Abstract
1, 3, 4- Oxadiazoles and quinazolinones are privileged structures with extensive biological activities. On account of reported anticancer activity of them, in this study, a multi-step reaction procedure has been developed for the synthesis of some quinazolinone-1, 3, 4-oxadiazole derivatives. Reaction of the synthesized 3-amino-4(3H) quinazolinone derivatives with chloroacetyl chloride in the presence of dichloromethane/triethylamine yielded 2-chloro -N-(4-oxo-2-quinazolin3 (3H)-yl) acetamide derivatives as intermediate. Treatment of the resultants with 5- (4-chlorophenyl) 1, 3, 4-oxadiazole-2-thiol in dry acetone and potassium carbonate gave coupled derivatives of quinazolinone-1, 3, 4-oxadiazole. The cytotoxic effect of final compounds was tested against MCF-7 and HeLa cell lines using MTT assay. Compound 2-(5-(4-chlorophenyl)-1,3,4-oxadiazol-2-ylthio) N-(4-oxo-2-propylquinazolin)3(4H)acatamide 6a exhibited remarkable cytotoxic activity at 10 and 100 μM against HeLa cell line. The alteration of substituents on C2 of quinazolinone ring revealed that the introduction of propyl moeity improved cytotoxic activity against HeLa cell line.
Keywords: Cytotoxicity, Quinazolinone, Oxadiazole
INTRODUCTION
Amongst heterocyclic compounds, oxadiazole is one of the attractive constructions for the development of new drugs. In drug discovery program, oxadiazole ring can be used as a main pharmacophore interfering with receptor, an aromatic linker, modulating molecular properties, and bioisosteres of amides and esters (1-5).
There are four known isomers of this five-membered ring including: 1,2,4-, 1,2,3-, 1,2,5-, and 1,3,4-oxadiazole. 2,5 Disubstituted 1, 3, 4 oxadiazole isomer is associated with diverse pharmacological activities such as anticancer, antibacterial, antifungal, antitubercular, anticonvulsant, and anti-inflammatory effects (5,6,7,8). The proposed anticancer mechanisms for oxadiazoles include inhibition of tubulin polymerization and epidermal growth factor receptor (EGFR). Quinazolinone is another nitrogenated scaffold that was extensively used in drug development programs and its derivatives showed diverse biological activities as anticancer, antifungal, anti-inflammatory, and antimicrobial (9,10,11,12,13,14,15). Quinazolinone-based structures exert their anticancer activity through different mechanisms including inhibition of the DNA repair enzyme system, inhibition of EGFR (9), thymidylate enzyme inhibition, and inhibitory effects for tubulin polymerize (14). The efficacy of 1,3,4-oxadiazole and quinazolinone derivatives has been demonstrated in many literatures. Also quinazolinone-oxadiazole hybrid structures with antimicrobial, anti-inflammatory, antioxidant, anticancer, and analgesic effects have been reported (5,10,16,17,18,19,20).
Because of the remarkable cytotoxic effects of 1, 3, 4-oxadiazole (6,7,8) and quinazolinone (9,10,11,12,13) derivatives, and in the hope of achieving synergistic response due to the presence of both quinazolinone and 1,3,4-oxadiazole moieties, in this study, some of new quinazoline derivatives containing the 1, 3, 4-oxadiazole were synthesized and evaluated for their cytotoxic activity.
MATERIALS AND METHODS
Instrumentation
All starting materials, reagents, and solvents were purchased from commercial suppliers like Merck (Germany) and Aldrich (USA) companies. Merck silica gel 60 F254 plates (Germany) were applied for analytical thin layer chromatography (TLC). Proton nuclear magnetic resonance (HNMR) spectra were recorded using a Bruker 400 MHz spectrometer (Germany), and chemical shifts are expressed as ppm with tetramethylsilane (TMS) as internal standard. Infrared (IR) (KBr discs) was recorded with a WQF-510 fourier-transform IR (FT-IR) spectrophotometer (China). Melting points were determined using electrothermal 9200 melting point apparatus (United Kingdom) and are uncorrected.
