Abstract
Background.
In response to a mumps outbreak at the University of Iowa and surrounding community, university, state, and local health officials implemented a vaccination campaign targeting students <25 years of age with an additional dose of measles-mumps-rubella (MMR) vaccine. More than 4700 vaccine campaign doses were administered; 97% were documented third doses. We describe the epidemiology of the outbreak before and after the campaign, focusing on cases in university students.
Methods.
Mumps cases were identified from reportable disease databases and university health system records. Detailed information on student cases was obtained from interviews, medical chart abstractions, university and state vaccination records, and state public health laboratory results. Pre- and postcampaign incidence among students, university faculty/staff, and community members <25 vs ≥25 years old were compared using Fisher exact test. Multivariable regression modeling was performed to identify variables associated with a positive mumps polymerase chain reaction test.
Results.
Of 453 cases in the county, 301 (66%) occurred in university students. Student cases were primarily undergraduates (90%) and highly vaccinated (86% had 2 MMR doses, and 12% had 3 MMR doses). Fewer cases occurred in students after the campaign (75 [25%]) than before (226 [75%]). Cases in the target group (students <25 years of age) declined 9% postcampaign (P = .01). A positive mumps polymerase chain reaction test was associated with the presence of parotitis and early sample collection, and inversely associated with recent receipt of MMR vaccine.
Conclusions.
Following a large additional dose MMR vaccination campaign, fewer mumps cases occurred overall and in the target population.
Keywords: mumps, parotitis, outbreak, measles-mumps-rubella vaccine, MMR
Mumps is a vaccine-preventable illness that classically presents with parotitis and can result in meningitis, hearing loss, orchitis, oophoritis, and mastitis [1, 2]. Since the 1989 recommendation of 2 doses of measles-mumps-rubella (MMR) vaccine by the Advisory Committee on Immunization Practices (ACIP) [3], reported cases of mumps have declined by 99%. A resurgence in US mumps cases has occurred in the past decade; in 2016, >6300 cases were reported in 47 states [4–6]. Outbreaks frequently affect people in close-contact settings, including universities [7].
Students attending postsecondary educational institutions are at increased risk for exposure to or transmission of mumps. ACIP recommends that all students entering these settings receive 2 doses of MMR vaccine, or have other evidence of immunity before enrollment [8]. Campaigns to administer additional doses of MMR vaccine have been implemented previously in mumps outbreaks among highly vaccinated populations, but data on effectiveness are limited [9–11].
On 20 July 2015, 2 cases of mumps were reported in male students at the University of Iowa. The case count increased to 12 students by the start of the fall semester, and 6 cases were identified in county residents without university affiliation. The university, county, and state health departments coordinated outbreak response including early case recognition, testing, and isolation. For example, a case in a community daycare staff member prompted letters to parents and coworkers about mumps signs and symptoms, prevention, and the importance of vaccination. University students not up to date with 2 doses of MMR were sent frequent reminders to obtain the vaccine, and a complimentary vaccine dose was provided to approximately 120 students who requested a third MMR dose early during the outbreak. Additionally, a targeted MMR vaccine dose intervention was attempted in 1 fraternity in early October 2015 (24 of 100 members were vaccinated). Despite these efforts, by 1 November 2015, there were 114 student cases throughout the university, primarily in students <25 years old, prompting officials to launch a widespread MMR vaccination campaign.
We describe the outbreak response investigation for student cases, examine case age distribution before and after the campaign, and assess predictors of positive mumps reverse-transcription polymerase chain reaction (RT-PCR) testing.
METHODS
Outbreak Setting
Johnson County, Iowa, has a population of almost 140 000 residents. The University of Iowa is located in Iowa City within Johnson County; >22 000 undergraduates (and 30 000 total students) are enrolled. The university’s mandatory 2-dose MMR vaccination policy was introduced in 2003. In 2012, the university fully implemented an electronic compliance system that requires clinician documentation of 2 MMR vaccine doses or evidence of immunity to register for spring semester courses.
Measles-Mumps-Rubella Vaccination Campaign
University, local, and state health officials held regular meetings to coordinate outbreak response, including deliberating and ultimately deciding to implement an MMR vaccination campaign targeted to students <25 years old. The campaign consisted of 8 free vaccination clinics held at diverse and central locations on campus, during daytime and evening hours, from 10 to 19 November 2015. During the campaign, 4782 total MMR doses were administered, of which 97% were documented third MMR doses. Of 19 705 university students aged 18–24 years with 2 MMR doses at the start of the fall semester, 24% received a third dose during the 2015–2016 academic calendar year [12].
