Abstract
Background
Preterm birth has been linked to increased risk of autism spectrum disorders (ASD), but how this risk changes with gestational age at birth has not been well-characterized, especially with regard to co-occurring intellectual disability (ID).
Methods
Register-based cohort study of singleton births in 1984–2007 in Stockholm County, Sweden (N total: 480,728; n ASD: 10,025). We assessed overall and sex-specific gestational week specific prevalence estimates and risk ratios of ASD with and without ID.
Results
Preterm and postterm births were associated with elevated risk of ASD, and the relationship between gestational age at birth and ASD with and without ID differed in males and females. Risk of ASD without ID was higher in preterm births among both sexes and decreased continuously with increasing length of gestation. Risk of ASD with ID was higher in both preterm and postterm births among both sexes, with postterm birth in females being more highly associated with ASD with ID than that in males.
Conclusions
The relationship between gestational age at birth and ASD differs by the presence/absence of co-occurring ID and fetal sex. Both preterm and postterm birth are associated with increased risk of ASD. Risk of ASD is not constant within conventionally defined gestational age at birth periods. Further research on mechanism underlying these associations is needed.
Keywords: Autism Spectrum Disorders, Intellectual Disability, Gestational Age, Pre-term, Post-term, Generalized Additive Model
Introduction
Autism spectrum disorders (ASD) are early-onset neurodevelopmental conditions with diverse behavioral presentations and multifactorial etiology.1 ASD can be reliably diagnosed by two years of age.1 It has been suggested that the development of ASD without co-occurring intellectual disability (ID, defined as IQ < 70) may involve a stronger familial component than that of ASD with ID.2 Influences on the gestational environment, including fetal growth deviance,3 infection,4 inflammation,5 and maternal exposure to environmental toxins6 have been proposed to play a role in the etiology of ASD and co-occurring ID. In a recent study of a Stockholm-based cohort, we estimated that approximately 26% of those with ASD have co-occurring ID.7 The presence/absence of ID is considered to be the most critical factor affecting outcomes in this population.8
Gestational age at birth measures the duration of pregnancy and also is an established indicator of fetal growth maturity at birth. Preterm (gestational age at birth < 37 weeks) and postterm (gestational age at birth ≥ 42 weeks) births account for about 10% and 7% of all births worldwide, respectively.9,10 Although the causes of preterm and postterm births have not been fully identified, genetics, maternal reproductive history, maternal health before and during pregnancy, as well as child characteristics including fetal sex have been reported to influence pregnancy duration.9–12 Both preterm and postterm births have been linked to elevated risk of ASD. For example, one Danish population-based study of over 1.7 million births reported that compared to individuals born at 40 weeks, the risk of ASD among those born at 24–27 weeks and 42–43 weeks were 3–14.7 and 1.0–1.2 times higher, respectively.13 A recent meta-analysis identified 14 original research articles investigating the association between postterm birth and risk of ASD.14 Of these, 7 reported null findings, 6 reported a positive association, and 1 reported a negative association. The pooled estimate suggested a slight increase in relative risk (RR) of ASD associated with postterm birth, although the 95% confidence interval (CI) was wide (RR 1.14; 95% CI: 0.58, 2.24). Furthermore, phenotypic differences by sex have been noted in ASD,15 and pregnancies with boys tend to be shorter.11 However, prior investigations have not examined how associations may differ by sex for ASD with and without co-occurring ID in a large sample.16–18
Though the relationship between preterm birth and risk of ASD has been suggested with categorical parametrizations of gestational age,14 less is known about how risk of ASD changes across the full range of gestational age, especially among postterm births. In this study, we investigated the association between gestational age at birth and ASD with and without ID, and estimated overall and sex-specific prevalence at each week of gestational age at birth for deliveries at 27–43 weeks, using data from a large Swedish population-based record-linkage study. We hypothesized that the relationship between gestational age at birth and ASD without ID would be different from that between gestational age at birth and ASD with ID, and these relationships might be different in males and females.
Methods
Study population
This study was nested within the Stockholm Youth Cohort, an ongoing longitudinal register-linkage cohort study of the total child population aged 0–17 years residing in Stockholm County, Sweden, who were born between 1984 and 2007. The last follow-up date in the study period was December 31, 2011. A full description of the Stockholm Youth Cohort design is available elsewhere.19 Data on ante- and perinatal factors were obtained from the Swedish Medical Birth Register (MBR). Diagnoses of ASD and ID were ascertained using International Classification of Diseases (ICD) and Diagnosis and Statistical Manual of Mental Disorders (DSM) codes (ASD: ICD-9 299, ICD-10 F84, DSM-IV 299; ID: ICD-9 317–319, ICD-10 F70–F79, DSM-IV 317–319) and health services usage records from birth to the latest register update in December 2011. Family relations were identified from the Multi‐Generation Register. Demographic and financial data were obtained from the Integrated Database for Labor Market Research. Ethical approval was obtained from the research ethics committee at Karolinska Institutet.