General procedures for synthesis of compounds
2-Amido-benzoic acid derivatives (2a-2c) were prepared by reaction of anthranilic acid (1) with acylchloride derivatives. The reaction was followed by dehydrative cyclization of 2- amido-benzoic acid derivatives to form benzoxazinone intermediate (3a-3c). Reaction between benzoxazinone and hydrazine hydrate in ethanol under reflux condition produced 3-amino quinazolinone derivatives in high yield (4a-4c). Treatment of 3-amino quinazolinone with chloro acetylchloride in the presence of dichloromethane/triethylamine afforded 2-chloro -N-(4-oxo-2-quinazolin3 (3H)-yl) acetamide derivatives (5a-5c). Final compounds (6a-6c) obtained through the nucleophilic displacement of the chloride with thiol of 5-(4-chlorophenyl) 1, 3, 4- oxadiazole-2-thiol in dry acetone and potassium carbonate (Scheme 1) (21,22).
Scheme 1.
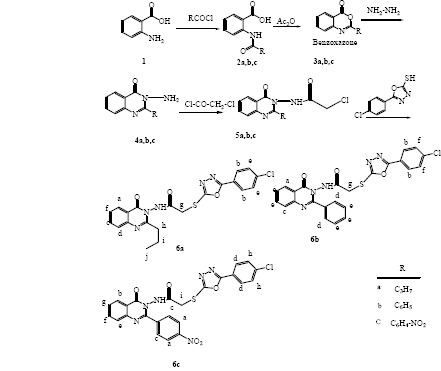
Synthesis of the target compounds (6a-6c).
Synthesis of 2- amido-benzoic acid derivatives (2a-2c)
To a magnetically stirred solution of anthranilic acid (1) (0.04 mol) in dimethyl formamide (35 mL) was added dropwise a solution of acylchloride (butyryl chloride, benzoyl chloride and 4-nitrobenzoyl chloride) (0.045 mol) over 15 min. The mixture was stirred at room temperature for 3 h until a solid product was formed. Then the mixture was poured into water and the precipitate was collected by filtration, washed with water, and dried under reduced pressure to achieve compounds 2a-2c (Scheme 1) (23).
Synthesis of benzoxazone derivatives (3a-3c)
A solution of compounds 2a-2c (0.01 mol) in acetic anhydride (30 mL) was heated for 1 h with vigorous stirring. After completion of the reaction which confirmed by TLC, the solvent was removed by distillation under reduced pressure to obtain derivatives 3a-3c (Scheme 1)(23).
Synthesis of 3-aminoquinazolinone derivatives (4a-4c)
A mixture of related benzoxazone 3a-3c (0.01 mol) and hydrazine hydrate (0.02 mol) in ethanol was refluxed for 3 h. After the reaction was completed, the mixture was cooled and the separated solid was collected by filtration and recrystallized from ethanol or isopropanol (21,22).
Synthesis of 2-chloro -N-(4-oxo-2-quinazolin3 (3H)-yl) acetamide derivatives (5a-5c)
Chloroacetylchloride (0.01 mol) was added to a solution of 3-amino-quinazoline derivatives 4a-4c (0.01 mol) in dry dichloromethane (20 mL) and triethylamine (0.01 mol), mixture was stirred at room temperature for 30 min. Then the reaction mixture was poured into ice water and extracted with dichloromethane and ethyl acetate. The extracted ethyl acetate was washed with sodium bicarbonate solution (3%) and dried over anhydrous magnesium sulfate, which upon evaporation afforded the products 5a-5c (21,22).
Synthesis of quinazolinone-oxadiazole hybrid derivatives (6a-6c)
Title compounds 6a-6c were synthesized by refluxing 2-chloro -N-(4-oxo-2-quinazolin3 (3H)-yl) acetamide derivatives 5a-5c (0.01 mol) with 5-(4-chlorophenyl) 1, 3, 4-oxadazole-2- thiol in dry acetone (20 mL) and anhydrous potassium carbonate (0.01 mmol) for 6 h. The reaction mixture was filtered while hot. The organic solution was concentrated and purified by preparative TLC (21,22).
