Abstract
Iron delivery to the brain is essential for multiple neurological processes such as myelination, neurotransmitter synthesis, and energy production. Loss of brain iron homeostasis is a significant factor in multiple neurological disorders. Understanding the mechanism by which the transport of iron across the blood–brain barrier (BBB) is regulated is crucial to address the impact of iron deficiency on brain development and excessive accumulation of iron in neurodegenerative diseases. Using induced pluripotent stem cell (iPSC)-derived brain endothelial cells (huECs) as a human BBB model, we demonstrate the ability of transferrin, hepcidin, and DMT1 to impact iron transport and release. Our model reveals a new function for H-ferritin to transport iron across the BBB by binding to the T-cell immunoglobulin and mucin receptor 1. We show that huECs secrete both transferrin and H-ferritin, which can serve as iron sources for the brain. Based on our data, brain iron status can exert control of iron transport across the endothelial cells that constitute the BBB. These data address a number of pertinent questions such as how brain iron uptake is regulated at the regional level, the source of iron delivery to the brain, and the clinical strategies for attempting to treat brain iron deficiency.
Keywords: Blood–brain barrier, H-ferritin, iron, transferrin, transferrin receptor
Introduction
The blood–brain barrier (BBB) is formed by tight junctions between endothelial cells which confer selective permeability of molecules entering the brain from the bloodstream.1 This selectivity is essential to the protection and optimal performance of the brain in normal physiological conditions. However, the presence of the tight junctions between endothelial cells requires that critical nutrients for optimal brain function be transported across the endothelial cells and released into the brain. These transport processes must also meet the requirements of the endothelial cells for these same nutrients.
A key nutrient for normal brain development and adult function is iron. Iron serves as a cofactor in a number of crucial cellular processes, such as oxygen transport, DNA synthesis, and in the brain, myelination, making it one of the most vital micronutrients present in the body.2 During development, iron deficiency may result in long standing and severe phenotypes, leading to poor cognitive function, motor deficits, and other neurological disorders.3–6 Conversely, excessive iron may cause cellular cytotoxicity through induction of oxidative stress leading to widespread neurodegeneration.7 Despite this importance, the exact mechanism of regulation of iron uptake into the brain is currently not known and is further complicated by the fact that there are distinct regional differences within the brain in the amount of iron present, suggesting local regulation of uptake or release of iron.
Studies into regulation of iron transport across the BBB have stagnated due to the acceptance of the transferrin (Tf) transcytosis paradigm.8 This model suggests that circulating holo-Tf binds to transferrin receptors (TfR) on the luminal side of the endothelial cells, is endocytosed as a complex into the cell and is subsequently exocytosed from the abluminal membrane, releasing unaltered holo-Tf into the brain extracellular space. This model is particularly problematic because it does not address the substantial iron needs of the endothelial cells themselves, nor does it address how iron uptake into the brain is regulated. Current research by our laboratory and others has suggested that endothelial cells can store iron, which is released into the brain upon demand, and has further suggested alternative transport models for Tf and possibly ferritin, challenging the current paradigm of Tf transcytosis.9–13 We have recently proposed an alternative model for iron transport and uptake into the brain.8 In this model, Tf undergoes endocytosis, and the iron is released from Tf in the endosome and is transported out of the endosome via the divalent metal transporter 1 (DMT1).14,15 Subsequently, this iron can enter the labile iron pool to be used by the endothelial cells, stored in ferritin, secreted, or released as free iron into the brain through the iron exporter ferroportin.16,17 This novel concept demonstrates how brain endothelial cells act as crucial regulators of cerebral iron homeostasis.
BBB research has commonly used models from primary animal sources, such as cows, pigs, and rats. While these models recapitulate most key BBB functions, including moderate transendothelial electrical resistance (TEER; a measure of tight junction formation), low passive permeability, and active transporter function, they are not necessarily representative of human BBB properties, which hamper the ability of the research to be translated to humans. However, the majority of human BBB models suffer from poor barrier properties, which limits their utility for discovery-based explorations. In this study, we use novel human-induced pluripotent stem cell (iPSC)-derived brain endothelial cells (huECs) to model the human BBB.18–20 IPSC-derived huECs exhibit excellent barrier properties, including high TEER (typically above 3000 Ω×cm2 in monoculture and above 6000 Ω×cm2 after co-culture with astrocytes and pericytes), low permeability to small molecule tracers such as sodium fluorescein, and expression of active efflux transporters such as p-glycoprotein and breast cancer resistance protein (BCRP). Moreover, iPSC-derived huECs have recently been used to model genetic diseases that impact BBB transporter functions.21,22 As such, the purpose of this study was to utilize iPSC-derived huECs to examine the mechanism(s) of iron uptake into and across the BBB.
Our central hypothesis for this work is that a mechanism exists to provide iron to brain microvasculature and that iron can be used within the endothelial cells or released to the brain in response to signals. This hypothesis required novel consideration of how Tf and iron are transported across the BBB, and in the process of pursuing the hypothesis we introduce H-ferritin (Hft) as a significant iron transporter. Ultimately, the data presented herein serve to establish BBB endothelial cells as a critical checkpoint and regulators of brain iron uptake.
Materials and methods
Human endothelial cell differentiation and cryopreservation
Human endothelial cells (huECs) were differentiated from CC3 iPSCs as described previously.18–20,23,24 CC3 iPSCs were maintained in E8 medium (prepared in-house) on growth factor reduced Matrigel (Corning).25 For maintenance, iPSCs were passaged using Versene (Thermo Fisher Scientific) upon reaching 60–80% confluence. Cells intended for differentiation were washed one time with DPBS (Thermo Fisher Scientific) and dissociated to single cells during a 3-min incubation with Accutase (Stem Cell Technologies) at 37℃. Cells were collected via centrifugation, and cell count and viability were assessed using a Trypan Blue stain and Countess II automated cell counter (Thermo Fisher Scientific). Cells were plated at a live density of 15,600 cells per square centimeter in E8 medium supplemented with 10 μM Rho-associated, coiled-coil containing protein kinase (ROCK) inhibitor Y27632 (R&D Systems). Differentiation was initiated 24 h after plating with a complete medium change to E6 medium.26 E6 medium was changed approximately every 24 h for four days. Cells were then expanded in human endothelial serum-free medium (hESFM; Thermo Fisher Scientific) containing 1% platelet poor plasma-derived serum (PDS; Alfa Aesar), collectively referred to as EC medium, 20 ng/mL basic fibroblast growth factor (bFGF; Peprotech), and 10 μM all-trans retinoic acid (RA; Sigma Aldrich). Medium was not changed for 48 h. Following this incubation, cells were washed once with DPBS and incubated with Accutase at 37℃ for approximately 25 min, or until cells fully dissociated from the plate. Cells were collected via centrifugation and resuspended in 60% EC medium with 20 ng/mL bFGF, 30% fetal bovine serum (Thermo Fisher Scientific), 10% Hybri-Max DMSO (Sigma), 10 μM RA, and 10 μM Y27632 at a ratio of 1 mL of freeze medium per well of a 6-well plate collected. Vials were frozen overnight at −80℃ in a freezing container (Thermo Fisher Scientific) followed by transfer to liquid nitrogen for long-term storage.
Hft preparation
We have previously described the methodology for recombinant Hft preparation.11,27,28 Briefly, wild-type human Hft containing a poly-His tag was subcloned into pET30a(+), to be produced in BL21 Escherichia coli. Isopropyl-β-D-thio-galactoside (IPTG) was used to induce expression. Following this, bacteria were lysed, and Hft protein was purified on a nickel column using standard techniques (GE Healthcare Bio-Sciences).
