Abstract
Background:
One of the most effective parameters in the progression of the prostate cancer is interleukin (IL)-6 through affecting pSTAT3, pERK1/2, and pAKT cell signaling proteins. Carvacrol is an herbal antioxidant with antitumor effects. The purpose of this study was to investigate the effects of carvacrol on IL-6 gene expression, pSTAT3, pAKT, pERK1/2 cellular signaling proteins, and invasion in human prostate cancer PC3 cells.
Methods:
PC3 cell viability was evaluated by MTT assay with different concentrations of carvacrol (0–800 μM). IL-6 gene expression and cellular concentration of pSTAT3, pERK1/2, and pAKT were investigated using the real-time reverse transcription quantitative polymerase chain reaction (RT-qPCR) and western blotting technic, respectively. PC3 cell invasion was determined by invasion assay test.
Results:
Carvacrol IC50 for PC3 prostate cancer cells was 360 μM. Carvacrol led to a significant reduction (P < 0.05) for IL-6 gene expression in a dose-dependent manner compared to control. IL-6 protein reduced 41.5% and 52.7% when compared with control cells at 360 and 420 μM of carvacrol, respectively. Carvacrol led to a decline in pSTAT3, pAKT, and pERK1/2 above 360 μM compared to control. PC3 potential invasion was significantly reduced after treatment with carvacrol in a dose-dependent manner.
Conclusions:
Decreased IL-6 protein level by carvacrol resulted in diminishing of pSTAT3, pERK1/2, and pAKT signaling proteins, which leads to the reduction of the cell survival, proliferation, and invasion in PC3 cells.
Keywords: Cell survival, interleukin-6, prostatic neoplasms, STAT3 protein, terpenes
Introduction
Nowadays, prostate cancer is known as second leading cause of cancer death in the US.[1] The proliferation of prostate cancer cells in the early stage is androgen dependent, so androgen deprivation therapy can prevent the progression of prostate cancer in this stage,[1,2] but after a period of time, prostate cancer cells become resistant to this treatment and tumor cells begin to grow again. Studies have shown the role of some cytokines including interleukin-6 (IL-6) in the process of growth, differentiation, and apoptosis of prostate cancer cells.[2,3,4] Several studies have shown that IL-6 causes growth of prostate cancer cells.[4] Many researches have reported that IL-6 contributes to prostate cancer progression through activation of several important signaling pathways including ERK, JAK-STAT, Src, MAPK, β-catenin, erbB1-3, and PI3k-AKTs in prostate cancer cells.[5] Therefore, examining IL-6 expression and inhibition of its signaling pathways may be helpful for treating prostate cancer.
Carvacrol is a monoterpenoid phenolic compound which is approved by the Food and Drug Administration and the Council of Europe for food use, category B, in the list of chemical flavorings.[6] The therapeutic effects of antioxidants and plant extracts have been proven in numerous researches.[7,8,9,10] Carvacrol has antioxidant, antimutagenic, antiplatelet, analgesic, anti-inflammatory, antiangiogenic, antielastase, insecticidal, and antiparasitic properties.[11] Furthermore, carvacrol has antiproliferative effects on many cancer cell lines such as gastric adenocarcinoma, HeLa, SiHa, Caco-2, HepG2, LNCaP, and MCF-7.[11,12,13,14,15] Other investigations have shown that carvacrol increases regeneration of liver cells after hepatectomy in mice and decreases the expression of IL-6 and tumor necrosis factor alpha (TNF-α) by anti-inflammatory properties.[11] Furthermore, it has been observed that carvacrol can suppress the growth and proliferation of human hepatocellular carcinoma cells (HepG2) by reducing phosphorylated ERK1/2.[11] Based on the latest studies, carvacrol reduces cell survival through induction of apoptosis in prostate cancer cells.[15] Therefore, in this study, we evaluated the possibility effects of carvacrol on the expression of IL-6, phosphorylated cellular signaling proteins (pAKT, pSTAT3, and pERK1/2), and invasion of PC3 prostate cancer cells.
