Abstract
Tinospora cordifolia is a popular medicinal plant which is used in several traditional medicines to cure various diseases. The common names are Amrita and Guduchi and belong to the family of Menispermaceae. It is considered an essential herbal plant of Indian system of medicine (ISM) and has been used in the treatment of fever, urinary problem, dysentery, skin diseases leprosy, diabetes, and many more diseases. The plant reported containing chemical compound including Alkaloids, Terpenoids, Lignans, Steroids and others that establish the phytochemistry and pharmacological activity of Tinospora cordifolia. The present review highlights the pharmacological importance viz antioxidant activity, antimicrobial activity, antibacterial activity, antifungal activity, anti-diabetic activity, antistress activity, hypolipidaemic effect, hepatic disorder, anticancer anti HIV potential, antiosteoporotic effects, antitoxic effects, wound healing, anticomplementary activity, and immunomodulating activity, systemic infection and Parkinson's disease.
Keywords: Natural product chemistry, Pharmaceutical science, Pharmaceutical chemistry, Tinospora cordifolia, Menispermaceae, Pharmacognostic description, Chemical constituent, Pharmacological activity
1. Introduction
Herbal formulations are medicinal preparation of one or more herbs present in specified quantities to give the benefits meant for cosmetic, diagnose and to mitigate diseases of human beings or animals [1]. It is also known as botanical medicine or phytomedicine. Earlier in the twentieth century, herbal medicine was the prime medication system as antibiotics or analgesics were not available. Increasing use of an allopathic system of medicine due to its fast therapeutic action and herbal medicine gradually lost their popularity among the people. For example, Curcuma is used in Traditional Chinese Medicine for more than two thousand years to treat anti-inflammatory and robust antioxidant [2, 3]. About 70–80% of people are still using herbal medicine for their primary health because of the less side effect and better compatibility with the human body [4]. Herbal medicine has gained momentum and is more effective as compared to synthetic drugs.
T. cordifolia (synonym: Tinospora sinensis (Lour.) Merr.) is also known as Guduchi/Amrita and its names in Latin: Tinospora cordifolia (Wild) Hook. f. & Thomson, English: Tinospora Gulancha/Indian Tinospora, Hindi: Giloya. It belongs to the family of Menispermaceae and is found in Myanmar, Sri Lanka, and China [5].
The plant is commonly used as traditional ayurvedic medicine and has several therapeutic properties [6, 7] such as jaundice, rheumatism, urinary disorder, skin diseases, diabetes, anemia, inflammation, allergic condition, anti-periodic, radioprotective properties, etc. [8, 9] The root of Giloya (T. cordifolia) is used as potent emetic and for bowel obstruction. The starch of this plant serves a beneficial household remedy for chronic fever, relieves burning sensation, increases energy and appetite.
Giloya is useful in the treatment of helminthiasis, heart diseases, leprosy, rheumatoid arthritis, support the immune system, the body's resistance to infections, supports standard white blood cell structure, function, and levels [10]. It also helps in digestive ailments such as hyperacidity, colitis, worm infestations, loss of appetite, abdominal pain, excessive thirst, and vomiting, and even liver disorders like hepatitis [11, 12].This pharmacological activities of the plant is due to its chemical constituents like diterpenoid lactones, glycosides, steroids, sesquiterpenoid, phenolics, aliphatic compounds, essential oils, a mixture of fatty acids, and polysaccharides and is present in a different part of the plant body, including root, stem, and whole part [13].
2. Main text
2.1. Pharmacognostic description
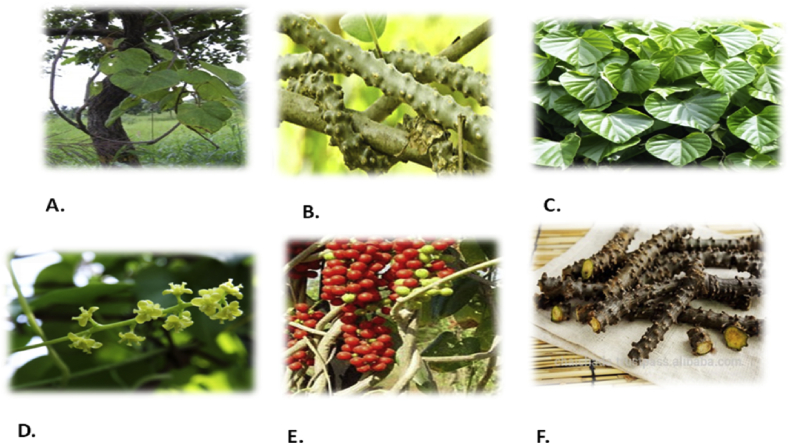
It is a large deciduous, extensively spreading climbing shrub with several coiled branches with a different type of morphology. The stem of the plant is filiform, fleshy and climbing in nature; bark is white to gray [14]. Powder of the stem is creamish brown or dark brown, characteristic odor, bitter taste and is used in dyspepsia, fever, and urinary diseases [15]. The starch obtained from the stem known as “Guduchi-satva.” It is highly nutritive and digestive. Leaves of this plant are simple, alternate, long-petioled (approximately 15 cm); round, pulvinate, heart-shaped, twisted partially and halfway around. Lamina is ovate, 10–20 cm long, seven nerved and deeply cordate at the base and membranous [16]. Flowers are unisexual, axillary position, 2–9 cm long leaflet branches and greenish-yellow in colour, male flowers are clustered, female usually solitary [17]. Its fruits are single-seeded, fruits during the winter and flowers grow during the summer [18]. The root is thread-like, aerial, squairshin, sometimes continuously lengthening touch the ground [19], aerial roots are characterized by tetra to penta arch primary structure [20]. The seeds are curved shape [21], and endocarp is variously ornamented, which provides critical taxonomic characters. morphology of the plants has been depicted in (Fig. 1).
Fig. 1.
Morphology of Tinospora cordifolia A) steam B) root C) leaves D) flower E) fruit F) seed.
2.2. Chemical constituent
The chemical constituents of T. cordifolia belong to different classes such as alkaloids, glycosides, steroids, phenolics, aliphatic compounds, polysaccharides, leaves are rich in protein (11.2%), calcium and phosphorus [22]. The stem contains clerodane furono diterpene glucoside (amritoside A, B, C, and D) and the structure has been established by different spectroscopic studies [23, 24, 25]. Some of the essential constituents reported in Table 1 and major constituents in Fig. 2 whereas the structure of the active chemical constituent for Tinospora cordifolia has been depicted in (see Fig. 3).
Table 1.
