Abstract
Ischemia/reperfusion (I/R) is a major cause of acute kidney injury. Several studies have shown that renin angiotensin (Ang) system and activation of Ang II type 1 receptor (AT1) are involved in various forms of kidney diseases. Likewise, Ang 1-7 as a physiologic antagonist of AT1 and losartan could possibly protect the kidney against I/R damage. Therefore, we investigated renal injury by administering the drugs before and after I/R. Fifty-four male Wistar rats were randomly assigned to five groups as follows. 1, Sham operated; 2, saline group (as a control group); 3, losartan group; 4, Ang 1-7group; and 5, Ang 1-7 + losartan simultaneously. It should be noted that groups 2-5 consisted of two separate I/R-induced subgroups both receiving medication where the first groups received the treatment 15 min before induction of I/R while the medications were given to the second groups immediately after induction of I/R. Twenty four h after I/R, blood samples were collected, and then levels of serum urea nitrogen (BUN), creatinine (Cr), nitrite, malondialdehyde (MDA), lactate dehydrogenase (LDH) and total antioxidant capacity (TAC) were measured. Likewise, nitrite, MDA and TAC were measured in the homogenized kidney tissues. After the induction of I/R, the BUN, Cr, LDH, and kidney tissue damage score increased. Administration of Ang 1-7 alone or simultaneously with losartan decreased the levels of aforementioned factors. Also, kidney MDA and nitrate levels significantly increased after I/R induction (P < 0.05). According to the results of this study, it can be claimed that the effect of losartan in the presence of Mas receptor is statistically significant and kidney damage dramatically decreases.
Keywords: Angiotensin 1-7, Ischemia/reperfusion, Losartan, Renal damage
INTRODUCTION
Ischemia/reperfusion (I/R) is a major cause of acute kidney injury (1). I/R model can be seen in the kidney transplant, septic shock, and cardiovascular surgery (2). This injury often causes serious kidney damage, ultimately leading to the chronic kidney diseases (3,4). The pathology of this complaint is very complex and includes the effects of hypoxia, the death of tubular and interstitial kidney cells, hemodynamic factors, and inflammatory processes (5). Recent evidences suggest that inflammation plays an important role in I/R injury (6). Likewise, several studies have shown that renin angiotensin (Ang) system (RAS) is involved in various forms of kidney diseases. On the other hand, one of the best methods for treatment of these disorders is controlling the effects of angiotensin II (Ang II). Ang II is normally associated with vascular contraction, oxidative stress, inflammation, and fibrosis (7,8). In a similar way, Ang II also plays an important role in I/R injury. Previous studies on renal I/R in rats have shown that RAS is activated after I/R and increases angiotensin II renal level (9). Activation of Ang II type 1 (AT1) receptor has several biological effects, including sodium retention, vascular contraction, glomerular filtration, mesangial cell hypertrophy, and renal damages (10).
Therefore, Ang-converting enzyme inhibitors (ACEi) and AT1 receptor blockers (ARBs) have been administered as the most frequently used therapies to reduce the progression of chronic kidney diseases (11).
The discovery of ACE2, a homologous ACE, and the Mas receptor attached to angiotensin 1-7 are new aspects of RAS. ACE2 converts Ang II to Ang 1-7. Then, Ang 1-7, the active heptapeptide of RAS, plays an important role in kidney function(1). The Mas receptor is usually considered as AT1 receptor antagonist (12,13). Ang 1-7 has vasodilatory, antihypertensive and antiprolifrative activities on the coronary artery (12,14). Moreover, it decreases the pressure response to Ang II in renal afferent arterioles of rabbits (15). Some previous studies reported that infusion of Ang 1-7 can increase renal blood flow without changing blood pressure in rats(14). Other studies have shown that the removal or inhibition of the ACE2 leads to exacerbating kidney diseases, such as diabetic nephropathy, ureteral obstruction, etc (16,17).
Considering the role of Mas and AT1 receptors in the RAS, it is assumed that Ang 1-7 and losartan could protect the kidney against I/R damage. Therefore, in line with the instructions for testing this assumption, this research investigated the effects of losartan, Ang 1-7 or their combination on renal injury.
