Abstract
In this study, we evaluated the effects of seven oligosaccharides on the growth rate and stress tolerance of Lactobacillus plantarum ZLP001 in vitro, and the potential synbiotic effects of the most effective oligosaccharide [fructo-oligosaccharide (FOS)] and L. plantarum ZLP001 on the growth performance, apparent nutrient digestibility, fecal microbiota, and serum immune index in weaning piglets. Most oligosaccharides were utilized as carbohydrate sources by L. plantarum ZLP001, but we observed obvious differences in the bacterial growth depending on oligosaccharide type and concentration. Oligosaccharides and glucose significantly alleviated the decrease in L. plantarum ZLP001 viability in artificial gastric fluid, whereas none of the sugars affected viability in artificial intestinal fluid. FOS and galacto-oligosaccharide significantly improved the viability of L. plantarum ZLP001 under heat stress (65 °C for 15 and 30 min). FOS and soybean oligosaccharide significantly increased the viability of L. plantarum ZLP001 in response to cold stress (4 °C for 30 and 60 days). On the basis of the findings of in vitro experiments, we selected FOS for in vivo studies. Eighty-four weaned piglets were randomly assigned to one of the following groups: control (basal diet, no additives), freeze-dried L. plantarum ZLP001 (4.2 × 109 CFU/g, 2 g/kg diet), FOS (5 g/kg diet), and combination (0.2% L. plantarum ZLP001 + 0.5% FOS). Body weight and feed consumption were recorded for determinations of the average daily gain (ADG), average daily feed intake (ADFI), and feed-to-gain ratio (F/G). On day 28, fresh fecal samples were collected to evaluate the apparent digestibility of nutrients and microbiota, and serum samples were collected to determine the immune status. L. plantarum ZLP001 plus FOS significantly increased ADG and decreased the F/G ratio compared with the no-additive control. The combination treatment also increased the apparent nutrient digestibility of dry matter and crude protein. Compared with the control and single supplementation, the combination treatment had a significant regulatory effect on the intestinal microbiota, as evidenced by increases in Lactobacillus spp. and a decrease in Enterobacteriaceae. In addition, the combination treatment increased the concentrations of serum IFN-γ and immunoglobulin G. In conclusion, FOS can be utilized well by L. plantarum ZLP001 and can be combined with it as a potential synbiotic that shows synergistic effects in weaning piglets.
Keywords: Lactobacillus plantarum, oligosaccharide, carbohydrate source, stress tolerance, synbiotic, weaning piglet
Introduction
Probiotics have positive effects on feed efficiency and average body weight gain, and can enhance immune responses and intestinal health in various livestock animals (Uyeno et al., 2015; Liao and Nyachoti, 2017; Al-Khalaifah, 2018). Their beneficial effects in weaning piglets have also been widely reported (Barba-Vidal et al., 2018; Yi et al., 2018). The combined effects of probiotics with other nutrients, particularly prebiotics, on host intestine and health have gained increasing research interest. Studies on the effects of prebiotics on the intestinal microbiota of weaning piglets have shown that prebiotics can selectively increase Lactobacillus populations (Jiao et al., 2014) and reduce the populations of certain potentially harmful bacteria, such as Clostridium and Enterobacteriaceae (Castillo et al., 2008). Thus, the use of synbiotics to improve weaning piglet intestinal health and performance shows considerable potential (Markowiak and Śliżewska, 2018). However, it has been demonstrated that specific probiotic strains show clear differences in their abilities to ferment different oligosaccharides to support their growth (Sims et al., 2014), thereby indicating that the use of random combinations of probiotic and prebiotic agents might not always have the anticipated effects. In our previous studies, we identified Lactobacillus plantarum ZLP001 as an effective probiotic strain. Its probiotic potential has been demonstrated in intestinal pig epithelial cells (IPEC-J2) cells (Wang et al., 2018a, 2018b) as well as in weaning piglets (Wang et al., 2011, 2012). However, the potential relationship with functional oligosaccharides and their combined effect in piglets are still under investigation. In this study, we evaluated seven oligosaccharides for their influence on the growth and stress tolerance of L. plantarum ZLP001 in comparison with glucose or an absence of supplemental sugar, and subsequently evaluated the potential synbiotic effects of treatments using L. plantarum ZLP001 combined with the selected fructo-oligosaccharide on weaning piglets.
MATERIALS AND METHODS
Ethical Statement
This study was conducted in accordance with the guidelines established by the Animal Care and Use Committee of the Institute of Animal Husbandry and Veterinary Medicine, Beijing Academy of Agriculture and Forestry Sciences (IAHVM-BAAFS).
Probiotic Strain, Culture, and Freeze-drying
Lactobacillus plantarum ZLP001, which was originally isolated from the intestines of healthy piglets in our laboratory, has been officially identified by the China Center of Industrial Culture Collection (Beijing, China) and is preserved in the China General Microbiological Culture Collection Center (CGMCC No. 7370). L. plantarum ZLP001 was grown in improved De Man Rogosa Sharpe (MRS) liquid medium (10 g peptone, 5 g yeast powder, 20 g glucose, 10 g beef extract, 5 g sodium acetate, 2 g dibasic ammonium citrate, 2 g dipotassium phosphate, 0.58 g magnesium sulfate, 0.19 g manganese sulfate, 1 mL of Tween-80, and water to 1 L; pH 6.5) at 37 °C under anaerobic conditions. To obtain freeze-dried L. plantarum ZLP001, bacterial cells were collected by centrifugation at 4,000 × g for 10 min. The bacterial sludge was mixed well with a protective medium (12 g skimmed milk, 10 g dextrin, 4 g lactose, 1.5 g sodium glutamate, and 0.5 g L cysteine per 100 mL). The mixture was subsequently transferred to a freeze-drying plate and incubated at −70 °C for 2 h, and then dried in a freeze-dryer at 1 Pa and –80 °C for 24 h. The viable cell count of the freeze-dried powder was 4.2 × 109 CFU/g.
Oligosaccharides
Seven commercially available oligosaccharides, recognized as promising candidates for use as prebiotics or synbiotics in animal production, were investigated for their potential as a carbohydrate source for L. plantarum ZLP001. Information on the oligosaccharides is presented in Table 1.
Table 1.
Oligosaccharide substrates used in this study
| Product | Description | Purity | Supplier |
|---|---|---|---|
| MOS | Mannan-oligosaccharide | 90% | Hongkong Sheli, Ltd. |
| GOS | Galacto-oligosaccharides | 90% | Quantum Hi-Tech (China) Biological Co., Ltd. |
| FOS | Fructo-oligosaccharide | 95% | Quantum Hi-Tech (China) Biological Co., Ltd. |
| XOS | Xylo-oligosaccharide | 95% | Shandong Longlive Bio-Technology, Ltd. |
| SBOS | Soybean oligosaccharide | 90% | Linuo Biotechnology Co., Ltd. |
| RFO | Raffinose family oligosaccharide | 90% | Zhongmian-ziguang Biotechnology Co., Ltd. |
| Inulin | Inulin powder | 95% | Linuo Biotechnology Co., Ltd. |
Growth Assay
Carbohydrate utilization by L. plantarum ZLP001 was evaluated by growth assays. MRS broth containing glucose or one of the seven aforementioned oligosaccharides was prepared, and MRS broth without added sugar was used as a control medium (NS). The oligosaccharides were added at concentrations of 1%, 2%, 4%, and 8%, respectively, and glucose was added at 2% based on our previously optimized MRS ingredients for L. plantarum ZLP001. L. plantarum ZLP001 was inoculated into each prepared medium at 1% and cultured in a Bioscreen fully automatic growth curve analyzer [Vizai International Holdings (Hong Kong), Ltd., Hong Kong, China] at 37 °C. To asses bacterial growth, the optical density (OD) at 600 nm was determined at hourly intervals for 24 h.