Some of the essential constituents of T. cordifolia.
| Active Component | Compounds | References |
|---|---|---|
| Terpenoids | Tinosporide, Furanolactone diterpene, Furanolactone clerodane diterpene, furanoid diterpene, Tinosporaside, ecdysterone makisterone and several glucosides isolated as poly acetate, phenylpropene disaccharides cordifolioside A, B and C, cordifoliside D and E, Tinocordioside, cordioside, palmatosides C and F, Sesquiterpene glucoside tinocordifolioside, Sesquiterpene tinocordifolin. | [26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36] |
| Alkaloids | Tinosporine, (S), Magnoflorine, (S), Berberine, (S), Choline, (S), Jatrorrhizine, (S), 1,2-Substituted pyrrolidine(S), Alkaloids, viz. jatrorrhizine, palmatine, beberine, tembeterine, choline. | [37, 38, 39, 40, 41] |
| Lignans | 3 (a, 4-dihydroxy-3-methoxybenzyl)-4-(4-hydroxy-3-methoxybenzyl), (S) | [42] |
| Steroids | Giloinsterol, (S), ß-Sitosterol, (S), 20a- Hydroxy ecdysone, (S). | [43, 44, 45, 46] |
| Others | Giloin, Tinosporan acetate, Tinosporal acetate, Tinosporidine, Heptacosanol, Octacosanol, sinapic acid, Tinosponone, two phytoecdysones, an immunologically active arabinogalactan. | [47, 48, 49, 50, 51] |
Fig. 2.
Major constituent of Tinospora cordifolia: terpenoid, alkaloid, lignans, steroids.
Fig. 3.
Structure of the chemical constituent of T. cordifolia.
2.3. Pharmacological activities
Different pharmacological activities of T. cordifolia has been reported by the researcher, which has been described:
2.3.1. Antioxidant activity
Mehra et al., prepared the formulation and evaluated its antioxidant activity by DPPH (1-diphenyl-2-picrylhydrazyl) free radical scavenging method. They estimated the total flavonol and total phenolic content. Using the result of the formulation showed potent antioxidant activity and inhibitory concentration (IC50) at 5 μg/ml as compared to standard drug ascorbic acid [52]. George et al., reported the methanolic, ethanolic, and water extracts of T. cordifolia for their antioxidant activity, in which the stemic ethanol extract increased the erythrocytes membrane lipid peroxide, catalase activity and decrease the superoxide dismutase, glutathione peroxidase in alloxan-induced diabetic rats. The leaves extract of methanol, partitioned in water with ethyl acetate and butanol at 250 mg/ml, and showed their antioxidant activity, extracts of methanol phosphomolybdenum and metal chelating activity were high followed by ethyl acetate, butanol, and water extract [53]. It also decrease level of free radical species of diabetic rat and up-regulate the anti-oxidant enzyme [54, 55, 56], scavenging activity for free radical of methanol extract was high compared with phenol extract [57]. This plant modifies the different enzymatic system which controls the production of these reactive species and maintains the oxidative load by regulating the lipid peroxidation process and glutathione level [58, 59]. Premnath et al., dried the leave of T. cordifolia and powdered and extracted with chloroform, methanol, ethanol hexane, and water. Antioxidant assay by different in-vitro models, lipid peroxidation inhibitory activity, DPPH radical scavenged, and superoxide radical scavenging activity. Other solvent extracts showed weak antioxidant activity, whereas ethanol extract had high antioxidant activity. The results suggested that the antioxidant compound are better in ethanol extract, and there is a direct correlation between the total polyphenols extracted and its anti-oxidant activity [60].
2.3.2. Antimicrobial activity
Antimicrobial activity of the T. cordifolia with different solvents on different micro-organism, showed good antifungal and antibacterial activity [61]. Jeyachandran et al., reported the antimicrobial activity of stem extracts by in-vitro analysis against both gram-positive and gram-negative bacteria and showed good therapeutic activity on the infectious disease. It has taken a methanolic extract of T. cordifolia against both bacteria group [62]. Narayanan et al., have reported antibacterial activity of plants to extract against Escherichia coli, Proteus vulgaris, Salmonella typhi, Salmonella paratyphi, Salmonella typhimurium, Klebsiella pneumoniae Pseudomonas aeruginosa, Enterobacter aerogene, Shigella flexneri, Staphylococcus aureus and Serratia marcesenses (Gram-positive bacteria) [63]. The aqueous, ethanol and acetone extract of T. cordifolia inhibited the activity on clinical isolates of urinary pathogens Klebsiella pneumoniae and Pseudomonas aeruginosa. [64] Singh et al., has reported silver nanoparticles from the stem of T. cordifolia, which possess antibacterial activity against the different strains of bacterias [65]. Allemailem et al., have reported the antifungal activity of T. cordifolia, which was determined using the agar well plate diffusion method. The aqueous extract of T. cordifolia showed potent activity against A. fumigatus, Aspergillus flavus, and Aspergilles nigar (fungus) in the study [66]. Agarwal et al., studied in-vitro extract of T. cordifolia was obtained using 100% ethanol by maceration process. They prepared etholic extract seven different concentrations and tested against S. mutans in brain–heart infusion agar medium. Plates were incubated aerobically at 37 °C for 48 h, using Vernier caliper and measured the zone of inhibition. 0.2% chlorhexidine and dimethylformamide were used as positive and negative controls, respectively. This experiment data were analysed by descriptive-analytic tests. Which showed the maximum antibacterial activity of T. cordifolia a volume of 40 μl at 2% concentration with a zone of inhibition of 19 mm. A 30 μl volume of 0.2% chlorhexidine showed a zone of inhibition of 28 mm, and dimethylformamide showed no zone of inhibition [67]. Khan et al., reported the antifungal activity TCAE (Tinospora cordifolia aqueous extract) was tested for in-vitro against the isolates of different Aspergillus species. To evaluate in vivo activity, different doses (10, 25, and 50 mg/kg) of TCAE were orally administered in A. fumigatus-infected mice for seven days for the evaluation of in vivo activity. The effectiveness of aqueous extract on the basic survival rate and assessing the fungal burden in the kidney of the treated mice [68]. Prasad et al., studied the anti-oxidant and antimicrobial properties phenolic extract of T. cordifolia stem and root. Total reducing power, hydrogen peroxide scavenging activity assay, and hydroxyl radical scavenging activity were checked using different in-vitro assays. The ethanolic extract showed maximum 87.2% and 91.0% free radical scavenging activity concerning H2O2 scavenging and hydroxyl free radical scavenging assay [69].