MATERIALS AND METHODS
Animals
In this research, 54 male Wistar rats, weighting 184.4 ± 7.2 g, were housed at room temperature of 23-25 °C with 12/12-h light/dark cycles and were allowed to acclimatize to the conditions for a week. The animals were fed with rat chow and water ad libitum. The protocol of experiment was approved in advance by the Zahedan University of Medical Sciences Ethics Committee (Ethic No. IR.ZAUMS.REC. 1395.187).
Experimental protocol
Fifty-four male Wistar rats were randomly assigned to five experimental groups. Each of these groups consiststed of 6 animals; namely 1, sham operated group (sham); 2, saline group (as a control group); 3, losartan (Sigma St. Louis, MO, USA) group that received 10 mg/kg interaperitoneally based on the protocol (18); 4, Ang 1-7 (Sigma St. Louis, MO, USA) group treated with Ang 1-7, 50 μg/kg (interaperitoneally) (19); and 5, Ang 1-7 + losartan received these two agents simultaneously. It should be noted that groups 2-5 consisted of two separate I/R-induced subgroups both receiving medication where the first groups received the treatment 15 min before induction of I/R while the medications were given to the second groups immediately after induction of I/R. On the day of I/R induction, the animals were anesthetized with the mixture of xylaxine (10 mg/kg, interaperitoneally) and ketamine (75 mg/kg, interaperitoneally). The skin was incised prior to the removal of lumbar tissues and then the kidneys were cautiously accessible to avoid being hurt. To achieve kidney I/R in animals, the renal artery and vein were instantaneously obstructed in both kidneys, using clamps on the vessels for 45 min. Next, the clamps were detached carefully to ensure establishing blood circulation into the kidneys. The same surgical procedure was done on the animals in all groups except the sham group in which the vessels were not clamped. Twenty four h after the surgical procedures, blood samples were taken from the heart under anesthetization, and the right kidneys were excised and homogenized. Next, left kidney tissues were fixed in 10% formalin for pathological investigations.
Biochemical analyses
The level of serum urea nitrogen (BUN), creatinine (Cr), and lactate dehydrogenase (LDH) was determined using quantitative diagnostic kits (Pars Azmoon, I.R. Iran). The levels of nitrite (stable NO metabolite) in serum and the supernatant from the homogenized tissue were determined using a colorimetric assay kit (Zelbio, Germany) involving the Griess reaction. Malondialdehyde (MDA) levels of serum and the supernatant from the homogenized tissue were measured based on the manual methodology (20,21). Also total antioxidant capacity (TAC) was determined using a colorimetric assay kit (Zelbio, Germany). After removal, the kidneys were fixed in 10% formalin solution.
The hematoxylin and eosin staining was performed to test the tissue damage. Based on the extent of renal damage, the samples were scored 1- 4, where zero was assigned to the normal tissue.
Statistical analysis
The data are expressed as mean ± SEM. The levels of BUN, Cr, MDA, nitrite, LDH, TAC, and kidney weights were analyzed using one-way analysis of variance (ANOVA) followed by the LSD test. Likewise, the groups were compared by the Kruskal-Wallis or Mann-Whitney U tests with regard to the kidney tissue damage score (KTDS). P ≤ 0.05 was considered statistically significant using SPSS version 16 (Chicago, IL, USA).
RESULTS
Effect of ischemia/reperfusion on kidney weight
According to the findings, the mean kidney weight, one kidney of each animal, per 100 g of body mass was quantified. The following measures are the results of weighing before and after the induction of I/R, respectively.
Control, 0.33 ± 0.06 and 0.45 ± 0.03; losartan, 0.51 ± 0.042 and 0.44 ± 0.05; Ang 1-7, 0.46 ± 0.04 and 0.39 ± 0.02; Ang1-7 + losartan, 0.43 ± 0.02 and 0.52 ± 0.06; and sham, 0.44 ± 0.01. The comparisons did not show any significant differences.
The effect of ischemia/reperfusion on serum biochemicals and KTDS
After induction of I/R, the serum BUN and Cr increased in the control group compared to the sham group. Intergroup comparisons did not show any significant differences in BUN, Cr, LDH, MDA, nitrite, TAC, and KTDS between groups receiving treatments before or after induction of I/R. Ang 1-7 alone or along with losartan were associated with a decrease in serum BUN levels compared with control groups and losartan group (P < 0.001). Meanwhile, the serum Cr level indicated a statistically significant decrease after administration of losartan, Ang 1-7 alone, as well as their simultaneous administration, compared to the control group (Fig. 1).