Case Identification and Classification
Case-finding activities included review of local and state health department reportable illness databases, State Hygienic Laboratory at the University of Iowa (SHL) mumps RT-PCR test results, and university health system records. University and health department records were used to categorize cases as university students, staff, or Johnson County residents without university affiliation (community cases). Cases were classified as confirmed or probable using the Council of State and Territorial Epidemiologists case definition [13].
Student Case Investigation
As most cases were reported in the university student population, the investigation elicited detailed demographic, exposure, clinical symptoms, and complication information on student cases with phone interviews and medical chart abstractions. Student case vaccination status was verified using the university’s proprietary electronic vaccination database, as well as Iowa’s Immunization Registry Information System and university health system electronic medical records. Vaccination data were examined to ensure age-appropriate administration, adherence to minimum intervals between doses, and to avoid duplication of dates; when necessary, copies of original vaccination records were reviewed manually.
Laboratory Testing
SHL performed mumps RT-PCR on buccal swab specimens using a laboratory-developed test based on a short hydrophobic (SH) primer assay developed at the Centers for Disease Control and Prevention (CDC) [14]. Specimens were collected by massaging the parotid gland area for 30 seconds before collecting secretions with a Universal Transport Media/Viral Transport Kit rigid straight flocked swab. SHL also performed serologic mumps testing for immunoglobulin M (IgM) antibodies using an indirect fluorescent antibody assay (PanBio, EMD Millipore, Billerica, Massachusetts). A positive mumps test was determined by either positive buccal swab RT-PCR test or positive mumps IgM serologic result. Ten RT-PCR–positive samples were genotyped by the Minnesota Public Health Laboratory using previously described methods [15–17].
Statistical Analyses
The analysis described here focuses on mumps cases both at the University of Iowa as well as in the community of Johnson County; the vaccine effectiveness of the third MMR dose in the student body population is reported elsewhere [12]. To examine case count overall and by age, before and after the immunization campaign, we defined the preintervention period as beginning with the first case on 13 July 2015, and ending on 10 December 2015, 21 days after the final mass immunization clinic. The 21-day postvaccination interval has been used in prior outbreaks to assess effectiveness of additional vaccine doses, and covers the average mumps incubation period of 16–18 days (range, 12–25 days) [9, 10, 18, 19]. We defined the postintervention period as 11 December 2015 through the final day of the spring semester on 13 May 2016. The postintervention period included a winter break (19 December 2015–18 January 2016), when students left campus. Some students, however, did remain on campus for athletic training and events and winter session classes. Cases continued to be reported during this period. To standardize the pre- and postintervention comparison, age was calculated as of the date of the first vaccination campaign (10 November 2015). We compared the median age of cases and the proportion of cases in the campaign-targeted age range (<25 years old) pre- vs postintervention using Wilcoxon rank-sum test and Fisher exact test, respectively.
Predictors of a positive mumps RT-PCR test were examined using multivariable logistic regression. Predictor variables considered in the model were age; presence of parotitis, fever, illness duration, complication, and/or severe mumps illness (defined as illness resulting in a complication and/or >1 healthcare visit); time between illness onset and laboratory sample collection; time between illness onset and most recent vaccination; and vaccination within 180 days prior to illness onset.
Ethical Review
The outbreak response activities were determined to be public health nonresearch by the Iowa Department of Public Health and CDC.
RESULTS
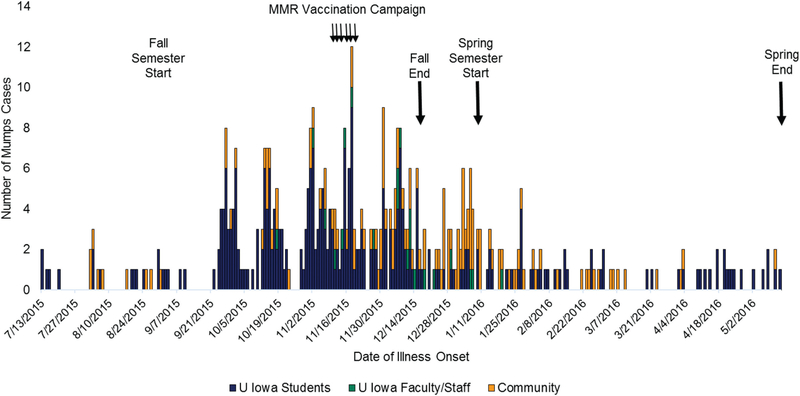
From 13 July 2015 to 13 May 2016, 453 mumps cases were reported in Johnson County: 301 (66%) were university students, 21 (5%) were nonstudent university faculty or staff, and 131 (29%) were community members (Figure 1). Three hundred cases (66%) were confirmed and 153 (34%) were probable.
Figure 1.