Radiolabeling of Tf and Hft
59Fe (Perkin Elmer) was complexed with nitrilotriacetic acid (NTA), ferric chloride (FeCl3), and sodium bicarbonate (NaHCO3) at a ratio of 100 µL NTA: 6.7 µL FeCl3: 23.3 µL NaHCO3: 50 µCi 59FeCl3 to form the 59Fe-NTA complex. This ratio was adjusted accordingly for cases where more/less than 50 µCi 59FeCl3 was needed. After complexing, 59Fe-NTA was subsequently incubated with apo-Tf (Sigma) or Hft for 30 min to allow for iron loading.8,29 Free iron was separated from the total complex using Sephadex-G50 QuickSpin columns following manufacturer's instructions (Roche).
Transport studies
Initially, the apical chamber of 12-well Transwell plates (Costar Transwell, 0.4 µm pore, Corning) was coated with collagen IV (Sigma) and fibronectin (Sigma) at a ratio of five parts sterile ddH2O mixed with four parts 1 mg/mL collagen IV and 1 part 1 mg/mL fibronectin. A total of 250 µL was used to coat plates overnight at 37℃. Following coating, 25,000 huECs were thawed and plated into the apical chamber of a 12-well Transwell plate in 500 µL hESFM (Thermo Fisher) plus 1% platelet poor PDS ( Fisher Scientific), 10 µM of a ROCK inhibitor Y27632 (R&D Systems), 20 ng/mL basic fibroblast growth factor (bFGF, Peprotech), and 10 µM RA ( Sigma). The basal chamber was filled with 1.5 mL of the same media. After allowing the cells to attach overnight at 37℃, we changed the media in both chambers to hESFM, supplemented with 1% PDS, 10 µM Y27632, but lacking bFGF and RA. Cells were incubated at 37℃ overnight to allow growth and tight junction formation, after which all transport studies were performed. Immediately before the experiment was performed, a complete media exchange was conducted by washing three times with 1 × DPBS to remove serum; 500 µL of serum-free media was added to the apical chamber, while 1.5 mL was added to the basal chamber. After media addition, trans-endothelial electrical resistance measurements were taken using an Epithelial Volt/Ohm Meter for TEER (EVOM2, STX2, World Precision Instruments). Blank (media only) TEER readings were obtained and subtracted from all other TEER measurements. Across all experimental conditions, we report an average TEER value of 1562 ± 516 Ω × cm2.
RITC-Dextran (70 kDa, Sigma) was added at the beginning of the experiment in order to monitor tight junction formation and barrier permeability30; 10 µCi of either the 59Fe-NTA-Tf (1 mg/mL) or 59Fe-NTA-Hft (100 µg/mL) complex was added to the apical chamber and cells were incubated at 37℃, 5% CO2. The following regulatory molecules were added to the basal chamber: (1) Apo-Tf was added at 1 mg/mL, (2) holo-Tf was loaded with non-radioactive FeCl3 and added at 1 mg/mL, (3) Hepcidin (Peptides International, PLP-4392-s) was added at 500 nM. Furthermore, 104 nM of a DMT1 inhibitor (XEN602, generously provided by Xenon Pharmaceuticals)31 was added to the apical chamber concurrently with Tf or Hft addition. For all experimental conditions, aliquots were sampled from the basal chamber at 0, 2, 4, 6, 8, and 24 h. These aliquots were further separated into protein bound and non-protein bound samples using Sephadex-G50 QuickSpin columns (Roche). Final 59Fe activity in the apical chamber was also measured at the 24-h time point. At the end of the experiment, media was aspirated, cells were washed three times with 1 × DPBS (Corning), trypsinized, and 59Fe activity counted. All 59Fe counts were measured using a Beckman Gamma 4000 gamma counter (Beckman Coulter) and recorded as counts per minute (CPM). Blank media and background counts were obtained, averaged, and subtracted from all experimental counts. Additionally, at each time point, samples were taken from the basal chamber to be assessed for barrier permeability. RITC fluorescence (excitation: 555 nm, emission: 580 nm) was measured on a SpectraMax Gemini EM plate reader (Molecular Devices). Across all experiments, no permeability was seen using the RITC-dextran control.
Release studies
We have previously described the methodology for performing release assays.8,29 Briefly, huECs in serum-free media plated in a 24-well Transwell format were loaded with 10 µCi 59Fe-NTA complex and incubated overnight at 37℃. Following incubation, cells and chambers were rinsed three times with 1 × DPBS and serum-free media was added, along with apo-Tf (1 mg/mL), holo-Tf (1 mg/mL), or hepcidin (500 nM). These concentrations were selected based on estimated Tf in the cerebrospinal fluid (0.5 µM) and hepcidin levels in the blood.29 59Fe release was measured by taking 100 µL aliquots at 0, 2, 4, 6, 8, and 24 h, further separated into protein-bound and free 59Fe fractions using Sephadex-G50 QuickSpin columns, and quantified via a Beckman Gamma 4000 gamma counter. As described before, tight junction formation and barrier permeability were measured via TEER and RITC signal.
Autoradiograph
Release studies were performed as described previously, except no periodic aliquots were taken. Instead, all basal chamber media was collected at the 24-h time point. Subsequently, samples were concentrated using a Vacufuge (Eppendorf) and reconstituted in 33 µL 1 × PBS. Next, samples were boiled for 10 min with 1 × samples buffer, loaded onto a 4–20% Criterion TGX Precast Protein Gel (Bio-Rad), and run at 250 V for 23 min. Gels were covered with plastic-wrap and loaded into an autoradiography cassette (FisherBiotech) with HyBlot CL Autoradiography film (Denville Scientific) and an intensifying screen for 2.5 h after which film was developed.
Western blot
Protein expression was detected via immunoblot as previously described.8,29 Briefly, huECs were digested in a mixture of RIPA buffer (Sigma) and protease inhibitor cocktail (PIC, Sigma) for 1 h at room temperature. Subsequently, total protein was quantified by bicinchoninic assay (BCA, Pierce) and loaded onto a 4–20% Criterion TGX Precast Protein Gel (Bio-Rad). Protein was transferred onto a nitrocellulose membrane and probed for ferroportin (Alomone Labs, 1:500, AIT-001), Hft (Cell Signaling, 1:500, 4393S), Tf (Abcam, 1:5000, ab82411) or beta-actin (Sigma, 1:3000, A5441). Corresponding secondary antibody conjugated to HRP was used (1:5000, GE Amersham) and bands were visualized using ECL reagents (Perkin-Elmer) on an Amersham Imager 600 (GE Amersham).
Immunofluorescence
Fluorescent quantum dots (620 nm, Ocean Nanotech) were conjugated to Hft protein (Hft-QD) according to the manufacturer's instructions. As a negative control (Ctrl-QD), the same protocol was carried out substituting Hft for PBS. Prior to cell plating, the collagen IV/fibronectin/ddH2O coating mixture was used to coat four well chamber slides (Fisher Scientific) for 4 h, after which chambers were allowed to dry; 25,000 huECs/chamber were then plated overnight. Next, 50 µg Hft-QD or an equivalent volume Ctrl-QD was added to plated cells for 3 h at 37℃. Cells were washed three times with 1 × DPBS and subsequently fixed in 4% paraformaldehyde for 20 min at room temperature, followed by washing with 1 × DPBS three times. Cells were blocked in 1% milk for 1 h and stained overnight with an anti-Tim-1 antibody (R&D Systems, MAB1750) at 1:100. Following this, fluorescent anti-mouse secondary antibody (Thermo Fisher Scientific) was used at 1:200, followed by a counterstain with DAPI. Cells were viewed with confocal microscopy (Leica).
Statistical analysis
Statistical analyses were performed by one-way or two-way ANOVA using time and treatment as the two factors, with Bonferroni post hoc test for significance using GraphPad Prism 4 software (GraphPad Software, Inc., San Diego, CA, USA). Data from three independent replicates were averaged and are expressed as the mean ± standard deviation (SD). A p-value of < 0.05 was considered significant.