The growth performance of L. plantarum ZLP001 in the presence of the different oligosaccharides as carbohydrate sources was evaluated by inoculating MRS agar plates with serial dilutions of bacterial culture and counting the subsequently grown bacterial colonies after incubation for 48 h at 37 °C. The pH of the culture medium was also measured at this time point, and the experiment was repeated three times.
Viability of L. plantarum ZLP001 in Artificial Gastric and Intestinal Fluids
Artificial gastric and intestinal fluids were prepared as described by Kim et al. (2008), with some modifications. MRS broth containing glucose or one of the different oligosaccharides and MRS broth without added sugar were prepared. The pH was adjusted to 2 with 5 mol/L hydrochloric acid and the media were sterilized. Artificial gastric fluid was prepared by suspending 1 mL of pepsin (1,000 units/mL, Sigma) in 99 mL of each of the aforementioned MRS media, and filter-sterilizing using a 0.22-µm membrane filter. Simulated small intestinal fluid was prepared by suspending 0.8 g bile salts (Sigma) in 100 mL of sterile MRS medium containing oligosaccharides, glucose, or no added sugar. The pH was adjusted to 8.0 with 1 mol/L sodium hydroxide. Trypsin solution (500 unit/mL; Sigma) was added to the prepared solution and the mixture was filter-sterilized through a 0.22-µm membrane filter.
Lactobacillus plantarum ZLP001 was cultured in the presence of the different oligosaccharides at optimal concentrations determined by the growth assay. The bacterial culture was inoculated at 1% into simulated gastric fluid (pH 2.0) or simulated intestinal fluid (pH 8.0). The mixtures were vortexed for 10 s and then incubated at 37 °C under anaerobic conditions for 3 h. One milliliter of the bacterial suspension was then taken and centrifuged at 4,000 × g for 10 min. The pelleted bacteria were washed twice with phosphate buffer (pH 7.2) and then resuspended in an equivalent volume. Viable cell counts were performed for colonies growing on MRS agar inoculated with serial dilutions.
Heat Stress and Cold Storage Tolerance
We examined the effects of oligosaccharides on L. plantarum ZLP001 survival under heat stress and cold storage. To measure heat resistance, 10 mL of bacterial culture was centrifuged at 4,000 × g for 10 min and the cells were resuspended in 10 mL of MRS broth (containing oligosaccharides, glucose, or no added sugar) preheated at 65 °C. Each of oligosaccharides was supplemented at its optimal concentration, as determined by the aforementioned growth assay. Samples were heat-treated at 65 °C for 15 or 30 min and then cooled on ice. One milliliter samples were serially diluted and spread onto MRS agar plates to determine the CFUs after incubation for 48 h at 37 °C. To determine cold storage tolerance, a bacterial culture was centrifuged and cells were resuspended in 10 mL of MRS broth (containing oligosaccharides, glucose, or no added sugar) precooled at 4 °C. Samples were cold-treated at 4 °C for 30 or 60 days and thereafter allowed to warm to room temperature. One milliliter samples were serially diluted and spread onto MRS agar plates to determine CFUs after incubation for 48 h at 37 °C. The experiments were replicated three times.
Animals and Diets
Eighty-four post-weaning piglets (Large White × Landrace) with an initial body weight of 7.19 ± 0.45 kg were randomly assigned to one of four groups. Each treatment group comprised seven piglets per pen and treatments were performed in triplicate. Animals were raised at the Beijing Fangshan Pig Breeding Farm (Beijing, China) in a separate room that was decontaminated before the study. Each pen was equipped with mesh floor, one feeder, and one water nipple. The pig barn was maintained at 20–25 °C. All pigs had free access to feed and water throughout the feeding trial. Piglets received a complete feed mainly consisting of maize and soybean meal, which was formulated according to the NRC (2012) guidelines and the Feeding Standard of Swine (2004). The ingredients and chemical composition of the basal diet are given in Table 2. The dietary treatments consisted of basal diet with no additives, basal diet supplemented with freeze-dried L. plantarum ZLP001 (4.2 × 109 CFU/g, 2 g/kg diet), basal diet supplemented with 0.5% fructo-oligosaccharide (5 g/kg diet), and basal diet supplemented with 0.2% freeze-dried L. plantarum ZLP001 plus 0.5% fructo-oligosaccharide. The concentrations of L. plantarum ZLP001 and FOS used in this study were based on those determined in our previous studies and a preliminary experiment. The trial consisted of a 3-day pre-experimental period followed by a 28-day experimental period. Evaluations were carried out by trained personnel blinded to the treatments.
Table 2.
Ingredient composition and chemical analysis of the basal diet
| Ingredients | Content, g/kg |
|---|---|
| Corn | 600 |
| Soybean meal | 230 |
| Wheat bran | 50 |
| Fish meal | 20 |
| Whey | 50 |
| Soybean oil | 10 |
| Premix1 | 40 |
| Chemical compositions | |
| Digestible energy2, MJ/kg | 13.80 |
| Metabolizable energy2, MJ/kg | 12.77 |
| Crude protein3, % | 18.50 |
| Lysine3, % | 1.18 |
| Methionine3, % | 0.32 |
| Calcium3, % | 0.76 |
| Total phosphorus3, % | 0.56 |
1Provided per kg of complete diet: vitamin A, 10,000 IU; vitamin D3, 2,700 IU; vitamin E, 28 mg; vitamin K3, 2 mg/kg; vitamin B1, 2 mg/kg; vitamin B2, 5 mg; vitamin B6, 2.5 mg; vitamin B12, 0.04 mg; niacin, 30 mg; pantothenic acid, 12 mg; folic acid, 1 mg; biotin, 0.5 mg; Fe, 130 mg; Zn, 130 mg; Cu, 160 mg; Mn, 20 mg; I, 0. 5 mg; Se, 0.3 mg.
2Calculated nutrient levels.
3Measured nutrient levels.
Measurement of Production Performance and Apparent Nutrient Digestibility
Individual piglet body weight was measured at the start and the end of the experiment. Feed consumption per pen was determined at the end of the experiment. The average daily gain (ADG), average daily feed intake (ADFI), and feed-to-gain ratio (F/G) were calculated. At the end of the animal trial, one piglet from each pen was selected for a 7-day nutrient digestibility analysis. Approximately 200 g of fecal sample was collected from each pig, to which 20 mL of 10% hydrochloric acid was added. Fecal samples collected from each pig over 3 days were pooled, dried in a forced air oven at 65 °C for 72 h, and then ground to pass through a 1-mm screen. The apparent nutrient digestibility was measured using acid insoluble ash (AIA) as an indicator. The nutrient contents of the samples were analyzed according to AOAC (2005) international methods for dry matter (DM: method 930.15), crude protein (CP: method 968.06), calcium (Ca: method 984.01), and phosphorus (P: method 965.17). The AIA content was determined in accordance with the method specified by the National Standards of the People’s Republic of China (GB/T 23742-2009, 2009). The apparent nutrient digestibility was calculated as follows: digestibility (%) = [1 − (Nf × AIAd)/(Nd × AIAf)] ×100, where Nf is the nutrient concentration in feces (% DM), Nd is the nutrient concentration in the diet (% DM), AIAd is the AIA concentration in the diet (% DM), and AIAf is the AIA concentration in feces (% DM).