Mumps cases in Johnson County, Iowa, 13 July 2015–13 May 2016. Adapted from [12]. Abbreviation: MMR, measles-mumps-rubella.
Student Case Investigation
At the time of illness, 36 student cases (12%) had 3 MMR doses, 260 (86%) had 2 doses, 2 (0.7%) had 1 dose, and 2 (0.7%) had 0 doses; 1 had an unknown vaccination history. Of the 36 cases with a third MMR dose, 14 became ill 1–21 days after the third dose, and 18 became ill 22–122 days after the third dose.
Demographic and academic information was obtained from university records (Table 1). A slightly higher percentage of mumps cases were undergraduates (90%) than in the fall semester student population (75% undergraduates). For the other characteristics examined, mumps cases were representative of the underlying student population.
Table 1.
Characteristics of University of Iowa Student Mumps Cases, 13 July 2015–13 May 2016 (n = 301)
| Student Cases | No. (%) |
|---|---|
| Gender | |
| Male | 146 (48) |
| Female | 155 (52) |
| Race | |
| White non-Hispanic | 237 (79) |
| African American | 7 (2) |
| Hispanic/Latino | 19 (6) |
| Asian | 4 (1) |
| Multiracial | 11 (4) |
| Unknown | 23 (8) |
| Age, y | |
| Median | 21 |
| Range | 18–32 |
| Interquartile range | 20–22 |
| Level of study | |
| Undergraduate | 271 (90) |
| Graduate | 11 (4) |
| Professional | 19 (6) |
| College | |
| Liberal Arts | 205 (68) |
| Business | 33 (11) |
| Engineering | 28 (9) |
| Other | 35 (12) |
Of 219 students who provided data regarding housing, activities, and mumps contacts (Table 2), 207 (94%) lived with a roommate or housemate. A minority reported living in university-owned dormitories (n = 33 [15%]) or a fraternity or sorority house (n = 12 [5%]), although a larger percentage were members of a fraternity or sorority (n = 75 [34%]). Seventy-six (34%) reported contact with at least 1 other person who had mumps, including 47 with prolonged face-to-face contact (defined as <2 feet for >2 hours), and 20 who reported direct saliva exchange.
Table 2.
Potential Exposures for Mumps Infection Among University of Iowa Student Mumps Cases, 13 July 2015–13 May 2016 (n = 219)
| Student Cases With Exposure Responses | No. (%) |
|---|---|
| Household size | |
| Shared (roommate or housemate) | 207 (94) |
| Median No. of roommates (range)a | 1 (1–3) |
| Median No. of housemates (range) | 3 (1–8) |
| Single occupant (solo) | 12 (6) |
| Housing type | |
| Apartment | 118 (54) |
| House | 56 (26) |
| Dormitory | 33 (15) |
| Fraternity or sorority house | 12 (5) |
| University activitiesb | |
| Fraternity or sorority membership | 75 (34) |
| Sports (collegiate or intramural) | 38 (17) |
| Clubs and organizations | 34 (16) |
| Contact with person with mumps | |
| Yes | 76 (34) |
| Including extended contactb,c | 47 |
| Including shared saliva contactb | 20 |
| No | 94 (43) |
| No response | 49 (22) |
| Employment | |
| University employment | 55 (25) |
| Nonuniversity employment | 62 (28) |
| None | 102 (47) |
Data regarding number of housemates were excluded for individuals who reported residence in a dormitory or fraternity/sorority house.
Responses were not mutually exclusive.
Contact within 2 feet for >2 hours.
Further clinical information was obtained from case interviews (n = 223 [74%]) and/or medical chart abstractions (n = 269 [89%]) (Table 3). Parotitis and fever were present in 280 (98%) and 160 (56%) cases, respectively. Complications included meningitis (n = 1), transient hearing loss (n = 3), orchitis (n = 15), and mastitis (n = 2) [20]. Median illness duration, defined as time from symptom onset to full resolution, was 8 days (interquartile range [IQR], 6–10 days), and median period of self-isolation after diagnosis was 6 days (IQR, 5–7 days). Two hundred seventy-five (96%) students were recommended to isolate by their provider, as documented in the medical record (n = 232) and/or determined by case recall (n = 211).
Table 3.