Fig. 1.
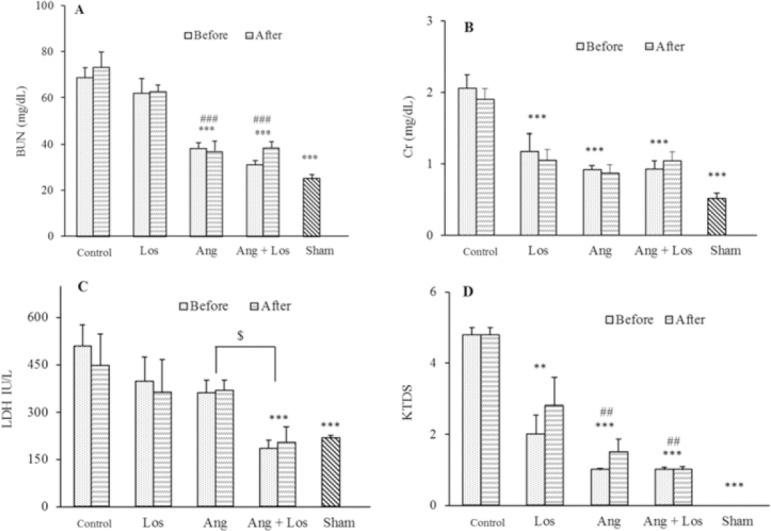
Evaluation of (A) BUN, (B) Cr, (C) LDH, and (D) KTDS levels of serum. The groups received, saline, losartan 10 mg/kg, angiotensin 1-7 50 mg/kg, and angiotensin 1-7 + losartan in animals with or without I/R. ** (P ≤ 0.01) and *** (P ≤ 0.001) indicate significant differences compared to control group (saline); ## (P ≤ 0.01) and ### (P ≤ 0.001) represent significant differences in comparison with losartan; and $ (P ≤ 0.05) represents significant differences between indicated groups. BUN, Serum urea nitrogen; Cr, creatinine; LDH, lactate dehydrogenase; KTDS, kidney tissue damage score; Los, losartan; Ang, angiotensin; I/R; ischemia/reperfusion.
The serum levels of LDH amongst groups indicated a significant increase after the induction of I/R in control group compared to the sham group (P < 0.001). Co-administration of Ang 1-7 with losartan significantly reduced LDH serum levels between groups receiving treatments before or after I/R induction compared to the control group (P < 0.001), as shown in Fig. 1.
As shown in Fig. 1, the results of KTDS indicated a severe damage to the kidneys after I/R in the control group when compared with the sham group. On the other hand, the administration of losartan and Ang 1-7 separately or concurrently resulted in a significant reduction of tissue damage in comparison with control group (P < 0.05) which was consistent with the results of the pathological examinations shown in (Fig. 2).
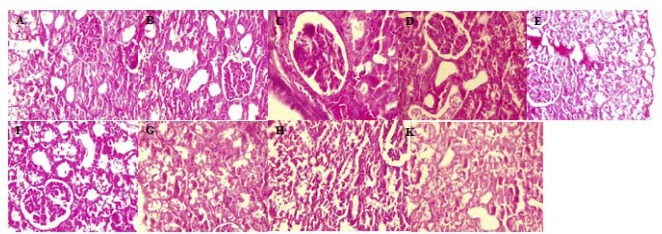
Fig. 2.
The pathology images (magnification ×400) of kidney tissue in nine experimental groups. The groups received (A and B) saline; (C and D) losartan, 10 mg/kg; (E and F) angiotensin 1-7, 50 mg/kg; (G and H) angiotensin 1-7 + losartan; and sham (K). The effect of each treatment was evaluated in healthy animals and after induction ischemia/reperfusion.
The mean of serum levels in biochemical factors such as MDA, nitrite, and TAC did not show any significant differences among the groups as displayed in Table 1.
Table 1.
