Abstract
Pulmonary veins (PVs) are the major origin of atrial fibrillation. Recently, we recorded hyperpolarization-activated Cl− current (ICl, h) in rat PV cardiomyocytes. Unlike the well-known chloride channel protein 2 (CLCN2) current, the activation curve of ICl, h was hyperpolarized as the Cl− ion concentration ([Cl−]i) increased. This current could account for spontaneous activity in PV cardiomyocytes linked to atrial fibrillation. In this study, we aimed to identify the channel underlying ICl, h. Using RT-PCR amplification specific for Clcn2 or its homologs, a chloride channel was cloned from rat PV and detected in rat PV cardiomyocytes using immunocytochemistry. The gene sequence and electrophysiological functions of the protein were identical to those previously reported for Clcn2, with protein activity observed as a hyperpolarization-activated current by the patch-clamp method. However, the [Cl−]i dependence of activation was entirely different from the observed ICl, h of PV cardiomyocytes; the activation curve of the Clcn2-transfected cells shifted toward positive potential with increased [Cl−]i, whereas the ICl, h of PV and left ventricular cardiomyocytes showed a leftward shift. Therefore, we used MS to explore the possibility of additional proteins interacting with CLCN2 and identified an individual 71-kDa protein, HSPA8, that was strongly expressed in rat PV cardiomyocytes. With co-expression of HSPA8 in HEK293 and PC12 cells, the CLCN2 current showed voltage-dependent activation and shifted to negative potential with increasing [Cl−]i. Molecular docking simulations further support an interaction between CLCN2 and HSPA8. These findings suggest that CLCN2 in rat heart contains HSPA8 as a unique accessory protein.
Keywords: cardiomyocyte, cardiovascular disease, chloride channel, mass spectrometry (MS), heat-shock protein (HSP), heart
Introduction
Ectopic excitability originating in pulmonary veins (PVs)2 causes symptomatic atrial fibrillation (AF) (1, 2). A number of researchers have recorded spontaneous excitability in isolated PV tissues. Several potential causes of spontaneous activity have been reported in these cases, including independent pace-making activity in muscular tunics of guinea pig PVs (3), α- and β-adrenergic stimulation by noradrenaline (3–5), Ca2+ overload in intracellular storage sites induced by digitalis (6), and intracellular Ca2+ leakage (7). The progression of atrial fibrosis is also considered to be an essential factor in structural remodeling in AF (8–12). Genome-wide analysis indicated that chromosomal loci associated with Ca2+-activated K+ channels and hyperpolarization-activated cation channels are linked to AF (13). Furthermore, transient receptor potential channel 3 protein expressed in atrial fibroblasts is also involved in structural remodeling of the atria (14). In addition, we previously identified a hyperpolarization-activated Cl− current (ICl, h) that appears to be involved in the ectopic automaticity of rat PV cardiomyocytes (15).
Several types of Cl− channels, including the cAMP-activated cystic fibrosis transmembrane conductance regulator (CFTR) (16), the Ca2+-activated Cl− channel (17), and the hyperpolarization-activated chloride channel protein 2 (CLCN2) channel (18), have been identified in mammalian cardiomyocytes. CLCN2 is activated by both hyperpolarization and hypoosmotic stress. Interestingly, Duan et al. (19) detected a minor splice variant of CLCN2 that is unique to the rat heart. However, like the full-length protein, this variant was activated by both hyperpolarization and hypoosmolality. In contrast, ICl, h was accepted as a novel type of hyperpolarization-activated Cl− current, because its response to intracellular Cl− and extracellular osmolarity differed from those of CLCN2 (15).
In this study, we attempted to identify the Cl− channel responsible for ICl, h using a molecular cloning and proteomic analysis-based approach. Our results revealed that a member of the heat-shock protein (HSP) 70 superfamily, HSPA8, acts as an auxiliary protein of the CLCN2 Cl− channel. Thus, we propose that CLCN2, in association with HSPA8, is a leading candidate for the molecular architecture underlying ICl, h.
Results
Functional cloning of full-length Clcn2 from rat PV cardiomyocytes
To identify a Cl− channel that could be attributed to the previously reported ICl, h in rat PV cardiomyocytes (15), we attempted to isolate cDNA corresponding to the Cl− channel by RT-PCR. Total RNA extracted from rat PV cardiomyocytes was reverse-transcribed and amplified using a set of specific primers (Full_rClcn2_F/Full_rClcn2_R, Table 1). Based on the hypothesis that the Cl− channel-encoding gene of interest should be homologous to Clcn2, the primers were designed to span the region encompassing the start and end codons of Clcn2 (i.e. the complete coding sequence). As shown in Fig. 1A, the reverse transcript from the small intestine indicated that the intrinsic full-length Clcn2 gene was 2811 bp in length. Transcripts from PV cardiomyocytes were apparently identical in size to this Clcn2 sequence. Gel bands corresponding to the Clcn2 cDNA amplicons transcribed from PVs were digested, purified, and inserted into the pcDNA3.1 expression vector using appropriate restriction enzymes. Sanger sequencing confirmed that the inserted cDNA sequences were identical to the published Clcn2 sequence (Fig. S1). The smaller transcripts below the Clcn2 bands (Fig. 1A) are products of nonspecific amplification.
Table 1.
List of cloning primers
The top pair of primers was used in the cloning of full-length rat Clcn2. The lower pair was used in the subcloning of rat Hspa8. The restriction enzyme cutting sites are indicated in lowercase characters.
| Primer name | Sequences (5′–3′) |
|---|---|
| Full_rClcn2_F | 5′-ACAaagcttAGAAGCAAGAGGAGGCAAGAGG-3′ |
| Full_rClcn2_R | 5′-ACACAgaattcCTTCTGGTCACTGCTGGCTTGC-3′ |
| EcoRI–Hspa8–forward | 5′-AAgaattcTCTGTGTGGTCTCGTCATCAGC-3′ |
| XbaI–Hspa8–reverse | 5′-AAtctagaGGGTCCCTGTGGAACAATGC-3′ |
Figure 1.
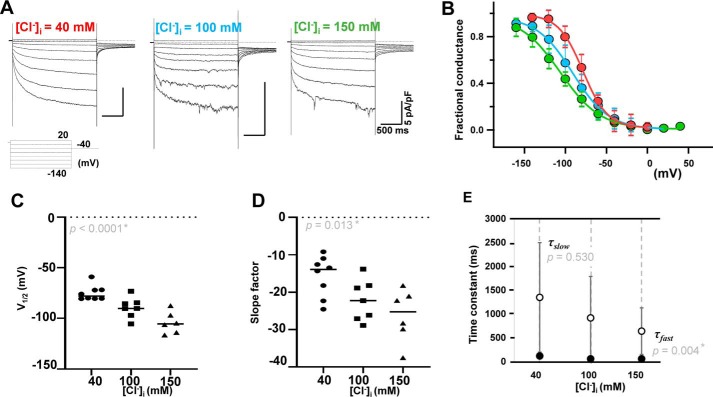
Functional cloning of Clcn2 from rat PV. A, detection of full-length 2811-bp rat Clcn2 in the small intestine (SI), used as a positive control, and in PV. The DNA sizes are indicated on the left. The red arrow indicates the exact size of CLCN2. Each pair of adjacent lanes is derived from the same rat (i.e. the PV of four individual rats is shown on the gel). The two most intense bands in the middle of the gel were extracted for gene cloning. B, hyperpolarization-activated Cl− currents of cloned Clcn2 were recorded under chloride ion concentrations ([Cl−]i) of 40, 100, and 150 mm, with [Cl−]o = 148.9 mm. The pulse protocol is indicated at the bottom of the figure. Dashed lines indicate current levels of 0. C, steady-state activation curves under different [Cl−]i evaluated by current amplitudes of tail currents at 40 mV. Magenta, marine blue, and green indicate data obtained under [Cl−]i of 40, 100, and 150 mm, respectively. n = 7 for each [Cl−]i. V1/2 and S values determined by Boltzmann fitting are plotted against [Cl−]i in D and E, respectively. Statistically significant difference was calculated from Kruskal-Wallis test. F, slow (τslow) and fast (τfast) time constants at −120 mV obtained by fitting the raw data with a biexponential function are plotted against [Cl−]i.