Microbiological Determination
To determine the microbiological status of piglets, fecal samples were collected on the last day of the trial from three randomly selected piglets in each pen. DNA from each fecal sample was extracted and purified using a QIAamp DNA Stool Mini Kit (Qiagen, Crawley, West Sussex, UK). Total bacteria, Lactobacillus spp., and Enterobacteriaceae in fecal samples were quantified by real-time PCR using SYBR green dye according to the method described by Castillo et al. (2006). The primers used in this study were as follows: forward: 5′-GCAGGCCTAACACATGCAAGTC-3′ and reverse: 5′-CTGCTGCCTCCCGTAGGAGT-3′ for total bacteria, forward: 5′-GCAGCAGTAGGGAATCTTCCA-3′ and reverse: 5′-GCATTYCACCGCTACACATG-3′ for Lactobacillus spp. and forward: 5′-ATGGCTGTCGTCAGCTCGT-3′ and reverse: 5′-CCTACTTCTTTTGCAACCCACTC-3′ for Enterobacteriaceae. All microbial enumerations were expressed as log 16S rRNA copies per gram of feces.
Serum Immune Indices Determination
At the end of the trial, two medium-weight piglets were randomly selected from each pen and blood samples were collected from the jugular vein into vacuum blood collection tubes. After 6–8 h of coagulation at room temperature, the samples were centrifuged at 3,000 × g for 15 min to separate the serum. The concentrations of interferon (IFN)-γ (Thermo Fisher Scientific, Inc., Waltham, MA) and haptoglobin (Immunology Consultants Laboratory, Inc., Newberg, OR) were measured by enzyme-linked immunosorbent assays (ELISAs). Serum levels of immunoglobulin A (IgA), immunoglobulin G (IgG), and immunoglobulin M (IgM) were determined using porcine-specific ELISA kits (Bethyl Laboratories, Inc., Montgomery, AL).
Statistical Analysis
For assessment of oligosaccharides in vitro, the results are presented as treatment means ± the standard error of the mean (SEM). Differences between oligosaccharides/glucose and treatment with no added sugar (control) were evaluated using a Student’s t-test. Data from the feeding trial, presented as the group means ± SEM, were analyzed using the PROC general linear model procedure in the SAS statistical software package version 9.3 (SAS Institute, Inc., Cary, NC). The pen was considered the experimental unit. Differences among means were examined using Duncan’s multiple range test. P < 0.05 was considered to indicate a statistical significance.
RESULTS
Carbohydrate Utilization by L. plantarum ZLP001
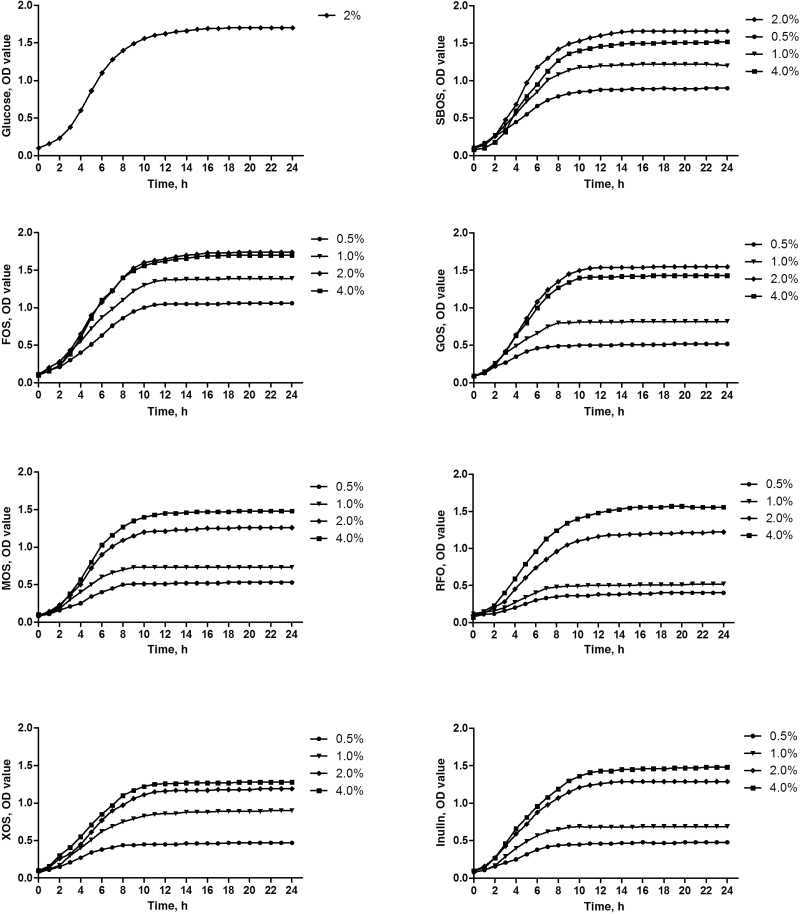
To evaluate carbohydrate utilization by L. plantarum ZLP001, we examined the growth of this strain in the presence of seven oligosaccharides and glucose by measuring the OD at 600 nm over a period of 24 h. The growth curves shown in Figure 1 indicate that L. plantarum ZLP001 can use all the assessed oligosaccharides as carbon sources; however, growth rates clearly differed depending on oligosaccharide type and concentration. For most oligosaccharides, the growth-promoting effect increased with increasing concentration, except for soybean oligosaccharide (SBOS), fructo-oligosaccharide (FOS), and galacto-oligosaccharide (GOS), which yielded the highest OD at a concentration of 2%. Compared with glucose, SBOS and FOS had similar or higher OD values when the culture reached the plateau phase, indicating that L. plantarum ZLP001 can use both SBOS and FOS as efficiently as glucose to support growth. Some oligosaccharides were less effectively utilized by L. plantarum ZLP001, even at maximum concentrations. For example, in the presence of mannan-oligosaccharide (MOS), xylo-oligosaccharide (XOS), and inulin, the OD did not reach 1.5 during the culture period, regardless of concentration.
Figure 1.
Growth curves for Lactobacillus plantarum ZLP001 grown in MRS broth containing glucose, FOS (fructo-oligosaccharide), MOS (mannan-oligosaccharide), XOS (xylo-oligosaccharide), SBOS (soybean oligosaccharide), GOS (galacto-oligosaccharides), RFO (raffinose family oligosaccharide), and inulin. The growth curves were generated based on measurement of the OD at 600 nm every hour for 24 h. The concentration of glucose was 2%, and other oligosaccharides were added at 0.5%, 1%, 2%, and 4%.