The serum levels of MDA, nitrite, and TAC. Data are expressed as mean ± SEM. The groups received treatments, saline (as a control group); losartan, 10 mg/kg; Ang 1-7, 50 mg/kg; and Ang 1-7 + losartan, before or after I/R induction.
| Groups | MDA (μmol/L) | Nitrite (μmol/L) | TAC (mmol/L) | |||
|---|---|---|---|---|---|---|
| Before I/R | After I/R | Before I/R | After I/R | Before I/R | After I/R | |
| Control | 15.85 ± 0.52 | 15.55 ± 1.07 | 36.95 ± 0.34 | 32.38 ± 2.64 | 0.37 ± 0.01 | 0.35 ± 0.05 |
| Losartan (10 mg/kg) | 12.87 ± 2.04 | 14.81 ± 0.99 | 28.29 ± 2.72 | 28.43 ± 2.28 | 0.34 ± 0.01 | 0.32 ± 0.03 |
| Ang 1-7 (50 mg/kg) | 13.98 ± 0.68 | 13.91 ± 0.71 | 38.45 ± 4.17 | 30.01 ± 4.63 | 0.32 ± 0.01 | 0.38 ± 0.04 |
| Ang1-7 + losartan | 12.25 ± 0.48 | 11.79 ± 2.18 | 32.67 ± 3.96 | 34.37 ± 4.29 | 0.34 ± 0.06 | 0.31 ± 0.02 |
| Sham | 14.61 ± 2.70 | 36.45 ± 3.03 | 0.38 ± 0.04 | |||
MDA, Malondialdehyde; TAC, total antioxidant capacity; Ang, angiotensin; I/R; ischemia/reperfusion.
The effect of ischemia/reperfusion on kidney MDA, nitrite, and TAC levels
Comparison of MDA, nitrite, and TAC levels amongst different groups receiving treatments before or after I/R induction showed no significant differences as displayed in Fig. 3. Following I/R induction, MDA level showed a significant increase in control group compared to the sham group (P < 0.001). The administration of losartan and Ang 1-7 separately or simultaneously reduced lipid peroxidation and thus MDA level (P < 0.001) shown in Fig. 3, which is in alignment with tissue damage findings shown is Figs. 1 and 2.
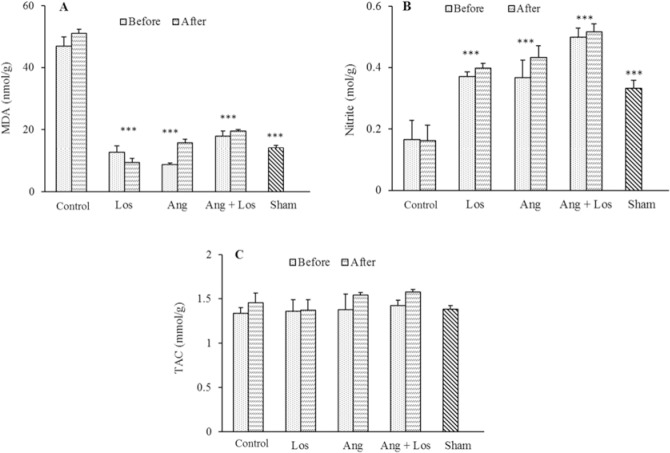
Fig. 3.
Kidney levels of (A) MDA, (B) nitrite, and (C) TAC. The groups received, saline, losartan 10 mg/kg, angiotensin 1-7 50 mg/kg, and angiotensin 1-7 + losartan before and after I/R. *** Indicates significant differences (P ≤ 0.001) compared to control group (saline) MDA, malondialdehyde; TAC, total antioxidant capacity; Los, losartan; Ang, angiotensin; I/R; ischemia/reperfusion.
I/R significantly reduced kidney nitrite levels in the control group compared to the sham group (P < 0.001). Losartan, Ang1-7, and their co-administration increased nitrite levels compared to control group (P < 0.001) as depicted in Fig. 3. It is worth mentioning that the mean kidney levels of TAC among the groups did not show any significant differences (Fig. 3).
DISCUSSION
Based on this research we found that prescribing losartan and Ang1-7 in brfore or after induction of I/R has the same effects on the process of injury also, the administration of Ang1-7 alone or in combination with losartan has a better effect on renal function and improves the levels of urea, Cr, LDH, MDA, nitrite and KTDS.
It has been shown that Ang 1-7 causes its vasodilator effects through stimulating the release of nitric oxide. This effect was reversed by inhibiting nitric oxide synthase. It is worth nothing that this effect depends on the amount of kinine presence (22). Ueda et al. also reported that Ang 1-7 vasodilator effects are due to bradykinin (BK) and the mechanisms involves the release of nitric oxide (23). Similarly, Almeida et al. have shown that a possible pathway for Ang 1-7 is strengthening the effects of BK (24). Hence, the findings of the aforementioned studies can justify the improvement of kidney’s function as it was significantly observed in our study.