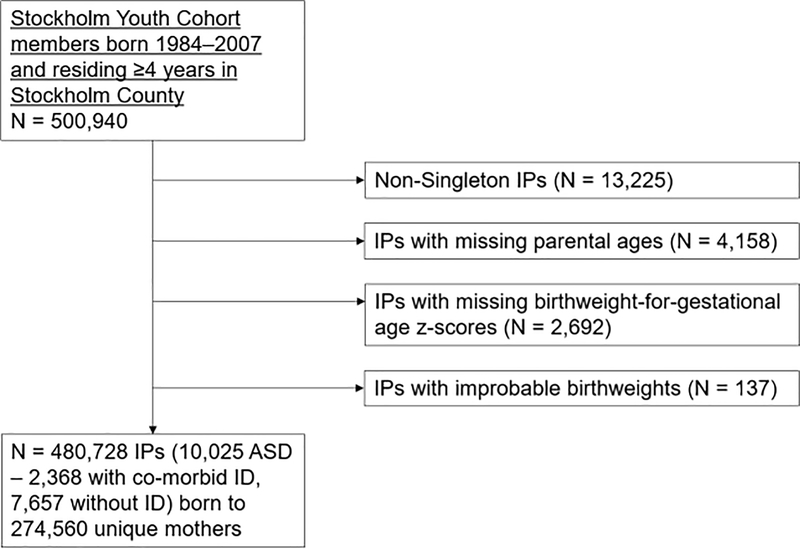
We examined singleton live births with complete data on ASD, ID, gestational age at birth and sex (Ntotal = 480,852, NASD = 10,029). Data on all other variables except maternal smoking were complete. Maternal smoking data were available for 90% of individuals. Figure 1 shows the derivation of the study sample. Because perinatal risk profiles including mortality, gestational age at birth and birthweight are very different between singleton and multiple births,1 we excluded multiple births from the study. Additionally, we excluded 137 individuals with improbable birthweights using methods we have applied in a previous study.20
Figure 1.
Flow chart describing the cohort composition. IP: index person. ID: intellectual disability.
Variables
Gestational age, gestational diabetes, hypertension, parity, birthweight and maternal smoking were obtained from the MBR. The MBR uses information from several variables to hierarchically determine gestational age—first from ultrasound-based estimate, then from date of last menstrual period, pediatric record, and birthweight. About 90% of gestational age at birth data on births during our study period were based on ultrasound results, followed by about 8% that were based on date of last menstrual period.21 Gestational age in weeks rounded to the nearest tenth was used in the analysis. Gestational diabetes (ICD-10 O24.4, ICD-9 648W) and hypertension (ICD-10 I10-I13 and O10-O11, ICD-9 401–404, 642A-642C, ICD-8 400, 401, 402, 403, 404) were identified using ICD codes.19 Parity was categorized as 1, 2 and ≥ 3 in the analysis. Birthweight-for-gestational age z-scores were calculated from birthweight and gestational age at birth data using separate national references for boys and girls.3 Paternal and maternal ages at delivery were calculated based on each parent’s date of birth. Parental history of neurologic and psychiatric disorders data were extracted from regional and national registers as previously described and coded as one binary variable (yes/no history) for each parent.22 Disposable family income data were obtained from the Integrated Database for Labour Market Research, were adjusted for family size and inflation and accounted for all sources of income. This variable was categorized into quintiles based on national data.22 Maternal country of birth data were obtained from the Total Population Register and was categorized as mother “born in Sweden”, “in another European country, or “outside Europe”. Maternal self-reported smoking during pregnancy were obtained from the MBR and were categorized as “yes”, “no” or “unknown”.
Statistical analysis
We used cubic splines in generalised additive models (GAM) to model the association of gestational age at birth with the risk of ASD,23 adjusting for potential confounders identified a priori from the literature.6,14,24 Covariates included in the GAM were: sex-adjusted birthweight-for-gestational age z-scores, offspring sex, birth year, paternal and maternal ages and their interaction22, maternal smoking, paternal and maternal neuropsychiatric histories, parity, gestational diabetes, hypertension, family income quintiles and maternal country of origin. We included a random intercept for sibship in the model to account for clustering of unmeasured familial components in ASD25, preterm12 and postterm26 birth, and conducted a sensitivity analysis only on non-siblings, (i.e., one child from each family was chosen at random to be included in this analysis), using the same variables and model specifications as those used in the analysis of the total sample. Sex-specific associations were assessed using the likelihood ratio test comparing nested models with and without an interaction term between sex and gestational age. In sex-stratified analyses, we used the same model specification to estimate the association of gestational age at birth and risk of ASD with and without co-occurring ID separately in males and females. From expected probabilities of ASD based on the multivariable GAM model, we calculated gestational age at birth--specific prevalence proportions associated with each week.