Results
Tf and Hft function as iron carriers
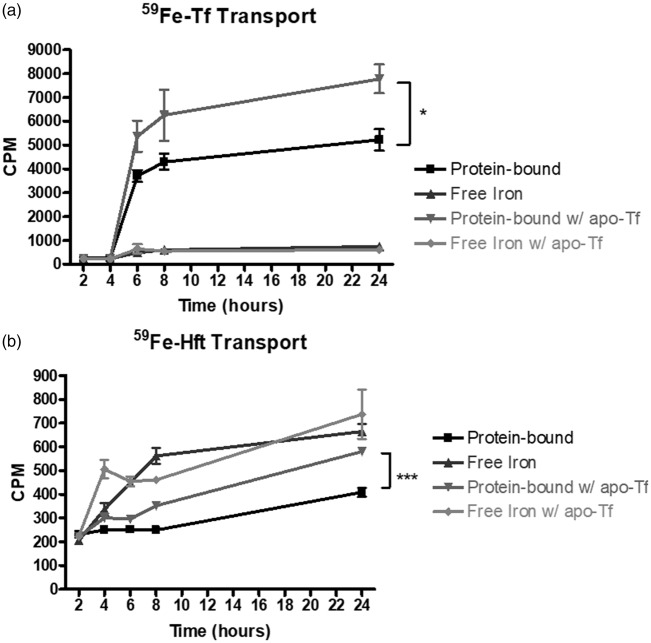
The first experiment was designed to test the ability of Tf and Hft to deliver iron across this BBB model. HuECs were plated and incubated with 1 mg/mL 59Fe-Tf or 100 µg/mL 59Fe-Hft. Sampling from the basal chamber every 2 h allowed us to determine the quantity of iron transport over 24 h. Tf-bound iron was predominantly transported across the huECs in a protein-bound form, although there was a measurable amount of free iron released (Figure 1(a)). Conversely, 59Fe delivered by Hft uptake was primarily released from the huECs as free iron, with a modest amount of protein-bound iron transported (Figure 1(b)).
Figure 1.
Effect of apo-Tf on iron transport across the huECs. (a) 1 mg/mL 59Fe-Tf robustly delivers iron across huECs in both protein-bound and free forms. Tf-bound is the preferred method of transport. Apo-Tf significantly increased iron transport across the huECs in the 59Fe-Tf bound form. Transport of free iron was unaffected by apo-Tf addition. (b) 100 µg/mL 59Fe-Hft also delivers iron across huECs, but the preferred method of transport is via free iron. Apo-Tf caused significant increase in protein-bound 59Fe that was found in the basal chamber. Free 59Fe transport was unaffected by apo-Tf addition. Means of three biological replicates ± SD from the treatment groups were evaluated against the “Protein-bound” or “Free Iron” conditions using two-way ANOVA with Bonferroni's posttest for significance. *p < 0.05, ***p < 0.001.
Apo-Tf affects protein-bound iron transport across the BBB
Next, to test the hypothesis that there is regulatory control of iron transport, 1 mg/mL apo-Tf (iron-poor Tf) was added to the basal chamber during the transport experiment. When apo-Tf was added to the basal chamber, there was a similar pattern of protein bound and free 59Fe transport (Figure 1(a)); however, the amount of protein bound iron transported was increased by 48% after 24 h (p < 0.05). The addition of apo-Tf to the basal chamber did not affect the amount of free 59Fe transported via Hft (Figure 1(b)), but did induce a 42% increase (p < 0.001) in the amount of protein-bound 59Fe iron transported after 24 h (Figure 1(b)).
Holo-Tf affects protein-bound iron transport across the BBB
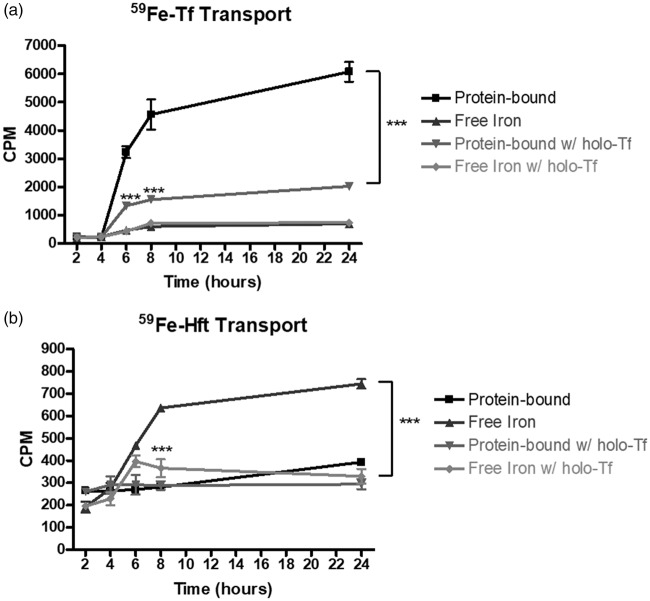
Given that apo-Tf altered iron transport in huECs, we next tested the hypothesis that holo-Tf (iron-rich Tf) decreases the amount of iron transported across the BBB. To test this hypothesis, we added 1 mg/mL holo-Tf to the basal chamber during our transport assay. As a result, the amount of protein bound 59Fe transported was significantly depressed at 6, 8, and 24 h, showing a 67% reduction after 24 h (Figure 2(a), p < 0.001). When holo-Tf was coupled with the 59Fe-Hft transport condition, the amount of free 59Fe transported was decreased by 56% (Figure 2(b), p < 0.001).
Figure 2.
Effect of holo-Tf on iron transport across the huECs. 1 mg/mL holo-Tf was added to the basal chamber during 59Fe-Tf or 59Fe-Hft transport. (a) Holo-Tf significantly decreased iron transport across the huECs in the protein-bound form. Transport of free iron was unaffected by holo-Tf addition. (b) Holo-Tf caused a significant decrease in free 59Fe that was found in the basal chamber. 59Fe-Hft transport was unaffected by holo-Tf addition. Means of three biological replicates ± SD from the treatment groups were evaluated against the “Protein-bound” or “Free Iron” conditions using two-way ANOVA with Bonferroni's posttest for significance. ***p < 0.001.
Hepcidin affects free iron transport efflux from huECs
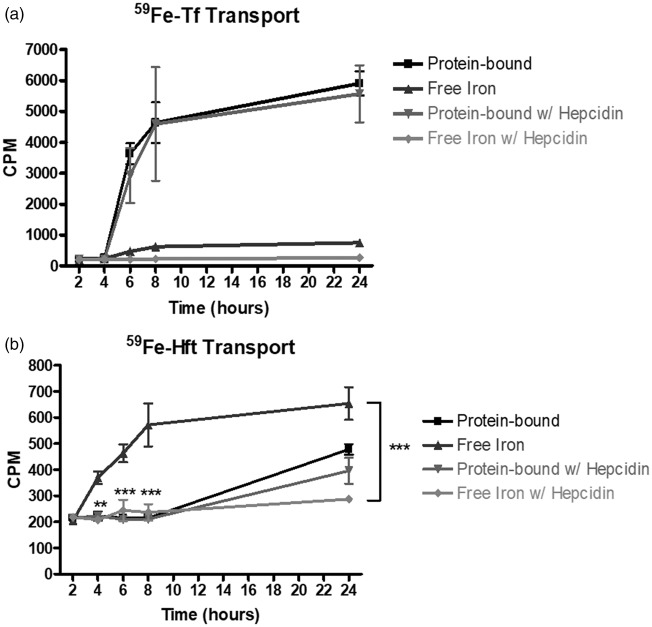
To begin to determine how free 59Fe was transported across the huECs, we examined the only known exporter of iron in vertebrates, ferroportin, and its regulator, hepcidin. Hepcidin is a hormone produced by the liver which binds to ferroportin and causes its internalization and degradation.32,33 We tested the hypothesis that addition of hepcidin to the basal chamber will cause a reduction in the amount of free 59Fe transported across the huECs. We added 500 nM hepcidin to the basal chamber of each well and added 59Fe-Tf or 59Fe-Hft to the apical chamber (Figure 3). For the control, we added 59Fe-Tf or 59Fe-Hft to the apical chamber with no hepcidin added to the basal chamber. In this experiment, we found that addition of hepcidin did not affect the amount of protein-bound 59Fe transported via Tf or Hft (Figure 3(a) and (b). However, addition of hepcidin lowered the amount of free iron transported after 24 h by 64% after Tf addition (p = 0.157). In addition, there were significant reductions in free iron transported 8 and 24 h after Hft addition, with a 24-h reduction of 56% after Hft addition (p < 0.001).
