Abstract
A hallmark of non-rapid eye movement (REM) sleep is the decreased brain activity as measured by global reductions in cerebral blood flow, oxygen metabolism, and glucose metabolism. It is unknown whether the blood oxygen level dependent (BOLD) signal undergoes similar changes. Here we show that, in contrast to the decreases in blood flow and metabolism, the mean global BOLD signal increases with sleep depth in a regionally non-uniform manner throughout gray matter. We relate our findings to the circulatory and metabolic processes influencing the BOLD signal and conclude that because oxygen consumption decreases proportionately more than blood flow in sleep, the resulting decrease in paramagnetic deoxyhemoglobin accounts for the increase in mean global BOLD signal.
Keywords: Global signal, sleep, fMRI BOLD, oxyhemoglobin dissociation curve, Bohr effect
Introduction
The descent from wakefulness to slow wave sleep is accompanied by global reductions in cerebral blood flow, oxygen metabolism (Table 1) and glucose metabolism as measured with the Kety–Schmidt technique1–3 and positron emission tomography.4–9 These methods utilize direct sampling from arterial and cerebral venous catheters10 or a modeled function11–14 to assess physiological changes. In contrast, the blood oxygen level dependent (BOLD) signal measures the concentration of deoxyhemoglobin which is paramagnetic and under the combined influence of blood flow and oxygen consumption.15–20 Furthermore, oxygen delivery to the brain is not only under the influence of blood flow but also the dynamics of oxygen dissociation within hemoglobin,21 a remarkably precise process whereby the brain fine tunes its oxygenation to its metabolic needs.22
Table 1.
Change in OEF, CBF, and CMRO2 during sleep.
| Study | Sleep depth | (Sleep − Wake)/Wake × 100% |
||
|---|---|---|---|---|
| OEF | CBF | CMRO2 | ||
| Mangold et al.72 | Sleep spindles and delta waves (six subjects) | −10.7 | 9.8 | −2.8 |
| Madsen et al.3 | Slow wave sleep (eight subjects) Slow wave and light sleep (one subject) | −7.3 | −18.6 | −24.6 |
| Madsen et al.2 | Light sleep (six subjects) Slow wave and light sleep (two subjects) | −5.6 | 0.7 | −4.9 |
| Boyle et al.1 | Light and slow wave sleep (seven subjects) REM (one subject) | −5.6 | −11.3 | −16.3 |
OEF: oxygen extraction fraction; CBF: cerebral blood flow; CMRO2: cerebral metabolic rate of oxygen.
A continued source of controversy in resting-state BOLD functional magnetic resonance imaging (fMRI) has been the whole brain mean signal, termed the global signal, as its removal affects the topography among and relationship between the brain’s systems in studies of intrinsic functional connectivity.23,24 Although the global signal includes nonneuronal components reflecting cardiac signals,25 respiratory signals,26 and head movement,27 simultaneous electrophysiological and fMRI recordings in nonhuman primates suggest the presence of an important neuronal component.28,29
Recent work has found that hemispherical differences in the global BOLD signal’s spontaneous fluctuations can be exploited to map the human brain’s lateralized organization30 which changes during non-REM sleep.31 In addition, the amplitude of the global BOLD signal’s spontaneous fluctuations is modulated by arousal,29 vigilance,32 sleep depth,31 and sleep deprivation.33 By examining changes in the spontaneous fluctuations, these previous studies examined changes in variance. Mean global signal changes were not examined.
BOLD studies of non-REM sleep have investigated spontaneous fluctuations extensively, including changes in amplitude,31,34,35 temporal dynamics,36–38 and functional connectivity,31,39–45 but the mean signal which accompanies these fluctuations has yet to be explored. Do tonic changes in the global signal mirror the reported metabolic decreases? To answer that question, we extracted the global component of the ongoing BOLD signal and show that the mean global signal in gray matter increases significantly with sleep depth.
Material and methods
Subjects, EEG-fMRI acquisition, and artifact correction
The experiments were conducted according to the Helsinki Declaration of 1975 (and as revised in 1983) and were approved by the Ethik-Kommission des Fachbereichs Medizin der Goethe-Universität Frankfurt am Main (Geschaefts-Nr.: 305/07). Written informed consent was obtained from all subjects. Seventy-one right-handed (mean ± SD age 24.3 ± 4.7 years, range 19–48, 44 females) non-sleep-deprived subjects were scanned in the evening at ∼8:00 PM. Subjects were instructed to close their eyes and lie still and relaxed. No specific instruction was given to fall asleep or maintain wakefulness. Acquisition parameters and details for these data have been published previously.46 Briefly, fMRI was acquired in a single 52.2 min scan using a 3 T Siemens Trio (Erlangen, Germany) (1,505 volumes of T2*-weighted echo planar images, TR/TE = 2.080 ms/30 ms, matrix 64 × 64, voxel size 3 × 3 × 2 mm3, distance factor 50%; FOV 192 mm2) with an optimized polysomnographic setting (chin and tibial EMG, ECG, EOG recorded bipolarly—sampling rate 5 kHz, low-pass filter 1 kHz) simultaneously with 30 electroencephalographic (EEG) channels recorded via a cap (modified BrainCapMR, Easycap, Herrsching, Germany; 5 kOhm safety resistors in all electrodes) with FCz as the reference (sampling rate 5 kHz, low-pass filter 250 Hz, high pass filter 0.016 Hz) using MR compatible amplifiers (BrainAmp MR+, BrainAmp ExG; Brain Products, Gilching, Germany), pulse oximetry, and respiration recorded via sensors from the Trio (sampling rate 50 Hz). The threshold for electrode impedances was 35 kOhm, though impedances typically remained below 30 kOhm. MRI and pulse artifact correction were performed based on the average artifact subtraction method47 as implemented in Vision Analyzer2 (Brain Products, Germany) followed by independent component analysis based rejection of residual artifact components (Cardioballistogram Correction parameters; Vision Analyzer). Sleep stages were scored in 30 s epochs by an expert according to the American Academy of Sleep Medicine criteria.48
Image preprocessing
Image preprocessing included the following steps: (1) compensation for slice-dependent time shifts, (2) elimination of odd/even slice intensity differences due to interleaved acquisition, and (3) realignment of all data acquired in each subject within and across runs to compensate for rigid body motion.49 The functional data were transformed into atlas space50 by computing a sequence of affine transformations (first frame of BOLD run to MP-RAGE to atlas representative target without compensation for local distortions between echo planar images and anatomy) which were combined by matrix multiplication, resampling to a 2 mm isotropic grid. For cross-modal (i.e. functional to structural) image registration, a locally developed algorithm was used.51
Modeling and extracting the global signal
The BOLD time series was modeled with a general linear model that included terms for the global signal (global), white matter (WM), and ventricles (CSF: cerebrospinal fluid) that were separated by sleep stage (W, N1, N2, and N3) and whether the scored epoch was less than 60 s (short and long). The global signal regressor was formed from a whole brain mask that included the cerebellum, WM, and ventricles but not extra-axial CSF, while WM and ventricular regressors were formed from each individual’s eroded WM and ventricular masks.52 Regressors were computed by removing the linear trend and constant at each voxel, averaging over voxels within the respective mask, separated into scored epochs then assigned to the appropriate short regressor if the epoch was less than 60 s or long if otherwise. Although the general linear model included regressors for all sleep stages, during a given epoch as determined by the scoring, only a single regressor had values representing the BOLD fluctuations of that region (i.e. global, WM, and CSF), while the others were set to zero. For example, during an epoch scored as “wakefulness,” the global, WM, and CSF “wakefulness” regressors held nonzero values, while the N1, N2, and N3 sleep regressors held zeros. Thus, the regressors for the different stages were orthogonal. This is clarified in Figure 1 which shows a portion of a design matrix.