Methods
The human PC3 prostate cancer cells were prepared from Pasteur Institute of Iran (Tehran, Iran). Carvacrol, Pen/Strep, trypan blue, dimethyl sulfoxide, and 3-(4, 5-dimethylthiaztol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Fetus bovine serum and RPMI1640 medium were obtained from Gibco (Rockville, MD, USA). Antibodies were prepared from Abcam (San Francisco, CA, USA). All other chemicals used were of analytical grade quality.
Cell culture and viability
Cell viability was evaluated with media containing different concentrations of carvacrol (0–800 μM) for 48 h as described previously.[7] The percentage viability was calculated as follows: Percentage viability = A (sample)/A (control) × 100.
Real-time quantitative polymerase chain reaction for interleukin 6
RT-qPCR for IL-6 was conducted as described previously[16] with 0, 230, 360, and 420 μM of carvacrol for 48 h in the presence of specific primers for IL-6 (Forward: 5’-AAGCCAGAGCTGTGCAGATGAGTA-3’; Reverse: 5’ TGTCCTGCAGCCACTGGTTC-3’) and GAPDH (Forward: 5′-ACACCCACTCCTCCACCTTTG-3′; Reverse: 5′-CCACCACCCTGTTGCTGTAG-3′). The temperature profile for the reaction was an initial denaturation stage of 95°C at 10 min; then, a three-step program was developed for 40 cycles including 95°C for 15 s, 60°C for 20 s, and 72°C for 25 s, respectively. GAPDH was used as an endogenous control gene for the normalization of IL-6 gene expression.
Western blotting
PC3 cells were treated with different concentrations of carvacrol (0, 230, 360, and 420 μM) for 48 h. Then, western blot analysis for pERK1/2, pSTAT3, and pAKT cellular signaling proteins were conducted as described previously.[16]
Invasion assay
Invasion assay was conducted with different concentrations of carvacrol (0, 130, and 360 μM) for 48 h in 24 well plates as described previously.[7] Briefly, transwell with 8 μm pores, 24-well plates, and matrigel were used for invasion assay. PC3-treated cells (5 × 104 per well) were added into the upper chamber of the transwell and were incubated at 37°C in 5% CO2 for 24 h. Then, cells on the bottom side of the transwell (migrated cells) were fixed by 5% glutaraldehyde and were stained with 0.5% toluidine blue solution. Finally, the fixed cells in the low chamber of the transwell were counted using inverted microscope.
Measurement of interleukin-6
PC3 cells were seeded with different concentrations of carvacrol (0, 230, 360, and 420 μM) for 48 h at a density of 2 × 105 cells/well in 6-well plates at 37°C in a 5% CO2 incubator. Then, the secreted IL-6 protein in the supernatants was assessed using enzyme-linked immunosorbent assay (ELISA) kit (AViBion Human IL-6 ELISA kit) according to the manufacturer's instructions.
Statistical analysis
Data are shown as mean ± standard deviation. Statistical analysis carried out with SPSS software version 20.0 (SPSS, Chicago, IL, USA) and GraphPad Prism 5 software (GraphPad Software Inc., San Diego, CA). Group means were compared by Kruskal–Wallis nonparametric analysis of variance and P < 0.05 was considered statistically significant. Inhibitory concentration of 50% (IC50) was calculated by the Probit procedure using SPSS. The relative level gene expression was calculated with DDCT method, and the data were expressed as fold change. Melting curves were generated to ensure the purity of the amplification product of each reaction. Western blot experiments were repeated three times.
Results
The effects of carvacrol on PC3 cell viability
Figure 1 shows the effects of carvacrol on PC3 cells in different concentrations (0–800 μM) after 48 h. Cell proliferation and viability in carvacrol-treated PC3 decreased in a dose-dependent manner [Figures 1 and 2]. The IC50 calculated for carvacrol was 360 μM on PC3 cells.
Figure 1.