The electrophysiological properties of the cloned Clcn2 gene were analyzed in HEK293 cells using the patch-clamp method. Without CLCN2 transfection, time-dependent current was negligible (Fig. S2A). To evaluate the effects of different Cl− ion concentrations ([Cl−]i) on the voltage-gating, steady-state activation under various [Cl−]i values (40, 100, or 150 mm) was analyzed by measuring the amplitude of the tail current at 40 mV. The relationship between the test potentials and the relative amplitude of the tail current was fitted with the Boltzmann equation and plotted (Fig. 1C). As [Cl−]i increased, the steady-state activation curves shifted toward positive potential and maintained similar slopes. The V1/2 and the slope factors were −72.3 ± 6.6 and −29.6 ± 3.3 mV, respectively, at a [Cl−]i of 40 mm (n = 5), −58.3 ± 15.4 and −30.6 ± 9.5 mV, respectively, at a [Cl−]i of 100 mm (n = 7), and −52.1 ± 10.4 and −28.3 ± 7.2 mV, respectively, at a [Cl−]i of 150 mm (n = 7) (Fig. 1, D and E). There was a statistically significant difference in V1/2 (Kruskal-Wallis test, p = 0.0025) but no difference in slope factors. These results are consistent with previous reports (20). The time course of activation is summarized in Fig. 1F with fast and slow time constants (τfast and τslow). In some cases, the time constants had to be interfered with by the apparent inactivation, most likely as a result of Cl− depletion.
Cell localization of CLCN2 in rat PV and LV cardiomyocytes
CLCN2 is reported to be expressed in the rat left ventricle (LV) (21). We confirmed the cellular localization of CLCN2 in LV and PV cardiomyocytes using immunocytochemistry. Fig. 2 shows representative confocal images of cardiomyocytes isolated from these tissues and labeled with a set of CLCN2 (green) and Na-K pump (red), or RyR (green) and CLCN2 (red) antibodies. Immunostaining of the Na-K pump indicates a plasma membrane pattern, whereas RyR represents a typical cell structure of cardiomyocyte. Overlay image of CLCN2 with Na/K pump reveals that the Cl− channel expresses the cell surface in addition to intracellular spaces. CLCN2 is also expressed in the periphery of LV myocyte.
Figure 2.
Immunocytochemical analysis of cardiomyocytes isolated from PV (left, middle) and LV (right). PV myocyte is labeled with a pair of CLCN2 (green) and Na/K pump (red) and a pair of RyRs (green) and CLCN2s (red). Immunostaining of Na/K pump performed as a plasma membrane marker, and co-localization between CLCN2 and Na/K pump is indicated by yellow color in the overlay image. RyR indicates striated myocardial pattern. LV myocytes are stained with antibodies against RyR and CLCN2.
Hyperpolarization-activated Cl− current in rat LV cardiomyocytes
Next, we examined whether the Cl− current in LV myocytes also exhibited ICl, h-like properties. The effects of different [Cl−]i (40, 100, or 150 mm; n = 8, 7, and 6 for each, respectively) on voltage-gating were evaluated using the patch-clamp method. As [Cl−]i increased, the steady-state activation curves shifted toward negative potential with more gradual slopes. The V1/2 and the slope factors were −75.0 ± 7.4 and −15.7 ± 5.5 mV, respectively, at a [Cl−]i of 40 mm and −89.4 ± 10.2 and −22.2 ± 5.5 mV, respectively, at a [Cl−]i of 100 mm and −104.3 ± 10.8 and −26.2 ± 7.1 mV, respectively, and at a [Cl−]i of 150 mm (Fig. 3, B–D). The values of both V1/2 and slope factor were decreased as [Cl−]i increased. The time course of activation at −120 mV was analyzed by fitting the data to a first-order biexponential function. Both τslow and τfast were plotted (Fig. 3E). τslow values were 1346.6 ± 1150.0, 915.4 ± 869.5, and 636.5 ± 489.8 ms of [Cl−]i and 40, 100, and 150 mm, respectively (Fig. 3E, open circles). The τfast values were 114.9 ± 35.9, 55.7 ± 20.3, and 55.8 ± 10.9 ms at [Cl−]i and 40, 100, and 150 mm, respectively (Fig. 3E, filled circles), and significantly decreased by increasing [Cl−]i (p = 0.004).
Figure 3.
Effect of the chloride ion concentration ([Cl−]i) on the voltage dependence of the hyperpolarization-activated Cl− current (ICl, h) in left ventricle cardiomyocytes. A, raw traces recorded at [Cl−]i of 40, 100, and 150 mm [Cl−]i, with [Cl−]o = 148.9 mm. Horizontal bars (500 ms) and vertical bars (5 pA/picofarad) are shown for all traces. The pulse protocol is shown. B, steady-state activation curves evaluated by tail currents under different [Cl−]i at 40 mV. The color-coding is the same as in Fig. 1. V1/2 and S values are plotted against [Cl−]i in C and D, respectively. E, slow (τslow, open circles) and fast (τfast, closed circles) time constants at −120 mV obtained by fitting the raw data with a biexponential function are plotted against [Cl−]i.
Molecular identification of HSPA8 as an accessory protein of CLCN2
The electrophysiological function of endogenous CLCN2 in rat LV and PV cardiomyocytes was observed as ICl, h. The observed [Cl−]i-dependent gating of ICl, h was largely different from the properties of the well-known CLCN2 current (ClC-2). When Clcn2 is introduced alone in HEK293 cells, the steady-state activation curve of ClC-2 shifts toward positive potential with increasing [Cl−]i (20, 22). We hypothesized that CLCN2 is regulated by an unidentified molecule, which would modulate the [Cl−]i dependence of the channel. We therefore explored CLCN2-binding proteins by immunoprecipitation. Solubilized membrane proteins from rat PV and LV cardiomyocytes were precipitated with an anti-CLCN2 antibody and separated by electrophoresis. Precipitants were detected at ∼90, ∼70, ∼50, and ∼25 kDa on Coomassie-stained gels (Fig. 4A). CLCN2 was identified as the band running at ∼90 kDa. A band of weak intensity at ∼180 kDa was presumed to be residual CLCN2 dimer. Bands at ∼50 and ∼25 kDa are consistent with the heavy and light chains of immunoglobulin used in the immunoprecipitation. However, the residual ∼70-kDa bands (marked with arrows in Fig. 4A) are unknown proteins, indicating an accessory protein of CLCN2. These bands were excised, digested with trypsin, and eluted with 0.5% TFA. The eluted peptides were then analyzed using MS. Of the eluted peptides, 62 were identified as being of rat origin (Fig. S3). Only eight of the peptides had been recognized as a part of functional protein, and all corresponded to constitutive heat-shock protein HSPA8 (71 kDa) of rats (Fig. 4B and Fig. S3). Immunoblotting of the precipitate indicated that HSPA8 corresponded to the CLCN2-interacting protein (Fig. 4C, left), and the interaction between HSPA8 and CLCN2 was confirmed by reverse co-immunoprecipitation using an anti-HSPA8 antibody (Fig. 4C, right). The molecular interaction was confirmed by immunoblotting more than three times in both PV and LV samples. Fig. 4C is the representative example. Hspa8 was abundantly expressed in rat PV cardiomyocytes (Fig. 5A) and was successfully subcloned from rat PV cardiomyocytes (Fig. S3) using specific primers (Table 1). Immunocytochemistry discovered that HSPA8 was present on the cell surface of PV and LV cardiomyocytes (Fig. 5, B and C), implying co-localization with CLCN2.
Figure 4.
Identification of HSPA8 as a CLCN2-interacting protein. A, detection of CLCN2-interacting proteins in the membrane fraction by Coomassie Brilliant Blue staining. The membrane protein fractions from lysed PV and LV cardiomyocytes were immunologically purified by co-immunoprecipitation (IP) using an anti-CLCN2 antibody and separated by SDS-PAGE. Protein size is indicated on the left. CLCN2 and immunoglobulin heavy and light chain proteins were detected at ∼90, 50, and 25 kDa, respectively. The protein at ∼70 kDa (indicated with arrows) is an unidentified molecule. Precipitant with 10 μg of mouse IgG was loaded in as a negative control. B, determination of the amino acid sequence of the unknown molecule by LC-tandem MS analysis. Mass spectrometry analysis identified eight peptides of rat origin, all of which corresponded to a single protein, HSPA8. The complete 646-amino acid sequence of HSPA8 is displayed. The 90 amino acids used in the identification of the eight peptides are indicated in bold, underlined, italic characters. Coverage was calculated as the ratio of the identified 90 amino acids to the 646 total amino acids. C, confirmation of the protein–protein interaction. Precipitants obtained from the crude membrane fractions of PV or LV using either CLCN2 (left panel) or HSPA8 (right panel) antibody are blotted with the other antibody. Origins of loaded proteins (i.e. PV or LV) are indicated above the corresponding gel lanes. Precipitant with 10 μg of rabbit IgG was also loaded as a negative control on the right panel. IB, immunoblot.