Results
Sample characteristics
The study sample included 480,728 individuals. A total of 10,025 (2.1%) persons were diagnosed with ASD; of these, 2,368 (23.6%) and 7,657 (76.4%) were diagnosed with and without co-occurring ID, respectively.
There were some differences in sample characteristics by case status (Table 1). For example, individuals with ASD were more likely to be male, first-born, from families with lower income, and born preterm or postterm. Individuals with ASD were also more likely to have mothers with gestational diabetes and hypertension, and parents with a history of neuropsychiatric disorders. The prevalence proportions of preterm and post-term birth were higher among individuals with ASD, especially those with co-occurring ID, than among those without ASD.
Table 1.
Characteristics of the Stockholm Youth Cohort, born 1984–2007.
| Characteristics | No ASD (N = 470,703) | ASD (N = 10,025) | ASD without ID (N = 7,657) | ASD with ID (N = 2,368) |
|---|---|---|---|---|
| Male, n (%) | 239330 (50.8) | 7103 (70.9) | 5406 (70.6) | 1697 (71.7) |
| Birthweight-for-gestational age z-score, mean (SD a) | 0 (1) | −0.1 (1.1) | 0 (1.1) | −0.2 (1.2) |
| Maternal age (years) at delivery, mean (SD) | 29.8 (5.2) | 29.7 (5.5) | 29.7 (5.5) | 29.8 (5.6) |
| Paternal age (years) at delivery, mean (SD) | 32.8 (6.3) | 32.8 (6.7) | 32.5 (6.6) | 33.5 (6.9) |
| Gestational age at birth b, n (%) | ||||
| Extremely preterm (<28 weeks) | 580 (0.1) | 49 (0.5) | 28 (0.4) | 21 (0.9) |
| Very preterm (28 – <32 weeks) | 1193 (0.2) | 51 (0.5) | 34 (0.4) | 17 (0.7) |
| Moderate to late preterm (32 – <37 weeks) | 18943 (4.0) | 541 (5.4) | 397 (5.2) | 144 (6.1) |
| Term (37 – <42 weeks) | 413721 (87.9) | 8458 (85.2) | 6577 (85.9) | 1971 (83.2) |
| Postterm (≥42 weeks) | 36386 (7.7) | 840 (8.4) | 624 (8.1) | 216 (9.1) |
| Parity, n (%) | ||||
| 1 | 211913 (45.0) | 4863 (48.5) | 3866 (50.5) | 997 (42.1) |
| 2 | 170729 (36.3) | 3299 (32.9) | 2451 (32.0) | 848 (35.8) |
| ≥3 | 88061 (18.7) | 1863 (18.6) | 1340 (17.5) | 523 (22.1) |
| Gestational hypertension, n (%) | 14140 (3.0) | 424 (4.2) | 345 (4.5) | 79 (3.3) |
| Gestational diabetes, n (%) | 2986 (0.6) | 110 (1.1) | 80 (1.0) | 30 (1.3) |
| Maternal smoking during pregnancy c, n (%) | 65867 (14.0) | 1634 (16.3) | 1298 (17.0) | 336 (14.2) |
| Maternal neuropsychiatric history, n (%) | 29773 (6.3) | 916 (9.1) | 741 (9.7) | 175 (7.4) |
| Paternal neuropsychiatric history, n (%) | 22096 (4.7) | 666 (6.6) | 526 (6.9) | 140 (5.9) |
| Family income, n (%) | ||||
| Q1 (Lowest) | 68602 (14.6) | 1585 (15.8) | 1072 (14.0) | 513 (21.7) |
| Q2 | 73429 (15.6) | 1835 (18.3) | 1371 (17.9) | 464 (19.6) |
| Q3 | 74611 (15.9) | 1810 (18.1) | 1423 (18.6) | 387 (16.3) |
| Q4 | 94207 (20.0) | 1976 (19.7) | 1581 (20.7) | 395 (16.7) |
| Q5 (Highest) | 159854 (34.0) | 2819 (28.1) | 2210 (28.9) | 609 (25.7) |
| Maternal region of origin, n (%) | ||||
| Born in Sweden | 358164 (76.1) | 7743 (77.2) | 6191 (80.9) | 1552 (65.5) |
| Born in Europe outside Sweden | 39276 (8.3) | 895 (8.9) | 657 (8.6) | 238 (10.1) |
| Born outside Europe | 73263 (15.6) | 1387 (13.8) | 809 (10.6) | 578 (24.4) |
| Birth year d, n (%) | ||||
| 1984 – 1988 | 83504 (98.5) | 1249 (1.5) | 890 (1.1) | 359 (0.4) |
| 1989 – 1993 | 108302 (97.8) | 2474 (2.2) | 1816 (1.6) | 658 (0.6) |
| 1994 – 1998 | 94569 (97.1) | 2871 (2.9) | 2206 (2.3) | 665 (0.6) |
| 1999 – 2003 | 94486 (97.7) | 2266 (2.3) | 1814 (1.9) | 452 (0.4) |
| 2004 – 2007 | 89842 (98.7) | 1165 (1.3) | 931 (1.0) | 234 (0.3) |
SD: standard deviation.