Cytotoxicity assay
Sample and culture media preparation
MCF-7 (breast cancer), and HeLa (cervical cancer) cells were purchased from pasture institute of Iran (Tehran, I.R. Iran) and maintained at 37 °C in a humidified atmosphere (90%) containing 5% CO2. Both cell lines were grown in RPMI 1640 completed with 5% v/v fetal bovine serum, 100 U/mL penicillin, and 100 mg/mL streptomycin. After 2-3 subcultures, 180 μL of the cell suspensions (5 × 104 cells/mL) were seeded in 96well plates and incubated for 24 h.
The stock solutions of compounds (10 mM, 1 mL) were prepared in minimum volume of dimethyl sulfoxide (DMSO) and diluted with the medium to obtain 10, 100, 1000 μM concentrations. After 24 h incubation, 20 μL of different concentrations of the derivatives were added\and the microplates were further incubated for 48 h. Paclitaxel was used as positive control. To evaluate cell survival, 20 μL of MTT solution (5 mg/mL in phosphate buffer solution) was added to each well and incubated for 4 h. Afterwards, the media in each well was gently replaced with 150 μL DMSO to dissolve formazan crystals. The absorbance of each well was measured at 540 nm using an ELISA plate reader. Each experiment was repeated three times. Analysis of variance (ANOVA) followed by Tukey test was used to determine the differences between various groups.
Cell viability calculated using the following equation:
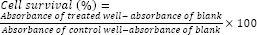
RESULTS
2-(5-(4-Chlorophenyl)-1, 3, 4-oxadiazol-2- ylthio)N-(4-oxo-2-propylquinazolin)3(4H) acatamide (6a)
Yield: 31%, m.p. 162.5-163 °C, IR νmax, 3243(NH), 2931 (C-H), 1677 (C=O), 1611(C=N), cm-1; 1HNMR: (400 MHz; CDCl3): δ 9.79 (b, NH), 8.07 (1H, dd, J = 8 Hz, J = 4 Hz, Ha), 7.87 (2H, d, J =8 Hz, Hb), 7.66 (1H, m, Hc), 7.59 (1H, d, J =8 Hz, Hd), 7.44(2H, d, J = 8 Hz, He), 7.34 (1H, t, J = 8 Hz, Hf), 4.17-4.21 (1H, d, J = 16 Hz, CH2g), 3.94-3.98 (1H, d, J =16 Hz, CH2g), 2.64 (2H, t, J = 8 Hz, CH2h), 1.72 (2H, m, CH2i), 0.92 (3H, t, J = 8 Hz, CH3 J).
2-(5-(4-Chlorophenyl)-1,3,4-oxadiazol-2- ylthio)N-(4-oxo-2-phenylquinazolin)3(4H) acatamide (6b)
Yield: 30%, m.p.150-151 °C, IR νmax, 3216 (NH), 1696 (C=O) cm-1; 1HNMR δ: (400 MHz; CDCl3), 8.73 (1H, s, NH), 8.23 (1H, d, J = 8 Hz, Ha), 7.72-7. 78 (3H, m, Hb,c), 7.57(2H, d, J = 8 Hz, Hd), 7.38-7.50(7H, m, He,f), 4.08-4.12(1H, d, J = 16 Hz,CH2 g),3.87-3.91(1H, d, J = 16Hz, CH2 g).
2-(5-(4-Chlorophenyl)-1,3 ,4 - oxadiazol – 2 - ylthio)N(2-4nitrophenyl)4-oxoquinazolin) 3(4H)acatamide(6C)
Yield: 33%, m.p.177 °C (decomposed), IR νmax, 3313 (NH), 1696 (C=O), 1596, 1349 NO2 cm-1; 1HNMR δ: (400 MHz; CDCb), 8.74 (1H, s, NH), 8.24-8.28 (3H, m, Ha’b), 7.73-7.83 (5H, m, Hc,d,e), 7.53 (1H, t, J = 8 Hz, Hf), 7.1-7.24 (3H, m, Hg,h), 4.12-4.16 (1H, d, J = 16 Hz,CH2i), 3.91 -3.95 (1H, d, J = 16 Hz, CH2i).