2.3.3. Anti-toxic effects
Gupta et al., reported the extract to scavenge free radicals generated during aflatoxicosis. It showed protective effects of T. cordifolia on thiobarbituric acid reactive substances (TBARS) levels and increase the level of GSH, ascorbic acid, protein, and the activities of anti-oxidant enzymes viz., Superoxide Dismutase (SOD), Catalase (CAT), GPx enzyme, Glutathione S-transferase (GST) and glutathione reductase (GR) in kidney. The alkaloids such as choline, tinosporin, isocolumbin, palmatine, tetrahydropalmatine, and magnoflorine present in the plant of T. cordifolia showed protection against aflatoxin-induced nephrotoxicity.http://www.ancientscienceoflife.org/article.asp?issn=0257-7941;year=2012;volume=31;issue=4;spage=151;epage=159;aulast=Saha [70] Sharma et al., studied the stem and leaves extract of the plant has shown hepatoprotective effect in Swiss albino male mice against lead nitrate induced toxicity [71]. Oral administration of plant extracts prevented the occurrence of lead nitrate induced liver damage [72]. They also checked the decreased level of SOD, CAT and increased level of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphate (ALP), and acid phosphates (ACP). http://www.ancientscienceoflife.org/article.asp?issn=0257-7941;year=2012;volume=31;issue=4;spage=151;epage=159;aulast=Saha [72] Synergistic administration of aqueous extract of stem and leaf increased the activities of SOD and CAT and decreased the levels of AST, ALT, ALP, and ACP enzymes. http://www.ancientscienceoflife.org/article.asp?issn=0257-7941;year=2012;volume=31;issue=4;spage=151;epage=159;aulast=Saha [72] Protective role of aqueous extract of the plant stem and leaves overcoming the toxic effects was also reported by them. http://www.ancientscienceoflife.org/article.asp?issn=0257-7941;year=2012;volume=31;issue=4;spage=151;epage=159;aulast=Saha [71].
2.3.4. Antidiabetic activity
The anti-diabetic activities is due to alkaloids (Magnoflorine, Palmetine, Jatrorrhizine), tannins, cardiac glycosides, flavonoids, saponins, etc. [73] The crude extract of the stem in ethyl acetate, dichloromethane (CDM), chloroform and hexane was studied for inhibition of the alpha-glucosidase enzyme. The activity of the enzyme inhibited hypoglysomic action in diabetic animal and normal animals. The aqueous extract was studied in the rats, without the addition of Tinospora cordifolia extract increase in glucose by 21.3%, insulin by 51.5%, triglycerides by 54.12%, and glucose-insulin index by 59.8 when plant containing extract was given. The fructose-induced abnormalities in the liver involving lipid peroxidation, protein carbonyl groups, GSH levels, and enzymatic antioxidants decreased [74]. Methew et al., have reported invivo studies of different extracts of the plants on a diabetic patient. Sedimental extract of Tinospora on the subject was studied at 30 d ay. Different doses (200and 400 mg/kg b. w) of Ethanolic extract of T. cordifolia leaves were prepared. The doses were administered orally for ten days and 30 days in streptozotocin-diabetic albino rats. T. cordifolia showed the antidiabetic activity in diabetic animals an efficacy an 50%–70% compared to insulin [75].
From Guduchi Prasant et al., isolated alkaloids, cardiac glycosides, saponins, flavonoids, tannins, and steroids that contains anti-diabetic property. Alkaloids from this plants showed insulin-mediated actions due to insulin hormone [76]. Gestational diabetes can increase the GSH content and other reactive species that can act as a threat to the mother as well as the fetus. The study based upon the pregnant rat using T. cordifolia was incorporated in the daily diet to a diabetic-pregnant rat (streptozocin-induced diabetes), which showed a protective effect by reducing the oxidative load thereby preventing the relative incidence of diseases and any birth defect [77]. In a diabetic rat model, T. cordifolia root extracts of Guduchi attenuated the brain mediated lipid level and down-regulated the blood glucose and urinary glucose level emphasizing its anti-diabetic and lipid-lowering activity [78].
The root extract of Guduchi showed an antihyperglycemic effect in the alloxan-induced diabetic model by decreasing its excess glucose level in urine as well as in normal [79]. Certain herbal preparation, including Guduchi like Ilogen-Excel, Hyponidd, and Dihar have been tested in diabetic rat models, the anti-diabetic activity of T. cordifolia was observed. The effects by Ilogen Excel down the level of excess glucose in the blood and enhance the insulin efficiency by increasing its amount in the systemic circulation. Hyponidd is reported, and it maintained the oxidative load by decreasing reactive species and reduced the glucose-mediated hemoglobin count. when the tested of ‘Dihar’ for one and a half month in streptozotocin-induced diabetic model decreased the urea as well as creatinine amount in the blood with an increase in enzyme activities [54, 80, 81, 82].
2.3.5. Antistress activity
Sarma et al., reported ethanolic extract of T. cordifolia at the dose of 100 mg/kg gives significant anti-stress activity in all parameters compared with standard drug diazepam (dose of 2.5 mg/kg) [83]. The plant extract gives a moderate degree of behavior disorders and mental deficit response. The clinical research showed the improved I. Q level of patients. In Ayurveda, it acts as Medhya Rasayana or brain tonic by increasing mind power like memory and recollection [84].
2.3.6. Hypolipidemic effect
Stanely et al., studied the hypolipidemic effect of an aqueous extract of the root on the rats weighing 2.5 and 5.0 g/kg body weight on sixth weeks, that resulted in decrease tissue cholesterol, reduction in serum, phospholipids, and free fatty acid in alloxan diabetic rats. The dose of root extract 5.0 g/kg body weight showed the highest hypolipidaemic effect. When the level of serum lipids in diabetes increased, they represented coronary heart disease, lower the serum lipids level decreased the risk of vascular disease. The ability of T. cordifolia root extract to reduce the level of serum or tissue lipids in diabetics animals have never been studied before till then [85].
2.3.7. Hepatic disorder
Protective Effects of Tinospora cordifolia water extract (TCE) on Hepatic and Gastrointestinal Toxicity was reported by Sharma et al., a significant increase in the levels of gamma-glutamyl transferase, aspartate transaminase, alanine transaminase, Triglyceride, Cholesterol, HDL and LDL (P < 0.05) in alcoholic sample whereas their level get downregulated after TCE intervention, patients showed the normalized liver function of T. cordifolia stand to relieve the symptoms [86].