The minimum and maximum gestational age at birth was 23 and 45 weeks, respectively.
10.7% and 11.0% of maternal self-reported smoking during pregnancy data were missing among individuals with and without ASD diagnosis, respectively.
Birth year was summarized in this table as birth year intervals and included in the GAM model as a continuous variable. Column-wise proportions were calculated to present prevalence of ASD with and without ID over birth year periods.
The pattern of case distribution by gestational age at birth differed by sex (Supplementary Table 1), although caution is warranted due to smaller strata sizes. Fewer females than males were affected by ASD with and without ID. Preterm and postterm births were more prevalent in both sexes among persons with ASD with and without ID.
Gestational age at birth and risk of ASD with and without ID
As shown in Table 2, the estimated prevalence of ASD decreased monotonically in births between 27 and 40 weeks, and then increased monotonically in births between 41 and 43 weeks. In Supplementary Table 2, we present results from sensitivity analysis on non-siblings. Prevalence estimates were slightly attenuated after accounting for unmeasured familial factors, but the trend of elevated risk in preterm and postterm births persisted. Estimated ASD prevalence was substantially higher for very preterm and postterm births compared to infants born at 40 weeks. For example, in Table 2, among individuals born at 27 and 43 weeks, the expected prevalence of ASD was 52.4 (95% CI: 42.3, 64.6) and 23.1 (95% CI: 21.3, 25.0) per 1,000, respectively; among those born at 40 weeks, the prevalence was estimated to be 19.8 (95% CI: 19.3, 20.3) per 1,000. Also, the risk of co-occurring ID among cases increased with decreasing length of gestation. Among births at 27 weeks, of the expected 52.4 (95% CI: 42.3, 64.6) per 1,000 affected individuals, about 41% of the cases were also expected to be affected by ID; in births at 40 weeks, the expected prevalence of co-occurring ID among cases was about 23%. A visualization of these estimates is provided in Supplementary Figure 1.
Table 2.
Prevalence estimates and associated 95% confidence intervals (CI) by weeks of gestational age at birth for ASD (with or without ID), ASD without ID, and ASD with ID.
| Gestational age at birth (weeks) | ASD Cases per 1,000 (95% CI) | ASD without ID Cases per 1,000 (95% CI) | ASD with ID Cases per 1,000 (95% CI) |
|---|---|---|---|
| 27 | 52.4 (42.3, 64.6) | 32.9 (25.6, 42.5) | 21.3 (14.6, 29.7) |
| 28 | 44.1 (36.4, 53.2) | 29.8 (24.1, 36.8) | 16.0 (11.5, 21.8) |
| 29 | 37.7 (31.7, 44.6) | 27.5 (23.0, 33.3) | 12.7 (9.2, 16.5) |
| 30 | 33.1 (27.7, 38.9) | 25.3 (21.5, 29.7) | 10.4 (7.7, 13.8) |
| 31 | 30.0 (25.1, 35.0) | 23.7 (20.3, 27.7) | 8.9 (6.5, 11.9) |
| 32 | 28.1 (23.9, 32.7) | 22.6 (19.6, 26.2) | 8.1 (6.1, 10.8) |
| 33 | 27.0 (23.7, 30.8) | 21.5 (19.0, 24.3) | 7.7 (6.1, 10.0) |
| 34 | 26.7 (24.0, 29.4) | 20.6 (18.8, 22.5) | 7.6 (6.3, 9.3) |
| 35 | 26.4 (24.5, 28.4) | 19.8 (18.3, 21.3) | 7.5 (6.6, 8.8) |
| 36 | 25.8 (24.3, 27.3) | 18.9 (17.8, 20.1) | 7.3 (6.4, 8.1) |
| 37 | 24.4 (23.3, 25.6) | 18.0 (17.2, 19.0) | 6.6 (6.0, 7.3) |
| 38 | 22.5 (21.7, 23.3) | 17.1 (16.5, 17.7) | 5.7 (5.4, 6.2) |
| 39 | 20.9 (20.3, 21.4) | 16.3 (15.8, 16.7) | 5.0 (4.7, 5.3) |
| 40 | 19.8 (19.3, 20.3) | 15.7 (15.3, 16.1) | 4.6 (4.3, 4.8) |
| 41 | 19.8 (19.3, 20.3) | 15.5 (15.1, 16.0) | 4.6 (4.4, 4.8) |
| 42 | 20.9 (20.1, 21.6) | 15.7 (15.0, 16.3) | 5.1 (4.7, 5.5) |
| 43 | 23.1 (21.3, 25.0) | 16.1 (15.1, 17.3) | 6.2 (5.3, 7.3) |
Gestational age at birth and risk of ASD with/without ID in girls and boys