Clinical Symptoms, Complications, Healthcare Use, and Laboratory Testing for University of Iowa Student Mumps Cases, 13 July 2015–13 May 2016
| Characteristic | Self-Reported (n = 223) | Clinician-Documented (n = 269) | Self-Reported or Clinician-Documented (n = 287) |
|---|---|---|---|
| Symptoms | |||
| Parotid swellinga | 203 (91) | 247 (92) | 280 (98) |
| Parotid pain | 189 (85) | 235 (87) | 270 (94) |
| Fever | 125 (56) | 77 (29) | 160 (56) |
| Complications | |||
| Orchitisb | 14 | 2 | 15 (11) |
| Meningitisc | 0 | 1 | 1 (0.3) |
| Transient hearing lossd | 3 | 1 | 3 (1) |
| Mastitise | 2 | 0 | 2 (0.7) |
| Duration of illness, d | |||
| Median (interquartile range) | 8 (6–10) | ||
| Minimum and maximum | 2–32 | ||
| Period of self-isolation, d | |||
| Median (interquartile range) | 6 (5–7) | ||
| Minimum and maximum | 1–32 | ||
| Healthcare utilization related to mumps diagnosisf | |||
| Additional healthcare visit for mumps symptoms <1 week prior to diagnosis | 15 (5) | ||
| Additional healthcare visit after diagnosis for continued/worsening symptoms | 10 (3) | ||
| Emergency room follow-up for mumps-related illness | 5 (2) |
Data are presented as No. (%) unless otherwise indicated.
The 7 cases that did not have parotid swelling presented with parotid pain [5], jaw pain [3], and/or other salivary gland swelling [3].
Of 107 male cases interviewed and asked if they had “pain, swelling or inflammation of the testes,” 9 cases reported testicular swelling and 5 cases reported testicular pain without swelling. Of 131 male cases with medical chart abstractions, 2 clinicians documented orchitis.
Confirmed by cerebrospinal fluid analysis.
One case had hearing loss documented by a formal hearing test; this case was treated with oral corticosteroids and reported full resolution of hearing loss after a few days.
Cases were asked if they had “pain, swelling or infection of the breast tissue.”
Healthcare utilization totals derived from a combination of case interviews and medical records. Medical records were not available to confirm all reported visits.
Medical records were reviewed for additional clinical visits and management of complications. Fifteen cases had >1 clinical visit for the same or similar symptoms <1 week prior to their mumps diagnosis visit, but were not formally diagnosed until the second visit; 10 were seen after their diagnosis visit for continued or worsening symptoms. Five cases had a follow-up emergency room visit: 1 for meningitis, 1 for a suspected parotid abscess (computed tomography scan was negative for abscess, and showed acute right parotitis and inflammation of right submandibular gland), and 3 for pain control and dehydration resulting from poor oral intake secondary to parotitis. Two cases were hospitalized after mumps diagnosis, 1 for dehydration 10 days later, and 1 for lower extremity weakness 30 days later, though neither hospitalization was ultimately attributed to mumps.
Impact of Vaccination Campaign
Fewer cases among students occurred in the 5 months after the intervention (n = 75 [25%]) than the 5 months before the intervention (n = 226 [75%]) (Table 4). Postintervention student cases were less likely to be in the campaign-targeted age group of <25 years (92%) than preintervention cases (98%) (P = .018). Conversely, while there were fewer cases overall among nonstudents, there was no postintervention decrease in cases nor in the proportion of cases in faculty/staff (P = 1.0) and community (P = .27) among persons <25 years old.
Table 4.
Mumps Cases in the Pre- and Postintervention Periods, by Campaign-Targeted Age Group—Johnson County, Iowa, 13 July 2015–13 May 2016a
| Characteristic | Precampaign Period (13 July 2015–10 December 2015) | Postcampaign Period (11 December 2015–13 May 2016) | P Value |
|---|---|---|---|
| Students, No. | 226 | 75 | |
| Median age (range) | 21 (18–27) | 21 (18–30) | .05 |
| Age <25 y, No. (%) | 222 (98) | 69 (92) | .018 |
| Faculty/staff, No. | 11 | 10 | |
| Median age (range) | 24 (20–43) | 24 (22–54) | .60 |
| Age <25 y, No. (%) | 6 (55) | 6 (60) | 1 |
| Community, No. | 59 | 72 | |
| Median age (range) | 23 (7–67) | 24 (12–73) | .17 |
| Age <25 y, No. (%) | 36 (61) | 37 (51) | .27 |
University students <25 years old were targeted for an outbreak dose of measles-mumps-rubella vaccine in a vaccination campaign held 10–19 November 2015. Age at the start of the vaccination campaign (10 November 2015) used for analysis.
Laboratory Testing
Laboratory tests were performed for 249 (83%) student cases; 245 had RT-PCR testing and 13 had IgM serologic testing. Ten RT-PCR positive specimens were genotyped and found to have identical sequences; all were genotype G, by far the most frequently detected mumps genotype reported from 45 US states in 2015–2016 (Rota and McNall, unpublished data).