Figure 3.
Effect of Hepcidin on iron transport across the huECs; 500 nM Hepcidin was added to the basal chamber during 59Fe-Tf or 59Fe-Hft transport. (a) Hepcidin decreased free iron transport across the huECs. Protein-bound 59Fe transport was unaffected by hepcidin addition. (b) Hepcidin caused significant decrease in free 59Fe that was found in the basal chamber. 59Fe-Hft transport was unaffected by hepcidin addition. Means of three biological replicates ± SD from the treatment groups were evaluated against the “Protein-bound” or “Free Iron” conditions using two-way ANOVA with Bonferroni's posttest for significance. **p < 0.01, ***p < 0.001.
DMT1 inhibition affects iron transport
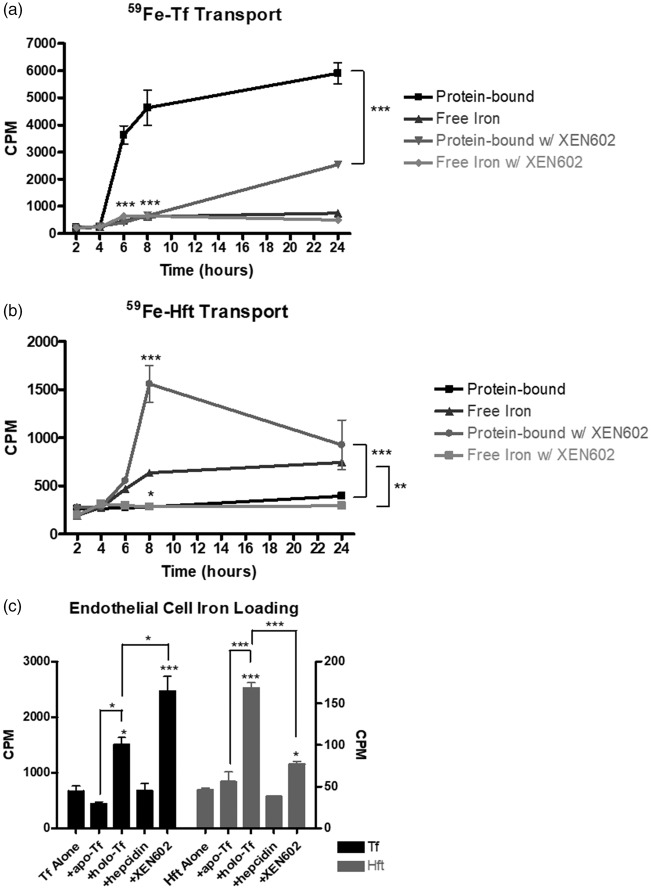
As previously mentioned, DMT1 is responsible for endosomal release of iron. However, the role of DMT1 has yet to be characterized in iron delivered by Hft, and its role in the transport of free iron across the BBB is not universally accepted.29,34 To interrogate the role of DMT1 in our model, we utilized a DMT1 inhibitor, XEN602. We added 104 nM XEN602 to the apical chamber at the start of the experiment, concurrent with 59Fe-Tf or 59Fe-Hft addition (Figure 4). In the Tf transport experiment, addition of XEN602 not only lowered the amount of protein bound iron transported by 56% after 24 h (p < 0.001), but also altered the rate at which iron was transported across the huECs (Figure 4(a)). Free 59Fe transport was unaffected. In the 59Fe-Hft transport condition, XEN602 addition reversed the standard transportation method for Hft (Figure 4(b)). When compared to the control condition, there was a 236% increase in the amount of protein-bound 59Fe transported, with a concurrent 61% decrease in the amount of 59Fe transported after 24 h (Figure 4(b), p < 0.001). These data suggest a significant and novel role for DMT1 in the delivery of iron to the brain via Hft.
Figure 4.
Effect of the DMT1 inhibitor, XEN602, on iron transport across the huECs; 100 µM XEN602 was added to the basal chamber during 59Fe-Tf or 59Fe-Hft transport. (a) Inhibition of DMT1 significantly decreased protein-bound iron transport across the huECs. Free 59Fe transport was unaffected by XEN602 addition. (b) XEN602 caused significant decrease in free 59Fe that was found in the basal chamber. Protein-bound transport was also significantly increased by XEN602 addition. Means of three biological replicates ± SD from the treatment groups were evaluated against the “Protein-bound” or “Free Iron” conditions using two-way ANOVA with Bonferroni's posttest for significance. (c) Human endothelial cells were loaded using either 59Fe-Tf (black bars) or 59Fe-Hft (gray bars) and subsequently collected for counting. 59Fe-Tf shown by the left y-axis, while 59Fe-Hft shown by the right y-axis. Holo-Tf and XEN602 significantly increased the amount of 59Fe retained inside the endothelial cell. Means of three biological replicates ± SD from the treatment groups were evaluated against the “Tf alone” or “Hft alone” conditions for the cell loading experiments using one-way ANOVA with Bonferroni's posttest for significance. *p < 0.05, **p < 0.01, ***p < 0.001.
Iron loading of huECs
At the 24-h time point for the transport assay, cells from each condition were collected and intracellular 59Fe content was examined (Figure 4(c)). In the control transport condition, cells exposed to 59Fe-Tf contained 669.45 ± 167.45 CPM, while cells incubated with 59Fe-Hft contained 46.44 ± 5.12 CPM. When apo-Tf was added to the basal chamber, cells exposed to 59Fe-Tf added had 436.03 ± 67.31 CPM, while cells incubated with 59Fe-Hft had 55.97 ± 20.51 CPM. Though not statistically significant (p = 0.13), this represented a 35% decrease in the iron content after loading the cells with 59Fe-Tf and a 20% increase after loading with 59Fe-Hft (p = 0.35). When incubated with holo-Tf in the basal chamber, cells loaded with 59Fe-Tf added contained 1502.65 ± 230.14 CPM (p < 0.05), while cells loaded with 59Fe-Hft contained 168.23 ± 11.16 CPM (p < 0.001). When incubated with hepcidin in the basal chamber, cells loaded with 59Fe-Tf recorded an average CPM of 675.17 ± 128.85 CPM, while cells loaded with 59Fe-Hft recorded an average CPM of 38.3 ± 0.53. When incubated with XEN602 in the basal chamber, cells loaded with 59Fe-Tf recorded an average CPM of 2464.4 ± 474.28 CPM (p < 0.001), while cells loaded with 59Fe-Hft recorded an average CPM of 76.97 ± 5.26 (p < 0.05). Data from these studies provide evidence for the regulation of iron loading of huECs by Tf and Hft.