Cytotoxic effects of the derivatives (6a-6c)
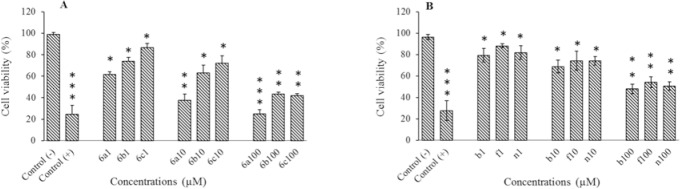
The cytotoxicity of compounds were evaluated against HeLa and MCF-7 cell lines at different concentrations (1, 10, and 100 μM) using MTT assay. Results are shown in Fig. 1A, 1B, and Table 1.
Fig. 1.
Cytotoxic effect of compounds 6a-6c on (A) HeLa and (B) MCF-7 cells following exposure to different concentrations (1, 10, and 100 μM). Data are presented as mean ± SD, n = 3. *P < 0.05, **P < 0.01, and *** P < 0.001 Shows significant differences in comparison with negative control group, Paclitaxel was used as positive control.
Table 1.
The IC50 of tested compounds against MCF-7 and HeLa cell lines.
| Target compounds | R | IC50 (μM) MCF-7 | IC50 (μM) HeLa |
|---|---|---|---|
| 6a | Propyl | 82.18 ± 3 | 7.52 ± 0.6 |
| 6b | Phenyl | 97.17 ± 4 | 79.74 ± 3 |
| 6c | Nitro phenyl | 101.47 ± 4 | 79.32 ± 3 |
Compound 6a exhibited remarkable cytotoxic effect at all tested concentrations (1, 10, and 100 μM) on HeLa cell line and cell viability reduced to about 61%, 37% and 24% respectively. Compounds 6b and 6c indicated similar effects in the same concentrations on HeLa cell line. These two compounds reduced cell viability to about 42% at 100 μM.
Remarkable differences were not observed between the cytotoxicity of these compounds on MCF-7 cell line. These compounds displayed the highest cytotoxic activities against MCF-7 cells at 100 μM that cell viability reduced to about 50%.
These compounds exhibited significant differences in viability compared to the negative control on both cell lines which presented in Fig. 1A and 1B.
DISCUSSION
1, 3, 4-Oxadiazole heterocycle as good bioisosteres of amides and esters, can improve pharmacological activity via hydrogen bonding interactions with the receptors (6,24). Literature survey revealed that little changes in the structure of substituted 1, 3, 4-oxadiazole can lead to quantitative and qualitative alterations in their biological activities (3).
A series of 2, 5-disubstituted-1, 3, 4- oxadiazoles has been reported as tubulin polymerization inhibitors (8,25). Moreover, 1, 3, 4-oxadiazole derivatives possessing 1,4-benzodioxan moiety have been introduced as potential anticancer agents (8,26). Besides, quinazolinone is a heterocyclic scaffold with extensive biological effects, in particular, anticancer activity. Derivatives of substituted quinazolinone at 2, 3 or 2, 4 positions have been reported as anticancer agents (22,27,28,29). Literature surveys have shown many reports on cytotoxic activities of quinqzolinone (9,22,27) and oxadiazoles (1,2,6,8).
Hybrid structures of quinazolinone- oxadiazole have presented anticancer (5,14,19) and antimicrobial (14,16,20) activities. Some of the 4-alkoxyquinazoline derivatives containing the 1, 3, 4-oxadiazole scaffold showed potent inhibitory activity against HeLa and MCF-7 cell lines (19).