2.3.8. Anticancer activity
Ali et al., studied the anticancer activity of T. cordifolia palmatine extract in animal models, alkaloid using response surface methodology (RSM). The extract indicates the anticancer potential in 7,12-dimethylbenz(a)anthracene DMBA induced skin cancer model in mice [87]. Rahul et al., prepared the extract of 200, 400, 600 mg/kg dry weight in a dose depend upon manners. 50% methanolic extract of cordifolia to C57 BI mice for 30 days at a dose of 750 mg/kg body weight the tumor size reduced life span [88]. Mishra et al., showed the anti-brain cancer potential, 50% ethanolic extract of T. cordifolia (TCE) using C6 glioma cells significantly induced differentiation in C6 glioma cells, and reduced cell proliferation [89].
2.3.9. Anti-HIV potential
Kalikae et al., showed that the root extract of T. cordifolia affects the immune system of HIV positive patient. The stem extract of Tinospora cordifolia reduces the ability of eosinophil count, stimulation of B lymphocytes, macrophages, level of hemoglobin, and polymorphonuclear leucocytes [90, 91].
2.3.10. Wound healing
Shanbhag T et al., The present study was aimed at evaluating the wound healing profile of alcoholic extract of T. cordifolia and its effect on dexamethasone suppressed healing. Incision, excision, and dead space of the wound models were employed to investigate the wound healing potential of the plant increased tensile strength extract of T. cordifolia may be attributed to the promotion of collagen synthesis. The extract of T. cordifolia did not reverse dexamethasone suppressed wound healing [92].
2.3.11. Anti-osteoporotic effects
Abiramasundari et al., reported T. cordifolia affect the proliferation, differentiation, and mineralization of bone-like matrix on osteoblast model systems in-vitro and hence finds potential application as an anti-osteoporotic agent. Alcoholic extract of T. cordifolia has been shown to stimulate the growth of osteoblasts, increasing the differentiation of cells into the osteoblastic lineage and also increasing the mineralization of bone-like matrix [93]. Ecdysteroids isolated from the plant have been reported of protein anabolic and anti-osteoporotic effects in mammals. Beta-Ecdysone (Ecd) from T. cordifolia extracts have been reported to induce a significant increase in the thickness of joint cartilage, induce the osteogenic differentiation in mouse mesenchymal stem cells [94] and to relieve osteoporosis in osteoporotic animal models [62, 94]. Further 20-OH-β-Ecd isolated from T. cordifolia has been reported for its anti-osteoporotic effects [93], thus highlighting the role of Tinospora cordifolia in the treatment of osteoporosis and osteoarthritis [95].
2.3.12. Anticomplement activity and immunomodulating activity
Kapil et al., Studied the syringin (TC-4) and cordiol (TC-7) isolate from T. cordifolia inhibited the in-vitro immune hemolysis of antibody-coated sheep erythrocytes by guinea pig serum. Immune hemolysis was reduced due to inhibition of the C3-convertase of the classical complement pathway. The compounds of T. cordifolia rise to significant increases in IgG antibodies in guinea pig serum. Cordioside (TC-2), cordiofolioside A (TC-5) and cordiol (TC-7) activated macrophase with increasing incubation times [96]. Sharma et al., isolated and characterised different classes of active compounds reported their mmunomodulatory activity [7].
2.3.13. Parkinson's disease
Birla et al., reported T. cordifolia extract is highly attractive against the Parkinosonism. They observed the anti-inflammatory activity of aqueous extract in 1-methyl-4-phenyl-1,2,3,6-tetra hydropyridine (MPTP)-intoxicated Parkinsonian mouse model. The extract reversed the behavior of the target MPTP-intoxicated mice and its suggest that T. cordifolia protected dopaminergic neurons by suppressing neuroinflammation in MPTP-induced Parkinsonian mouse model [97].
The plant exhibited multiple biological activities due to diverse chemical constituents present in it. The biologically active chemical molecules are present in different parts of the T. Cordifolia. That is the explanation for curing various ailments in human being using a different part of the miraculous plant from the ancient ages (Table 2).
Table 2.
Biological activities of T. Cardifolia concerning different parts of the plant.
| Active compound | Biological activity |
|---|---|
| Terpenoids | Stem: Respiratory track infection [98], skin disease [5],Anti-hyperglycemic property [99]. |
| Alkaloids | Stem and root of plant: Anti-cancer property [100], Antioxidant activity [101]. |
| Lignans | The root of plant: Anti-neoplastic property [102], Antioxidant activity [101]. |
| Steroids | Arial part of stem: Anti-stress activity [83]. |
| Other | The whole part of Plant: Antidote to snakebite and scorpion sting, Analgesic and Neuropharmacological activities, Diabetes, Rheumatoid arthritis, Gout, Cancer, high cholesterol content, antipyretic, antileprotic, radioprotective [8,103, 104, 105, 106, 107]. |
N.B. Biological activities of different parts of T. Cardifolia. The number denoted on the disease are the references cited.
3. Conclusions
T. cordifolia is a medicinal plant having various type of compounds. The different bioactive compounds, including alkaloids, steroids, glycosides, sesquiterpenoids, etc have been discussed. Present review spotlights the artistic antifungal activity, antioxidant activity, antimicrobial activity, antibacterial activity, hypolipidaemic effect, hepatic disorder, anticancer, Anti HIV potential, Antiosteoporotic effects, Antitoxic effects, Wound healing, anticomplementary activity, immunomodulating activity, systemic Infection and Parkinson's disease of T. Cordifolia. It has been used successfully in Ayurvedic medicine from the ancient era, and its products are used for their better economic and therapeutic utilization. In this regard, further studies need to be carried out to explore T. cordifolia for its potential in preventing and treating diseases. This review can be used for further research investigations as well as clinical purpose in the development of novel drugs.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and All authors listed have significantly contributed to the development and the writing of this article. the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
"Authors would like to acknowledge Dr. Neeraj Pizar, Scientific Writing Cell, Shoolini University for the support provided towards language editing of the manuscript".
Contributor Information
Ashutosh K. Dash, Email: ashutosh.dash10@gmail.com.
Deepak Kumar, Email: guptadeepak002@gmail.com.