Results from the sex-stratified analysis are presented in Table 3. The relationship between gestational age at birth and ASD without ID did not differ by sex (likelihood ratio test p-value = 1), but the association between gestational age at birth and ASD with ID was different between males and females (likelihood ratio test p-value = 0.02). Expected prevalence of ASD without ID decreased continuously as gestational age at birth increased in both sexes. Risk of ASD with ID increased among postterm births in both males and females, with the strength of association in females being stronger. The expected prevalence of ASD with ID was 6.7 (95% CI: 6.3, 7.1) and 8.0 (95% CI: 6.7, 9.5) per 1,000 among males born at 40 and 43 weeks, respectively; in other words, the risk of ASD with ID among males born at 43 weeks was about 1.2 times as high as the risk of ASD with ID among boys born at 40 weeks. This risk ratio (RR) among females born in these two gestational age at birth weeks was 1.4. In Supplementary Table 3, we presented this RR and its associated 95% CI, as well as RR estimates for births in other weeks of gestation between the 27th and the 43rd weeks. Visualizations for these estimates are provided in Supplementary Figure 2.
Table 3.
Prevalence estimates and associated 95% confidence intervals (CI) by weeks of gestational age at birth for ASD overall, and ASD with and without ID in males and females.
| Gestational age at birth (weeks) | ASD Cases per 1,000 (95% CI) | ASD without ID Cases per 1,000 (95% CI) | ASD with ID Cases per 1,000 (95% CI) | |||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| 27 | 72.1 (56.4, 92.4) | 29.8 (19.5, 44.7) | 46.7 (34, 63.7) | 17.7 (13.3, 23.5) | 29.1 (19.5, 45) | 11.2 (5.7, 22.1) |
| 28 | 59.9 (47.9, 73.9) | 25.8 (17.9, 36.8) | 42.6 (32.6, 55.9) | 16.9 (13, 22.3) | 21.9 (15.3, 32.1) | 8.6 (4.8, 15.8) |
| 29 | 49.8 (41.1, 61.4) | 22.8 (16.6, 31.2) | 39.2 (31.4, 49.7) | 16.2 (12.5, 20.9) | 16.7 (11.6, 23.9) | 7.3 (4.1, 12.8) |
| 30 | 43.2 (34.5, 54.3) | 20.7 (15.3, 28.1) | 36.3 (29.7, 43.9) | 15.4 (12.2, 19.7) | 13.3 (9, 19.2) | 6.3 (3.4, 11.5) |
| 31 | 39.1 (32.1, 47.7) | 19.5 (14.2, 26.5) | 33.6 (28.0, 40.3) | 14.7 (12.1, 18.0) | 11.2 (7.8, 17.1) | 5.9 (3.3, 10.3) |
| 32 | 36.8 (30.8, 44.3) | 18.7 (14.5, 24.8) | 31.2 (27.0, 36.2) | 14.0 (11.8, 16.7) | 10.1 (7.3, 14.2) | 5.9 (3.6, 9.7) |
| 33 | 35.7 (31.0, 40.8) | 18.4 (14.6, 22.8) | 29.5 (26.1, 33.6) | 13.3 (11.4, 15.6) | 9.9 (7.5, 13.1) | 6.1 (4.1, 9.1) |
| 34 | 35.5 (31.6, 39.8) | 18.1 (15.3, 21.5) | 27.9 (25.1, 30.9) | 12.7 (11.2, 14.8) | 9.7 (7.8, 12) | 6.2 (4.5, 8.3) |
| 35 | 35.1 (31.9, 38.4) | 17.7 (15.5, 20.2) | 26.6 (24.5, 29.1) | 12.2 (10.9, 13.6) | 9.6 (8.1, 11.5) | 6.1 (4.7, 7.6) |
| 36 | 34.2 (31.9, 36.7) | 16.7 (14.9, 18.8) | 25.4 (23.6, 27.3) | 11.6 (10.7, 12.7) | 9.4 (8, 10.9) | 5.5 (4.3, 6.7) |
| 37 | 32.6 (30.7, 34.4) | 15.4 (14.2, 16.8) | 24.3 (23.0, 25.6) | 11.1 (10.2, 12) | 8.7 (7.7, 9.8) | 4.5 (3.8, 5.3) |
| 38 | 30.6 (29.3, 31.9) | 13.9 (13.1, 14.9) | 23.3 (22.3, 24.4) | 10.6 (10, 11.2) | 7.8 (7.2, 8.5) | 3.6 (3.2, 4.1) |
| 39 | 28.9 (28.0, 29.9) | 12.7 (12.1, 13.3) | 22.6 (21.7, 23.3) | 10.1 (9.7, 10.6) | 7.1 (6.7, 7.6) | 2.9 (2.7, 3.2) |
| 40 | 27.9 (26.9, 28.7) | 11.9 (11.4, 12.4) | 22.0 (21.3, 22.6) | 9.7 (9.3, 10.1) | 6.7 (6.3, 7.1) | 2.6 (2.3, 2.8) |
| 41 | 27.7 (26.8, 28.5) | 11.6 (11.1, 12.2) | 21.6 (20.9, 22.3) | 9.2 (8.8, 9.7) | 6.7 (6.3, 7.1) | 2.6 (2.3, 2.8) |
| 42 | 28.4 (27.0, 29.8) | 11.9 (11.0, 12.8) | 21.5 (20.5, 22.6) | 8.8 (8.2, 9.5) | 7.2 (6.5, 7.8) | 2.9 (2.5, 3.3) |
| 43 | 29.9 (27.7, 32.7) | 12.6 (10.9, 14.4) | 21.5 (19.9, 23.5) | 8.4 (7.7, 9.2) | 8.0 (6.7, 9.5) | 3.6 (2.7, 4.8) |
Comment
Principal findings
