Abstract
Dynamic cerebral autoregulation (dCA) has been shown to be impaired in cerebrovascular diseases, but there is a lack of consistency across different studies and the different metrics that have been proposed for assessment. We performed a systematic review and meta-analyses involving assessment of dCA in ischemic and hemorrhagic stroke. Thirty-three articles describing assessment of dCA with transfer function analysis (TFA) were included, with meta-analyses performed for derived parameters of gain, phase and autoregulation index (ARI). A total of 1233 patients were pooled from 12 studies on acute ischemic stroke (AIS) and two studies on intracerebral hemorrhage (ICH). In comparison with controls, TFA phase of AIS was significantly reduced (nine studies), in both hemispheres (P < 0.0001). TFA gain provided inconsistent results, with reduced values in relation to controls, for both hemispheres. The ARI (six studies) was reduced compared to controls, in both hemispheres (P < 0.005). In ICH, gain showed higher values compared to controls for the unaffected (P = 0.01), but not for the affected hemisphere. Meta-analyses in AIS have demonstrated that phase and the ARI index can show highly significant differences in comparison with healthy controls, while ICH have been limited by the scarcity of studies and the diversity of units adopted for gain.
Keywords: Cerebral blood flow, dynamic cerebral autoregulation, ischemic stroke, intracranial hemorrhage, transfer function analysis
Introduction
Dynamic cerebral autoregulation (dCA) is an important physiological marker in acute neurovascular pathology, with both prognostic and therapeutic significance, and is impaired in up to one-third of patients following acute stroke.1,2 Impaired dCA has been studied in many cases of moderate to severe ischemic and hemorrhagic stroke, demonstrating damaged cerebral arterioles and capillaries, with related endothelial dysfunction, receptor impairment, and smooth muscle deactivation.3,4 These structural and functional changes lead to impaired vasomotor regulation and may contribute to the risks of hyper- or hypoperfusion, leading to secondary injury and worse outcomes.5
Different cerebral blood flow (CBF) measurement modalities have been used for dCA assessment such as near-infrared spectroscopy (NIRS) and transcranial Doppler ultrasound (TCD). Most studies of dCA in stroke used TCD to obtain estimates of CBF by measuring CBF velocity (CBFV) in the middle cerebral artery (MCA) or other intra-cranial vessels.6 Although the thigh cuff maneuver was the original method used to demonstrate the phenomenon of dCA,7 and several other protocols have since been advocated,8 the most common approach in clinical studies has been based on transfer function analysis (TFA), in conjunction with spontaneous fluctuations in arterial blood pressure (BP) and CBFV.6,9 By using BP as the ‘input’ and CBFV as ‘output’, TFA yields estimates of the amplitude (‘gain’) and phase of the BP-CBFV relationship as a function of the frequency of oscillations. The gain reflects how much of the amplitude of BP oscillations are attenuated by a working dCA at each frequency, while the phase expresses the time delay of CBFV changes following the BP oscillations. The autoregulation index (ARI), initially introduced to quantify the CBFV response to a thigh cuff maneuver,10 can also be extracted by TFA from spontaneous fluctuations of BP and CBFV.11,12 TFA parameters, including the ARI, have been reported to be altered in stroke patients.5,12,13 A recent international consensus White Paper14 is likely to improve inter-centre consistency for estimates of gain and phase, but their reliability for detecting loss of dCA function in cerebrovascular disease is largely unknown. To address this gap in knowledge, we performed a systematic review of the literature, including meta-analyses of gain, phase and ARI in studies of both ischemic and hemorrhagic stroke. Although some recent reviews have made reference to the application of TFA in studies of stroke,5,15,16 to our knowledge, this study is the first to perform a systematic review of this topic, with meta-analyses of gain, phase and ARI. The aims of this review were to assess the quality and quantity of evidence for TFA parameters from dCA studies in acute stroke, to assess the heterogeneity of available evidence, and to identify knowledge gaps for future research.
Materials and methods
Medline (1946-present), Embase (1947-present), Web of Science (1970-present), Psychinfo (1984-present), CIHNAL (1976-present) and The Cochrane Library (1993-present) were searched using the strategy provided in Supplementary Material. The search was limited to human studies in English, after 1990, as TFA application to dCA was first proposed by Giller et al.17 in this year. The equations of TFA parameters (gain, phase and coherence) and ARI are described in Appendix 1. The initial database search was conducted in August 2018, and was updated in May 2019 prior to submission. In addition, reference lists from all included studies and related papers were screened in PubMed for additional relevant articles. The protocol was registered on PROSPERO for this review prior to initiation (CRD42018109435). The PRISMA flow diagram of articles in this review is provided in Figure 1. Studies on neurological diseases, subjected to qualitative analysis (Figure 1) were further limited to ischemic and hemorrhagic stroke for the purposes of meta-analysis and detailed systematic review. Studies were screened initially by title and abstract by one reviewer (KI), and 10% were checked by a second reviewer (LCB). Included studies were independently evaluated as full papers by two reviewers against the inclusion and exclusion criteria (KI, LCB). Quality assessment was undertaken using pre-defined criteria which have been published previously,16,18 and summary charts and tables for risk of bias were constructed using the quality assessment criteria in RevMan© version 5.3 for Windows (Supplementary Figures I and II). All data were extracted into Microsoft Excel for Windows by one reviewer (KI), and were then quality evaluated by a second reviewer (LCB).