We reported the synthesis of a novel series of quinazolin-4(3H)-one derivatives bearing oxadiazole, in the 3-position of the quinazolinone nucleus in a multiple-step reaction procedure. Amongst tested compounds, 6a showed the highest cytotoxic activity against HeLa cell line at all tested concentrations while compounds 6b and 6c indicated mild cytotoxic effects against HeLa cell line at highest tested concentration. Three compounds displayed the highest cytotoxic activities against MCF-7 cells at 100 μM concentration.
According to the results shown in Table 1, compounds 6b and 6c bearing aromatic substituents on C2 of the quinazolinone ring showed lowest cytotoxic activities on both cell lines, while compound 6a containing aliphatic substituent on C2 was more active on HeLa cell line with IC50 value 7.52 μM.
Khodarahmi et al. reported the synthesis and cytotoxic evaluation of quinazolinone- benzimidazole hybrid derivatives against MCF-7 and HeLa cell lines. Cytotoxicity results revealed that compounds with phenyl and nitrophenyl substitutes on C2 of the quinazolinone ring had the lowest cytotoxic activity against both cell lines and compounds with aliphatic substituents in this position had the highest potency (30). Other hybrids of quinazolinone-triazole were recently reported by Jafari et al. Cytotoxicity results exhibited that the presence of electron donating substituents on C2 of the quinazolinone ring could be in favor of the activity for these compounds (22). Collectively it could be assumed that the presence of electron-donating groups such as propyl substitution on C2 of quinazolinon ring could improve activity for these compounds while electron withdrawing groups such as phenyl and nitrophenyl substitutes have opposite effects.
CONCLUSION
In the present study, some of the conjugated oxadiazole-quinazolinone derivatives with amide linker were synthesized and evaluated for their cytotoxicity against HeLa and MCF-7 cell lines. Compound 6a showed the highest cytotoxic activities with the IC50 value of 7.52 μM against HeLa cell line. Substitution of propyl group at 2 position of quinazolinone improved the cytotoxic activity against HeLa possibly due to electronic effects.
ACKNOWLEDGMENTS
The content of this paper was extracted from the Pharm.D thesis submitted by Azadeh Sharifzadeh which was financially supported (Grant No. 396382) by the Vice Chancellor of Research of Isfahan University of Medical Sciences, Isfahan, I.R. Iran.
REFERENCES
- 1.Puthiyapurayil P, Poojary B, Chikkanna C, Buridipad SK. Design, synthesis and biological evaluation of a novel series of 1,3,4-oxadiazole bearing N-methyl-4- (trifluoromethyl)phenyl pyrazole moiety as cytotoxic agents. Eur J Med Chem. 2012;53:203–210. doi: 10.1016/j.ejmech.2012.03.056. [DOI] [PubMed] [Google Scholar]
- 2.Singh R, Chouhan A. Various approaches for synthesis 3, 4-oxadiazole derivative and their pharmacological activity. World J Pharm Pharm Sci. 2014;3(10):1474–1505. [Google Scholar]
- 3.Siddiqui SZ, Rehman A, Abbasi MA, Abbas N, Khan KM, Ashraf M, et al. Synthesis, characterization and biological screening of N-substituted derivatives of 5- benzyl-1,3,4-oxadiazole-2yl-2’'-sulfanyl acetamide. Pak J Pharm Sci. 2013;26(3):455–463. [PubMed] [Google Scholar]