Figure 1.
Two portions of a design matrix from a single subject illustrating the layout of the global, white matter and CSF regressors and their separation into long and short epochs. This subject lacked a wakefulness epoch less than 60s hence the omission of the Wshort regressor. Time increases downward. Solid gray areas are zero valued. Vertical blocks with changing grayscale contrast reflect varying signal intensity and the extent of the regressor. The solid white horizontal block separates the two portions.
Subject-specific general linear models53 were fit to BOLD time series at each voxel as shown schematically below
| (1) |
where the notation represents eight terms. Head movement was accounted for by motion, 24 time series formed from measured head shifts and angular displacements in three dimensions (i.e. X, Y, Z, pitch, yaw, and roll) along with their squares, derivatives, and squared derivatives.54 The low-pass filter was implemented with a Fourier basis set that consisted of sine and cosine pairs modeling full cycles from the cutoff of 0.08 Hz to the Nyquist frequency,55,56 thus low-pass filtering was accomplished with the inclusion of high-frequency terms. Additional terms included the constant c0 and linear trend to model slow drifts. The partial correlation model of equation (1) was solved via ordinary least squares yielding estimated weights for all regressors.
The global signal was extracted from the BOLD time series by regressing all terms in equation (1) except those pertaining to the global signal.
The mean global signal was computed by averaging over all volumes that were part of a long epoch, then normalizing by the constant c0. For example, the mean global signal for wakefulness was
where the notation represents a volume from a wakefulness epoch of at least 60 s duration and is the total number of wakefulness volumes from epochs of at least 60 s duration. We emphasize that our measure of mean global signal is not an absolute measure, rather relative to the four stages of non-REM sleep.
Considering each stage, all 71 subjects had at least a single long epoch (≥60 s) of wakefulness, 58 subjects had at least a single long epoch of N1 sleep, 42 subjects had at least a single long epoch of N2 sleep, and 19 subjects had at least a single long epoch of N3 sleep. Sufficient short epochs were not present for further analysis.31 For voxelwise analyses, this was followed by spatial smoothing with a 4 mm full width at half maximum three-dimensional Gaussian kernel to blur individual differences in brain anatomy.
Mean global signal: Voxelwise direct statistical comparisons of differential effects
Since only 19 of the 71 subjects were observed with at least a single long epoch for each sleep stage, the direct statistical comparison of the mean global signal with sleep depth over all 71 subjects was one of unbalanced data that were solved with a linear mixed effects model.57 This random intercept model (i.e. different intercepts for each subject) implements an analysis of variance (ANOVA) with a random factor of subjects at 71 levels and a fixed factor of sleep stage at 4 levels (W, N1, N2, and N3). This analysis produced an F statistic for the main effect of sleep stage reflecting the direct comparison of the four stages of non-REM sleep. The F statistic was z-transformed and corrected for multiple comparisons (|z| ≥ 3.0, minimum 45 face connected voxels, P < 0.05 corrected) with a Monte Carlo-based method.58,59
Mean global signal: Principal component analysis
Group averaged mean global signal maps were formed for each sleep stage. These maps comprised the four features (W, N1, N2, and N3) with all significant voxels from the main effect of sleep stage as the observations for a principal component analysis. The first component was then projected back on the brain by computing the inner product with the group averaged mean global signal maps at each voxel.
Minimally processed signal
Subject-specific general linear models53 were fit to the BOLD time series at each voxel with a model that included only the constant c0 and linear trend to model slow drifts. The minimally processed signal was extracted from the BOLD time series by regressing both these terms, then normalizing by the constant c0.
We wished to compare the mean global signal which is modeled with a complete set of terms (equation (1)) considered appropriate for the analysis of resting-state data52 to a minimally processed signal that lacks the regression of WM, ventricular and motion signals along with the filtering of signals above 0.08 Hz. For the latter, respiratory and cardiac fluctuations are aliased through the entire frequency spectrum of the BOLD signal, however, above 0.08 Hz the amplitude of these aliased signals is greatly increased.56 Thus, the minimally processed signal includes a greater contribution from nonneuronal sources.60
Global-signal-regressed signal
The global-signal-regressed (GSR) signal was extracted from the BOLD time series by regressing all terms in equation (1) including the global signal. This signal is equivalent to that commonly used in resting-state functional connectivity studies.52 The statistical analysis of the GSR signal paralleled that of the global signal.
Post hoc comparisons: Regional direct statistical comparisons of differential effects
Post hoc comparisons included three regional group level linear mixed effects analyses: wakefulness vs. N1 sleep (71 subjects), N2 vs. N2 sleep (58 subjects), and N2 vs. N3 sleep (42 subjects). Use of linear mixed effects allows the analysis of all subjects even if they experienced only one of the two stages under comparison (i.e. unbalanced data).57 All analyses used a random intercept model (i.e. different intercepts for each subject) implementing an ANOVA with a random factor of subjects and a fixed factor of sleep stage at the two levels under comparison.
If the region was defined from a different mean signal, then a group-level linear mixed effects analysis over all four stages of non-REM sleep (71 subjects) was initially computed, and the three pairwise post hoc comparisons were performed only if the direct comparison of all four sleep stages was significant. For example, regions defined from the voxelwise, multiple comparisons corrected main effect of sleep stage of the mean global signal were applied to the mean minimally processed signal. Similarly, regions defined from the voxelwise, multiple comparisons corrected main effect of sleep stage of the mean GSR signal were applied to the mean global and minimally processed signals.