Figure 1.
PRISMA flow diagram.
Inclusion criteria
(1) Adults aged ≥18 years; (2) Diagnosis of cerebrovascular diseases as defined by standard criteria; (3) Transfer function analysis metrics (gain, phase, coherence, or ARI) present; (4) dCA assessment in clinical population.
Exclusion criteria
(1) Adults aged under 18 years; (2) Healthy population; (3) No TFA measures as listed above; (4) Non-cerebral autoregulation assessment.
RevMan5 software© for Windows, using the inverse variance method with random effects model was used to perform meta-analyses for possible heterogeneity of studies, based on the DerSimonian and Laird approach.19,20 These methods can be found in detail in the Cochrane Handbook for Systematic Reviews of Interventions19 and statistical algorithms for Review Manager 5.21 In brief, this approach assumes that the included studies are estimating different but related effects.19 Using the inverse variance method, an estimate of the between-study variance is calculated (Tau2), where Tau represents the estimated standard deviation of the underlying effects from the studies included in the analysis.19 The between-study variance is determined by comparing the fixed effect, inverse variance result for each individual study.21 The 95% confidence intervals for the range of these underlying effects are calculated as 2 × Tau above and below the pooled estimate.19 The inverse variance method adjusts the contribution of each individual study to the pooled estimate by applying weighting to each study dependent on the variation and heterogeneity (the reciprocal of the study variance or square of the standard error).19,21 The units of phase were expressed in degrees, converting from radians whenever necessary, and the mean differences (MD) between patients and controls were calculated. Since gain had heterogeneous units that could not be converted to a common unit (absolute or normalised unit) as recommended in the White paper,14 the standardised mean differences (SMDs) were calculated. The heterogeneity of studies were described as I2 index which indicates the percentage of variation.22 Heterogeneity was low when I2 < 25%, moderate for I2 = 26 to 74% and I2 ≥ 75% was considered high and significant if P < 0.01.22
Pre-defined criteria for meta-analyses were: at least two studies with the same clinical population, same TFA parameter (phase, gain or ARI), and with sufficient homogeneity between the two or more studies (heterogeneity I2 score < 75%). Meta-analysis was not performed if there were less than two measurements in the same vessel for the same parameter, standard deviation was not provided, or data were not provided for controls and patients. Where studies measured both MCAs, affected and unaffected were separately categorised to prevent double-counting participants. In studies where only one control group was present,12,23–25 the same controls were used for each of the patient groups within a single study. Sensitivity analyses were carried out where the control participants were double counted.
Results
Summary of included studies
Thirty-three studies were included comprising 18 on AIS (large artery stroke, one study that also included transient ischemic attack (TIA)),12,13,26–41 6 on ICH,42–47 3 on small artery stroke, including lacunar infarction,48–50 and 6 on AIS both large and small artery stroke.2,23–25,51,52
Study quality
The quality of included studies ranged from 10 to 15 (median 14, IQR 13–15) (Supplementary Table I).
Participant characteristics
From 33 studies of cerebrovascular disease (AIS including small and large artery and ICH), there was a total of 1233 participants (1010 subjects for AIS and 223 cases of ICH). In the AIS group, both large (n = 735 cases) and small artery (n = 275) strokes were included. The majority of studies reported the National Institutes of Health Stroke Scale (NIHSS) for categorising stroke severity, mean ranging from 0.7 to 22,2,12,13,23,24,26,27,29,34–40,49,51,52 three studies used degree of intracranial artery stenosis28,32,41 and there were four studies where severity remained unclear.25,30,33,50 Eleven studies presented the modified Rankin Scale (mRS).13,24,27,29,32,35–38,43,46 Moreover, the majority of participants were recruited within 6 to 72 h from stroke onset,2,12,13,24–27,29,34–40,42,43,45–47,49,51 including within ultra-acute 6 h (n = 2),26,27 24 h (acute) were studied in four studies,37,42,43,46 three studies examined dCA < 48 h,12,13,38 dCA was assessed at 48 h (n = 8),2,25,34–36,39,47,49 five studies assessed at 72 h (all subacute),24,29,40,45,51 other four studies recruited participants after first week and longer of stroke onset (16 days; chronic)32,33,48,52 and one study examined recovery at six months.49 The location of the lesion was identified in the MCA (n = 13),23,26–28,33–37,40,41,49,51 posterior cerebral artery (n = 3),31,32,49 and anterior cerebral artery territories (n = 3),2,25,52 though eight studies did not specify the precise localization of the lesion.12,13,24,29,38,39,48,50 Five studies of ICH recruited participants for dCA testing within 48 h of onset,42,43,45–47 with the exception of one study which assessed at four to six days after onset.44