References
- 1.Olabiyi A.S., Nkemehule F.E., Odukoya O.A., Samuel T.A., Ogbonnia S.O. Inhibition of glycosylation as an index of activity in plants with antidiabetic potentials. Biochem. Pharmacol. 2013;2:181. [Google Scholar]
- 2.Singletary K. Turmeric: an overview of potential health benefits. Nutr. Today. 2010;45:216–225. [Google Scholar]
- 3.Wilken R., Veena M.S., Wang M.B., Srivatsan E.S. Curcumin: a review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer. 2011;10:1–19. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pooja A., Nagesh L. Murlikrishnan, Evaluation of the antimicrobial activity of various concentrations of tulsi (Ocimum sanctum) extracts against Streptococcus mutans: an in-vitro study. Indian J. Dent. Res. 2010;21:357–359. doi: 10.4103/0970-9290.70800. [DOI] [PubMed] [Google Scholar]
- 5.Saha S., Ghosh S. Tinospora cordifolia: one plant, many roles. Ancient Sci. Life. 2012;31:151–159. doi: 10.4103/0257-7941.107344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meena A.K., Singh A., Panda P., Mishra S., Rao M.M. Tinospora cordifolia: its bioactivities & evaluation of physicochemical properties. IJPPR. 2010;2:50–55. [Google Scholar]
- 7.Sharma U., Bala M., Kumar N., Singh B., Munshi R.K., Bhalerao S. Immunomodulatory active compounds from Tinospora cordifolia. J. Ethnopharmacol. 2012;141:918–926. doi: 10.1016/j.jep.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 8.Goel H.C., Prasad J., Singh S., Sagar R.K., Agrawala P.K., Bala M., Sinha A.K., Dogra R. Radioprotective potential of an herbal extract of Tinospora cordifolia. J. Radiat. Res. 2004;45:61–68. doi: 10.1269/jrr.45.61. [DOI] [PubMed] [Google Scholar]
- 9.Sonkamble V.V., Kamble L.H. Antidiabetic potential and identification of phytochemicals from Tinospora cordifolia. Am. J. Phytomed. Clin. Ther. 2015;3:97–110. [Google Scholar]
- 10.Sinha K., Mishra N.P., Singh J., Khanuja S.P.S. Tinospora cordifolia (Guduchi) a reservoir plant for therapeutic applications. Indian J. Tradit. Knowle. 2004;3:257–270. [Google Scholar]
- 11.Salkar K., Chotalia C., Salvi R. Tinospora cordifolia: an antimicrobial and immunity enhancer plant. Int. J. Sci. Res. 2017;6:1603–1607. [Google Scholar]
- 12.Upreti P., Chauhan R.S. Effect of leaf powder of giloy (Tinospora cordifolia) in fish feed on survival and growth of post larvae of Catla catla. J. Appl. Nat. Sci. 2018;10:144–148. [Google Scholar]
- 13.Khan M.M., dul Haque M.S., Chowdhury M.S. Medicinal use of the unique plant Tinospora cordifolia: evidence from the traditional medicine and recent research. Asian J. Med. Biol. Res. 2016;2:508–512. [Google Scholar]
- 14.Upadhyay A.K., Kumar K., Kumar A., Mishra H.S. Tinospora cordifolia (Wild.) Hook. f. and Thoms. (Guduchi)–Validation of the ayurvedic pharmacology through experimental and clinical studies. Int. J. Ayurveda Res. 2010;1:112–121. doi: 10.4103/0974-7788.64405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tiwari P., Nayak P., Prusty S.K., Sahu P.K. Phytochemistry and pharmacology of Tinospora cordifolia. Syst. Rev. Pharm. 2018;9:70–78. [Google Scholar]
- 16.Gupta A.K. 1sted. Vol. 1. 2003. pp. 212–218. (Anonymous:Quality Standards of Indian Medicinal Plants, NewDelhi). [Google Scholar]
- 17.Arul V., Miyazaki S., Dhananjayan R. Studies on the anti-inflammatory, antipyretic and analgesic properties of the leaves of Aegle marmelos Corr. J. Ethnopharmacol. 2005;96:159–163. doi: 10.1016/j.jep.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Spandana U., Liakhat Ali S.L., Nirmala T., Santhi M., Babu S.D. A review on Tinospora cordifolia. IJCPR. 2013;4:61–68. [Google Scholar]
- 19.Sinha A., Sharma H.P. A medicinal plant: micropropagation and phytochemical screening of Tinospora cordifolia (Wild.) Miers ex Hook F & Thoms. IJAPBC. 2015;4:114–121. [Google Scholar]
- 20.Singh S.S., Pandey S.C., Srivastava S., Gupta V.S., Patro B., Ghosh A.C. Chemistry moreover, medicinal properties of Tinospora cordifolia (Guduchi) Indian J. Pharmacol. 2003;35:83–91. [Google Scholar]
- 21.Misra B., Prakash B. Vol. 1. 1969. p. 26. (Study of Medicinal Plant and Drug, Bhava Prakash Nighantu). [Google Scholar]
- 22.Chaudhary N., Siddiqui M.B., Khatoon S. Pharmacognostical evaluation of Tinospora cordifolia (Willd) Meirs and identification of biomarkers. J. Res. Indian Med. 2014;13:543–550. [Google Scholar]
- 23.Maurya R., Manhas L.R., Gupta P., Mishra P.K., Singh G., Yadav P.P. Amritosides A, B, C and D: clerodane furano diterpene glucosides from Tinospora cordifolia. Phytochemistry. 2004;5:2051–2055. doi: 10.1016/j.phytochem.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Abhijeet R., Mokat D. On vegetative propagation through stem cuttings in medicinally lucrative Tinospora species. J. Pharmacogn. Phytochem. 2018;2:2313–2318. [Google Scholar]
- 25.Sumran G., Aggarwal A. Prospect of Indian herbs as sources of antioxidants incombating oxidative stress. Chem. Biol. Interface. 2019;9:1–20. [Google Scholar]
- 26.Khuda M.Q.I., Khaleque A., Ray N. Tinospora cordifolia constituents of plants fresh from the field. Sci. Res. 1964;1:177–183. [Google Scholar]
- 27.Hanuman J.B., Bhatt R.K., Sabata B.K. A diterpenoid furanolactone from Tinospora cordifolia. Phytochemistry. 1986;25:1677–1680. [Google Scholar]
- 28.Bhatt R.K., Hanuman J.B., Sabata B.K. A new clerodane derivative from Tinospora cordifolia. Phytochemistry. 1988;27:1212–1216. [Google Scholar]
- 29.Hanuman J.B., Bhatt R.K., Sabata B. A clerodane furano-diterpene from Tinospora cordifolia. J. Nat. Prod. 1988;51:197–201. [Google Scholar]