Figure 5.
Expression and localization of Hspa8 in cardiomyocytes. A, RT-PCR detects full-length 2027-bp Hspa8 (SI; small intestine). The cDNA is subcloned. B, immunocytochemistry of PV cardiomyocytes against HSPA8 (green) and Na/K pump (red). Immunostaining of Na/K pump indicates the location of the cell surface. The overlay image (bottom) emphasizes co-localization of HSPA8 with the plasma membrane marker. Scale bar is 10 μm. C, immunocytochemistry of LV cardiomyocytes against HSPA8 (green) and Na/K pump (red). HSPA8 is located on the cell surfaces. Of note, immunostaining of Na/K pump detects enriched transverse tubule. Scale bar is 10 μm. D, immunoprecipitants in PC12 cells reconfirm the interaction between cloned CLCN2 and HSPA8 again. IP, immunoprecipitation; IB, immunoblot.
Functional characterization of HSPA8 by patch-clamping in HEK293 and PC12 cells
Cloned Hspa8 was co-transfected into HEK293 cells with Clcn2, and the ClC-2 current was recorded using the conventional whole-cell patch-clamp method (Fig. 6). At a [Cl−]i of 40 mm, the V1/2 was −71 ± 20.0 mV, and the slope factor was −23.9 ± 3.1 (n = 5). The V1/2 was not statistically changed (p = 0.38), while the slope factor was affected by transfection of cloned Hspa8 (p = 0.03). However, with a [Cl−]i of 150 mm, the voltage dependence of the ClC-2 current produced by co-transfected Hspa8 and Clcn2 appeared not to fit a single Boltzmann function, but it was satisfactorily approximated by the sum of the two components. The V1/2 and slope factor values for one component were −120.4 ± 12.3 and −12.9 ± 6.2 mV, respectively. They were significantly affected by co-transfection of HSPA8 (p = 0.004 for V1/2 and p = 0.009 for slope factor with Mann-Whitney test).The V1/2 and slope factor values for the other component were −25.1 ± 25.4 and 21.2 ± 8.6 mV, respectively, without a statistically significant difference from those values in the absence of Hspa8 (p = 0.126 and 0.247 for V1/2 and slope factor). The relative magnitudes of the two components are 0.38 ± 0.14 for the former (low voltage) and 0.62 ± 0.14 for the latter (high voltage) components, respectively (n = 5, Fig. 6, A–C). Reportedly, rat CLCN2 binds to another heat-shock protein, human HSP90, in HEK293 cells (24). To diminish the effect of human HSP90 on voltage-dependent gating, we carried out an identical patch-clamp protocol using the rat-derived cell line PC12. The cells are known to possesses voltage-gated K+ currents (25), while the Cl− current was undetectable as indicated in Fig. S2B. When Clcn2 was transfected alone into PC12 cells, the V1/2 and slope factor values of the ClC-2 current were −55. 1 ± 9.4 and −19.5 ± 1.8 mV, respectively, at a [Cl−]i of 40 mm, and −56.3 ± 24.7 and −22.7 ± 5.9 mV, respectively, at a [Cl−]i of 150 mm (n = 4 for each, Fig. 7, A–C). Statistically significant differences between [Cl−]i of 40 and 150 mm in both V1/2 and slope factor were unobserved. By contrast, when Hspa8 was overexpressed with Clcn2 in PC12 cells, the activation curves deviated from a single Boltzmann function and were fitted to the sum of two Boltzmann components, i.e. low and high voltage–activated components (Fig. 7, D–F). At a [Cl−]i of 40 mm (n = 6), the V1/2 and slope factor values of the low voltage–activated component were −110.9 ± 12.7 and −14.9 ± 7.9 mV, respectively. The values for the high voltage–activated component were −40.3 ± 23.5 and −14.8 ± 3.0 mV, respectively. When [Cl−]i was increased to 150 mm (n = 5), the activation of the low voltage–activated component shifted toward negative potential (p = 0.002). The V1/2 and slope factor values of the low voltage–activated component at a [Cl−]i of 150 mm were −182.0 ± 13.6 and −22.9 ± 6.1 mV, respectively, whereas those of the high voltage–activated component were −17.1 ± 10.2 and −12.5 ± 4.5 mV, respectively. Statistical analysis revealed that the V1/2 of low-voltage component after the co-transfection was substantially shifted toward negative potential by increasing [Cl−]i (p = 0.002), while the other component was unaffected (p = 0.09). The relative magnitudes of the low voltage–activated component are overviewed in Fig. 7G. To evaluate the effect of HSPA8 on time-dependent kinetics, we measured the time constant of the activated currents and tail currents subsequent to the activation by hyperpolarization to −160 mV. The influence of the presence or absence of HSPA8 was compared in the both condition of [Cl−]i = 40 and 150 mm. As a result, there are no significant impact of HSPA8 on the time constants (Fig. 7, H and I). Electrophysiological properties other than the voltage dependences and time constants were also evaluated (Fig. 8). Consistent effects of Hspa8 expression were not uncovered in those parameters.
Figure 6.
Effect of Hspa8 expression on the chloride ion concentration ([Cl−]i)-dependent voltage-gating in HEK293 cells. Hspa8 was co-transfected with Clcn2. A, [Cl−]i was 40 mm (left) and 150 mm (right). The pulse protocol is shown in the inset. Activation curves were evaluated based on the amplitude of tail currents at 40 mV. The initial portion of the tail current is shown in expanded scale in B. Experimental results are summarized by Boltzmann fitting in C. V1/2 and S values are plotted against [Cl−]i in D and E, respectively. Of note, the activation curve at [Cl−]i is divided into two components, and the low voltage–activated component is statistically significantly different from the activation curve at 40 mm [Cl−]i.
Figure 7.
Effect of Hspa8 overexpression on the [Cl−]i-dependent voltage-gating in PC12 cells. A, chloride ion concentration ([Cl−]i)-dependent voltage-gating CLCN2 current in PC12 cells without co-transfection of Hspa8. Current traces were obtained using the pulse protocol shown in the inset. The [Cl−]i was 40 mm (left) and 150 mm (right). Activation curves were evaluated based on the amplitude of tail currents at 40 mV. The tail current is shown in expanded scale in B. The color-coding of the results is the same as in Fig. 1. C, activation curves are gained by Boltzmann fittings. D, CLCN2 current in PC12 cells with co-transfection of Hspa8. The pulse protocol is same as A. The tail current and activation curve are shown in E. The activation curves are fitted with a double-Boltzmann function in F, and contributions of the low voltage–activated component are overviewed in G. H, time constants of the Cl− currents activated −160 to −80 mV. The currents were evoked in PC12 cells with or without overexpression of Hspa8 in both conditions of [Cl−]i = 40 and 150 mm. The two-way ANOVA undetected impact of Hspa8 on the value is shown. I, τfast (closed circles) and τslow (open circles) of tail currents at 40 mV following activation by hyperpolarization of −160 mV are measured in PC12 cells with or without overexpression of Hspa8 in both conditions of [Cl−]i = 40 and 150 mm.
Figure 8.
Other electrophysiological parameters for HEK293 and PC12 cells. The amplitudes of the CLCN2 currents were evaluated by tail currents at 40 mV following the hyperpolarization to −120 mV. The data obtained using HEK293 cells are summarized in A, and PC12 data are summarized in B. The data are classified by experimental conditions, i.e. [Cl−]i, cell type, and co-expression of Hspa8, as indicated. C and D summarize cell capacitance (Cm) of cells. According to the Mann-Whitney test for each experimental condition (cell type and [Cl−]i), Hspa8 expression affects current amplitude at 40 mm [Cl−]i in HEK293 cells; otherwise, no statistical significance was detected.