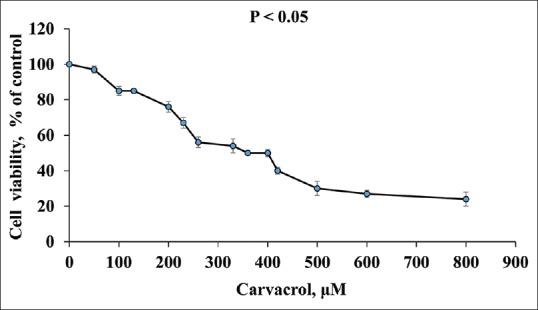
Effect of carvacrol on cell viability and proliferation of PC3 cells after 48 h. Data indicate mean ± standard deviation, n = 3
Figure 2.
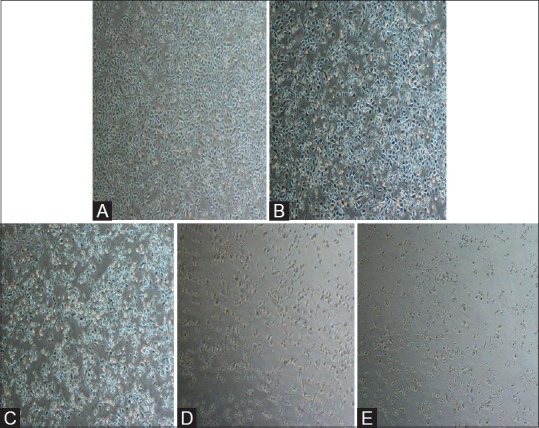
Morphological changes of PC3 cells after treatment with various concentrations of carvacrol (A, 0; B, 130; C, 230; D, 360; and E, 420 μM, respectively) for 48 h
The effects of carvacrol on interleukin-6 gene expression and interleukin-6 synthesis
Table 1 shows the effects of carvacrol on IL-6 gene expression and IL-6 synthesis in PC3 cells. Carvacrol at 230, 360, and 420 μM resulted in a significant reduction (P < 0.05) in IL-6 protein synthesis (37.1, 41.5, and 52.7%, respectively) in a dose-dependent manner compared to control. Furthermore, IL-6 gene expression was significantly declined (P < 0.05) at 420 μM of carvacrol compared to other concentrations.
Table 1.
Effect of carvacrol on the secretion and expression of interleukin-6 in prostate cancer cells
| Carvacrol (µM) | IL-6 (pg/mL) | IL-6 expression (2−ΔΔCt) |
|---|---|---|
| 0 | 402.33±2.71 | 1 |
| 230 | 253.53±5.41a | 15.02±2.14a |
| 360 | 235.41±5.11a,b | 1.75±0.60a,b |
| 420 | 190.21±4.18a,b,c | 0.85±0.10a,b,c |
aP<0.05 compared to the control cells, bP<0.05 compared to 230 μM, cP<0.05 compared to 360 μM. IL-6=Interleukin-6, PC3=Prostate cancer
The effects of carvacrol on the cellular signaling pathways
Figure 3 shows the cellular levels of phosphorylated STAT3, ERK, and AKT signaling proteins in carvacrol-treated PC3. Carvacrol partially reduced the levels of pSTAT3 and pAKT as opposed to the control cells. Moreover, a noticeable reduction in the cellular levels of pSTAT3 and pAKT was seen after treatment by carvacrol at 360 and 420 μM compared to control.
Figure 3.
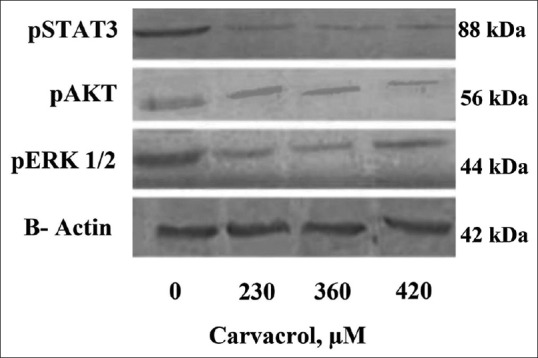
The effects of carvacrol with different concentrations on the levels of signaling proteins in PC3 cells that were treated with increasing doses of carvacrol for 48 h
The effects of carvacrol on PC3 cell invasion
The potential invasion of carvacrol-treated PC3 cells was significantly declined (P < 0.05) in a dose-dependent pattern [Figure 4]. Carvacrol at 130 and 360 μM significantly reduced PC3 cell invasion by 52.38% and 79.42% compared to control cells, respectively.