Iron and protein release by huECs
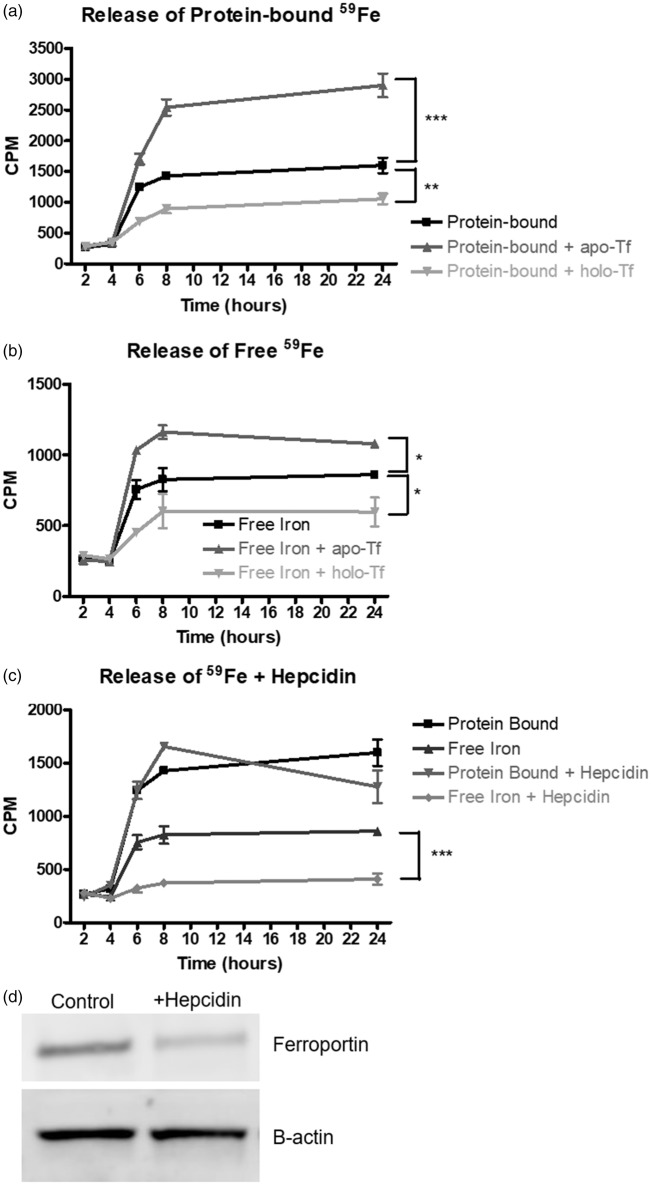
The next series of studies evaluated the ability of huECs to release 59Fe in a protein-free loading paradigm. All of the release assays were performed under serum-free conditions and the huECs were loaded directly with 59Fe-NTA instead of 59Fe-Tf or 59Fe-Hft8. We first established that iron is released over a 24-h period both bound to a protein and as free iron (Figure 5(a)). When huECs were incubated with 1 mg/mL apo-Tf in the basal chamber for 24 h after being loaded with 59Fe-NTA overnight, there was an 81% increase (p < 0.001) in protein-bound 59Fe and free 59Fe released by the huECs, whereas there was a 35% decrease (p < 0.01) after incubation with 1 mg/mL holo-Tf (Figure 5(a) and (b)). Furthermore, when 500 nM hepcidin was added to the basal chamber of the huECs after iron loading, a 52% decrease in free 59Fe released (p < 0.001) was seen after 24 h; protein-bound 59Fe was unaffected (Figure 5(c)). In order to probe the mechanism for this effect, we incubated hepcidin with huECs for 24 h and found a 64% decrease (p < 0.05) in ferroportin (Figure 5(d)).
Figure 5.
Iron release from human endothelial cells. Human endothelial cells were loaded with 10 µCi 59Fe-NTA overnight. (a) Release of protein-bound 59Fe significantly increased after incubation with 1 mg/mL apo-Tf and significantly decreased after incubation with 1 mg/mL holo-Tf. (b) Release of free 59Fe by huECs was significantly increased after incubation with apo-Tf and significantly decreased after incubation with holo-Tf. (c) 500 nM hepcidin treatment for 24 h significantly decreases free 59Fe release. Release of protein-bound 59Fe is unaffected by hepcidin addition. (d) Representative Western blot demonstrating incubation of huECs with 500 nM hepcidin for 24 h results in a 64% decrease in ferroportin expression. Means of three biological replicates ± SD from the treatment groups were evaluated against the “Protein-bound” or “Free Iron” conditions using two-way ANOVA with Bonferroni's posttest for significance. *p < 0.05, **p < 0.01, ***p < 0.001.
The release of iron bound to protein into the basal chamber following iron loading of the cells indicated that iron was being bound to a protein synthesized by the huECs. Thus, we undertook the next experiments to identity the protein secreted by endothelial cells. We performed the iron release assay as before, but collected and concentrated the entirety of the basal chamber media. Gel electrophoresis was performed on the concentrated basal chamber media with 59Fe-Tf and 59Fe-Hft applied as standards. Using autoradiography techniques on a SDS-PAGE gel, we demonstrate that the 59Fe is associated with Tf (Figure 6(a)). Moreover, when the basal chamber media was subjected to an immunoblot analysis, Tf was detected in the basal chamber. Furthermore, the amount of Tf secreted changes in response to apo-Tf or holo-Tf (Figure 6(b)). Hft is also secreted into the basal chamber and is responsive to apo-Tf and holo-Tf (Figure 6(c)).
Figure 6.

Protein release from human endothelial cells. (a) Autoradiograph results demonstrating 59Fe iron released by huECs is associated with transferrin. 59Fe associated with Hft was undetectable. (b) Immunoblot demonstrating release of transferrin by huECs is comparable to 59Fe-Tf standard. (c) Immunoblot demonstrating release of Hft by huECs comparable to 59Fe-Hft standard. Lanes are as follows: (1) 59Fe-Hft standard (Hft Std), (2) 59Fe-Tf standard (Tf Std), (3) Control release experiment (Ctrl), (4) Release experiment with apo-Tf (+apo-Tf), (5) Release experiment with holo-Tf (+holo-Tf).
huECs use Tim-1 to take up Hft
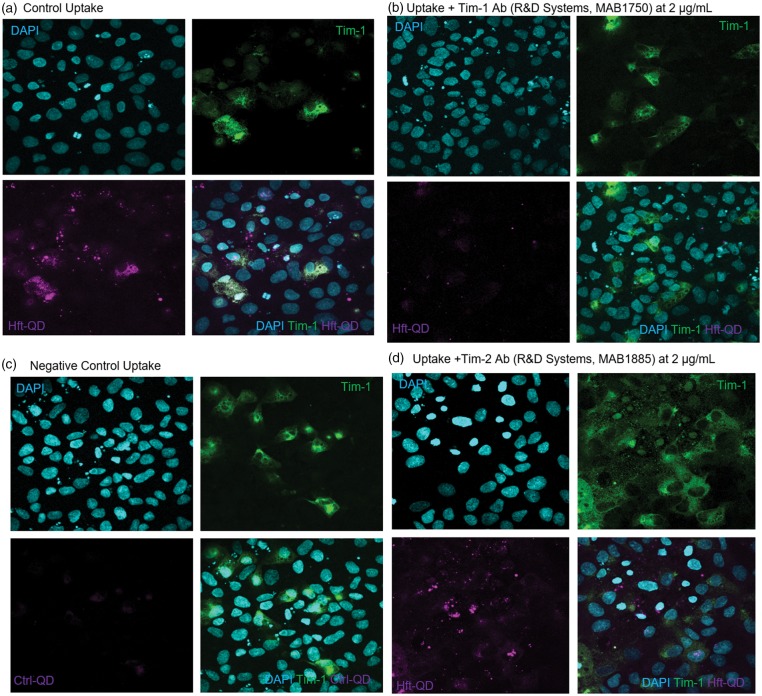
Lastly, although we have demonstrated Hft uptake into the brain in animal studies,11,28 Hft uptake is a novel concept in human BBB studies. Therefore, we examined the mechanism by which endothelial cells take up Hft, hypothesizing that the T-cell immunoglobulin and mucin receptor, Tim-1, facilitate Hft uptake. This is based upon previous literature describing Tim-2, the rodent homolog to Tim-1, as a binding partner to Hft.28 We used immunocytochemistry to explore colocalization between fluorescently labeled Hft (Hft-QD) and Tim-1 (Figure 7). As demonstrated in Figure 7(a), Hft-QD are robustly taken up by huECs. Furthermore, this Hft-QD uptake localizes with Tim-1. When Hft-QD are incubated concurrently with 2 µg/mL of an anti-Tim-1 antibody, there is a marked decrease in the amount of Hft-QD uptake (Figure 7(b)). As a negative control for this experiment, we used Ctrl-QD (Figure 7(c)). Additionally, we attempted to compete off the Hft-QD interaction with Tim-1 by using 2 µg/mL of an anti-Tim-2 antibody, but found no decrease in Hft-QD uptake (Figure 7(d)).