Transfer function analysis parameters
The results of TFA are usually interpreted with metrics of gain and phase or ARI.17 The majority of studies (n = 20) showed the results of gain and phase,23,27–29,31–33,35,38–41,43,46–50,52 six studies also included the ARI,2,12,13,25,38,52 five studies showed only phase24,26,34,42,44 and one article presented only gain for interpreting dCA45 (Supplementary Table II). TFA results were presented in different units; the majority of studies (n = 9) reporting unit of gain as cm/s/mmHg,23,32,35,38,41,47,49,50,52 six studies as %/%,29,31,33,39,43,46 four studies as %/mmHg,27,45,48,50 with dB and amplitude used for one study each.28,40 Two units were used for phase with 24 studies presenting degrees23,24,26–36,39,41–44,46,47,49,51,52 and 4 studies using radians.38,40,48,50
Phase by affected and unaffected hemispheres
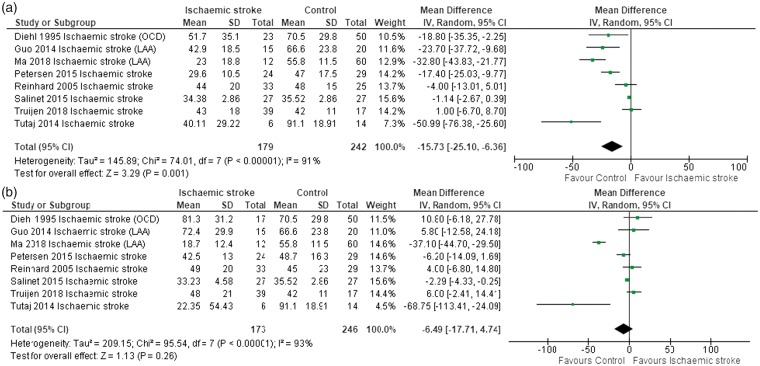
Data were pooled from nine studies by using the mean difference (MD). Phase was significantly lower in AIS compared to controls, for both the affected (Figure 2(a); MD: −20.16, CI: −28.80 to −11.52, P < 0.00001) and unaffected hemispheres (Figure 2(b); MD: −14.61, CI: −24.21 to −5.01, P = 0.003). Moreover, with large vessel occlusion (n = 8), phase was significantly lower in the affected AIS hemisphere, compared to controls (Figure 3(a); MD: −15.73, CI: −25.10 to −6.36, P = 0.001), but not in the unaffected hemisphere (Figure 3(b)). In a pooled analysis of small artery stroke in four studies, phase was significantly lower in AIS, compared to controls in both the affected (Figure 4(a); MD: −26.63, CI: −33.96 to −19.30, P < 0.00001) and unaffected hemispheres (Figure 4(b); MD: −27.11, CI: −32.17 to −22.04, P < 0.00001).
Figure 2.
(a) Forest plot of phase for the affected side in nine studies of AIS. Heterogeneity is high and also significant (I2 = 94%, P < 0.00001). (b) Phase for the unaffected side of nine studies. Heterogeneity is high and significant (I2 = 94%, P < 0.00001). In a sensitivity analysis, removing studies23,24 which used the same control participants did not alter the results.
Figure 3.
(a) Forest plot of phase for a sub-group of eight studies on the affected side in large artery stroke. Heterogeneity is high and also significant (I2 = 91%, P < 0.00001). (b) Phase for a sub-group of eight studies of the unaffected side in large vessel disease. Heterogeneity is high and also significant (I2 = 93%, P < 0.00001).
Figure 4.
(a) Forest plot of phase for a sub-group of four studies on the affected side in small artery stroke. Heterogeneity is moderate and non-significant (I2 = 62%, P = 0.05). (b) Phase for a sub-group of four studies on the unaffected side in small vessel disease. Heterogeneity is low and non-significant (I2 = 25%, P = 0.26).
Gain by affected and unaffected hemispheres
Six studies were included for meta-analysis. Gain was significantly lower in AIS compared to controls for both the affected (Figure 5(a); SMD: −0.75, CI: −1.49 to −0.02, P = 0.04) and unaffected hemispheres (Figure 5(b); SMD: −0.85, CI: −1.61 to −0.09, P = 0.03). On subgroup analysis, gain was significantly lower in patients with small vessel disease compared to controls for both the affected (n = 3) (Figure 6(a); SMD: −0.33, CI: −0.063 to 0.03, P = 0.03) and unaffected hemispheres (Figure 6(b); SMD: −0.44, CI: −0.75 to −0.14, P = 0.004). However, there were no significant differences in five studies of large artery stroke in both hemispheres (Supplementary Figure III).
Figure 5.
(a) Forest plot of gain for the affected side in six studies of AIS. Heterogeneity is high and significant (I2 = 91%, P < 0.00001). (b) Gain for the unaffected side of six studies of AIS. Heterogeneity is high and also significant (I2 = 91%, P < 0.00001).
Figure 6.
(a) Forest plot of gain for a sub-group of two studies on the affected side in small artery stroke. Heterogeneity is low and non-significant (I2 = 0%, P = 0.50). (b) Gain for a sub-group of two studies on the unaffected side in small vessel disease. Heterogeneity is low and non-significant (I2 = 0%, P = 0.61).