Figure 4.
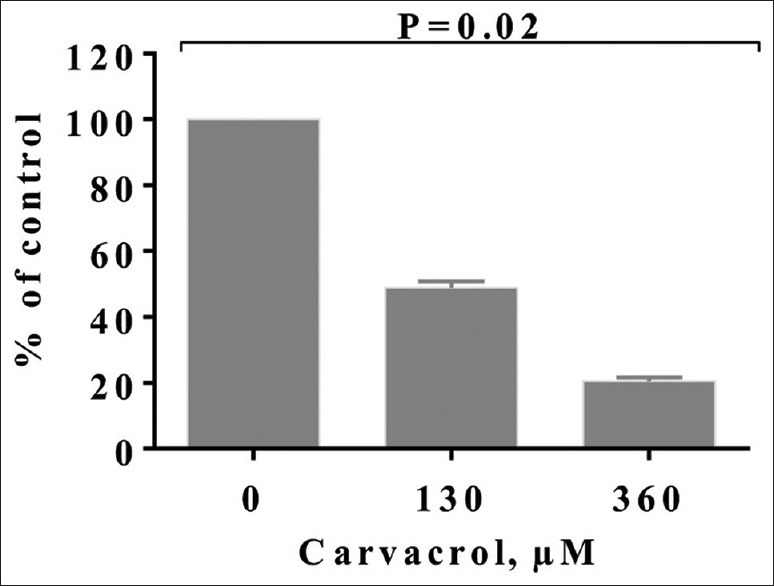
The effect of carvacrol on the invasion of PC3 cells after 48 h. Bars represent the mean ± standard deviation of triplicate determinations
Discussion
Nowadays, cancer chemotherapy using synthetic drugs has demonstrated many side effects. Therefore, researchers have done extensive studies on the effects of antioxidants and compounds derived from medicinal herbs to modulate cancer progression.[14,15,16] Based on our results, PC3 cell viability was decreased from 56% (carvacrol at 230 μM) to 50% after carvacrol treatment in concentration of 360 μM (IC50) which is close to IC50 of carvacrol for H1299 cells.[17] Figures 1 and 2 show that PC3 cell death and morphology changes increased gradually by the addition of carvacrol concentration. These changes are conspicuous after exposure to carvacrol at 360 and 420 μM. Furthermore, in our study, the effects of carvacrol on IL-6 gene expression and its protein synthesis are reported in Table 1. Our results indicate that treatment with lower concentration of carvacrol resulted in a significant increase in IL-6 gene expression. Many previous published papers have demonstrated that prostate cancer cells can acquire resistance to apoptosis by increasing the expression of IL-6 protein.[18,19] Therefore, in our study, elevation of IL-6 in PC3 cells that were treated with low concentration of carvacrol can be an internal reaction of cancer cells against apoptotic effects of carvacrol. On the other hand, these results show a reduction in IL-6 protein levels at 360 and 420 μM of carvacrol (41.5% and 52.7%, respectively) in treated PC3 cells compared to control cells. Nevertheless, previous studies have proven that carvacrol can upregulate the expression of pro-inflammatory factors such as TNF-α, cyclooxygenase-2, IL-6, and nuclear factor-kappaB (NF-kB) in D-galactosamine-induced hepatotoxic rats due to its anti-inflammatory effects[20] which are consistent with our findings. Recently, the effects of other antioxidants on the reduction of IL-6 gene expression in DU-145 cells were confirmed.[21] Furthermore, previous studies related to pigs have shown that carvacrol can inhibit the secretion of TNF-α and IL-1 β from macrophages.[22] According to our results, diminished IL-6 secretion of carvacrol-treated PC3 cells [Table 1] may be, at least partly, due to anti-inflammatory potential of carvacrol.