Homology modeling of protein structures and protein–protein docking simulations
To compare the protein–protein docking simulation between CLCN2 and either HSP90 or HSPA8, protein structures of rat CLCN2, rat HSPA8, and human HSP90 were homologically simulated using the homology-modeling server SWISS-MODEL. The Cl− channel structure from human CLC-1 (26) was used as a template for the model of rat CLCN2. The QMEAN score and sequence identity of the model were −1.43 and 53.71%, respectively. QMEAN is a type of Z-score: values around zero indicate good agreement between model and experimental structures of similar size, and scores of −4.0 or less indicate models of low quality (27). A sequence identity of greater than 40% is preferable when building an appropriate model. The X-ray crystallography structure of bovine HSPA8 was used as the a template for rat HSPA8 (28). The QMEAN score and sequence identity were −0.70 and 99.64%, respectively. Human HSP90 was modeled based on data from cryo-EM (29). The QMEAN score and sequence identity of the model were −1.74 and 86.58%, respectively. Protein–protein docking between each subunit of the homodimeric CLCN2 (green and cyan) and its interaction proteins (wheat and olive) were built in 1:1 stoichiometry using ClusPro 2.0. The weighted energy of the top-ranked cluster was −1690.2 C for the CLCN2–HSP90 interaction, and −1497.8 C for the CLCN2–HSPA8 interaction, indicating enough energies to form protein–protein interactions. The highest priority models for CLCN2–HSPA8 and CLCN2–HSP90 are represented in Figs. 9 and 10, respectively. The anion selectivity filter is indicated in red in Figs. 9A and 10A; the estimated molecular surfaces of both models are overviewed in Figs. 9B and 10B, and the electrostatically tight interaction between CLCN2 and HSP90 or HSPA8 is shown in a blue–red color gradient in Figs. 9C and 10C. According to the simulation model, HSP90 (Fig. 9, A–C) and HSPA8 (Fig. 10, A–C) bind to CLCN2 in different ways. HSP90 surrounds CLCN2 and binds to the lateral side of each CLCN2 subunit in 1:1 stoichiometry. The dimer interface remains unaffected (Fig. 9C). In contrast, HSPA8 protrudes straight from the boundary part between the two CLCN2 subunits and tightly binds to the interface of the homodimer, i.e. one HSPA8 binds to both CLCN2 subunits (Fig. 10C).
Figure 9.
High-throughput simulation of protein–protein docking between rat CLCN2 and human HSP90. Protein structures of CLCN2 (cyan and green) and HSP90 (olive and wheat) were homologically modeled by SWISS-MODEL. Docking states were calculated using ClusPro 2.0. The highest priority model is visualized as a cartoon ribbon structure (A) and with protein surface (B). The upper panels in A and B are side views of the model with a tentative plasma membrane (gray lines). The corresponding lower panels are bottom views, which illustrate the intracellular side of the subunit assembly. Anion-selective protopore gates are indicated in red. C, surface electrostatic potential is color-coded by the kT/e unit. One of the HSP90 proteins (olive color in the top panel) is subtracted in the middle, displaying an electrostatically strong docking site (dotted line). The cartoon structure (bottom) indicates that the docking site does not affect the homodimeric interface.
Figure 10.
Protein–protein docking between rat CLCN2 and HSPA8. Protein structures of CLCN2 (cyan and green) and HSPA8 (olive and wheat) were homologically modeled, and docking states were calculated using ClusPro 2.0. The highest priority model is visualized with a tentative plasma membrane (gray lines). The upper and lower panels in A and B display side and bottom views, respectively. The anion-selective protopore gates are colored red. C, surface electrostatic potential is color-coded by the kT/e unit. One of the HSPA8 proteins (olive color in the top panel) is subtracted in the middle, displaying an electrostatically strong docking site (dotted line). The cartoon structure (bottom) indicates that the docking site interacts with the homodimeric interface.
Discussion
Major findings
This study demonstrates that CLCN2 is functionally expressed in cardiomyocytes and interacts with HSPA8 at the cell membrane. Because other possible proteins that contribute to the hyperpolarization-activated Cl− current are undetectable, the CLCN2–HSPA8 complex is a leading candidate for the molecular architecture of the previously reported ICl, h. The ClC-2 current induced by the co-transfection of Clcn2 and Hspa8 into HEK293 and PC12 cells showed unique [Cl−]i-dependent properties. Voltage-dependent activation revealed two components with Boltzmann fitting, i.e. low- and high-voltage–activated components. Statistical analyses revealed that essential impact of Hspa8 appears to be the low-voltage–activated component, which shifts to hyperpolarized potentials by increasing [Cl−]i. V1/2 of the high-voltage–activated component and other physiological parameters in the presence of Hspa8 are hardly different from those in the absence of it.
It is known that as [Cl−]i increases, the steady-state activation curve of CLCN2 shifts toward positive potential (Fig. 1, C–E) (20), and such [Cl−]i dependence is caused by the association of HSP90 with CLCN2. Hinzpeter et al. (24) reported that human HSP90 binds to rat CLCN2 in HEK293 cells and that pharmacological inhibition of HSP90 reduced ClC-2 currents and impaired the [Cl−]i-dependent rightward shift of the fractional conductance. Previously, we recorded a ClC-2–like current, ICl, h, and in this study, we confirmed the expression of CLCN2 in the cells (Figs. 1–5). Interestingly, the activation curve of ICl, h shifted toward negative potential with increasing [Cl−]i values in PV cardiomyocytes. Here, another CLCN2-interacting partner, HSPA8, was identified (Fig. 4). Homology modeling of the protein structure and protein–protein docking simulations demonstrated that both heat-shock proteins, human HSP90 and rat HSPA8, interact with the same channel-pore subunit, CLCN2, but in different ways (Figs. 9 and 10). Each HSP90 binds to one of the dimeric subunits of CLCN2, without affecting the interface of the homodimer (Fig. 9), while HSPA8 binds to both CLCN2s at the boundary between the homodimer subunits. These structural differences may underlie the different [Cl−]i dependences, i.e. the ClC-2 current produced by CLCN2–HSP8 shows a [Cl−]i-dependent leftward shift in the activation curve, as was observed for ICl, h in LV and PV cardiomyocytes (Fig. 3) (15). However, in this study, we could not perfectly reproduce the [Cl−]i-dependent leftward shift of the activation curve when Hspa8 and Clcn2 were co-transfected into HEK293 or PC12 cells (Figs. 6 and 7). This may indicate that HSPA8 and endogenous CLCN2-interacting proteins, such as HSP90, hinder each other when associating with CLCN2, thereby counteracting the [Cl−]i-dependent shift of the activation curve. Theoretically, two Boltzmann components suggest that HSPA8 and the other CLCN2-interacting molecules produce two ClC-2 currents with different kinetic properties, one of which is activated at a negative potential, and the other is activated at a more positive potential under high [Cl−]i conditions (Fig. 7, D–G). In this respect, this study suggests that HEK293 cells use HSP90 as a CLCN2-binding partner, whereas PC12 cells also contain a subunit that is functionally distinct from HSPA8.
CLCN2 belongs to the CLC Cl− channel/transporter family, which includes homodimeric channels with two independent Cl− permeation pathways. The negatively-charged carboxyl side chain of a glutamate residue located within the permeation pathway forms a protopore (30). The CLC family possesses two different ionic gates: a fast-acting protopore gate and a slow-acting common gate. The fast gate controls each protopore, and a common gate simultaneously controls both protopores. Detailed mechanisms of these gates are still being debated. It was recently suggested that the voltage-dependent activation of the protopore gates is not governed by the movement of voltage-sensitive domains (31); instead, the hyperpolarization pushes the intracellular Cl− ions into binding sites within the pore, resulting in the opening of the protopore gate. This hypothesis favors the [Cl−]i-dependent rightward shift of the activation curve of the ClC-2 current observed in HEK293 cells. Indeed, the CLCN2–HSP90 association (observed in Fig. 9) hardly obstructs the nature of the protopores. At the same time, it has been reported that neutralization of Glu-217, the gating glutamate of guinea pig CLCN2, results in ClC-2 channels without a protopore gate and that the activation curve of these channels shows a rightward shift with decreasing [Cl−]i (32). These findings indicate that [Cl−]i affects the protopore gate and the common gate in opposite ways and that the overall [Cl−]i dependence usually masks the effect on the protopore gate in the case of ClC-2 current. Accordingly, we speculate that HSPA8 affects the [Cl−]i dependence by enhancing the [Cl−]i-dependent property of the common gate. Structural analysis (Fig. 10) indicates that the CLCN2–HSPA8 complex interacts with the dimeric interface across both individual subunits. It is likely that the common gate is stabilized by the CLCN2–HSPA8 interaction, and its characteristics may be unmasked. This finding is consistent with our speculation, and we may safely conclude that HSPA8 is an auxiliary subunit that modulates the [Cl−]i dependence of CLCN2.