Results
Mean global signal and sleep depth
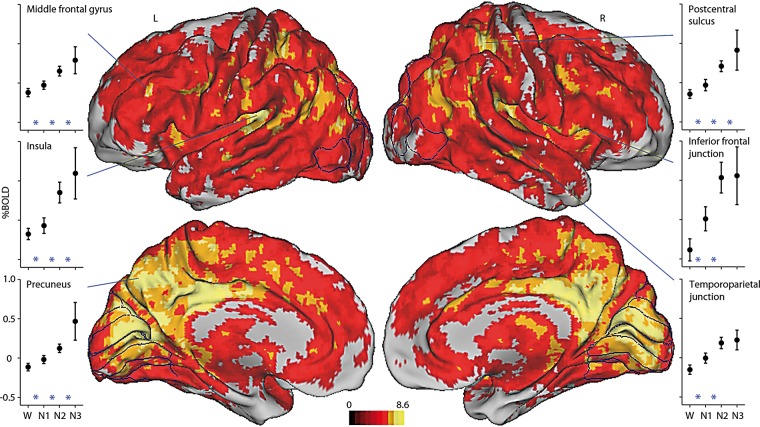
Our null hypothesis is that any changes in mean global signal with sleep depth are within the noise of the BOLD signal. This was tested with direct statistical comparison of mean global signal values across wakefulness, N1, N2, and N3 sleep via a group level linear mixed effects analysis.57 Figure 2 shows the main effect of sleep stage highlighting regions with changes in mean global signal that differ significantly across the four stages. The group averaged mean signal levels for each stage are plotted. The mean global signal increases with sleep depth over most of gray matter. These increases are not absolute measures but relative to the four stages of non-REM sleep.
Figure 2.
The mean global signal increases with sleep depth. Direct statistical comparison of the mean global signal over the four non-REM sleep stages. Shown is the main effect of sleep stage from a group level linear mixed effects analysis of 71 subjects. Displayed regions exhibit significant mean global signal changes across W, N1, N2, and N3 sleep. Color bar reflects the value of gaussianized F statistics corrected for multiple comparisons (P < 0.05). Lateral surfaces are displayed in the first row and medial surfaces in the second row. Highlighted regions include the middle frontal gyrus, insula, and precuneus in the left hemisphere, and postcentral sulcus, inferior frontal junction, and temporoparietal junction in the right hemisphere. Plotted are group averaged mean global signals for W (71 subjects), N1 sleep (58 subjects), N2 sleep (42 subjects), and N3 sleep (19 subjects). Percent BOLD signal was computed for each subject by normalizing to the value of the constant term, then multiplying by 100. Error bars reflect the 95% confidence interval of the means. Included in the plots are three regional post hoc, group-level linear mixed effects analyses: W vs. N1 (71 subjects), N1 vs. N2 (58 subjects), and N2 vs. N3 (42 subjects), with * indicating P < 0.05 for the two stages it is placed between.
Principal component analysis of the mean global signal
We next sought to quantify these changes in mean signal by performing a principal component analysis across the four stages with each significant voxel from the main effect of sleep stage serving as an observation. The first principal component recapitulated the observed signal increases from wakefulness through N3 sleep, accounting for 83.9% of the variance. Figure 3 shows the projection of the first component. While medial and temporal cortical regions map positively onto the first component, ventricular regions map negatively. This mapping results from increasing mean global signals in cortical regions, consistent with those plotted in Figure 2 and decreases in ventricular regions.61 In the last column, minimally processed mean signals are plotted. Reduced increases and decreases are observed for all four regions compared to the mean global signals.
Figure 3.
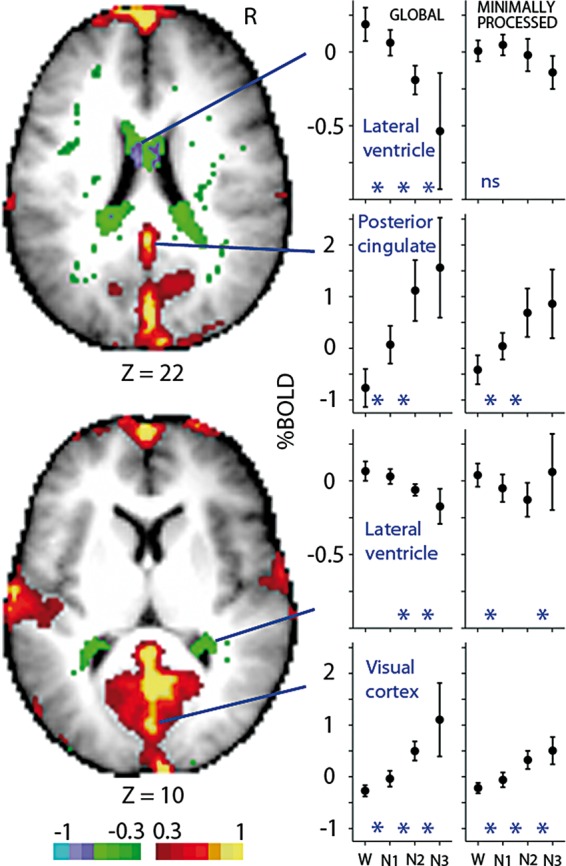
Principal component analysis of the mean global signal. The first component accounts for 83.9% of the variance attributed to modulation of the mean global signal by sleep depth. Shown in the axial slices is the projection of the first principal component. Color bar reflects the inner product of the first principal component and the group averaged mean global signal of each sleep stage. Images have been thresholded to highlight regions most strongly correlated with the first principal component. Highlighted are two cortical and ventricular regions. Plotted are mean global and minimally processed (last column) signals reflecting group averages of 71 subjects (W), 58 subjects (N1), 42 subjects (N2), and 19 subjects (N3). In contrast to cortex, the mean global signal decreases with sleep depth in ventricular regions. Percent BOLD signal was computed for each subject by normalizing to the value of the constant term, then multiplying by 100. Error bars reflect the 95% confidence interval of the means. Included in the plots are three regional post hoc, group-level linear mixed effects analyses: W vs. N1 (71 subjects), N1 vs. N2 (58 subjects) and N2 vs. N3 (42 subjects), with * indicating P < 0.05 for the two stages it is placed between. ns, main effect of sleep stage not significant (P > 0.05).