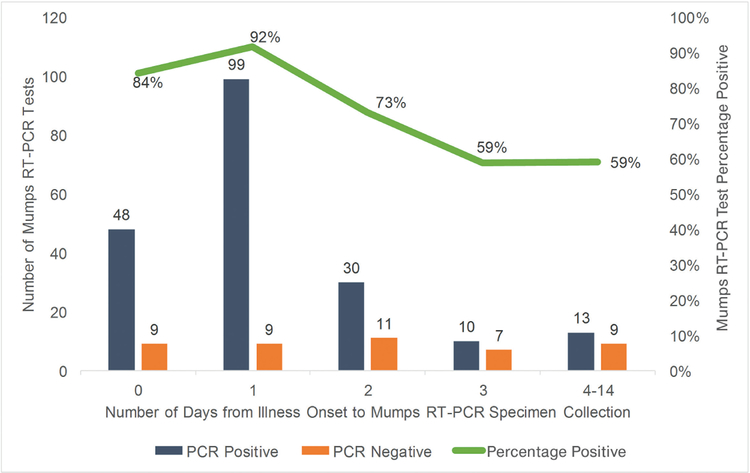
Among the 245 specimens tested for mumps with RT-PCR, 206 (84%) were tested within the first 3 days of symptom onset (Figure 2). Specimens collected 0–2 days after symptom onset were significantly more likely to test positive (86%) than specimens collected 3–14 days after symptom onset (59%) (P < .0001).
Figure 2.
Mumps reverse-transcription polymerase chain reaction (RT-PCR) test results, by days of illness between symptom onset and sample collection.
In multivariable logistic regression examining predictors of a positive RT-PCR test, statistically significant predictors included parotitis, less time from illness onset to specimen collection, and the absence of recent MMR vaccination (defined as <180 days prior to illness onset) (Table 5). Cases with parotitis had 11 times greater odds of a positive RT-PCR test than cases without parotitis (adjusted odds ratio [AOR], 11.2; 95% confidence interval [CI], 2.0–85.6; P = .008). Cases with delayed collection of specimens had lower odds of a positive PCR result; each additional day that passed from illness onset to specimen collection decreased the odds of a positive RT-PCR test by 23% (AOR 0.77; 95% CI, .65–.89; P = .002). Cases that received a recent (<180 days) MMR vaccination had one-third the odds of a positive RT-PCR result (AOR, 0.34; 95% CI, .13–.92; P = .03).
Table 5.
Predictors of a Positive Mumps Reverse-Transcription Polymerase Chain Reaction Test in Students With Probable or Confirmed Mumps, University of Iowa Student Mumps Cases, 13 July 2015–13 May 2016a
| Binary Regression | Multivariable Regression | |||||
|---|---|---|---|---|---|---|
| Predictor Variable | OR | (95% CI) | P Value | AOR | (95% CI) | P Value |
| Parotitis | 12.0 | (2.3–64.0) | .004 | 11.2 | (2.0–85.6) | .008 |
| Fever | 1.6 | (.86–3.1) | .14 | |||
| Any complication | 1.3 | (.35–4.5) | .73 | |||
| Illness length (each additional day) | 1.03 | (.94–1.2) | .55 | |||
| Severe illness | 1.09 | (.47–2.5) | .85 | |||
| Time to laboratory collection (each additional day) | 0.77 | (.65–.90) | .002 | 0.77 | (.65–.89) | .002 |
| Time between last MMR vaccine and illness (each additional year) | 1.05 | (.99–1.1) | .09 | |||
| Last MMR vaccination <180 d prior to illness onset | 0.38 | (.16–.93) | .03 | 0.34 | (.13–.92) | .03 |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; MMR, measles-mumps-rubella; OR, odds ratio.
Two hundred twenty-one University of Iowa students had a mumps polymerase chain reaction test result.
CONCLUSIONS
From 13 July 2015 to 13 May 2016, Johnson County, Iowa, experienced a mumps outbreak with 453 cases; 70% were in persons affiliated with the University of Iowa, and 30% in Johnson County residents. Student cases, similar to the overall student population, were highly vaccinated: 98% had ≥2 doses of MMR prior to the onset of illness. Following a wide-scale vaccination campaign targeting students <25 years of age that began just prior to the highest peak of the outbreak, postintervention cases among students were less likely to be in the campaign-targeted age group of <25 years, and the overall case count declined in the spring semester.
The outbreak in Johnson County was part of the largest statewide outbreak in Iowa since 2006, when 1963 cases were reported [6, 21]. Prior to 2015, Iowa reported <40 mumps cases annually from 2007 to 2014. The number of cases in Johnson County would likely have been much larger in the absence of several key factors. The university maintains extremely high vaccination coverage in students via its compliance system, which mandates documentation of 2 MMR doses for registration in spring semester courses. The university and local health department rapidly increased outbreak awareness through provider alerts and communication with students, parents, community health providers, and community organizations. Relatedly, the university health system deployed effective triage, testing, and isolation protocols, decreasing transmission. University policies contributed to the high adherence to isolation recommendations by students. The uninfected roommates of student cases residing in dormitories were removed so case patients could isolate in their current residence. Student cases could order food online for delivery to dormitories, houses, and apartments to ensure isolation was not compromised. Professors were instructed to accept student self-report of mumps illness as justification to miss class or reschedule exams.