To the best of our knowledge, this is the first report to identify HSPA8 as an accessory subunit of an ion channel. HSPA8 is a clathrin-uncoating ATPase (33) and is classified as a member the HSP70 family (34). Most HSPs are stress-inducible, whereas HSAP8 constitutively localizes to the cytoplasm and lysosome. As a chaperone protein, HSPA8 is substantially recruited in the cell-cycle G1/S transition (35, 36), and it positively regulates chaperone-mediated autophagy (37–39). With these cellular functions, HSPA8 is thought to play a crucial role in cell differentiation and embryonic development (40–42). Additionally, the ability of HSPA8 to interact with ion channels on the cell membrane has been reported in the process of ubiquitin-mediated proteasomal degradation. For example, HSPA8 cooperates with co-chaperones, such as the C-terminal heat-shock cognate 70-interacting protein and Bag1, in the ubiquitination of CFTR (43), Kv1.5 (44), and the human ether-a-go-go–related gene K+ channel (44). In this study, expression of HSPA8 was nearly restricted to the cell surface, and a corresponding co-chaperone was not observed in the Coomassie-stained SDS-polyacrylamide gels (Fig. 4A). Thus, HSPA8 is supposed to bind directly to CLCN2 on the membrane and to regulate channel properties rather than engage the maturation of the channel. Hinzpeter et al. (24) reported that the association of HSP90 with ClC-2 results in higher channel activity as a result of increased cell-surface channel expression, facilitation of channel opening, and enhanced channel sensitivity to intracellular [Cl−]i. In this study, Clcn2 was transiently transfected with or without co-transfection of Hspa8, and the level of gene expression was not stably controlled. The density of ClC-2 current in HEK293 and PC12 cells varied from cell to cell (Fig. 8); thus, we could not determine whether HSPA8 aids in the expression of CLCN2 channels. It should be noted that HEK293 and PC12 cells possess CLCN2-interacting proteins, and therefore, the positive effect of HSPA8 on channel expression may have been masked by these endogenous molecules.
Accessory proteins for CLCN2 appear to differ by cell type. In glial cells, CLCN2 interacts with GlialCAM (45), which was originally identified as a major partner of MLC1, the protein responsible for autosomal-recessive inheritance disease megalencephalic leukoencephalopathy with subcortical cysts (46). GlialCAM directs the CLCN2 channel to the cell–cell junctions, enhances the current amplitude (47), and changes the activation properties by affecting the common gate (48). We propose that CLCN2 requires accessory proteins for proper expression and function at cell membranes and that HSPA8 fills this role in the heart.
Limitations
It was considerably challenging to reproduce the same time-dependent kinetics in ClC-2 current in different biological materials, such as Xenopus oocytes, cultured cells, and isolated cardiomyocytes. Reportedly, the time constant in activation currents of ClC-2 is <200 ms for HEK293 (49), ∼2000 ms for cardiomyocytes (18, 19), and ≫5000 ms for Xenopus oocytes (23). In other words, the activation kinetics are prolonged as the cell size increased. One of the reasons is considered to be the amount of cytoskeleton inside cells. Ahmed et al. (23) have reported that disruptions of the cytoskeletons dramatically enhanced the activation process of the current. Further efforts will be required to determine the molecular reasons for cell type–dependent differences in the time dependence of the current.
Conclusions
This study identified an accessory protein of the ClC-2 channel in rat cardiomyocytes. This regulatory protein is likely to be responsible for the molecular characteristics of ICl, h in voltage-dependent gating. The results may contribute to the development of drugs that pharmacologically suppress the hyperpolarization-activated current that precedes diastolic depolarization in arrhythmia.
Materials and methods
Ethical approval
The protocols used in this study were approved by the Animal Ethics Committees of the Yamagata University Faculty of Medicine, Japan, and the Akita University School of Medicine, Japan. All experiments were performed at Yamagata University or Akita University under personal and project licenses following appropriate institutional reviewing.
Preparation of rat hearts
Male Wistar rats (8–14 weeks) were purchased from Charles River Laboratories or CLEA Japan, Inc. To isolate cardiomyocytes from PV and LV, rats were anesthetized by intraperitoneal injection of mixed anesthesia (0.15 mg/kg medetomidine, 2.0 mg/kg midazolam, and 2.5 mg/kg butorphanol). After checking suppression of the nociceptive reflex, the chest cavity was opened under artificial respiration, and the aorta was cannulated in situ to perfuse the coronary arteries. The heart and lung were excised in block, mounted on a Langendorff apparatus, and then perfused sequentially with the following buffers: 1) normal Tyrode's solution for ∼3 min, to wash blood out from heart and lung; 2) nominally Ca2+-free Tyrode's solution for 5 min; and 3) Ca2+-free Tyrode's solution containing 0.05% (w/v) collagenase and 0.005% (w/v) elastase (Wako Pure Chemicals, Osaka, Japan) for 30 min. The composition of the normal Tyrode's solution was as follows (in mm): NaCl, 136.9; KCl, 5.4; CaCl2, 1.8; MgCl2, 0.5; NaH2PO4, 0.33; HEPES, 5.0; and glucose, 5.5 (pH 7.4 adjusted with NaOH). After digestion, the heart was perfused with 50 ml of a high-K+, low-Cl− solution composed of the following (in mm): l-glutamic acid, 70; KOH, 70; KCl, 30; KH2PO4 10; MgCl2, 1; taurine, 20; glucose, 10; EGTA, 0.3; and HEPES, 10 (pH 7.4 adjusted with KOH). We then trimmed off the soft tissue containing the vagus nerves, adipose tissue, and the pulmonary artery under a stereomicroscope. The LV was dissected, and the left PV was excised from the digested block. The LV and left PV were then minced gently in the high-K+, low-Cl− solution before being gently agitated to dissociate the cells. The two cell suspensions were stored at 4 °C for later use.
PV preparation for molecular cloning of Clcn2 was obtained by a similar procedure to that described above, except that the enzymatic digestion was not performed. In this study, samples of PV were collected from regions, including PV ostia and the left PV trunk.
Molecular cloning of Clcn2 and Hspa8 from rat PV cardiomyocytes
Total RNA was extracted from rat PV using a RNeasy mini kit (Qiagen, Hilden, Germany). cDNA was transcribed using a PrimeScript II 1st Strand cDNA synthesis kit (Takara Bio Inc., Kusatsu, Japan). The Clcn2 and Hspa8 complete coding sequences were then amplified from the extracted cDNA using specific primers (see Table 1) containing HindIII and EcoRI and EcoRI and XbaI restriction sites, respectively. The following thermal cycler protocol was used to amplify the cDNA regions: 35 cycles of 98 °C for 10 s, 55 °C for 5 s, and 72 °C for 3 min, followed by a final extension at 72 °C for 7 min. All reactions were performed in a Dice thermal cycler (Takara). Amplicons were gel-purified and subcloned into the pcDNA3.1 vector (Thermo Fisher Scientific, Waltham, MA) using the appropriate restriction enzymes and a DNA ligation kit, Long (Takara). Recombinant plasmids were purified, and their DNA sequences were confirmed by Sanger sequencing (model 310; Applied Biosystems, Foster City, CA).
Transfection of Clcn2 and Hspa8 into HEK293 and PC12 cells
For functional analysis, cloned Clcn2 was transiently transfected with or without subcloned Hspa8 into HEK293 and PC12 cells using Lipofectamine 2000 transfection reagent (Thermo Fisher Scientific) according to the manufacturer's instructions. Enhanced green fluorescence protein was co-transfected to label transfected cells. The transfection efficiency was 40–50%. Cells were settled on poly-d-lysine–coated coverslips and subcultured for 48 h prior to electrophysiological recording.