Figure 3 shows the levels of signaling proteins, especially phosphorylated STAT3 which significantly decreased after treatment with increasing concentrations of carvacrol (360 and 420 μM) in PC3 cells. IL6 can interfere in progression of prostate cancer through STAT3, AKT, and MAPK signaling pathways.[3,4,23] In this study, carvacrol in the higher concentration of 360 μM led to decrease in the levels of phosphorylated forms of STAT3 and AKT [Figure 3]. Recent published papers have proven that phosphorylated AKT plays a major role in prevention of apoptosis in cancer cells. Furthermore, it has been demonstrated that pSTAT3 protein contributes to the growth and survival of cells by increasing the expression of antiapoptotic factors such as Bcl-2 and Bcl-XL.[24] In addition, in a study on human hepatocellular carcinoma cell line (HepG-2), it was observed that carvacrol can inhibit cancer cell growth by decreasing phosphorylated ERK1/2 in a dose-dependent manner. Carvacrol also induces apoptosis in HepG-2 cells by increasing the level of P38 and activating MAPK pathway.[11] Therefore, based on our results, carvacrol led to a reduction in the induced effects by IL-6, activation apoptosis, and reduction of cell viability in PC3 cells through downregulating the expression of pERK1/2, pSTAT3, and pAKT. Therefore, it seems that inhibition of the IL-6 signaling pathways can be considered, at least in part, a promising target for the treatment of prostate cancer.
In this research, carvacrol inhibited the ability of invasion and migration of PC3 cells by 52.38% and 79.42% at 360 μM and 130 μM, respectively, compared to control cells [Figure 4]. Nevertheless, it is reported that P2Y agonists can stimulate PC3 cells invasion by activating ERK1/2 and P38 protein kinases.[25] On the other hand, antioxidant compounds can decrease the metastatic ability of cancer cells by reducing P38, MAPK, and AKT signaling pathways.[21,26] Other researches have reported that gallic acid decreases cancer cell invasion in PC3, cervical, and glioma cancer cells through reducing the pERK 1/2, pAKT, and Ras/MAPK.[27,28,29] Thus, the reduction of signaling protein pathways and downregulation of IL-6 by carvacrol in this study resulted in diminishing of PC3 cell invasion.
In our study, we did not evaluate the other mechanisms which probably play a role in the effects of carvacrol on PC3 cells such as apoptosis/necrosis or proapoptotic characters such as NF-κB, protein expression of cleaved caspase 3, Bax, and downregulated Bcl-2 expression. Thus, we suggest that future studies focus on other possible mechanisms of carvacrol on the mentioned principle factors in PC3 cells.
Conclusions
Our findings indicate that IL-6 downregulation and reduction of IL-6 protein level in PC3 cells by carvacrol led to a reduction of pSTAT3, pERK1/2, and pAKT cellular signaling proteins which resulted in the reduction of cell proliferation, viability, and invasion in PC3 cells. Therefore, it seems that carvacrol can be considered as an anticancer agent and we suggest that future studies focus on the effects of carvacrol on other cell signaling and effector proteins.
Financial support and sponsorship
This study was funded by the Shahrekord University of Medical Sciences (grant no. 1203), Shahrekord, Iran.
Conflicts of interest
The authors report no conflict of interest.
Acknowledgments
We would like to express our gratitude to those who have helped us in Clinical Biochemistry Research Center of Shahrekord University of Medical Sciences. The results described in this paper were the MS dissertation of Ms. Mahnaz Keloushadi.
References
- 1.American Cancer Society. Cancer facts and figures. [Last accessed 2019 Apr 30]. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-andstatistics/annual-cancer-facts-and-figures/2019/cancer-facts-andfigures-2019.pdf .