Mean GSR signal and sleep depth
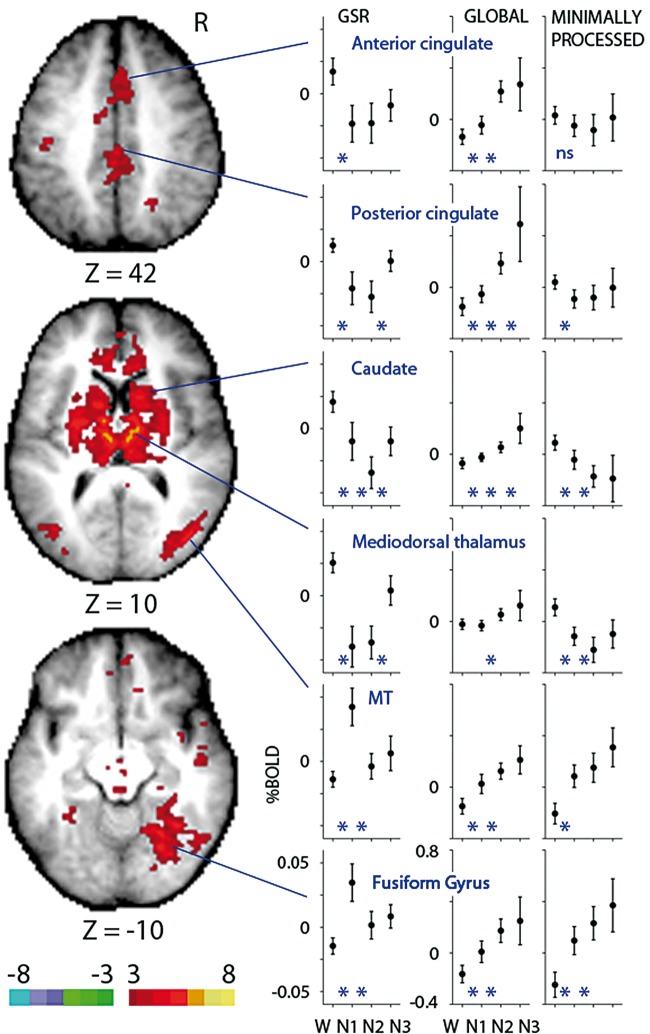
It is possible that the observed global signal increases in gray matter are dominated by much larger local effects. All terms of equation (1) were regressed including those of the global signal. We tested the null hypothesis that changes in this mean GSR signal with sleep depth are within the noise of the BOLD signal with a group-level linear mixed effects analysis.57 Figure 4 shows the main effect of sleep stage highlighting regions with significant mean GSR signal changes across the four stages. Group-averaged GSR, global and minimally processed signals are plotted. The mean GSR signal is somewhat variable from state to state decreasing from wakefulness in midline and subcortical regions and increasing in extrastriate regions. In contrast, the mean global signal is remarkably consistent across regions as evidenced by increases with sleep depth that are an order of magnitude larger than their GSR counterparts.
Figure 4.
The mean global-signal-regressed (GSR) signal decreases with sleep depth in midline and subcortical regions and increases in extrastriate regions. Direct statistical comparison of the mean GSR signal over the four non-REM sleep stages. The main effect of sleep stage from a group-level linear mixed effects analysis of 71 subjects is shown. Displayed regions exhibit significant mean GSR signal changes across W, N1, N2, and N3 sleep. Color bar reflects the value of gaussianized F statistics corrected for multiple comparisons (P < 0.05). Highlighted regions include the anterior cingulate (first row), posterior cingulate, caudate, mediodorsal thalamus, MT and fusiform gyrus (last row). Plotted are mean GSR, global and minimally processed (last column) signals reflecting group averages of 71 subjects (W), 58 subjects (N1), 42 subjects (N2) and 19 subjects (N3). Note different ordinate scales between GSR and global/minimally processed signals. Percent BOLD signal was computed for each subject by normalizing to the value of the constant term, then multiplying by 100. Error bars reflect the 95% confidence interval of the means. Included in the plots are three regional post hoc, group-level linear mixed effects analyses: W vs. N1 (71 subjects), N1 vs. N2 (58 subjects) and N2 vs. N3 (42 subjects), with * indicating P < 0.05 for the two stages it is placed between. ns: main effect of sleep stage not significant (P > 0.05).
Although magnitudes are directly comparable between GSR and global signals, one must be cautious with comparison to the minimally processed signal (Figures 3 and 4). Unlike the GSR and global signals which are normalized to the same constant weight from equation (1), the constant weight used for normalization of the minimally processed signal is estimated by a reduced model that adds only a linear trend term.
Discussion
Global blood flow and oxygen metabolism in non-REM sleep
During non-REM sleep compared to wakefulness, measurements of global brain blood flow and oxygen consumption have consistently demonstrated that oxygen consumption, which always decreases, does so to a greater extent than blood flow (Table 1). The consequence of this is a decrease in the fraction of oxygen removed from flowing blood in the brain, the oxygen extraction fraction. As a result, the concentration of oxygen-depleted red blood cells in the brain vasculature decreases, thus the concentration of deoxyhemoglobin decreases. Since the BOLD signal is biophysically a measurement of local deoxyhemoglobin concentration,21 a decrease in deoxyhemoglobin compared to oxyhemoglobin results in an increase in BOLD signal, while an increase in deoxyhemoglobin compared to oxyhemoglobin results in a decrease in BOLD signal. Another way to think of this is that a decrease in BOLD signal reflects a greater proportion of oxygen-depleted red blood cells, while an increase in BOLD signal reflects a lesser proportion of oxygen-depleted red blood cells.
While regional brain blood flow and oxygen consumption are highly correlated in awake, resting, adult human subjects62,63 that relationship can vary depending upon the circumstances under which it is measured. For example, task-induced regional activity in the human brain is accompanied by an increase in blood flow without an increase in oxygen consumption,64,65 an observation that provided the basis for fMRI.15–18 Furthermore, while anoxia can render a person unconscious within seconds,66 the blood flow response of the human brain to graded hypoxia is a threshold phenomenon which is not initiated until the arterial oxygen tension has reached approximately 50% of its normal level.67 Under such circumstances, the brain relies first on its ability to increase its oxygen extraction from circulating hemoglobin (i.e. oxygen rich red blood cells). During non-REM sleep alveolar ventilation decreases,68–71 however, the resulting hypoxia,69 if any,68,72 is likely insufficient to initiate a blood flow response in normal, healthy individuals.73 Moreover, decreased alveolar ventilation predicts a decrease in the evoked respiratory signal74,75 as breathing becomes shallower and a corresponding decrease in mean global BOLD signal.
Another feature of sleep-induced alveolar under-ventilation68–71 is an increase in alveolar carbon dioxide tension.68,70,76 Abundant evidence accumulated over many years has established the exquisite sensitivity of the brain vasculature to changes in arterial carbon dioxide tension,77,78 which predictably affects the BOLD signal,21 but the only measurements we have of arterial carbon dioxide tension during sleep indicate that there is no change.72 The acidosis,68 if any,72 from increased alveolar carbon dioxide tension predict a decrease in mean global BOLD signal (Figure 5).
Figure 5.
Oxyhemoglobin dissociation curve. As the brain moves from wakefulness to N3 sleep, carbon dioxide production drops, increasing the pH of capillary blood, a leftward shift of the oxyhemoglobin dissociation curve by the Bohr effect and an increase in the ratio of oxyhemogloblin to deoxyhemoglobin as less oxygen is extracted from the alkaline erythrocyte resulting in an increase in mean global BOLD signal.