- 4.Jafari E, Mohammadi T, Jahanian-Najafabadi A, Hassanzadeh F. Synthesis and antimicrobial evaluation of some 2,5 disubstituted 1,3,4-oxadiazole derivatives. Res Pharm Sci. 2017;12(4):330–336. doi: 10.4103/1735-5362.212051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar A, Gupta A, Kashaw V, Shukla G, Mishra V, Kashaw SK. Synthesis and anticancer evaluation of some novel 3-[5-(4-Substituted) Phenyl1,3,4- oxadiazole2yl-]-2-phenylquinazoline4(3H)-ones. Int J Pharm Pharm Sci. 2012;4(suppl 1):502–506. [Google Scholar]
- 6.de Oliveira CS, Lira BF, Barbosa-Filho JM, Lorenzo JG, de Athayde-Filho PF. Synthetic approaches and pharmacological activity of 1,3,4-oxadiazoles: a review of the literature from 2000-2012. Molecules. 2012;17(9):10192–10231. doi: 10.3390/molecules170910192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh R, Chouhan A. Biological importance of 1, 3, 4- oxadiazole derivatives. IJABR. 2013;3(2):140–149. [Google Scholar]
- 8.Ramazani A, Khoobi M, Torkaman A, Nasrabadi FZ, Forootanfar H, Shakibaie M, et al. One-pot, four- component synthesis of novel cytotoxic agents1-(5- aryl-1,3,4-oxadiazol-2-yl)-1-(1 H-pyrrol-2- yl)methanamines. Eur J Med Chem. 2014;78:151–156. doi: 10.1016/j.ejmech.2014.03.049. [DOI] [PubMed] [Google Scholar]
- 9.Noolvi MN, Patel HM, Bhardwaj V, Chauhan A. Synthesis and in vitro antitumor activity of substituted quinazoline and quinoxaline derivatives: search for anticancer agent. Eur J Med Chem. 2011;46(6):2327–2346. doi: 10.1016/j.ejmech.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Chawla A, Batra Ch. Recent advances of quinazolinone derivatives as marker for various biological activities. Int Res J Pharm. 2013;4(3):49–58. [Google Scholar]
- 11.Khodarahmi GA, Rahmani Khajouei M, Hakimelahi GH, Abedi D, Jafari E, Hassanzadeh F. Antibacterial, antifungal and cytotoxic evaluation of some new 2, 3- disubstituted 4(3H)-quinazolinone derivatives. Res Pharm Sci. 2012;7(3):151–158. [PMC free article] [PubMed] [Google Scholar]
- 12.Khodarahmi GA, Jafari E, Hakimelahi GH, Abedi D, Rahmani Khajouei M, Hassanzadeh F. Synthesis of some new quinazolinone derivatives and evaluation of their antimicrobial activities. Iran J Pharm Res. 2012;11(3):789–797. [PMC free article] [PubMed] [Google Scholar]
- 13.Rezaee Nasab R, Mansourian M, Hassanzadeh F. Synthesis, antimicrobial evaluation and docking studies of some novel quinazolinone Schiff base derivatives. Res Pharm Sci. 2018;13(3):213–221. doi: 10.4103/1735-5362.228942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jafari E, Rahmani Khajouei M, Hassanzadeh F, Hakimelahi GH, Khodarahmi GA. Quinazolinone and quinazoline derivatives: recent structures with potent antimicrobial and cytotoxic activities. Res Pharm Sci. 2016;11(1):1–14. [PMC free article] [PubMed] [Google Scholar]
- 15.Vaseghi G, Jafari E, Hassanzadeh F, Haghjooy-Javanmard Sh, Dana N, Rafi eian-Kopaei M. Cytotoxic evaluation of some fused pyridazino- and pyrrolo- quinazolinones derivatives on melanoma and prostate cell lines. Adv Biomed Res. 2017;6:76. doi: 10.4103/2277-9175.209049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sowjanya C, RamaBharathi V, Devi GK, Rajitha G. Synthesis and evaluation of some novel 3-[5-phenyl- 1,3,4-oxadiazole-2-yl]-2(substitutedstyryl)- quinazoline-4(3H)-ones for antibacterial activity. J Chem Pharm Res. 2011;3(6):212–216. [Google Scholar]
- 17.Rajasekaran S, Gopalkrishna R. Synthesis and anti-denaturation activity of some substituted quinazolinone analogs. Int J Chem Tech Res. 2012;4(3):1207–1211. [Google Scholar]