ARI by affected and unaffected hemispheres
ARI of mild AIS decreased in the affected hemisphere at 36 h post-event and was worse in both hemispheres at 96 h after onset,12 remaining lower at two weeks after onset13 and apparently diminished in a group of coexisting large and small artery stroke compared with individual large or small artery stroke.52 However, ARI did not show a significant difference between affected and unaffected hemispheres in patients with TIA12 and AIS.25,38 When pooling four studies, the affected side showed highly significantly reduced ARI in AIS compared to controls (Figure 7(a); MD: −0.79, CI: −1.28 to −0.30, P = 0.002), and also on unaffected side (Figure 7(b); MD: −0.52, CI: −0.88 to −0.16, P = 0.005).
Figure 7.
(a) Forest plot of ARI for the affected side in four studies of AIS. Heterogeneity was low and non-significant (I2 = 37%, P = 0.16). (b) ARI for the unaffected side in four studies of AIS. Heterogeneity was low and non-significant (I2 = 0%, P = 0.99). In a sensitivity analysis, removing studies12,25 which used the same control participants did not significantly alter the results.
TFA examined over serial measurements in ischemic stroke patients
Six studies performed serial assessments of dCA in AIS. Four studies measured dCA at similar time points: 0–2 days and 3–7 days, of which three reported only phase24,34,36 and one reported phase and gain.35 However, one study measured dCA at one week, six weeks and three months follow-up,33 and another did not present the results of phase37 and these were therefore excluded from meta-analyses. Pooled analyses of the affected hemisphere demonstrated phase did not significantly differ in all participants between 0–2 days and 3–7 days after stroke onset (Supplementary Figure IV(a); MD: 3.61, CI: −0.38 to 7.59, P = 0.08). On the unaffected side, phase showed no significant differences between serial measurements (Supplementary Figure IV(b)). However, a tendency towards higher phase on 0–2 days in both hemispheres was observed in large artery stroke and one study showed bilaterally lower gain at 0–2 days.35 Nevertheless, two studies on the affected and unaffected hemispheres found a highly rebounding phase,34 and Kwan et al.33 also reported that there was a trend of increasing phase in both hemispheres when measurements were taken after seven days from onset.
Dynamic CA in ICH
Two studies were selected for meta-analysis, with one study reporting both phase and gain43 and the other only gain.45 Excluded studies addressed vasospasm and delayed cerebral ischemia with ICH,47 did not report SD,46 used the same data from another study,43,44 or did not identify the side of the lesion.42 Therefore, two articles were included for gain but none for phase. In the affected hemisphere, gain did not show a significant difference between ICH and controls (Figure 8(a), SMD: 0.68, CI: −0.05 to 1.40, P = 0.07), but tended to favour ICH patients. However, in the unaffected hemisphere, gain was significantly higher in patients when compared to controls (Figure 8(b), SMD: 0.98, CI: 0.21 to 1.74, P = 0.01). Although phase could not be meta-analysed, individual studies showed a significantly lower phase in ICH when compared to controls.42–44
Figure 8.
(a) Forest plots of gain for the affected side in two studies of ICH. Heterogeneity was high but non-significant (I2 = 70%, P = 0.07). (b) Gain for the unaffected side in two studies of ICH. Heterogeneity was high but also non-significant (I2 = 71%, P = 0.06).
Discussion
The majority of existing studies on dCA disturbances during the acute stage of stroke report that worse dCA within a week after onset may be predictive of poor clinical outcomes.27,29,32,36,37,43,46 Although there are currently standardized recommendations for measuring TFA in the context of dCA research,14 this tends to apply only to healthy studies. TFA is a non-invasive technique that can be applied safely and easily to a wide range of clinical conditions, but there is currently no systematic review with associated meta-analysis of TFA for assessment of dCA in neurological applications.
Main findings
To our knowledge this is the first systematic review and meta-analysis to demonstrate that TFA is an effective tool for quantitative and qualitative assessment of dCA in acute stroke. TFA parameters consistently detected changes in dCA in AIS. TFA parameters (phase and ARI) demonstrated significantly impaired autoregulation in AIS patients compared to controls in meta-analysis, especially analyses in all stroke types, large and small artery stroke, and in both affected and unaffected hemispheres. However, dCA remained largely intact in the unaffected hemisphere in large territory stroke. There was significant heterogeneity on meta-analysis of the included studies, with variation in study setting, stroke population, and parameters measured. Despite this, results remained consistent between studies, and were statistically significant on pooled analyses, concluding that impaired autoregulation is present in AIS across stroke sub-types.
Phase
Phase was the parameter used by the majority of studies in this review to assess autoregulation, and also showed the most significant disturbance in AIS compared to controls. In terms of stroke sub-types, phase was significantly reduced in the affected hemisphere in large artery stroke, and bilaterally in small vessel stroke. Studies that conducted serial measurements demonstrated a tendency towards lower phase at three to seven days compared to 0–2 post-event.34–36 Given that phase was the most consistently reported parameter, showing the greatest change, and with the ability to discriminate between stroke sub-types, this would suggest phase is advantageous over other commonly reported TFA parameters for the assessment of dCA in AIS.