University, local, and state health departments met weekly to coordinate efforts to plan and implement the MMR vaccination campaign that likely further limited the spread of the outbreak. Iowa health officials provided >4700 free MMR doses to the population most affected (university students <25 years old) during early and mid-November. Five of the 6 mass vaccination clinics occurred before the highest peak in the outbreak, and the campaign was followed temporally by a substantial decline in cases, particularly among students in the campaign-targeted age group. In contrast, case counts did not decline in faculty/ staff or community members of all ages, nor among those <25 years old who were not targeted by the campaign. Studies of 2 previous outbreaks, in Guam and New York in 2009–2010, reported declines in the case count after outbreak dose interventions [9, 10] but, in both instances, the campaigns occurred after the outbreak peak.
Breakthrough mumps cases are not unexpected, even in highly vaccinated populations. The estimated median vaccine effectiveness of the MMR vaccine in preventing mumps is 78% for 1 dose (range, 49%–91%) and 88% for 2 doses (range, 66%–95%) [8]. Waning immunity in young adults vaccinated as children has been demonstrated in the literature [22–27]. Universities provide a setting with close contact, shared living conditions, and social behaviors that contribute to a high intensity of exposure that may increase risk of transmission and propagation of outbreaks. In this outbreak, most student cases shared housing with a roommate or housemate. One-third of cases among students interviewed reported contact with someone who was ill with mumps. Though many reported either extended face-to-face contact or direct saliva contact with a person who had mumps, the true contact rate may be underestimated due to contact with asymptomatic cases and a reluctance to report close contact.
Almost 30% of cases occurred in Johnson County residents. These individuals’ vaccination statuses are unknown; it is possible they were not as highly vaccinated as students, which could have contributed to propagation of the outbreak. Students and community members also likely mixed in daily interactions, suggested by the high proportion of students residing in apartments and houses alongside community members and who reported working outside the university.
These findings provide insight into the clinical manifestations and complications of mumps in a highly vaccinated population. Most cases were mild, and 15 cases were given alternative diagnoses prior to mumps, which could delay outbreak recognition and control. Complication rates for orchitis (11%), hearing loss (1%), and meningitis (0.3%) were consistent with other vaccine-era outbreaks [8, 9, 28, 29]. Although lower than prevaccine era rates, these are a reminder that mumps infection can lead to serious complications [19]. Our case interviews identified more cases with complications, particularly orchitis and hearing loss, than would have otherwise been obtained from medical records, suggesting that patients do not always seek care even in the event of a complication.
The outbreak investigation provides further information to inform laboratory testing during mumps outbreaks. Collectively, these findings can help clinicians and health departments determine testing protocols and interpretation of test results in the setting of a mumps outbreak.
Our model suggests that 3 factors affect the outcome of RT-PCR testing: parotitis, time from symptom onset, and no recent MMR vaccination. A negative RT-PCR test in the absence of parotitis may reflect that the concentration of mumps RNA in the clinical sample was below the limit of detection for the RT-PCR assay or case misclassification (the person not truly having mumps). Each additional day from symptom onset to specimen collection reduced the test positivity rate by 23%, with a notable decline in sensitivity when samples were collected after the first 3 days of illness, consistent with RT-PCR test performance in prior outbreaks [30]. Our findings support the recommendation to collect buccal swab specimens for RT-PCR testing as soon as possible after symptom onset [31].
The impact of recent vaccination on RT-PCR test results could have several explanations. Recent vaccination could have protected individuals from mumps virus, in which case their symptoms could have an alternate etiology—such as Epstein-Barr virus or influenza—among other viruses detected in sporadic, though infrequently epidemic, cases of parotitis [32]. Alternatively, despite recent vaccination, individuals could have been infected with mumps virus, but experienced shorter or less viral shedding (or both) resulting from the vaccination immune boost.