Growth Performance of L. plantarum ZLP001
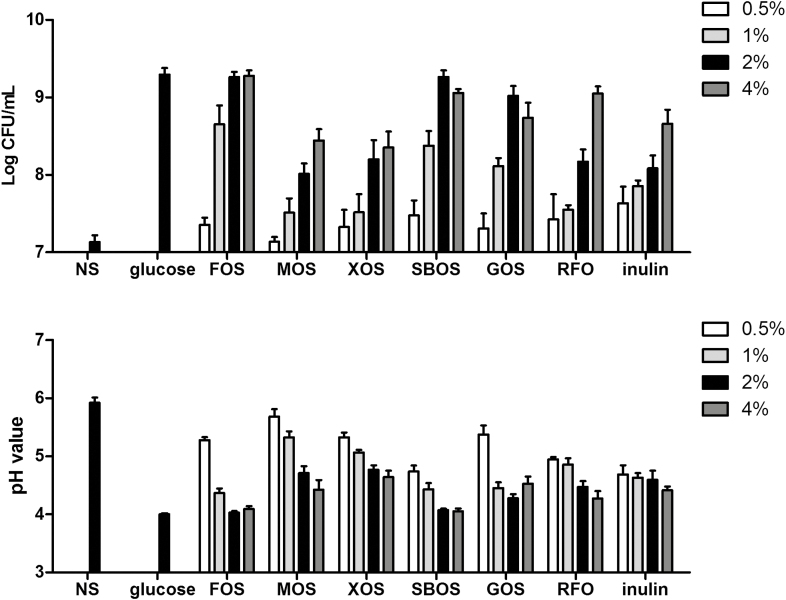
To investigate the growth performance of L. plantarum ZLP001 in the presence of the different oligosaccharides, we determined the viable cell counts after a 24-h fermentation. As shown in Figure 2, viable counts varied considerably depending on the carbon source and its concentration, with different oligosaccharides yielding the highest viable counts at different concentrations (2% or 4%). For all oligosaccharides, viable counts were lower at the lowest concentrations (0.5% or 1%). The viable counts in the presence of FOS and SBOS reached up to 109 CFU at the higher concentrations, which was similar to the viable count obtained for 2% glucose. With regards to pH, most of the oligosaccharides produced a significant decrease in pH values after culture for 24 h, particularly when supplemented at their higher concentrations. Compared with glucose, FOS- and SBOS-supplemented cultures showed similar pH values at their higher concentrations.
Figure 2.
Growth performance and pH value of Lactobacillus plantarum ZLP001 in the presence of glucose, FOS (fructo-oligosaccharide), MOS (mannan-oligosaccharide), XOS (xylo-oligosaccharide), SBOS (soybean oligosaccharide), GOS (galacto-oligosaccharides), RFO (raffinose family oligosaccharide), and inulin after 24-h incubation. Growth performance was determined by counting viable bacteria on MRS agar plates (mean ± SEM, n = 3). NS, MRS medium without any added sugar. The concentration of glucose was 2%, and other oligosaccharides were added at 0.5%, 1%, 2%, and 4%.
Viability in Artificial Gastric and Intestinal Fluids
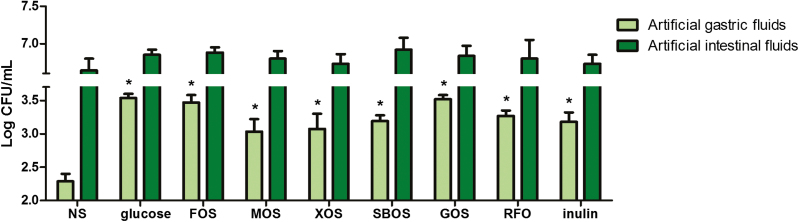
To evaluate the influence of the different oligosaccharides on the tolerance of L. plantarum ZLP001 to artificial gastric and intestinal fluids, we evaluated the viability of L. plantarum ZLP001 in artificial gastric fluid (pH 2.0) and artificial intestinal fluid (pH 8.0) after a 3-h exposure (Figure 3). Although L. plantarum ZLP001 viability had decreased significantly after exposure to artificial gastric fluid for 3 h, the addition of oligosaccharides and glucose significantly improved viability compared with the control group (P < 0.05). FOS and GOS improved viability to a similar level as glucose, whereas other oligosaccharides were observed to be less effective than glucose. There was no significant difference in the viability of L. plantarum ZLP001 in artificial intestinal fluid, regardless of the treatment.
Figure 3.
Survival of Lactobacillus plantarum ZLP001 cultured in the presence of glucose, FOS (fructo-oligosaccharide), MOS (mannan-oligosaccharide), XOS (xylo-oligosaccharide), SBOS (soybean oligosaccharide), GOS (galacto-oligosaccharides), RFO (raffinose family oligosaccharide), and inulin after 3-h exposure to artificial gastric or intestinal fluid. Survival was evaluated by counting viable bacteria on MRS agar plates (mean ± SEM, n = 3). NS, MRS medium without any added sugar. The concentration of each oligosaccharides are the optimal concentrations obtained by growth assay. The concentration were 2% for glucose, FOS, SBOS, and GOS, while the concentration were 4% for MOS, XOS, RFO, and inulin.
Viability under Conditions of Heat Stress and Cold Storage
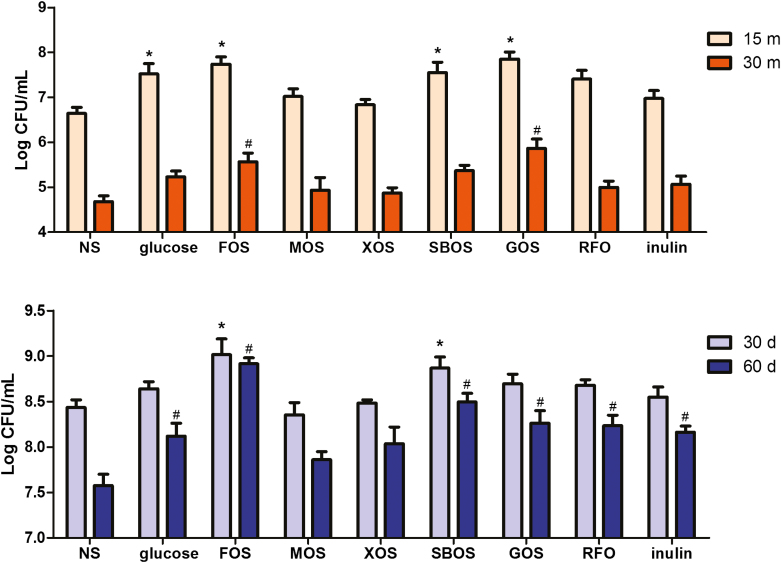
Figure 4A shows the survival of L. plantarum ZLP001 after exposure to heat stress at 65 °C for 15 or 30 min. The viable counts of L. plantarum ZLP001 decreased markedly in response to the longer exposure to heat stress, with the lowest survival being observed in the no sugar control treatment, in which bacterial cell viability had decrease by 50% after 30 min. After both 15 min and 30 min of heat stress, bacteria cultured in the presence of FOS and GOS showed significantly higher viable counts when compared with the control (P < 0.05). Similarly, the L. plantarum ZLP001 survival rate clearly decreased with an increasing amount of time in cold storage (Figure 4B), particularly in the no sugar control treatment. After 30 days of cold storage, we observed that FOS and SBOS had significantly enhanced bacterial viability compared with the control (P < 0.05), whereas, with the exception of MOS and XOS, all oligosaccharides had significantly promoted survival after 60 days of cold storage (P < 0.05) compared with the control.
Figure 4.
Survival of Lactobacillus plantarum ZLP001 cultured in the presence of glucose, FOS (fructo-oligosaccharide), MOS (mannan-oligosaccharide), XOS (xylo-oligosaccharide), SBOS (soybean oligosaccharide), GOS (galacto-oligosaccharides), RFO (raffinose family oligosaccharide), and inulin after exposure to heat stress (65 °C; 15 or 30 min) or cold storage (4 °C; 30 or 60 days). Survival was evaluated by counting viable bacteria on MRS agar plates (mean ± SEM, n = 3). NS, MRS medium without any added sugar. The concentration of each oligosaccharides are the optimal concentrations obtained by growth assay. The concentration were 2% for glucose, FOS, SBOS, and GOS, while the concentration were 4% for MOS, XOS, RFO, and inulin.