- 30.Bhatt R.K., Sabata B.K. A furanoid diterpene glucoside from Tinospora cordifolia. Phytochemistry. 1989;28:2419–2422. [Google Scholar]
- 31.Khan M.A., Gray I.A., Waterman P.G. Tinosporaside an 18-norclerodane glucoside from Tinospora cordifolia. Phytochemistry. 1989;28:273–275. [Google Scholar]
- 32.Gangan V.D., Arjun P.P., Sipahimalani T., Banerji A., Cardifolisides A., B C. Norditerpene furon glucoside from Tinospora cordifolia. Phytochemistry. 1994;37:781–786. doi: 10.1016/s0031-9422(00)90358-3. [DOI] [PubMed] [Google Scholar]
- 33.Maurya R., Handa S.S. Tinocordifolin, a sesquiterpene from Tinospora cordifolia. Phytochemistry. 1998;44:1343–1345. [Google Scholar]
- 34.Gangan V.D., Arjun P.P., Sipahimalani A.T., Banerji A. Norditerpene furon glucoside from Tinospora cordifolia. Phytochemistry. 1995;39:1139–1142. doi: 10.1016/s0031-9422(00)90358-3. [DOI] [PubMed] [Google Scholar]
- 35.Wazir V., Maurya R., Kapil R.S. A clerodane furano diterpene glucoside from Tinospora cordifolia. Phytochemistry. 1995;38:447–449. doi: 10.1016/j.phytochem.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 36.Gagan V.D., Pradhan P., Sipahimalan A.T., Banerji A., Palmatosides C F. Diterpene furan glucosides from Tinospora cordifolia-structural elucidation by 2D NMR spectroscopy. Indian J. Chem. 1996;35B:630–634. [Google Scholar]
- 37.Choudhary N., Siddiqui M.B., Azmat S., Khatoon S. Tinospora cordifolia: ethnobotany, phytopharmacology and phytochemistry aspects. IJPSR. 2013;4:891. [Google Scholar]
- 38.Bisset N.G., Nwaiwu J. Quaternary alkaloids of Tinospora species. Planta Med. 1983;48:275–279. doi: 10.1055/s-2007-969933. [DOI] [PubMed] [Google Scholar]
- 39.Mahajan V.R., Jolly C.I., Kundnani K.M. A new hypoglycaemic agent from Tinospora cordifolia. Indian Drugs. 1985;23:119–120. [Google Scholar]
- 40.Sarma D.N.K., Khosa R.L., Chansauria J.P.N., Ray A.K. The effect of Tinospora cordifolia on brain neurotransmitters in the stressed rat. Fitoterapia. 1995;66:421–422. [Google Scholar]
- 41.Pathak A.K., Agarwal A.K., Jain D.C., Sharma R.P., Howarth O.W. NMR studies of 20 hydroxyecdysones, a steroid isolated from Tinospora cordifolia. Indian J. Chem. 1995;34:674–676. [Google Scholar]
- 42.Hanuman J.B., Mishra A.K., Sabata B. A natural phenolic lignan from Tinospora cordifolia Miers. J. Chem. Soc. 1986:1181–1185. [Google Scholar]
- 43.Kidwai A.R., Salooja K.C., Sharma V.N., Siddiqui S. Chemical examination of Tinospora cordifolia. J. Sci. Indian Res. 1949;8:115–118. [Google Scholar]
- 44.Khaleque A., Maith M.A.W., Huq M.S., K Abul B. Tinospora cordifolia IV. Isolation of heptacosanol, ß sitosterol and three other compounds tinosporine, cordifol and cordifolone. Pakistan J. Sci.Industry Res. 1970;14:481–483. [Google Scholar]
- 45.Dixit S.N., Khosa R.L. Chemical investigations on Tinospora cordifolia (wild.) miers. Indian. J. Appl. Chem. 1971;34:46–47. [Google Scholar]
- 46.Pathak A.K., Jain D.C., Sharma P.R. Chemistry and biological activities of the genus Tinospora. Int. J. Pharmacogn. 1995;33:277–287. [Google Scholar]
- 47.Khuda M.Q., Khaleque A., Basar K.A., Rouf M.A., Khan M.A., Roy N. Studies on Tinospora cordifolia II: isolation of tinosporine, tinosporic acid and tinosporol from the fresh creeper. Sci. Res. 1966;3:9–12. [Google Scholar]
- 48.Khaleque A., Maith M.A.W., Huq M.S., Tinospora cordifolia K.A., III Isolation of tinosporine, heptacosanol, ß sitosterol. Pakistan J . Sci. Industry Res. 1971;14:481–483. [Google Scholar]
- 49.Maurya R., Wazir V., Tyagi A., Kapil R.S. Clerodane diterpene from Tinospora cordifolia. Phytochemistry. 1995;38:659–661. [Google Scholar]
- 50.Pradhan P., Gangan V.D., Sipahimalani A.T., Banerji A. Two phytoecdysones from Tinospora cordifolia: structural assignment by 2D NMR spectroscopy. Indian J. Chem. 1997;36B:958–962. [Google Scholar]
- 51.Chintalwar G., Jain A., Sipahimalani A., Banerji A., Sumariwalla P., Ramakrishnan R., Sainis K. An Immunologically active arabinogalactan from Tinospora cordifolia. Phytochemistry. 1999;52:1089–1093. doi: 10.1016/s0031-9422(99)00386-6. [DOI] [PubMed] [Google Scholar]
- 52.Mehra R., Naved T., Arora M., Madan S. Standardization and evaluation of formulation parameters of Tinospora cordifolia tablet. J. Adv. Pharm. Educ. Res. 2013;3:440–449. [Google Scholar]
- 53.George M., Josepha L., Mathew M. A research on screening of learning and memory enhancing the activity of whole plant extract of Tinospora cordifolia (Willd) Pharma Innovation. 2016;5:104–107. [Google Scholar]
- 54.Prince S.M., Menon V.P. Hypoglycaemic and hypolipidaemic action of alcohol extract of Tinospora cordifolia roots in chemical induced diabetes in rats. Phytother Res. 2003;17:410–413. doi: 10.1002/ptr.1130. [DOI] [PubMed] [Google Scholar]
- 55.Sivakumar V., Rajan M.S.D. Antioxidant effect of Tinospora cordifolia extracts in alloxan-induced diabetic rats. Indian J. Pharm. Sci. 2010;72:795. doi: 10.4103/0250-474X.84600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prince S.M., Menon V.P. Antioxidant action of Tinospora cordifolia root extract in alloxan diabetic rats. Phytother Res. 2001;15:213–218. doi: 10.1002/ptr.707. [DOI] [PubMed] [Google Scholar]
- 57.Upadhyay N., Ganie S.A., Agnihotri R.K., Sharma R. Free radical scavenging activity of Tinospora cordifolia (Wild.) Mier. J. Pharmacogn. Phytochem. 2014;3:63–69. [Google Scholar]