A more complete way to understand the increase in the mean global BOLD signal is to examine events likely occurring in the capillary. Carbon dioxide entering the capillary from brain oxidative metabolism is hydrated in erythrocytes (i.e. red blood cells). In the process, hydrogen ions are produced reducing the pH of the erythrocytes, which in turn decreases the oxygen affinity of hemoglobin facilitating the release of oxygen to the tissue.79 When brain oxidative metabolism decreases, as it does in non-REM sleep, this process is reversed: the amount of carbon dioxide produced by the brain decreases leading to an increase in erythrocyte pH as governed by the Henderson-Hasselbalch equation80 and increased oxygen affinity of hemoglobin resulting in a leftward shift in the oxyhemoglobin dissociation curve (Figure 5). As a result, deoxyhemoglobin decreases and the BOLD signal increases. The importance of this process is dramatically demonstrated when the critical enzyme responsible for carbon dioxide hydration, carbonic anhydrase, is inhibited. This causes an immediate increase in brain blood flow and a decrease in oxygen consumption.81 Accompanying the sleep-induced changes attenuating oxygen release from hemoglobin is an accompanying increase in brain pH due to a reduction in metabolically produced carbon dioxide. The brain compensates for this by reducing its blood flow in order to maintain pH homeostasis.82
It is important to remember that while the body rests on the flat part of the oxyhemoglobin dissociation curve68 where it suffers little affect from small changes in blood pH, as it should to robustly maintain a high level of oxygen saturation, the brain resides on the steep portion83 where brain oxygenation is precisely regulated.84 The brain fine tunes oxygen delivery at the capillary level by simply adjusting the extraction of oxygen from the circulating blood taking advantage of the oxyhemoglobin dissociation curve’s steepness and the Bohr effect.79 This keeps tissue levels of oxygen at an optimum regardless of whether it is oversupplied, as is the case of non-REM sleep, or undersupplied when the blood supply is compromised or the environmental oxygen falls (e.g. during a sojourn at altitude). With increasing depth of non-REM sleep, the brain’s demand for oxygen drops resulting in decreased oxygen extraction (Table 1),1–3,72 decreased capillary deoxyhemoglobin, and an increase in the global BOLD signal.
Dissociated gray matter and ventricular signals
In contrast to the mean global BOLD signal increases in gray matter, decreases with sleep depth were observed in large vessels (Figure 3). This dissociation between gray matter and ventricular regions is apparent even with the minimally processed signal (Figure 3) suggesting it is not a result of modelling (equation (1)). As noted above, carbon dioxide is a potent cerebral vasodilator.77,78 Although measurements of arterial carbon dioxide tension found only a trend toward an increase in non-REM sleep,72 alveolar measurements have consistently reported significant increases.68,70,76 One may speculate that the increase in carbon dioxide tension leads to an increase in cerebral blood volume as a result of vasodilation.77 In gray matter (and throughout parenchyma), this cerebral blood volume increase is balanced by a decrease in the volume of brain tissue.61 The ventricles, however, lack brain tissue so the increase in cerebral blood volume is accommodated by a volume decrease in cerebrospinal fluid,61 which may, in part, shift to spinal regions.85 In summary, while we attribute mean global signal increases in gray matter to decreases in oxygen extraction, the decreases in ventricular regions we speculate reflect an increase in cerebral blood volume.86 Furthermore, if changes in gray matter were cogent with those in ventricular regions, this would suggest at least the partial influence of pulsatile effects from cardiac cycles.25
Mean GSR signal and non-REM sleep
Although an order of magnitude smaller than the mean global signal, the GSR signal separates into a midline/subcortical and an extrastriate pattern (Figure 4). Many of the thalamic and extrastriate regions have been previously identified from mean signal changes of simply opening and closing the eyes.87 While the mean BOLD signal decreases in thalamic regions upon eye closure, increases are seen in extrastriate cortex.87 Consistent with this dissociation are the GSR signal increases in extrastriate cortex and decreases in thalamic regions in non-REM sleep. Considering the midline subcortical regions, the thalamus has an important role in the electrophysiological signals that define the different stages of non-REM sleep,88 and the neighboring basal ganglia (e.g. caudate) are believed to play a significant role in regulating the sleep-wake cycle as damage is associated with disturbed sleep.89 Midline cortical regions may also have an important role in non-REM sleep as task state fMRI with simultaneous electrocardiography has implicated the anterior cingulate with state changes of the cardiovascular system during task performance compared to rest.90 Recent speculation concerning the dorsal posterior cingulate has suggested it has a prominent role in adjusting the global focus of the brain as modulated by arousal, attention, and environmental changes.91 In addition, decreased gray matter volume92 and increased regional cerebral blood flow during N3 sleep93 in posterior cingulate have been associated with sleep walking. Taken together, regions that show changes in mean GSR signal with sleep depth may interact with the whole brain (and body) in a way that promotes non-REM sleep. Future work should seek to establish the relationship between the global and GSR signal as this has the potential to illuminate the influence of individual areas on global brain function.
Conclusion
Our data suggest that mean changes in the global BOLD signal shed light on important brain physiology as represented by the relationship between cerebral blood flow and oxygen metabolism. Although these changes are not easily explained by head motion or respiratory effects, there is good evidence that the global signal includes contributions from these sources.52 Our method of modeling the global signal (Eq. 1) is unique in that the full complement of resting-state processing is implemented in a single regression rather than in a series of regressions and filtering steps,23,24,52 as a single regression will account for the greatest amount of variance.94 The focus of resting-state fMRI methodology has been how to process the data so that the so-called resting-state networks can be identified most effectively.23,24,52 In this context, the global signal has been eschewed as dominated by nonneuronal effects.24,52 Little effort has been devoted to process the global BOLD signal with the goal of uncovering meaningful physiology despite increasing evidence of important signals of neural origin.28,29
It is possible that the increases in mean global BOLD signal are just a curious observation limited to non-REM sleep, but the possibility also exists for a signal of great biological interest. Our global signal measure, although a relative one, can be used comparatively in a pseudo-quantitative manner given that the data were collected in a single scan. The most outstanding feature of Figure 2 is the broad swath bilaterally on the medial surfaces extending from posterior cingulate caudally into precuneus and ventrally into visual cortex. Posterior cingulate and precuneus are among the most metabolically active regions with blood flow and oxygen metabolism significantly greater than the brain’s global mean in wakeful rest.95 They are key regions in the so-called Default Mode Network,95 play an important role in memory96–99 and attentional91,100 processing and are currently of great interest in the study of neurodegenerative diseases, as atrophy96,101 hypometabolism,96,101–103 tau pathology,104,105 and amyloid deposition96,99,101,106,107 precede the rest of the brain. Curiously, dementia patients occasionally show greater pathology in visual cortex.105 Investigating whether the mean global BOLD signal changes with aging and disease progression, and its possible association with other measures would increase our knowledge of the underlying biology of both healthy aging and impaired cognition.