- 2.Nguyen DP, Li J, Tewari AK. Inflammation and prostate cancer: The role of interleukin 6 (IL-6) BJU Int. 2014;113:986–92. doi: 10.1111/bju.12452. [DOI] [PubMed] [Google Scholar]
- 3.Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26:54–74. doi: 10.1016/j.smim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Culig Z. Proinflammatory cytokine interleukin-6 in prostate carcinogenesis. Am J Clin Exp Urol. 2014;2:231–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Perner S, Cronauer MV, Schrader AJ, Klocker H, Culig Z, Baniahmad A, et al. Adaptive responses of androgen receptor signaling in castration-resistant prostate cancer. Oncotarget. 2015;6:35542–55. doi: 10.18632/oncotarget.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Vincenzi M, Stammati A, De Vincenzi A, Silano M. Constituents of aromatic plants: Carvacrol. Fitoterapia. 2004;75:801–4. doi: 10.1016/j.fitote.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Rezaei A, Heidarian E. Co-administration of trientine and flaxseed oil on oxidative stress, serum lipids and heart structure in diabetic rats. Indian J Exp Biol. 2013;51:646–52. [PubMed] [Google Scholar]
- 8.Heidarian E, Saffari J, Jafari-Dehkordi E. Hepatoprotective action of Echinophora platyloba DC leaves against acute toxicity of acetaminophen in rats. J Diet Suppl. 2014;11:53–63. doi: 10.3109/19390211.2013.859217. [DOI] [PubMed] [Google Scholar]
- 9.Heidarian E, Rafieian-Kopaei M, Khoshdel A, Bakhshesh M. Metabolic effects of berberine on liver phosphatidate phosphohydrolase in rats fed on high lipogenic diet: An additional mechanism for the hypolipidemic effects of berberine. Asian Pac J Trop Biomed. 2014;4:S429–35. doi: 10.12980/APJTB.4.2014C474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heidarian E, Jafari-Dehkordi E, Seidkhani-Nahal A. Lipid lowering effect of artichoke on liver phosphatidate phosphohydrolase and plasma lipids in hyperlipidemic rats. J Med Plant Res. 2011;5:4918–24. doi: 10.1016/j.fct.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Yin QH, Yan FX, Zu XY, Wu YH, Wu XP, Liao MC, et al. Anti-proliferative and pro-apoptotic effect of carvacrol on human hepatocellular carcinoma cell line HepG-2. Cytotechnology. 2012;64:43–51. doi: 10.1007/s10616-011-9389-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Günes-Bayir A, Kiziltan HS, Kocyigit A, Güler EM, Karataş E, Toprak A, et al. Effects of natural phenolic compound carvacrol on the human gastric adenocarcinoma (AGS) cells in vitro. Anticancer Drugs. 2017;28:522–30. doi: 10.1097/CAD.0000000000000491. [DOI] [PubMed] [Google Scholar]
- 13.Llana-Ruiz-Cabello M, Gutiérrez-Praena D, Pichardo S, Moreno FJ, Bermúdez JM, Aucejo S, et al. Cytotoxicity and morphological effects induced by carvacrol and thymol on the human cell line caco-2. Food Chem Toxicol. 2014;64:281–90. doi: 10.1016/j.fct.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Al-Fatlawi AA, Rahisuddin AA. Cytotoxicity and pro-apoptotic activity of carvacrol on human breast cancer cell line MCF-7. World J Pharm Sci. 2014;2:1218–23. [Google Scholar]
- 15.Patel B, Shah VR, Bavadekar SA. Anti-proliferative effects of carvacrol on human prostate cancer cell line, LNCaP. FASEB J. 2012;26:1037–5. [Google Scholar]
- 16.Eskandari E, Heidarian E, Amini SA, Saffari-Chaleshtori J. Evaluating the effects of ellagic acid on pSTAT3, pAKT, and pERK1/2 signaling pathways in prostate cancer PC3 cells. J Cancer Res Ther. 2016;12:1266–71. doi: 10.4103/0973-1482.165873. [DOI] [PubMed] [Google Scholar]
- 17.Ozkan A, Erdogan A. A comparative study of the antioxidant/prooxidant effects of carvacrol and thymol at various concentrations on membrane and DNA of parental and drug resistant H1299 cells. Nat Prod Commun. 2012;7:1557–60. [PubMed] [Google Scholar]