Effects of L. plantarum ZLP001 and FOS on the growth performance of weaned piglets
On the basis of the findings of the aforementioned in vitro experiments, FOS appeared to have the strongest beneficial effects on L. plantarum ZLP001, and therefore we selected FOS for in vivo analyses. The effects of L. plantarum ZLP001, FOS, and their combination on the growth performance of weaned pigs are given in Table 3. We found that the combination of L. plantarum ZLP001 and FOS significantly increased the ADG (P < 0.05) after 4 weeks of dietary supplementation. Although when supplemented individually, both L. plantarum ZLP001 and FOS increased the ADG, by 6.03% and 6.28%, respectively, the differences observed being non-significant (P > 0.05). Similarly, none of the three treatments had an effect on ADFI (P > 0.05). However, administration of L. plantarum ZLP001 alone or in combination with FOS significantly decreased the F/G ratio (P < 0.05), whereas no significant different was observed for FOS supplementation alone.
Table 3.
Effects of Lactobacillus plantarum ZLP001 and FOS on growth performance in weaning piglets1
| Items2 | No additive | ZLP001 | FOS | ZLP001 + FOS | SEM | P-value |
|---|---|---|---|---|---|---|
| Initial weight, kg | 7.05 | 7.14 | 7.07 | 7.08 | 0.12 | 0.853 |
| Final weight, kg | 20.97 | 22.17 | 21.26 | 21.88 | 0.75 | 0.466 |
| ADG, g | 398.11b | 422.46ab | 423.32ab | 439.15a | 5.90 | 0.043 |
| ADFI, g | 816.33 | 780.06 | 805.20 | 754.82 | 15.14 | 0.590 |
| F/G | 2.05a | 1.81b | 1.90ab | 1.73b | 0.04 | 0.025 |
1ZLP001, L. plantarum ZLP001; FOS, fructo-oligosaccharides
2F/G, feed-to-gain ratio.
a,bMeans within rows with different superscript letters differ (P < 0.05).
Effects of L. plantarum ZLP001 and FOS on Apparent Nutrient Digestibility in Weaning Piglets
We used the AIA method to estimate the apparent nutrient digestibility of DM, CP, Ca, and P in weaning piglets after dietary supplementation with L. plantarum and FOS, both individually and combined. The apparent digestibility of DM was increased (P < 0.05) by all three treatments (Table 4), whereas for CP, the combination of L. plantarum ZLP001 and FOS yielded the highest apparent digestibility, which was significantly higher than that in the basal diet control (P < 0.05). Regardless of treatment, no significant differences were observed with respect to the apparent digestibility of Ca or P.
Table 4.
Effects of Lactobacillus plantarum ZLP001 and FOS on apparent nutrient digestibility in weaning piglets (%)1
| Items | No additive | ZLP001 | FOS | ZLP001 + FOS | SEM | P-value |
|---|---|---|---|---|---|---|
| DM | 81.42b | 84.98a | 85.36a | 86.51a | 1.09 | 0.034 |
| CP | 78.54b | 83.51ab | 80.28ab | 85.89a | 2.65 | 0.022 |
| Ca | 51.63 | 53.17 | 50.88 | 52.80 | 1.78 | 0.657 |
| P | 46.21 | 50.40 | 47.83 | 51.39 | 2.53 | 0.337 |
1ZLP001, L. plantarum ZLP001; FOS, fructo-oligosaccharides
a,bMeans within rows with different superscript letters differ (P < 0.05).
Effects of L. plantarum ZLP001 and FOS on Fecal Microbiota in Weaning Piglets
We found that fecal abundance of Lactobacillus spp. was significantly increased by L. plantarum ZLP001 and FOS treatments alone or in combination when compared with the basal diet control (P < 0.05, Table 5). In contrast, fecal Enterobacteriaceae counts were significantly decreased in response to all three treatments (P < 0.05), with the combined treatment having a significantly stronger effect than either single treatments (P < 0.05). Although fecal microbial shedding of total bacteria was not significantly (P > 0.05) affected by the dietary treatments at the end of this experiment, supplementation with L. plantarum ZLP001 and FOS tended to enhance the total bacterial abundance.
Table 5.
Effects of Lactobacillus plantarum ZLP001 and FOS on fecal microbiota in weaning piglets (Log10 copies/gram contents)
| Items | No additive | ZLP001 | FOS | ZLP001 + FOS | SEM | P-value |
|---|---|---|---|---|---|---|
| Total bacteria | 7.22 | 7.56 | 7.35 | 7.40 | 0.25 | 0.255 |
| Lactobacillus spp. | 4.13b | 4.44a | 4.41a | 4.56a | 0.16 | 0.041 |
| Enterobacteriaceae | 4.14a | 3.41b | 3.78b | 3.01c | 0.11 | 0.028 |
ZLP001, L. plantarum ZLP001
a,b,cMeans within rows with different superscript letters differ (P < 0.05).
Effects of L. plantarum ZLP001 and FOS on Serum Immune Index in Weaning Piglets
Combined supplementation of L. plantarum ZLP001 and FOS was observed to significantly increase the serum IFN-γ concentration in piglets compared with the control (P < 0.05; Table 6). The serum IgG level was significantly increased by supplementation with L. plantarum ZLP001 or FOS, or their combination (P < 0.05), whereas in contrast serum haptoglobin, IgA and IgE were not significantly (P >0.05) altered by the dietary treatments assessed in the present study.
Table 6.
Effects of Lactobacillus plantarum ZLP001 and FOS on serum immune index in weaning piglets1
| Items | No additive | ZLP001 | FOS | ZLP001 + FOS | SEM | P-value |
|---|---|---|---|---|---|---|
| IFN-γ, pg/mL | 6.10b | 6.50ab | 6.45ab | 6.62a | 0.08 | 0.029 |
| Haptoglobin, μg/mL | 222.91 | 209.70 | 210.49 | 201.44 | 2.62 | 0.068 |
| IgA, μg/mL | 46.16 | 43.35 | 45.54 | 46.27 | 0.66 | 0.948 |
| IgG, μg/mL | 359.22b | 402.67a | 421.95a | 400.49a | 8.71 | 0.020 |
| IgE, μg/mL | 101.34 | 102.45 | 105.18 | 111.72 | 4.82 | 0.288 |
1ZLP001, L. plantarum ZLP001; FOS, fructo-oligosaccharides after ZLP001
a,bMeans within rows with different superscript letters differ (P < 0.05).
