Salinet ASM, Panerai RB, et al. Does hypercapnia-induced impairment of cerebral autoregulation affect neurovascular coupling? A functional TCD study. J Appl Physiol 2013; 115: 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moody Michelle, Ronney BP, Penelope JE, et al. Cerebral and systemic hemodynamic changes during cognitive and motor activation paradigms. Am J Physiol Regul Integr Comp Physiol 2005; 288: R1581–R1588. [DOI] [PubMed] [Google Scholar]
- 35.Caldas JR, Panerai RB, Salinet ASM, et al. Dynamic cerebral autoregulation is impaired during sub-maximal isometric handgrip in patients with heart failure. Am J Physiol Heart Circ Physiol 2018; 315: H254–H261. [DOI] [PubMed] [Google Scholar]