- 58.Jayaprakash R., Ramesh V., Sridhar M.P., Sasikala C. Antioxidant activity of ethanolic extract of Tinospora cordifolia on N-nitrosodiethylamine (diethyl nitrosamine) induced liver cancer in male wister albino rats. J. Pharm. BioAllied Sci. 2015;7(S1):40–45. doi: 10.4103/0975-7406.155791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bafna P.A., Balaraman R. Anti-ulcer and anti-oxidant activity of pepticare, a herbomineral formulation. Phytomedicine. 2005;12:264–270. doi: 10.1016/j.phymed.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 60.Premanath R., Lakshmidevi N. Studies on the anti-oxidant activity of Tinospora cordifolia (Miers.) leaves using in-vitro models. J. Am. Sci. 2010;6:736–743. [Google Scholar]
- 61.Duraipandiyan V., Ignacimuthu S., Balakrishna K., Aaharbi N.A. Antimicrobial activity of Tinospora cordifolia: an ethnomedicinal plant. Asean J. Trad. Knowldge. 2012;7:59–65. [Google Scholar]
- 62.Jeyachandran R., Xavier T.F., Anand S.P. Antibacterial activity of stem extracts of Tinospora cordifolia (willd) hook. Anc. Sci. Life. Res. 2003;25:40–43. [PMC free article] [PubMed] [Google Scholar]
- 63.Narayanan A.S., Raja S.S., Ponmurugan K., Kandekar S.C., Natarajaseenivasan K., Maripandi A., Mandeel Q.A. Antibacterial activity of selected medicinal plants against multiple antibiotic resistant uropathogens. Benef. Microbes. 2011;2:235–243. doi: 10.3920/BM2010.0033. [DOI] [PubMed] [Google Scholar]
- 64.Shanthi V., Nelson R. Antibacterial activity of Tinospora cordifolia (Willd) Hook. F. Thoms on urinary tract pathogens. Int. J. Curr. Microbiol. App. Sci. 2013;2:190–194. [Google Scholar]
- 65.Singh K., Panghal M., Kadyan S., Chaudhary U., Yadav J.P. Antibacterial activity of synthesized silver nanoparticles from Tinospora cordifolia against multi-drug resistant strains of pseudomonas aeruginosa isolated from burn patients. J. Nanomed. Nanotechnol. 2014;5:1–6. [Google Scholar]
- 66.Allemailem K.S., Almatroudi A., Alsahli M.A., Khan A., Khan M.A. Tinospora cordifolia aqueous extract alleviates cyclophosphamide-induced immune suppression, toxicity and systemic candidiasis in immunosuppressed mice: in vivo study in comparison to antifungal drug fluconazole. Curr. Pharmaceut. Biotechnol. 2019;20:1–5. doi: 10.2174/1389201019666190722151126. [DOI] [PubMed] [Google Scholar]
- 67.Agarwal S., Ramamurthy P.H., Fernandes B., Rath A., Sidhu P. Assessment of antimicrobial activity of different concentrations of Tinospora cordifolia against Streptococcus mutans: an in-vitro study. Dent. Res. J. 2019;16:24–28. [PMC free article] [PubMed] [Google Scholar]
- 68.Khan M.A. Tinospora cordifolia aqueous extract ameliorates the systemic infection of aspergillus fumigatus in balb/c mice. Asian. J. Pharm. Clin. Res. 2019;12:525–528. [Google Scholar]
- 69.Prasad B., Chauhan A. Anti-Oxidant and antimicrobial studies of Tinospora cordifolia (Guduchi/Giloy) stems and roots under in-vitro condition. Int. J. Adv. Microbiol. Health. Res. 2019;3:1–10. [Google Scholar]
- 70.Gupta R., Sharma V. A meliorative effects of Tinospora cordifolia root extract on histopathological and biochemical changes induced by aflatoxin-b in mice kidney. Toxicol. Int. 2011;18:94–98. doi: 10.4103/0971-6580.84259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharma V., Pandey D. Beneficial effects of Tinospora cordifolia on blood profiles in male mice exposed to lead. Toxicol. Int. 2010;17:8–11. doi: 10.4103/0971-6580.68341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharma V., Pandey D. Protective role of Tinospora cordifolia against lead-induced hepatotoxicity. Toxicol. Int. 2010;17:12–17. doi: 10.4103/0971-6580.68343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anonymous, the Ayurvedic Pharmacopoeia of India. Part I. first ed.. Vol. 1, Department of AYUSH, Ministry of Health and FW, New Delhi (2001) 53-55.
- 74.Sudha P., Zinjarde S., Bhargava S.Y., Kumar A.R. Potent α-amylase inhibitory activity of Indian ayurvedic medicinal plants. BMC Complement Altern. Med. 2011;11:1–10. doi: 10.1186/1472-6882-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chougale A.D., Ghadyale V.A., Panaskar S.N., Arvindekar A.U. Alpha-glucosidase inhibition by stem extract of Tinospora cordifolia. J. Enzym. Inhib. Med. Chem. 2009;24:998–1001. doi: 10.1080/14756360802565346. [DOI] [PubMed] [Google Scholar]
- 76.Patel M.B., Mishra S. Hypoglycemic activity of alkaloidal fraction of Tinospora cordifolia. Pharma Innovation. 2016;5:104. doi: 10.1016/j.phymed.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 77.Patel M.B., Mishra S.M. Magnoflorine from Tinospora cordifolia stem inhibits α-glucosidase and its antiglycemic in rats. J. Funct. Foods. 2012;4:79–86. [Google Scholar]
- 78.Shivananjappa M.M., Muralidhara M. Abrogation of maternal and fetal oxidative stress in the streptozotocin-induced diabetic rat by dietary supplements of Tinospora cordifolia. Phytomedicine. 2011;18:1045–1052. doi: 10.1016/j.nut.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 79.Singh D., Chaudhuri P.K. Chemistry and pharmacology of Tinospora cordifolia. Nat. Prod. Commun. 2017;12:299–308. [PubMed] [Google Scholar]
- 80.Umamaheswari S., P Mainzen S.P. Antihyperglycemic effect of ‘Ilogen-Excel,’ an ayurvedic herbal formulation in streptozotocin-induced diabetes mellitus. Acta Pol. Pharm. 2007;64:53–61. [PubMed] [Google Scholar]
- 81.Babu P.S., Stanely P.P.M. Antihyperglycaemic and antioxidant effect of hyponid, an ayurvedic herbo mineral formulation in streptozotocin induced diabetic rats. J. Pharm. Pharmacol. 2004;56:1435–1442. doi: 10.1211/0022357044607. [DOI] [PubMed] [Google Scholar]