Figure 7.
Human endothelial cells take up H-ferritin through Tim-1. (a) HuECs incubated with Hft-QD for 3 h and stained for Tim-1 display colocalization between Hft-QD (magenta) and Tim-1 (green). Cell nuclei are stained with DAPI. (b) Hft-QD uptake is greatly decreased after incubating huECs with Hft-QD and an anti-Tim-1 antibody (2 µg/mL) concurrently. (c) Using Ctrl-QD as a negative control, huECs do not take up non-specific quantum dots. (d) To discern whether Hft-QD is specific to Tim-1, an anti-Tim-2 antibody (2 µg/mL) was used in an attempt to compete off Hft-QD. No reduction in signal was observed.
Discussion
The aim of this study was to demonstrate the active transport of Tf and Hft in a human BBB model, and how this uptake and transport might be modulated. As previously mentioned, the Tf transcytosis paradigm has numerous flaws that do not address the endothelial cells' iron requirements. The existing paradigm also fails to consider the possibility that iron uptake by the brain is regulated by the brain itself; this concept is critical to the debate regarding whether iron supplementation will increase brain iron in development or contribute to late onset neurodegenerative disease.35 In this study, we have expanded upon our previous work using bovine brain and retinal primary endothelial cells by utilizing a recently characterized novel model of the human BBB that recapitulates tight barrier properties and active transport functions.18 The data with the huECs and our previous studies with primary bovine endothelial cells revealed similar results regarding iron transport across the endothelial cells in the BBB model and the regulatory factors that may impact this transport.8,29 Furthermore, using the huEC as a model, we have demonstrated a novel function for Hft to deliver iron through Tim-1 to the endothelial cells. In addition, our current results identify a significant role for holo-Tf, hepcidin, and DMT1 in the regulation of uptake and transport/release of both Tf-bound and Hft-bound iron, as well as free iron.
We provide evidence that Hft, a protein thought to be solely used for iron storage, can serve as an iron delivery protein and is secreted from BBB endothelium as a source of iron for the brain. In addition to the demonstration of Hft-mediated iron delivery to the brain, we also demonstrate that Hft can load the endothelial cells with iron and this process is sensitive to regulation. The delivery of iron to both the developing and mature brain is critical, especially when considering the amount of iron required for specific and highly demanding metabolic brain processes such as myelination, growth, and development.9,36,37 The ability of Hft to deliver iron to the brain and other organs has been previously reported in cell and animal models.11 In addition to iron delivery, ferritin has been employed as a potential delivery protein for chemotherapeutic agents and contrast agents in the brain.38–40 Our data suggest that the protein can be transported intact across the BBB, thus highlighting the potential for ferritin to directly target sites in the brain. Our data further suggest the uptake mechanism for Hft is mediated by Tim-1, the sequential human homolog to the rodent Tim-2, a member of the T cell immunoglobulin and mucin domain family, which has been shown to robustly bind to Hft and deliver iron to oligodendrocytes.27,28,41–43
The data we present here demonstrate the ability of proteins found in the brain to significantly affect iron transport at the level of the endothelial cells. This point is especially important because it suggests that signaling from specific regions of the brain may regulate iron release from endothelial cells accounting for local regulation consistent with reports of regional iron uptake in animal models following iron deprivation.44 This concept of local regulation becomes increasingly crucial in many neurological diseases that have an altered brain iron profile specific to various regions of the brain, such as the substantia nigra in Parkinson's disease45 and restless leg syndrome,46,47 the hippocampus in Alzheimer's disease,48 as well as white matter in multiple sclerosis.49,50 Here, we present data that support a role for apo-Tf, holo-Tf, and hepcidin in regulating the amount of iron transported across the BBB and released from the endothelial cells. This regulation directly impacts not only the maximal amount of iron transported but also the rate of transport. While we cannot rule out that the observed increase in protein-bound iron could result from increased free iron binding to available apo-Tf in the basal chamber, there is no ferroxidase activity in the basal media that would promote a conversion of ferrous to ferric iron for apo-Tf binding. Nonetheless, this does not change the interpretation that apo-Tf promotes increased release of iron from the endothelial cells.
Our data not only demonstrate regulation of transport, but also suggest that BBB endothelium can store iron and release it in a regulated manner, thus acting as a reservoir of iron for the brain. The ability to store iron in endothelial cells is consistent with our previous reports that ferritin is present in both animal and human brain microvasculature.3 We demonstrate in this study that both apo-Tf and holo-Tf have significant effects on the level of iron storage in the endothelial cells, with apo-Tf decreasing the amount of iron stored while holo-Tf increased the amount of stored. These data, along with our previous reports in animal studies51 provide convincing evidence that the BBB is not a simple conduit for iron transport. Moreover, the data showing apo-Tf and holo-Tf display significant and opposing effects on iron transport and endothelial cell storage of iron suggests the ratio of apo-Tf/holo-Tf is responsible for the regional regulation of iron.
In addition, we demonstrate that hepcidin acts as a regulatory mechanism for the release of free iron from the endothelial cells. Hepcidin functions by causing the only known iron exporter ferroportin to internalize and subsequently be degraded.13,32,52 Our data suggest that hepcidin from the brain parenchyma can robustly decrease the amount of free iron that is released from the endothelial cells. Notably, the regulation by hepcidin seems to affect only the export of free iron, regardless of which protein is used to initially transport iron. This would be consistent with the existing concept for iron release via ferroportin but clearly indicates there is an additional mechanism for protein bound iron release from BBB endothelium. That protein bound iron release is regulated suggests a novel interaction between Tf and ferroportin on the abluminal side of the BBB that will be explored in future studies.
To release free iron via ferroportin, there must be a mechanism within the BBB endothelium to remove iron from both Tf and Hft. The DMT1 was originally characterized to transport a broad range of ions53; however, it has been shown to be a robust endosomal iron transporter in a variety of cell types.14,17 Here, our data reveal that pharmacological inhibition of DMT1 results in separate intracellular fates for Tf and Hft. After receptor-mediated endocytosis of Tf, the Fe-Tf complex is directed to the endosome, where the pH decrease causes Fe3+ to be released from Tf and reduced to Fe2+. Subsequently, the free Fe is exported out of the endosome by DMT1 to be used in a variety of cellular processes, stored within ferritin, exported as free iron via ferroportin, or exported from the cell as Tf-bound iron.8 Our data support these pathways for iron, as inhibition of DMT1 dramatically lowers the level of protein-bound iron transported through the BBB endothelium while dramatically increasing the amount of iron retained in the endothelial cells. Furthermore, DMT1 inhibition results in drastic increases in the amount of iron retained within the cell during transport, providing further evidence for involvement of DMT1 in intracellular pathways. The interrogation of a relationship between DMT1 and Hft iron delivery has not been explored in any cell type to our knowledge. Here, we provide evidence that Hft-mediated transport of iron is significantly impacted by DMT1 inhibition. The data reveal that DMT1 inhibition pushes the intracellular steady-state towards transport of protein-bound iron, which suggests multiple fates for Hft within the endothelial cell after internalization. After endocytosis, Hft is likely localized to the endo-lysosome to be degraded, where the iron contained within is exported from the endosome via DMT1 and from the cell via ferroportin. However, blockage of DMT1 may result in a lack of degradation of Hft, thereby causing the entire protein being exported from the cell. Though we have provided strong evidence for the existence of a novel mechanism of Hft transport, further studies are required to fully characterize the intracellular pathways.