In this large population-based study, we investigated the relationship between gestational age at birth and ASD with and without ID and estimated ASD prevalence for births in each week of gestational age at birth. We also investigated whether these relationships differed in males and females. In summary, preterm and postterm births were associated with elevated risk of ASD with and without ID, and the pattern of these associations differed by sex. Risk of ASD without ID was higher in preterm births in both sexes and decreased continuously with increasing length of gestation. Risk of ASD with ID was higher in both preterm and postterm births in both sexes, with postterm birth in females being more highly associated with ASD with ID than that in males.
Interpretation
Consistent with previous findings,13,16 we observed a positive association between preterm birth and higher risk of ASD. Atladóttir reported an inverse association between gestational age at birth and ASD with a risk curve whose shape was very similar to the observations reported here; however, the investigators did not separately consider ASD with and without ID.16 D’Onofrio investigated the association of gestational age at birth with ASD and other morbidities using data on over 3 million singleton births in Sweden between 1973 and 2008.13 In analyses using categories of gestational age, the investigators reported that compared to the risk of ASD for individuals born at 37–42 weeks, the risk for those born at 23–27 weeks, 28–30 weeks, 31–32 weeks, and 33–36 weeks were 3.2, 2.4, 1.7, and 1.3 times higher, respectively, also consistent with our results. Potential mechanisms that could explain this association include maternal health factors such as maternal pre-pregnancy obesity or infection during pregnancy,4,6 but this remains speculative.
These findings support a positive association between postterm birth and higher risk of ASD. Gardener pooled results from 14 studies and reported an expected increase in the risk of ASD by 14% among postterm births.14 In our study, individuals born in the 43rd week were 13% more likely to be affected by ASD in reference to those born in the 40th week. Though yet to be replicated, the increase in expected prevalence of ASD was driven mainly by that of ASD with ID in our sample. Potential mechanisms underlying this association may include a greater likelihood of birth complications resulting in brain injuries leading to increased risk of ASD and ID during postterm delivery.11,12 The strength of association between postterm birth and risk of ASD with ID was modest, and our continuous, flexible parameterization of gestational age at birth offered advantages in detecting weaker associations. Prior studies using categorical gestational age at birth intervals might have had limited statistical power to estimate this association.27–31 Alternatively, different reproductive and obstetric practices, as well as variations in ascertainment of gestational age at birth, ASD and ID across countries may also have influenced the observed relationships.28–32
In the analysis, we did not observe sex differences in patterns of association between gestational age at birth and risk of ASD without ID. Expected prevalence of ASD regardless of ID status were consistently higher in males than that in females. We did, however, observe different patterns of association between gestational age at birth and ASD with ID in males versus females. Females born postterm were at somewhat higher risk of ASD with ID than males born in the same week. It has been previously reported that ASD with ID might be genetically distinct from ASD with ID,2 and that specific cognitive skills and other ASD phenotypes may vary by sex.15 Our findings suggest that the etiology of ASD with ID may be different in males and females. However, as ASD prevalence is known to exhibit strong male predominance, there were few females with ASD in our sample, so our estimates for females are imprecise and need to be interpreted with caution.