Discussion
A number of oligosaccharides have been reported to be good carbohydrate sources that can be used to enhance the growth of Lactobacillus (Mäkeläinen et al., 2010; Muthaiyan et al., 2012). In the current study, we found that most of the oligosaccharides we assessed were utilized as a carbohydrate source by L. plantarum ZLP001, regardless of the concentration applied. This may be partially associated with that fact that L. plantarum ZLP001 has numerous genes that play roles in carbohydrate transport and metabolism (Zhang et al., 2018). However, the growth of probiotic strains on particular oligosaccharides is considered to be strain specific, and strains show different abilities to use different oligosaccharides as a carbohydrate source (Holt et al., 2005; Sims et al., 2014). Among the oligosaccharides examined in the present study, we found that FOS at concentrations of 2% and 4% promoted growth to a similar level as glucose, followed in terms of effectiveness by SBOS at 2%. Consistently, previous studies have shown that FOS can be fermented by a wide range of probiotic species, including those in the genera Bifidobacterium and Lactobacillus (Sims et al., 2014). The utilization of SBOS by Lactobacillus was not confirmed in pure culture, but in an in vitro inoculums system, SBOS significantly increased the microbial diversity and the population of Bifidobacterium and Lactobacillus (Zhou et al., 2012). These in vitro results indicate that supplementation of animal feed with these specific oligosaccharides can potentially promote the growth of L. plantarum ZLP001 in vivo when supplemented simultaneously. However, whether L. plantarum ZLP001 can metabolize these oligosaccharides in the presence of the complex milieu of intestinal microbiota remains to be investigated.
To exert a strong beneficial effect, probiotic strains should possess high viability. Thus, it is important that probiotic strains have the capacity to survive survive extreme environment and technological processing. In this regard, it has been demonstrated that different sugars and other culture medium components can exert protective effects by preventing membrane damage, protein denaturation, and other cell injuries (Vitali et al., 2016). Therefore, we evaluated the protective role of the seven oligosaccharides on L. plantarum ZLP001 under different stress conditions. Resistance to the extreme environment of the gastrointestinal tract, which is likely to be inimical to the survival of probiotic strains, is one of the essential characteristics of potential probiotic organisms (Liong and Shah, 2005). Thus, for a probiotic agent to be effective, it should ideally exhibit a strong tolerance to acidic conditions and bile salts (Shokryazdan et al., 2017). In the present study, we found that several oligosaccharides showed a clear protective effect on L. plantarum ZLP001 survival in artificial gastric fluid. Similar protective effects of FOS and XOS on Lactobacillus under artificial gastric conditions have been reported by Pan et al. (2009). Although we didn’t observed the effect of oligosaccharides on artificial intestinal fluid in present study, FOS has also been verified to confer a higher resistance to bile salts than either glucose or fructose in Bifidobacterium (Perrin et al., 2000). Heat stability is a further essential characteristic of probiotic strains, particularly with respect to surviving the high-temperature conditions typically experienced during industrial processing, like spray drying necessary to produce marketable products. In this regard, our data indicated that L. plantarum ZLP001 is sensitive to heat (65 °C) for 15 or 30 min. However, some of the oligosaccharides we examined, especially FOS and GOS, conferred a certain extent protection against heat stress. Similar protection effect of several oligosaccharides were observed by Pan et al. (2009). At the opposite end of the temperature range, cold storage tolerance is another important property desirable in probiotic strains, notably with respect to extending shelf life. The results of our cold tolerance experiment revealed that oligosaccharides, especially for relative long time, enhanced the survival rate of L. plantarum ZLP001 stored at 4 °C, indicating that some oligosaccharides may have a good cryoprotective effect. The possible reason of this protect effect conferred by oligosaccharides may be related to carbon catabolite repression, which is correlated with the cold shock response (Beaufils et al., 2007). Some genes associated with stress response in Lactobacillus species have been identified, such as the molecular chaperone groESL (Walker et al., 1999), the regulation of oligosaccharides on these gene which may result in the subsequent protection effect need further investigation. Moreover, it has been extensively demonstrated that metabolic processes of bacteria are altered to varying extents in response to different stresses, resulting in the production of specific stress metabolites, such as guanosine phosphate, guanosine tetraphosphate (ppGpp) and pentaphosphate (pppGpp), and phosphate, which may be associated with the synthesis of stress-related proteins (Yousef and Juneja, 2003; Hosseini Nezhad et al., 2015). Accordingly, further studies are also needed to ascertain whether oligosaccharides enhance probiotic strain survival under stress conditions via the regulation of certain stress metabolites.
Lactic acid bacteria have beneficial effects on animal production owing to their special probiotic properties (Shokryazdan et al., 2017). And the use of FOS in animal production also has been extensively studied (Pan et al., 2018; Schokker et al., 2018). Although to date, studies have shown that FOS supports the growth of L. plantarum No. 14 in the luminal contents of mice when these are administered concurrently (Takemura et al., 2010), there have been relatively few studies that have investigated the combined use of L. plantarum and FOS in weaning piglets.
In piglets, the main purpose of administering probiotics, prebiotics, and synbiotics is to reduce the incidence of weaning-related diarrhea and to improve body health and production performance (Rhouma et al., 2017), and the beneficial effects of these agents on animal performance, including piglets, have been extensively studied (Markowiak and Śliżewska, 2018; Méndez-Palacios et al., 2018). The combination of prebiotics and probiotics is believed to be more efficient than use of either prebiotics or probiotics alone. In the present study, we found that both L. plantarum ZLP001 and FOS increased the ADG in piglets, whereas their combined administration significantly increased ADG compared with the no additive control. Together with our F/G ratio data, these results are suggestive of a certain synergistic effect of L. plantarum ZLP001 and FOS. The improved apparent intestinal digestibility of DM and CP in response to probiotic and FOS addition appears to mirror the positive effects on piglet growth performance. With regards to CP in particular, a synergistic effect could explain the effect of the combination on body weight gain. However, synbiotic effects are not invariably observed upon combined supplementation of prebiotic and probiotics. For example, Liu et al. (2018) were unable to detect any interaction between dietary supplementation of probiotics and XOS with regards to growth performance and nutrient digestibility in weanling pigs. An increase in beneficial Lactobacillus and a concomitant decrease in pathogenic Enterobacteriaceae in fecal samples upon probiotic and oligosaccharide dietary supplementation reflects the modulation of gut microbial composition in response to this treatment, and may contribute to explaining the improvement in production performance and nutrient digestibility (Meng et al., 2010). Such a modulation effect of L. plantarum ZLP001 and FOS on piglet intestinal microbiota was observed in present study, and may have partially contributed to an enhanced piglet production performance. Similar previous studies have also noted significant decreases in total coliform bacteria and increases in bifidobacteria populations in response to dietary supplementation with probiotics, oligosaccharides, or their combination (Shim et al., 2005). In this regard, we suspect that sampling of the contents of different segments of intestine in conjunction with sequence analyses would contribute to enhancing our current understanding of the relationship between the synergistic effects of probiotics and oligosaccharides and the composition of intestinal microbiota. Probiotics and fructo-oligosaccharide have previously been demonstrated to have modulating effects on different immune parameters (Ashraf and Shah, 2014; Pan et al., 2018). In the present study, we accordingly observed an increase in the production of IFN-γ and IgG in pigs in response to dietary supplementation with L. plantarum ZLP001 alone, FOS alone, or their combination. IFN-γ, an important activator of macrophages, is a broad-spectrum antiviral agent, which can inhibit virus replication by inducing cellular antiviral protein production. Recent studies have shown that pseudorabies virus (PRV) reduces IFN-γ production in weaned pigs, whereas dietary pectic oligosaccharide can alleviate PRV-induced diarrhea by increasing the levels of IFN-γ in the jejunal and ileal mucosa of pigs (Chen et al., 2017). IgG is known to induce a protective immune response against various pathogen and viral infections, and supplementation of piglet diets with either L. plantarum B2984 or lactulose alone, or in combination in a synbiotic diet, has been shown to enhance the serum IgG response to Salmonella typhimurium infection (Naqid et al., 2015). Our results provide further evidence of the beneficial effects of these agents and their supplementation as strategies to enhance pig immune responses, which may enhance protection against enteric infection. Moreover, activation of the immune system may also account for the enhanced performance and better health condition of weaned piglets.