Our findings are subject to limitations. First, as our investigation was observational, the association between the vaccination campaign and the decline in cases is temporal, and we cannot directly attribute case count declines to the intervention. Other factors, including the natural evolution of the outbreak, dispersal of students during winter break, and changes in individual risk behavior as the outbreak progressed, could have contributed to the decreased cases after the campaign. Similarly, as nonstudents may have different opportunities for mumps exposure and risks of transmission, the observed difference in the student and nonstudent age distribution is not necessarily due to the presence or absence of the vaccination campaign. Second, some individuals with mumps illness may not have visited a healthcare provider, leading to underreporting and possible underestimation of case counts. Third, data on clinical symptoms, complications, and vaccination were only available for university students, not university faculty/staff and community members. Nonetheless, the breadth of clinical and demographic data collected and the timing of the outbreak dose campaign for university students in a context of high 2-dose vaccination coverage provide unique and useful information to understand vaccine-era mumps outbreaks.
In conclusion, the mumps outbreak in Johnson County, Iowa, included cases in university student, faculty/staff, and community members, and a wide-scale vaccination campaign was implemented in the university just prior to the highest peak of the outbreak. Shared housing, close contact, and social mixing among university students were common and likely contributed to outbreak propagation; high 2-MMR-dose coverage and coordinated outbreak response by university, local, and state officials likely helped reduce outbreak size. After the outbreak dose campaign, overall and age-targeted student cases declined. As mumps outbreaks continue to occur in highly vaccinated populations similar to this university setting, further evaluations of the impact of vaccination campaigns are needed to guide public health response.
Acknowledgments.
We thank the following University of Iowa students: Amy Hoehne, Geneva Wilson, Kelsey Fillman, Danielle Riley, Claire Weidman, Kelsey Saddoris, Jessica Smith, Brittney Donovan, Daniel Suh, Blake Smith, Maya Ramaswamy, Erin Renfrew, Shad Shadwilliam, Eleanor Ginn, Nicole Westergaard, Adrijana Pusnik, Chase Kooyam, Mitchell Lefebvre, Morgan Bobb, Ravish Patel, Christie De Vito, Abigail Narayan, Anna Rizvi-Toner, Bryan Anderson; Jessica Rudd, at the Centers for Disease Control and Prevention (CDC); Dave Koch, at the Johnson County Public Health Department; Kelli Smith, at the Iowa Department of Public Health; and many members of the Johnson County Public Health Department, Iowa Department of Public Health, Iowa State Hygienic Laboratory and Minnesota Public Health Laboratory.
Financial support. This investigation did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Footnotes
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Rubin SA, Plotkin S. Mumps vaccine. In: Plotkin O, Orenstein W, Offit PA, eds. Vaccines. 6th ed. Philadelphia, PA: Saunders, 2012:419–46. [Google Scholar]
- 2.Fiebelkorn AB, Albert B, Hickman C, Bellini W. Chapter 9: Mumps. Manual for the surveillance of vaccine-preventable diseases, 5th ed. Atlanta, GA: Centers for Disease Control and Prevention, 2012. [Google Scholar]
- 3.Measles prevention. MMWR Suppl 1989; 38:1–18. [PubMed] [Google Scholar]
- 4.Dayan GH, Quinlisk MP, Parker AA, et al. Recent resurgence of mumps in the United States. N Engl J Med 2008; 358:1580–9. [DOI] [PubMed] [Google Scholar]
- 5.Barskey AE, Glasser JW, LeBaron CW. Mumps resurgences in the United States: a historical perspective on unexpected elements. Vaccine 2009; 27:6186–95. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention, Division of Health Informatics and Surveillance. National notifiable diseases surveillance system. Atlanta, GA: Available at: https://www.cdc.gov/mmwr/mmwr_nd/nd_data_tables.html. Accessed 28 June 2017. [Google Scholar]
- 7.Centers for Disease Control and Prevention. Mumps cases and outbreaks Available at: https://www.cdc.gov/mumps/outbreaks.html. Accessed 25 February 2017.
- 8.McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2013; 62:1–34. [PubMed] [Google Scholar]
- 9.Nelson GE, Aguon A, Valencia E, et al. Epidemiology of a mumps outbreak in a highly vaccinated island population and use of a third dose of measles-mumps-rubella vaccine for outbreak control—Guam 2009 to 2010. Pediatr Infect Dis J 2013; 32:374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogbuanu IU, Kutty PK, Hudson JM, et al. Impact of a third dose of measles-mumps-rubella vaccine on a mumps outbreak. Pediatrics 2012; 130:e1567–74. [DOI] [PubMed] [Google Scholar]
- 11.Albertson JP, Clegg WJ, Reid HD, et al. Mumps outbreak at a university and recommendation for a third dose of measles-mumps-rubella vaccine—Illinois, 2015–2016. MMWR Morb Mortal Wkly Rep 2016; 65:731–4. [DOI] [PubMed] [Google Scholar]
- 12.Cardemil CV, Dahl R, James L, Wannemuehler K, Gary HE Jr, Shah M, Marin M, Riley J, Feikin D, Patel M, Quinlisk P. Effectiveness of a third dose of MMR vaccine for mumps outbreak control. NEJM 2017; In press. [DOI] [PMC free article] [PubMed]
- 13.Council of State and Territorial Epidemiologists. Mumps 2012 case definition. Available at: https://wwwn.cdc.gov/nndss/conditions/mumps/case-definition/2012/. Accessed 25 February 2017.
