To the Editor: We read with interest the report by Johnson et al1 on the remarkable response to dupilumab in a 5-year-old patient with severe, recalcitrant atopic dermatitis (AD). This case report highlighted the utility of dupilumab for use in the pediatric population. We aim to expand on this finding by reporting on the success of dupilumab to achieve clearance of a 22-month-old boy with severe AD.
A 22-month-old African-American boy with medical history of obstructive hypertrophic cardiomyopathy, hypertension, hydronephrosis, gastroesophageal reflux disease, asthma, developmental delay, oropharyngeal dysphagia, and congenital subglottic stenosis presented with a 6-month history of poorly controlled AD, with generalized scaly, excoriated patches, worse on the head and extremities. He was unsuccessfully treated with hydrocortisone 2.5% ointment to his face and triamcinolone 0.1% ointment to his body as needed, oral diphenhydramine, bleach baths biweekly, and aggressive moisturization.
Given the failure of topical therapy, we were challenged to find a systemic treatment that would maximize adherence and minimize side effects given this patient's serious comorbidities, especially hypertension. Phototherapy, although found to be effective in the treatment of pediatric AD with minimal side effects, has a relatively slow onset of action and requires frequent office visits. Methotrexate, a systemic immunomodulator, is found to be efficacious in the treatment of pediatric AD but can cause immunosuppression, bone marrow suppression, hepatotoxicity, and gastrointestinal upset.2 Cyclosporine is found to be effective in achieving remission in children with severe AD3 but is known to be nephrotoxic and would not be ideal in a patient who already suffers from hydronephrosis and hypertension. Although not approved for use in children younger than 12 years, dupilumab's pharmacodynamics provide little physiologic basis to expect side effects with use in a younger age group than that for which it is already approved by the US Food and Drug Administration. Moreover, evidence suggests that it may help improve the symptoms of our patient's asthma.4
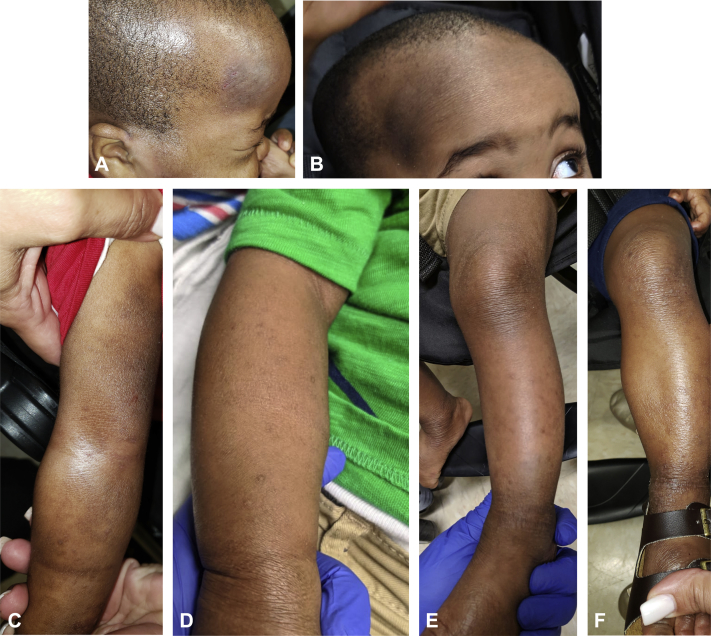
The patient was started on dupilumab at a loading dose of 114 mg (10 mg/kg) on day 0, followed by 57 mg (5 mg/kg) subcutaneously every 2 weeks beginning on day 15. After 4 weeks of treatment (3 doses), his AD improved dramatically (Fig 1). Notably, the parent commented that the pruritus appeared to resolve completely within 48 hours of initiating treatment. This case supports dupilumab's utility in the young pediatric population with AD. Clinical trials are underway to evaluate the use of dupilumab in children between 6 months and 12 years of age. We hope this letter adds further value to the case report by Johnson et al1 and prompts studies for even younger ages in light of dupilumab's safety benefits relative to existing options.
Fig 1.
Before and after 3 doses of dupilumab. A and B, Improvement of a hyperpigmented thin plaque on the patient's forehead. C and D, Improvement of eczematous lesions of the patient's right forearm. E and F, Improvement of eczematous lesions of the patient's left leg and ankle.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Johnson B.B., Beck L.A., Mustafa S.S. Remarkable response to dupilumab in a 5-year-old patient with severe, recalcitrant atopic dermatitis. JAAD Case Rep. 2019;5(7):605–608. doi: 10.1016/j.jdcr.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson K., Putterman E., Rogers R.S., Patel D., Treat J.R., Castelo-Soccio L. Treatment of severe pediatric atopic dermatitis with methotrexate: a retrospective review. Pediatr Dermatol. 2019;36(3):298–302. doi: 10.1111/pde.13781. [DOI] [PubMed] [Google Scholar]
- 3.Berth-Jones J., Finlay A.Y., Zaki I. Cyclosporine in severe childhood atopic dermatitis: a multicenter study. J Am Acad Dermatol. 1996;34(6):1016–1021. doi: 10.1016/s0190-9622(96)90281-9. [DOI] [PubMed] [Google Scholar]
- 4.Beck L.A., Thaçi D., Hamilton J.D. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371(2):130–139. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]