Acknowledgments
The authors thank Dmitriy A Yablonskiy and Anish Mitra for helpful discussions.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health (NINDS NS080675 to M.E.R.), Bundesministerium für Bildung und Forschung (01EV0703 to H.L. and E.T.), LOEWE Neuronale Koordination Forschungsschwerpunkt Frankfurt (H.L. and E.T.) and in part by the Neuroimaging Informatics and Analysis Center at Washington University led by Dan Marcus (1P30NS09857701).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and publication of this article.
Authors’ contributions
MPM analyzed the BOLD data. HL and ET designed and conducted the research and analyzed the EEG data. MPM, HL, and MER wrote the manuscript. All reviewed the manuscript.
References
- 1.Boyle PJ, Scott JC, Krentz AJ, et al. Diminished brain glucose metabolism is a significant determinant for falling rates of systemic glucose utilization during sleep in normal humans. J Clin Invest 1994; 93: 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madsen PL, Schmidt JF, Holm S, et al. Cerebral oxygen metabolism and cerebral blood flow in man during light sleep (stage 2). Brain Res 1991; 557: 217–220. [DOI] [PubMed] [Google Scholar]
- 3.Madsen PL, Schmidt JF, Wildschiodtz G, et al. Cerebral O2 metabolism and cerebral blood flow in humans during deep and rapid-eye-movement sleep. J Appl Physiol 1991; 70: 2597–2601. [DOI] [PubMed] [Google Scholar]
- 4.Braun AR, Balkin TJ, Wesenten NJ, et al. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain 1997; 120: 1173–1197. [DOI] [PubMed] [Google Scholar]
- 5.Buchsbaum MS, Gillin JC, Wu J, et al. Regional cerebral glucose metabolic rate in human sleep assessed by positron emission tomography. Life Sci 1989; 45: 1349–1356. [DOI] [PubMed] [Google Scholar]
- 6.Kajimura N, Uchiyama M, Takayama Y, et al. Activity of midbrain reticular formation and neocortex during the progression of human non-rapid eye movement sleep. J Neurosci 1999; 19: 10065–10073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nofzinger EA, Buysse DJ, Miewald JM, et al. Human regional cerebral glucose metabolism during non-rapid eye movement sleep in relation to waking. Brain 2002; 125: 1105–1115. [DOI] [PubMed] [Google Scholar]
- 8.Maquet P, Dive D, Salmon E, et al. Cerebral glucose utilization during sleep-wake cycle in man determined by positron emission tomography and [18F]2-fluoro-2-deoxy-D-glucose method. Brain Res 1990; 513: 136–143. [DOI] [PubMed] [Google Scholar]
- 9.Maquet P, Dive D, Salmon E, et al. Cerebral glucose utilization during stage 2 sleep in man. Brain Res 1992; 571: 149–153. [DOI] [PubMed] [Google Scholar]
- 10.Kety S, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure and normal values. J Clin Investig 1948; 27: 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su Y, Arbelaez AM, Benzinger TL, et al. Noninvasive estimation of the arterial input function in positron emission tomography imaging of cerebral blood flow. J Cereb Blood Flow Metab 2012; 33: 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sari H, Erlandsson K, Law I, et al. Estimation of an image derived input function with MR-defined carotid arteries in FDG-PET human studies using a novel partial volume correction method. J Cereb Blood Flow Metab 2017; 37: 1398–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khalighi MM, Deller TW, Fan AP, et al. Image-derived input function estimation on a TOF-enabled PET/MR for cerebral blood flow mapping. J Cereb Blood Flow Metab 2017; 38: 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Islam MM, Tsujikawa T, Mori T, et al. Estimation of arterial input by a noninvasive image derived method in brain H215O PET study: confirmation of arterial location using MR angiography. Phys Med Biol 2017; 62: 4514–4524. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa S, Tank DW, Menon R, et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A 1992; 89: 5951–5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frahm J, Bruhn H, Merboldt KD, et al. Dynamic MR imaging of human brain oxygenation during rest and photic stimulation. J Magn Reson Imaging 1992; 2: 501–505. [DOI] [PubMed] [Google Scholar]
- 17.Kwong KK, Belliveau JW, Chesler DA, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A 1992; 89: 5675–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bandettini PA, Wong EC, Hinks RS, et al. Time course EPI of human brain function during task activation. Magn Reson Med 1992; 25: 390–397. [DOI] [PubMed] [Google Scholar]
- 19.Mark CI, Mazerolle EL, Chen JJ. Metabolic and vascular origins of the BOLD effect: implications for imaging pathology and resting-state brain function. J Magn Reson Imaging 2015; 42: 231–246. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa S, Lee TM, Nayak AS, et al. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med 1990; 14: 68–78. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa S, Lee TM, Kay AR, et al. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A 1990; 87: 9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashi T, Watabe H, Kudomi N, et al. A theoretical model of oxygen delivery and metabolism for physiologic interpretation of quantitative cerebral blood flow and metabolic rate of oxygen. J Cereb Blood Flow Metab 2003; 23: 1314–1323. [DOI] [PubMed] [Google Scholar]
- 23.Weissenbacher A, Kasess C, Gerstl F, et al. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage 2009; 47: 1408–1416. [DOI] [PubMed] [Google Scholar]
- 24.Fox MD, Zhang D, Snyder AZ, et al. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 2009; 101: 3270–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shmueli K, van Gelderen P, de Zwart JA, et al. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. Neuroimage 2007; 38: 306–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wise RG, Ide K, Poulin MJ, et al. Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. Neuroimage 2004; 21: 1652–1664. [DOI] [PubMed] [Google Scholar]
- 27.Zeng L-L, Wang D, Fox MD, et al. Neurobiological basis of head motion in brain imaging. Proc Natl Acad Sci U S A 2014; 111: 6058–6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scholvinck ML, Maier A, Ye FQ, et al. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci U S A 2010; 107: 10238–10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, de Zwart JA, Scholvinck ML, et al. Subcortical evidence for a contribution of arousal to fMRI studies of brain activity. Nat Commun 2018; 9: 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McAvoy M, Mitra A, Coalson RS, et al. Unmasking language lateralization in human brain intrinsic activity. Cereb Cortex 2016; 26: 1733–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McAvoy M, Mitra A, Tagliazucchi E, et al. Mapping visual dominance in human sleep. NeuroImage 2017; 150: 250–261. [DOI] [PubMed] [Google Scholar]