Strengths
The access to a large, population-based, high quality, prospectively collected data on prenatal and parental health, family income and other important measures is a main strength of this study. Multi-register data-linkage of a large volume of prospectively collect data enabled accurate and comprehensive capture of data on gestational age at birth and other pre- and perinatal variables, diagnoses, and parents’ health. Characteristics of our study sample (such as the male predominance in ASD prevalence, higher proportion of persons affected by ASD having parents with neuropsychiatric disorders, and mothers with gestational diabetes and hypertension) were in agreement with existing findings.14,24,29 Also, we were able to analyze the non-linearity of the association between gestational age at birth and ASD using GAMs.
Limitations of the data
As with most observational epidemiologic data, some measurement or misclassification error is expected for gestational age at birth and other variables. However, nearly all gestational age at birth measures were based on ultrasound measures during the prenatal period, so the best estimates were used in the analysis and any measurement error is likely to be small and non-differential with respect to outcome. We restricted our sample to singletons, so it is important to note that our findings cannot necessarily be generalized to individuals from multiple births. Finally, we did not examine potential mediation by general perinatal and neonatal risk factors, such as mode of delivery and more detailed birth complications. Such perinatal factors may increase risk of ASD and therefore may be on the causal pathway between gestational age at birth and ASD.14
Conclusions
We observed that preterm and postterm births were associated with elevated risk of ASD regardless of presence or absence of co-occurring ID, and the relationship between gestational age at birth and ASD with and without ID differed in males and females. Risk of ASD without ID was higher in preterm births, but not postterm births in both sexes. Risk of ASD with ID was higher in both preterm and postterm births in both sexes, with postterm birth in females being more highly associated with ASD with ID than that in males. These findings should be replicated using comparable approaches in other large data sets.
Supplementary Material
Acknowledgements
This study was supported by National Institutes of Health, Grant No. NIH 1 R21 ES023760-01A1, Baily Thomas Charitable Fund, Grant No. TRUST/RNA/AC/KW/3115/5780, and the Swedish Research Council.
Reference
- 1.Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, et al. The epidemiology of autism spectrum disorders. Annual Review of Public Health 2007;28:235–258. [DOI] [PubMed] [Google Scholar]
- 2.Robinson EB, Samocha KE, Kosmicki JA, McGrath L, Neale BM, Perlis RH, et al. Autism spectrum disorder severity reflects the average contribution of de novo and familial influences. Proceedings of the National Academy of Sciences of the United States of America 2014;111:15161–15165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abel KM, Dalman C, Svensson AC, Susser E, Dal H, Idring S, et al. Deviance in fetal growth and risk of autism spectrum disorder. The American Journal of Psychiatry 2013;170:391–398. [DOI] [PubMed] [Google Scholar]
- 4.Lee BK, Magnusson C, Gardner RM, Blomström Å, Newschaffer CJ, Burstyn I, et al. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain, Behavior, and Immunity 2015;44:100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goines PE, Croen LA, Braunschweig D, Yoshida CK, Grether J, Hansen R, et al. Increased midgestational IFN-γ, IL-4 and IL-5 in women bearing a child with autism: A case-control study. Molecular Autism 2011;2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyall K, Schmidt RJ, Hertz-Picciotto I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. International Journal of Epidemiology 2014;43:443–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Idring S, Lundberg M, Sturm H, Dalman C, Gumpert C, Rai D, et al. Changes in prevalence of autism spectrum disorders in 2001–2011: findings from the Stockholm youth cohort. Journal of Autism and Developmental Disorders 2015;45:1766–1773. [DOI] [PubMed] [Google Scholar]
- 8.Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines 2004;45:212–229. [DOI] [PubMed] [Google Scholar]
- 9.Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bulletin of the World Health Organization 2010;88:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galal M, Symonds I, Murray H, Petraglia F, Smith R. Postterm pregnancy. Facts, Views & Vision in ObGyn 2012;4:175–187. [PMC free article] [PubMed] [Google Scholar]