- 82.Patel S.S., Shah R.S., Goyal R.K. Antihyperglycemic, antihyperlipidemic, and antioxidant effects of Dihar, a polyherbal ayurvedic formulation in streptozotocin-induced diabetic rats. Indian J. Exp. Biol. 2009;47:564–570. [PubMed] [Google Scholar]
- 83.Sarma D.N.K., Khosa R.L., Chaurasia J.P.N., Sahai M. Antistress activity of Tinospora cordifolia and Centella asiatica extracts. Phytother Res. 1996;10:181–184. [Google Scholar]
- 84.Baghel P. Plant of versatile vroperties of Tinospora cordifolia (Guduchi) IJAIR. 2017;5:751–753. [Google Scholar]
- 85.Stanely P.P.M., Menon V.P., Gunasekharam G. Hypolipidaemic action of Tinospora cordifolia roots in alloxan-induced diabetic rats. J. Ethnopharmacol. 1999;64:53–57. doi: 10.1016/s0378-8741(98)00106-8. [DOI] [PubMed] [Google Scholar]
- 86.Sharma B., Dabur R. Protective effects of Tinospora cordifolia on hepatic and gastrointestinal toxicity induced by chronic and moderate alcoholism. Alcohol Alcohol. 2016;51:1–10. doi: 10.1093/alcalc/agv130. [DOI] [PubMed] [Google Scholar]
- 87.Ali H., Dixit S. Extraction optimization of Tinospora cordifolia and assessment of the anticancer activity of its alkaloid palmatine. Sci. World J. 2013;28:1–10. doi: 10.1155/2013/376216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Verma R., Chaudhary H.S., Agrawal R.C. Evaluation of antcarcinogenic and antmutagenic effect of Tinospora cordifolia in experimental animals. J. Chem. Pharm. Res. 2011;3:877–881. [Google Scholar]
- 89.Mishra R., Kaur G. Aqueous ethanolic extract of Tinospora cordifolia as a potential candidate for differentiation based therapy of glioblastomas. PLoS One. 2013;8 doi: 10.1371/journal.pone.0078764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kalikaer M.V., Thawani V.R., Varadpande U.K., Santakke S.D., Singh R.P., Khiyani R.K. Immunomodulatory effect of Tinospora cordifolia extracts in HIV positive patients. Indian J. Pharmacol. 2008;40:107–110. doi: 10.4103/0253-7613.42302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Akhtar S. Use of Tinospora cordifolia in HIV infection. Indian J. Pharmacol. 2010;42:57–63. doi: 10.4103/0253-7613.62402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shanbhag T., Shenoy S., Rao M.C. Wound healing profile of Tinospora cordifolia. Indian Drugs. 2005;42:217–221. [Google Scholar]
- 93.Abiramasundari G., Sumalatha K.R., Sreepriya M. Effects of Tinospora cordifolia (Menispermaceae) on the proliferation, osteogenic differentiation and mineralization of osteoblast model systems in-vitro. J. Ethnopharmacol. 2012;141:474–480. doi: 10.1016/j.jep.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 94.Gao L., Cai G. Beta-ecdysterone induces osteogenic differentiation in mouse mesenchymal stem cells and relieves osteoporosis. Biol. Pharm. Bull. 2008;31 doi: 10.1248/bpb.31.2245. 2245-9. [DOI] [PubMed] [Google Scholar]
- 95.Kapur P., Wuttke W., Jarry H., Seidlova D.W. Beneficial effects of beta-ecdysone on the joint, epiphyseal cartilage tissue and trabecular bone in ovariectomized rats. Phytomedicine. 2010;17 doi: 10.1016/j.phymed.2010.01.005. 350-5. [DOI] [PubMed] [Google Scholar]
- 96.Kapil A., Sharma S. Immunopotentiating compounds from Tinospora cordifolia. J. Ethnopharmacol. 1997;58:89–95. doi: 10.1016/s0378-8741(97)00086-x. [DOI] [PubMed] [Google Scholar]
- 97.Birla H., Rai S.N., Singh S.S., Zahra W., Rawat A., Tiwari N., Singh R.K., Pathak A., Singh S.P. Tinospora cordifolia suppresses neuroinflammation in Parkinsonian mouse model. NeuroMolecular Med. 2019;21:42–53. doi: 10.1007/s12017-018-08521-7. [DOI] [PubMed] [Google Scholar]
- 98.Antul K., Amandeep P., Gurwinder S., Anuj C. Review on pharmacological profile of medicinal vine: Tinospora cordifolia. CJAST. 2019;35:1–11. [Google Scholar]
- 99.Khanal P., Mandar B., Patil B., Hullatti K. In silico antidiabetic screening of borapetoside C, cordifolioside A and magnoflorine. Indian J. Pharm. Sci. 2019;81:550–555. [Google Scholar]
- 100.Shamsuzzaman M., Hasan M.R. A review of anticancer potential medicinal plants. J. sci. Facts. 2019;5:1–4. [Google Scholar]
- 101.Kumar V., Singh S., Singh A., Dixit A.K., Srivastava B., Sidhu G.K., Singh R., Meena A.K., Singh R.P., Subhose V., Prakash O. Phytochemical, antioxidant, antimicrobial, and protein binding qualities of hydro-ethanolic extract of Tinospora cordifolia. JBAPN. 2018;8:192–200. [Google Scholar]
- 102.Uren A.G., Rourke K.O., Aravind L.A., Pisabarro M.T., Seshagiri S., Koonin E.V., Dixit V.M. Identification of paracaspases and metacaspases: two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol. Cell. 2000;6:961–967. doi: 10.1016/s1097-2765(00)00094-0. [DOI] [PubMed] [Google Scholar]
- 103.Spandana U., Ali S.L., Nirmala T., Santhi M., Babu S. A review on Tinospora cordifolia. Int. J. Curr. Pharm. Res. 2013;4:61–68. [Google Scholar]
- 104.Soman D., Kundagol M.C., Chacko J. Ayurvedic management of gouty arthritis: a case report. J. Ayur. Herb. Med. 2018;4:154–157. [Google Scholar]
- 105.Vedavathy S., Rao K.N. Antipyretic activity of six indigenous medicinal plants. J. Ethnopharmacol. 1991;33:193–196. doi: 10.1016/0378-8741(91)90178-g. [DOI] [PubMed] [Google Scholar]
- 106.Asthana J.G., Jain S., Mishra A., Vijaykant M.S. Evaluation of antileprotic herbal drug combinations and their combination with Dapsone. Indian Drugs. 2001;38:82–86. [Google Scholar]
- 107.Nagaraja P.K., Kammar K.F., Devi S. Modulation of morphology and some gluconeogenic enzymes activity by Tinospora cordifolia (Willd.) in diabetic rat kidney. Biomed. Res. 2007;18:179–183. [Google Scholar]