ARI
ARI was significantly reduced in both hemispheres in all studies of AIS compared to controls.12,13,25,38 Although individual studies did not demonstrate significant changes in ARI, this was highly significant on pooled analyses, suggesting thus far that studies have been under-powered to detect these changes. In comparison to phase and gain, the ARI was also disadvantaged by studies dominated by strokes of lesser severity. An important consideration is the fact that calculation of the ARI includes all the information from phase and gain,6 and therefore has the potential to perform equally or better than these parameters. Moreover, the ARI can be seen as an inter-method ‘currency’ that can be adopted with other protocols, such as the thigh cuff maneuver, that do not utilise TFA.6,8,10,25,38
Gain
Only two studies demonstrated a higher gain on the affected23,40 and unaffected hemispheres23 in AIS patients. However, on pooled analyses, gain was significantly lower (signalling better autoregulation) in AIS compared to control. This is in contrast to the findings on pooled analyses of phase and ARI of poorer autoregulation in AIS compared to control. These results are unexpected, as the gain would be anticipated to be high in AIS pathology, particularly in the affected hemisphere, as a result of reduced ability of the brain to dampen the effects of BP on CBFV due to impaired vasomotor activity.53 There are a number of potential explanations for this unexpected result. Firstly, there was significant heterogeneity in the pooled analysis as a result of frequency range for dCA assessment (Supplemental Table II). Secondly, the results reported here are SMD due to variation in the units reported between studies. Thirdly, the reliability of gain as a marker of impaired autoregulation may not be as robust as that of phase and ARI.6 This is as a result of methodological variations in the calculation and reporting of gain as a parameter, leading to significant concerns in its interpretability and validity as a measure of dCA.6 Finally, nonstationary dCA behaviour might be more strongly manifested in longitudinal changes in gain, when compared to other parameters such as phase or ARI.54
ICH
The results of ICH studies showed a lower phase in both hemispheres. Phase was also significantly lower during the early stage (1–6 days), and had a prolonged decreasing phase with a further deterioration 7–13 days after ICH onset when compared with healthy controls.42–44 Autoregulatory capability appears to rebound by 30 days but does not fully recover.43 Perihematomal edema and hematoma size, as well as impaired Glasgow Coma Scale, may contribute to dCA impairment between 10 and 20 days after ictus.16,55 Moreover, meta-analysis in few ICH studies showed higher gain in both hemispheres of ICH patients,43,45 which reflects the poor dampening mechanism of regulators, though further studies are required for confirmation. Nonetheless, it appears that using TFA for assessment of dCA in clinical applications is valuable prior to management of blood pressure to prevent hypoperfusion leading to increased ischemic penumbra and hyperperfusion inducing cerebral edema.
Limitations of the study
The major limitations of this review are: small sample sizes, a wide range of time points for TFA assessment of dCA after stroke onset, methodology and statistical analysis changing over time across studies, lack of detail regarding the location of the brain lesion in some studies, significant heterogeneity of pooled analyses and units of TFA measurement, inclusion of papers that did not follow the Cerebral Autoregulation Research Network (CARNet)’s White Paper recommendations,14 and lack of consideration of other modalities, such as NIRS, that could have greater sensitivity to detect affected tissue perfusion in certain brain regions. Although this review has demonstrated clear and consistent abnormalities in dCA on pooled analyses, gain did not follow the expected pattern of results, and further work is required to investigate the reliability and validity of this parameter in comparison to that of phase and ARI in acute stroke. Importantly, not all studies clearly followed the recommended methodology for gain, limiting the interpretation of the results reported here. Despite clear recommendations on the conduct and reporting of studies of TFA, few of those included in this review were in line with these guidelines.14 Future studies of TFA in clinical applications should adhere to this guidance to improve the quality of conduct and reporting of studies, and facilitate better quality meta-analyses with lower heterogeneity.
Further work
Further prospective work on standardised TFA settings is necessary to reduce the heterogeneity of outcomes. The diversity of timings for follow-up assessments of dCA should also be addressed. Dedicated studies with more frequent assessments of dCA, ranging from the ultra-acute (hours) to the medium-term post stroke (weeks), would improve our knowledge of the longitudinal course of dCA. Of considerable relevance, thresholds for impairment of dCA in both AIS and ICH need to be established to allow better patient stratification for patient prognostication and management and more robust assessments of the role of dCA in patient outcomes.
Robust techniques and metrics are needed to move assessment of dCA from the research arena into routine patient care. In AIS, meta-analyses of TFA parameters, obtained from spontaneous fluctuations of BP at rest, have demonstrated that phase and the ARI index can show highly significant differences in comparison with healthy controls, while less clear-cut results were obtained for gain. Meta-analyses of TFA applications to ICH have been limited by the scarcity of studies and the diversity of units of measurement adopted for gain. Evaluation of dCA in the acute phase of stroke and other cerebrovascular diseases is essential for informed clinical management. This review has demonstrated that TFA has considerable potential as a diagnostic tool, although further work is needed to assess its prognostic value. Further work is also needed to establish the ability of TFA parameters to distinguish between different types, locations and severity of stroke, and to establish ranges of normality that take into account different phenotypes.