CONCLUSION
In conclusion, our findings confirmed that the use of oligosaccharides by L. plantarum ZLP001 as a carbohydrate source is type specific and concentration dependent. Among the different oligosaccharides we assessed, FOS was found to exhibit highest beneficial effect to L. plantarum ZLP001, as evidenced by the high viability conferred and resistance to stressors. Moreover, the synergistic efficacy of L. plantarum ZLP001 and FOS provides convincing evidence that these agents may have potential utility as synbiotics that can be applied to enhance weanling pig performance by increasing digestibility, improving intestinal health, and strengthening immune function. Further research is, nevertheless, needed to determine the mechanisms whereby FOS facilitates the growth and stress resistance of L. plantarum ZLP001, as well as the mechanisms underlying their synergistic action in weaning piglets.
Footnotes
This research was financially supported by the Beijing Innovation Consortium of Agriculture Research System (BAIC02-2019) and the Special Program on Science and Technology Innovation Capacity Building of BAAFS (KJCX201914 and KJCX20161503). The authors report no conflict of interest.
LITERATURE CITED
- Al-Khalaifah H. S. 2018. Benefits of probiotics and/or prebiotics for antibiotic-reduced poultry. Poult. Sci. 97:3807–3815. doi: 10.3382/ps/pey160 [DOI] [PubMed] [Google Scholar]
- AOAC. 2005. Official methods of analysis of AOAC international. 18th ed Arlington, VA: Association of Official Analytical Chemists. [Google Scholar]
- Ashraf R., and Shah N. P.. . 2014. Immune system stimulation by probiotic microorganisms. Crit. Rev. Food Sci. Nutr. 54:938–956. doi: 10.1080/10408398.2011.619671 [DOI] [PubMed] [Google Scholar]
- Barba-Vidal E., Martín-Orúe S. M., and Castillejos L.. . 2018. Review: are we using probiotics correctly in post-weaning piglets? Animal 12:2489–2498. doi: 10.1017/S1751731118000873 [DOI] [PubMed] [Google Scholar]
- Beaufils S., Sauvageot N., Mazé A., Laplace J. M., Auffray Y., Deutscher J., and Hartke A.. . 2007. The cold shock response of Lactobacillus casei: relation between HPr phosphorylation and resistance to freeze/thaw cycles. J. Mol. Microbiol. Biotechnol. 13:65–75. doi: 10.1159/000103598 [DOI] [PubMed] [Google Scholar]
- Castillo M., Martín-Orúe S. M., Manzanilla E. G., Badiola I., Martín M., and Gasa J.. . 2006. Quantification of total bacteria, enterobacteria and lactobacilli populations in pig digesta by real-time PCR. Vet. Microbiol. 114:165–170. doi: 10.1016/j.vetmic.2005.11.055 [DOI] [PubMed] [Google Scholar]
- Castillo M., Martín-Orúe S. M., Taylor-Pickard J. A., Pérez J. F., and Gasa J.. . 2008. Use of mannanoligosaccharides and zinc chelate as growth promoters and diarrhea preventative in weaning pigs: effects on microbiota and gut function. J. Anim. Sci. 86:94–101. doi: 10.2527/jas.2005-686 [DOI] [PubMed] [Google Scholar]
- Chen H., Hu H., Chen D., Tang J., Yu B., Luo J., He J., Luo Y., Yu J., and Mao X.. . 2017. Dietary pectic oligosaccharide administration improves growth performance and immunity in weaned pigs infected by rotavirus. J. Agric. Food Chem. 65(14):2923–2929. doi: 10.1021/acs.jafc.7b00039 [DOI] [PubMed] [Google Scholar]
- GB 2009. Animal feeding stuffs-determination of ash insoluble in hydrochloric acid (GB/T 23742-2009). Beijing: Standards Press of China. [Google Scholar]
- Holt S. M., Miller-Fosmore C. M., and Côté G. L.. . 2005. Growth of various intestinal bacteria on alternansucrase-derived oligosaccharides. Lett. Appl. Microbiol. 40:385–390. doi: 10.1111/j.1472-765X.2005.01681.x [DOI] [PubMed] [Google Scholar]
- Hosseini Nezhad M., Hussain M. A., and Britz M. L.. . 2015. Stress responses in probiotic Lactobacillus casei. Crit. Rev. Food Sci. Nutr. 55:740–749. doi: 10.1080/10408398.2012.675601 [DOI] [PubMed] [Google Scholar]
- Jiao L. F., Song Z. H., Ke Y. L., Xiao K., Hu C. H., and Shi B.. . 2014. Cello-oligasaccharides influences intestinal microflora, mucosal architecture and nutrient transport in weaned pigs. Anim. Feed Sci. Technol. 195:85–91. doi: 10.1016/j.anifeedsci.2014.05.014 [DOI] [Google Scholar]
- Kim S. J., Cho S. Y., Kim S. H.,Song O. J., Shin I. S., Cha D. S., and Park H. J.. . 2008. Effect of microencapsulation on viability and other characteristics in Lactobacillus acidophilus TCC 43121. LWT-Food Sci. Technol. 41:493–500. doi: 10.1016/j.lwt.2007.03.025 [DOI] [Google Scholar]
- Liao S. F., and Nyachoti M.. . 2017. Using probiotics to improve swine gut health and nutrient utilization. Anim. Nutr. 3:331–343. doi: 10.1016/j.aninu.2017.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liong M. T., and Shah N. P.. . 2005. Acid and bile tolerance and cholesterol removal ability of lactobacilli strains. J. Dairy Sci. 88:55–66. doi: 10.3168/jds.S0022-0302(05)72662-X [DOI] [PubMed] [Google Scholar]
- Liu J. B., Cao S. C., Liu J., Xie Y. N., and Zhang H. F.. . 2018. Effect of probiotics and xylo-oligosaccharide supplementation on nutrient digestibility, intestinal health and noxious gas emission in weanling pigs. Asian-Australas. J. Anim. Sci. 31:1660–1669. doi: 10.5713/ajas.17.0908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkeläinen H., Saarinen M., Stowell J., Rautonen N., and Ouwehand A. C.. . 2010. Xylo-oligosaccharides and lactitol promote the growth of Bifidobacterium lactis and Lactobacillus species in pure cultures. Benef. Microbes 1:139–148. doi: 10.3920/BM2009.0029 [DOI] [PubMed] [Google Scholar]
- Markowiak P., and Śliżewska K.. . 2018. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 10:21. doi: 10.1186/s13099-018-0250-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez-Palacios N., Méndez-Mendoza M., Vázquez-Flores F., Castro-Colombres J. G., and Ramírez-Bribiesca J. E.. . 2018. Productive and economic parameters of pigs supplemented from weaning to finishing with prebiotic and probiotic feed additives. Anim. Sci. J. 89(7):994–1001. doi: 10.1111/asj.13008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q. W., Yan L., Ao X., Zhou T. X., Wang J. P., Lee J. H., and Kim I. H.. . 2010. Influence of probiotics in different energy and nutrient density diets on growth performance, nutrient digestibility, meat quality, and blood characteristics in growing-finishing pigs. J. Anim. Sci. 88:3320–3326. doi: 10.2527/jas.2009-2308 [DOI] [PubMed] [Google Scholar]