Electrophysiological analysis by patch-clamping
The whole-cell patch-clamp method was used to record membrane currents (patch-clamp amplifier Axopatch 200B; Molecular Devices, Chicago). Borosilicate glass electrodes had tip resistances between 2.0 and 5.0 megohms when filled with internal solution. For Cl− current recording, KCl of Tyrode's solution was substituted by equimolar CsCl. Membrane currents were recorded under voltage-clamp conditions at 37 ± 1 °C. Pulse protocols and data acquisition and storage were managed using CLAMPEX (Molecular Devices). The sampling frequency was 1 kHz and low-pass filtering was performed at 500 Hz. The cell membrane capacitance was determined by applying a 30-ms hyperpolarizing voltage-clamp step from a holding potential of 0 to −5 mV and integrating the area under the capacitive transient. All patch-clamp data were analyzed using IGOR software (Wavemetrics, Portland, OR). The junction potential between Cs-Tyrode's solution and the intracellular solution were corrected numerically using previously measured values (15), because solutions used in this study were identical. The compositions of internal solutions for recording Cl− currents are presented in Table 2. To evaluate the voltage-dependent current availability, the current was activated by applying 2-s hyperpolarizing pulses to various test potentials from the holding potential of 0 mV, followed by depolarization to 40 mV. The relationship between the test potentials and the relative amplitude of the tail current was fitted using Boltzmann Equation 1,
| (Eq. 1) |
where IMax is the predicted maximum current amplitude; Vm is the membrane voltage; S is the scaling factor; and V1/2 is the Vm needed to reach half the I/IMax. Summations of two Boltzmann functions were applied to analyze the activation curves of two-component currents in HSPA8-expressing cells. Time-dependent kinetics of the ICl, h of LV cardiomyocytes was analyzed by fitting the current with a first-order biexponential function as shown in Equation 2,
| (Eq. 2) |
where τfast and τslow are fast and slow time constants, respectively.
Table 2.
Composition of internal solutions used in Cl− current recording
Solution 1 is [Cl−]i = 40 mm; solution 2 is [Cl−]i = 100 mm; and solution 3 is [Cl−]i = 150 mm. The pH value of all internal solutions was adjusted with CsOH.
| Internal solutions |
|||
|---|---|---|---|
| 1 | 2 | 3 | |
| CsCl | 30.0 | 90.0 | 140.0 |
| CsOH | 110.0 | 50.0 | |
| Aspartic acid | 110.0 | 50.0 | |
| Na2-ATP | 5.0 | 5.0 | 5.0 |
| MgCl2 | 5.0 | 5.0 | 5.0 |
| EGTA | 5.0 | 5.0 | 5.0 |
| HEPES | 5.0 | 5.0 | 5.0 |
| GTP-Na2 | 0.1 | 0.1 | 0.1 |
Immunocytochemistry and confocal imaging
Isolated PV and LV cardiomyocytes settled on poly-d-lysine–coated coverslips were incubated for 30 min at room temperature and subsequently fixed with 4% paraformaldehyde for 30 min at room temperature. The cells were then permeabilized using PBS containing 0.2% (w/v) Triton X-100 for 15 min and subsequently incubated with blocking buffer (PBS containing 5% BSA). Cells were then stained with antibodies against ryanodine receptor (RyR; Abcam, Cambridge, UK; catalog no. ab2827), Na/K pump (Abcam, catalog no. ab76020), CLCN2 (Santa Cruz Biotechnology, Dallas, TX, catalog no. sc-377284; Alomone Labs, Jerusalem, Israel, catalog no. ACL-002), and HSPA8 (Abcam; catalog no. ab2788). All primary antibodies were diluted 1:500 in antibody buffer/PBS containing 1% (w/v) BSA. Appropriate secondary antibodies were then used (either anti-mouse or anti-rabbit, conjugated with either Alexa Fluor 488 or Alexa Fluor 594) at a 1:1000 dilution. Cells were mounted on glass slides using Permount (Thermo Fisher Scientific, Hampton, NH). Single-slice and/or image stacks were obtained using a Zeiss 700 confocal microscope (×63, pinhole size of an arbitrary unit). Captured images were processed using ZEN Image software (Zeiss).
Co-immunoprecipitation and MS
To prepare crude membrane protein fractions from rat hearts, the LV and PV were dissected from rats as described above in Ca2+-free Tyrode's solution at 4 °C and then flash-frozen in liquid nitrogen before being stored at −80 °C for later use. To extract membrane proteins, the heart samples were resuspended in a solution containing 50 mm Tris-HCl (pH 7.5), 250 mm sucrose, and proteinase inhibitor mixture (cOmplete Mini, Thermo Fisher Scientific) and then homogenized. The homogenates were centrifuged at 800 × g for 10 min to remove the nuclear proteins and then recentrifuged at 15,000 × g for 15 min to remove lysosomes and mitochondria. Supernatants were pelleted at 100,000 × g for 1 h at 4 °C. The resultant pellets were used as the plasma membrane–enriched fraction. For solubilization, the pellets were incubated for 1 h at 4 °C in cell lysis buffer composed of 10 mm Tris (pH 7.5), 1% (w/v) Triton X-100, 150 mm NaCl, 5 mm EDTA, 10% glycerol, and proteinase inhibitor mixture. Aliquots (0.5 ml) of the solubilized samples containing membrane proteins (500 or 1000 μg quantified by the Bradford assay) were incubated for 1 h with 2 μg of mouse anti-CLCN2 (Santa Cruz Biotechnology, catalog no. sc-377284) or rabbit anti-HSPA8 (Proteintech, Chicago; catalog no. 10654-1-AP) coupled with Dynabeads protein G superparamagnetic beads (Thermo Fisher Scientific). Precipitants with 10 μg of normal mouse or rabbit IgG were used as negative controls. After being washed three times, bound proteins were eluted in Laemmli buffer and run on 10% SDS-polyacrylamide gels. For LC-tandem MS analysis (LC-MS/MS), Coomassie Brilliant Blue-stained gel lanes were excised, and proteins were digested with high-grade trypsin (Promega, Fitchburg, WI). Trypsinized peptides were resolved in 0.5% TFA and then loaded onto a pre-column (C18 PepMap100, 5 μm; Dionex, Idstein, Germany). After demineralization of the loaded sample, LC was performed on an EASY-nLC1000 LC system (Thermo Fisher Scientific) and directly electrosprayed into a Q-Exactive mass spectrometer (Thermo Fisher Scientific). MS/MS spectra were analyzed using Proteome Discoverer 1.3 (Thermo Fisher Scientific).
SDS-PAGE and western blotting
Proteins extracted from PV, LV, and PC12 cells were separated by electrophoresis on 10% SDS-polyacrylamide gels using the Mini-PROTEAN system (Bio-Rad) and then transferred onto polyvinylidene fluoride membranes. The membranes were blotted using mouse anti-CLCN2 (Santa Cruz Biotechnology, catalog no. sc-377284) or rabbit anti-HSPA8 (Proteintech, catalog no. 10654-1-AP) primary antibodies at a 1:1000 dilution. Appropriate secondary antibodies were then used (either anti-mouse or anti-rabbit, conjugated with horseradish peroxidase (HRP), at 1:10,000 dilution)). Housekeeping protein GAPDH was blotted using anti-GAPDH antibody (Cell Signaling, catalog no. 2118), if necessary. The HRP reaction was carried out using ECL Prime Detection Reagent (GE Healthcare). Results were visualized using a ChemiDoc XRS 255 imaging system (Bio-Rad) or ImageStation 2000R (Eastman Kodak Co.).
Homology modeling of protein structure and protein–protein docking simulation
Template-based homology modeling of rat CLCN2 and HSPA8 was performed using the SWISS-MODEL bioinformatic modeling server (https://swissmodel.expasy.org/). Human CLC-1 (26) and HSP90 (29) and bovine HSPA8 (28) were used as templates. Protein models were simulated as both dimers and monomers, downloaded as PDB files, and uploaded to Cluspro 2.0 (https://cluspro.org/).3 This computer server calculates interaction energies of billions of conformation models and outputs 10 protein–protein docking states. The top-ranked docking state was used as the CLCN2–HSPA8 docking model. The model was visualized using PyMOL software (Schrödinger, LLC).
Statistical analysis
Results are expressed as the mean ± S.E. The number of cells (n) used in each experiment is indicated in the figures and text. Statistical significance was determined using Mann-Whitney's U-tests, one-way ANOVA, Kruskal-Wallis test, or two-way ANOVA appropriately using Prism 8.1.0 (GraphPad Software, San Diego). A p value less than 0.05 was considered statistically significant and indicated with an asterisk in figures.
Author contributions
Y. Okamoto, Y. N., K. I., and K. O. conceptualization; Y. Okamoto resources; Y. Okamoto and Y. N. data curation; Y. Okamoto formal analysis; Y. Okamoto funding acquisition; Y. Okamoto investigation; Y. Okamoto, Y. N., Y. Obara, K. I., and D. T. methodology; Y. Okamoto and K. O. writing original draft; K. O. supervision; K. O. project administration; K. O. writing review and editing.