Supplemental Material
Supplemental Material for Assessment of cerebral autoregulation in stroke: A systematic review and meta-analysis of studies at rest by Kannakorn Intharakham, Lucy Beishon, Ronney B Panerai, Victoria J Haunton and Thompson G Robinson in Journal of Cerebral Blood Flow & Metabolism
Appendix 1. Equations for calculation of TFA gain, phase and ARI
Using beat-to-beat values of BP as input and corresponding values of CBFV as output, the fast Fourier transform is applied with Welch’s method to produce estimates of the auto- and cross-spectra of BP and CBFV, represented as Sxx(f) (BP), Syy(f) (CBFV) and Sxy(f), respectively. From these quantities, the complex transfer function, H(f) is calculated as
| (1) |
which can be expressed as the amplitude (or ‘gain’) and phase frequency responses, given by
| (2) |
| (3) |
where G(f) is the gain and Φ(f) is the phase as a function of frequency and HR(f) and HI(f) are the real and imaginary parts of H(f), respectively.
Finally, the magnitude-squared coherence (MSC) function, which reflects the degree of linear association between input and output is given by
| (4) |
ARI equations
The autoregulation index (ARI) was introduced by Tiecks et al.,10 to quantify the CBFV response to a rapid change in BP, induced by the sudden release of pressurised thigh cuffs. A second-order linear differential equation was proposed to predict the CBFV signal V(t) following a pressure change P(t). Initially, the pressure change is normalised as
| (5) |
where CrCP represents the critical closing pressure. The velocity response can be estimated as
| (6) |
where K is a parameter of gain and x2(t) is a state variable obtained from the following state equation system representing a second-order linear differential equation
| (7) |
| (8) |
where f is the sampling frequency, T represents a time constant, and D is the damping factor. The parameter values of gain , time constant and damping factor are combined in a table,10 corresponding to 10 values of ARI, ranging from 0 (absence of CA) to 9 (best observed CA). When P(t) is represented by a sudden change in BP, or a step function, equations (6) to (8) can then be used to generate 10 theoretical CBFV step responses, each corresponding to a value of ARI.
CBFV step responses can be obtained by the inverse FFT transform of equation (1), and then compared to each of the 10 template step responses proposed by Tiecks et al.,10 using the least square procedure, to derive a value of ARI associated with spontaneous fluctuations in BP and CBFV.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: KI is supported by PhD scholarship of the Ministry of Science and Technology of the Royal Thai Government. LB is funded by the Dunhill Medical Trust (RTF1806\27). TGR is an NIHR Senior Investigator.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
KI and LB performed literature search and extracted information. KI performed meta-analyses. VJS, RBP and TGR conceptualised and planned study. KI, LB and RBP drafted manuscript. All authors revised draft and accepted final version of the manuscript.
Supplemental material
Supplemental material for this article is available online.
References
- 1.Markus HS. Cerebral perfusion and stroke. J Neurol Neurosurg Psychiatry 2004; 75: 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llwyd O, Salinet ASM, Panerai RB, et al. Cerebral haemodynamics following acute ischaemic stroke: effects of stroke severity and stroke subtype. Cerebrovasc Dis Extra 2018; 8: 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.del Zoppo GJ, Hallenbeck JM. Advances in the vascular pathophysiology of ischemic stroke. Thromb Res 2000; 98: 73–81. [DOI] [PubMed] [Google Scholar]
- 4.Immink RV, van den Born BJ, van Montfrans GA, et al. Impaired cerebral autoregulation in patients with malignant hypertension. Circulation 2004; 110: 2241–2245. [DOI] [PubMed] [Google Scholar]
- 5.Aries MJ, Elting JW, De Keyser J, et al. Cerebral autoregulation in stroke: a review of transcranial Doppler studies. Stroke 2010; 41: 2697–2704. [DOI] [PubMed] [Google Scholar]
- 6.Panerai RB. Cerebral autoregulation: from models to clinical applications. Cardiovasc Eng 2008; 8: 42–59. [DOI] [PubMed] [Google Scholar]
- 7.Aaslid R, Lindegaard KF, Sorteberg W, et al. Cerebral autoregulation dynamics in humans. Stroke 1989; 20: 45–52. [DOI] [PubMed] [Google Scholar]
- 8.Simpson D, Claassen J. CrossTalk opposing view: dynamic cerebral autoregulation should be quantified using induced (rather than spontaneous) blood pressure fluctuations. J Physiol 2018; 596: 7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzeng YC, Panerai RB. CrossTalk proposal: dynamic cerebral autoregulation should be quantified using spontaneous blood pressure fluctuations. J Physiol 2018; 596: 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiecks FP, Lam AM, Aaslid R, et al. Comparison of static and dynamic cerebral autoregulation measurements. Stroke 1995; 26: 1014–1019. [DOI] [PubMed] [Google Scholar]