It has been indicated that BK bioavailability and also its receptor susceptibility has increased in the presence of Ang1-7 (23). Many studies have reported the vascular actions of Ang 1-7 is involved in increasing vasodilator prostanoids, NO, and endothelium-derived hyperpolarizing factor. Moreover, a crosstalk between Mas reseptor with other receptors including AT2 and BK may also activate these routes. For example, blocking the BK receptor eliminates Ang 1-7 vasodilator effects (22,25).
Another study revealed that a five-day administration of Ang 1-7 decreases proteinuria and improves glomerulosclerosis in the glomerulonephritis model (26). Whereas, Van der Wouden et al. reported that Ang 1-7 cannot reduce proteinuria in adriamycin-induced nephropathy (ADR- nephropathy) (27). Likewise, Velkoska et al. has shown that a ten-day Ang 1-7 infusion in subtotal nephrectomy model in rats is related to malignant effects on blood pressure, heart, increasing the ACE and decreasing activity of the ACE2 (28). This discrepancy, however, needs further detailed explorations. Regarding this discrepancy, it is probable that Ang 1-7 effects in the kidney are significantly influenced by the experimental conditions and level of RAS activities. In fact, differences between species, local, and systemic Ang1-7 concentrations, nephron segments, activity levels of RAS, together with water and electrolyte status can be responsible for the various angiotensin effects on kidney function.
Immunohistochemical studies indicate that there is a wide distribution of the Mas receptor in various nephron segments such as juxtaglomerular apparatus, proximal tubule, collecting ducts (29), and in the cortical and central regions of kidney of rats (30). The most important biological effects of Ang 1-7 is mediated through the Mas receptor (4). The deficiency of this receptor causes fluid retention, hypertension, proteinuria, collagen deposition, increased expression mRNA of AT1, and TGFβ in the kidney tissues (29). Therefore, the loss of Mas receptor disturbs the ACE/Ang II/AT1 axis and consequently exerts its destructive effects on the kidneys. Here, the reason is not clearly understood through which pathway Ang 1-7 reduces proteinuria and attenuates the kidney damage.
Matsusaka et al. reported that ARBs reduce podocyte damage, decrease proteinuria and glomerulosclerosis in the NEP25 model (31). Similarly, this protective effect depends on the inhibition of AT1 receptor. Natio’s report emphasizes that not only the inhibition of AT1 receptor, but also the effect of Ang II through AT2 receptor lead to the protection of podocytes in the nephrectomy 5/6 model, following ARBs administration (32).
CONCLUSION
Although AT1 receptor blockers (ARBs) play important roles in the treatment of kidney diseases, according to the results of this study, our data suggest a synergistic effect of losartan and Ang 1-7 on renal function improvement. It is clear that part of this effect depends on Mas receptor, which can be confirmed in subsequent studies using A779 as Mas receptor antagonist. Therefore, it is suggested that therapeutic attention be given to Ang 1-7. Considering the above findings regarding the protective role of Ang1-7 and losartan on I/R injury, it is suggested that the role of TGFβ, BK, and other mechanisms involved in this effect be taken into more detailed consideration in the future studies.
ACKNOWLEDGMENTS
The research was financially supported by Zahedan University of Medical Sciences, Zahedan, I.R. Iran (Grant No. 8001).