- Muthaiyan A., Hernandez-Hernandez O., Moreno F. J., Sanz M. L., and Ricke S. C.. . 2012. Hydrolyzed caseinomacropeptide conjugated galactooligosaccharides support the growth and enhance the bile tolerance in Lactobacillus strains. J. Agric. Food Chem. 60:6839–6845. doi: 10.1021/jf301392y [DOI] [PubMed] [Google Scholar]
- Naqid I. A., Owen J. P., Maddison B. C., Gardner D. S., Foster N., Tchórzewska M. A., La Ragione R. M., and Gough K. C.. . 2015. Prebiotic and probiotic agents enhance antibody-based immune responses to Salmonella typhimurium infection in pigs. Anim. Feed Sci. Technol. 201:57–65. doi: 10.1016/j.anifeedsci.2014.12.005 [DOI] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed Washington, DC: National Academies Press. [Google Scholar]
- NY 2004. Feeding standard of swine (NY/T 65–2004). Beijing: China Agriculture Press. [Google Scholar]
- Pan L., Farouk M. H., Qin G., Zhao Y., and Bao N.. . 2018. The influences of soybean agglutinin and functional oligosaccharides on the intestinal tract of monogastric animals. Int. J. Mol. Sci. 19:554–571. doi: 10.3390/ijms19020554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Wu T., Zhang L., Cai L., and Song Z.. . 2009. Influence of oligosaccharides on the growth and tolerance capacity of lactobacilli to simulated stress environment. Lett. Appl. Microbiol. 48:362–367. doi: 10.1111/j.1472-765X.2008.02539.x [DOI] [PubMed] [Google Scholar]
- Perrin S., Grill J. P., and Schneider F.. . 2000. Effects of fructooligosaccharides and their monomeric components on bile salt resistance in three species of Bifidobacteria. J. Appl. Microbiol. 88:968–974. doi: 10.1046/j.1365-2672.2000.01070.x [DOI] [PubMed] [Google Scholar]
- Rhouma M., Fairbrother J. M., Beaudry F., and Letellier A.. . 2017. Post weaning diarrhea in pigs: risk factors and non-colistin-based control strategies. Acta Vet. Scand. 59:31. doi: 10.1186/s13028-017-0299-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schokker D., Fledderus J., Jansen R., Vastenhouw S. A., de Bree F. M., Smits M. A., and Jansman A. A. J. M.. . 2018. Supplementation of fructooligosaccharides to suckling piglets affects intestinal microbiota colonization and immune development. J. Anim. Sci. 96:2139–2153. doi: 10.1093/jas/sky110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim, S. B., M. W. A. Verstegen, I. H. Kim, O. S. Kwon, and J. M. A. J. Verdonk. 2005. Effects of feeding antibiotic-free creep feed supplemented with oligofructose, probiotics or synbiotics to suckling piglets increases the preweaning weight gain and composition of intestinal microbiota. Arch. Anim. Nutr. 59(6): 419–427. doi: 10.1080/17450390500353234 [DOI] [PubMed] [Google Scholar]
- Shokryazdan P., Faseleh Jahromi M., Liang J. B., and Ho Y. W.. . 2017. Probiotics: from isolation to application. J. Am. Coll. Nutr. 36:666–676. doi: 10.1080/07315724.2017.1337529 [DOI] [PubMed] [Google Scholar]
- Sims I. M., Ryan J. L., and Kim S. H.. . 2014. In vitro fermentation of prebiotic oligosaccharides by Bifidobacterium lactis HN019 and Lactobacillus spp. Anaerobe 25:11–17. doi: 10.1016/j.anaerobe.2013.11.001 [DOI] [PubMed] [Google Scholar]
- Takemura N., Ozawa K., Kimura N., Watanabe J., and Sonoyama K.. . 2010. Inulin-type fructans stimulated the growth of exogenously administered Lactobacillus plantarum No. 14 in the mouse gastrointestinal tract. Biosci. Biotechnol. Biochem. 74:375–381. doi: 10.1271/bbb.90794 [DOI] [PubMed] [Google Scholar]
- Uyeno Y., Shigemori S., and Shimosato T.. . 2015. Effect of probiotics/prebiotics on cattle health and productivity. Microbes Environ. 30:126–132. doi: 10.1264/jsme2.ME14176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitali B., Abruzzo A., Parolin C., Palomino R. A., Dalena F., Bigucci F., Cerchiara T., and Luppi B.. . 2016. Association of Lactobacillus crispatus with fructo-oligosaccharides and ascorbic acid in hydroxypropyl methylcellulose vaginal insert. Carbohydr. Polym. 136:1161–1169. doi: 10.1016/j.carbpol.2015.10.035 [DOI] [PubMed] [Google Scholar]
- Walker D. C., Girgis H. S., and Klaenhammer T. R.. . 1999. The groESL chaperone operon of Lactobacillus johnsonii. Appl. Environ. Microbiol. 65:3033–3041. doi: 10.1016/j.matdes.2008.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Ji H. F., Wang S. X., Liu H., Zhang W., Zhang D. Y., and Wang Y. M.. . 2018a. Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front. Microbiol. 9:1953. doi: 10.3389/fmicb.2018.01953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Ji H. F., Wang S. X., Zhang D. Y., Liu H., Shan D. C., and Wang Y. M.. . 2012. Lactobacillus plantarum ZLP001: in vitro assessment of antioxidant capacity and effect on growth performance and antioxidant status in weaning piglets. Asian-Australas. J. Anim. Sci. 25:1153–1158. doi: 10.5713/ajas.2012.12079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Ji H. F., Zhang D. Y., Liu H., Wang S. X., Shan D. C., and Wang Y. M.. . 2011. Assessment of probiotic properties of Lactobacillus plantarum ZLP001 isolated from gastrointestinal tract of weaning pigs. Afr. J. Biotechnol. 10:11303–11308. [Google Scholar]
- Wang J., Zeng Y. X., Wang S. X., Liu H., Zhang D. Y., Zhang W., Wang Y. M., and Ji H. F.. . 2018b. Swine-derived probiotic Lactobacillus plantarum inhibits growth and adhesion of enterotoxigenic escherichia coli and mediates host defense. Front. Microbiol. 9:1364. doi: 10.3389/fmicb.2018.01364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H., Wang L., Xiong Y., Wen X., Wang Z., Yang X., Gao K., and Jiang Z.. . 2018. Effects of Lactobacillus reuteri LR1 on the growth performance, intestinal morphology, and intestinal barrier function in weaned pigs. J. Anim. Sci. 96:2342–2351. doi: 10.1093/jas/sky129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef A. E., and Juneja V. K.. . 2003. Microbial stress adaptation and food safety. Boca Raton (FL): CRC Press. [Google Scholar]
- Zhang W., Ji H., Zhang D., Liu H., Wang S., Wang J., and Wang Y.. . 2018. Complete genome sequencing of Lactobacillus plantarum ZLP001, a potential probiotic that enhances intestinal epithelial barrier function and defense against pathogens in pigs. Front. Physiol. 9:1689. doi: 10.3389/fphys.2018.01689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X. L., Kong X. F., Yang X. J., and Yin Y. L.. . 2012. Soybean oligosaccharides alter colon short-chain fatty acid production and microbial population in vitro. J. Anim. Sci. 90(Suppl. 4):37–39. doi: 10.2527/jas.50269 [DOI] [PubMed] [Google Scholar]