- 11.Panerai RB, White RP, Markus HS, et al. Grading of cerebral dynamic autoregulation from spontaneous fluctuations in arterial blood pressure. Stroke 1998; 29: 2341–2346. [DOI] [PubMed] [Google Scholar]
- 12.Atkins ER, Brodie FG, Rafelt SE, et al. Dynamic cerebral autoregulation is compromised acutely following mild ischaemic stroke but not transient ischaemic attack. Cerebrovasc Dis 2010; 29: 228–235. [DOI] [PubMed] [Google Scholar]
- 13.Salinet AS, Panerai RB, Robinson TG. The longitudinal evolution of cerebral blood flow regulation after acute ischaemic stroke. Cerebrovasc Dis Extra 2014; 4: 186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Claassen JA, Meel-van den Abeelen AS, Simpson DM, et al. Transfer function analysis of dynamic cerebral autoregulation: a white paper from the International Cerebral Autoregulation Research Network. J Cereb Blood Flow Metab 2016; 36: 665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castro P, Azevedo E, Sorond F. Cerebral autoregulation in stroke. Curr Atheroscler Rep 2018; 20: 37. [DOI] [PubMed] [Google Scholar]
- 16.Minhas JS, Panerai RB, Ghaly G, et al. Cerebral autoregulation in hemorrhagic stroke: a systematic review and meta-analysis of transcranial Doppler ultrasonography studies. J Clin Ultrasound 2019; 47: 14–21. [DOI] [PubMed] [Google Scholar]
- 17.Giller CA. The frequency-dependent behavior of cerebral autoregulation. Neurosurgery 1990; 27: 362–368. [DOI] [PubMed] [Google Scholar]
- 18.Beishon L, Haunton VJ, Panerai RB, et al. Cerebral hemodynamics in mild cognitive impairment: a systematic review. J Alzheimers Dis 2017; 59: 369–385. [DOI] [PubMed] [Google Scholar]
- 19.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions, 5.1.0 ed London: The Cochrane Collaboration, 2011. [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 21.Deeks JJ, Higgins JP. Statistical algorithms in review manager 5, London: The Cochrane Collaboration, 2010. [Google Scholar]
- 22.Green S and Higgins J. Cochrane handbook for systematic reviews of interventions. 4.2.5 ed. London: The Cochrane Collaboration, 2005.
- 23.Guo ZN, Liu J, Xing Y, et al. Dynamic cerebral autoregulation is heterogeneous in different subtypes of acute ischemic stroke. PLoS One 2014; 9: e93213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma H, Guo ZN, Jin H, et al. Preliminary study of dynamic cerebral autoregulation in acute ischemic stroke: association with clinical factors. Front Neurol 2018; 9: 1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saeed NP, Panerai RB, Horsfield MA, et al. Does stroke subtype and measurement technique influence estimation of cerebral autoregulation in acute ischaemic stroke? Cerebrovasc Dis 2013; 35: 257–261. [DOI] [PubMed] [Google Scholar]
- 26.Castro P, Azevedo E, Serrador J, et al. Hemorrhagic transformation and cerebral edema in acute ischemic stroke: link to cerebral autoregulation. J Neurol Sci 2017; 372: 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castro P, Serrador JM, Rocha I, et al. Efficacy of cerebral autoregulation in early ischemic stroke predicts smaller infarcts and better outcome. Front Neurol 2017; 8: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Liu J, Xu WH, et al. Impaired dynamic cerebral autoregulation and cerebrovascular reactivity in middle cerebral artery stenosis. PLoS One 2014; 9: e88232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chi NF, Hu HH, Wang CY, et al. Dynamic cerebral autoregulation is an independent functional outcome predictor of mild acute ischemic stroke. Stroke 2018; 49: 2605–2611. [DOI] [PubMed] [Google Scholar]
- 30.Diehl RR, Linden D, Lucke D, et al. Phase relationship between cerebral blood-flow velocity and blood-pressure – a clinical-test of autoregulation. Stroke 1995; 26: 1801–1804. [DOI] [PubMed] [Google Scholar]
- 31.Fritzsch C, Rosengarten B, Guschlbauer B, et al. Neurovascular coupling and cerebral autoregulation in patients with stenosis of the posterior cerebral artery. J Neuroimaging 2010; 20: 368–372. [DOI] [PubMed] [Google Scholar]
- 32.Gong X, Liu J, Dong P, et al. Assessment of dynamic cerebral autoregulation in patients with basilar artery stenosis. PLoS One 2013; 8: e77802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwan J, Lunt M, Jenkinson D. Assessing dynamic cerebral autoregulation after stroke using a novel technique of combining transcranial Doppler ultrasonography and rhythmic handgrip. Blood Press Monit 2004; 9: 3–8. [DOI] [PubMed] [Google Scholar]