REFERENCES
- 1.Fang F, Liu GC, Zhou X, Yang S, Reich HN, Williams V, et al. Loss of ACE2 exacerbates murine renal ischemia-reperfusion injury. PloS One. 2013;8(8):e71433. doi: 10.1371/journal.pone.0071433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bazzano T, Restel TI, Porfirio LC, Souza AS, Silva IS. Renal biomarkers of male and female Wistar rats (Rattus norvegicus) undergoing renal ischemia and reperfusion. Acta Cir Bras. 2015;30(4):277–288. doi: 10.1590/S0102-865020150040000007. [DOI] [PubMed] [Google Scholar]
- 3.Lai CF, Wu VC, Huang TM, Yeh YC, Wang KC, Han YY, et al. Kidney function decline after a non-dialysis-requiring acute kidney injury is associated with higher long-term mortality in critically ill survivors. Crit Care. 2012;16(4):R123–R132. doi: 10.1186/cc11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peeters P, Terryn W, Vanholder R, Lameire N. Delayed graft function in renal transplantation. Curr Opin Crit Care. 2004;10(6):489–498. doi: 10.1097/01.ccx.0000146119.46547.05. [DOI] [PubMed] [Google Scholar]
- 5.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17(6):1503–1520. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 6.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66(2):480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz-Ortega M, Rupérez M, Esteban V, Rodríguez-Vita J, Sanchez-Lopez E, Carvajal G, et al. Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol Dial Transplant. 2006;21(1):16–20. doi: 10.1093/ndt/gfi265. [DOI] [PubMed] [Google Scholar]
- 8.Rüster C, Wolf G. Renin-angiotensin-aldosterone system and progression of renal disease. J Am Soc Nephrol. 2006;17(11):2985–2991. doi: 10.1681/ASN.2006040356. [DOI] [PubMed] [Google Scholar]
- 9.Allred AJ, Chappell MC, Ferrario CM, Diz DI. Differential actions of renal ischemic injury on the intrarenal angiotensin system. Am J Physiol Renal Physiol. 2000;279(4):F636–F645. doi: 10.1152/ajprenal.2000.279.4.F636. [DOI] [PubMed] [Google Scholar]
- 10.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52(4):639–672. [PubMed] [Google Scholar]
- 11.Chan JC, Ko GT, Leung DH, Cheung RC, Cheung MY, So WY, et al. Long-term effects of angiotensin-converting enzyme inhibition and metabolic control in hypertensive type 2 diabetic patients. Kidney Int. 2000;57(2):590–600. doi: 10.1046/j.1523-1755.2000.00879.x. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira AJ, Santos RA, Bradford CN, Mecca AP, Sumners C, Katovich MJ, et al. Therapeutic implications of the vasoprotective axis of the renin-angiotensin system in cardiovascular diseases. Hypertension. 2010;55(2):207–213. doi: 10.1161/HYPERTENSIONAHA.109.140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos RA, Ferreira AJ, e Silva ACS. Recent advances in the angiotensin-converting enzyme 2-angiotensin (1-7)-Mas axis. Exp Physiol. 2008;93(5):519–527. doi: 10.1113/expphysiol.2008.042002. [DOI] [PubMed] [Google Scholar]
- 14.Nematbakhsh M, Safari T. Role of Mas receptor in renal blood flow response to angiotensin (1-7) in male and female rats. Gen Physiol Biophys. 2014;33(3):365–372. doi: 10.4149/gpb_2014008. [DOI] [PubMed] [Google Scholar]
- 15.Ren Y, Garvin JL, Carretero OA. Vasodilator action of angiotensin-(1-7) on isolated rabbit afferent arterioles. Hypertension. 2002;39(3):799–802. doi: 10.1161/hy0302.104673. [DOI] [PubMed] [Google Scholar]
- 16.Wong DW, Oudit GY, Reich H, Kassiri Z, Zhou J, Liu QC, et al. Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am J Pathol. 2007;171(2):438–451. doi: 10.2353/ajpath.2007.060977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassanshahi J, Maleki M, Nematbakhsh M. Renin-angiotensin system and unilateral ureteral obstruction. Physiol Pharmacol. 2017;21(4):266–278. [Google Scholar]
- 18.Haghighi M, Nematbakhsh M, Talebi A, Nasri H, Ashrafi F, Roshanaei K, et al. The role of angiotensin II receptor 1 (AT1) blockade in cisplatin-induced nephrotoxicity in rats: gender- related differences. Ren Fail. 2012;34(8):1046–1051. doi: 10.3109/0886022X.2012.700886. [DOI] [PubMed] [Google Scholar]