- 14.Boddicker JD, Rota PA, Kreman T, et al. Real-time reverse transcription-PCR assay for detection of mumps virus RNA in clinical specimens. J Clin Microbiol 2007; 45:2902–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin L, Beard S, Brown DW. Genetic heterogeneity of mumps virus in the United Kingdom: identification of two new genotypes. J Infect Dis 1999; 180:829–33. [DOI] [PubMed] [Google Scholar]
- 16.Mumps virus nomenclature update: 2012. Wkly Epidemiol Rec 2012; 87:217–24. [PubMed] [Google Scholar]
- 17.Jin L, Örvell C, Myers R, et al. Genomic diversity of mumps virus and global distribution of the 12 genotypes. Rev Med Virol 2015; 25:85–101. [DOI] [PubMed] [Google Scholar]
- 18.Wharton M, Cochi SL, Hutcheson RH, Bistowish JM, Schaffner W. A large outbreak of mumps in the postvaccine era. J Infect Dis 1988; 158:1253–60. [DOI] [PubMed] [Google Scholar]
- 19.Fiebelkorn AP, Lawler J, Curns AT, Brandeburg C, Wallace GS. Mumps postex-posure prophylaxis with a third dose of measles-mumps-rubella vaccine, Orange County, New York, USA. Emerg Infect Dis 2013; 19:1411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donahue M, Schneider A, Ukegbu U, et al. Notes from the field: complications of mumps during a university outbreak among students who had received 2 doses of measles-mumps-rubella vaccine—Iowa, July 2015-May 2016. MMWR Morb Mortal Wkly Rep 2017; 66:390–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Center for Acute Disease Epidemiology, Iowa Department of Public Health. Iowa Disease Surveillance System (IDSS). Des Moines: Iowa Department of Public Health, Center for Acute Disease Epidemiology. [Google Scholar]
- 22.Cohen C, White JM, Savage EJ, et al. Vaccine effectiveness estimates, 2004–2005 mumps outbreak, England. Emerg Infect Dis 2007; 13:12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LeBaron CW, Forghani B, Beck C, et al. Persistence of mumps antibodies after 2 doses of measles-mumps-rubella vaccine. J Infect Dis 2009; 199:552–60. [DOI] [PubMed] [Google Scholar]
- 24.Davidkin I, Jokinen S, Broman M, Leinikki P, Peltola H. Persistence of measles, mumps, and rubella antibodies in an MMR-vaccinated cohort: a 20-year follow-up. J Infect Dis 2008; 197:950–6. [DOI] [PubMed] [Google Scholar]
- 25.Cortese MM, Jordan HT, Curns AT, et al. Mumps vaccine performance among university students during a mumps outbreak. Clin Infect Dis 2008; 46:1172–80. [DOI] [PubMed] [Google Scholar]
- 26.Vygen S, Fischer A, Meurice L, et al. Waning immunity against mumps in vaccinated young adults, France 2013. Euro Surveill 2016; 21:30156. [DOI] [PubMed] [Google Scholar]
- 27.Rubin S, Kennedy R, Poland G. Emerging mumps infection. Pediatr Infect Dis J 2016; 35:799–801. [DOI] [PubMed] [Google Scholar]
- 28.Barskey AE, Schulte C, Rosen JB, et al. Mumps outbreak in Orthodox Jewish communities in the United States. N Engl J Med 2012; 367:1704–13. [DOI] [PubMed] [Google Scholar]
- 29.Gouma S, Hahné SJ, Gijselaar DB, Koopmans MP, van Binnendijk RS. Severity of mumps disease is related to MMR vaccination status and viral shedding. Vaccine 2016; 34:1868–73. [DOI] [PubMed] [Google Scholar]
- 30.Rota JS, Rosen JB, Doll MK, et al. Comparison of the sensitivity of laboratory diagnostic methods from a well-characterized outbreak of mumps in New York city in 2009. Clin Vaccine Immunol 2013; 20:391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Laboratory testing for mumps infection. Available at: https://www.cdc.gov/mumps/lab/qa-lab-test-infect.html. Accessed 16 June 2016.
- 32.Barskey AE, Juieng P, Whitaker BL, et al. Viruses detected among sporadic cases of parotitis, United States, 2009–2011. J Infect Dis 2013; 208:1979–86. [DOI] [PubMed] [Google Scholar]