- 34.Petersen NH, Ortega-Gutierrez S, Reccius A, et al. Dynamic cerebral autoregulation is transiently impaired for one week after large-vessel acute ischemic stroke. Cerebrovasc Dis 2015; 39: 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reinhard M, Roth M, Guschlbauer B, et al. Dynamic cerebral autoregulation in acute ischemic stroke assessed from spontaneous blood pressure fluctuations. Stroke 2005; 36: 1684–1689. [DOI] [PubMed] [Google Scholar]
- 36.Reinhard M, Rutsch S, Lambeck J, et al. Dynamic cerebral autoregulation associates with infarct size and outcome after ischemic stroke. Acta Neurol Scand 2012; 125: 156–162. [DOI] [PubMed] [Google Scholar]
- 37.Reinhard M, Wihler C, Roth M, et al. Cerebral autoregulation dynamics in acute ischemic stroke after rtPA thrombolysis. Cerebrovasc Dis 2008; 26: 147–155. [DOI] [PubMed] [Google Scholar]
- 38.Salinet AS, Robinson TG, Panerai RB. Effects of cerebral ischemia on human neurovascular coupling, CO2 reactivity, and dynamic cerebral autoregulation. J Appl Physiol 2015; 118: 170–177. [DOI] [PubMed] [Google Scholar]
- 39.Truijen J, Rasmussen LS, Kim YS, et al. Cerebral autoregulatory performance and the cerebrovascular response to head-of-bed positioning in acute ischaemic stroke. Eur J Neurol 2018; 25: 1365–e1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tutaj M, Miller M, Krakowska-Stasiak M, et al. Dynamic cerebral autoregulation is compromised in ischaemic stroke of undetermined aetiology only in the non-affected hemisphere. Neurol Neurochir Pol 2014; 48: 91–97. [DOI] [PubMed] [Google Scholar]
- 41.Wang S, Guo ZN, Xing Y, et al. Dynamic cerebral autoregulation in asymptomatic patients with unilateral middle cerebral artery stenosis. Medicine 2015; 94: e2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lang EW, Diehl RR, Mehdorn HM. Cerebral autoregulation testing after aneurysmal subarachnoid hemorrhage: the phase relationship between arterial blood pressure and cerebral blood flow velocity. Crit Care Med 2001; 29: 158–163. [DOI] [PubMed] [Google Scholar]
- 43.Ma H, Guo ZN, Liu J, et al. Temporal course of dynamic cerebral autoregulation in patients with intracerebral hemorrhage. Stroke 2016; 47: 674–681. [DOI] [PubMed] [Google Scholar]
- 44.Ma H, Guo ZN, Sun X, et al. Hematoma volume is a predictive factor of disturbed autoregulation after spontaneous intracerebral hemorrhage. J Neurol Sci 2017; 382: 96–100. [DOI] [PubMed] [Google Scholar]
- 45.Nakagawa K, Serrador JM, LaRose SL, et al. Dynamic cerebral autoregulation after intracerebral hemorrhage: a case-control study. BMC Neurol 2011; 11: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oeinck M, Neunhoeffer F, Buttler KJ, et al. Dynamic cerebral autoregulation in acute intracerebral hemorrhage. Stroke 2013; 44: 2722–2728. [DOI] [PubMed] [Google Scholar]
- 47.Otite F, Mink S, Tan CO, et al. Impaired cerebral autoregulation is associated with vasospasm and delayed cerebral ischemia in subarachnoid hemorrhage. Stroke 2014; 45: 677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gommer ED, Staals J, van Oostenbrugge RJ, et al. Dynamic cerebral autoregulation and cerebrovascular reactivity: a comparative study in lacunar infarct patients. Physiol Meas 2008; 29: 1293–1303. [DOI] [PubMed] [Google Scholar]
- 49.Guo ZN, Xing Y, Wang S, et al. Characteristics of dynamic cerebral autoregulation in cerebral small vessel disease: diffuse and sustained. Sci Rep 2015; 5: 15269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muller M, Osterreich M, von Hessling A, et al. Incomplete recovery of cerebral blood flow dynamics in sufficiently treated high blood pressure. J Hypertens 2019; 37: 372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Immink RV, van Montfrans GA, Stam J, et al. Dynamic cerebral autoregulation in acute lacunar and middle cerebral artery territory ischemic stroke. Stroke 2005; 36: 2595–2600. [DOI] [PubMed] [Google Scholar]
- 52.Xiong L, Tian G, Lin W, et al. Is dynamic cerebral autoregulation bilaterally impaired after unilateral acute ischemic stroke? J Stroke Cerebrovasc Dis 2017; 26: 1081–1087. [DOI] [PubMed] [Google Scholar]
- 53.Meel-van den Abeelen AS, van Beek AH, Slump CH, et al. Transfer function analysis for the assessment of cerebral autoregulation using spontaneous oscillations in blood pressure and cerebral blood flow. Med Eng Phys 2014; 36: 563–575. [DOI] [PubMed] [Google Scholar]
- 54.Panerai RB. Nonstationarity of dynamic cerebral autoregulation. Med Eng Phys 2014; 36: 576–584. [DOI] [PubMed] [Google Scholar]
- 55.Xi G, Keep RF, Hoff JT. Pathophysiology of brain edema formation. Neurosurg Clin N Am 2002; 13: 371–383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Assessment of cerebral autoregulation in stroke: A systematic review and meta-analysis of studies at rest by Kannakorn Intharakham, Lucy Beishon, Ronney B Panerai, Victoria J Haunton and Thompson G Robinson in Journal of Cerebral Blood Flow & Metabolism