- 18.Rajasekaran S, Gopalkrishna R. Synthesis, antibacterial and antioxidant activity of some 2, 3-susbtituted quinazolin-4(3H)-ones. Der Pharmacia Lett. 2012;4(2):470–474. [Google Scholar]
- 19.Qiao F, Yin Y, Shen YN, Wang SF, Sha S, Wu X, et al. Synthesis, molecular modeling, and biological evaluation of quinazoline derivatives containing the 1,3,4-oxadiazole scaffold as novel inhibitors of VEGFR. RSC Adv. 2015;5(26):19914–19923. [Google Scholar]
- 20.Dhani R. Analgesic activity of 3-[(5-substitued) - 1, 3, 4 oxadiazole-2-yl) methylamino]-2-methyl quinazolin- 4(3h)-ones. Int J Anal Pharm Biomed Sci. 2012;1(4):30–33. [Google Scholar]
- 21.Alagarsamy V, Solomon VR, Sulthana MT, Vijay MS, Narendhar B. Design and synthesis of quinazolinyl acetamide for their analgesic and anti-inflammatory activities. Z Naturforsch. 2015;70(8):1–8. [Google Scholar]
- 22.Hassanzadeh F, Sadeghi-aliabadi H, Nikooei S, Jafari E, Vaseghi G. Synthesis and cytotoxic evaluation of some derivatives of triazole-quinazolinone hybrids. Res Pharm Sci. 2019;14(2):130–137. doi: 10.4103/1735-5362.253360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jafari E, Khodarahmi GA, Hakimelahi GH, Tsai FY, Hassanzadeh F. Synthesis of some new tricyclic 4(3H)- quinazolinone derivatives. Res Pharm Sci. 2011;6(2):93–100. [PMC free article] [PubMed] [Google Scholar]
- 24.Bajaj S, Asati V, Singh J, Roy PP. 1,3,4-Oxadiazoles: an emerging scaffold to target growth factors, enzymes and kinases as anticancer agents. Eur J Med Chem. 2015;97:124–141. doi: 10.1016/j.ejmech.2015.04.051. [DOI] [PubMed] [Google Scholar]
- 25.Ouyang X, Piatnitski EL, Pattaropong V, Chen X, He HY, Kiselyov AS, et al. Oxadiazole derivatives as a novel class of antimitotic agents: Synthesis, inhibition of tubulin polymerization, and activity in tumor cell line. Bioorg Med Chem Lett. 2006;16(5):1191–1196. doi: 10.1016/j.bmcl.2005.11.094. [DOI] [PubMed] [Google Scholar]
- 26.Zhang XM, Qiu M, Sun J, Zhang YB, Yang YS, Wang XL, et al. Synthesis, biological evaluation, and molecular docking studies of 1, 3,4-oxadiazole derivatives possessing 1,4-benzodioxan moiety as potential anticancer agents. Bioorg Med Chem. 2011;19(21):6518–6524. doi: 10.1016/j.bmc.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Tiwari AK, Singh VK, Bajpai A, Shukla G, Singh S, Mishra AK. Synthesis and biological properties of 4-(3H)-quinazolone derivatives. Eur J Med Chem. 2007;42(9):1234–1238. doi: 10.1016/j.ejmech.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed MF, Belal A. Synthesis, characterization, and biological evaluation of new quinazolin-4-one derivatives hybridized with pyridine or pyran moiety. Res Chem Inter med. 2016;42(2):659–671. [Google Scholar]
- 29.Alafeefy AM, Ahmad R, Abdulla M, Eldehna WM, Al- Tamimi AM, Abdel-Aziz HA, et al. Development of certain new 2-substituted-quinazolin-4- ylaminobenzenesulfonamide as potential antitumor agents. Eur J Med Chem. 2016;109:247–253. doi: 10.1016/j.ejmech.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Taherian E, Khodarahmi G, Rahmani Khajouei M, Hassanzadeh F, Dana N. Synthesis and cytotoxic evaluation of novel quinozalinone derivatives with substituted benzimidazole in position 3. Res Pharm Sci. 2019;14(3):247–254. doi: 10.4103/1735-5362.258493. [DOI] [PMC free article] [PubMed] [Google Scholar]