- 32.Wong CW, Olafsson V, Tal O, et al. The amplitude of the resting-state fMRI global signal is related to EEG vigilance measures. Neuroimage 2013; 83: 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeo BTT, Tandi J, Chee WLC. Functional connectivity during rested wakefulness predicts vulnerability to sleep deprivation. NeuroImage 2015; 111: 147–158. [DOI] [PubMed] [Google Scholar]
- 34.Fukunaga M, Horovitz SG, van Gelderen P, et al. Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magn Reson Imaging 2006; 24: 979–992. [DOI] [PubMed] [Google Scholar]
- 35.Horovitz SG, Fukunaga M, de Zwart JA, et al. Low frequency BOLD fluctuations during resting wakefulness and ligt sleep: a simultaneous EEG-fMRI study. Hum Brain Mapp 2008; 29: 671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis B, Tagliazucchi E, Jovicich J, et al. Progression to deep sleep is characterized by changes to BOLD dynamics in sensory cortices. NeuroImage 2016; 130: 293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitra A, Snyder AZ, Tagliazucchi E, et al. Propagaged infra-slow intrinsic brain activity reorganizes across wake and slow wave sleep. eLife 2015; 4: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tagliazucchi E, von Wenger F, Morzelewski A, et al. Breakdown of long-range temporal dependence in default mode and attention networks during deep sleep. PNAS 2013; 110: 15419–15424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chow HM, Horovitz SG, Carr WS, et al. Rhythmic alternating patterns of brain activity distinguish rapid eye movement sleep from other states of consciousness. Proc Natl Acad Sci U S A 2013; 110: 10300–10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spoormaker VI, Schroter MS, Gleiser PM, et al. Development of a large-scale functional brain network during human non-rapid eye movement sleep. J Neurosci 2010; 30: 11379–11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tagliazucchi E, Crossley N, Bullmore ET, et al. Deep sleep divides the cortex into opposite modes of anatomical-functional coupling. Brain Struct Funct 2016; 221: 4221–4234. [DOI] [PubMed] [Google Scholar]
- 42.Fransson P, Skiold B, Engstrom M, et al. Spontaneous brain activity in the newborn brain during natural sleep–an fMRI study in infants born at full term. Pediatr Res 2009; 66: 301–305. [DOI] [PubMed] [Google Scholar]
- 43.Horovitz SG, Braun AR, Carr WS, et al. Decoupling of the brain's default mode network during deep sleep. Proc Natl Acad Sci U S A 2009; 106: 11376–11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boly M, Perlbarg V, Marrelec G, et al. Hierarchical clustering of brain activity during human nonrapid eye movement sleep. Proc Natl Acad Sci U S A 2012; 109: 5856–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uehara T, Yamasaki T, Okamoto T, et al. Efficiency of a “small-world” brain network depends on consciousness level: a resting-state fMRI study. Cereb Cortex 2013; 24: 1529–1539. [DOI] [PubMed] [Google Scholar]
- 46.Tagliazucchi E, von Wegner F, Morzelewski A, et al. Automatic sleep staging using fMRI functional connectivity data. Neuroimage 2012; 63: 63–72. [DOI] [PubMed] [Google Scholar]
- 47.Allen PJ, Polizzi G, Krakow K, et al. Identification of EEG events in the MR scanner: the problem of pulse artifact and a method for its subtraction. NeuroImage 1998; 8: 229–239. [DOI] [PubMed] [Google Scholar]
- 48.Berry RB, Brooks R, Gamaldo CE, et al. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications, Darien, IL: American Academy of Sleep Medicine, 2017. [Google Scholar]
- 49.Ojemann JG, Akbudak E, Snyder AZ, et al. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage 1997; 6: 156–167. [DOI] [PubMed] [Google Scholar]
- 50.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain, New York: Thieme Medical Publishers, Inc., 1988. [Google Scholar]
- 51.Rowland DJ, Garbow JR, Laforest R, et al. Registration of [18F]FDG microPET and small-animal MRI. Nucl Med Biol 2005; 32: 567–572. [DOI] [PubMed] [Google Scholar]
- 52.Power JD, Mitra A, Laumann TO, et al. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 2014; 84: 320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Friston KJ, Holmes AP, Poline JB, et al. Analysis of fMRI time-series revisited. Neuroimage 1995; 2: 43–53. [DOI] [PubMed] [Google Scholar]
- 54.Friston KJ, Williams S, Howard R, et al. Movement-related effects in fMRI time-series. Magn Reson Med 1996; 35: 346–355. [DOI] [PubMed] [Google Scholar]
- 55.Biswal B, Yetkin FZ, Haughton VM, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995; 34: 537–541. [DOI] [PubMed] [Google Scholar]
- 56.Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage 1998; 7: 119–132. [DOI] [PubMed] [Google Scholar]
- 57.Bates D, Machler M, Bolker BM, et al. Fitting linear mixed-effects models using lme4. J Stat Soft 2015; 67: 1–48. [Google Scholar]
- 58.Forman SD, Cohen JD, Fitzgerald M, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 1995; 33: 636–647. [DOI] [PubMed] [Google Scholar]
- 59.McAvoy MP, Ollinger JM, Buckner RL. Cluster size thresholds for assessment of significant activation in fMRI. Neuroimage 2001; 13: S198. [Google Scholar]
- 60.Laufs H, Walker MC, Lund TE. Letter to the editor: ‘Brain activation and hypothalamic functional connectivity during human non-rapid eye movement sleep: an EEG/fMRI study’ – its limitations and an alternative approach. Brain 2007; 130: e75. [DOI] [PubMed] [Google Scholar]
- 61.Thomas BP, Liu P, Aslan S, et al. Physiologic underpinnings of negative BOLD cerebrovascular reactivity in brain ventricles. Neuroimage 2013; 83: 505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raichle ME, Grubb RL, Jr., Gado MH, et al. Correlation between regional cerebral blood flow and oxidative metabolism. In vivo studies in man. Arch Neurol 1976; 33: 523–526. [DOI] [PubMed] [Google Scholar]
- 63.Powers WJ, Videen TO, Markham J, et al. Metabolic control of resting hemispheric cerebral blood flow is oxidative, not glycolytic. J Cereb Blood Flow Metab 2011; 31: 1223–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A 1986; 83: 1140–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cooper R, Crow HJ, Walter WG, et al. Regional control of cerebral vascular reactivity and oxygen supply in man. Brain Res 1966; 3: 174–191. [DOI] [PubMed] [Google Scholar]
- 66.Moss J, Rockoff M. EEG monitoring during cardiac arrest and resuscitation. JAMA 1980; 244: 2750–2751. [PubMed] [Google Scholar]
- 67.Shimojyo S, Scheinberg P, Kogure K, et al. The effects of graded hypoxia upon transient cerebral blood flow and oxygen consumption. Neurology 1968; 18: 127–133. [DOI] [PubMed] [Google Scholar]