- 19.Forte BL, Slosky LM, Zhang H, Arnold MR, Staatz WD, Hay M, et al. Angiotensin-(1-7)/Mas receptor as an antinociceptive agent in cancer-induced bone pain. Pain. 2016;157(12):2709–2721. doi: 10.1097/j.pain.0000000000000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miri S, Safari T, Komeili GR, Nematbakhsh M, Niazi AA, Jahantigh M, et al. Sex difference in gentamicin-induced nephrotoxicity: influence of L- arginine in rat model. Int J Prev Med. 2018;9:108–123. doi: 10.4103/ijpvm.IJPVM_54_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Safari T, Miri S, Ghofran O, Fereidooni F, Niazi AA, Baghei H, et al. Gender differences in response to vitamin E and C in gentamicin induced nephrotoxicity in Wistar rats. J Nephropathol. 2017;6(4):338–345. [Google Scholar]
- 22.Heitsch H, Brovkovych S, Malinski T, Wiemer G. Angiotensin-(1-7)-stimulated nitric oxide and superoxide release from endothelial cells. Hypertension. 2001;37(1):72–76. doi: 10.1161/01.hyp.37.1.72. [DOI] [PubMed] [Google Scholar]
- 23.Ueda S, Masumori-Maemoto S, Wada A, Ishii M, Brosnihan KB, Umemura S. Angiotensin (1-7) potentiates bradykinin-induced vasodilatation in man. J Hypertens. 2001;19(11):2001–2009. doi: 10.1097/00004872-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Almeida AP, Frábregas BC, Madureira MM, Santos RJS, Campagnole-Santos MJ, Santos RAS. Angiotensin-(1-7) potentiates the coronary vasodilatory effect of bradykinin in the isolated rat heart. BRAZ J MED BIOL RES. 2000;33:709–713. doi: 10.1590/s0100-879x2000000600012. [DOI] [PubMed] [Google Scholar]
- 25.Santos RA, Campagnole-Santos MJ, Andrade SP. Angiotensin-(1-7): an update. Regul Pept. 2000;91(1-3):45–62. doi: 10.1016/s0167-0115(00)00138-5. [DOI] [PubMed] [Google Scholar]
- 26.van der Wouden EA, Henning RH, Deelman LE, Roks AJ, Boomsma F, de Zeeuw D. Does angiotensin (1-7) contribute to the antiproteinuric effect of ACE-inhibitors. J Renin Angiotensin Aldosterone Syst? 2005;6(2):96–101. doi: 10.3317/jraas.2005.016. [DOI] [PubMed] [Google Scholar]
- 27.Velkoska E, Dean RG, Griggs K, Burchill L, Burrell LM. Angiotensin-(1-7) infusion is associated with increased blood pressure and adverse cardiac remodelling in rats with subtotal nephrectomy. Clin Sci (Lond) 2011;120(8):335–345. doi: 10.1042/CS20100280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinheiro SVB, Ferreira AJ, Kitten GT, da Silveira KD, da Silva DA, Santos SHS, et al. Genetic deletion of the angiotensin-(1-7) receptor Mas leads to glomerular hyperfiltration and microalbuminuria. Kidney Int. 2009;75(11):1184–1193. doi: 10.1038/ki.2009.61. [DOI] [PubMed] [Google Scholar]
- 29.da Silveira KD, Bosco KSP, Diniz LR, Carmona AK, Cassali GD, Bruna-Romero O, et al. ACE2-angiotensin-(1-7)-Mas axis in renal ischaemia/reperfusion injury in rats. Clin Sci (Lond) 2010;119(9):385–394. doi: 10.1042/CS20090554. [DOI] [PubMed] [Google Scholar]
- 30.Pinheiro SV, Simoes e Silva AC, Sampaio WO, de Paula RD, Mendes EP, Bontempo ED, et al. Nonpeptide AVE 0991 is an angiotensin-(1-7) receptor Mas agonist in the mouse kidney. Hypertension. 2004;44(4):490–496. doi: 10.1161/01.HYP.0000141438.64887.42. [DOI] [PubMed] [Google Scholar]
- 31.Matsusaka T, Asano T, Niimura F, Kinomura M, Shimizu A, Shintani A, et al. Angiotensin receptor blocker protection against podocyte-induced sclerosis is podocyte angiotensin II type 1 receptor- independent. Hypertension. 2010;55(4):967–973. doi: 10.1161/HYPERTENSIONAHA.109.141994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naito T, Ma LJ, Yang H, Zuo Y, Tang Y, Han JY, et al. Angiotensin type 2 receptor actions contribute to angiotensin type 1 receptor blocker effects on kidney fibrosis. Am J Physiol Renal Physiol. 2010;298(3):F683–F691. doi: 10.1152/ajprenal.00503.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]