In this study, we also provide evidence that BBB endothelial cells can secrete Tf and Hft. These data could further significantly alter the paradigm for brain iron uptake as it suggests proteins synthesized in BBB endothelium bind iron within these cells and release it into the brain. The concept of Tf being synthesized for release by endothelial cells is consistent with our previous report that Tf mRNA is present in human brain microvasculature.3 The presence of mRNA for Hft has also been detected in human brain microvasculature and was considered as evidence for iron storage capacity of these cells, but now this concept may be expanded to secretion based on the findings in this study.54,55 Though it is clear from the immunoblot analysis that Hft is secreted by BBB endothelium, one explanation for why a similar result was not found in the autoradiograph may stem from the loss of 59Fe nucleation after protein denaturation. Clearly, synthesis and subsequent secretion of Tf and Hft by BBB endothelium demonstrates yet another level of regulation for iron transport to the brain and endothelial cells, which may function as a critical checkpoint.
The results of this study on iron transport at the level of the BBB have significant implications in treating iron-related brain disorders, as well as efforts to use the iron transport system in the brain to deliver therapeutics and contrast agents. Our data indicate that the brain has the ability to control the amount of iron transport and release and thus attempts to increase brain iron may be limited. This concept of “brain side” control could limit the effectiveness of iron supplementation strategies for overcoming behavioral and cognitive impairment associated with developmental iron deficiency56,57 and also for treating neurological disorders such as restless legs syndrome.58,59 Rather, strategies may instead need to focus on targeting release mechanisms in BBB endothelial cells. Moreover, technologies have been developed to harness the iron transport system in the brain to treat neurodegenerative diseases such as the use of bispecific antibodies against TfR in an attempt to alter deposition of β-amyloid in Alzheimer's disease,60,61 or deliver chemotherapeutic agents to treat glioblastoma.62 Both of these strategies are centered on the Tf transcytosis model that, based on our data, is not a direct transport model. However, the present data do increase support for the use of Hft as an effective protein for delivering agents to the brain.
In summary, these data present novel findings about regulation of brain iron uptake and transport across the BBB, providing evidence for Hft as a novel iron delivery protein and the beginning for mechanistic studies addressing this novel uptake mechanism. Finally, we have also identified a novel function of BBB endothelium to secrete Tf and Hft, further challenging the established transcytosis paradigm and revealing a heretofore unappreciated relationship between the BBB endothelium and brain iron uptake.
Acknowledgements
The authors would like to thank Drs. Kari Duck and Achuthamangalan Madhankumar for technical assistance. The authors would also like to thank the Microscopy Imaging Core Lab at Penn State College of Medicine for imaging assistance. The DMT1 inhibitor was provided by Xenon Pharmaceuticals.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this study was provided by NIH R01 NS077678 (JRC). The hiPSC lines used in this study were developed by funding from NIH/NIEHS RO1 ES016931 (ABB) and RO1 ES010563 (ABB). EHN is supported by a National Science Foundation Graduate Research Fellowship.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JC is the founder and chairman of the board of Sidero Biosciences LLC, a company with a product involving oral delivery of ferritin for management of iron deficiency.
Authors' contributions
BC performed all experiments; EHN, ABB, and ESL contributed cell lines and reagents; BC, IS, and JC designed research; All authors analyzed data, edited, and contributed to writing the paper.
References
- 1.Almutairi MMA, Gong C, Xu YG, et al. Factors controlling permeability of the blood–brain barrier. Cell Mol Life Sci 2016; 73: 57–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beard JL, Dawson H, Piñero DJ. Iron metabolism: a comprehensive review. Nutr Rev 1996; 54: 295–317. [DOI] [PubMed] [Google Scholar]
- 3.Connor JR, Ponnuru P, Wang X-S, et al. Profile of altered brain iron acquisition in restless legs syndrome. Brain 2011; 134: 959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hadziahmetovic M, Song Y, Ponnuru P, et al. Age-dependent retinal iron accumulation and degeneration in hepcidin knockout mice. Investig Ophthalmol Vis Sci 2011; 52: 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hulet S, Powers S, Connor J. Distribution of transferrin and ferritin binding in normal and multiple sclerotic human brains. J Neurol Sci 1999; 165: 48–55. [DOI] [PubMed] [Google Scholar]
- 6.Haider L. Inflammation, iron, energy failure, and oxidative stress in the pathogenesis of multiple sclerosis. Oxid Med Cell Longev 2015; 2015: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacKenzie EL, Iwasaki K, Tsuji Y. Intracellular iron transport and storage: from molecular mechanisms to health implications. Antioxid Redox Signal 2008; 10: 997–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simpson IA, Ponnuru P, Klinger ME, et al. A novel model for brain iron uptake: introducing the concept of regulation. J Cereb Blood Flow Metab 2015; 35: 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarthy RC, Kosman DJ. Iron transport across the blood–brain barrier: development, neurovascular regulation and cerebral amyloid angiopathy. Cell Mol Life Sci 2015; 72: 709–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiandra L, Mazzucchelli S, Truffi M, et al. In vitro permeation of FITC-loaded ferritins across a rat blood-brain barrier: a model to study the delivery of nanoformulated molecules. J Vis Exp 2016; 22: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher J, Devraj K, Ingram J, et al. Ferritin: a novel mechanism for delivery of iron to the brain and other organs. Am J Physiol Cell Physiol 2007; 293: C641–C649. [DOI] [PubMed] [Google Scholar]
- 12.Taylor EM, Morgan EH. Developmental changes in transferrin and iron uptake by the brain in the rat. Dev brain Res 1990; 55: 35–42. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy RC, Kosman DJ. Glial cell ceruloplasmin and hepcidin differentially regulate iron efflux from brain microvascular endothelial cells. PLoS One 2014; 9: e89003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleming MD, Romano MA, Su MA, et al. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci U S A 1998; 95: 1148–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trinder D, Oates PS, Thomas C, et al. Localisation of divalent metal transporter 1 (DMT1) to the microvillus membrane of rat duodenal enterocytes in iron deficiency, but to hepatocytes in iron overload. Gut 2000; 46: 270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X-B, Hill P, Haile DJ. Role of the ferroportin iron-responsive element in iron and nitric oxide dependent gene regulation. Blood Cells Mol Dis 2002; 29: 315–326. [DOI] [PubMed] [Google Scholar]