Supplementary Material
Acknowledgments
We thank Dr. Yuko Kozasa (Kurume University) for helpful comments and encouragement, and we thank Hayden Peacock (Edanz Group) for editing a draft of this manuscript.
This work was supported by JSPS KAKENHI (Grant-in-Aid for Research Activity Start-up Grant Number JP15H06006 to Y. Okamoto, and Grant-in-Aid for Young Scientists (B) Grant Number JP17H15554 to Y. Okamoto) by a grant-in-aid from the Nishinomiya Basic Research Foundation of Japan (to Y. Okamoto), and by collaboration with Ono Pharmaceutical Cooperation, Ltd. (Japan). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S4.
Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party hosted site.
- PV
- pulmonary vein
- AF
- atrial fibrillation
- CFTR
- cystic fibrosis transmembrane conductance regulator
- LV
- left ventricle
- ANOVA
- analysis of variance
- RyR
- ryanodine
- HRP
- horseradish peroxidase
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase.
References
- 1. Haïssaguerre M., Jaïs P., Shah D. C., Takahashi A., Hocini M., Quiniou G., Garrigue S., Le Mouroux A., Le Métayer P., and Clémenty J. (1998) Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 339, 659–666 10.1056/NEJM199809033391003 [DOI] [PubMed] [Google Scholar]
- 2. Chugh S. S., Havmoeller R., Narayanan K., Singh D., Rienstra M., Benjamin E. J., Gillum R. F., Kim Y. H., McAnulty J. H. Jr., Zheng Z. J., Forouzanfar M. H., Naghavi M., Mensah G. A., Ezzati M., and Murray C. J. (2014) Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation 129, 837–847 10.1161/CIRCULATIONAHA.113.005119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheung D. W. (1981) Electrical activity of the pulmonary vein and its interaction with the right atrium in the guinea pig. J. Physiol. 314, 445–456 10.1113/jphysiol.1981.sp013718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Doisne N., Maupoil V., Cosnay P., and Findlay I. (2009) Catecholaminergic automatic activity in the rat pulmonary vein: electrophysiological differences between cardiac muscle in the left atrium and pulmonary vein. Am. J. Physiol. Heart Circ. Physiol. 297, H102–H108 10.1152/ajpheart.00256.2009 [DOI] [PubMed] [Google Scholar]
- 5. Okamoto Y., Takano M., Ohba T., and Ono K. (2012) Arrhythmogenic coupling between the Na+–Ca2+ exchanger and inositol 1,4,5-triphosphate receptor in rat pulmonary vein cardiomyocytes. J. Mol. Cell. Cardiol. 52, 988–997 10.1016/j.yjmcc.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 6. Cheung D. W. (1981) Pulmonary vein as an ectopic focus in digitalis-induced arrhythmia. Nature 294, 582–584 10.1038/294582a0 [DOI] [PubMed] [Google Scholar]
- 7. Sood S., Chelu M. G., van Oort R. J., Skapura D., Santonastasi M., Dobrev D., and Wehrens X. H. (2008) Intracellular calcium leak due to FKBP12.6 deficiency in mice facilitates the inducibility of atrial fibrillation. Heart Rhythm. 5, 1047–1054 10.1016/j.hrthm.2008.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goette A., Staack T., Röcken C., Arndt M., Geller J. C., Huth C., Ansorge S., Klein H. U., and Lendeckel U. (2000) Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J. Am. Coll. Cardiol. 35, 1669–1677 10.1016/S0735-1097(00)00611-2 [DOI] [PubMed] [Google Scholar]
- 9. Shiroshita-Takeshita A., Brundel B. J., Burstein B., Leung T. K., Mitamura H., Ogawa S., and Nattel S. (2007) Effects of simvastatin on the development of the atrial fibrillation substrate in dogs with congestive heart failure. Cardiovasc. Res. 74, 75–84 10.1016/j.cardiores.2007.01.002 [DOI] [PubMed] [Google Scholar]
- 10. Zhang D., Chen X., Wang Q., Wu S., Zheng Y., and Liu X. (2017) Role of the MAPKs/TGF-β1/TRAF6 signaling pathway in postoperative atrial fibrillation. PLoS ONE 12, 1–12 10.1371/journal.pone.0173759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lian H., Wang X., Wang J., Liu N., Zhang L., Lu Y., Yang Y., and Zhang L. (2015) Heart-specific overexpression of (pro)renin receptor induces atrial fibrillation in mice. Int. J. Cardiol. 184, 28–35 10.1016/j.ijcard.2015.01.088 [DOI] [PubMed] [Google Scholar]
- 12. Li M., Yi X., Ma L., and Zhou Y. (2013) Hepatocyte growth factor and basic fibroblast growth factor regulate atrial fibrosis in patients with atrial fibrillation and rheumatic heart disease via the mitogen-activated protein kinase signaling pathway. Exp. Ther. Med. 6, 1121–1126 10.3892/etm.2013.1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ellinor P. T., Lunetta K. L., Albert C. M., Glazer N. L., Ritchie M. D., Smith A. V., Arking D. E., Müller-Nurasyid M., Krijthe B. P., Lubitz S. A., Bis J. C., Chung M. K., Dörr M., Ozaki K., Roberts J. D. et al. (2012) Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat. Genet. 44, 670–675 10.1038/ng.2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harada M., Luo X., Qi X. Y., Tadevosyan A., Maguy A., Ordog B., Ledoux J., Kato T., Naud P., Voigt N., Shi Y., Kamiya K., Murohara T., Kodama I., Tardif J. C., et al. (2012) Transient receptor potential canonical-3 channel-dependent fibroblast regulation in atrial fibrillation. Circulation 126, 2051–2064 10.1161/CIRCULATIONAHA.112.121830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Okamoto Y., Kawamura K., Nakamura Y., and Ono K. (2014) Pathological impact of hyperpolarization-activated chloride current peculiar to rat pulmonary vein cardiomyocytes. J. Mol. Cell. Cardiol. 66, 53–62 10.1016/j.yjmcc.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 16. Levesque P. C., Hart P. J., Hume J. R., Kenyon J. L., and Horowitz B. (1992) Expression of cystic fibrosis transmembrane regulator Cl− channels in heart. Circ. Res. 71, 1002–1007 10.1161/01.RES.71.4.1002 [DOI] [PubMed] [Google Scholar]
- 17. Verkerk A. O., Veldkamp M. W., Bouman L. N., and van Ginneken A. C. (2000) Calcium-activated Cl− current contributes to delayed after depolarizations in single Purkinje and ventricular myocytes. Circulation 101, 2639–2644 10.1161/01.CIR.101.22.2639 [DOI] [PubMed] [Google Scholar]
- 18. Duan D., Ye L., Britton F., Horowitz B., and Hume J. R. (2000) A novel anionic inward rectifier in native cardiac myocytes. Circ. Res. 86, E63–E71 [PubMed] [Google Scholar]
- 19. Britton F. C., Wang G.-L., Huang Z. M., Ye L., Horowitz B., Hume J. R., and Duan D. (2005) Functional characterization of novel alternatively spliced ClC-2 chloride channel variants in the heart. J. Biol. Chem. 280, 25871–25880 10.1074/jbc.M502826200 [DOI] [PubMed] [Google Scholar]
- 20. Sánchez-Rodríguez J. E., De Santiago-Castillo J. A., and Arreola J. (2010) Permeant anions contribute to voltage dependence of ClC-2 chloride channel by interacting with the protopore gate. J. Physiol. 588, 2545–2556 10.1113/jphysiol.2010.189175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Britton F. C., Hatton W. J., Rossow C. F., Duan D., Hume J. R., and Horowitz B. (2000) Molecular distribution of volume-regulated chloride channels (ClC-2 and ClC-3) in cardiac tissues. Am. J. Physiol. Heart Circ. Physiol. 279, H2225–H2233 10.1152/ajpheart.2000.279.5.H2225 [DOI] [PubMed] [Google Scholar]
- 22. Clark S., Jordt S. E., Jentsch T. J., and Mathie A. (1998) Characterization of the hyperpolarization-activated chloride current in dissociated rat sympathetic neurons. J. Physiol. 506, 665–678 10.1111/j.1469-7793.1998.665bv.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahmed N., Ramjeesingh M., Wong S., Varga A., Garami E., and Bear C. E. (2000) Chloride channel activity of ClC-2 is modified by the actin cytoskeleton. Biochem. J. 352, 789–794 [PMC free article] [PubMed] [Google Scholar]