- 11.Benson JB, Haith MM. Diseases and Disorders in infancy and Early Childhood. Academic Press; 2009. [Google Scholar]
- 12.Barth WH. Familial spontaneous preterm birth: further evidence of a complex genetic disease. Obstetrics and Gynecology 2010;115:1114–1115. [DOI] [PubMed] [Google Scholar]
- 13.D’Onofrio BM, Class QA, Rickert ME, Larsson H, Långström N, Lichtenstein P. Preterm birth and mortality and morbidity: a population-based quasi-experimental study. JAMA psychiatry 2013;70:1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics 2011;128:344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubenstein E, Wiggins LD, Lee L-C. A Review of the Differences in Developmental, Psychiatric, and Medical Endophenotypes Between Males and Females with Autism Spectrum Disorder. Journal of Developmental and Physical Disabilities 2015;27:119–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atladóttir HÓ, Schendel DE, Henriksen TB, Hjort L, Parner ET. Gestational Age and Autism Spectrum Disorder: Trends in Risk Over Time. Autism Research: Official Journal of the International Society for Autism Research 2016;9:224–231. [DOI] [PubMed] [Google Scholar]
- 17.Leavey A, Zwaigenbaum L, Heavner K, Burstyn I. Gestational age at birth and risk of autism spectrum disorders in Alberta, Canada. The Journal of Pediatrics 2013;162:361–368. [DOI] [PubMed] [Google Scholar]
- 18.Schendel D, Bhasin TK. Birth weight and gestational age characteristics of children with autism, including a comparison with other developmental disabilities. Pediatrics 2008;121:1155–1164. [DOI] [PubMed] [Google Scholar]
- 19.Idring S, Rai D, Dal H, Dalman C, Sturm H, Zander E, et al. Autism spectrum disorders in the Stockholm Youth Cohort: design, prevalence and validity. PloS One 2012;7:e41280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abel K, Heuvelman H, Wicks S, Rai D, Emsley R, Gardner R, et al. Gestational age at birth and academic performance: population-based cohort study. International journal of epidemiology 2017;46:324–335. [DOI] [PubMed] [Google Scholar]
- 21.National Board of Health, Welfare Centre for Epidemiology. The Swedish Medical Birth Register–a summary of content and quality. National Board of Health and Welfare Stockholm, Sweden; 2003. [Google Scholar]
- 22.Idring S, Magnusson C, Lundberg M, Ek M, Rai D, Svensson AC, et al. Parental age and the risk of autism spectrum disorders: findings from a Swedish population-based cohort. International journal of epidemiology 2014:dyt262. [DOI] [PubMed] [Google Scholar]
- 23.Wood S Generalized additive models: an introduction with R. CRC press; 2006. [Google Scholar]
- 24.Newschaffer CJ, Fallin D, Lee NL. Heritable and nonheritable risk factors for autism spectrum disorders. Epidemiologic Reviews 2002;24:137–153. [DOI] [PubMed] [Google Scholar]
- 25.Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. JAMA 2014;311:1770–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oberg AS, Frisell T, Svensson AC, Iliadou AN. Maternal and fetal genetic contributions to postterm birth: familial clustering in a population-based sample of 475,429 Swedish births. American Journal of Epidemiology 2013;177:531–537. [DOI] [PubMed] [Google Scholar]
- 27.Durkin MS, Maenner MJ, Newschaffer CJ, Lee L-C, Cunniff CM, Daniels JL, et al. Advanced parental age and the risk of autism spectrum disorder. American Journal of Epidemiology 2008;168:1268–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schieve LA, Clayton HB, Durkin MS, Wingate MS, Drews-Botsch C. Comparison of Perinatal Risk Factors Associated with Autism Spectrum Disorder (ASD), Intellectual Disability (ID), and Co-occurring ASD and ID. Journal of Autism and Developmental Disorders 2015;45:2361–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsson HJ, Eaton WW, Madsen KM, Vestergaard M, Olesen AV, Agerbo E, et al. Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. American Journal of Epidemiology 2005;161:916–925– 928. [DOI] [PubMed] [Google Scholar]
- 30.Sugie Y, Sugie H, Fukuda T, Ito M. Neonatal factors in infants with Autistic Disorder and typically developing infants. Autism: The International Journal of Research and Practice 2005;9:487–494. [DOI] [PubMed] [Google Scholar]
- 31.Hultman CM, Sparén P, Cnattingius S. Perinatal risk factors for infantile autism. Epidemiology (Cambridge, Mass.) 2002;13:417–423. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Lv C-C, Tian J, Miao R-J, Xi W, Hertz-Picciotto I, et al. Prenatal and perinatal risk factors for autism in China. Journal of Autism and Developmental Disorders 2010;40:1311–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.