- 17.Linder MC. Mobilization of stored iron in mammals: a review. Nutrients 2013; 5: 4022–4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lippmann ES, Azarin SM, Kay JE, et al. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat Biotechnol 2012; 30: 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lippmann ES, Al-Ahmad A, Azarin SM, et al. A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci Rep 2014; 4: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollmann EK, Bailey AK, Potharazu AV, et al. Accelerated differentiation of human induced pluripotent stem cells to blood–brain barrier endothelial cells. Fluids Barriers CNS 2017; 14: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vatine GD, Al-Ahmad A, Barriga BK, et al. Modeling psychomotor retardation using iPSCs from MCT8-deficient patients indicates a prominent role for the blood-brain barrier. Cell Stem Cell 2017; 20: 831–843.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim RG, Quan C, Reyes-Ortiz AM, et al. Huntington's disease iPSC-derived brain microvascular endothelial cells reveal WNT-mediated angiogenic and blood-brain barrier deficits. Cell Rep 2017; 19: 1365–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lippmann ES, Al-Ahmad A, Palecek SP, et al. Modeling the blood–brain barrier using stem cell sources. Fluids Barriers CNS 2013; 10: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar KK, Lowe EW, Aboud AA, et al. Cellular manganese content is developmentally regulated in human dopaminergic neurons. Sci Rep 2015; 4: 6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen G, Gulbranson DR, Hou Z, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Meth 2011; 8: 424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lippmann ES, Estevez-Silva MC, Ashton RS. Defined human pluripotent stem cell culture enables highly efficient neuroepithelium derivation without small molecule inhibitors. Stem Cells 2014; 32: 1032–42. [DOI] [PubMed] [Google Scholar]
- 27.Todorich B, Zhang X, Connor JR. H-ferritin is the major source of iron for oligodendrocytes. Glia 2011; 59: 927–935. [DOI] [PubMed] [Google Scholar]
- 28.Todorich B, Zhang X, Slagle-Webb B, et al. Tim-2 is the receptor for H-ferritin on oligodendrocytes. J Neurochem 2008; 107: 1495–1505. [DOI] [PubMed] [Google Scholar]
- 29.Duck KA, Simpson IA, Connor JR. Regulatory mechanisms for iron transport across the blood-brain barrier. Biochem Biophys Res Commun 2017; 494: 70–75. [DOI] [PubMed] [Google Scholar]
- 30.Srinivasan B, Kolli AR, Esch MB, et al. TEER measurement techniques for in vitro barrier model systems. J Lab Autom 2015; 20: 107–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cadieux JA, Zhang Z, Mattice M, et al. Synthesis and biological evaluation of substituted pyrazoles as blockers of divalent metal transporter 1 (DMT1). Bioorgan Med Chem Lett 2012; 22: 90–95. [DOI] [PubMed] [Google Scholar]
- 32.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004; 306: 2090–2093. [DOI] [PubMed] [Google Scholar]
- 33.Ganz T. Hepcidin and its role in regulating systemic iron metabolism. Hematology 2006; 2006: 29–35. [DOI] [PubMed] [Google Scholar]
- 34.Skjørringe T, Burkhart A, Johnsen KB, et al. Divalent metal transporter 1 (DMT1) in the brain: implications for a role in iron transport at the blood-brain barrier, and neuronal and glial pathology. Front Mol Neurosci 2015; 8: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Rensburg SJ, Kotze MJ, van Toorn R. The conundrum of iron in multiple sclerosis – time for an individualised approach. Metab Brain Dis 2012; 27: 239–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Todorich B, Pasquini JM, Garcia CI, et al. Oligodendrocytes and myelination: the role of iron. Glia 2009; 57: 467–478. [DOI] [PubMed] [Google Scholar]
- 37.Moos T, Morgan EH. A morphological study of the developmentally regulated transport of iron into the brain. Dev Neurosci 2002; 24: 99–105. [DOI] [PubMed] [Google Scholar]
- 38.Geninatti Crich S, Cadenazzi M, Lanzardo S, et al. Targeting ferritin receptors for the selective delivery of imaging and therapeutic agents to breast cancer cells. Nanoscale 2015; 7: 6527–33. [DOI] [PubMed] [Google Scholar]
- 39.Uchida M, Flenniken ML, Allen M, et al. Targeting of cancer cells with ferrimagnetic ferritin cage nanoparticles. J Am Chem Soc 2006; 128: 16626–16633. [DOI] [PubMed] [Google Scholar]
- 40.Bellini M, Mazzucchelli S, Galbiati E, et al. Protein nanocages for self-triggered nuclear delivery of DNA-targeted chemotherapeutics in cancer cells. J Control Rel 2014; 196: 184–196. [DOI] [PubMed] [Google Scholar]
- 41.Chen TT, Li L, Chung D-H, et al. TIM-2 is expressed on B cells and in liver and kidney and is a receptor for H-ferritin endocytosis. J Exp Med 2005; 202: 955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han J, Seaman WE, Di X, et al. Iron uptake mediated by binding of H-ferritin to the TIM-2 receptor in mouse cells. PLoS One 2011; 6: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chiou B, Lucassen E, Sather M, et al. Semaphorin4A and H-ferritin utilize Tim-1 on human oligodendrocytes: a novel neuro-immune axis. Glia 2018; 66: 1317–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Unger EL, Earley CJ, Thomsen LL, et al. Effects of IV iron isomaltoside-1000 treatment on regional brain iron status in an iron-deficient animal. Neuroscience 2013; 246: 179–185. [DOI] [PubMed] [Google Scholar]
- 45.Wypijewska A, Galazka-Friedman J, Bauminger ER, et al. Iron and reactive oxygen species activity in parkinsonian substantia nigra. Parkinson Relat Disord 2010; 16: 329–333. [DOI] [PubMed] [Google Scholar]
- 46.Snyder AM, Wang X, Patton SM, et al. mitochondrial ferritin in the substantia nigra in restless legs syndrome. J Neuropathol Exp Neurol 2009; 68: 1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Connor JR, Boyer PJ, Menzies SL, et al. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology 2003; 61: 304–309. [DOI] [PubMed] [Google Scholar]
- 48.Peters DG, Connor JR, Meadowcroft MD. The relationship between iron dyshomeostasis and amyloidogenesis in Alzheimer's disease: two sides of the same coin. Neurobiol Dis 2015; 81: 49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hametner S, Wimmer I, Haider L, et al. Iron and neurodegeneration in the multiple sclerosis brain. Ann Neurol 2013; 74: 848–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams R, Buchheit CL, Berman NEJ, et al. Pathogenic implications of iron accumulation in multiple sclerosis. J Neurochem 2012; 120: 7–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duck KA, Neely EB, Simpson IA, et al. A role for sex and a common HFE gene variant in brain iron uptake. J Cereb Blood Flow Metab 2018; 38: 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raha-Chowdhury R, Raha AA, Forostyak S, et al. Expression and cellular localization of hepcidin mRNA and protein in normal rat brain. BMC Neurosci 2015; 16: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gunshin H, Mackenzie B, Berger UV, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 1997; 388: 482–488. [DOI] [PubMed] [Google Scholar]
- 54.Han J, Day JR, Thomson K, et al. Iron deficiency alters H- and L-ferritin expression in rat brain. Cell Mol Biol 2000; 46: 517–528. [PubMed] [Google Scholar]
- 55.Han J, Day JR, Connor JR, et al. H and L ferritin subunit mRNA expression differs in brains of control and iron-deficient rats. J Nutr 2002; 132: 2769–2774. [DOI] [PubMed] [Google Scholar]
- 56.Hergüner S, Keleşoğlu FM, Tanıdır C, et al. Ferritin and iron levels in children with autistic disorder. Eur J Pediatr 2012; 171: 143–146. [DOI] [PubMed] [Google Scholar]
- 57.Camaschella C. Iron deficiency: new insights into diagnosis and treatment. Hematology 2015; 2015: 8–13. [DOI] [PubMed] [Google Scholar]
- 58.Li X, Allen RP, Earley CJ, et al. Brain iron deficiency in idiopathic restless legs syndrome measured by quantitative magnetic susceptibility at 7 tesla. Sleep Med 2016; 22: 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mehmood T, Auerbach M, Earley CJ, et al. Response to intravenous iron in patients with iron deficiency anemia (IDA) and restless leg syndrome (Willis–Ekbom disease). Sleep Med 2014; 15: 1473–1476. [DOI] [PubMed] [Google Scholar]
- 60.Yu YJ, Atwal JK, Zhang Y, et al. Therapeutic bispecific antibodies cross the blood-brain barrier in nonhuman primates. Sci Transl Med 2014; 6: 1–10. [DOI] [PubMed] [Google Scholar]
- 61.Zuchero YJY, Chen X, Bien-Ly N, et al. Discovery of novel blood-brain barrier targets to enhance brain uptake of therapeutic antibodies. Neuron 2016; 89: 70–82. [DOI] [PubMed] [Google Scholar]
- 62.Voth B, Nagasawa DT, Pelargos PE, et al. Transferrin receptors and glioblastoma multiforme: current findings and potential for treatment. J Clin Neurosci 2015; 22: 1071–1076. [DOI] [PubMed] [Google Scholar]