- 24. Hinzpeter A., Lipecka J., Brouillard F., Baudoin-Legros M., Dadlez M., Edelman A., and Fritsch J. (2006) Association between Hsp90 and the ClC-2 chloride channel upregulates channel function. Am. J. Physiol. Cell Physiol. 290, C45–C56 10.1152/ajpcell.00209.2005 [DOI] [PubMed] [Google Scholar]
- 25. Kashino Y., Obara Y., Okamoto Y., Saneyoshi T., Hayashi Y., and Ishii K. (2018) ERK5 phosphorylates Kv4.2 and inhibits inactivation of the A-type current in PC12 cells. Int. J. Mol. Sci. 19, 2008 10.3390/ijms19072008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park E., and MacKinnon R. (2018) Structure of the CLC-1 chloride channel from Homo sapiens. Elife 7, 1–24 10.7554/eLife.36629.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benkert P., Biasini M., and Schwede T. (2011) Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 27, 343–350 10.1093/bioinformatics/btq662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jiang J., Prasad K., Lafer E. M., and Sousa R. (2005) Structural basis of interdomain communication in the Hsc70 chaperone. Mol. Cell. 20, 513–524 10.1016/j.molcel.2005.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Verba K. A., Wang R. Y., Arakawa A., Liu Y., Shirouzu M., Yokoyama S., and Agard D. A. (2016) Atomic structure of Hsp90–Cdc37–Cdk4 reveals that Hsp90 traps and stabilizes an unfolded kinase. Science 352, 1542–1547 10.1126/science.aaf5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dutzler R., Campbell E. B., Cadene M., Chait B. T., and MacKinnon R. (2002) X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature 415, 287–294 10.1038/415287a [DOI] [PubMed] [Google Scholar]
- 31. De Jesús Pérez J. J., Castro-Chong A., Shieh R., Hernández Carballo C. Y., de Santiago Castillos J. A., and Arreola J. (2016) Gating the glutamate gate of CLC-2 chloride channel by pore occupancy. J. Gen. Physiol. 1, 25–37 10.1085/jgp.201511424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Niemeyer M. I., Cid L. P., Zúñiga L., Catalán M., and Sepúlveda F. V. (2003) A conserved pore-lining glutamate as a voltage- and chloride-dependent gate in the ClC-2 chloride channel. J. Physiol. 553, 873–879 10.1113/jphysiol.2003.055988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Davis J. Q., and Bennett V. (1985) Human erythrocyte clathrin and clatrin-uncoating protein. J. Biol. Chem. 260, 14850–14856 [PubMed] [Google Scholar]
- 34. Chappell T. G., Welch W. J., Schlossman D. M., Palter K. B., Schlesinger M. J., and Rothman J. E. (1986) Uncoating ATPase is a member of the 70 kilodalton family of stress proteins. Cell 45, 3–13 10.1016/0092-8674(86)90532-5 [DOI] [PubMed] [Google Scholar]
- 35. Gevers M., Fracella F., and Rensing L. (1997) Nuclear translocation of constitutive heat-shock protein 70 during S phase in synchronous macroplasmodia of Physarum polycephalum. FEMS Microbiol. Lett. 152, 89–94 10.1111/j.1574-6968.1997.tb10413.x [DOI] [PubMed] [Google Scholar]
- 36. Diehl J. A., Yang W., Rimerman R. A., Xiao H., and Emili A. (2003) Hsc70 regulates accumulation of cyclin D1 and cyclin D1-dependent protein kinase. Mol. Cell. Biol. 23, 1764–1774 10.1128/MCB.23.5.1764-1774.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang Q., She H., Gearing M., Colla E., Lee M., Shacka J. J., and Mao Z. (2009) Regulation of neuronal survival factor MEF2D by chaperone-mediated autophagy. Science 323, 124–127 10.1126/science.1166088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mak S. K., McCormack A. L., Manning-Bog A. B., Cuervo A. M., and Di Monte D. A. (2010) Lysosomal degradation of α-synuclein in vivo. J. Biol. Chem. 285, 13621–13629 10.1074/jbc.M109.074617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hubbi M. E., Hu H., Kshitiz Ahmed I., Levchenko A., and Semenza G. L. (2013) Chaperone-mediated autophagy targets hypoxia-inducible factor-1α (HIF-1α) for lysosomal degradation. J. Biol. Chem. 288, 10703–10714 10.1074/jbc.M112.414771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patterson S. T., Li J., Kang J. A., Wickrema A., Williams D. B., and Reithmeier R. A. (2009) Loss of specific chaperones involved in membrane glycoprotein biosynthesis during the maturation of human erythroid progenitor cells. J. Biol. Chem. 284, 14547–14557 10.1074/jbc.M809076200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Faucette A. N., Maher V. A., Gutierrez M. A., Jucker J. M., Yates D. C., Welsh T. H. Jr., Amstalden M., Newton G. R., Nuti L. C., Forrest D. W., and Ing N. H. (2014) Temporal changes in histomorphology and gene expression in goat testes during postnatal development. J. Anim. Sci. 92, 4440–4448 10.2527/jas.2014-7903 [DOI] [PubMed] [Google Scholar]
- 42. DeGeer J., Kaplan A., Mattar P., Morabito M., Stochaj U., Kennedy T. E., Debant A., Cayouette M., Fournier A. E., and Lamarche-Vane N. (2015) Hsc70 chaperone activity underlies Trio GEF function in axon growth and guidance induced by netrin-1. J. Cell Biol. 210, 817–832 10.1083/jcb.201505084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meacham G. C., Patterson C., Zhang W., Younger J. M., and Cyr D. M. (2001) The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nat. Cell Biol. 3, 100–105 10.1038/35050509 [DOI] [PubMed] [Google Scholar]
- 44. Li P., Kurata Y., Maharani N., Mahati E., Higaki K., Hasegawa A., Shirayoshi Y., Yoshida A., Kondo T., Kurozawa Y., Yamamoto K., Ninomiya H., and Hisatome I. (2015) E3 ligase CHIP and Hsc70 regulate Kv1.5 protein expression and function in mammalian cells. J. Mol. Cell. Cardiol. 86, 138–146 10.1016/j.yjmcc.2015.07.018 [DOI] [PubMed] [Google Scholar]
- 45. Jeworutzki E., López-Hernández T., Capdevila-Nortes X., Sirisi S., Bengtsson L., Montolio M., Zifarelli G., Arnedo T., Müller C. S., Schulte U., Nunes V., Martínez A., Jentsch T. J., Gasull X., Pusch M., and Estévez R. (2012) GlialCAM, a protein defective in a leukodystrophy, serves as a ClC-2 Cl− channel auxiliary subunit. Neuron 73, 951–961 10.1016/j.neuron.2011.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Leegwater P. A., Yuan B. Q., van der Steen J., Mulders J., Könst A. A., Boor P. K., Mejaski-Bosnjak V., van der Maarel S. M., Frants R. R., Oudejans C. B., Schutgens R. B., Pronk J. C., and van der Knaap M. S. (2001) Mutations of MLC1 (KIAA0027), encoding a putative membrane protein, cause megalencephalic leukoencephalopathy with subcortical cysts. Am. J. Hum. Genet. 68, 831–838 10.1086/319519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Capdevila-Nortes X., Jeworutzki E., Elorza-Vidal X., Barrallo-Gimeno A., Pusch M., and Estévez R. (2015) Structural determinants of interaction, trafficking and function in the ClC-2/MLC1 subunit GlialCAM involved in leukodystrophy. J. Physiol. 593, 4165–4180 10.1113/JP270467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jeworutzki E., Lagostena L., Elorza-Vidal X., López-Hernández Estévez R., and Pusch M. (2014) GlialCAM, a CLC-2 Cl− channel subunit, activates the slow gate of CLC chloride channels. Biophys. J. 107, 1105–1116 10.1016/j.bpj.2014.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yamada T., Krzeminski M., Bozoky Z., Forman-Kay J. D., and Strange K. (2016) Role of CBS and Bateman domains in phosphorylation-dependent regulation of a CLC anion channel. Biophys. J. 111, 1876–1886 10.1016/j.bpj.2016.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.