- 18.Domingo-Domenech J, Oliva C, Rovira A, Codony-Servat J, Bosch M, Filella X, et al. Interleukin 6, a nuclear factor-kappaB target, predicts resistance to docetaxel in hormone-independent prostate cancer and nuclear factor-kappaB inhibition by PS-1145 enhances docetaxel antitumor activity. Clin Cancer Res. 2006;12:5578–86. doi: 10.1158/1078-0432.CCR-05-2767. [DOI] [PubMed] [Google Scholar]
- 19.Dorff TB, Goldman B, Pinski JK, Mack PC, Lara PN, Jr, Van Veldhuizen PJ, Jr, et al. Clinical and correlative results of SWOG S0354: A phase II trial of CNTO328 (siltuximab), a monoclonal antibody against interleukin-6, in chemotherapy-pretreated patients with castration-resistant prostate cancer. Clin Cancer Res. 2010;16:3028–34. doi: 10.1158/1078-0432.CCR-09-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aristatile B, Al-Assaf AH, Pugalendi KV. Carvacrol suppresses the expression of inflammatory marker genes in D-galactosamine-hepatotoxic rats. Asian Pac J Trop Med. 2013;6:205–11. doi: 10.1016/S1995-7645(13)60024-3. [DOI] [PubMed] [Google Scholar]
- 21.Chun JY, Tummala R, Nadiminty N, Lou W, Liu C, Yang J, et al. Andrographolide, an herbal medicine, inhibits interleukin-6 expression and suppresses prostate cancer cell growth. Genes Cancer. 2010;1:868–76. doi: 10.1177/1947601910383416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Song M, Che TM, Bravo D, Pettigrew JE. Anti-inflammatory effects of several plant extracts on porcine alveolar macrophages in vitro . J Anim Sci. 2012;90:2774–83. doi: 10.2527/jas.2011-4304. [DOI] [PubMed] [Google Scholar]
- 23.Santoni M, Massari F, Del Re M, Ciccarese C, Piva F, Principato G, et al. Investigational therapies targeting signal transducer and activator of transcription 3 for the treatment of cancer. Expert Opin Investig Drugs. 2015;24:809–24. doi: 10.1517/13543784.2015.1020370. [DOI] [PubMed] [Google Scholar]
- 24.Cui Y, Lu P, Song G, Liu Q, Zhu D, Liu X, et al. Involvement of PI3K/Akt, ERK and p38 signaling pathways in emodin-mediated extrinsic and intrinsic human hepatoblastoma cell apoptosis. Food Chem Toxicol. 2016;92:26–37. doi: 10.1016/j.fct.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Chen L, He HY, Li HM, Zheng J, Heng WJ, You JF, et al. ERK1/2 and p38 pathways are required for P2Y receptor-mediated prostate cancer invasion. Cancer Lett. 2004;215:239–47. doi: 10.1016/j.canlet.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 26.Shen KH, Hung SH, Yin LT, Huang CS, Chao CH, Liu CL, et al. Acacetin, a flavonoid, inhibits the invasion and migration of human prostate cancer DU145 cells via inactivation of the p38 MAPK signaling pathway. Mol Cell Biochem. 2010;333:279–91. doi: 10.1007/s11010-009-0229-8. [DOI] [PubMed] [Google Scholar]
- 27.Heidarian E, Keloushadi M, Ghatreh-Samani K, Valipour P. The reduction of IL-6 gene expression, pAKT, pERK1/2, pSTAT3 signaling pathways and invasion activity by gallic acid in prostate cancer PC3 cells. Biomed Pharmacother. 2016;84:264–9. doi: 10.1016/j.biopha.2016.09.046. [DOI] [PubMed] [Google Scholar]
- 28.Zhao B, Hu M. Gallic acid reduces cell viability, proliferation, invasion and angiogenesis in human cervical cancer cells. Oncol Lett. 2013;6:1749–55. doi: 10.3892/ol.2013.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu Y, Jiang F, Jiang H, Wu K, Zheng X, Cai Y, et al. Gallic acid suppresses cell viability, proliferation, invasion and angiogenesis in human glioma cells. Eur J Pharmacol. 2010;641:102–7. doi: 10.1016/j.ejphar.2010.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]