- 68.Robin ED, Whaley RD, Crump CH, et al. Alveolar gas tensions, pulmonary ventilation and blood pH during physiologic sleep in normal subjects. J Clin Invest 1958; 37: 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stradling JR, Chadwick GA, Frew AJ. Changes in ventilation and its components in normal subjects during sleep. Thorax 1985; 40: 364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Douglas NJ, White DP, Pickett CK, et al. Respiration during sleep in normal man. Thorax 1982; 37: 840–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.White DP, Weil JV, Zwillich CW. Metabolic rate and breathing during sleep. J Appl Physiol 1985; 59: 384–391. [DOI] [PubMed] [Google Scholar]
- 72.Mangold R, Sokoloff L, Conner E, et al. The effects of sleep and lack of sleep on the cerebral circulation and metabolism of normal young men. J Clin Invest 1955; 34: 1092–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Owens RL. Supplemental oxygen needs during sleep. Who benefits? Respir Care 2013; 58: 32–47. [DOI] [PubMed] [Google Scholar]
- 74.Chang C, Cunningham JP, Glover GH. Influence of heart rate on the BOLD signal: the cardiac response function. Neuroimage 2009; 44: 857–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Birn RM, Smith MA, Jones TB, et al. The respiration response function: the temporal dynamics of fMRI signal fluctuations related to changes in respiration. Neuroimage 2008; 40: 644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Naifeh KH, Kamiya J. The nature of respiratory changes associated with sleep onset. Sleep 1981; 4: 49–59. [DOI] [PubMed] [Google Scholar]
- 77.Grubb RL, Jr., Raichle ME, Eichling JO, et al. The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke 1974; 5: 630–639. [DOI] [PubMed] [Google Scholar]
- 78.Fujishima M, Scheinberg P, Busto R, et al. The relation between cerebral oxygen consumption and cerebral vascular reactivity to carbon dioxide. Stroke 1971; 2: 251–257. [DOI] [PubMed] [Google Scholar]
- 79.Bohr CHR, Hasselbalch K, Krogh A. Concerning a biologically important relationship – the influence of the carbon dioxide content of blood on its oxygen binding. Skand Arch Physiol 1904; 16: 401–412. [Google Scholar]
- 80.Bray J. Estimating plasma pH. Lecture notes on human physiology, 4th ed Malden, MA: Blackwell Science, 1999, pp. 556. [Google Scholar]
- 81.Laux BE, Raichle ME. The effect of acetazolamide on cerebral blood flow and oxygen utilization in the rhesus monkey. J Clin Invest 1978; 62: 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raichle ME, Posner JB, Plum F. Cerebral blood flow during and after hyperventilation. Arch Neurol 1970; 23: 394–403. [DOI] [PubMed] [Google Scholar]
- 83.Sanotskaya NV. Changes in the oxygen tension of the brain and skeletal muscle in hyper- and hypocapnia. Bull Exp Biol Med 1962; 53: 3–8. [PubMed] [Google Scholar]
- 84.Jones MD, Traystman RJ, Simmons MA, et al. Effects of changes in arterial O2 content on cerebral blood flow in the lamb. Am J Physiol-Heart Circul Physiol 1981; 240: H209–H215. [DOI] [PubMed] [Google Scholar]
- 85.Grant R, Condon B, Patterson J, et al. Changes in cranial CSF volume during hypercapnia and hypocapnia. J Neurol Neurosurg Psychiatry 1989; 52: 218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bianciardi M, Fukunaga M, van Gelderen P, et al. Negative BOLD-fMRI signals in large cerebral veins. Journal of Cerebral Blood Flow and Metabolism 2011; 31: 401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McAvoy M, Larson-Prior L, Ludwikow M, et al. Dissociated mean and functional connectivity BOLD signals in visual cortex during eyes closed and fixation. J Neurophysiol 2012; 108: 2363–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science 1993; 262: 679–685. [DOI] [PubMed] [Google Scholar]
- 89.Vetrivelan R, Qiu MH, Chang C, et al. Role of Basal Ganglia in sleep-wake regulation: neural circuitry and clinical significance. Front Neuroanat 2010; 4: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Critchley HD, Mathias CJ, Josephs O, et al. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain 2003; 126: 2139–2152. [DOI] [PubMed] [Google Scholar]
- 91.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain 2014; 137: 12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heidbreder A, Stefani A, Brandauer E, et al. Gray matter abnormalities of the dorsal posterior cingulate in sleep walking. Sleep Med 2017; 36: 152–155. [DOI] [PubMed] [Google Scholar]
- 93.Bassetti C, Vella S, Donati F, et al. SPECT during sleepwalking. The Lancet 2000; 356: 484–485. [DOI] [PubMed] [Google Scholar]
- 94.Snedecor GW, Cochran WG. Statistical methods, 8th ed Ames, IA: Iowa State University Press, 1989. [Google Scholar]
- 95.Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci U S A 2001; 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 2005; 25: 7709–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pearson JM, Heilbronner SR, Barack DL, et al. Posterior cingulate cortex: adapting behavior to a changing world. Trends Cogn Sci 2011; 15: 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 2006; 129: 564–583. [DOI] [PubMed] [Google Scholar]
- 99.Sperling RA, Laviolette PS, O’Keefe K, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 2009; 63: 178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Small DM, Gitelman DR, Gregory MD, et al. The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. Neuroimage 2003; 18: 633–641. [DOI] [PubMed] [Google Scholar]
- 101.Rabinovici GD, Jagust WJ. Amyloid imaging in aging and dementia: testing the amyloid hypothesis in vivo. Behav Neurol 2009; 21: 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Minoshima S, Giordani B, Berent S, et al. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol 1997; 42: 85–94. [DOI] [PubMed] [Google Scholar]
- 103.Matsuda H. Cerebral blood flow and metabolic abnormalities in Alzheimer’s disease. Ann Nucl Med 2001; 15: 85–92. [DOI] [PubMed] [Google Scholar]
- 104.Maarouf CL, Kokjohn TA, Walker DG, et al. Biochemical assessment of precuneus and posterior cingulate gyrus in the context of brain aging and Alzheimer’s disease. PLoS One 2014; 9: e105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Johnson KA, Schultz A, Betensky RA, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol 2016; 79: 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vlassenko AG, Benzinger TL, Morris JC. PET amyloid-beta imaging in preclinical Alzheimer’s disease. Biochim Biophys Acta 2012; 1822: 370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rowe CC, Ackerman U, Browne W, et al. Imaging of amyloid β in Alzheimer’s disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol 2008; 7: 129–135. [DOI] [PubMed] [Google Scholar]